Abstract
Background
Numerous psychiatric and behavioral disorders such as Autism, Attention Deficit Disorder and Schizophrenia may involve disruptions in the development of the mesocortical dopamine pathway, consisting of dopaminergic projections from the midbrain ventral tegmental area (VTA) to the medial prefrontal cortex (mPFC). Nuclear steroid hormone receptors are powerful transcription factors and can profoundly and permanently alter fundamental processes of neural development. Nuclear progesterone receptor (PR) is transiently expressed in both the VTA and the PFC of rodents during perinatal life, suggesting that PR may regulate the normal development of this important behavioral circuit.
Methods and Results
Here, we demonstrate that virtually all PR immunoreactive (PRir) cells in the VTA also express tyrosine hydroxylase-ir (THir). In addition, retrograde tract tracing reveals that many PRir cells in the VTA project to the mPFC. Administration of a PR antagonist to rats during the neonatal period decreased THir fiber density in prelimbic mPFC of juveniles (P25) and decreased levels of THir in the VTA of adults. Neonatal treatment with a PR antagonist impaired adult performance on a passive inhibitory avoidance task and an attentional set shift task, measures of behavioral inhibition/impulsivity and cognitive flexibility, respectively. THir levels in VTA were reduced and cognitive flexibility was impaired in PR knockout mice as well.
Conclusions
These findings provide novel insights into a potential role for PR in the developmental etiology of behavioral disorders that involve impairments in complex cognitive behaviors and have implications for the use of synthetic progestins in humans during critical neurodevelopmental periods.
Keywords: progesterone receptor, dopamine, development, cognitive flexibility
Introduction
The mesocortical dopamine pathway consists of dopaminergic projections from the midbrain ventral tegmental area (VTA; A10) to cortical areas, especially the prefrontal cortex (PFC). Dopamine release in the PFC is critical for ‘executive functions,’ such as attention, working memory, behavioral inhibition and cognitive flexibility. Even slight perturbations during critical periods of development can cause permanent alterations in the function of this pathway and may underlie some of the cognitive deficits in behavioral disorders such as schizophrenia [1, 2] and attention deficit disorder [3-7].
The mesocortical dopamine pathway may be sensitive to steroid hormone action during development. Nuclear steroid hormone receptors are powerful transcription factors and regulate fundamental processes of neural development. Progesterone receptor (PR) is expressed throughout the perinatal rat brain including brain regions associated with cognitive function [8-10]. PR immunoreactivity (PRir) and PR mRNA are transiently expressed in the subplate and pyramidal cell layers of all functional regions (frontal, motor, somatosensory, auditory, visual) of the fetal and neonatal cortex, including prefrontal cortex [10,11]. Preliminary data also suggests that PR is transiently expressed in the neonatal mPFC. In addition, PR is expressed during perinatal life in several midbrain nuclei including the VTA [8,12]. The timing of PR expression in VTA and PFC corresponds with periods of significant dendritic maturation of mPFC neurons and synaptogenesis for dopaminergic axons within the frontal cortex [13-15], suggesting that PR activity may be important for establishing functional connectivity. Consistent with this idea, progesterone treatment during postnatal life increased the number of midbrain neurons expressing tyrosine hydroxylase (TH), the rate limiting enzyme in the synthesis of dopamine [16] and altered levels of dopamine and its metabolites in the prefrontal cortex in adulthood [17].
The present study characterizes the full ontogeny and phenotype of PRir cells in the mesocortical pathway and tests the hypothesis that PR activity during perinatal life plays a critical role in the development of dopaminergic innervation of mPFC and subsequent mPFC-dependent cognitive behaviors in adulthood. Results demonstrate that PR is expressed in dopaminergic cells of the VTA and within VTA cells that project to the mPFC in neonates, and that inhibition of PR activity decreases dopaminergic innervation of prelimbic mPFC, decreases TH expression in the VTA and impairs cognitive flexibility and behavioral inhibition in adulthood. These results provide valuable insight into the normal development and potential susceptibilities of this critical behavioral circuit and further elucidate the importance of steroid hormones and their receptors in neural development.
Methods
Animals
All animal procedures were approved by the Institutional Animal Care and Use Committee at the University at Albany. Pregnant female Sprague-Dawley rats were purchased from Taconic Laboratories (Germantown, NY) at gestational day 16 and were allowed to deliver pups normally. The day of birth was designated as postnatal day 1 (P1). Mice used in this study were of a mixed background (C129SvEv X C57Bl), and were either homozygous for an insertional mutation in the PR gene (PRKO) or were homozygous for the wildtype PR gene (WT) and were bred at UAlbany by crossing heterozygous males and females. PRKO mice were created by the insertion of a neomycin resistance gene and a lacZ reporter gene into Exon 1, thereby inhibiting the synthesis of functional PR transcripts and preventing the expression of both PRA and PRB isoforms of the receptor protein [18]. All animals were housed on a reverse 12-h light, 12-h dark cycle at a constant temperature of 25 ± 2° C, with food and water available ad libitum.
RU486 administration
Male rat pups were treated daily with the PR antagonist RU486 (20mg/kg, s.c. in sesame oil [19, 20]; Mifepristone, Sigma Aldrich, St. Lois, MO) or an equal volume of the oil vehicle alone from P1-P14, the period spanning peak PR expression in the VTA and PFC.
Determination of genotype
For mice, ear tissue was collected by ear punch for determination of genotype. Tissue was first lysed using proteinase K in a water bath heated to 55°C overnight, after which total genomic DNA was purified and amplified using the Qiagen DNeasy Blood and Tissue kit. PCR was performed on purified DNA using three separate primers for PR and Neomycin (PR: 5’- TAGACAGTGTCTTAGACTCGTTGTTG-3’; 5’-GATGGGCACATGGATGAAAC-3’; Neomycin: 5’-GCATGCTCCAGACTGCCTTGGGAAA-3’). Using gel electrophoresis, genotype was determined by the molecular weight of the extracted PCR product. Treatment groups were counterbalanced by litter, such that WT and PRKO mice from at least five different litters were represented in each group to avoid possible litter effects.
Tissue collection
All rats and mice in these experiments were sexed using anogenital distance as a marker. Neonatal rats were anesthetized by hypothermia, while juvenile and adult rats and mice were administered a lethal injection of sodium pentobarbital (Sleepaway; Zoetis). All animals were euthanized by decapitation, and brains were rapidly removed and immersed in 5% acrolein in 0.1M phosphate buffer (pH 7.6) for 6 hours, then cryoprotected in 30% sucrose in 0.1M phosphate buffer for 72h. Brains were sectioned in the coronal plane at 50μm on a freezing microtome and stored in cryoprotectant (30% sucrose, 0.1% polyvinyl-pyrrolidone-40 in ethylene glycol and 0.1M phosphate buffer) until immunocytochemical processing.
Progesterone receptor immunocytochemistry
Free-floating sections were rinsed in 0.05M tris-buffered saline (TBS), incubated in 1% sodium borohydride in TBS for 10 min, and blocked in TBS containing 20% normal goat serum (NGS), 1% hydrogen peroxide and 1% bovine serum albumin (BSA) for 30 min. Sections were then incubated in primary antisera against PR (rabbit polyclonal; DAKO, Carpinteria, CA) (1:1000 in TTG: TBS containing 0.3% Triton-X-100 and 2% NGS) for 72 hours. This rabbit polyclonal antisera was raised against the following sequence of the PR protein, amino acids 533-547, which is the DNA binding domain of the human PR protein and is contained in both A and B isoforms of the receptor [21]. The specificity of this antisera has been previously demonstrated by our lab [8, 10]. Nuclear immunoreactivity is completely eliminated by preabsorption of the PR antiserum overnight with either the antigen peptide or a 10-fold molar excess of human PRA and PRB proteins [20, 22] or when the primary antiserum was omitted. In addition, all immunoreactivity is absent in PRKO mice [10].
Following primary antibody incubation, sections were rinsed 3 times in TTG (5 min each) and placed in a solution containing 5μg/ml goat anti-rabbit biotinylated secondary antibody in TTG for 90 min at room temperature, followed by incubation in Avidin-Biotin Complex in TBS (Vectastain Elite Kit, Vector Labs) for 1 hour. Sections were then rinsed three times in TBS, then incubated in TBS containing 0.05% diaminobenzadine, 0.75nM nickel ammonium sulfate, 0.15% β-D glucose, 0.04% ammonium chloride and 0.001% glucose oxidase for 10 minutes. After being rinsed in TBS, sections were mounted on gelatin-coated slides and coverslipped with Permount (Fisher Scientific, Pittsburgh, PA).
Tyrosine hydroxylase immunocytochemistry
Immunocytochemical procedures were the same as described above, except tissue was incubated in TH antisera (rabbit polyclonal; Millipore) for 48h at 1:50,000 for THir cell bodies in the VTA and at 1:1000 for THir fibers in the mPFC.
Double immunocytochemistry
For the colocalization of PRir and THir and the colocalization of PRir and Fluorogold (FG), free-floating sections were incubated in 1% sodium borohydride in TBS for 10 min, then blocked in 20% normal goat serum, 1% BSA and 1% hydrogen peroxide in TBS for 30 min. Sections were incubated in both antisera against PR (1:500, rabbit polyclonal, DAKO corp.) and antisera against TH (1:50,000, mouse monoclonal, Millipore), or antisera against PR (1:500) and antisera against Fluorogold (1:500, guinea pig monoclonal, Protos Bio-tech) diluted in TTG for 72 hours. For adequate visualization of PR, the immunohistochemical signal was enhanced using the Tyramide Signal Amplification (TSA) method (PerkinElmer Inc.). Briefly, after primary antibody incubation, the tissue was incubated in biotinylated goat-anti-rabbit secondary antibody (5μg/ml) in TTG for 90 min at room temperature, followed by streptavidin-horseradish peroxidase (SA-HRP) and Biotinyl Tyramide solutions. Finally, tissue was incubated for 1 hour in a solution containing streptavidin-conjugated fluorophore to detect PR (1:200, Alexa Fluor 488, Invitrogen Inc.), along with a goat-anti-mouse fluorescently-tagged secondary antibody for the detection of TH, or goat-anti-guinea pig for Fluorogold (1:200, Alexa Fluor 568), both diluted in TBS. Sections were then mounted on slides and coverslipped with ProLong Gold Antifade reagent (Invitrogen, Carlsbad, CA).
