Abstract
Activation of nuclear β-catenin and expression of its transcriptional targets promotes chronic myeloid leukemia (CML) progression, tyrosine kinase inhibitor (TKI) resistance, and leukemic stem cell self-renewal. We report that nuclear β-catenin plays a role in leukemia cell-intrinsic but not -extrinsic BCR-ABL1 kinase-independent TKI resistance. Upon imatinib inhibition of BCR-ABL1 kinase activity, β-catenin expression was maintained in intrinsically resistant cells grown in suspension culture and sensitive cells cultured in direct contact (DC) with bone marrow (BM) stromal cells. Thus, TKI resistance uncouples β-catenin expression from BCR-ABL1 kinase activity. In β-catenin reporter assays, intrinsically resistant cells showed increased transcriptional activity versus parental TKI-sensitive controls, and this was associated with restored expression of β-catenin target genes. In contrast, DC with BM stromal cells promoted TKI resistance, but had little effects on Lef/Tcf reporter activity and no consistent effects on cytoplasmic β-catenin levels, arguing against a role for β-catenin in extrinsic TKI resistance. N-cadherin or H-cadherin blocking antibodies abrogated DC-based resistance despite increasing Lef/Tcf reporter activity, suggesting that factors other than β-catenin contribute to extrinsic, BM-derived TKI resistance. Our data indicate that, while nuclear β-catenin enhances survival of intrinsically TKI-resistant CML progenitors, it is not required for extrinsic resistance mediated by the BM microenvironment.
Keywords: Chronic myeloid leukemia, β-catenin, Tyrosine kinase inhibitors, Bone marrow microenvironment
INTRODUCTION
Chronic myeloid leukemia (CML) is driven by the BCR-ABL1 oncoprotein, resulting from the t(9;22)(q34;q11) chromosomal translocation.1 CML typically presents in chronic phase (CP-CML), and without treatment, CP-CML progresses to an acute leukemia termed blastic phase (BP-CML).(2) Tyrosine kinase inhibitors (TKIs) such as imatinib, nilotinib and dasatinib, induce durable responses in most patients with CP-CML.3–5 However, 10–20% of newly diagnosed CP-CML patients exhibit primary or acquired resistance to frontline TKI therapy, and responses in patients with BP-CML are usually transient.6–8 TKI resistance is often due to reactivation of BCR-ABL1 through point mutations that impair drug binding.9,10 Additional patients develop TKI resistance despite sustained suppression of BCR-ABL1 kinase activity, termed BCR-ABL1 kinase-independent resistance.2,11 Conceptually, activation of alternative signaling pathways may occur through cell-autonomous mechanisms (intrinsic to the leukemia cells) or by extrinsic factors from the bone marrow (BM) microenvironment. Consistent with the latter, CML cells co-cultured with human BM stromal cells are partially protected from the effects of BCR-ABL1 kinase inhibition.12–15 Various pathways are implicated in extrinsic resistance, including JAK/STAT11–13 and CXCR4/CXCL12,16,17 and evidence suggests that extrinsic factors promote survival of primitive CML cells despite TKI inhibition of BCR-ABL1, resulting in disease persistence.18,19
The Wnt-β-catenin pathway is a central signaling system in mammalian development and cellular homeostasis.20 β-catenin exists in distinct nuclear and cytoplasmic pools that fulfill multiple roles.20 Nuclear β-catenin acts as a transcriptional co-activator in complex with lymphoid enhancing factor (Lef)/T-cell factor (Tcf), thereby activating target genes such as axin2.21 Cytoplasmic β-catenin connects the intracellular portion of classical cadherins to α-catenin and actin filaments, thereby connecting cadherins engaged in cell-cell junctions to the cytoskeleton.22
Wnt-β-catenin signaling is involved in several critical aspects of CML pathogenesis. Murine studies implicate β-catenin in CML stem cell maintenance in the presence of TKIs.23,24 Granulocyte-macrophage progenitor cells (GMPs) from patients with myeloid BP-CML can acquire self-renewal capacity through nuclear β-catenin signaling.25,26 Activation of a β-catenin-directed transcriptional program has also been linked to TKI resistance and CML persistence in murine disease models and primary human CML cells.14,26–30 However, the mechanism β-catenin activation in each of these scenarios is not well understood. In BP-CML, expression of an abnormally spliced glycogen synthase kinase 3β (GSK3β) lacking catalytic activity leads to β-catenin stabilization.31 Another intrinsic mechanism of β-catenin activation is increased expression of GAS2, an inhibitor of the protease, calpain, resulting in cleavage of β-catenin.32 In primitive CML cells, BCR-ABL1 can activate JAK2 in a kinase-independent manner, resulting in β-catenin stabilization,30 and Alox5 upregulates β-catenin expression in a kinase-independent manner in murine models of CML.28 Finally, it has been reported that microenvironmental factors activate nuclear β-catenin, including N-cadherin and engagement of CD27 by its ligand, CD70.33,34
Here, we use shRNA-mediated β-catenin knockdown, Lef/Tcf reporter assays, immunofluorescence, and nucleocytoplasmic fractionation in isogenic imatinib-sensitive and -resistant CML cell lines, as well as imatinib-naïve and -resistant primary CML CD34+ cells, to dissect the role β-catenin in BCR-ABL1 kinase-independent TKI resistance. Our results implicate nuclear β-catenin in intrinsic, cell autonomous TKI resistance, but not extrinsic, bone marrow-derived TKI resistance.
MATERIALS AND METHODS
Cell cultures and primary cells
TKI-resistant K562R and AR230R cell lines have been described and were maintained in continuous 1.0 μM imatinib (TKI-sensitive K562S and AR230S cells served as controls).11 Derivative lines were generated by retroviral or lentiviral infection, followed by antibiotic selection or sorting for GFP+ or RFP+ cells. All cell lines were confirmed to be negative for mycoplasma contamination using the MycoAlert Mycoplasma Detection Kit (Lonza, Walkersville, MD, USA). The CD34+ fraction (purity >90%, Mitenyi Biotech, San Diego, CA, USA) of peripheral blood (PB) mononuclear cells (MNCs) from patients with CP-CML was selected and kept overnight with cytokines (1X CC100; StemCell Technologies, Vancouver, BC, Canada) prior to use in inhibitor treatment assays. Isolation of primary mesenchymal stromal cells (MSCs) have been previously described.35 Donors gave informed consent and studies were approved by The University of Utah Institutional Review Board (IRB). For details on primary specimens see Supplementary Table S1.
Stromal cell protection assays
K562S and AR230S cell lines (1.5x105 cells/mL) or CMLCD34+ progenitors from newly diagnosed patients (106 cells/mL) were cultured in regular medium (RM), HS-5 conditioned medium (CM), or in direct contact (DC) with HS-5 cells for 36 h or as otherwise indicated, followed by plating of equal numbers of viable cells in methylcellulose semisolid medium and/or immunoblot analyses.12,13 Where indicated, similar assays were performed in DC with primary MSCs harvested from normal donors for allogeneic stem cell transplantation.35 Anti-N-cadherin (GC-4, Sigma, St. Louis, MO), anti-H-cadherin (LifeSpan Biosciences, Inc., Seattle, WA), or isotype-matched control antibodies (Santa Cruz Biotechnology, Inc., Dallas, TX) were added at 40 μg/mL and pre-incubated with both HS-5 cells and CML cells for 3 h prior to plating in co-cultures. Antibodies remained in the medium throughout the 36 h protection assay prior to functional analyses. For details see Supplementary Materials and Methods.
