Abstract
Hyperactivity is a key symptom and the most observable manifestation of Attention-Deficit/Hyperactivity Disorder (ADHD). The over-activity associated with ADHD can cause specific challenges in academic settings, extracurricular activities and social relationships. Cognitive control challenges are also well-established in ADHD. The current study included 44 children between the ages of 10 and 17 diagnosed with ADHD or who were typically developing (TD), all of whom had no psychiatric co-morbidity or significant learning disorders. Participants wore an actometer on their ankle while performing a flanker paradigm in order to objectively measure their rates of activity in association with cognitive control. Analyses assessed the relationship between frequency and intensity of activity to task accuracy on trial by trial basis. A significant interaction effect between group and performance revealed that more intense movement was associated with better performance in the ADHD, but not TD group. The ADHD group demonstrated more intense activity than the TD group during correct (but not error) trials. Within-group, children with ADHD generated higher intensity movements in their correct trials compared to their error trials, whereas the TD group did not demonstrate any within-group differences. These findings suggest that excessive motoric activity associated with clinically significant ADHD symptoms may reflect compensatory efforts to modulate attention and alertness. Future research should systematically explore the relationship between motion in ADHD and how it might be used to improve cognitive performance.
Keywords: ADHD, cognitive control, physical activity, actigraphy, flanker
Introduction/Background
Hyperactivity is considered a core symptom of attention-deficit/hyperactivity disorder (ADHD) and is the most observable manifestation of the disorder. It is perhaps the earliest symptom associated with the disorder in a child’s life and can cause challenges in academic settings, extracurricular activities and social relationships. Yet, the role of hyperactivity in many current models of ADHD is minimized, viewed as incidental and unrelated to the cognitive challenges associated with the disorder (c.f. Rapport et al., 2009 for a review). The relationship between elevated rates of activity and ADHD is pervasive as the disorder is associated with increased movement and fidgeting (Corkum, Tannock, & Moldofsky, 1998; Rapport et al., 2009), even during sleep, (Corkum et al., 1998; Porrino et al., 1983) in both children and adults (Teicher, Polcari, Fourligas, Vitaliano, & Navalta, 2012). Several recent studies have documented increased rates of activity during the performance of cognitively-demanding tasks, such as working memory, stop-signal or choice tasks (Alderson, Rapport, Kasper, Sarver, & Kofler, 2012; Hudec, Alderson, Kasper, & Patros, 2014; Rapport et al., 2009). Rapport et al. (2009) hypothesized that increased movement may serve a compensatory function to augment under-arousal inherent to ADHD. In this model, hyperactivity is a secondary symptom reflective of unconscious efforts to increase arousal to meet cognitive demands. The model predicts that increased hyperactivity is associated with better cognitive performance for persons with ADHD.
It is especially important to define the relationship between movement and cognitive performance in ADHD due to the abundant evidence of cognitive control impairments in ADHD (de Zeeuw, Weusten, van Dijk, van Belle, & Durston, 2012; Dramsdahl, Westerhausen, Haavik, Hugdahl, & Plessen, 2011; Tsal, Shalev, & Mevorach, 2005). Cognitive control refers to processes that allow information processing and behavior to vary adaptively depending on current goals and includes working memory, attentional bias, inhibition and planning.
The purpose of this study was to examine the relationship between motor activity and accuracy, on a trial by trial basis, in cognitive control performance (e.g., flanker task) in ADHD and typically developing (TD) children. This is the first study we know of to assess the relationship between activity with task performance on a trial by trial basis in ADHD.
Method
We included 18 TD children and 26 children with either ADHD-Combined Presentation (ADHD-CO) or ADHD-Inattentive Presentation (ADHD-IA) after obtaining both informed, written parental consent and written assent. Participants were 10 – 17 years of age. The project was approved by the Institutional Review Board of the University of California, Davis.