Behavioral testing
Male rat subjects were raised/treated as described above. At P90, all experimental animals underwent daily pre-test handling (1 min per day) for one week prior to testing. One cohort of rats was tested on the Inhibitory Avoidance Task. To test the effects of RU486 on cognitive flexibility, an additional cohort of rats was tested on the Attentional Set-Shift Task. Last, a cohort of adult WT and PRKO mice was used to test cognitive flexibility in the adapted water maze task [23]. In these mice, testing commenced at P60. All testing procedures were conducted by an experimenter blind to treatment group.
Experiment 1: ontogeny of progesterone receptor expression in the rat ventral tegmental area and mPFC
For the VTA ontogeny, caesarean sections were performed to collect fetal tissue on embryonic day 16 (E16), E18 and E20 (three litters per age group). Briefly, pregnant females were given a lethal dose of sodium pentobarbital. The abdominal cavity was opened and the uterine horns were removed and placed on ice. Following removal from the uterine horn, each fetus was placed on ice prior to sacrifice by decapitation. Neonatal brains were collected at P1, P7, P14 and P28 and fixed in 5% acrolein. All tissue was fixed, cut and processed for immunocytochemistry for PR as described above. For the mPFC ontogeny, animals were sacrificed at P2, P6, P10, P15, P20, P25 and P30. For both the VTA and mPFC ontogenies, tissue from 5 males and 5 females was collected for each age group. For both the VTA and mPFC ontogeny, the exact dates were chosen based on previous studies from our laboratory [9, 11].
A representative, anatomically-matched section from the VTA and mPFC of each animal was selected for image analysis. Microscopic images were captured with a Nikon Eclipse E600 microscope fitted with a SPOT Insight camera connected to a Dell Inspiron computer. Scion Image software (Frederick, MD) was used to analyze captured images. The relative amount of PRir was determined as described previously [20, 24] by measuring the area (μm2) covered by “thresholded” pixels [i.e. those pixels with a gray level higher than a defined threshold density (specific immunoreactive staining)]. “Threshold” was determined as a constant function of the background optical density defined as the mean optical density three to five SDs higher than the mean background density. The mean background density was measured in a region devoid of PRir immediately adjacent to the analyzed region containing PRir. Statistical analyses were performed for each region using a two-way ANOVA (sex × age), followed by pre-planned, post-hoc comparisons using Student-Newman-Keuls (p<0.05).
Experiment 2: characterization of the phenotype of PRir cells in the neonatal rat VTA
Experiment 2a: PR expression in dopaminergic cells of the VTA during development
Tissue was collected from 5 male and 5 female rats at P1 and P7, and processed for double immunocytochemistry for PRir and THir as described above. Analysis of the co-expression of PR and TH was conducted using a Zeiss LSM 510 confocal microscope. Images were taken sequentially at 63X magnification using an Argon laser for 488 wavelengths (for PR detection) and a HeNe laser for 568 wavelengths (for TH detection). The z-stack consisted of 21 sequential images at 1μm each. For each animal, three matching representative brain sections through the VTA were observed. For each of the three sections per animal, 10 cells which were immunoreactive for PR were sampled at 63x from two different sites per section, from the parabrachial and paranigral subregions of the VTA, such that approximately 30 cells for each animal were considered for this analysis. From this sample the percentage of cells that were immunoreactive for both PR and TH was estimated.
Experiment 2b: PR expression in VTA cells projecting to mPFC
Male and female pups (n=20) were anesthetized by hypothermia on P3 and their heads secured using a stereotaxic apparatus adapted for neonatal rats (David Kopf Instruments, Tujunga, CA). Using neonatal rat stereotaxic coordinates (1.5-2.5mm ventral, 2.0mm rostral to bregma) [25], a 1.0μl Hamilton syringe needle containing FG (Hydroxystilbamidine: 0.2μl of 2% FG in sterile water), was lowered through the skull and into the frontal cortex targeting the prelimbic (PL) and infralimbic (IL) mPFC. FG was injected using a microinfusion pump (World Precision Instruments) at 0.005μl per second. Following recovery from anesthesia, pups were returned to their home cages for a three-day survival period, after which brain tissue was collected at P6, as described above, and immersion fixed in 5% acrolein. FG fluorescence was analyzed on sections through the mPFC to determine the accuracy and the extent of the FG injections. Only animals whose FG injections were limited to the PL and IL mPFC were included for this analysis. While all injections hit the mPFC, a total of 7 animals were excluded from the study due to FG infiltration tissue outside of the PL/IL. In addition, as a control for FG specificity, two animals intentionally received injections into the striatum. In these subjects, it was confirmed that FG-labeled cells were observed in the substantia nigra and not the VTA. Analysis of PR and FG colocalization was conducted on a remaining 11 animals (6 male, 5 female).
Sections through the VTA were immunocytochemically processed for FG and for PRir as described above. Three representative matching sections from the VTA were chosen for each animal. Confocal images at 63X magnification were taken sequentially using an Argon laser for 488 wavelengths (for PR detection) and a HeNe laser for 568 wavelengths (for FG detection). Analysis was limited to regions of the VTA that contained both PR and FG staining. From the three sections used, approximately 10 PRir cells per section were randomly selected for a total of 30 cells per animal. A semi-quantitative assessment of the percentage of PRir cells in the VTA that were co-localized with FG was conducted.
Experiment 3: PR activity during development and levels of tyrosine hydroxylase in the VTA
Because no sex differences were observed in expression of PR or in the phenotype of PRir cell, the following studies were conducted in male rats treated with sesame oil or RU486, and in male WT and PRKO mice. Brain tissue was collected from male rats (RU486 and oil treated) at P25 and P90 and from male mice (WT and PRKO) at P25 and P70 (n=9 or 10 per group). P25 represents the developmental period prior to significant synaptic pruning in mPFC and P70 (mice) and P90 (rats) represents an adult stage of full maturity of mPFC [26-29]. Tissue was processed for TH immunocytochemistry as described above. The density of TH-expressing cells was determined in one matching representative section from each animal. Labeled THir cells were counted in one representative section containing the parabrachial and paranigral VTA using a 10 × 10 grid reticule attached to the eye piece of a Nikon Eclipse E600 microscope. This grid was large enough such that all THir cells within the representative section were counted.
Additionally, using Scion Image software, TH optical density was measured in the VTA to determine relative levels of TH expression as opposed to the number of cells expressing TH. Using Scion Image software, the VTA from one representative anatomically matched section per animal was digitally traced, defined by borders outlined in Paxinos & Watson (1998) [30]. Using a 20x objective, photographs were acquired as described in Experiment 1. The optical density scale used to measure relative TH immunoreactivity was inverted such that zero was white and 254 was black, (i.e., a higher number indicating higher relative levels of THir. T-tests were used for experiments using rats and mice for TH cell counts and TH optical density.
Experiment 4: PR activity during development and dopaminergic innervation of the mPFC
Sections containing PFC were collected/processed from animals used in Experiment 3, and processed for TH immunocytochemistry as described above. Analysis of TH fiber density in the mPFC was accomplished using previously established methods [31]. Briefly, representative, anatomically-matched sections through the mPFC were used to capture photographs in darkfield illumination using a Model 1.3.0 SPOT digital camera (Diagnostic Instruments) attached to a Nikon E600 microscope using a 20x objective. The density of THir fibers (area covered by thresholded pixels per square unit area) was quantified using Scion Image as described previously. Thresholded pixels were distinguished as being above a predetermined background level that was devoid of TH staining. Separate photographs were acquired from layers I-II and from layers V-VI. Separation of these layers is made identifiable based on THir fiber orientation [32, 33]. The PL mPFC was identified based on proximity to the forceps minor corpus callosum, easily identifiable by its white matter composition [30]. Treatment groups were compared using separate Students t-tests for layers II and V of each mPFC subregion.
Experiment 5: PR activity during neonatal life in rats and behavioral inhibition in adulthood
Neonatal rats were treated as described above with either RU486 or sesame oil (n=9 per group). In adulthood, animals underwent a two-trial passive inhibitory avoidance task. This task measures both long-term memory of negative consequences and behavior inhibition and may be influenced by activity in the mPFC [34-36]. In trial one, rats were placed into a chamber lit by ambient room light, with access to an adjoining enclosed, dark chamber, which is accessible via the opening of an electronic door after a 30s habituation period. One second after the rats entered the dark chamber they received a mild, but aversive, inescapable foot shock (0.5mA, 3 sec). In Trial 2, 48hr later, rats were placed back into the lit chamber and the latency to re-enter the dark chamber was measured, with a maximum latency of 900 seconds. A shorter latency to reenter the dark chamber is indicative of impaired memory and/or a deficit in behavioral inhibition. A two-way ANOVA (Day × Treatment) will be used to assess performance across days, followed by a nonparametric Mann-Whitney U-test was used to compare latencies on the second day of the task.
Experiment 6: inhibition of PR activity during neonatal life and cognitive flexibility in adulthood
Attentional set shift task in rats
Neonatal rats were treated as described above with RU486 or sesame oil (n=9 per group) and tested in the attentional set-shift task at P90, which assesses cognitive flexibility in rats. Prior to testing, food was restricted to 15g per day, maintaining body weight at 80-90% of free-feeding weight to ensure motivation for a food reward. Animals were handled and weighed daily to ensure that body weights did not drop below 80% free feeding weight. The testing apparatus is an adaptation of a plus maze, in which the arms of the maze are painted either white or black and the floors have either a rough or smooth texture. All experimental procedures are identical to those described in Stefani and Moghaddam, (2006) [37].
