Abstract
Silkworm has great potential as production system of recombinant mammalian proteins. When the protein products are used for medical purpose, it is required to reduce the risk of an allergy, the content of core alpha 1,3-fucosyl residue attached to the N-glycan of proteins, for example. We isolated the gene of an enzyme responsible for the transfer of core alpha 1,3-fucosyl residue, core alpha 1,3-fucosyltransferase (Fuc-T C3), from silkworm. A candidate cDNA for silkworm Fuc-T C3 was isolated as a homolog of the fruit fly enzyme gene fucTA. The gene was located on chromosome 7 of the silkworm genome and was composed of seven exons, which spanned approximately 10 kb on the genome. The coding region of the gene was 1,350 bp and encoded a 450-amino acid protein with a molecular mass of 52.2 kDa. Deduced amino acid sequence of the coding region showed one transmembrane domain in its N-terminal and typical motifs common to fucosyltransferases including Fuc-T C3s of other organisms in its C-terminal. The extract of CHO cells transfected with the cDNA showed Fuc-T C3 activity using GDP-fucose and DABS-GnGn peptide as substrates. These results showed this cDNA clone actually encodes silkworm Fuc-T C3.
Keywords: Bombyx mori, fucose, glycosylation, N-glycan, transferase
Recombinant mammalian glycoproteins for human medical use are produced by using mammalian cell culture method (Ludwig et al. 1995). However, bovine serum, which is usually used for an ingredient of the culture medium, has a contamination risk of pathogens such as viruses harmful to humans, besides it is so expensive. Silkworm (Bombyx mori), one of the Lepidopteran species, has been noticed for a safe and cost-effective tool for this purpose, because no harmful virus are known in this organism so far and relatively large amounts of recombinant proteins can be easily obtained comparing with the mammalian cell culture method (Smith et al. 1983). Two production systems are known with silkworm, a baculovirus expression system and a germline transgenic system. In the former system, a recombinant baculovirus is infected to the insect larvae and then a recombinant protein is accumulated in its body fluid (Luckow and Summers 1989, Drugmand et al. 2012). In the latter system, recombinant proteins are generated in silk glands and secreted into sericin layer or fibroin fiber of a cocoon (Tomita et al. 2003, 2007).
Although variety of recombinant mammalian proteins have been shown to be produced by both systems, there is a large concern about their N-glycan profile, which differs from that of mammalians. N- glycans of recombinant glycoproteins produced by the baculovirus expression system showed typical insect-type profiles: high contents of paucimannosidic, oligomannosidic (ranging from Man5 GlcNAc2 to Man9 GlcNAc2) (Kuroda et al. 1990, Chen et al. 1991, Williams et al. 1991), and core alpha 1, 3-fucosyl residue (Staudacher et al. 1992, Kubelka et al. 1993). Most of the mammalian N-glycans are complex type and their nonreducing terminals are usually sialylated. In addition, the mammalian-type core fucosyl linkage to N-glycans is only alpha 1,6 (Longmore and Schachter 1982). These structural differences in N-glycans may alter the properties of glycoproteins; biological activity, or in vivo stability (Geisow 1992). Moreover, there is a concern that core alpha 1,3 fucosyl-residues on N-glycans could cause an allergic reaction for humans (Tretter et al. 1993). In this point, the transgenic system has an advantage, because N-glycans of a recombinant mouse IgG produced with the system carried no core-fucosyl (1, 3 and 1, 6 linked core fucoses) residues (Iizuka et al. 2009).
To eliminate completely the allergy risk of core alpha 1,3-fucosyl residues from the N-glycans on recombinant glycoproteins produced by silkworm, inhibition of the biosynthesis of the fucosyl residue by using gene knockout or RNA interference technologies is preferable. For this purpose, we isolated the gene of a key enzyme in this pathway, Fuc-T C3, as the first step. Fuc-T C3s are distributed in insects, plants, and snail and catalyze the alpha 1, 3-linkage of fucosyl moiety to the GlcNAc at the reducing end of N-glycans (Lerouge et al. 1998). Insect Fuc-T C3 cDNAs have already been cloned from fruit fly (Drosophila melanogaster) and honey bee (Apis mellifera) (Fabini et al. 2001, Rendic et al. 2007), and the enzyme activity of the expression product was confirmed in fruit fly.
In this study, we described the isolation of silkworm Fuc-T C3 gene and its characteristics; gene structure, deduced amino acid sequence, and enzyme activity. These results would lead to develop the technologies to block completely the core alpha 1,3-fucosylation of N-glycans in silkworm protein production system.