We excluded participants if they met any of the following criteria: (a) IQ score < 85; (b) a math or reading learning disability; (c) history of head trauma, neurological disorder or major medical problem; (d) prescribed psychoactive medication besides ADHD medications (i.e., stimulants or atomoxetine) in the ADHD group; (e) meeting DSM-IV-TR criteria for any other Axis I diagnosis besides ADHD or oppositional defiant disorder in the ADHD group and any Axis I disorder including ADHD in the TD group. TD children were excluded for t-scores ≥ 65 on the Conners’ Rating Scale-Revised: Long Version (CPRS-R:L,(Conners et al., 1997)). Participants with ADHD were included if they had a Conners’ Total ADHD score ≥ 65 according to a parent in conjunction with a significant indication of ADHD on a structured clinical interview. We attempted to collect teacher ratings to substantiate diagnoses beyond the parent report to assess the pervasiveness of the symptoms across settings for children with ADHD or absence of significant symptoms in TDs; however, teacher reports were only available for only 11 children. Children who were unclear or borderline on diagnostic measures and did not have teacher ratings were not included in the study. All participants prescribed medication did not take it for 24 hours before the experimental testing session. Of the 26 ADHD participants, 18 had a history of taking stimulant medication (15 were currently prescribed stimulant medication) and 4 had a history of taking non-stimulant medication (1 was currently prescribed nonstimulant medication). Participants started taking medication on average at 10 years of age.
The parent version of the computerized Diagnostic Interview for Children and Adolescents (DICA-IV (Reich, 2000)) provided additional information to confirm the ADHD diagnosis and screen for other Axis I psychiatric disorders. Either the Wechsler Abbreviated Scale of Intelligence (WASI (Wechsler, 1999)) or Wechsler Intelligence Scale for Children (WISC-IV (Wechsler, 2003)) (n = 5) assessed participants’ intellectual abilities. The Letter-Word Identification and Calculation scales of the Woodcock-Johnson Tests of Achievement-Third Edition NU (WJ-III NU (Woodcock, McGrew, & Mather, 2001)) assessed reading and mathematical performance. Evidence of a math/reading disorder was considered present if a child performed below 80 on an achievement test and if there was a ≥1.5 SD difference between IQ and WJ-III scores.
Two licensed psychologists with extensive experience in ADHD determined eligibility and subtype according to Diagnostic and Statistical Manual of Mental Disorders, text revision (DSM-IV-TR) based on parent ratings, interview observations and any supplemental interview or information (i.e., teacher ratings, previous report cards or psychological evaluations). The presence of other psychiatric diagnoses was based on the DICA-IV and follow-up interviews.
Participants performed a cued variant of the Eriksen flanker paradigm (Fig. 1(Eriksen & Eriksen, 1974)) with stimuli presented using E-Prime (Psychology Software Tools, Inc., Sharpsburg, PA). Each trial began with a fixation point (crosshair) (500 ms) followed by a cue (1000 ms), which was one of three types: null, response preparation or warning cue, represented by a pair of blue and/or yellow cartoon hands with the color providing cue information. We collapsed across cue types for analyses reported in this manuscript, as cue types was not a variable of interest in this study. Following the cue and a subsequent fixation point (800 ms), participants were presented with the flanker stimuli (1300 ms). Trials were separated by a 400 ms intertrial interval with a total of 56 neutral, 56 congruent, and 92 incongruent stimuli.
Figure 1.