Pre-testing procedures
All animals underwent extensive pre-test handling and habituation to the apparatus for 11 days prior to testing. Food restriction was continued throughout the testing procedure. On days 1-5, each animal was handled by the experimenter for one minute, then given 3-4 sucrose pellets (45mg, Dustless Precision Pellets, Bio-Serv, Frenchtown, NJ) in their home cages. On days 6-8, rats underwent maze habituation in which all 4 arms of the maze were baited with sucrose pellets. Habituation trials lasted until all pellets were eaten or a maximum of 5 minutes. On days 9- 11, one arm of the maze was blocked, and animals were trained to make an arm “choice” by leaving the start arm and entering one of two open arms of the maze. For these days, there were 8 consecutive training trials in which 50% of their “choices” were rewarded in a random manner.
Testing Procedures
Testing was conducted on two consecutive days, consisting of multiple trials on each day (“Sets” 1 and 2). For both sets, the maze was arranged as it was in the training trials such that animals placed in alternating start arms always had an option of turning right or left into one of two target arms. In set 1, animals were trained to enter baited arms using one specific cue (based on arm color or texture) to earn a food reward. Set 1 ended when a performance criteria of 8 consecutive correct trials was met. Animals unable to reach this criterion within 120 trials would be excluded from the study. In set 2, (24 hours later) the “rule” or contingency for the reward was changed to a different sensory modality (e.g. food reward switched from light arms to rough arms). In set 2, all animals completed 80 trials regardless of performance. This extra-dimensional shift (from one sensory domain to another) is dependent on dopaminergic activity in the mPFC [38-42]. The number of trials to achieve criterion on test day 1, the percent of trials with correct choices across trial “blocks” (8 consecutive trials per block) on test day 2 and the number of perseverative errors (i.e., incorrect responses based on the reward contingency from test day 1) were analyzed. A perseveration error is defined as making a choice indicative of the first rule association after the rule has changed in set 2. “Omission” errors, incorrect choices that are not representative of either rule 1 or rule 2, were also counted. Because of the cap on the maximum number of trials, trials to criterion in Set 1 were analyzed using the Mann-Whitney U test. A two-way ANOVA (treatment × trial block) was used to analyze percent correct across set two. Additionally, separate two-way ANOVAs were conducted for the first half (trial blocks 1-5) and the second half (trial blocks 1-6). Student's t-tests were used to compare the number of perseveration and omission errors between groups on Set 2.
Water maze task in adult WT and PRKO mice
WT and PRKO mice (n=10 per group) were tested in a water maze task at P60. Procedures for this adapted water maze test were adopted from Karatsoreos et al. (2011) [23] to assess cognitive flexibility in mice. Male WT and PRKO mice were housed with littermates throughout the duration of testing, and all tests were conducted during the animals’ dark phase to enhance activity level. Testing was conducted in a plastic tank filled with water (26 °C) made opaque with powdered milk. Trials were recorded using ANY-maze behavior-tracking software (Stoelting, Wood Dale, IL) by an experimenter blind to treatment group.
The task occurred over 5 consecutive days (4 trials per day). In each trial, the latency to reach a “safety” platform was recorded with a maximum latency of 60s. On day 1, to assess swimming ability and motivation, animals were placed into the maze with a visible platform. On days 2 and 3, the platform remained in the same location in the maze, but was submerged below the water surface and was no longer visible, requiring spatial memory of the location of the platform. Latency to reach the platform on days 2 and 3 assess long-term spatial memory. On day 4, the location of the platform was moved to an opposite quadrant of the maze requiring animals to learn this new location. On day 5, the platform is in the same location as on day 4. Latency to reach the platform on days 4 and 5 represent cognitive flexibility, the ability to learn the position of the new platform location while inhibiting the tendency to revert back to the first location of the safety platform. On days 4 and 5, the number of entries into the “old quadrant” (the quadrant where the platform was located on days 1-3) was recorded as perseveration errors [23]. Swim speed was also recorded for all trials as a control to ensure that differences in latency are indicative of differences in cognition and not motor abnormalities. Differences in the latency to the platform across days was analyzed using two-way repeated measures ANOVA (genotype × day). Students t-tests were used for between group comparisons of swim speed (cm/second) and entries into the old quadrant.
Results
Experiment 1: PR is transiently expressed in the rat VTA and mPFC during neonatal life
Preliminary evidence suggested that PR was expressed in both the VTA and the mPFC during development in the rat. In the first experiment, we sought to determine the developmental window during which PR immunoreactive cells are present in the VTA and mPFC.
VTA
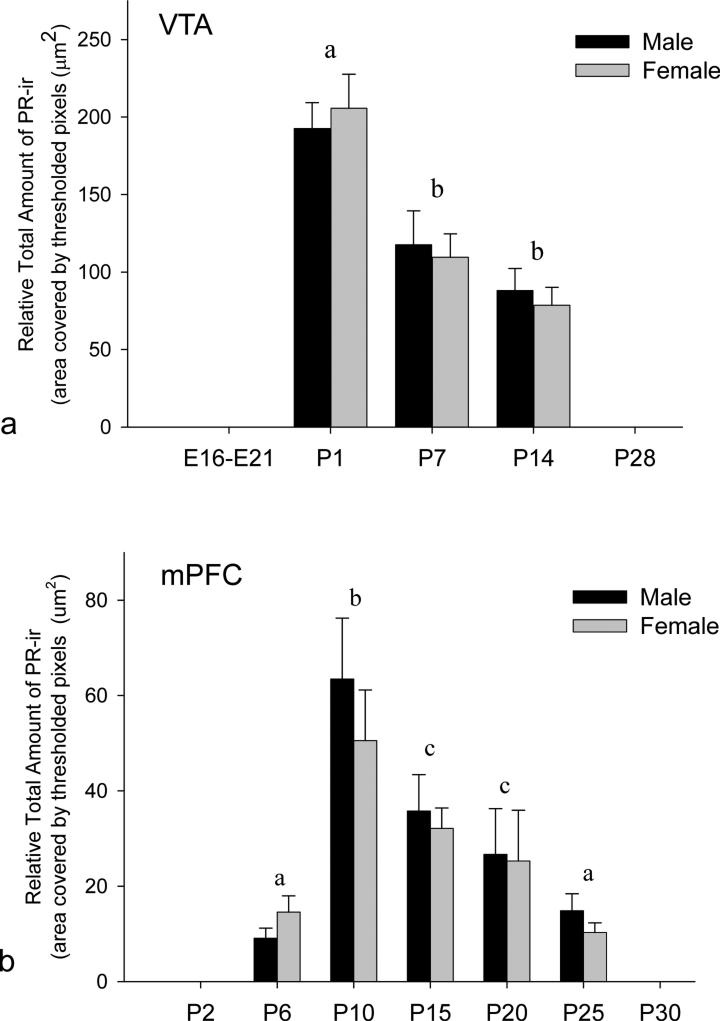
PR immunoreactivity was not observed at E16, E18 or E21. However, PRir was present within the parabrachial and paranigral VTA on P1, P7 and P14, but absent by P28 (fig. 1a). For the relative amount of PRir in the VTA, two-way ANOVA (age × sex) revealed a significant main effect of age (F2,29=21.988, p<0.001), but no significant effect of sex (p=0.923) and no significant interaction (p=0.780). PR expression in the VTA was highest on P1 in males and females, and thereafter, declined on P7 and P14 (p< 0.05) through P14 and was virtually absent by P28. Student Newman Keuls post hoc analysis revealed a significant difference between P1 and both P7 and P14 (p<0.05).
Figure 1. Transient expression of PR in the rat VTA and mPFC during development.
The mean (+/− sem) relevant total amount of nuclear PRir in (a) the VTA and (b) the mPFC (prelimbic). (a) Nuclear PRir was not detectable in the VTA prenatally (E16-E21), but was transiently expressed from the day of birth (P1) through at least P14, and absent by P28. PRir levels were highest on P1 with a significant decline at P7 and P14. (b) In the mPFC, PRir was not detectable on P2, but was expressed from P6 through P25, peaking around P10 and absent by P30. Postnatal ages with differing letters were significantly different from one another (p<0.05). There was no significant effect of sex at any age in either region.
mPFC
PRir was present in layers II/III of mPFC. PR expression was first detected on P6, peaked on P10, then progressively declined and was undetectable at P30 (fig. 1b). A two-way ANOVA (Age × Sex) revealed a main effect of age (F4,50=11.623, p<0.001), no effect of sex (p=0.512) and no age by sex interaction (p=0.837). Post hoc analysis revealed a significant difference between P10 and every other age examined. No PRir cells were detected in any other mPFC layers. PRir was not detected at P2 or P30.
Experiment 2: PR is expressed in dopaminergic cells and cells projecting to mPFC in the rat VTA
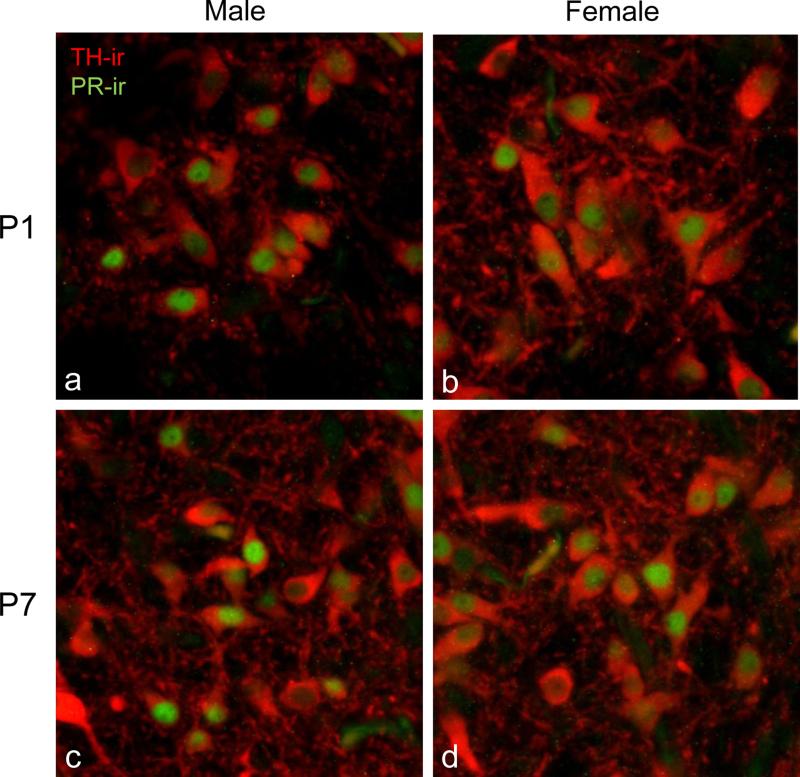
In order to determine if PR was expressed in dopaminergic cells of the VTA, we examined the colocalization of PRir and tyrosine hydroxylase-ir (THir) in males and females on P1 and P7 using confocal microscopy. In both sexes, more than 90% of PR immunoreactive cells also contained TH-ir at P1 and P7 in the parabrachial and paranigral subdivisions of the VTA (fig. 2) THir was limited to the cytoplasm and dendrites, allowing for clear visualization of PRir in the nucleus of individual cells.