Materials and Methods
Experimental Animals
Silkworm strain w1-pnd was obtained from the National Institute of Agrobiological Sciences (Tsukuba, Japan) and was used throughout the experiments. The larvae were reared at 25°C on an artificial feed (Silk Mate PM, Nosan Corp., Yokohama, Japan).
Cloning of Silkworm Fuc-T C3 cDNA
A candidate genomic sequence for silkworm Fuc-T C3 was obtained by searching the silkworm genomic database in genomic BLAST (http://www.ncbi.nlm.nih.gov/sutils/genom_table.cgi?organism=insects) for a protein homolog of the fruit fly FucTA (core alpha 1,3-fucosyltransferase) (NCBI number: AJ302045). One contig (NCBI number: AADK01016505) showed a homology and its deduced cDNA sequence was used as a clue to obtain a full length coding region of the cDNA. Silkworm RNA was prepared from a mixture of silk glands and fat bodies derived from the fifth instar larvae using TRIzol reagent (Life Technologies Japan, Tokyo, Japan), and mRNA was isolated from the RNA with FastTrack MAG mRNA Isolation Kits (Life Technologies Japan).
The 3’-end of the contig sequence was extended by reverse transcription with oligo-dT-3 sites Adaptor Primer (3’-Full RACE Core Set TAKARA BIO INC., Ootsu, Japan) using the RNA as the template. The target cDNA fragment was amplified by polymerase chain reaction (PCR) with the same primer and the A1 primer, 5’-GCAGCAGT GGCTC CAC GTAATTCG-3’. The product was purified by Wizard SV Gel and PCR Clean-up System (Promega Corp., Tokyo, Japan) and a nested PCR was performed with the same primer and the A2 primer, 5’-CTGATCTAGAGGTACC GGATCC GGTCC TGAAGAACTCGC T G CGTACTTACATCG-3’ (restriction sites in bold). The purified PCR product was introduced into pBluescript II for sequence analysis.
The 5’-end of the contig sequence was extended by reverse transcription with 5’-phosphorylated primer B1, 5’-GTAAGGTGCTAC-3’, using the mRNA as the template. The cDNA produced was circularized, and the first PCR was performed with the primers B2, 5’-4CTTGGAATGTCCTTACCACACG-3', and B3, 5’-GTCTATGGGG CACTTGTTTC-3’ (5’- Full RACE Core Set TAKARA BIO INC.). A nested PCR was performed with the B3 primer and B4, 5’-GGACTGCAACTTACCGCAGAGAC-3’. The nested PCR product was introduced into the pCR2.1 TA cloning vector for sequence analysis (Life Technologies Japan).
Eventually, the full length coding region of the cDNA was amplified with the primers, 5’-CGCGGATCCACCATGCGGGTGA GGGC GG CTCG-3’ and 5’- GCGTCTAGATTATCCATTATCTTTAGTATCAG ATTCC-3’ (restriction sites in bold), using the product as the template and it was inserted into the BamHI and XbaI site of pcDNA3.1-Hygro mammalian expression vector (Life Technologies Japan) to construct pcDNA3.1-Hygro Fuc-T C3.
DNA Sequencing
DNA sequence was obtained using ABI PRISM BigDye Terminator sequencing ready mix and ABI PRISM 310 genetic analyzer (PerkinElmer Japan Co., Ltd., Yokohama, Japan).
Hydrophobic Cluster Analysis
Hydrophobic cluster analysis (HCA) is a graphical protein sequence comparison method, based on the detection of hydrophobic clusters containing the amino acids Val, Ile, Leu, Phe, Met, Trp, and Tyr, which are presumed to correspond to the regular secondary structure elements constituting the hydrophobic core of globular proteins (Gaboriaud et al. 1987). And its plotting was performed by a Drawhca server (provided by Ressource Parisienne en Bioinformatique Structurale, France) on the internet (http://mobyle.rpbs.univ-paris-diderot.fr/cgi-bin/portal.py?form=HCA).
Sequence Determination of Silkworm Fuc-T C3 Genomic DNA
A genomic DNA was prepared from the fifth-instar larvae using SepaGene (Eidia Co., Ltd., Tokyo, Japan), and the coding region of the Fuc-T C3 cDNA was obtained by PCR. Approximately 10 kb of genomic DNA sequence of the region was determined.
