Abstract
Objectives
Switching from maintenance of general anesthesia with an ether anesthetic to maintenance with high-dose (concentration > 50% and total gas flow rate > 4 liters per minute) nitrous oxide is a common practice used to facilitate emergence from general anesthesia. The transition from the ether anesthetic to nitrous oxide is associated with a switch in the putative mechanisms and sites of anesthetic action. We investigated whether there is an electroencephalogram (EEG) marker of this transition.
Methods
We retrospectively studied the ether anesthetic to nitrous oxide transition in 19 patients with EEG monitoring receiving sevoflurane, oxygen and air for general anesthesia maintenance.
Results
Following the transition to nitrous oxide, the alpha (8 to 12 Hz) oscillations associated with sevoflurane dissipated within 3 to 12 minutes (median 6 minutes) and were replaced by highly coherent large-amplitude slow-delta (0.1 to 4 Hz) oscillations that persisted for 2 to 12 minutes (median 3 minutes).
Conclusions
Administration of high-dose nitrous oxide is associated with transient, large amplitude slow-delta oscillations.
Significance
We postulate that these slow-delta oscillations may result from nitrous oxide-induced blockade of major excitatory inputs (NMDA glutamate projections) from the brainstem (parabrachial nucleus and medial pontine reticular formation) to the thalamus and cortex. This EEG signature of high-dose nitrous oxide may offer new insights into brain states during general anesthesia.
Keywords: Slow waves, EEG, general anesthesia, NMDA-receptor blockade, parabrachial nucleus
Introduction
Use of nitrous oxide dates back to the early 1800s, making it the oldest anesthetic currently administered (Andrews, 1868). Because administration of nitrous oxide alone is not sufficiently potent to produce general anesthesia, today it is used most often as an adjunct during general anesthesia to reduce the quantity administered and hence, the side effects of the potent ether anesthetics. For example, use of nitrous oxide with ether anesthetics, such as sevoflurane, desflurane or isoflurane, in combination with an oxygen and air mixture, a muscle relaxant and analgesics is a standard anesthetic combination (Eger et al., 1997). During closure of the surgical incision, a common practice is to switch from the ether anesthetic to nitrous oxide to facilitate recovery from general anesthesia. Administration of high concentration nitrous oxide (> 60% with ~40% oxygen) at high flow rates causes its rapid uptake in the lungs, circulation and the brain. At the same time, stopping the ether anesthetic terminates its steady state within the brain and the lungs, and shifts its gradient towards removal. The switch from the ether anesthetic to nitrous oxide shifts the anesthetic state from maintenance of unconsciousness sufficient for surgery to a state of sedation from which a patient can be more rapidly awakened.
The sedative effects of nitrous oxide are believed to be mediated through blockade of N-methyl-D-aspartate (NMDA) glutamatergic receptors (Jevtovic-Todorovic et al., 1998, Mennerick et al., 1998, Hemmings et al., 2005). The electroencephalogram (EEG) under nitrous oxide is commonly associated oscillations in the beta (12 to 25 Hz) and the gamma (> 25 Hz) ranges and possibly a decrease in power in the delta range (Yamamura et al., 1981). Recently, it has been reported that at inspired concentrations of 40%, nitrous oxide actually maintains activity in the beta and gamma bands while reducing activity in the delta (1 to 4 Hz) and in the theta (5 to 8 Hz) bands (Foster et al., 2011, 2013). The link between the actions at glutamatergic synapses and the EEG patterns has not been clearly established. Characterizing the EEG activity of nitrous oxide concentrations higher than 40% proved challenging because the higher concentrations induced significant nausea and emesis (Foster et al., 2011).
The EEG observed during the administration of ether anesthetics shows large alpha, theta and slow-delta oscillations when patients are sufficiently unconscious to conduct surgery (Akeju et al., 2014, Brown et al., 2014). Because these patterns are similar to those seen with propofol and given that both the ether anesthetics and propofol (Feshchenko et al., 2004, Cimenser et al., 2011, Purdon et al., 2013) are known to act at γ-aminobutyric acid A (GABAA) receptors, it is likely that the EEG patterns for the ether anesthetics are related to the same GABAergic circuit mechanisms as propofol (Bai et al., 1999, Hemmings et al., 2005, Ching et al., 2010, Cimenser et al., 2011, Purdon et al., 2013). It has been reported that the transition from an ether anesthetic to high-dose nitrous oxide is marked by EEG slow-delta oscillations rather than by beta and gamma oscillations (Hagihira et al., 2012). The differences in the EEG signatures between the ether anesthetic, low-dose nitrous oxide and high-dose nitrous oxide suggests that there may be different mechanisms and sites of anesthetic action in the three cases. Therefore, we investigated the ether anesthetic to nitrous oxide transition using spectral analysis to develop a detailed quantitative assessment of the EEG signatures associated with high-dose nitrous oxide.
We studied retrospectively the ether anesthetic to high-dose nitrous oxide transition in 19 patients receiving sevoflurane, oxygen and air for maintenance of general anesthesia. We report that high-dose nitrous oxide is associated with the disappearance of oscillations from 5 Hz and above over 3 to 12 minutes and the appearance of large amplitude, highly coherent slow (0.1 to 1 Hz) and delta (1 to 4 Hz) oscillations. The slow-delta oscillations persist for 2 to 12 minutes and then transition to high-frequency, low-amplitude beta and gamma oscillations. We propose transient blockade of glutamatergic inputs from the brainstem to the thalamus and to the basal forebrain as a neural circuit mechanism for this EEG signature associated with high-dose nitrous oxide administration.
Materials and Methods
Study Protocol
This retrospective observational study was approved by the Human Research Committee at Massachusetts General Hospital. We reviewed our database of 954 cases of general anesthesia and simultaneous EEG recordings collected between September 1, 2011 and October 30, 2014. We identified 169 cases in which sevoflurane and nitrous oxide were administered. From these we removed 68 cases in which the patients had received sevoflurane and nitrous oxide as their entire anesthetic. Of the remaining 101 cases, we identified 26 cases that met the criteria of general anesthesia maintenance with sevoflurane and oxygen with a conversion to nitrous oxide at a concentration > 50% during skin closure. Of the 26 cases, we removed 7 cases: 4 cases due to train-of-four monitor artifacts, and 3 cases due to incomplete EEG records. Therefore, we chose for analysis 19 cases, involving 19 different patients. Table 1 summarizes the patient characteristics.
Table 1.