Figure 2. PR is expressed in dopaminergic cells of the rat VTA.
Colocalization of PRir (green; nuclear) and tyrosine hydroxylase-ir (THir) (red; cytoplasmic) in the neonatal VTA. (a) P1 male (b) P1 female (c) P7 male (d) P7 female. More than 90% of the PRir cells in the parabrachial VTA were dopaminergic.
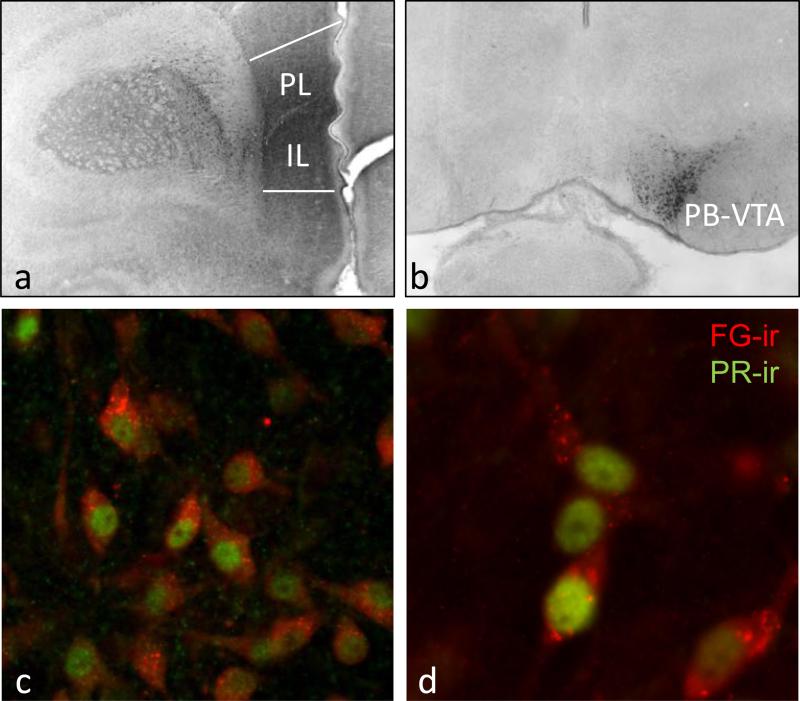
Because PR was expressed in dopaminergic projection neurons of VTA, we next examined whether PR was expressed in VTA cells that project to mPFC. The retrograde tracer Fluorogold was injected into the PL and IL mPFC on P3 and confocal microscopy was used to examine the colocalization of FG and PRir in VTA on P6. FG injections that were limited to the PL/IL mPFC (fig. 3a) labeled many cells of the VTA (fig. 3b). FG injections that missed PL/IL mPFC resulted in no FG labeling of the VTA. As an additional specificity control, FG injections intentionally made into the striatum labelled cells in the substantia nigra, but not the VTA. Approximately 50% of the FG labeled cells in VTA also expressed nuclear PRir (fig. 3c,d). It is important to note that in addition to the PR/FG co-localized cells, there were also approximately 50% of the PRir cells were not immunoreactive for FG, and approximately 50-60% of the FGir cells did not express PR. Taken together, these results suggest that PR is transiently expressed during development in midbrain VTA cells of the mesocortical dopaminergic pathway to mPFC.
Figure 3. PR is expressed in VTA cells that project to mPFC in neonatal rats also immunoreactive for PR.
The retrograde tracer Fluorogold (FG) was injected into the prelimbic (PL) and infralimbic (IL) mPFC, and labeled cells of the VTA that were immunoreactive for PR. (a) FG immunoreactivity within the IL and PL mPFC at the injection site (b) retrogradely labelled FG immunoreactive cells within the parabrachial VTA (PB-VTA) (c, d) colocalization of PRir (green; nuclear) and FGir (red; cytoplasmic) in the VTA of a male at P6.
Experiment 3: PR activity during development regulates levels of tyrosine hydroxylase in VTA
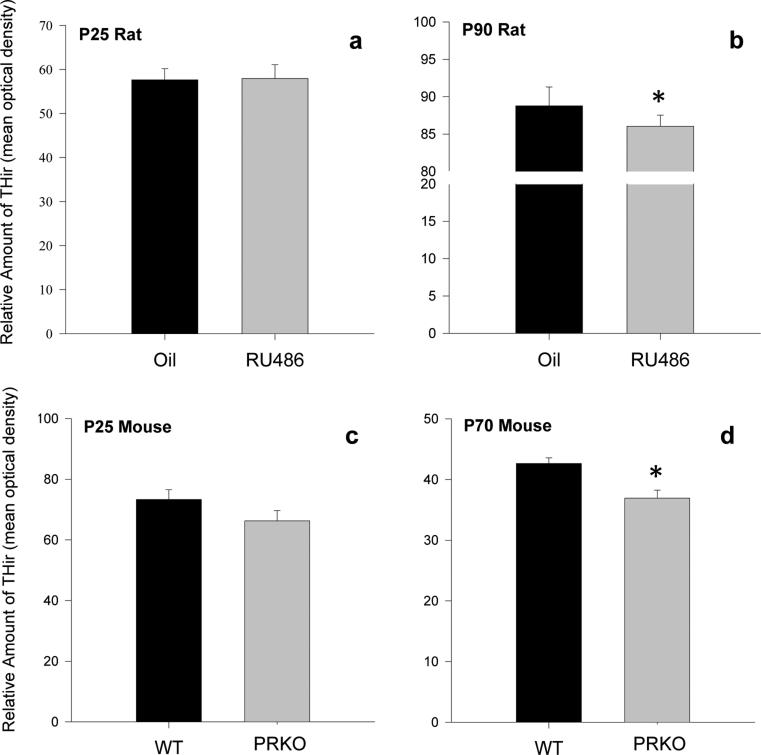
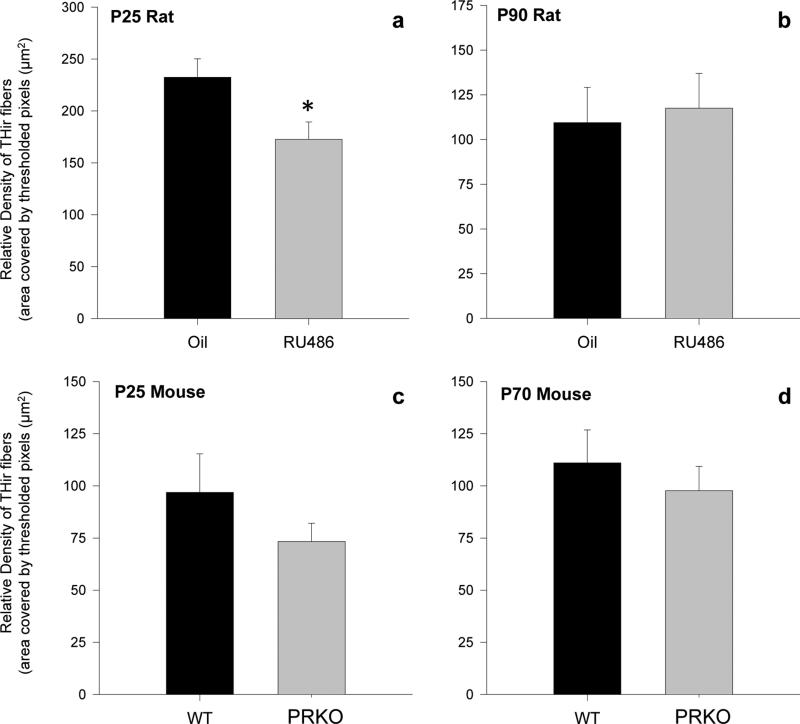
PR is transiently expressed in dopaminergic cells of the VTA during postnatal development. To determine whether PR activity may regulate dopamine synthesis later in life, we examined mean levels of THir per cell in the VTA in rats treated with a PR antagonist during neonatal life and in PRKO mice in both juveniles and adults. At P25, there was no effect of RU486 treatment in rats, or genotype in mice on relative levels of THir in the VTA (optical density). (fig. 4a,c). In contrast, levels of THir were significantly lower in the VTA of RU486 rats (t=2.681, p=0.017) and PRKO mice (t=2.587, p=0.012) compared to controls in adulthood (P90 and P70 respectively) (fig. 4b,d). There was no effect of treatment or genotype on the number of THir cells in VTA at P25 or in adulthood in either rats or mice (data not shown). These results suggest that disruption of PR activity during early life alters the development of the VTA in such a way as to decrease dopamine synthesis sometime after, but not prior to, adolescence.
Figure 4. Inhibition of PR activity during development decreases tyrosine hydroxylase levels in the adult VTA.
Relative total amount of tyrosine hydroxylase-ir (THir) in the VTA of (a) P25 rats or (b) P90 rats following administration of the PR antagonist RU486 (20mg/kg) or the oil vehicle during neonatal life or in the VTA of (c) P25 or (d) P70 wildtype or PR knockout mice * significantly different from same-age counterpart. Data are presented as mean + s.e.m., *p<0.05.