Expression of Silkworm Fuc-T C3 in Chinese hamster ovary (CHO) Cells
The DNA (2 µg) of pcDNA3.1/Hygro Fuc-T C3 was transfected into CHO cells using FuGENE 6 (Roche Diagnostics K.K., Tokyo, Japan), and hygromycin B resistant transformants were selected. The culture selected was maintained for over a month, and stable transformants were used in the following experiments. Transcription from the Fuc-T C3 cDNA in transformants was confirmed by detecting the Reverse Transcription Polymerase Chain Reaction (RT-PCR) fragment (144 bp), which was amplified with the primers, 5’-GACCGGTG TC ATCTTACG-3’ and 5’-CGTGTGGTAA GGACAT TCC-3’ (Fig. 1), using the total RNA of the cells as the template.
Fig. 1.

cDNA and protein sequences of silkworm Fuc-T C3. The nucleotide and its deduced 450 amino acid sequences of Fuc-T C3 are shown. The predicted transmembrane domain is underlined. Double underline indicates consensus sites for asparagine-linked glycosylation. Arrows indicate the primers used to confirm the transcription of this cDNA in transformants by RT-PCR.
Assay of Core Alpha Fucosyltransferases (Fuc-T Cs) in CHO Cells
Approximately 1.0 by 107 of CHO cells (transfectant or nontransfectant control) were washed with Phosphate buffered saline (PBS) and suspended in the same buffer containing 1 mM Phenylmethylsulfonyl fluoride (PMSF) and 0.1% NP-40. Cells were disrupted by sonic treatment on ice for 30 s twice, and crude cell extracts were prepared by centrifugation of the disrupted cells at 20,000 × g for 10 min at 4°C. Fuc-T C activity was measured in the following assay mixture (20 µl): crude cell extract (22.5 µg), 5 nmol of Dabsylated (DABS)-GnGn peptide (Dabsylated tetrapeptide, asialo, agalacto, biantenary: Merck Serono Co., Ltd. Tokyo, Japan), 0.5 mM GDP-fucose, 100 mM MES/NaOH, pH 7.0, 15 mM MnCl2, and 0.1% Triton X-100. The mixtures were incubated for 17 h at 37°C, and then the reaction was stopped by heating for 5 min at 60°C.
High Performance Liquid Chromatography (HPLC) Analyses of the Reaction Products of Fuc-T C Assay
The reaction products were separated on an ODS column (Develosil ODS-HG-3, i.d. 3.0 by 250 mm, Nomura Chemical Co., Ltd., Seto, Japan) at a flow rate of 0.4 ml/min at 40°C using the solvent A (10 mM sodium phosphate pH 6.4, 5% (v/v) acetonitrile) and the solvent B (10 mM sodium phosphate pH 6.4, 50% (v/v) acetonitrile). The separation condition was 30% of the solvent B for 4 min, followed by elution with a linear gradient 30–37% of the solvent B for 35 min. Elution of reaction products was monitored by detecting the absorption of DABS at 465 nm.
Structural Analysis of N-Glycans of the Reaction Products of Fuc-T C Assay
The Fuc-T C reaction mixture was treated with chloroform and diethyl ether to remove glycoproteins derived from CHO cells. N-glycans on DABS-GnGn peptide were released with N-glycosidase A and then pyridylaminated (PA) oligosaccharides were prepared by reacting the reducing ends to 2-aminopyridine. Each PA-oligosaccharide was identified by comparing its retention time on HPLC using both ODS and amide columns as well as its MS data with that of standard one on the GALAXY database (Yagi et al. 2005). Additionally, the identity was confirmed by analyzing the mixture of the PA-oligosaccharide and standard one by HPLC, giving a single peak on the chromatogram.
Expression of the Fuc-T C3 Gene in Silkworm
Each organ was isolated from the fifth-instar larvae and its total RNA was prepared according to the same method as described above. Reverse transcription PCR for Fuc-T C3 mRNA was performed with the same primers 5’-GACCGGTGTCATCTTACG-3’ and 5’-CGTGTGGTAA GGAC ATT C C -3 ’ using a reverse transcription kit (SuperScript one-step RT-PCR, Life Technologies Japan).
Results
Isolation of Silkworm Fuc-T C3 cDNA
A silkworm homolog sequence of fruit fly Fuc-T C3 (Fuc-TA) (NCBI number: AJ302045, protein, CAC41641), which was identified on the silkworm genomic database, KAIKOBLAST, was a 1,057 bp of a contig (NCBI number: AADK01016505). On the basis of this contig sequence, we isolated a cDNA clone carrying the full length coding region of silkworm Fuc-T C3 (NCBI number: AB551654). The 1,350 bp of the coding region encoded a 450 amino acid protein with a predicted molecular mass of 52.2 kDa (Fig. 1).