Patient characteristics.
| Patient | Age (years) | Sex | Height (cm) | Weight (kg) | Surgical Procedure | Length of Surgical Procedure (min) | Paravertebral Block = B None = N |
|---|---|---|---|---|---|---|---|
| A | 48 | F | 155 | 75 | MASTOPEXY WITH AUGMENTATION, BILATERAL | 583 | N |
| B | 44 | F | 165 | 79 | BILATERAL MASTECTOMY WITH BILATERAL IMPLANT | 313 | B |
| C | 22 | F | 168 | 118 | LAPAROSCOPIC APPENDECTOMY | 85 | N |
| D | 50 | F | 168 | 81 | ABDOMINOPLASTY | 268 | N |
| E | 63 | M | 178 | 108 | KIDNEY TRANSPLANT | 266 | N |
| F | 70 | F | 157 | 53 | LAPAROSCOPIC DISTAL PANCREATECTOMY | 196 | B |
| G | 61 | F | 163 | 77 | LAPAROSCOPIC TRANSVERSE COLECTOMY | 261 | N |
| H | 54 | F | 178 | 111 | LAPAROSCOPIC CHOLECYSTECTOMY | 105 | N |
| I | 47 | F | 178 | 85 | TOTAL ABDOMINAL HYSTERECTOMY | 181 | N |
| J | 65 | M | 170 | 94 | THYROIDECTOMY | 378 | N |
| K | 56 | F | 160 | 103 | THYROIDECTOMY TOTAL | 196 | N |
| L | 81 | F | 152 | 72 | LEFT MASTECTOMY WITH SENTINEL NODE BIOPSY | 101 | N |
| M | 62 | F | 160 | 68 | LAPAROSCOPIC SIGMOID COLECTOMY; LIVER BIOPSY | 162 | N |
| N | 37 | F | 160 | 82 | ABDOMINOPLASTY | 218 | N |
| O | 37 | F | 157 | 64 | ABDOMINOPLASTY | 179 | N |
| P | 75 | M | 178 | 75 | LAPAROSCOPIC ESOPHAGEAL HERNIA REPAIR | 191 | N |
| Q | 69 | F | 163 | 66 | L MASTECTOMY SIMPLE | 137 | N |
| R | 34 | F | 173 | 61 | LAP MYOMECTOMY | 194 | N |
| S | 38 | M | 188 | 98 | KIDNEY TRANSPLANT | 210 | N |
| Mean (sd) | 53 (16) | M = 4 F = 15 |
167 (10) | 83 (18) | 222 (114) | B = 2 N = 17 |
For all 19 patients, anesthetic concentrations, oxygen delivery, heart rate, blood pressure, and oxygen saturation were automatically recorded in the electronic medical record (MetaVision, Dedham, MA) at one minute intervals. Intravenous drug doses and times were recorded by the anesthesia caregivers. General anesthesia was induced with propofol (mean ± standard deviation; 184 ± 48 mg) and intubation was carried out using succinylcholine, cisatracurium, vecuronium or rocuronium for muscle relaxation. General anesthesia was maintained with sevoflurane, oxygen and air along with either intravenous narcotics (17 cases), or paravertebral block using local anesthetics and/or opioids (2 cases). Muscle relaxation was maintained with cis-atracurium, vecuronium or rocuronium. The maintenance concentration of expired sevoflurane ranged from 1.3% to 2.4% (median 1.7%). Air was added to the flow of oxygen to maintain a 40–80% fraction of inspired oxygen. During maintenance, the total gas flow was maintained between 0.9 to 8.0 liters per minute (median 1.8 liters per minute) up through fascial closure. For skin closure, total gas flow was maintained between 2.0 to 14.0 liters per minute (median 5.7 liters per minute), the maximum expired concentration of nitrous oxide achieved was 51 to 79% (median 70%) and the sevoflurane concentration was decreased to 1% or less. At case completion, sevoflurane and nitrous oxide were discontinued and 100% oxygen was administered, typically at 8 liters per minute or higher to allow the patients to emerge.
EEG Recordings and Statistical Analysis
The EEG was continuously recorded in each patient during general anesthesia using the Sedline monitor (Masimo, Irvine, CA) with the standard six-electrode frontal montage. The standard Sedline Sedtrace electrode array records from electrodes located approximately at positions Fp1, Fp2, F7, and F8, with ground electrode at Fpz and reference electrode approximately 1 cm above Fpz. Electrode impedance was less than 5kΩ in each channel. We report here the analyses using Fp1. The analyses were identical for Fp1 and Fp2. The EEG recording began 3 to 5 minutes prior to induction of general anesthesia and was continued briefly after extubation.
To compare the EEG differences between the sevoflurane and nitrous oxide states, we identified from each patient’s electronic medical record two representative periods, each 60 seconds in duration. To represent steady-state sevoflurane general anesthesia, we chose a 60-second EEG period beginning 15 minutes prior to the initiation of nitrous oxide. For each subject, we identified the slow-delta oscillation onset and offset times. We chose a period that was representative of the nitrous oxide effect by taking a 60-second segment, beginning 30 seconds after the onset of the slow-delta oscillation. We also computed the total cumulative dose of nitrous oxide to relate the slow-delta oscillation appearance to the dose of nitrous oxide received by the patient.
For each patient, we plotted the time-domain EEG signal. We used standard multitaper spectral methods (Percival et al., 1993, Babadi et al., 2014) implemented in the Chronux toolbox (http://chronux.org) to compute the spectrogram (Mitra et al., 2008). The spectrogram is the decomposition of the EEG signal into its spectral components (power by frequency) across time, where power is the log base 10 of the EEG signal amplitude squared. The parameters for the multitaper spectral analysis were: window length T = 2s with no overlap, time-bandwidth product TW = 3, number of tapers K = 5, and spectral resolution of 3 Hz. We computed group-level spectrograms for the sevoflurane and the nitrous oxide periods by taking the median spectrogram across all patients in each period.
We conducted for each patient a coherence analysis in the sevoflurane and the nitrous oxide periods using the multitaper settings chosen to compute the spectra (Mitra et al., 2008). The coherence is the correlation between two EEG signals at a given frequency which we computed as the magnitude of the cross-spectrum between the two signals divided by the square root of the product of the spectra of the two signals. We chose the frontal EEG electrodes, F7 and F8, to compute coherence during the sevoflurane and the nitrous oxide periods (Percival et al., 1993). We calculated the coherence between F7 and F8 because these electrodes have greater spatial separation than electrodes F1 and F2.
To analyze the difference in power (coherence) between the sevoflurane period and the nitrous oxide period we computed within subject the difference in power (coherence) between the two anesthetic states in three frequency bands: slow-delta (0.1 to 4 Hz), alpha (8–12 Hz) and beta-gamma (13–40 Hz). We estimated the difference in the spectral properties of the two anesthetic states across the 19 patients by computing the median difference in power (coherence) in each frequency band and the 95% confidence interval for the median. We use the median in our analysis because the sample size is only 19 patients in our analysis. The median provides a robust, nonparametric estimate of the magnitude of the difference and the 95% confidence intervals provide a conservative estimate of the uncertainty in the difference (Mosteller, 1973). Because each comparison involved making an inference about a median power difference in a frequency band, we considered a result to be statistically significant if the 95% confidence interval did not contain 0. Because there are 19 patients, the coverage probability for the 95% confidence intervals will be at least, 0.98 instead of 0.95.