Experiment 4: PR activity during development influences dopaminergic innervation of the mPFC
PR is transiently expressed in dopaminergic cells of the VTA during a period of dopaminergic synaptogenesis and pruning in the prefrontal cortex. We therefore tested the hypothesis that PR activity during neonatal life is important for dopaminergic innervation of the mPFC. Neonatal administration of RU486 in rats significantly decreased the density of THir fibers within layer II of the prelimbic mPFC in rats at P25 (t= 2.44, p=0.024), but not at P90 (fig. 5a,b). RU486 had no significant effect on THir fiber density in Layer V at either age in rats (Data not shown). In mice, there were no effects of genotype on THir fiber density in either layer V or layer II of the P25 or P70 mouse mPFC (fig. 5c,d).
Figure 5. Inhibition of PR activity during development alters dopaminergic innervation of mPFC in adolescent rats.
The relative density of tyrosine hydroxylase-ir (THir) fibers within layer II of prelimbic mPFC in rats at P25 (a) or P90 (b) following administration of the PR antagonist or the oil vehicle during neonatal life .There was a trend toward reduced THir fiber density in mPFC in PRKO mice at P25 (c), there were significant no differences in the mouse mPFC at either age (c, d). Data are presented as mean + s.e.m., *p<0.05.
Experiment 5: Inhibition of PR activity during development increases impulsivity in adulthood
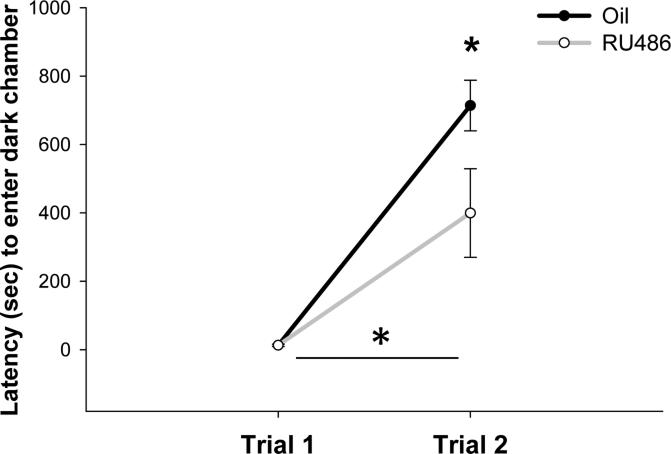
Evidence thus far suggested that PR is transiently expressed in both the mPFC and in dopaminergic cells of the VTA that project to the mPFC generating the hypothesis that PR activity during development may be important for cognitive behaviors in which the mesocortical dopamine pathway is critical. In the first behavioral experiment, we examined behavioral inhibition/impulsivity using the active avoidance task in adult rats treated with the PR antagonist as neonates. A two-way ANOVA revealed a significant main effect of Treatment (F1,17=4.938, p=0.035), a main effect of Day (F1,17=59.165, p=0.034) and a significant Treatment by Day interaction (F1,17=4.942, p=0.034) (fig. 6). Two follow-up non parametric Mann-Whitney rank sum tests showed that there was no difference between groups on Day 1, but there was a significant difference in latency on Day 2 (T= 38.0, p=0.04). Control rats approached maximum latency, whereas RU486 treated rats entered the dark chamber in about half the time. These results suggest that disruption of PR activity during development impairs long term memory for an aversive event, and/or behavioral inhibition, the ability to inhibit a motivated behavior despite a previous negative consequence. This may also be interpreted as an increase in impulsivity (i.e., engaging in a behavior without considering potential risks.
Figure 6. Inhibition of PR activity during neonatal life decreases behavioral inhibition/increases impulsivity in adulthood.
Neonatal administration of the PR antagonist RU486 impaired performance on the inhibitory avoidance task compared to oil treated controls. The latency (sec) to enter the dark chamber was not significantly different between the groups in Trial 1. In Trial 2, both groups had significantly longer latencies compared to Trial 1, in effect, an avoidance of the dark chamber indicating a memory for the aversive event in Trial 1 in both groups. However, latency to enter the dark chamber was significantly shorter for RU486 treated rats in Trial 2 compared to controls suggesting an impaired ability to inhibit a motivated response or an increase in impulsivity. Data are presented as mean + s.e.m., *p<0.05.
Experiment 6: Disruption of PR activity during development impairs cognitive flexibility and increases perseveration in adult rats and mice
To examine the role of PR activity in cognitive flexibility, which is dependent upon dopamine release in the mPFC, we utilized the Set Shift Task in adult rats treated neonatally with the PR antagonist or we used an adapted water maze in adult PRKO mice.
Set shift task in rats
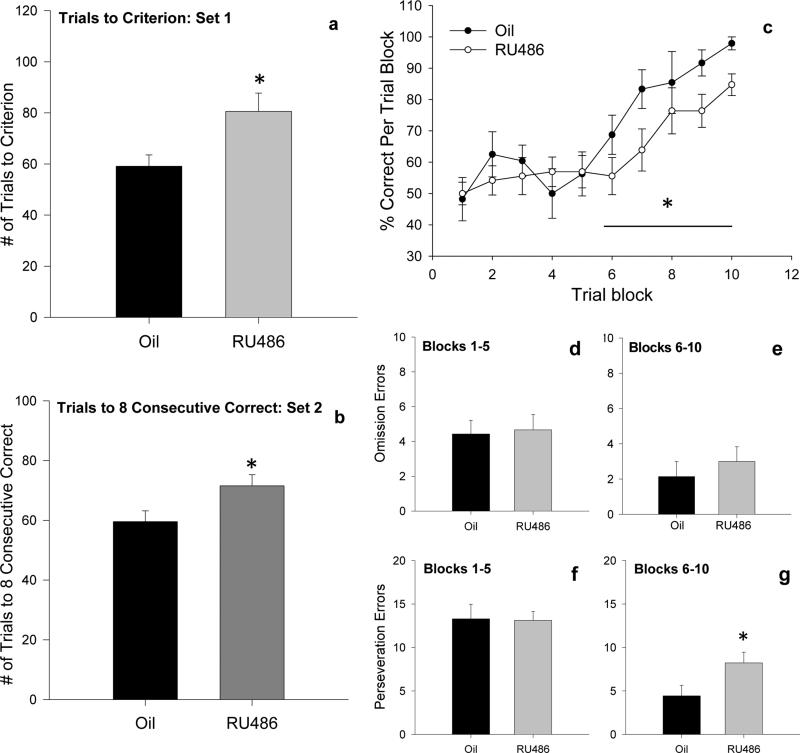
In the initial rule-learning association trials (Set 1), neonatal RU486 administration increased the number of trials required to reach the criterion of eight consecutive correct choices compared to controls (t=−2.462, p=0.026) (fig. 7a), suggesting that RU486 treated rats were slower to learn the initial rule. Additionally, one animal from the RU486 group failed to reach criterion within 120 trials and was excluded from the study. Nonetheless, all rats in both groups reached criterion and therefore went into Set 2 at similar levels of performance on the first rule. RU486-treated animals again required significantly more trials to reach 8 consecutive correct choices in set 2 (t=−2.275, p=0.039) (fig. 7b).
Figure 7. Inhibition of PR activity during neonatal life impairs cognitive flexibility and increases perseverative errors in adult rats.
(a,b) RU486 treated rats required a significantly greater number of trials to reach 8 consecutive correct responses in (a) Set 1 and in (b) Set 2 compared to controls. (c) The percent correct responses in Set 2 following the rule change for Trial Blocks 1-10 (8 trials each) in RU486 treated rats or oil controls. There was no significant difference in performance between treatment groups in Trial Blocks 1-5. However, in Trial Blocks 6-10, RU486 treated rats were slower to acquire the new rule (i.e., made significantly fewer correct responses) and never reach the performance of controls. (d,e) There was no effect of treatment on the number of omission errors (i.e., errors made using neither the first rule nor the new rule) in either Trails Blocks 1-5 or 6-10. (f,g) Both groups made a high number of perseverative errors (i.e., errors made using the first rule) in Trial Blocks 1-5, RU486 treated rats continued to make a significantly greater number of perseverative errors in Trial Blocks 6-10 compared to controls. Data are presented as mean + s.e.m., *p<0.05.
In Set 2, the rule was changed to a different sensory modality (e.g., from light to rough arms baited; an extradimensional shift). Both groups performed at about baseline levels for Trial Blocks 1-5. However, at about Trial Block 6, control rats made the “shift” to the new rule and achieved greater than 90% correct responses by Trial Block 10. In contrast, RU486 treated rats were slower to make the shift to the new rule and never quite performed as well as controls (fig. 7c). Two way repeated measures ANOVA on Trial Blocks 1-5 revealed no significant main effect for Trial Block, no significant effect of treatment and no significant interaction. However, in Trials Blocks 6-10, there was a significant main effect of Trial Block (F9,159=11.760, p<0.001) and a significant main effect of treatment (F1,159=4.756, p=0.031 (fig. 7c) with no significant interaction between Trial Block and treatment.
The number of perseveration errors (incorrect responses made using the old rule) did not differ between the groups in Trial Blocks 1-5 (both groups displayed a high number of perseveration errors). However, in Trial Blocks 6-10, RU486 treated rats continued to make perseveration errors and made significantly more perseveration errors than controls (t=−2.152, p=0.049) (fig. 7f,g). In contrast, the number of omission errors (incorrect responses using neither the old rule nor the new rule) did not differ between the groups in either Trials Blocks 1-5 or 6-10 (fig. 7d,e). Taken together, these results suggest that disruption of PR activity during development impairs cognitive flexibility and increases perseverative behavior.