Analysis of the Deduced Amino Acid Sequence of Silkworm Fuc-T C3
The deduced amino acid sequence of the silkworm Fuc-T C3 showed the following characteristics. One transmembrane domain was observed in its N-terminal region, between Val-15 and Leu-37, by computer-aided analyses using both SOSUI (provided by Nagoya Univ., Japan) and “TMpred” (provided by EMBnet, Switzerland) (Figs. 1 and 2). The former program also predicted that this transmembrane domain forms an alpha helix structure. Three consensus asparagine-linked glycosylation sites were noticed (Figs. 1 and 2). In its C-terminal region, from Asp-224 to Ala-408, typical motifs for alpha 1,3-fucosyltransferases (1,3-Fuc-Ts), including Fuc-T C3, (I or V) DXYG, YKFXLAFENS, DY (I or V) TEK and CXXC (Val-284 to Cys-408) (Breton et al. 1998, Oriol et al. 1999), and a Fuc-T C3-specific motif, AXF(I or V)SNCX ARNXRLQ (Ala-259 to Gln-273) (Fabini et al. 2001), were found. A postulated Mn(II) binding motif, DXD (SSD in plant), which is observed in many glycosyltransferases, was also observed as DSD (Asp-224 to Asp-226) (Wilson et al. 2001) (Fig. 3).
Fig. 2.
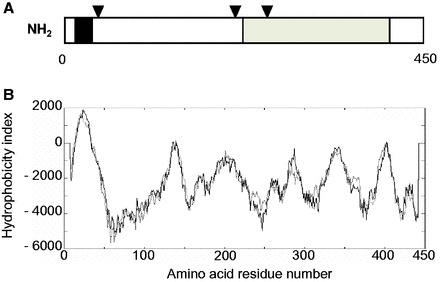
Predicted protein structure of silkworm Fuc-T C3. (A) Predicted structure of silkworm Fuc-T C3. The open reading frame encodes a 450-amino acid protein with molecular mass 52.2 kDa. The black area indicates the putative transmembrane domain (Val-15 to Leu-37) predicted by “TM pred.” Arrowheads indicate the positions of potential N-glycosylation sites. The shaded area (Asp-224 to Ala-408) indicates the region containing typical motifs for 1,3-Fuc-Ts. (The more details are described in Fig. 3.) (B) Hydropathy profile of silkworm Fuc-T C3. The hydropathy plot was calculated by “TM pred.”
Fig. 3.

Comparison of the C-terminal region of Fuc-T C3s. Amino acid sequences conserved in Fuc-T C3s of silkworm, fruit fly, honey bee, and mung bean were aligned. White letters on black background depict amino acid conserved among these four organisms. Letters on gray background depict amino acid conserved among three organisms. Dashed lines represent gaps required for the alignment of these sequences. Motifs specific for 1,3-Fuc-Ts, (I or V)DXYG, YKFXLAFENS, DY(I or V)TEK, and CXXC are underlined by solid line. A long stretch of identity AXF(I or V)SNCXARNXRLQ, common to Fuc-T C3s, is underlined by dashed line. Deduced Mn (II) binding site, DXD (SSD in plant), is underlined by double line.
HCA Comparison of C-Terminal Regions of Silkworm and Fruit Fly Fuc-T C3s
The HCA patterns of the amino acid sequences of the C- terminal region of Fuc-T C3 compared between silkworm and fruit fly were almost the same, showing the two-dimensional structures of the region is highly conserved between the two organisms (Fig. 4).
Fig. 4.
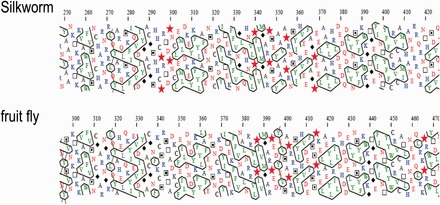
HCA plots of Fuc-T C3 C-terminal regions of silkworm and fruit fly. HCA of Fuc-T C3 C-terminal regions of silkworm (Ala-259 to Ala-408) (top) and fruit fly (Ala-305 to Ala-455) (bottom) was done by using Drawhca program. To make the visual inspection of the pattern easier, the hydrophobic clusters were encircled. The one-letter code for amino acids is used except for Gly, Pro, Ser, and Thr, which are represented by diamonds, stars, squares with solid dots, and open squares, respectively. Only four particular amino acids were chosen to highlight is following below. A diamond for Gly which in contrast confers the largest freedom to the chain. A star for Pro which confers the greatest constraint to the polypeptide chain. Squares for Ser and Thr, respectively, as these two small polar amino acids can mask their polarity through H-bonding with the carbonyls of the main chain, particularly within helices.