Results
Spectral Analysis of the Sevoflurane to Nitrous Oxide Transition
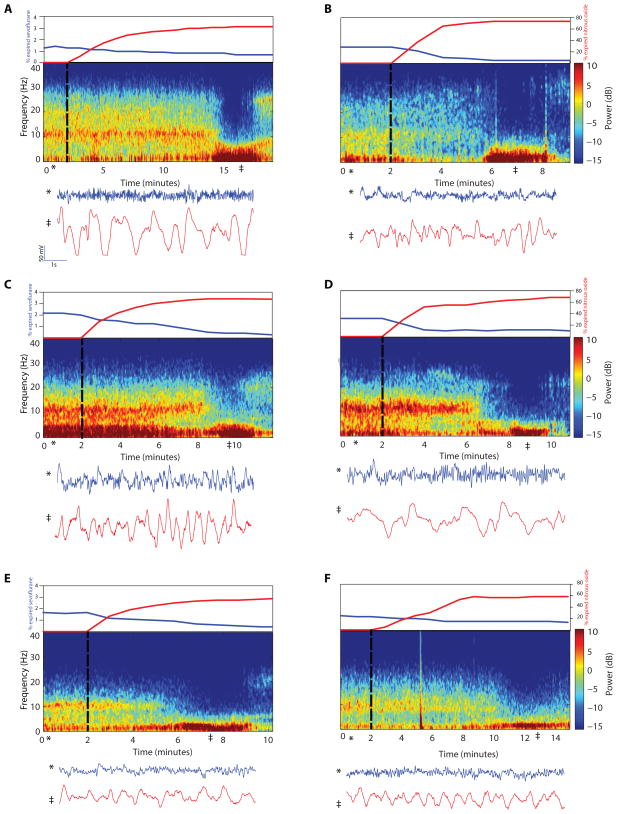
Several minutes were required for both the decrease in the sevoflurane concentration (Fig. 1, upper subpanels, blue curves) and the increase in the nitrous oxide concentration (Fig. 1, upper subpanels, red curves) to reach the target levels. For example, Patient A (Fig. 1A) required 7 minutes to both reach the target nitrous oxide concentration and for the sevoflurane concentration to reach 1% or less. In contrast, Patient C (Fig. 1C) required 4 minutes to reach the target nitrous oxide concentration and 7 minutes to achieve the sevoflurane decrease. The median time to achieve a 50% expired nitrous oxide target concentration was 3 minutes with a range of 2 to 16 minutes, whereas the median time to decline to 1% expired sevoflurane or less was 3 minutes with a range of 1 to 20 minutes. During the transition, spectral power in the alpha band dissipated (Fig. 1 and Supplementary Figure S1, middle subpanels). This was followed by a substantial decrease in power across all frequencies above 5 Hz with a concomitant increase in the slow-delta power (Fig. 1 and Supplementary Figure S1, middle subpanels). The dramatic differences in the raw EEG traces before and after administration of nitrous oxide are illustrated in the lower subpanels of Fig. 1 and Supplementary Figure S1. The time required for the appearance of the slow-delta oscillations and the disappearance of the alpha oscillations was 3 to 12 minutes (median 6 minutes). The slow-delta oscillations persisted for 2 to 12 minutes (median 3 minutes) and then transformed eventually into higher frequency beta-gamma (13–40 Hz) oscillations (Fig. 1 middle subpanels A–F, Supplementary Figure S1 middle subpanels G–S).
Figure 1.
Time courses of anesthetic concentrations (upper subpanels); spectrograms (middle subpanels); and the time-domain signature of sevoflurane and the time-domain signature of nitrous oxide-induced slow-delta oscillations (lower subpanels) for Patients A–F. The start of nitrous oxide and the decrease of sevoflurane are indicated by the black dotted vertical line. The time courses of the anesthetic concentrations, sevoflurane (blue curve) and nitrous oxide (red curve), are shown in the upper subpanels. The spectrograms (middle subpanels) during the sevoflurane period show large oscillations in the alpha frequency band (8–12 Hz) that dissipate with the transition to nitrous oxide. After the nitrous oxide concentration plateaus, the spectrograms show increased power in the slow-delta frequency band (0.1–4 Hz) that persists for several minutes. Ten-second EEG traces recorded during the sevoflurane period (*) and during the midpoint of the nitrous oxide-induced slow-delta oscillation period (‡) are shown in the lower subpanels. Slow-delta oscillations are present in both periods. However, the slow-delta oscillations are significantly enhanced after the transition to nitrous oxide (See Figure 2D). The vertical line in panel F (minute 5) is either an electrocautery or movement artifact.
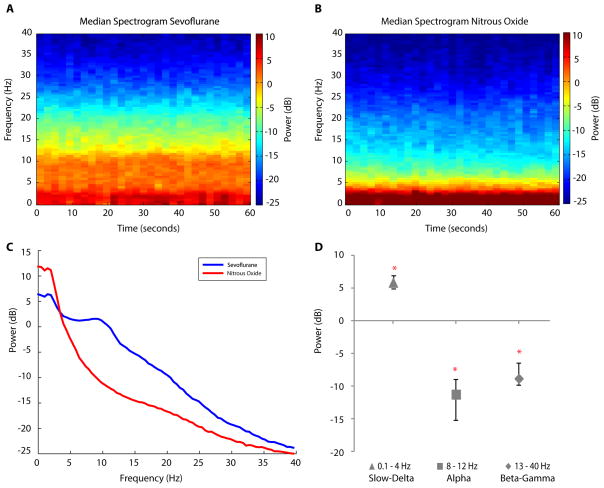
To display the group difference in spectral dynamics between the sevoflurane and nitrous oxide periods, we computed across patients the median spectrogram for the two periods (Fig. 2A, 2B). The median spectrogram of the sevoflurane period (Fig. 2A) showed substantial power in the alpha and slow-delta frequency bands. In contrast, the median spectrogram of the nitrous oxide period showed greater power in the slow-delta frequency range, and less power in the alpha frequency range compared with the median sevoflurane spectrogram (Fig. 2B). There was substantially less power at all frequencies above 5 Hz in the median nitrous oxide spectrogram (Fig. 2B) relative to the median sevoflurane spectrogram (Fig. 2A).
Figure 2.
Group-level spectral analysis during the sevoflurane period, defined as the 1-minute segment, 15 minutes prior to the start of nitrous oxide, and during the nitrous oxide period defined as the 1-minute segment around the midpoint of the slow-delta oscillation period. A. Median spectrogram during the sevoflurane period, showing power in the slow-delta and alpha frequency bands. B. Median spectrogram during the nitrous oxide period, showing increased power in the slow-delta frequency band and decreased power in the alpha frequency band compared with the sevoflurane period. C. Median spectra for the sevoflurane (blue) and nitrous oxide (red) periods with 95% confidence intervals (shaded regions) showing the power in the slow-delta frequency band for nitrous oxide is greater than that for sevoflurane. D. Median difference in power (nitrous oxide – sevoflurane) and 95% confidence intervals computed in the slow-delta (triangle), alpha (square), and beta-gamma (diamond) ranges. All three power differences are statistically significant (indicated by *) as none of the 95% confidence intervals cover zero.
To quantify these differences in spectral features between the two periods we computed for each patient the average spectrum (average of 30 spectra computed in 2-sec windows) in each period. We computed the group-level summary within each period as the median of the average spectrum across subjects (Fig. 2C). For the sevoflurane period, the median EEG power in the alpha band was 2.6 dB [0.96 dB, 3.54 dB 95% confidence interval], whereas the median EEG power in the slow-delta band was 7.82 dB [4.73 dB, 9.01 dB]. For nitrous oxide, the EEG power in the alpha band was −8.64 dB [−10.69 dB, −6.65 dB], whereas the EEG power in the slow-delta band was 13.71 dB [10.50 dB, 15.99 dB] (Fig. 2C).