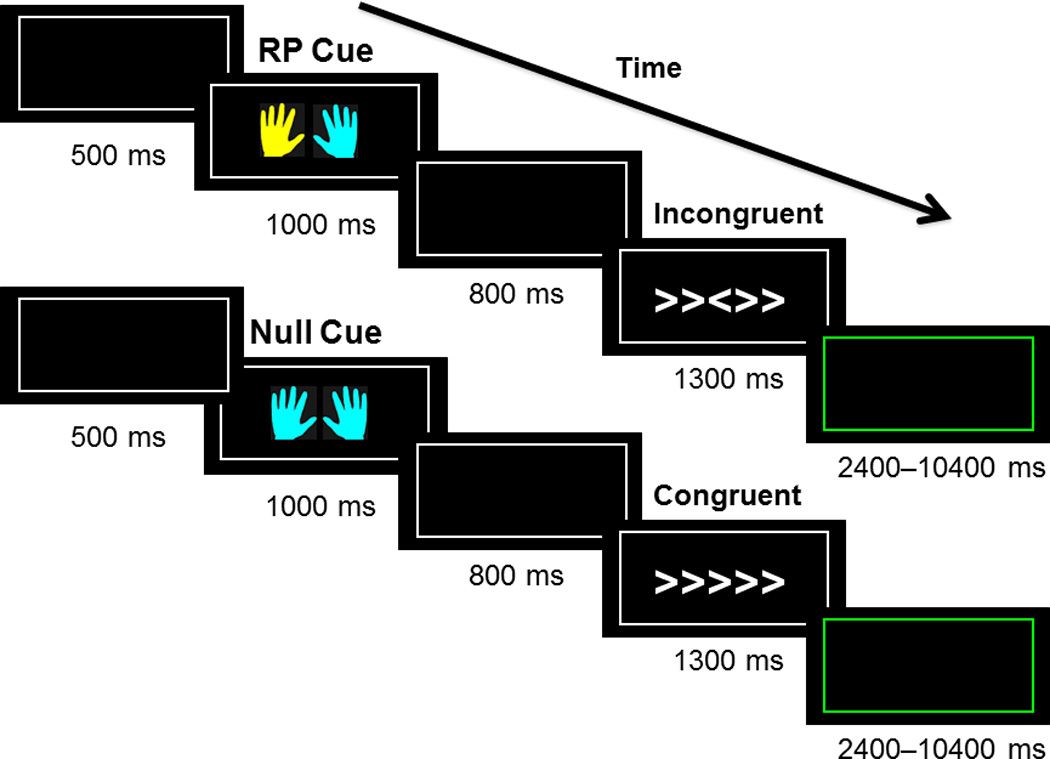
Depiction of the order in which the stimuli are presented to study participants. A fixation stimulus (black square) is presented, then a cue in the form of yellow and/or blue hands, a subsequent fixation stimulus and then the flanker stimuli. The cue color signaled which hand was likely to be the correct response for the upcoming target. Participants are instructed to respond with a button press in either their right or left hand corresponding with the direction of the central arrow head in the flanker stimuli. Trials could be congruent (five arrows all facing the same direction), incongruent (five arrows in which the central arrow is facing the opposite direction of the four flanking arrows), or neutral (an arrow in the middle with four flanking plus signs). Participants have 1300 ms to respond to the flanker stimuli before it proceeds to the next trial.
Participants were instructed to identify the orientation of the central symbol while ignoring the flanking symbols, indicating their response by pressing a button with the corresponding hand. Participant instructions emphasized both speed and accuracy. Trials could be congruent (five arrows all facing the same direction), incongruent (five arrows in which the central arrow is facing the opposite direction of the flanking arrows), or neutral (an arrow in the middle with four flanking plus signs). Our dependent variable was percent correct versus incorrect on the flanker trials.
We measured movement for each participant by affixing a Motionlogger® (Ambulatory Monitoring, Inc.) actometer to their ankle during task performance. The actometer is a wristwatch-like device that measures movement by creating a voltage signal proportion to the forces acting upon the device. The signal is then compared to a sensitivity threshold in the device and the comparison of these two values is mapped across time. We collected two movement measures. Proportional integrity mode (PIM) measured intensity of movement (i.e., area under the curve) and zero crossing mode (ZCM) measured frequency of movement (i.e., number of times that the accelerometer waveform crossed 0).
Flanker and actometer data were synced with each other to allow trial-by-trial analyses. Actometer data were averaged across each trial with movements for each 4-sec trial determined by the average of two 2-sec actometer samples. We first examined movement across cues separately for correct and incorrect trials. This preliminary analysis did not reveal any evidence that the pattern of group differences varied by cue. Therefore, we averaged the movement measures for correct and error trials across cues for each individual.