Water maze task in adult WT and PRKO mice
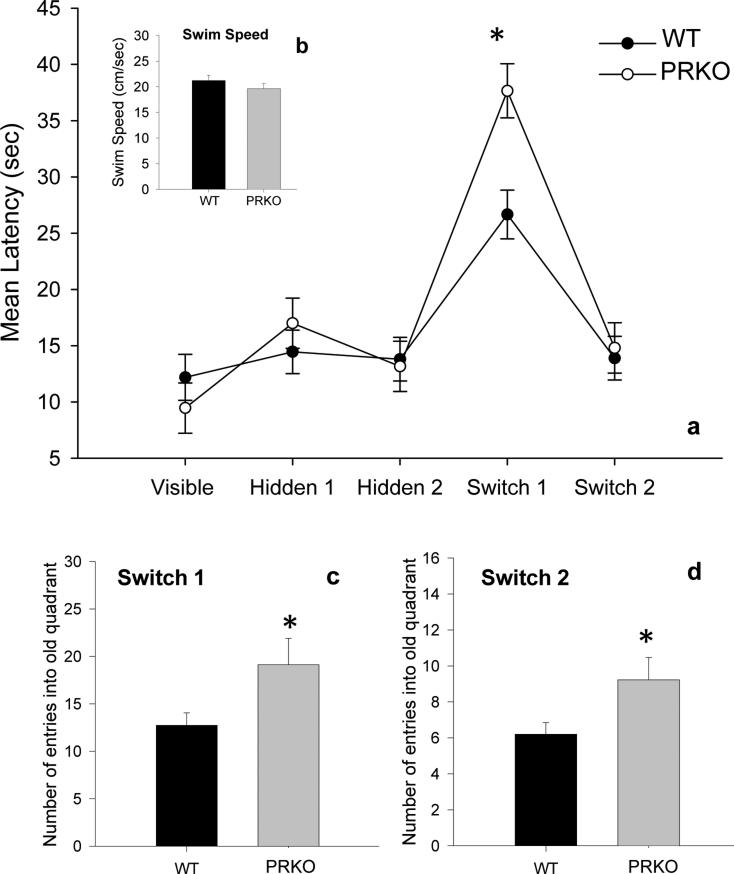
We next examined the role of PR in cognitive flexibility using PRKO mice in an adapted water maze. WT and PRKO mice did not differ in their ability to learn the placement of a hidden platform. However, when the location of the hidden platform was changed, PRKO mice were slower to shift to the new location and made significantly more perseveration errors. Repeated measures two way ANOVA revealed a significant interaction between genotype and test day (F4,100=2.770, p=0.034), a significant main effect of test day (F4,100=28.439, p<0.001) and no significant main effect of genotype (fig. 8a). Post hoc tests revealed that PRKO mice had a significantly longer latency to reach the platform on test day 4 (p<0.05), the first day the platform was moved to the new quadrant. There were no significant effects of genotype on any other test day. In addition, PRKO mice committed significantly more perseveration errors (i.e., entries into the previously correct quadrant) on test days 4 (t=2.271, p=0.036) and 5 (t=2.221, p=0.04) (fig. 8c,d). There were no significant differences in swim speed between PRKO and WT mice across all test days (fig. 8b). Results from PRKO mice are consistent with the findings in rats and suggest that PR expression is important in cognitive flexibility. An absence of functional PR expression increases perseverative behavior.
Figure 8. Cognitive flexibility is impaired in progesterone receptor knockout mice.
(a) There was no significant effect of genotype on the mean latency (sec) to find a visible platform (Visible) or to learn the location of a hidden platform (Hidden 1 and Hidden 2). However, PRKO mice had a significantly longer latency to find the hidden platform after it was moved to a new quadrant (Switch 1), but reduced their latency to the level of controls during the second trial (Switch 2), suggesting that PRKO mice were capable of learning the new location, but slower to make the change (i.e., reduced cognitive flexibility). (b) There was no significant difference in swim speed across all trials between PRKO and WT mice. (c,d) PRKO mice made a significantly greater number of perseveration errors (entering the quadrant in which the platform was previously hidden) on both Switch 1 and Switch 2. Data are presented as mean + s.e.m., *p<0.05.
Discussion
Fundamental processes of early neurodevelopment are critical for the proper maturation of mesocortical connectivity and for subsequent cognitive function [26, 27, 43]. The present results support the idea that the activity of PR, a powerful transcription factor, during neonatal life plays a previously overlooked role in the normal development of the mesocortical dopaminergic pathway and in normal complex cognitive behaviors in adulthood. PR is transiently expressed during development in both the source cells and target cells of the mesocortical pathway. PRir is observed in the parabrachial and paranigral subregions of the VTA during the first two weeks of life, with peak levels of expression occurring just after birth, continuing through at least P14 and absent by P28. The vast majority of PR expressing cells in the neonatal VTA of rats are dopaminergic, co-expressing tyrosine hydroxylase. Indeed, many of the VTA PRir cells project directly to the prelimbic and infralimbic mPFC. PR is also transiently expressed within layers II/III of the mPFC beginning at P6, with a peak in expression around P10, and absent by P30. Inhibition of PR activity during neonatal life significantly reduced THir fiber density in layer II of the prelimbic mPFC in pre-adolescent rats (with a similar trend in PRKO mice) and decreased THir-ir levels in the VTA of both rats and PRKO mice. Lastly, inhibition of PR activity during development altered behavioral inhibition/impulsivity in adult rats and impaired cognitive flexibility in rats and PR knockout mice in adulthood.
To our knowledge, the present findings are the first to report the presence of steroid receptors in cortically-projecting, dopaminergic cells of the VTA during development, although these results are consistent with previous reports from our laboratory demonstrating developmentally transient expression of PRir in the VTA and substantia nigra, as well as other areas of the midbrain and hindbrain [9]. In adulthood, androgen receptor (AR) and estrogen receptor β (ERβ), but not ERα, were colocalized with TH in VTA [44, 45] and were found in VTA cells projecting to prefrontal cortex [46]. However, the transient expression of PR the VTA during an important period of dopamine cell maturation [27] identifies PR as a potential contributor to the proper development of dopaminergic efferents from midbrain.
Neonatal inhibition of PR with RU486, while not altering the number of dopaminergic cells in the VTA, decreased relative total levels of TH protein, suggesting overall reduced rates of dopamine synthesis. The timing of peak PR expression in dopaminergic VTA cells during the first postnatal week coincides with transient alterations in the number of THir neurons in the VTA [27]. Interestingly, in both rats and mice, a decrease in TH levels was only observed in adulthood but not at P25, suggesting that PR activity in early life might influence further developmental processes in the mesocortical pathway that occur during adolescence. This is consistent with findings that estradiol exposure on the day of birth altered adult levels of dopamine in VTA as measured by HPLC [47] and with reports that in undifferentiated embryonic cultured stem cells, progesterone increased the number of TH positive cells and virtually all cells induced to become dopaminergic expressed PR [16]. In addition, progesterone increased promoter activity in the TH gene in cells transfected with the gene for PRB, suggesting that ligand bound PRB can be a transcriptional activator of TH gene expression [48]. PR activity in the VTA during development may induce transient changes in TH gene expression, leading to long term alterations in dopamine synthesis in adult VTA.
PR was also transiently expressed in layers II/III of the mPFC during the period in which afferents arrive in mPFC and form mature patterns of connectivity in superficial cortical layers, similar to the developmentally- and anatomically-specific pattern of PR expression in all other functional regions of cortex [10, 11, 49]. RU486 treatment neonatally decreased the density of THir fibers in the PL mPFC at P25, but not in adulthood, suggesting that PR activity earlier in development may set the stage for subsequent maturation that occurs in prefrontal cortex during adolescence, potentially permanently altering subsequent dopaminergic activity. Indeed, previous work shows that neonatal progesterone administration increased the dopamine metabolites DOPAC and HVA in the adult PFC [17], indicating an increase in dopamine turnover.
There are several potential mechanisms by which PR activity might alter the ‘attractiveness’ of mPFC target cells to influence the development of mesocortical circuits. For example, it is generally accepted that the extension and branching of the dendritic arbor and the density of dendritic spines on target cells are integral to the process of synaptogenesis [50, 51] and steroid hormones can regulate dendritic structure in both the adult and developing brain [52-55]. In developing pyramidal cells of the hippocampus, testosterone increased dendritic length and increased the number of dendritic branches [56]. In neonatal Purkinje cells, both progesterone and estradiol can promote dendritic outgrowth and increase the density of dendritic spines [55, 57, 58]. In developing cortical pyramidal cells, progesterone given to pregnant rats increased dendritic branching and increased the density of dendritic spines [59]. Another possibility, PR may regulate the expression of trophic factors in developing cortex. Indeed, there is a wealth of evidence that progesterone and its metabolites can regulate BDNF expression in numerous brain regions in adulthood [reviewed in 60], although little evidence exists regarding early development. However, in dissociated cell cultures of P3 prefrontal cortex, progesterone increased brain derived neurotrophic factor (BDNF) expression, and this increase was attenuated by RU486 and in PRKO mice [61]. PR regulation of mPFC target cell phenotype might influence synaptogenesis amongst arriving afferents, thereby potentially altering the strength and spatial patterning of connectivity.
Consistent with alterations in mesocortical dopamine circuitry, PR inhibition during neonatal life impaired complex cognitive behaviors that are dependent on dopaminergic activity in the mPFC [31, 62, 63]. First, RU486 treatment during the first two weeks of life decreased behavioral inhibition and/or increased impulsivity. In a passive inhibitory avoidance task, RU486 treated rats were quicker to enter the dark chamber compared to controls despite having received an aversive foot shock in that chamber previously. Both groups were equally highly motivated to enter the dark chamber on the first trial, and RU486 treated rats had a significantly longer latency to enter the dark chamber on the second trail compared to the first trial, suggesting that memory for the aversive stimuli was intact. Rather, the findings suggest that RU486 treatment impaired the ability to inhibit a motivated response (i.e., increased impulsivity), even in light of negative consequences.