To make inferences about the power changes between the sevoflurane and the nitrous periods we computed the spectra for the two periods and analyzed the median difference in power in three specific bands across subjects (Fig. 2C). The median difference (nitrous oxide – sevoflurane) in slow-delta power (Fig. 2D, triangle) was 5.89 dB [5.77 dB, 6.98 dB]. The median difference in alpha power (Fig. 2D, square) and in power in the beta-gamma range (Fig. 2D, diamond) were respectively, −11.24 dB [−15.16 dB, −8.94 dB] and −8.82 dB [−9.76 dB, −6.38 dB]. Each difference in power was statistically significant as none of the 95% confidence intervals covered zero (indicted by * in Fig. 2D).
Coherence Analysis of the Sevoflurane to Nitrous Oxide Transition
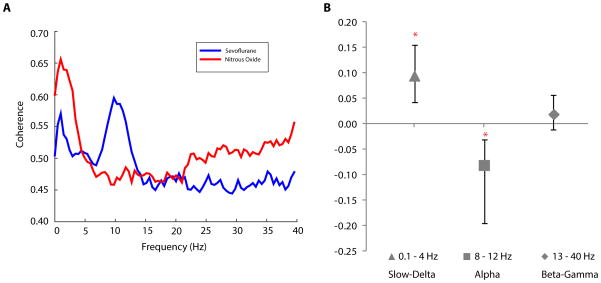
We computed across patients the median coherence during the sevoflurane period (Fig. 3A, blue curve) and during the nitrous oxide period (Fig. 3A, red curve). The group-level coherence of the sevoflurane period was strong in the slow-delta and alpha frequencies. During the sevoflurane period (Fig. 3A, blue curve) the alpha oscillation coherence was 0.61 [0.52, 0.66] and the slow-delta oscillation coherence was 0.57 [0.53, 0.60]. In contrast, the nitrous oxide period (Fig. 3A, red curve) showed a strong coherence only in the slow-delta frequency range which was 0.65 [0.58, 0.80].
Figure 3.
Group-level coherence analysis during the sevoflurane and during the nitrous oxide periods. A. Median coherence during the sevoflurane (blue) and during the nitrous oxide (red) periods with 95% confidence intervals (shaded regions). B. Median differences in coherence (nitrous oxide – sevoflurane) and 95% confidence intervals computed in the slow-delta (triangle), alpha (square), and beta-gamma (diamond) ranges. The coherence differences in the slow-delta and in the alpha bands are statistically significant (indicated by *) as none of the 95% confidence intervals cover zero.
The median differences (nitrous oxide – sevoflurane) in coherence were: 0.09 [0.04, 0.16] for the slow-delta band (Fig. 3B, triangle); −0.08 [−0.20, −0.03] for the alpha band (Fig. 3B, square); and 0.02 [−0.01, 0.06] for the beta-gamma band (Fig. 3B, diamond). The 95% confidence intervals for the median differences in coherence did not cover zero for the slow-delta and alpha bands indicating that these changes were statistically significant (indicted by * in Fig. 3B).
These results show that the transition to nitrous oxide from sevoflurane is marked by a substantial decrease in alpha power, a substantial increase in slow-delta power, a substantial decrease in beta-gamma power, loss of coherence in the alpha band, and enhanced coherence in the slow-delta band.
Discussion
Nitrous oxide is most commonly associated with increased beta and gamma oscillations (Yamamura et al., 1981) and more recently, with maintenance of beta and gamma oscillations and decrease in delta and theta oscillations (Foster et al., 2011, 2013). These studies used concentrations of nitrous oxide between 20 to 40%. Our findings give insight into the EEG dynamics induced by the higher nitrous oxide concentrations of 50–70% administered at high flow. We have demonstrated that switching from sevoflurane to nitrous oxide is associated with slow-delta oscillations. These slow-delta oscillations begin approximately 6 minutes after the switch and last from 2 to 11 minutes, before changing to the beta and gamma oscillations typically associated with nitrous oxide (Yamamura et al., 1981, Foster et al., 2011, 2013). The transition to nitrous oxide from sevoflurane is marked by a substantial decline in alpha power, a substantial increase in slow-delta power, and loss of coherence in the alpha band and enhanced coherence in the slow-delta band. Slow-delta oscillations have been reported when a 50:50 mixture of nitrous oxide and oxygen is administered at 2 atmospheres (Faulconer et al., 1949). Slow-delta oscillations have been reported in the transition from halothane, an alkane anesthetic to nitrous oxide (Avramov et al., 1990), as well as in the transition from the ether anesthetic, isoflurane to nitrous oxide (Hagihira et al., 2012). In both cases, the slow-delta oscillations were transient. Avramov and colleagues reported principally time-domain analyses, whereas Hagihira and colleagues used their own biocoherence-based index to conduct their analyses. Neither research group proposed a neural circuit hypothesis to explain these observations.
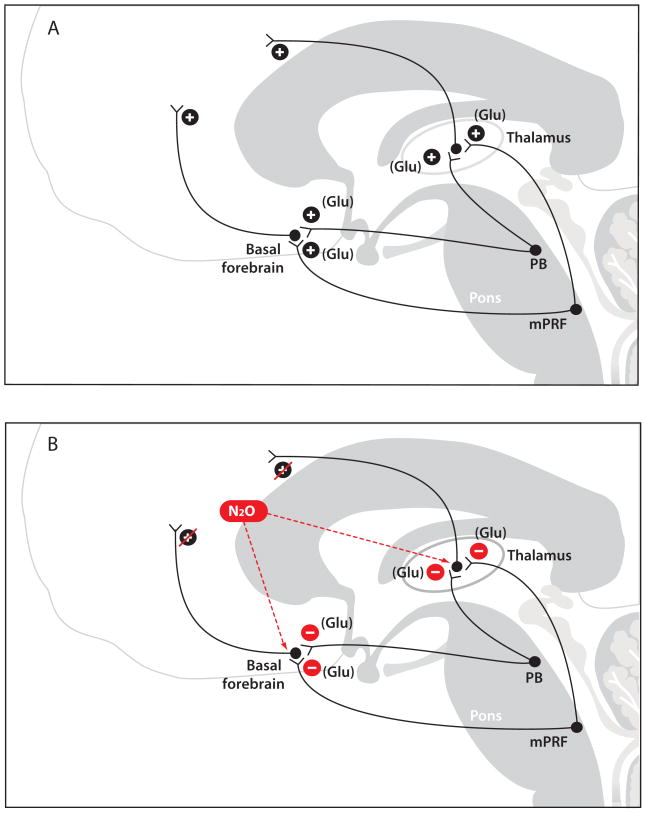
A Putative Mechanism of Nitrous Oxide-Induced Slow-Delta Oscillations
The mechanism of these slow-delta oscillations could be due to rapid delivery of high-dose (high concentrations at high flows) nitrous oxide to subcortical circuits and subsequent action of nitrous oxide at NMDA glutamate targets in major subcortical sites (Fig. 4). A putative subcortical target is the thalamus and the NMDA-mediated glutamatergic projections it receives from the parabrachial nucleus and from the medial pontine reticular formation, both of which lie in the pons (Boon et al., 2008, Fuller et al., 2011) (Fig. 4A). A second putative subcortical target is the basal forebrain and the glutamatergic projections it receives from the parabrachial nucleus. (Moga et al., 1990). These are the principal glutamatergic arousal pathways emanating from the brainstem (Saper et al., 1980, Fulwiler et al., 1984, Fuller et al., 2011, Kaur et al., 2013). When nitrous oxide is administered at high dose, it is taken up by the arterial circulation in the lungs. Blood containing the high nitrous oxide concentration reaches the brain through the internal carotid arteries bilaterally and the basilar artery posteriorly. This high dose of nitrous oxide reaches the thalamus via the thalamotuberal artery, the thalamoperforant artery, the thalamogeniculate artery, the anterior choroidal artery and the medial and lateral posterior choroidal arteries (Biller, 2009). The thalamotuberal artery arises from the posterior communicating artery; the thalamoperforant artery arises from the basilar artery; the thalamogeniculate artery arises from the posterior cerebral artery; the anterior choroidal artery arises from the internal carotid; and the medial and later posterior choroidal arteries arise from the posterior cerebral artery (Biller, 2009). Nitrous oxide reaches the basal forebrain from the anterior communicating arteries which arise from the middle cerebral artery (Biller, 2009).