Short hairpin RNA (shRNA) constructs
Apoptosis and proliferation assays
Following in vitro culture in RM or HS-5 DC, apoptosis was assayed by flow cytometric analyses. For details see Supplementary Materials and Methods.
Clonogenic assays
Methylcellulose colony assays were performed by plating CML cell lines or patient samples in 0.9% MethoCult (H4230; Stem Cell Technologies). For details see Supplementary Materials and Methods.
Immunoblot analysis
CML cell lines (1.5x105 cells/mL) or patient samples (1.0x106 cells/mL) were cultured in an equal volume of either RM or HS-5 CM alone or overlaid on HS-5 or primary MSC stroma (65% confluent), and treated with imatinib for 24–36 h without exogenous cytokines. Following TKI exposure, cells were lysed (0°C; 30 min.) in 30 μL RIPA buffer (150 mM NaCl, 1% NP40, 1% SDS, 50 mM Tris [pH 8.0]) containing protease (Complete Mini, Roche, Basel, Switzerland) and phosphatase (PhosStop, Roche) inhibitors, or lysed directly in 20 μL Laemmli buffer. Samples were denatured (100°C; 10 min) prior to SDS-PAGE and transferred to nitrocellulose membranes. Antibodies used were: mouse anti-β-catenin (#610154) and mouse anti-GRB2 (#610112; BD Transduction Laboratories); mouse anti-c-ABL (#OP20; Calbiochem); rabbit anti-pABL (#2865) and rabbit anti-WNT5A (#2392; Cell Signaling Technology, Danvers, MA, USA); mouse anti-α-tubulin (#T5168; Sigma-Aldrich); rabbit anti-lamin B (#ab41068; Abcam).
Gene expression microarrays
Amplified and labeled cDNA from HS-5, HS-23, and HS-27a cells were hybridized to a Human Gene 1.0 ST array. Image processing was performed using Affymetrix Command Console (AGCC) v.2.0.0.1029 software and expression analysis was performed using Affymetrix Expression Console v.1.1 software. Microarray assays were performed in the OHSU Gene Microarray Shared Resource (Portland, OR). For details see Supplementary Materials and Methods.
Nucleocytoplasmic fractionation
Cells were kept on ice and centrifugations were done at 4°C. 3x106–107 cells were washed twice with ice cold PBS followed by suspension in solution A (HEPES 10 mM, MgCl2 6H2O 1.5 mM, KCl 10 mM, DTT 0.5 mM, pH: 7.9, 10 min). Cells were centrifuged at 1000g and resuspended in 350 μl of solution A′ (Solution A plus 0.2% NP-40, 20 min) supplemented with protease inhibitors (Complete Mini, Roche). Lysis of cell membranes with preservation of nuclei was confirmed by microscopy. The cytoplasmic supernatant was collected after centrifugation at 13,000 rpm for 2 minutes. Nuclei were lysed in RIPA buffer (see above), and lysates were analyzed by immunoblot analyses. Antibodies against α-tubulin (Cell Signaling Technology) and lamin B (Abcam, Cambridge, MA, USA) were used as controls for the purity of cytoplasmic and nuclear fractions, respectively.
Immunofluorescence
Following the indicated treatment conditions, cells were fixed, permeabilized, and incubated with mouse anti-β-catenin (#2677; Cell Signaling Technology), followed by detection using an AlexaFluor 488-conjugated goat anti-mouse IgG (Invitrogen, Grand Island, NY, USA). Slides were examined using an Axioskop 2 mot plus equipped with an AxioCam microscope camera (Carl Zeiss Microscopy, LLC, Thornwood, NY, USA).
Lef/Tcf reporter assay
To detect endogenous β-catenin transcriptional activity, CML cell lines and CD34+ patient samples were lentivirally transduced with the pGreenFire Lenti-Reporter system (pGF1; System Biosciences, Mountain View, CA, USA) harboring eight sequential β-catenin-inducible elements or negative control sequences. For details see Supplementary Materials and Methods.
Statistical analyses
A two-tailed Student’s t test was used for assays with identical cell lines and CMLCD34+ patient samples. Data were considered statistically different when p values were <0.05. To ensure adequate statistical power, all data represent three independent experiments unless otherwise stated.
RESULTS
Imatinib does not reduce β-catenin protein levels in CML cells with intrinsic or extrinsic BCR-ABL1 kinase-independent TKI resistance
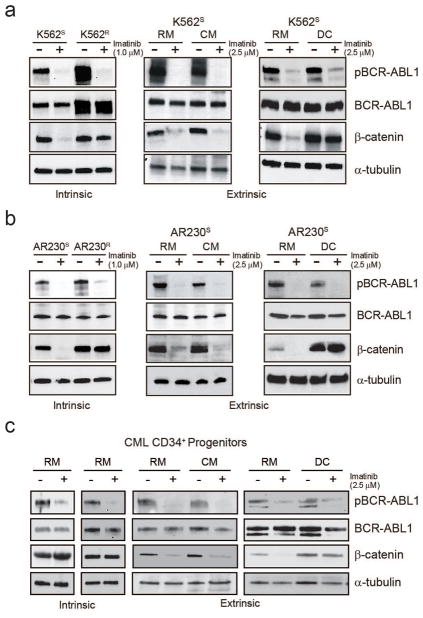
β-catenin is implicated in CML progression and TKI resistance, but underlying mechanisms remain under study.14,26,27,29,36 To dissect the contributions of β-catenin to intrinsic and extrinsic TKI resistance in CML, we began with pairs of isogenic CML cell lines. We modeled intrinsic resistance using K562R and AR230R cells, which are adapted for growth in 1.0 μM imatinib and also exhibit resistance to dasatinib and nilotinib.11 We also tested CD34+ progenitors from CP-CML patients who had failed treatment with ≥2 TKIs but lack clinically reported BCR-ABL1 kinase domain mutations. To model resistance imparted by the BM microenvironment, we cultured K562S and AR230S cells, as well as CD34+ progenitors from newly diagnosed CP-CML patients, in CM or DC with HS-5 BM stromal cells.11–13 Sequencing confirmed that all primary cells and cell lines expressed exclusively native BCR-ABL1.11 Immunoblot analysis revealed that imatinib treatment reduced β-catenin protein expression to low levels in TKI-sensitive K562S and AR230S cells. However, β-catenin protein expression was unaffected by imatinib treatment in intrinsically TKI-resistant K562R and AR230R cells (Figures 1a and 1b, left), as well as CD34+ cells from TKI-resistant patients (Figure 1c, left). In extrinsic resistance, K562S and AR230S cells maintained β-catenin expression when plated in DC with HS-5 cells in the presence of imatinib (Figures 1a and 1b, right), but not when cultured in RM or HS-5 CM (Figures 1a and 1b, middle), with similar results in CML CD34+ cells from newly diagnosed CML patients (Figure 1c, middle and right). These data demonstrate that β-catenin expression is maintained in both intrinsic and extrinsic BCR-ABL1 kinase-independent TKI resistance.14 Therefore, in contrast to imatinib-sensitive cells, imatinib-resistant cells have uncoupled β-catenin expression from BCR-ABL1 kinase activity.
Figure 1. β-catenin protein expression is upregulated in CML cells under conditions of TKI resistance.
a–c. Immunoblot analyses revealed increased levels of β-catenin protein in TKI-resistant K562R (a, n=6), AR230R (b, n=4), and CML CD34+ cells from TKI-resistant patients lacking BCR-ABL1 kinase domain mutations (c, n=3), all of which exhibit BCR-ABL1 kinase-independent TKI resistance (left). β-catenin protein levels are also maintained in parental K562S (a, n=3) and AR230S (b, n=3) cells, as well as CML CD34+ cells from newly diagnosed patients (c, n=3), when cultured in direct contact (DC) with HS-5 bone marrow (BM) stromal cells in the presence of 2.5 μM imatinib (right), but not when cultured in HS-5 conditioned medium (CM) (middle).