Neonatal RU486 treatment also impaired cognitive flexibility and increased perseveration in the attentional set-shift task. RU486 treated animals were slower than controls to make the shift to the new rule, and made significantly more perseverative errors, in effect, continuing to apply the old rule. Interestingly, RU486 treated animals required more trials to reach criterion to learn the initial rule, suggesting that PR expressed in developing hippocampus [8] or the mesocortical pathway may play a role in adult working memory as well. Nonetheless, both groups were performing at equal levels by the end of testing on the first day and made equivalent errors using the first rule on the beginning of the second day, suggesting that RU486 did not affect retention of the initial rule. RU486 is a potent PR antagonist, but also acts as an antagonist at glucocorticoid receptors (GR) but with lower affinity. However, GR expression is relatively low in the neonatal hippocampus and cortex [64, reviewed in 65], suggesting that the effects of RU486 in the present studies were most likely attributable to inhibition of PR activity specifically. Additionally, PRKO mice showed a similar deficit in cognitive flexibility on an adapted water maze task and also made more perseverative errors compared to controls. These results provide a convergence of evidence, using both pharmacological and genetic approaches, suggesting that PR activity during development is important for the normal development of mPFC-dependent cognitive behaviors.
Interestingly, the deficits induced by developmental PR inhibition are in many ways similar to those seen in a variety of human behavioral disorders. Disruptions of normal development can lead to permanent alterations in mesocortical structure and function which can manifest in behavioral disorders such as ADHD and schizophrenia [1-7], both of which are associated with deficits in cognitive flexibility and impaired impulse control. The present results implicate PR in the normal development of the circuits underlying these behaviors and raise the possibility that perturbations in the progesterone exposure or in the levels of PR expression during critical developmental windows may increase susceptibility to these disorders. Furthermore, these findings have implications for the use of synthetic progestins for the prevention of premature birth in at-risk pregnant women [reviewed in 66] and the potential effects on development of the fetal brain.
Acknowledgements
This work was supported by IOS1050367 and HD07643001 to CKW.
Footnotes
The authors declare no competing financial interests.
References
- 1.Goto Y, Grace AA. Alterations in medial prefrontal cortical activity and plasticity in rats with disruptions of cortical development. Biol Psychiat. 2006;60:1259–1267. doi: 10.1016/j.biopsych.2006.05.046. [DOI] [PubMed] [Google Scholar]
- 2.Floresco SB, Magyar O. Mesocortical dopamine modulation of executive functions: beyond working memory. Psychopharmacology. 2006;188:567–585. doi: 10.1007/s00213-006-0404-5. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan RM, Brake WG. What the rodent prefrontal cortex can teach us about attention deficit/hyperactivity disorder: the critical role of early developmental events on prefrontal function. Behav Brain Res. 2003;146:43–55. doi: 10.1016/j.bbr.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Viggiano D, Vallone D, Ruocco LA, Sadile AG. Behavioral, pharmacological, morpho-functional molecular studies reveal a hyperfunctioning mesocortical dopamine system in an animal model of attention deficit and hyperactivity disorder. Neurosci Biobehav R. 2003;27:683–689. doi: 10.1016/j.neubiorev.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Arnstein AFT, Li BM. Neurobiology of executive functions: Catecholamine influences on prefrontal cortical functions. Biol Psychiat. 2005;57:1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 6.Brennan AR, Arnstein AFT. Neuronal mechanisms underlying attention deficit hyperactivity disorder: The influence of arousal on prefrontal cortical function. New York Academy of Sciences. 2008:236–245. doi: 10.1196/annals.1417.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prince J. Catecholamine dysfunction in attention deficit hyperactivity disorder: An update. J Clin Psychopharm. 2008;28:S39–S45. doi: 10.1097/JCP.0b013e318174f92a. [DOI] [PubMed] [Google Scholar]
- 8.Quadros PS, Pfau JL, Wagner CK. Distribution of progesterone receptor immunoreactivity in the fetal and neonatal rat forebrain. J Comp Neurol. 2007;504:42–56. doi: 10.1002/cne.21427. [DOI] [PubMed] [Google Scholar]
- 9.Quadros PS, Schlueter LJ, Wagner CK. Distribution of progesterone receptor immunoreactivity in the midbrain and hindbrain of postnatal rats. Dev Neurobio. 2008;68:1378–1390. doi: 10.1002/dneu.20664. [DOI] [PubMed] [Google Scholar]
- 10.Lopez V, Wagner CK. Progestin receptor is transiently expressed perinatally in neurons of the rat isocortex. J Comp Neurol. 2009;512:124–139. doi: 10.1002/cne.21883. [DOI] [PubMed] [Google Scholar]
- 11.Jahagirdar V, Wagner CK. Ontogeny of progesterone receptor expression in the subplate of fetal and neonatal rat cortex. Cereb Cortex. 2010;20:1046–1052. doi: 10.1093/cercor/bhp165. [DOI] [PubMed] [Google Scholar]
- 12.Beyer C, Damm N, Brito V, Kuppers E. Developmental expression of progesterone receptor isoforms in the mouse midbrain. Dev Neurosci. 2002;13:877–880. doi: 10.1097/00001756-200205070-00028. [DOI] [PubMed] [Google Scholar]
- 13.De Brabander JM, Kramers JK, Uylings HBM. Layer-specific dendritic regression of pyramidal cells with aging in the human prefrontal cortex. Eur J Neurosci. 1998;10:1261–1269. doi: 10.1046/j.1460-9568.1998.00137.x. [DOI] [PubMed] [Google Scholar]
- 14.Petanjek Z, Judas M, Kostovic I, Uylings HBM. Lifespan alterations of basal dendritic trees of pyramidal neurons in the human prefrontal cortex: a layer-specific pattern. Cereb Cortex. 2008;18:915–929. doi: 10.1093/cercor/bhm124. [DOI] [PubMed] [Google Scholar]
- 15.Ernst M, Fudge JL. A developmental neurobiological model of motivated behavior: Anatomy, connectivity and ontogeny of the triadic nodes. Neurosci Biobehav R. 2009;33:367–382. doi: 10.1016/j.neubiorev.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diaz NF, Guerra-Arraiza C, Diaz-Martinez NE, Salazar P, Molina-Hernandez A, Camacho-Arroyo I, Velasco I. Changes in the content of estrogen and progesterone receptors during differentiation of mouse embryonic stem cells to dopamine neurons. Brain Res Bull. 2007;73:75–80. doi: 10.1016/j.brainresbull.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muneoka K, Kuwagata M, Ogawa T, Shioda S. Sex-specific effects of early neonatal progesterone treatment on dopamine and serotonin metabolism in rat striatum and frontal cortex. Life Sci. 2010;87:738–742. doi: 10.1016/j.lfs.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 18.Lydon J, DeMayo F, Funk C, Mani S, Hughes A, Montgomery C, Shyamala G, Conneely OM, O'Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Gene Dev. 1995;9:2255–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- 19.Lonstein JS, Quadros PS, Wagner CK. Effects of neonatal Ru486 on adult sexual, parental and fearful behaviors in rats. Behav Neurosci. 2001;115:58–70. doi: 10.1037/0735-7044.115.1.58. [DOI] [PubMed] [Google Scholar]
- 20.Quadros PS, Lopez V, De Vries GJ, Chung WC, Wagner CK. Progesterone receptors and the sexual differentiation of the medial preoptic nucleus. J Neurobio. 2002;51:24–32. doi: 10.1002/neu.10040. [DOI] [PubMed] [Google Scholar]
- 21.Traish AM, Wotiz HH. Monoclonal and polyclonal antibodies to human progesterone receptor peptide-(533-547) recognize a specific site in unactivated (8S) and activated (4S) progesterone receptor and distinguish between intact and proteolyzed receptors. Endocrinology. 1990;127:1167–1175. doi: 10.1210/endo-127-3-1167. [DOI] [PubMed] [Google Scholar]
- 22.Tetel MJ, Jung S, Carbajo P, Ladtkow T, Skafar DF, Edwards DP. Hinge and amino-terminal sequences contribute to solution dimerization of human progesterone receptor. Molecular Endocrinology. 1997;11:1114–1128. doi: 10.1210/mend.11.8.9963. [DOI] [PubMed] [Google Scholar]
- 23.Karatsoreos IN, Bhagat S, Bloss EB, Morrison JH, McEwen BS. Disruption of circadian clocks has ramifications for metabolism, brain and behavior. Proc Natl Acad Sci. 2011;108:1657–1662. doi: 10.1073/pnas.1018375108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzales KL, Tetel MJ, Wagner CK. Estrogen Receptor (ER) beta modulates ERalpha responses to estrogens in the developing rat ventromedial nucleus of the hypothalamus. Endocrinology. 2008;149:4615–4621. doi: 10.1210/en.2008-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heller A, Hutchens JO, Kirby ML, Karapas F, Fernandez C. Stereotaxic electrode placement in the neonatal rat. J Neurosci Meth. 1979;1:41–76. doi: 10.1016/0165-0270(79)90006-2. [DOI] [PubMed] [Google Scholar]
- 26.Kalsbeek A, Voorn P, Buijs RM, Pool CW, Uylings HB. Development of the dopaminergic innervation in the prefrontal cortex of the rat. J Comp Neurol. 1988;269:58–72. doi: 10.1002/cne.902690105. [DOI] [PubMed] [Google Scholar]
- 27.Lieb K, Anderson C, Lazarov N, Zienecker R, Urban I, Reisert I, Pilgrim C. Pre- and postnatal development of dopaminegic neuron numbers in the male and female mouse midbrain. Dev Brain Res. 1996;94:37–43. doi: 10.1016/0165-3806(96)00063-6. [DOI] [PubMed] [Google Scholar]