Figure 4.
Putative neurophysiological mechanisms of nitrous oxide-induced slow-delta oscillations. A. The parabrachial nucleus (PB) and the medial pontine reticular formation (mPRF) provide excitatory glutamatergic (Glu) projections to the basal forebrain and to the central thalamus. B. Delivery of a high concentration of nitrous oxide (N20) likely leads to an intense blockade of these major N-methyl-D-aspartate (NMDA) glutamatergic excitatory pathways from the PB and the mPRF to the thalamus and basal forebrain. This results in decreased excitatory inputs from the thalamus and the basal forebrain to the cortex that could contribute to the production of slow-delta oscillations.
Based on this neural circuitry and vascular anatomy, the slow-delta oscillations could be produced as follows. Delivery of a high-dose nitrous oxide could lead to an intense blockade of these major NMDA-glutamatergic excitatory pathways from the parabrachial nucleus and the medial pontine reticular formation to the thalamus and basal forebrain (Fig. 4B). Such a blockade would in turn produce decreased excitatory inputs from the thalamus to the cortex and from the basal forebrain to the cortex. As we discuss below, removal of substantial subcortical excitatory inputs to the cortex has been commonly associated with the production of EEG slow-delta oscillations.
Slow-Delta Oscillations in Other Brain States
Frédéric Bremer first demonstrated in the 1930s that slow-delta oscillations could be produced by removing significant brainstem inputs to the cortex (Posner et al., 2007). In cats, he showed that transection of the brainstem at the level of the midbrain resulted in EEG slow-delta and spindle oscillations, whereas transection of the brainstem at the level of intersection between the spinal cord and the medulla resulted in the persistence of active, awake-appearing EEG patterns. He termed the former pattern cerveau isolé and the latter pattern encéphale isolé (Posner et al., 2007). Slow-wave sleep, defined by its associated slow-wave and delta EEG patterns, is believed to result in part from the activation of GABA- and galanin-mediated inhibitory projections from the pre-optic area of the hypothalamus to the arousal centers in the midbrain pons and hypothalamus (Saper et al., 2005). Similarly, slow-delta oscillations and concomitant unconsciousness due to complex partial seizures originating in the temporal lobe have been linked to inhibition of thalamic, basal forebrain and brainstem arousal circuits (Norden et al., 2002, Englot et al., 2010).
Slow and delta oscillations are also produced by other anesthetics. Bolus administration of propofol to induce general anesthesia yields EEG slow-delta oscillations that appear within 20 to 30 seconds and are associated with loss of consciousness (Lewis et al., 2012). The mechanism of these slow waves is most likely GABAergic inhibition by propofol at the synapses of the pre-optic area projections onto the brainstem arousal centers producing decreased excitatory inputs to the cortex (Brown et al., 2010, Brown et al., 2011). Maintenance of unconsciousness with propofol is characterized by both alpha and slow-delta oscillations (Feshchenko et al., 2004, Cimenser et al., 2011, Lewis et al., 2012, Purdon et al., 2013). The EEG alpha oscillations are hypersynchronous across the front of the scalp and are believed to require GABAergic inhibition in both cortex and thalamus (Ching et al., 2010). The hypersynchronous alpha oscillations persist as long as propofol is continuously infused (Purdon et al., 2013). The slow oscillations also persist yet, unlike the alpha oscillations, are not spatially coherent, i.e., hypersynchronous across the scalp (Cimenser et al., 2011, Lewis et al., 2012, Purdon et al., 2013). The mechanisms of the slow oscillations during propofol maintenance is likely identical to the one for the bolus administration. Given that sevoflurane and propofol both act primarily at GABAergic circuits, the mechanisms for the alpha and slow-delta oscillations for sevoflurane are likely similar to those for propofol (Hemmings et al., 2005, Lewis et al., 2012, Purdon et al., 2013, Akeju et al., 2014).
Administration of dexmedetomidine to maintain deep sedation is also associated with EEG slow-wave oscillations (Akeju et al., 2014). This is because dexmedetomidine acts pre-synaptically to block release of norepinephrine from adrenergic nerve terminals projecting from the locus coeruleus (Correa-Sales et al., 1992, Jorm et al., 1993, Chiu et al., 1995, Mizobe et al., 1996, Nelson et al., 2002, Saper et al., 2005). These nerves terminate diffusely in the cortex, in the intralaminar nucleus of the thalamus, in the basal forebrain and in the pre-optic area of the hypothalamus (Brown et al., 2010, Brown et al., 2011). The mechanism of action of dexmedetomidine suggests that reduced cortical inputs achieved by inactivating one of the principal arousal pathways can result in EEG slow oscillations. Inactivation of dopaminergic centers can also result in EEG slow waves. In rats, lesions in the ventral tegemental area, the origin of the dopaminergic mesoocortical pathway, produced EEG slow waves and a profound state of decreased arousal (Jones et al., 1973). Direct injection of sodium thiopental into the brainstem of rats induced slow-delta oscillations and loss of consciousness (Devor et al., 2001) most likely by enhancing activity in GABAergic projections onto the major brainstem arousal centers (Brown et al., 2010, Brown et al., 2011).
If our hypothesis is correct, then nitrous oxide-mediated blockade of brainstem glutamatergic inputs to the thalamus and cortex would be another mechanism through which a decrease in brainstem excitatory inputs to the cortex leads to slow-delta oscillations. The neurophysiology of why decreases in major brainstem inputs to cortical and thalamic circuits favor the production of slow-delta oscillations remains an open question (Crunelli et al., 2010).