Nuclear β-catenin contributes to intrinsic BCR-ABL1 kinase-independent TKI resistance
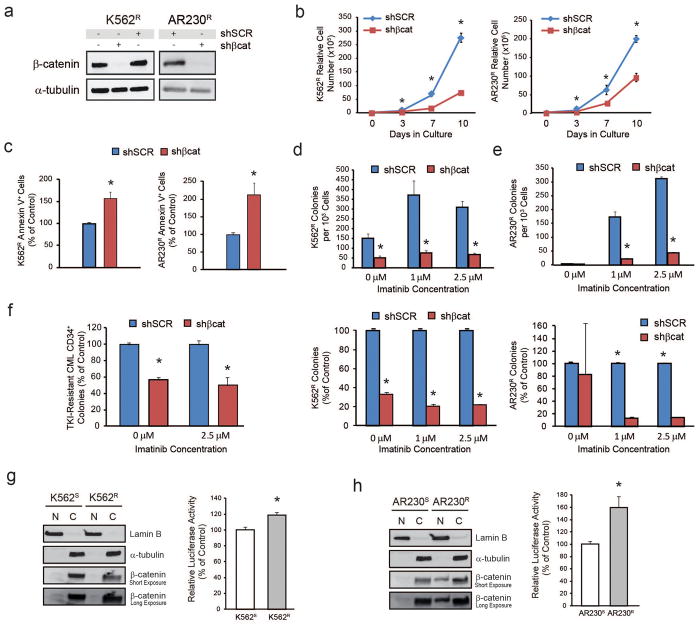
To further investigate β-catenin in TKI resistance, we constructed a lentiviral shRNA vector targeting β-catenin (shβcat) or a scrambled control (shSCR). Immunoblot confirmed >90% β-catenin knockdown in K562S and AR230S cells that was maintained for at least 10 days (Supplementary Figure S1a). shβcat reduced proliferation of K562S and AR230S cells cultured in exponential growth conditions (Supplementary Figure S1b), as well as increased apoptosis, and reduced clonogenicity (Supplementary Figures S1c–d). Despite the striking down-regulation of β-catenin expression in the presence of imatinib (Figure 1a and 1b, left), shβcat but not shSCR further increased apoptosis (Supplementary Figure S1c) and reduced colony formation (Supplementary Figure S1d), suggesting that residual β-catenin promotes survival in the presence of imatinib.
To assess the role of β-catenin in intrinsic TKI resistance, K562R and AR230R cells were infected with shβcat or shSCR. β-catenin knockdown (Figure 2a) reduced proliferation and clonogenicity and increased apoptosis (Figure 2b–e). Effects of shβcat appeared more pronounced in the presence of imatinib, although a direct comparison is difficult due to the detrimental impact of imatinib withdrawal, as described.11,37 shβcat also reduced colony formation by CD34+ cells from patients with clinical TKI resistance expressing native BCR-ABL1 (Figure 2f). These data implicate β-catenin in intrinsic TKI resistance of CML cell lines and primary cells.
Figure 2. shβcat reduces survival of CML cells with intrinsic BCR-ABL1 kinase-independent TKI resistance in the presence of imatinib.
a. Immunoblot analysis reveals near complete reduction (>90%) of β-catenin protein levels upon lentiviral delivery of shβcat into K562R (left, n=3) and AR230R (right, n=3) cells. b–e. shβcat results in reduced in vitro growth (b) and increased apoptosis (c) in the presence of 1 μM imatinib, as well as reduced colony formation (d–e) in semisolid medium in K562R (left, n=3) and AR230R (right, n=4) cells. f. shβcat also reduced colony formation of CML CD34+ cells from patients exhibiting clinical resistance to multiple TKIs but harboring no BCR-ABL1 kinase domain mutations both with and without imatinib (n=3). g–h. Nucleocytoplasmic fractionation and/or luciferase reporter assays revealed enhanced β-catenin transcriptional activity in intrinsically TKI resistant K562R (g, n=3) and AR230R (h, n=3) cell lines compared to parental TKI-sensitive controls. Bars represent standard error of the mean (SEM). *p<0.05.
To better understand the relative contributions of nuclear and cytoplasmic β-catenin to intrinsic TKI resistance, we performed nucleocytoplasmic fractionation. β-catenin was almost exclusively cytoplasmic in K562S and K562R cells (Figure 2g, left). However, in Lef/Tcf reporter assays, K562R cells exhibited a 19% increase of luciferase reporter activity compared to K562S cells (p<0.05) (Figure 2g, right). AR230R cells demonstrated higher expression of nuclear β-catenin than AR230S cells (Figure 2h, left) and a 60% increase of luciferase reporter activity (p<0.05) (Figure 2h, right). We also tested the mRNA expression of β-catenin target genes. Survivin expression was downregulated in K562S and AR230S cells treated with 1 μM imatinib, but restored in K562R and AR230R cells (Supplementary Figure S2a). A similar pattern was seen for axin2 expression in K562S and K562R cells (Supplementary Figure S2b). While axin2 was undetectable in AR230S cells, it was readily detectable in AR230R cells cultured in 1 μM imatinib (not shown). No consistent regulation was seen for cyclin D1 (Supplementary Figure S2c). Of these three known β-catenin transcriptional targets, only mRNA encoding survivin was consistently downregulated in shβcat- versus shSCR-expressing K562R and AR230R cells (Supplementary Figure S2d). Altogether, these data suggest that, upon inhibition of BCR-ABL1 kinase activity, intrinsically TKI resistant CML cells are able to maintain a high levels of nuclear β-catenin transcriptional activity, which promotes survival and colony formation (for a schematic diagram see Supplementary Figure S2e).
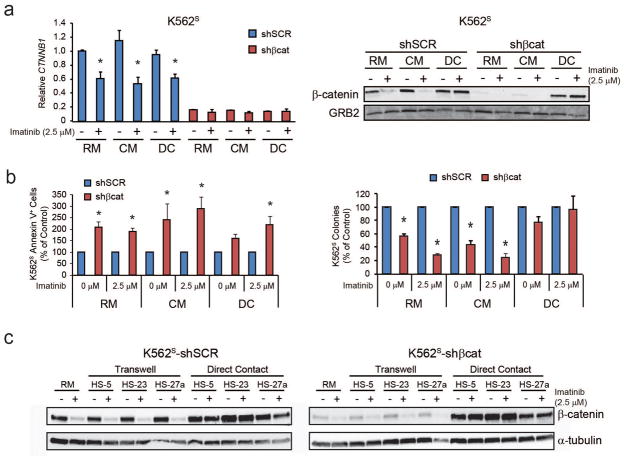
Direct contact with HS-5 stromal cells overrides shRNA-mediated β-catenin knockdown
To interrogate the role of β-catenin in TKI resistance mediated by the BM microenvironment, K562S cells were infected with shSCR or shβcat, and the resulting GFP+ cells were cultured in RM or HS-5 DC with and without 2.5 μM imatinib. Since HS-5 DC enhances β-catenin protein expression compared to cells grown in RM or HS-5 CM (Figure 1), we hypothesized that shRNA-mediated β-catenin knockdown would abolish the protective effects of HS-5 DC. As expected, shβcat reduced β-catenin mRNA to low levels across all conditions (Figure 3a, left). Unexpectedly, DC with HS-5 cells fully restored β-catenin protein levels in shβcat-expressing K562S cells (Figure 3a, right). While shβcat slightly increased apoptosis of K562S cells cultured in HS-5 DC with imatinib (Figure 3b, left), colony formation was completely restored (Figure 3b, right). Restoration of β-catenin protein expression in the presence of shβcat was not specific to HS-5 cells, and also occurred with the related HS-23 and HS-27a human BM stromal lines (Figure 3c).38 Cell-cell interactions mediated by integrins, selectins, and cadherins, but not immunoglobulin superfamily proteins such as CD34, are calcium-dependent.39,40 We therefore assayed DC-mediated β-catenin stabilization in K562S cells in the presence of EGTA and found that β-catenin stability was reduced (Supplementary Figure S3). Altogether, these data suggest that direct contact with BM stromal cells can stabilize β-catenin protein at the posttranscriptional level in a calcium-dependent manner, and that this stabilization is associated with imatinib resistance.