Statistical Analysis
Group differences in demographics, IQ, and Conners’ scores were assessed using Fisher’s exact tests for the categorical variables and Wilcoxon two-sample test for the continuous ones. The distributions for both PIM and ZCM were skewed to the right, thus they were squareroot transformed for the analyses. We used mixed-effects models (Laird & Ware, 1982) to characterize the relationship between diagnosis group, gender, and performance and intensity and frequency of movement. This approach allowed us to treat performance as a repeated effect, was flexible and could accommodate missing observations and heterogeneous variances (i.e., because the females were less variable than males). For each of the dependent variables, we first fitted a model with a main effect for group (ADHD or TD), performance (correct or incorrect), and their interaction, a term for gender and a random effect for person, to account for the correlation due to repeated measures on the same individual. A significant group by performance interaction indicates that the change in movement variable from correct to incorrect performance differs between the groups. If the interaction term was significant, we examined the simple contrasts of performance within levels of group (TD: Correct vs. Incorrect, ADHD: Correct vs. Incorrect), and simple comparisons of group within levels of performance (Correct: TD vs. ADHD, Incorrect: TD vs. ADHD). Standardized effect statistics (d) that accounted for the correlated nature of the data were derived from the mixed-effects analyses (Hedges, 2007). All tests were two-sided, with α = 0.05. All analyses were implemented using PROC MIXED in SAS Version 9.4 (SAS Institute Inc., Cary, NC).
Results and Discussion
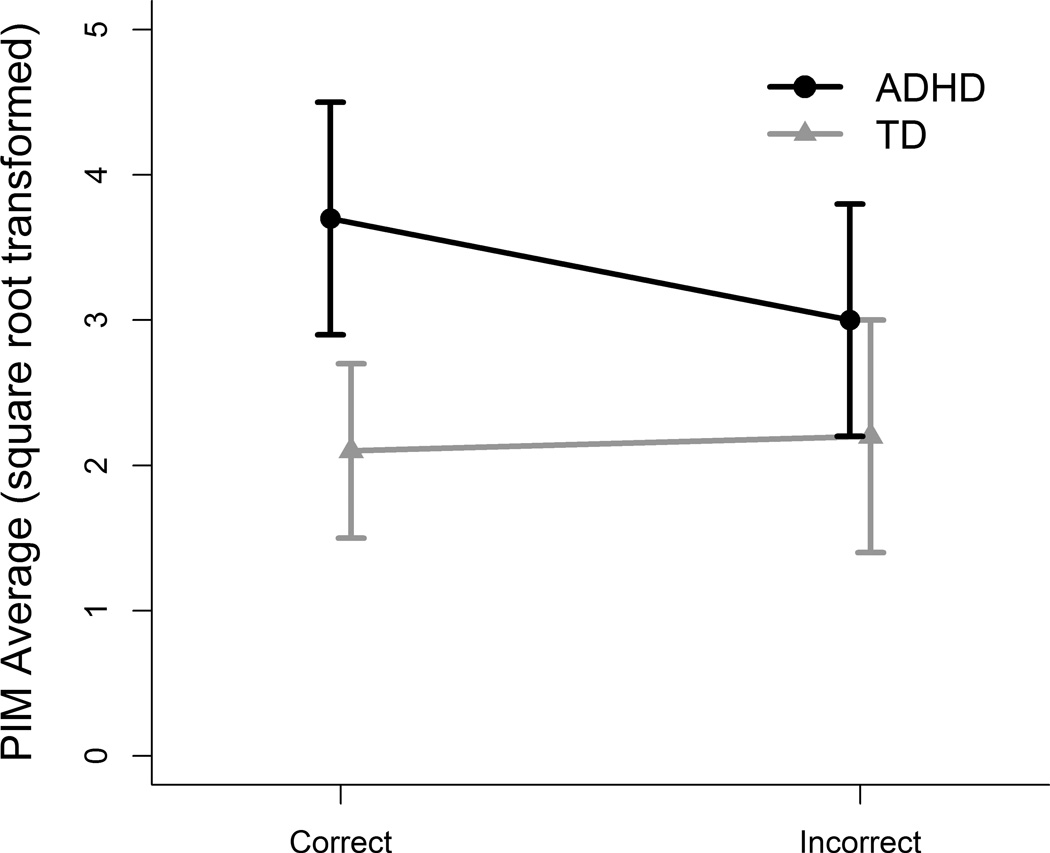
No significant differences between groups were found for age, IQ or gender. However, because the TD group had a higher percentage of females, gender was controlled for in the mixed-effects analyses. The results of the mixed effect models for performance for PIM revealed a significant diagnosis by performance interaction, F(1,40) = 4.23, p = .046. The ADHD group demonstrated significantly higher PIM during correct trials than the control group (estimated difference 1.52, SE = 0.55, d = 0.63, p = .009).The difference was not significant during incorrect trials (estimated difference 0.79, SE = 0.56, effect size = 0.33, p = .16). Within-group comparisons indicated that the ADHD group demonstrated significantly higher PIM during correct than incorrect trials (estimated difference 0.65, SE = 0.23, d= 0.37, p = .007) while the difference was not significant for the control group (estimated difference = −0.07, SE = 0.27, d = −0.04, p = .80).
The interaction between diagnosis and performance was not significant in the model predicting ZCM, F(1,40) = 1.35, p = .24. For this variable, the ADHD group showed higher values than the controls (estimated difference 0.40, SE = 0.17, d= 0.50, p = .03), regardless of the performance (correct or incorrect) of the trial. The main effect of performance was also not statistically significant F(1,41) = 0.01, p = .92.