Use of Anesthetics to Uncover Fundamental Brain Mechanisms
Our findings regarding nitrous oxide and slow-delta oscillations contribute to the growing body of work using anesthetics to uncover fundamental mechanisms of normal and pathological brain states. Several groups have used studies of general anesthesia to offer insights into mechanisms of consciousness and altered states of consciousness (Mashour, 2006, Alkire et al., 2008, Breshears et al., 2010, Hudetz, 2012, Casali et al., 2013, Mhuircheartaigh et al., 2013, Monti et al., 2013). Administration of ketamine has long served as a model for schizophrenia (Olney et al., 1995, Moghaddam et al., 1997), and more recently in low doses, as a novel immediate therapy for chronic depression (Zarate et al., 2006). Burst suppression, the profound state of brain inactivation and unconsciousness manifest in the EEG as periods of quiescence interspersed with periods of burst-like electrical activity, is associated with coma, developmental brain disorders and hypothermia yet, can be readily induced by administration of anesthetics (Amzica, 2009, Lewis et al., 2013). Studies of anesthetic-induced burst suppression are providing new ideas regarding the mechanisms that underlie this brain state that can be produced by many seemingly different phenomena (Amzica, 2009, Ching et al., 2012, Liley et al., 2013). In addition, controlled anesthetic-induced burst suppression is the approach used to achieve a medically-induced coma as treatment for intractable seizures or intracranial hypertension following a brain injury (Hunter et al., 2012, Ching et al., 2013, Shanechi et al., 2013). Both propofol-induced unconsciousness and alpha coma are associated with large alpha oscillations suggesting that unconsciousness under propofol-induced general anesthesia may help unravel the pathophysiology and poor prognosis of alpha coma (Cimenser et al., 2011, Sutter et al., 2012, Purdon et al., 2013). Finally, dexmedetomidine provides a pharmacological model for studying sleep mechanisms (Nelson et al., 2003, Huupponen et al., 2008, Akeju et al., 2014). Low-dose dexmedetomidine induces slow-wave oscillations and spindles (9–15 Hz oscillations) similar to those seen in non-REM stage II sleep, whereas higher doses of this sedative produce slow-wave oscillations similar to those found in non-REM stage III (slow-wave) sleep (Huupponen et al., 2008, Akeju et al., 2014).
Future Directions and Clinical Implications
This is a retrospective report. Therefore, our observations should be confirmed in a prospective investigation that allows precise anesthetic delivery, and thereby, relates more directly the time course of the nitrous oxide dosing and the EEG changes. It will also be important to include a behavioral paradigm to define clearly the level of consciousness during the nitrous oxide-induced slow oscillations (Purdon et al., 2013). Are the nitrous oxide slow-delta oscillations a marker of profound unconscious, as in the case of slow oscillations due to bolus administration of propofol? Or, are these oscillations a marker of a sedative state from which a patient can be readily aroused as in the case of dexmedetomidine sedation (Akeju et al., 2014)? Conducting a prospective study with a larger cohort of patients or study subjects will allow us to understand better the between-subject variation in time of onset and duration of slow-delta oscillations induced by nitrous oxide.
As reported by Hagihira and colleagues, we have also observed that switching to nitrous oxide from isoflurane also produces slow-delta oscillations (Hagihira et al., 2012). We have observed a similar EEG dynamics in the transition from the ether anesthetic, desflurane and from propofol. It will be beneficial to study prospectively the slow-delta oscillatory dynamics induced by the transition from these anesthetics as well. The transition to nitrous oxide from an ether anesthetic differs from the transition from propofol. High-flow delivery of nitrous oxide with oxygen and air facilitates emergence from an inhaled ether anesthetic by changing the flow gradients whereas switching from propofol, an intravenous anesthetic, produces no such change. Finally, if our NMDA-glutamatergic hypothesis is correct, then lesion, local pharmacological inhibition and/or optogenetic inactivation of glutamatergic circuits in the parabrachial nucleus and/or the medial pontine reticular formation should result in slow-delta oscillations and decreased arousal.
In summary, slow-delta oscillations can be produced by administering high-dose nitrous oxide. Further study of these oscillations should offer new insights into the neural circuit mechanisms of nitrous oxide and make possible more informed use of this agent during management of general anesthesia. These studies will also provide deeper insights into the neurophysiology of the brain’s arousal circuitry.
Supplementary Material
Time courses of anesthetic concentrations (upper subpanels); spectrograms (middle subpanels); and the time-domain signature of sevoflurane and the time-domain signature of nitrous oxide-induced slow-delta oscillations (lower subpanels) for Patients G–S. The start of nitrous oxide and the decrease of sevoflurane are indicated by the black dotted vertical line. The time courses of the anesthetic concentrations, sevoflurane (blue curve) and nitrous oxide (red curve), are shown in the upper subpanels. The spectrograms (middle subpanels) during the sevoflurane period show large oscillations in the alpha frequency band (8–12 Hz) that dissipate with the transition to nitrous oxide. After the nitrous oxide concentration plateaus, the spectrograms show increased power in the slow-delta frequency band (0.1–4 Hz) that persists for several minutes. Ten-second EEG traces recorded during the sevoflurane period (*) and during the midpoint of the nitrous oxide-induced slow-delta oscillation period (‡) are shown in the lower subpanels. Slow-delta oscillations are present in both periods. However, the slow-delta oscillations are significantly enhanced after the transition to nitrous oxide (See Figure 2D). The red vertical lines in panels G, J, O, R and S are either an electrocautery or movement artifact.
Highlights.
Slow-delta oscillations occur with the administration of high-dose (concentration > 60% and total gas flow rate > 4 liters per minute) nitrous oxide.
Our findings are in contrast to the beta oscillations commonly observed with lower concentrations (20–40%) of nitrous oxide.
These slow-delta oscillations may result from nitrous oxide-induced blockade of major excitatory inputs (NMDA glutamate projections) from the brainstem to the thalamus and cortex.
Acknowledgments
Supported by grants DP1-OD003646 (to ENB), DP2-OD006454 (to PLP) and TR01-GM104948 (to ENB) from the National Institutes of Health, Bethesda, Maryland; Foundation for Anesthesia Education and Research Award (to OA), Rochester, Minnesota; funds from the Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital, Boston, Massachusetts.