Figure 3. shRNA-mediated β-catenin knockdown is abolished at the protein but not mRNA level when K562S cells are co-cultured in direct contact (DC) with HS-5 BM stromal cells.
a. Immunoblot analysis demonstrates restored β-catenin protein levels in K562S-shβcat-expressing cells under conditions of co-culture in DC with HS-5 BM stromal cells (right, n=5). qRT-PCR confirmed maintenance of the knockdown at the mRNA level (left, n=2). Bars represent SEM and qRT-PCR data were normalized to expression of GUS. *p<0.05. b. shβcat increased apoptosis (left, n=3) and reduced colony formation (right, n=3) of K562S cells cultured in regular medium (RM) or HS-5 conditioned medium (CM), but these effects were reduced or abolished when cultured in DC with HS-5 BM stromal cells. Bars represent SEM. *p<0.05. c. Immunoblot analysis demonstrates restored β-catenin protein levels in K562S-shβcat-expressing cells under conditions of co-culture in DC with HS-23 and HS-27a BM stromal cells, which are closely related to the HS-5 cells used in these studies (n=2).
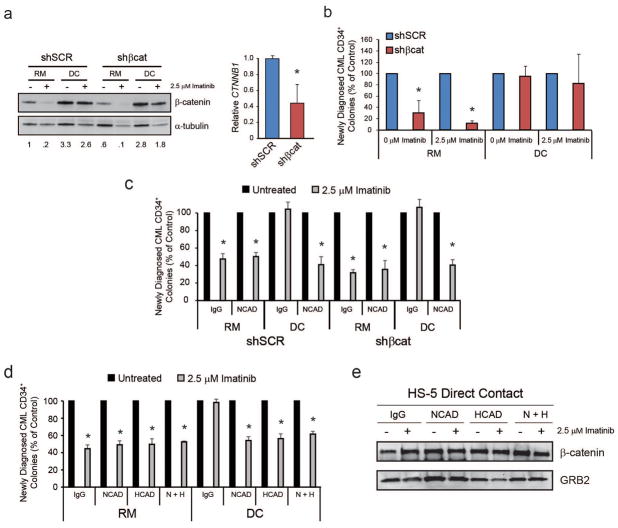
Blocking cadherin engagement restores the effects of imatinib and shβcat on CML CD34+ cells
β-catenin stabilization may occur through several cell-extrinsic mechanisms. In the canonical WNT/β-catenin pathway, activating WNT family members bind to cell surface receptors, including LRP/Frizzled, to sequester axin/GSK3β from the β-catenin destruction complex, thereby stabilizing β-catenin for entry into the nucleus.8 In contrast, when β-catenin engages the intracellular domain of classical cadherins, it is removed from the cytoplasmic destruction complex and becomes stabilized at the cell membrane.41,42 Non-classical cadherins exhibit different domain structures and not all have intracellular domains to bind β-catenin.42 To determine the mechanism of HS-5-mediated β-catenin stabilization, we performed mRNA microarray analysis on HS-5, HS-23 and HS-27a stromal cells, all of which stabilized β-catenin in the presence of imatinib (Figure 3c). N-cadherin and H-cadherin were highly expressed by all three stromal cell lines, while other cadherins were either absent or not consistently expressed (Supplementary Figure S4a). Of the WNT proteins, only WNT5A was highly expressed in all three stromal lines, while expression of activating WNT proteins (WNT1, WNT3, WNT3A, and WNT7A43) was low or absent (Supplementary Figure S4b). Expression of integrin family members was even across the three cell lines (Supplementary Figure S4c).
To confirm our cell line data in patient samples, CML CD34+ samples were transduced with either shSCR or shβcat and the knockdown was confirmed by immunoblot and/or qRT-PCR analyses (Figure 4a). Consistent with results in K562S cells, culture of shβcat-expressing CML CD34+ cells in HS-5 DC restored colony formation in the presence of imatinib (Figure 4b). Since N-cadherin was reported to interact with β-catenin in the context of BM-mediated TKI resistance, and is also highly expressed in our HS-5 stromal cell line (Supplementary Figure S4a), we next cultured CML CD34+ cells expressing shSCR or shβcat in RM or HS-5 DC in the presence or absence of 2.5 μM imatinib with either an N-cadherin blocking antibody or an IgG control. Addition of anti-N-cadherin to co-cultures of CML CD34+ cells expressing shβcat restored imatinib inhibition of colony formation (Figure 4c). As H-cadherin was also highly expressed on all of the stromal lines (Supplementary Figure 4a), we also tested the survival effects of blocking H-cadherin alone or in combination with N-cadherin. For these experiments, CML CD34+ cells from newly diagnosed patients and HS-5 BM stromal cells were pre-incubated for 3 h with anti-N- and/or anti-H-cadherin or an IgG control, and cultured in either RM or HS-5 DC for 36 h with and without imatinib followed by colony forming assays. H-cadherin block reduced colony formation to a similar degree as N-cadherin block, but the combination was no more efficient than each single antibody (Figure 4d), suggesting redundant roles for both cadherins in protecting CML cells from TKI-mediated cell death. However, neither H- nor N-cadherin block had any effect on β-catenin protein expression (Figure 4e).
Figure 4. The effects of HS-5 co-culture are confirmed in CD34+ cells from newly diagnosed CML patients, and treatment of cells with antibodies blocking N-cadherin or H-cadherin reduces survival under conditions of direct contact.
a. Immunoblot analysis revealed restored β-catenin protein expression in shβcat-expressing CML CD34+ cells cultured in HS-5 DC (left, n=3). qRT-PCR confirmed β-catenin knockdown at the mRNA level (right, n=3). Immunoblots were normalized to α-tubulin and qRT-PCR was normalized to GUS. b. Consistent with restored β-catenin protein levels, HS-5 DC also restored colony forming ability of shβcat-expressing CML CD34+ cells under scenarios of DC in the presence of imatinib (n=4). c. Addition of an N-cadherin blocking antibody to co-cultures restored the effects of imatinib and shβcat on colony formation of CML CD34+ cells grown in HS-5 DC (n=3). d,e. Addition of antibodies blocking N-cadherin (n=9), H-cadherin (n=8), or both (n=6), to HS-5 co-cultures restored the effects of imatinib on CML CD34+ cells cultured under HS-5 DC in the presence of imatinib (d), but did not have an effect on β-catenin protein levels as demonstrated by immunoblot analyses (e). Bars represent SEM. *p<0.05.