The goal of this study was to examine the relationship between cognitive control task performance and physical activity and how this relationship differs in children with ADHD compared to TD children. The groups differed in regard to the intensity of physical activity during correct trials but not incorrect trials, with the ADHD group evidencing more intense rates of activity only for correct trials. This finding suggests that cognitive control functioning in ADHD may be enhanced by more intense activity or that when a child with ADHD is using more cognitive resources the child is also more likely to engage in physical activity. Similarly to Dane (2000), we did not find any ADHD presentation group differences (CO vs IA). IA activity intensity fell between the TDs and ADHD-COs, lowering the overall activity rates in the ADHD group. It is not known whether excess movement in ADHD-IA and CO are associated with the same mechanism at differing intensities or whether the excess movement in these presentations is associated with different underlying causes. In an EEG-coherence study (Mazaheri et al., 2013), we found that cognitive control related-EEG functioning differed between the ADHD-CO and ADHD-IA groups, suggesting that underlying neural mechanisms may differ. Our modest sample size may not have been sufficient to identify statistical differences.
One possible mechanism for a relationship between movement and performance is that children with ADHD use movement to self-regulate alertness. An optimal level of arousal is required for peak cognitive performance (Yerkes & Dodson, 1908). Our data support Rapport et.al’s (2009) model that hyperactivity serves a “purposeful function” to compensate for underarousal and acts to improve cognitive performance. Howell et al. (2012) suggested a model where individuals with ADHD exhibit decreased tonic firing of the locus coeruleus-norepinephrine system (LC-NE) resulting in decreased cortical arousal and poor attentional performance. By increasing their activity rates, individuals with ADHD may be able to stimulate the LC-NE system resulting in increased arousal allowing them to work at optimal levels (Dishman, 1997).
Limitations and Future directions
We recommend follow-up studies with a larger sample size to assess for potential differences related to gender and ADHD presentation. We did not find a statistical difference between genders; however, a larger sample may be needed. This study was conducted in a controlled, laboratory setting with limited distractions and children with ADHD may manage their restlessness in different ways in a more naturalistic environment. Our study placed the actometer on one limb (i.e., the ankle), in contrast to the majority of other studies examining ADHD-related activity, in which data were collected from actometers placed on ankles and a non-dominant hand (Dane et al., 2000; Porrino et al., 1983; Rapport et al., 2009). Subsequent studies should assess if there are differences in findings related to whether data are collected from a hand or multiple sites.