Footnotes
Conflict of Interest
None of the authors have potential conflicts of interest to be disclosed.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akeju O, Westover MB, Pavone KJ, Sampson AL, Hartnack KE, Brown EN, et al. Effects of sevoflurane and propofol on frontal electroencephalogram power and coherence. Anesthesiology. 2014;121:990–8. doi: 10.1097/ALN.0000000000000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkire MT, Hudetz AG, Tononi G. Consciousness and anesthesia. Science. 2008;322:876–80. doi: 10.1126/science.1149213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amzica F. Basic physiology of burst-suppression. Epilepsia. 2009;50 (Suppl 12):38–9. doi: 10.1111/j.1528-1167.2009.02345.x. [DOI] [PubMed] [Google Scholar]
- Andrews E. The oxygen mixture, a new anesthetic combination. Chicago Medical Examiner. 1868;9:656–61. [PMC free article] [PubMed] [Google Scholar]
- Avramov MN, Shingu K, Mori K. Progressive changes in electroencephalographic responses to nitrous oxide in humans: a possible acute drug tolerance. Anesth Analg. 1990;70:369–74. doi: 10.1213/00000539-199004000-00005. [DOI] [PubMed] [Google Scholar]
- Babadi B, Brown EN. A review of multitaper spectral analysis. IEEE Trans Biomed Eng. 2014;61:1555–64. doi: 10.1109/TBME.2014.2311996. [DOI] [PubMed] [Google Scholar]
- Bai D, Pennefather PS, MacDonald JF, Orser BA. The general anesthetic propofol slows deactivation and desensitization of GABA(A) receptors. J Neurosci. 1999;19:10635–46. doi: 10.1523/JNEUROSCI.19-24-10635.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biller J. Stroke in children and young adults. Philadelphia, PA: Saunders; 2009. [Google Scholar]
- Boon JA, Milsom WK. NMDA receptor-mediated processes in the Parabrachial/Kolliker fuse complex influence respiratory responses directly and indirectly via changes in cortical activation state. Respir Physiol Neurobiol. 2008;162:63–72. doi: 10.1016/j.resp.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Breshears JD, Roland JL, Sharma M, Gaona CM, Freudenburg ZV, Tempelhoff R, et al. Stable and dynamic cortical electrophysiology of induction and emergence with propofol anesthesia. Proc Natl Acad Sci U S A. 2010;107:21170–5. doi: 10.1073/pnas.1011949107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EN, Lydic R, Schiff ND. General anesthesia, sleep, and coma. N Engl J Med. 2010;363:2638–50. doi: 10.1056/NEJMra0808281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EN, Purdon PL, Van Dort CJ. General anesthesia and altered states of arousal: a systems neuroscience analysis. Annu Rev Neurosci. 2011;34:601–28. doi: 10.1146/annurev-neuro-060909-153200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EN, Solt K, Purdon PL, Akeju O. Miller’s Anesthesia. 8. Elsevier; 2014. Monitoring the Depth of Anesthesia. [Google Scholar]
- Casali AG, Gosseries O, Rosanova M, Boly M, Sarasso S, Casali KR, et al. A theoretically based index of consciousness independent of sensory processing and behavior. Sci Transl Med. 2013;5:198ra05. doi: 10.1126/scitranslmed.3006294. [DOI] [PubMed] [Google Scholar]
- Ching S, Cimenser A, Purdon PL, Brown EN, Kopell NJ. Thalamocortical model for a propofol-induced alpha-rhythm associated with loss of consciousness. Proc Natl Acad Sci U S A. 2010;107:22665–70. doi: 10.1073/pnas.1017069108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching S, Liberman MY, Chemali JJ, Westover MB, Kenny JD, Solt K, et al. Real-time closed-loop control in a rodent model of medically induced coma using burst suppression. Anesthesiology. 2013;119:848–60. doi: 10.1097/ALN.0b013e31829d4ab4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching S, Purdon PL, Vijayan S, Kopell NJ, Brown EN. A neurophysiological-metabolic model for burst suppression. Proc Natl Acad Sci U S A. 2012;109:3095–100. doi: 10.1073/pnas.1121461109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu TH, Chen MJ, Yang YR, Yang JJ, Tang FI. Action of dexmedetomidine on rat locus coeruleus neurones: intracellular recording in vitro. Eur J Pharmacol. 1995;285:261–8. doi: 10.1016/0014-2999(95)00417-j. [DOI] [PubMed] [Google Scholar]
- Cimenser A, Purdon PL, Pierce ET, Walsh JL, Salazar-Gomez AF, Harrell PG, et al. Tracking brain states under general anesthesia by using global coherence analysis. Proc Natl Acad Sci U S A. 2011;108:8832–7. doi: 10.1073/pnas.1017041108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa-Sales C, Rabin BC, Maze M. A hypnotic response to dexmedetomidine, an alpha 2 agonist, is mediated in the locus coeruleus in rats. Anesthesiology. 1992;76:948–52. doi: 10.1097/00000542-199206000-00013. [DOI] [PubMed] [Google Scholar]
- Crunelli V, Hughes SW. The slow (<1 Hz) rhythm of non-REM sleep: a dialogue between three cardinal oscillators. Nat Neurosci. 2010;13:9–17. doi: 10.1038/nn.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor M, Zalkind V. Reversible analgesia, atonia, and loss of consciousness on bilateral intracerebral microinjection of pentobarbital. Pain. 2001;94:101–12. doi: 10.1016/S0304-3959(01)00345-1. [DOI] [PubMed] [Google Scholar]
- Eger EI, 2nd, Koblin DD, Harris RA, Kendig JJ, Pohorille A, Halsey MJ, et al. Hypothesis: inhaled anesthetics produce immobility and amnesia by different mechanisms at different sites. Anesth Analg. 1997;84:915–8. doi: 10.1097/00000539-199704000-00039. [DOI] [PubMed] [Google Scholar]
- Englot DJ, Yang L, Hamid H, Danielson N, Bai X, Marfeo A, et al. Impaired consciousness in temporal lobe seizures: role of cortical slow activity. Brain. 2010;133:3764–77. doi: 10.1093/brain/awq316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulconer A, Pender JW, Bickford RG. The influence of partial pressure of nitrous oxide on the depth of anesthesia and the electro-encephalogram in man. Anesthesiology. 1949;10:601–9. doi: 10.1097/00000542-194909000-00010. [DOI] [PubMed] [Google Scholar]
- Feshchenko VA, Veselis RA, Reinsel RA. Propofol-induced alpha rhythm. Neuropsychobiology. 2004;50:257–66. doi: 10.1159/000079981. [DOI] [PubMed] [Google Scholar]
- Foster BL, Liley DT. Nitrous oxide paradoxically modulates slow electroencephalogram oscillations: implications for anesthesia monitoring. Anesth Analg. 2011;113:758–65. doi: 10.1213/ANE.0b013e318227b688. [DOI] [PubMed] [Google Scholar]
- Foster BL, Liley DT. Effects of nitrous oxide sedation on resting electroencephalogram topography. Clin Neurophysiol. 2013;124:417–23. doi: 10.1016/j.clinph.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Fuller PM, Sherman D, Pedersen NP, Saper CB, Lu J. Reassessment of the structural basis of the ascending arousal system. J Comp Neurol. 2011;519:933–56. doi: 10.1002/cne.22559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulwiler CE, Saper CB. Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain. 1984;319:229–59. doi: 10.1016/0165-0173(84)90012-2. [DOI] [PubMed] [Google Scholar]
- Hagihira S, Takashina M, Mori T, Mashimo T. The impact of nitrous oxide on electroencephalographic bicoherence during isoflurane anesthesia. Anesth Analg. 2012;115:572–7. doi: 10.1213/ANE.0b013e3182575b70. [DOI] [PubMed] [Google Scholar]
- Hemmings HC, Jr, Akabas MH, Goldstein PA, Trudell JR, Orser BA, Harrison NL. Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol Sci. 2005;26:503–10. doi: 10.1016/j.tips.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hudetz AG. General anesthesia and human brain connectivity. Brain Connect. 2012;2:291–302. doi: 10.1089/brain.2012.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter G, Young GB. Status epilepticus: a review, with emphasis on refractory cases. Can J Neurol Sci. 2012;39:157–69. doi: 10.1017/s0317167100013160. [DOI] [PubMed] [Google Scholar]