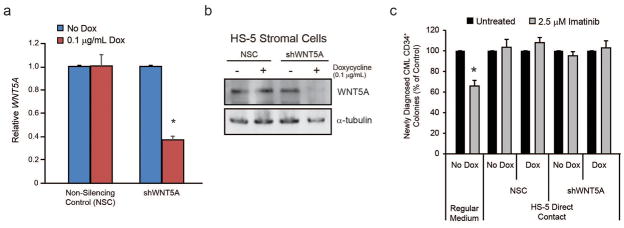
WNT signaling stabilizes β-catenin through the canonical pathway,44 and WNT5A was the only WNT protein highly expressed in all BM stromal cell lines (Supplementary Figure S4b). Depending on receptor context, WNT5A inhibits or activates canonical β-catenin signaling.43 We therefore expressed doxycycline-inducible shWNT5A in HS-5 stromal cells and tested whether WNT5A knockdown would impact DC-mediated TKI protection. WNT5A knockdown was confirmed by qRT-PCR and immunoblot analysis (Figures 5a and 5b). However, downregulation of WNT5A had no effect on HS-5-mediated protection of CML CD34+ cells cultured with 2.5 μM imatinib (Figure 5c). Together with the fact that HS-5 and related stromal lines do not express appreciable levels of activating WNT proteins, this suggests that the protective effects of HS-5 cells toward CML CD34+ cells are mediated by factors other than WNTs.
Figure 5. WNT5A has no effect on survival of CML CD34+ cells cultured in HS-5 DC.
a–b. shRNA-mediated knockdown of human WNT5A in HS-5 BM stromal cells was confirmed by both qRT-PCR (a, n=3) and immunoblot (b, n=2) analyses in the presence but not absence of doxycycline (0.1 μg/mL). As expected, the non-silencing control (NSC) did not induce a knockdown, whereas shWNT5A induced ~60% knockdown at both the mRNA and protein level. c. Co-culture of CML CD34+ cells with shWNT5A-expressing HS-5 cells in the presence of doxycycline had no effect on colony forming ability in the presence of imatinib (n=3). Bars represent SEM. *p<0.05.
Cadherin engagement reduces nuclear β-catenin activity in CML CD34+ cells grown in contact with HS-5 stromal cells
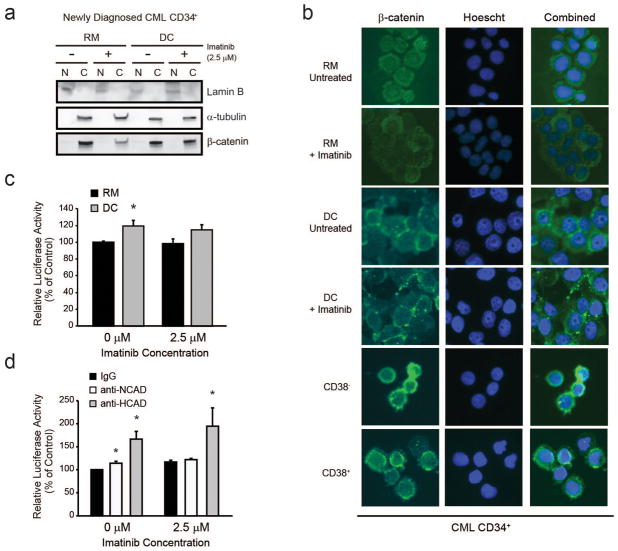
Canonical WNT/β-catenin signaling stabilizes nuclear β-catenin, thereby activating transcription of target genes. In contrast, the effects of cadherins on β-catenin transcriptional activity are dependent on the specific cadherin and cell context.45 We investigated the intracellular localization of β-catenin in extrinsic TKI resistance mediated by HS-5 cells. In three independent experiments, nucleocytoplasmic fractionation and immunofluorescence analyses revealed that β-catenin was found primarily within the cytoplasm of CD34+ cells from newly diagnosed CP-CML patients cultured in either RM or HS-5 DC (Figure 6a–b and Supplementary Figure S5a). Similar results were obtained in primitive CD34+38− CML stem cells and more committed CD34+38+ progenitors (Figure 6b). Nuclear β-catenin expression was also low in K562S cells (Supplementary Figure S5b) and other imatinib-sensitive CML cell lines, while nuclear β-catenin expression was high in HCT116 colon cancer cells (Supplementary Figure S5c).46 As expected, imatinib reduced cytoplasmic β-catenin in cells grown in RM but not in DC (Figure 6a, 6b, and Supplementary Figure S5a). In one patient specimen, low levels of nuclear β-catenin in cells growing in RM were increased in DC, but this increase was abolished by imatinib treatment (Supplementary Figure S5a, Patient #1). In the other patient specimens, nuclear β-catenin remained low to undetectable in all conditions (Figure 6a and Supplementary Figure S5a). We next expressed a β-catenin reporter construct in CML CD34+ cells and measured luciferase reporter activity when cells were cultured with and without HS-5 DC. Reporter activity was slightly increased by 21% in DC over controls in the absence of imatinib (Figure 6c, p<0.05, n=4) and by 11% in the presence of imatinib (Figure 6c, p=0.1, n=4). Thus, despite the pronounced HS-5-induced stabilization of total β-catenin, there was minimal change in β-catenin-dependent transcription. Also, HS-5 DC had no consistent effects on the mRNA expression of the β-catenin target genes axin2, survivin and cyclin D1 (data not shown). Lastly, we measured luciferase reporter activity in CML CD34+ cells cultured in HS-5 DC with anti-N- or anti-H-cadherin both with and without imatinib. In contrast to their inhibitory effects on colony formation (Figures 4c and 4d), N-cadherin blocking antibody caused a 10-15% (p<0.05) and H-cadherin blocking antibody a 30-70% (p<0.01) increase in luciferase reporter activity (Figure 6d), suggesting that cadherin engagement reduces nuclear β-catenin transcriptional activity. Accordingly, blocking of N- or H-cadherin engagement resulted in upregulation of the β-catenin target gene, survivin (Supplementary Figure S6). Altogether, these data suggest that the protective effects of HS-5 DC are dependent on N- and H-cadherin engagement, but not activation of nuclear β-catenin.
Figure 6. Nucleocytoplasmic fractionation, luciferase reporter activity, and immunofluorescence reveal minimal nuclear β-catenin transcriptional activity under conditions of extrinsic TKI resistance.
a,b. Nucleocytoplasmic fractionation (a, n=3) and immunofluorescence staining with a pan-β-catenin antibody (b, n=3) confirms that β-catenin is located primarily within the cytoplasm of CD34+ cells from newly diagnosed CML patients, when cultured in RM or HS-5 DC, and when sorted for the CD34+38− or CD34+38+ fractions. c,d. Luciferase reporter assays revealed that transcriptional activity is only minimally increased by HS-5 DC (b, n=5), and that addition of N-cadherin (n=3) or H-cadherin (n=3) blocking antibodies to HS-5 co-cultures results in a slight increase of luciferase reporter activity, while at the same time reducing colony forming ability (see Figure 4). d. Collectively, these data suggest that β-catenin transcriptional activity under conditions of DC does not correlate with survival. Bars represent SEM. *p<0.05.