Future research should investigate how opportunities for physical activities, particularly ones that are neither disruptive nor stigmatizing, can be used in academic settings to help children perform cognitively-demanding tasks. While there are currently tools on the market (e.g., fidget toys) that allow individuals to fidget without disrupting others, there has not been extensive research on their efficacy. Our findings also suggest that limiting movement in children with ADHD may potentially be detrimental to their cognitive performance.
Figure 2.
Diagnosis by performance interaction for intensity of movement (PIM). The ADHD group showed significantly more intense movements during correct vs. incorrect trials, whereas the TD group showed no significant difference in movement intensity during correct vs. incorrect trials. The ADHD group moved significantly more than the TD group during correct trials, but there was no difference between the diagnostic groups for incorrect trials.
Table 1.
Demographic and clinical characteristics for the participants
| TD (n = 18) |
ADHD (n = 26) |
|
|---|---|---|
| Age (years) | 14.5 (1.4) | 14.3 (2.3) |
| Female | 13 (72%) | 12 (46%) |
| IQ | 116.4 (10.3) | 114.0 (11.6) |
| Conners’ DSM Scores | ||
| Inattentive | 44.3 (3.1)* | 80.1 (10.9)* |
| Hyperactive-impulsive | 44.9 (3.0)* | 67.1 (18.9)* |
| Average PIM | ||
| Correct trials | 6.4 (8.9)* | 17.3 (17.1)* |
| Incorrect trials | 6.8 (8.1) | 12.4 (15.5) |
| Average ZCM | ||
| Correct trials | 0.9 (1.0)* | 2.0 (1.5)* |
| Incorrect trials | 1.2 (1.2)* | 1.9 (1.5)* |
Note: TD = typically developing;
Groups were compared using Fisher’s exact test for gender and Wilcoxon two-sample test for age, IQ, and Conner’s scores. Differences between ADHD and controls in average PIM and ZCM were estimated from mixed-effects regression models fitted to squared-root transformed data and adjusted for gender.
p< .05 for TD vs. ADHD comparison.
Acknowledgements
We would like to thank the children and their families for their participation. We are grateful to Catherine Fassbender, Ph.D., for the development of the version of the flanker task used in this study and to J. Faye Dixon, Ph.D., for her expertise in diagnostic issues. We also gratefully acknowledge the anonymous reviewers for their insightful suggestions that have considerably strengthened the manuscript. This work was supported by the National Institute of Mental Health (1R01MH091068) by a Children’s Miracle Network grant, and by a MIND Institute Pilot grant. Statistical support was provided by the MIND Institute Intellectual and Developmental Disabilities Research Center (U54 HD079125).