- 28.Park M, Kitahama K, Geffard M, Maeda T. Postnatal development of the dopaminergic neurons in the rat mesencephalon. Brain Dev. 2000;22:S38–S44. doi: 10.1016/s0387-7604(00)00145-5. [DOI] [PubMed] [Google Scholar]
- 29.Prakash N, Wurst W. Development of dopaminergic neurons in the mammalian brain. Cell Mol Life Sci. 2006;63:197–206. doi: 10.1007/s00018-005-5387-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego: 1998. [DOI] [PubMed] [Google Scholar]
- 31.Naneix F, Marchand AR, Di Scala D, Pape JR, Coutureau E. A role for medial prefrontal dopaminergic innervation in instrumental conditioning. J Neurosci. 2009;29:6599–6606. doi: 10.1523/JNEUROSCI.1234-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Eden CG, Uylings HBM. Cytoarchitectonic development of the prefrontal cortex in the rat. J Comp Neurol. 1985;241:253–267. doi: 10.1002/cne.902410302. [DOI] [PubMed] [Google Scholar]
- 33.Kritzer MF. Long term gonadectomy affects the density of tyrosine hydroxylase but not dopamine-β-hydroxylase, choline acetyltransferase or serotonin immunoreactive axons in the medial prefrontal cortices of adult male rats. Cereb Cortex. 2003;13:282–296. doi: 10.1093/cercor/13.3.282. [DOI] [PubMed] [Google Scholar]
- 34.Roozendaal B, McReynolds JR, Van der Zee EA, Lee S, McGaugh JL, McIntyre CK. Glucocorticoid effects on memory consolidation depend on functional interactions between the medial prefrontal cortex and basolateral amygdala. J Neurosci. 2009;29:14299–14308. doi: 10.1523/JNEUROSCI.3626-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McReynolds JR, Holloway-Erickson CM, Parmar TU, McIntyre CK. Corticosterone-induced enhancement of memory and synaptic Arc protein in the medial prefrontal cortex. Neurobiol Learn Mem. 2014;112:148–157. doi: 10.1016/j.nlm.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang FC, Liang KC. Interactions of the dorsal hippocampus, medial prefrontal cortex and nucleus accumbens in formation of fear memory: difference in inhibitory avoidance learning and contextual fear conditioning. Neurobiol Learn Mem. 2014;112:186–194. doi: 10.1016/j.nlm.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 37.Stefani MR, Moghaddam B. Rule learning and reward contingency are associated with dissociable patterns of dopamine activation in the rat prefrontal cortex, nucleus accumbens and dorsal striatum. J Neurosci. 2006;26:8810–8818. doi: 10.1523/JNEUROSCI.1656-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ragozzino ME, Detrick S, Kesner RP. Involvement of the prelimbic-infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. J Neurosci. 1999;19:4585–4594. doi: 10.1523/JNEUROSCI.19-11-04585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Birrell JM, Brown VJ. Medial prefrontal cortex mediates perceptual attentional set-shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ragozzino ME, Hassert KJ, Minniti N, Kiang C. The contribution of the rat prelimbicinfralimbic areas to different forms of task switching. Behav Neurosci. 2003;117:1054–1065. doi: 10.1037/0735-7044.117.5.1054. [DOI] [PubMed] [Google Scholar]
- 41.Floresco SB, Block AE, Tse MTL. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav Brain Res. 2008;190:85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 42.Rich EL, Shapiro M. Rat prefrontal cortical neurons selectively code strategy switches. J Neurosci. 2009;29:7208–7219. doi: 10.1523/JNEUROSCI.6068-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jahng JW, Ryu V, Yoo SB, Noh SJ, Kim JY, Lee JH. Mesolimbic dopaminergic activity responding to acute stress is blunted in adolescent rats that experienced neonatal maternal separation. Neurosci. 2010;171:144–152. doi: 10.1016/j.neuroscience.2010.08.063. [DOI] [PubMed] [Google Scholar]
- 44.Kritzer MF. Selective colocalization of immunoreactivity for intracellular gonadal hormone receptors and tyrosine hydroxylase in the ventral tegmental area, substantia nigra and retrorubral fields in the rat. J Comp Neurol. 1997;379:247–260. doi: 10.1002/(sici)1096-9861(19970310)379:2<247::aid-cne6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 45.Creutz LM, Kritzer MF. Mesostriatal and mesolimbic projections of midbrain neurons immunoreactive for estrogen receptor beta or androgen receptors in rats. J Comp Neurol. 2004;476:348–362. doi: 10.1002/cne.20229. [DOI] [PubMed] [Google Scholar]
- 46.Kritzer MF, Creutz LM. Region and sex differences in constituent dopamine neurons and immunoreactivity for intracellular estrogen and androgen receptors in mesocortical projections in rats. J Neurosci. 2008;28:9525–9535. doi: 10.1523/JNEUROSCI.2637-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cruz G, Riquelme R, Espinosa P, Jara P, Dagino-Subiabre A, Renard GM, Sotomayor-Zarate R. Neonatal exposure to estradiol valerate increases dopamine content in nigrostriatal pathway during adulthood in the rat. Horm Metab Res. 2014;46:322–327. doi: 10.1055/s-0033-1361159. [DOI] [PubMed] [Google Scholar]
- 48.Jensik PJ, Arbogast LA. Differential and interactive effects of ligand-bound progesterone receptor A and B isoforms on tyrosine hydroxylase promoter activity. J Neuroendocrinol. 2011;23:915–925. doi: 10.1111/j.1365-2826.2011.02197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Willing J, Wagner CK. Sensorimotor development in neonatal progesterone receptor knockout mice. Dev Neurobiol. 2014;74:16–24. doi: 10.1002/dneu.22124. [DOI] [PubMed] [Google Scholar]
- 50.Segal M, Murphy D. Estradiol Induces Formation of Dendritic Spines in Hippocampal Neurons: Functional Correlates. Horm behav. 2001;40:156–159. doi: 10.1006/hbeh.2001.1688. [DOI] [PubMed] [Google Scholar]
- 51.Yuste R, Bonhoeffer T. Morphological changes in dendritic spines associated with long term synaptic plasticity. Annu Rev Neurosci. 2001;24:1071–1089. doi: 10.1146/annurev.neuro.24.1.1071. [DOI] [PubMed] [Google Scholar]
- 52.Murphy DD, Segal M, Ninds NIH, Bethesda M, Cyclic AMP, Segal M. Progesterone Prevents Estradiol-Induced Dendritic Spine Formation in Cultured Hippocampal Neurons. Neuroendocrinology. 2000;72:133–143. doi: 10.1159/000054580. [DOI] [PubMed] [Google Scholar]
- 53.Woolley CS. Effects of oestradiol on hippocampal circuitry. Novartis Found Symp. 2000;230:173–180. doi: 10.1002/0470870818.ch13. [DOI] [PubMed] [Google Scholar]
- 54.Mong JA, Roberts RC, Kelly JJ, McCarthy MM. Gonadal steroids reduce the density of axospinous synapses in the developing rat arcuate nucleus: An electron microscopy analysis. J Comp Neurol. 2001;432:259–267. doi: 10.1002/cne.1101. [DOI] [PubMed] [Google Scholar]
- 55.Sakamoto H, Ukena K, Tsutsui K. Effects of progesterone synthesized de novo in the developing Purkinje cell on its dendritic growth and synaptogenesis. J Neurosci. 2001;21:6221–6232. doi: 10.1523/JNEUROSCI.21-16-06221.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Isgor C, Sengelaub DR. Effects of neonatal gonadal steroids on adult CA3 pyramidal neuron dendritic morphology and spatial memory in rats. J Neurobiol. 2003;55:179–190. doi: 10.1002/neu.10200. [DOI] [PubMed] [Google Scholar]
- 57.Sakamoto H, Ukena K, Tsutsui K. Dendritic spine formation in response to progesterone synthesized de novo in the developing Purkinje cell in rats. Neurosci Lett. 2002;322:111–115. doi: 10.1016/s0304-3940(02)00077-0. [DOI] [PubMed] [Google Scholar]
- 58.Sakamoto H, Mezaki Y, Shikimi H, Ukena K, Tsutsui K. Dendritic Growth and Spine Formation in Response to Estrogen in the Developing Purkinje Cell. Endocrinology. 2003;144:4466–4477. doi: 10.1210/en.2003-0307. [DOI] [PubMed] [Google Scholar]
- 59.Menzies KD, Drysdale DB, Waite PM. Effects of prenatal progesterone on the development of pyramidal cells in rat cerebral cortex. Exp Neurol. 1982;77:654–667. doi: 10.1016/0014-4886(82)90236-9. [DOI] [PubMed] [Google Scholar]
- 60.Pluchino N, Russo M, Santoro AN, Litta P, Cela V, Genazzani AR. Steroid hormones and BDNF. Neuroscience. 2013;239:271–279. doi: 10.1016/j.neuroscience.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 61.Jodhka PK, Kaur P, Underwood W, Lydon JP, Singh M. The differences in neuroprotective efficacy of progesterone and medroxyprogesterone acetate correlate with their effects on brain-derived neurotrophic factor expression. Endocrinology. 2009;150:3162–3168. doi: 10.1210/en.2008-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mello E, Souza T, Vianna MR, Rodrigues C, Quevedo J, Moleta BA, Izquierdo I. Involvement of the medial precentral prefrontal cortex in memory consolidation for inhibitory avoidance learning in rats. Pharmacol Biochem Behav. 2000;66:615–622. doi: 10.1016/s0091-3057(00)00277-x. [DOI] [PubMed] [Google Scholar]
- 63.Kritzer MF, Brewer A, Montalmant F, Davenport M, Robinson JK. Effects of gonadectomy and hormone replacement on operant tasks measuring prefrontal cortical function in adult male rats. Horm Behav. 2007;51:183–194. doi: 10.1016/j.yhbeh.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 64.Ordya NE, Galeeva AY, Pivina SG. Expression of glucocorticoid receptor in the brain of rats during postnatal ontogeny. Bull Exp Biol Med. 2008;146:176–179. doi: 10.1007/s10517-008-0248-6. [DOI] [PubMed] [Google Scholar]
- 65.Pryce CR. Postnatal ontogeny of expression of the corticosteroid receptor genes in mammalian brains: inter-species and intra-species differences. Brain Res Rev. 2008;57:596–605. doi: 10.1016/j.brainresrev.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 66.Schmouder VM, Prescott GM, Franco A, Fan-Havard P. The rebirth of progesterone in the prevention of preterm labor. Ann Pharmacotherapy. 2013;47:527–536. doi: 10.1345/aph.1R281. 2013. [DOI] [PubMed] [Google Scholar]