β-catenin protein is dispensable for BM-mediated protection of CML progenitor cells from the effects of imatinib
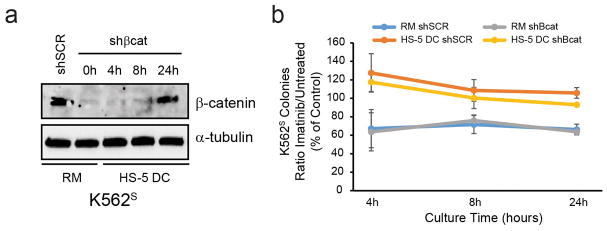
Since β-catenin expression at 24h was consistently restored in HS-5 DC despite expression of shβcat, we performed a time course experiment in K562S-shβcat cells to determine when β-catenin protein expression returned. Immunoblot analyses revealed that at 4 h and 8 h of co-culture with HS-5 cells, β-catenin protein was still efficiently downregulated (Figure 7a). Thus, we assessed whether HS-5 DC protects K562S-shβcat cells from TKI treatment at these time points. Despite the short-term co-culture and treatment with imatinib, 4 h and 8 h of culture with HS-5 cells was sufficient to protect cells from the effects of imatinib (Figure 7b), arguing against a critical role for β-catenin in extrinsic resistance. To expand on this result, we cultured CML CD34+ cells for 24 h with primary mesenchymal stromal cells (MSCs) harvested from normal bone marrow donors.35 Unlike HS-5 cells, primary MSCs did not stabilize β-catenin protein levels (Supplementary Figure S7a), while still protecting CML CD34+ cells from the effects of imatinib (Supplementary Figure S7b). Furthermore, MSC co-culture had no effect on Lef/Tcf reporter activity in CML CD34+ cells from newly diagnosed patients (Supplementary Figure S7c), suggesting that, while β-catenin protein is stabilized by HS-5 DC, it is not required for DC-mediated TKI protection.
Figure 7. HS-5 direct contact is sufficient to enhance survival of K562S cells in the presence of imatinib, despite efficient β-catenin knockdown at 4 h and 8 h of co-culture.
a,b. K562S cells were infected with either shSCR or shβcat and subject to culture in RM or HS-5 DC. A high dose of imatinib (5 μM) was used to push the system during short time points. At the indicated times, all cells were harvested and the GFP+ cells were isolated by FACS. Immunoblot analysis revealed that β-catenin stabilization takes at least 24 hours to occur and is not due to contamination by HS-5 stromal cells (a). Colony assays revealed that, despite efficient β-catenin knockdown at 4 h and 8 h of HS-5 co-culture, HS-5 DC still protected cells from the effects of short-term treatment with imatinib (b). Thus, while β-catenin protein is stabilized by HS-5 DC, it is not required for direct contact-mediated protection against TKI treatment. Bars represent SEM. *p<0.05.
DISCUSSION
BCR-ABL1 kinase-dependent TKI resistance in CML hinges on restoration of BCR-ABL1 kinase activity.10 In contrast, BCR-ABL1 kinase-independent resistance is based on activation of alternative signaling pathways that maintain viability and growth despite continued suppression of BCR-ABL1 kinase activity.2 The innate TKI resistance of CML stem cells could be considered a manifestation of BCR-ABL1 kinase-independent resistance that accounts for residual leukemia even in patients with profound TKI responses.18,19 BCR-ABL1 kinase-independent resistance may involve both intrinsic and extrinsic mechanisms, and several signaling molecules have been implicated both in CML persistence and overt resistance, including STAT3,11 PP2A30,47 and β-catenin.24,26,27,29 Targeting such universal resistance pathways would have broad applicability for CML therapy.
β-catenin has been implicated in several aspects of CML biology, including stem cell self-renewal, progression to BP-CML, and TKI resistance.14,23–25,27,29,34 How β-catenin is regulated in these various scenarios remains controversial. Zhao et al. reported reduced BCR-ABL1 protein levels in β-catenin-deficient murine CML, placing β-catenin upstream of BCR-ABL1.23 In contrast, Coluccia et al. identified β-catenin as a substrate of BCR-ABL1 in CML, where phosphorylation on tyrosines 86 and 654 leads to β-catenin stabilization.46 Other studies suggest that BCR-ABL1 upregulates and/or activates β-catenin in a kinase-independent manner.28,30 Two recent reports have suggested that extrinsic factors regulate nuclear β-catenin in CML. Zhang et al. implicated N-cadherin-mediated interaction with BM stromal cells in the activation of nuclear β-catenin in CML progenitor and stem cells,33 while Schurch et al. showed β-catenin activation by CD27/CD70 signaling.34
In the present study, we sought to understand the role of β-catenin in intrinsic versus extrinsic BCR-ABL1 kinase-independent TKI resistance. In cell lines and patient samples that are intrinsically resistant to TKIs, β-catenin protein expression was uncoupled from BCR-ABL1 kinase activity. Consistent with this, TKI-resistant cell lines had higher Lef/Tcf reporter activity than TKI-sensitive controls, which correlated with upregulation of β-catenin target genes. Although confirmatory Lef/Tcf reporter assays in CD34+ cells from TKI-resistant patients were precluded by the small numbers of cells available, β-catenin knockdown significantly reduced colony formation, suggesting that nuclear β-catenin promotes intrinsic BCR-ABL1 kinase-independent TKI resistance and may be a potential therapeutic target (Supplementary Figure S2e). These data are in line with our published gene expression signature which accurately predicts a patient’s response to imatinib, containing significant enrichment of β-catenin target genes in imatinib non-responders.29
In contrast with intrinsic TKI resistance, our data argue against a role for β-catenin in extrinsic, BM-derived TKI resistance. Similar to intrinsic resistance, β-catenin protein was highly stabilized when TKI-sensitive CML cells were cultured in direct contact with HS-5 stromal cells in the presence of imatinib. This was observed even in cells expressing shβcat, and correlated with restored colony forming ability in the presence of imatinib. However, despite the striking stabilization of β-catenin protein by HS-5 DC, which was calcium-dependent, we did not detect an increase of Lef/Tcf reporter activity in the presence of imatinib, neither by co-culture with HS-5 cells nor primary MSCs, and we saw no consistent changes in β-catenin target gene expression. Additionally, K562S cells expressing shβcat and plated in HS-5 contact were protected at 4 h and 8 h of co-culture, although β-catenin stabilization was not apparent at these time points.
A previous study had implicated N-cadherin in β-catenin stabilization by stromal cells and extrinsic resistance.33 However, while addition of N-cadherin and/or H-cadherin blocking antibodies to HS-5 co-cultures restored the effects of imatinib on CML cell survival, this had no effect on β-catenin protein stabilization, indicating that β-catenin stabilization is not required for N-cadherin or H-cadherin-mediated extrinsic resistance. Altogether, these data argue against a role for β-catenin in BM-mediated TKI resistance and are at odds with the report by Zhang et al.33 implicating nuclear β-catenin in stroma-mediated TKI resistance. One potential explanation for the discrepant results is the type of stromal cells used to support CML CD34+ cells (HS-5 cells in our study, hTERT-immortalized primary human marrow MSCs in Zhang et al.33). However, in the present study, co-culture of CML CD34+ cells with primary human MSCs, while protective against TKI treatment, had no effect on Lef/Tcf reporter activity, and did not result in β-catenin stabilization, further supporting our conclusions that stabilization of β-catenin protein and activation of nuclear β-catenin are not required in extrinsic TKI resistance, or that such an effect is highly dependent on the stromal line used.
In contrast to N-cadherin, H-cadherin is anchored in the cell membrane by glycophosphatidylinositol, has no cytoplasmic domain, and uses a different mechanism to form homotypic interactions. Additionally, H-cadherin is capable of engaging non-cadherin ligands such as β3 integrin, G protein-coupled receptors, and possibly receptor tyrosine kinases (reviewed in 42). Our data for the first time suggest that H-cadherin has pleiotropic effects on CML CD34+ cells grown on BM stroma, including negative regulation of nuclear β-catenin activity, and enhanced survival in the presence of imatinib, which may be due to activation of AKT or other downstream molecules48. Our data suggest that targeting H-cadherin may be therapeutically useful, but more experimentation will be required to dissect its diverse functions on leukemic and normal hematopoietic progenitor cells.