- Huupponen E, Maksimow A, Lapinlampi P, Sarkela M, Saastamoinen A, Snapir A, et al. Electroencephalogram spindle activity during dexmedetomidine sedation and physiological sleep. Acta Anaesthesiol Scand. 2008;52:289–94. doi: 10.1111/j.1399-6576.2007.01537.x. [DOI] [PubMed] [Google Scholar]
- Jevtovic-Todorovic V, Todorovic SM, Mennerick S, Powell S, Dikranian K, Benshoff N, et al. Nitrous oxide (laughing gas) is an NMDA antagonist, neuroprotectant and neurotoxin. Nat Med. 1998;4:460–3. doi: 10.1038/nm0498-460. [DOI] [PubMed] [Google Scholar]
- Jones BE, Bobillier P, Pin C, Jouvet M. The effect of lesions of catecholamine-containing neurons upon monoamine content of the brain and EEG and behavioral waking in the cat. Brain Res. 1973;58:157–77. doi: 10.1016/0006-8993(73)90830-5. [DOI] [PubMed] [Google Scholar]
- Jorm CM, Stamford JA. Actions of the hypnotic anaesthetic, dexmedetomidine, on noradrenaline release and cell firing in rat locus coeruleus slices. Br J Anaesth. 1993;71:447–9. doi: 10.1093/bja/71.3.447. [DOI] [PubMed] [Google Scholar]
- Kaur S, Pedersen NP, Yokota S, Hur EE, Fuller PM, Lazarus M, et al. Glutamatergic signaling from the parabrachial nucleus plays a critical role in hypercapnic arousal. J Neurosci. 2013;33:7627–40. doi: 10.1523/JNEUROSCI.0173-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis LD, Ching S, Weiner VS, Peterfreund RA, Eskandar EN, Cash SS, et al. Local cortical dynamics of burst suppression in the anaesthetized brain. Brain Res. 2013;136:2727–37. doi: 10.1093/brain/awt174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis LD, Weiner VS, Mukamel EA, Donoghue JA, Eskandar EN, Madsen JR, et al. Rapid fragmentation of neuronal networks at the onset of propofol-induced unconsciousness. Proc Natl Acad Sci U S A. 2012;109:E3377–86. doi: 10.1073/pnas.1210907109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liley DT, Walsh M. The Mesoscopic Modeling of Burst Suppression during Anesthesia. Front Comput Neurosci. 2013;7:46. doi: 10.3389/fncom.2013.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashour GA. Integrating the science of consciousness and anesthesia. Anesth Analg. 2006;103:975–82. doi: 10.1213/01.ane.0000232442.69757.4a. [DOI] [PubMed] [Google Scholar]
- Mennerick S, Jevtovic-Todorovic V, Todorovic SM, Shen W, Olney JW, Zorumski CF. Effect of nitrous oxide on excitatory and inhibitory synaptic transmission in hippocampal cultures. J Neurosci. 1998;18:9716–26. doi: 10.1523/JNEUROSCI.18-23-09716.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhuircheartaigh R, Warnaby C, Rogers R, Jbabdi S, Tracey I. Slow-wave activity saturation and thalamocortical isolation during propofol anesthesia in humans. Sci Transl Med. 2013;5:208ra148. doi: 10.1126/scitranslmed.3006007. [DOI] [PubMed] [Google Scholar]
- Mitra P, Bokil H. Observed brain dynamics. Oxford; New York: Oxford University Press; 2008. [Google Scholar]
- Mizobe T, Maghsoudi K, Sitwala K, Tianzhi G, Ou J, Maze M. Antisense technology reveals the alpha2A adrenoceptor to be the subtype mediating the hypnotic response to the highly selective agonist, dexmedetomidine, in the locus coeruleus of the rat. J Clin Invest. 1996;98:1076–80. doi: 10.1172/JCI118887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moga MM, Herbert H, Hurley KM, Yasui Y, Gray TS, Saper CB. Organization of cortical, basal forebrain, and hypothalamic afferents to the parabrachial nucleus in the rat. J Comp Neurol. 1990;295:624–61. doi: 10.1002/cne.902950408. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–7. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti MM, Lutkenhoff ES, Rubinov M, Boveroux P, Vanhaudenhuyse A, Gosseries O, et al. Dynamic change of global and local information processing in propofol-induced loss and recovery of consciousness. PLoS Comput Biol. 2013;9:e1003271. doi: 10.1371/journal.pcbi.1003271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosteller F, Rourke REK. Sturdy statistics: nonparametrics and order statistics. Reading, Mass: Addison-Wesley Pub. Co; 1973. [Google Scholar]
- Nelson LE, Guo TZ, Lu J, Saper CB, Franks NP, Maze M. The sedative component of anesthesia is mediated by GABA(A) receptors in an endogenous sleep pathway. Nat Neurosci. 2002;5:979–84. doi: 10.1038/nn913. [DOI] [PubMed] [Google Scholar]
- Nelson LE, Lu J, Guo T, Saper CB, Franks NP, Maze M. The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology. 2003;98:428–36. doi: 10.1097/00000542-200302000-00024. [DOI] [PubMed] [Google Scholar]
- Norden AD, Blumenfeld H. The role of subcortical structures in human epilepsy. Epilepsy Behav. 2002;3:219–31. doi: 10.1016/s1525-5050(02)00029-x. [DOI] [PubMed] [Google Scholar]
- Olney JW, Farber NB. NMDA antagonists as neurotherapeutic drugs, psychotogens, neurotoxins, and research tools for studying schizophrenia. Neuropsychopharmacology. 1995;13:335–45. doi: 10.1016/0893-133X(95)00079-S. [DOI] [PubMed] [Google Scholar]
- Percival DB, Walden AT. Spectral Analysis for Physical Applications. Cambridge University Press; 1993. [Google Scholar]
- Posner JB, Saper CB, Schiff N, Plum E. Plum and Posner’s Diagnosis of Stupor and Coma. Oxford University Press; USA: 2007. [Google Scholar]
- Purdon PL, Pierce ET, Mukamel EA, Prerau MJ, Walsh JL, Wong KF, et al. Electroencephalogram signatures of loss and recovery of consciousness from propofol. Proc Natl Acad Sci U S A. 2013;110:E1142–51. doi: 10.1073/pnas.1221180110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Loewy AD. Efferent connections of the parabrachial nucleus in the rat. Brain Res. 1980;197:291–317. doi: 10.1016/0006-8993(80)91117-8. [DOI] [PubMed] [Google Scholar]
- Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–63. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- Shanechi MM, Chemali JJ, Liberman M, Solt K, Brown EN. A brain-machine interface for control of medically-induced coma. PLoS Comput Biol. 2013;9:e1003284. doi: 10.1371/journal.pcbi.1003284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter R, Kaplan P. Electroencephalographic patterns in coma: when things slow down. Epileptologie. 2012;29:205–09. [Google Scholar]
- Yamamura T, Fukuda M, Takeya H, Goto Y, Furukawa K. Fast oscillatory EEG activity induced by analgesic concentrations of nitrous oxide in man. Anesth Analg. 1981;60:283–8. [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–64. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Time courses of anesthetic concentrations (upper subpanels); spectrograms (middle subpanels); and the time-domain signature of sevoflurane and the time-domain signature of nitrous oxide-induced slow-delta oscillations (lower subpanels) for Patients G–S. The start of nitrous oxide and the decrease of sevoflurane are indicated by the black dotted vertical line. The time courses of the anesthetic concentrations, sevoflurane (blue curve) and nitrous oxide (red curve), are shown in the upper subpanels. The spectrograms (middle subpanels) during the sevoflurane period show large oscillations in the alpha frequency band (8–12 Hz) that dissipate with the transition to nitrous oxide. After the nitrous oxide concentration plateaus, the spectrograms show increased power in the slow-delta frequency band (0.1–4 Hz) that persists for several minutes. Ten-second EEG traces recorded during the sevoflurane period (*) and during the midpoint of the nitrous oxide-induced slow-delta oscillation period (‡) are shown in the lower subpanels. Slow-delta oscillations are present in both periods. However, the slow-delta oscillations are significantly enhanced after the transition to nitrous oxide (See Figure 2D). The red vertical lines in panels G, J, O, R and S are either an electrocautery or movement artifact.