References
- Alderson RM, Rapport MD, Kasper LJ, Sarver DE, Kofler MJ. Hyperactivity in boys with attention deficit/hyperactivity disorder (ADHD): the association between deficient behavioral inhibition, attentional processes, and objectively measured activity. Child Neuropsychol. 2012;18(5):487–505. doi: 10.1080/09297049.2011.631905. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) Washington, D.C: American Psychiatric Publishing, Incorporated; 2013. [Google Scholar]
- Conners CK, Wells KC, Parker JD, Sitarenios G, Diamond JM, Powell JW. A new self-report scale for assessment of adolescent psychopathology: factor structure, reliability, validity, and diagnostic sensitivity. J Abnorm Child Psychol. 1997;25(6):487–497. doi: 10.1023/a:1022637815797. [DOI] [PubMed] [Google Scholar]
- Corkum P, Tannock R, Moldofsky H. Sleep disturbances in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1998;37(6):637–646. doi: 10.1097/00004583-199806000-00014. [DOI] [PubMed] [Google Scholar]
- Dane AV, Schachar RJ, Tannock R. Does actigraphy differentiate ADHD subtypes in a clinical research setting? J Am Acad Child Adolesc Psychiatry. 2000;39(6):752–760. doi: 10.1097/00004583-200006000-00014. [DOI] [PubMed] [Google Scholar]
- de Zeeuw P, Weusten J, van Dijk S, van Belle J, Durston S. Deficits in cognitive control, timing and reward sensitivity appear to be dissociable in ADHD. PLoS One. 2012;7(12):e51416. doi: 10.1371/journal.pone.0051416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishman RK. Brain monoamines, exercise, and behavioral stress: animal models. Med Sci Sports Exerc. 1997;29(1):63–74. doi: 10.1097/00005768-199701000-00010. [DOI] [PubMed] [Google Scholar]
- Dramsdahl M, Westerhausen R, Haavik J, Hugdahl K, Plessen KJ. Cognitive control in adults with attention-deficit/hyperactivity disorder. Psychiatry Res. 2011;188(3):406–410. doi: 10.1016/j.psychres.2011.04.014. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception and Psychophysics. 1974;16:143–149. [Google Scholar]
- Hedges LV. Effect Sizes in Cluster-Randomized Designs. J Education Behav Stat. 2007;32:341–370. [Google Scholar]
- Howells FM, Stein DJ, Russell VA. Synergistic tonic and phasic activity of the locus coeruleus norepinephrine (LC-NE) arousal system is required for optimal attentional performance. Metab Brain Dis. 2012;27(3):267–274. doi: 10.1007/s11011-012-9287-9. [DOI] [PubMed] [Google Scholar]
- Hudec KL, Alderson RM, Kasper LJ, Patros CH. Working memory contributes to elevated motor activity in adults with ADHD: an examination of the role of central executive and storage/rehearsal processes. J Atten Disord. 2014;18(4):357–368. doi: 10.1177/1087054713497398. [DOI] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38(4):963–974. [PubMed] [Google Scholar]
- Mazaheri A, Fassbender C, Coffey-Corina S, Hartanto TA, Schweitzer JB, Mangun GR. Differential Oscillatory Electroencephalogram Between Attention-Deficit/Hyperactivity Disorder Subtypes and Typically Developing Adolescents. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Rapoport JL, Behar D, Sceery W, Ismond DR, Bunney WE., Jr A naturalistic assessment of the motor activity of hyperactive boys. I. Comparison with normal controls. Arch Gen Psychiatry. 1983;40(6):681–687. doi: 10.1001/archpsyc.1983.04390010091012. [DOI] [PubMed] [Google Scholar]
- Rapport MD, Bolden J, Kofler MJ, Sarver DE, Raiker JS, Alderson RM. Hyperactivity in boys with attention-deficit/hyperactivity disorder (ADHD): a ubiquitous core symptom or manifestation of working memory deficits? J Abnorm Child Psychol. 2009;37(4):521–534. doi: 10.1007/s10802-008-9287-8. [DOI] [PubMed] [Google Scholar]
- Reich W. Diagnostic interview for children and adolescents (DICA) J Am Acad Child Adolesc Psychiatry. 2000;39(1):59–66. doi: 10.1097/00004583-200001000-00017. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Polcari A, Fourligas N, Vitaliano G, Navalta CP. Hyperactivity persists in male and female adults with ADHD and remains a highly discriminative feature of the disorder: a case-control study. BMC Psychiatry. 2012;12:190. doi: 10.1186/1471-244X-12-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsal Y, Shalev L, Mevorach C. The diversity of attention deficits in ADHD: the prevalence of four cognitive factors in ADHD versus controls. J Learn Disabil. 2005;38(2):142–157. doi: 10.1177/00222194050380020401. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio: Harcourt Assessment; 1999. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children: Fourth Edition. San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson III Battery. Itasca, IL: The Riverside Publishing Company; 2001. [Google Scholar]
- Yerkes R, Dodson J. The relation of strength of stimulus to rapidity of habit-forming. Journal of Comparative Neurology and Psychology. 1908;18(5):459–482. [Google Scholar]