Collectively, our data implicate nuclear β-catenin in intrinsic BCR-ABL1 kinase-independent TKI resistance, but argue against a direct role for β-catenin in BM-mediated (extrinsic) TKI resistance, with the caveat that even primary CML cells cultured ex vivo on primary MSCs may not fully recapitulate persistent leukemia cells in patients on long-term imatinib therapy, including heterogeneity across patients. Targeting nuclear β-catenin should be primarily considered in patients who fail TKIs in the absence of BCR-ABL1 mutations.
Supplementary Material
Acknowledgments
We gratefully acknowledge Drs. Michael Andreeff and Marina Konopleva (The University of Texas M. D. Anderson Cancer Center, Houston, TX, USA) for providing the primary human MSCs that were used for these studies, as well as Jenny Ottley for clerical assistance. This work was supported by a Translational Research Program Award (6086-12 to M.W.D) and a Specialized Center of Research Program Award (GCNCR0314A-UTAH to M.W.D) from The Leukemia & Lymphoma Society (LLS), National Institutes of Health (NIH) P01CA049639 (to M.W.D.), National Cancer Institute (NCI) R01CA178397 (to M.W.D. and T.O.), NCI 5P30CA042014-24, and by the V Foundation for Cancer Research (to M.W.D. and T.O.). A.M.E. was supported by a NCI T32CA093247, a Career Development Award from LLS (5090-12), and is currently funded through a Scholar Award from the American Society of Hematology (ASH). A.M.E. also acknowledges support from the NIH Loan Repayment Program. J.S.K. is a Special Fellow of LLS and was supported by a Translational Research Training in Hematology Award from ASH.
Footnotes
Supplementary information is available at Leukemia’s website.
CONFLICT OF INTEREST STATEMENT: M.W.D. is a consultant for BMS, Novartis, ARIAD, Pfizer and Incyte. His laboratory receives research funding from BMS and Novartis.
References
- 1.Rowley JD. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973 Jun 1;243(5405):290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- 2.O’Hare T, Zabriskie MS, Eiring AM, Deininger MW. Pushing the limits of targeted therapy in chronic myeloid leukaemia. Nat Rev Cancer. 2012 Aug;12(8):513–526. doi: 10.1038/nrc3317. [DOI] [PubMed] [Google Scholar]
- 3.Druker BJ, Guilhot F, O’Brien SG, Gathmann I, Kantarjian H, Gattermann N, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006 Dec 7;355(23):2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 4.Saglio G, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Lobo C, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010 Jun 17;362(24):2251–2259. doi: 10.1056/NEJMoa0912614. [DOI] [PubMed] [Google Scholar]
- 5.Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010 Jun 17;362(24):2260–2270. doi: 10.1056/NEJMoa1002315. [DOI] [PubMed] [Google Scholar]
- 6.de Lavallade H, Apperley JF, Khorashad JS, Milojkovic D, Reid AG, Bua M, et al. Imatinib for newly diagnosed patients with chronic myeloid leukemia: incidence of sustained responses in an intention-to-treat analysis. J Clin Oncol. 2008 Jul 10;26(20):3358–3363. doi: 10.1200/JCO.2007.15.8154. [DOI] [PubMed] [Google Scholar]
- 7.Sawyers CL, Hochhaus A, Feldman E, Goldman JM, Miller CB, Ottmann OG, et al. Imatinib induces hematologic and cytogenetic responses in patients with chronic myelogenous leukemia in myeloid blast crisis: results of a phase II study. Blood. 2002 May 15;99(10):3530–3539. doi: 10.1182/blood.v99.10.3530. [DOI] [PubMed] [Google Scholar]
- 8.Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre P, Paquette R, Chuah C, et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013 Nov 7;369(19):1783–1796. doi: 10.1056/NEJMoa1306494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, Rao PN, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001 Aug 3;293(5531):876–880. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- 10.Shah NP, Nicoll JM, Nagar B, Gorre ME, Paquette RL, Kuriyan J, et al. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell. 2002 Aug;2(2):117–125. doi: 10.1016/s1535-6108(02)00096-x. [DOI] [PubMed] [Google Scholar]
- 11.Eiring AM, Page BD, Kraft IL, Mason CC, Vellore NA, Resetca D, et al. Combined STAT3 and BCR-ABL1 inhibition induces synthetic lethality in therapy-resistant chronic myeloid leukemia. Leukemia. 2014 doi: 10.1038/leu.2014.245. [DOI] [PubMed] [Google Scholar]
- 12.Bewry NN, Nair RR, Emmons MF, Boulware D, Pinilla-Ibarz J, Hazlehurst LA. Stat3 contributes to resistance toward BCR-ABL inhibitors in a bone marrow microenvironment model of drug resistance. Mol Cancer Ther. 2008 Oct;7(10):3169–3175. doi: 10.1158/1535-7163.MCT-08-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Traer E, MacKenzie R, Snead J, Agarwal A, Eiring AM, O’Hare T, et al. Blockade of JAK2-mediated extrinsic survival signals restores sensitivity of CML cells to ABL inhibitors. Leukemia. 2012 May;26(5):1140–1143. doi: 10.1038/leu.2011.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim S, Saw TY, Zhang M, Janes MR, Nacro K, Hill J, et al. Targeting of the MNK-eIF4E axis in blast crisis chronic myeloid leukemia inhibits leukemia stem cell function. Proc Natl Acad Sci U S A. 2013 Jun 18;110(25):E2298–2307. doi: 10.1073/pnas.1301838110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Traer E, Javidi-Sharifi N, Agarwal A, Dunlap J, English I, Martinez J, et al. Ponatinib overcomes FGF2-mediated resistance in CML patients without kinase domain mutations. Blood. 2014 Mar 6;123(10):1516–1524. doi: 10.1182/blood-2013-07-518381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vianello F, Villanova F, Tisato V, Lymperi S, Ho KK, Gomes AR, et al. Bone marrow mesenchymal stromal cells non-selectively protect chronic myeloid leukemia cells from imatinib-induced apoptosis via the CXCR4/CXCL12 axis. Haematologica. 2010 Jul;95(7):1081–1089. doi: 10.3324/haematol.2009.017178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tabe Y, Jin L, Iwabuchi K, Wang RY, Ichikawa N, Miida T, et al. Role of stromal microenvironment in nonpharmacological resistance of CML to imatinib through Lyn/CXCR4 interactions in lipid rafts. Leukemia. 2012 May;26(5):883–892. doi: 10.1038/leu.2011.291. [DOI] [PubMed] [Google Scholar]
- 18.Corbin AS, Agarwal A, Loriaux M, Cortes J, Deininger MW, Druker BJ. Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity. J Clin Invest. 2011 Jan;121(1):396–409. doi: 10.1172/JCI35721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamilton A, Helgason GV, Schemionek M, Zhang B, Myssina S, Allan EK, et al. Chronic myeloid leukemia stem cells are not dependent on Bcr-Abl kinase activity for their survival. Blood. 2012 Feb 9;119(6):1501–1510. doi: 10.1182/blood-2010-12-326843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004 Sep;5(9):691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 21.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004 Mar 5;303(5663):1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jamora C, Fuchs E. Intercellular adhesion, signalling and the cytoskeleton. Nat Cell Biol. 2002 Apr;4(4):E101–108. doi: 10.1038/ncb0402-e101. [DOI] [PubMed] [Google Scholar]
- 23.Zhao C, Blum J, Chen A, Kwon HY, Jung SH, Cook JM, et al. Loss of beta-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell. 2007 Dec;12(6):528–541. doi: 10.1016/j.ccr.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heidel FH, Bullinger L, Feng Z, Wang Z, Neff TA, Stein L, et al. Genetic and pharmacologic inhibition of beta-catenin targets imatinib-resistant leukemia stem cells in CML. Cell Stem Cell. 2012 Apr 6;10(4):412–424. doi: 10.1016/j.stem.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jamieson CH, Ailles LE, Dylla SJ, Muijtjens M, Jones C, Zehnder JL, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. 2004 Aug 12;351(7):657–667. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- 26.Scheller M, Schonheit J, Zimmermann K, Leser U, Rosenbauer F, Leutz A. Cross talk between Wnt/beta-catenin and Irf8 in leukemia progression and drug resistance. J Exp Med. 2013 Oct 21;210(11):2239–2256. doi: 10.1084/jem.20130706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radich JP, Dai H, Mao M, Oehler V, Schelter J, Druker B, et al. Gene expression changes associated with progression and response in chronic myeloid leukemia. Proc Natl Acad Sci U S A. 2006 Feb 21;103(8):2794–2799. doi: 10.1073/pnas.0510423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y, Hu Y, Zhang H, Peng C, Li S. Loss of the Alox5 gene impairs leukemia stem cells and prevents chronic myeloid leukemia. Nat Genet. 2009 Jul;41(7):783–792. doi: 10.1038/ng.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McWeeney SK, Pemberton LC, Loriaux MM, Vartanian K, Willis SG, Yochum G, et al. A gene expression signature of CD34+ cells to predict major cytogenetic response in chronic-phase chronic myeloid leukemia patients treated with imatinib. Blood. 2010 Jan 14;115(2):315–325. doi: 10.1182/blood-2009-03-210732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neviani P, Harb JG, Oaks JJ, Santhanam R, Walker CJ, Ellis JJ, et al. PP2A-activating drugs selectively eradicate TKI-resistant chronic myeloid leukemic stem cells. J Cin Invest. 2013 Oct 1;123(10):4144–4157. doi: 10.1172/JCI68951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abrahamsson AE, Geron I, Gotlib J, Dao KH, Barroga CF, Newton IG, et al. Glycogen synthase kinase 3beta missplicing contributes to leukemia stem cell generation. Proc Natl Acad Sci U S A. 2009 Mar 10;106(10):3925–3929. doi: 10.1073/pnas.0900189106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang W, Zhou W, Saberwal G, Konieczna I, Horvath E, Katsoulidis E, et al. Interferon consensus sequence binding protein (ICSBP) decreases beta-catenin activity in myeloid cells by repressing GAS2 transcription. Mol Cell Biol. 2010 Oct;30(19):4575–4594. doi: 10.1128/MCB.01595-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang B, Li M, McDonald T, Holyoake TL, Moon RT, Campana D, et al. Microenvironmental protection of CML stem and progenitor cells from tyrosine kinase inhibitors through N-cadherin and Wnt-beta-catenin signaling. Blood. 2013 Mar 7;121(10):1824–1838. doi: 10.1182/blood-2012-02-412890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schurch C, Riether C, Matter MS, Tzankov A, Ochsenbein AF. CD27 signaling on chronic myelogenous leukemia stem cells activates Wnt target genes and promotes disease progression. J Clin Invest. 2012 Feb 1;122(2):624–638. doi: 10.1172/JCI45977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tabe Y, Konopleva M, Munsell MF, Marini FC, Zompetta C, McQueen T, et al. PML-RARalpha is associated with leptin-receptor induction: the role of mesenchymal stem cell-derived adipocytes in APL cell survival. Blood. 2004;103(5):1815–1822. doi: 10.1182/blood-2003-03-0802. [DOI] [PubMed] [Google Scholar]
- 36.Yong AS, Szydlo RM, Goldman JM, Apperley JF, Melo JV. Molecular profiling of CD34+ cells identifies low expression of CD7, along with high expression of proteinase 3 or elastase, as predictors of longer survival in patients with CML. Blood. 2006 Jan 1;107(1):205–212. doi: 10.1182/blood-2005-05-2155. [DOI] [PubMed] [Google Scholar]
- 37.Dengler MA, Staiger AM, Gutekunst M, Hofmann U, Doszczak M, Scheurich P, et al. Oncogenic stress induced by acute hyper-activation of Bcr-Abl leads to cell death upon induction of excessive aerobic glycolysis. PloS One. 2011;6(9):e25139. doi: 10.1371/journal.pone.0025139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roecklein BA, Torok-Storb B. Functionally distinct human marrow stromal cell lines immortalized by transduction with the human papilloma virus E6/E7 genes. Blood. 1995 Feb 15;85(4):997–1005. [PubMed] [Google Scholar]
- 39.Aplin AE, Howe A, Alahari SK, Juliano RL. Signal transduction and signal modulation by cell adhesion receptors: the role of integrins, cadherins, immunoglobulin-cell adhesion molecules, and selectins. Pharmacol Rev. 1998 Jun;50(2):197–263. [PubMed] [Google Scholar]
- 40.Juliano RL. Signal transduction by cell adhesion receptors and the cytoskeleton: functions of integrins, cadherins, selectins, and immunoglobulin-superfamily members. Ann Rev Pharmacol Toxicol. 2002;42:283–323. doi: 10.1146/annurev.pharmtox.42.090401.151133. [DOI] [PubMed] [Google Scholar]
- 41.Wang XJ, Li J, Fu BJ, Guo LL, Zhang JH, Huang SA. Methylation status of JunB and CDH13 gene promoter in CD34(+)CD38(−) chronic myelogenous leukemia cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2009 Dec;17(6):1405–1408. [PubMed] [Google Scholar]
- 42.Andreeva AV, Kutuzov MA. Cadherin 13 in cancer. Genes Chromosomes Cancer. 2010 Sep;49(9):775–790. doi: 10.1002/gcc.20787. [DOI] [PubMed] [Google Scholar]
- 43.Kikuchi A, Yamamoto H, Sato A, Matsumoto S. Wnt5a: its signalling, functions and implication in diseases. Acta Physiol. 2012 Jan;204(1):17–33. doi: 10.1111/j.1748-1716.2011.02294.x. [DOI] [PubMed] [Google Scholar]
- 44.Smalley MJ, Dale TC. Wnt signalling in mammalian development and cancer. Cancer Metastasis Rev. 1999;18(2):215–230. doi: 10.1023/a:1006369223282. [DOI] [PubMed] [Google Scholar]
- 45.Heuberger J, Birchmeier W. Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harb Perspect Biol. 2010 Feb;2(2):a002915. doi: 10.1101/cshperspect.a002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coluccia AM, Vacca A, Dunach M, Mologni L, Redaelli S, Bustos VH, et al. Bcr-Abl stabilizes beta-catenin in chronic myeloid leukemia through its tyrosine phosphorylation. EMBO J. 2007 Mar 7;26(5):1456–1466. doi: 10.1038/sj.emboj.7601485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neviani P, Santhanam R, Oaks JJ, Eiring AM, Notari M, Blaser BW, et al. FTY720, a new alternative for treating blast crisis chronic myelogenous leukemia and Philadelphia chromosome-positive acute lymphocytic leukemia. J Clin Invest. 2007 Sep;117(9):2408–2421. doi: 10.1172/JCI31095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bosserhoff AK, Ellmann L, Quast AS, Eberle J, Boyle GM, Kuphal S. Loss of T-cadherin (CDH-13) regulates AKT signaling and desensitizes cells to apoptosis in melanoma. Mol Carcinogen. 2014 Aug;53(8):635–647. doi: 10.1002/mc.22018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.