Analysis of Silkworm Fuc-T C3 Genomic DNA
The coding sequence of the silkworm Fuc-T C3 cDNA was aligned with the silkworm genomic DNA database and two nonoverlapped shotgun clones were identified: one was NCBI number AADK01016505, with which we had designed PCR primers at the start, and the other was AADK01011744. The Fuc-T C3 gene was located across these two shotgun clones (Fig. 5).
Fig. 5.
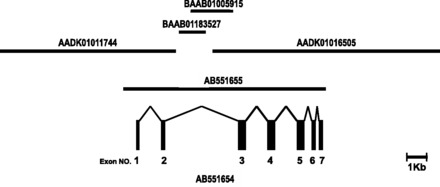
Exon–intron organization of silkworm Fuc-T C3 gene. The silkworm Fuc-T C3 cDNA (NCBI number AB551654) is coded by seven exons in the Fuc-T C3 gene, spanning approximately 10 kb (NCBI number AB551655) on the genome. These exons encode amino acid residues 1-60, 61-110, 111-190, 191-257, 258-335, 336-392, and 393-450, respectively. Four genomic contigs (NCBI numbers AADK01016505, AADK01011744, BAAB01183527, and BAAB01005915) were aligned to the genomic DNA sequence of silkworm Fuc-T C3.
To clarify the exon–intron structure of the Fuc-T C3 gene, genomic DNA sequence that covers the coding region of the cDNA was obtained as follows. First, the region between the two shotgun clones, AADK01016505 and AADK01011744, was amplified by PCR from the silkworm genomic DNA. A PCR fragment (2.2 kb) connected between the two shotgun clones. Two other contigs (NCBI number: BAAB01183527 and BAAB01005915) were identified on the fragment (Fig. 5). Finally, a Fuc-T C3 genomic DNA fragment of approximately 10 kb was amplified (NCBI number: AB551655). Analyses of the genomic sequence revealed that silkworm Fuc-T C3 gene composed of seven exons and is localized on chromosome 7 by searching the KAIKOBLAST database; http://kaikoblast.dna.affrc.go.jp/.
Detection of Fuc-T C3 Activity in the Transfected CHO Cell Extracts
HPLC analysis of the Fuc-T C reaction products with the extract of CHO cells (nontransfectant control or Fuc-T C3 cDNA transfectants) showed the following results. One product peak was observed beside the substrate (GnGn; DABS-GnGn peptide) peak in the control (Fig. 6, red line), whereas three product peaks were observed in the transfectants (Fig. 6, blue line). The only one product peak observed in control was deduced to be GnGnF6, which was produced by intrinsic core alpha 1,6-fucosyltransferase (Fuc-T C6) in the cells. One of the product peaks observed with the transfectants (CHO-Fuc-T C3 cell extract) was deduced to be GnGnF6 and the other two peaks were GnGnF3 and GnGnF3F6, which were produced by the silkworm Fuc-T C3 expressed in the cells.
Fig. 6.
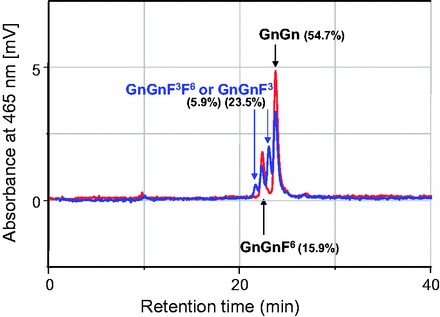
Products of core fucosyltransferase reactions. Reactions products of core fucosyltransferase were analyzed by HPLC. Red line shows the chromatogram of those with CHO cell extract (control experiment). Blue line shows those with CHO-Fuc-T C3 cell extract. HPLC peak area of each product in blue line was shown as percent in parentheses. In each reaction, 22.5 μg protein of cell extract was used, and the reaction mixture was incubated for 17 h at 37°C.
N-glycan Structure of the Reaction Products
N-glycan products identified as PA-oligosaccharides were GnGnF6 with control CHO cell extracts and GnGnF6, GnGnF3, and GnGnF3F6 with CHO-Fuc-T C3 cell extract (Table 1). These results confirmed that the expression product of the cDNA clone actually encodes silkworm Fuc-T C3. The abundance ratio of each products was GnGnF6 (10.8 %), GnGnF3 (31.9 %), and GnGnF3F6 (4.8 %) with CHO-Fuc-T C3 cell extract.
Table 1.
Abbreviation of N-glycan structure obtained from Fuc-T C reaction products
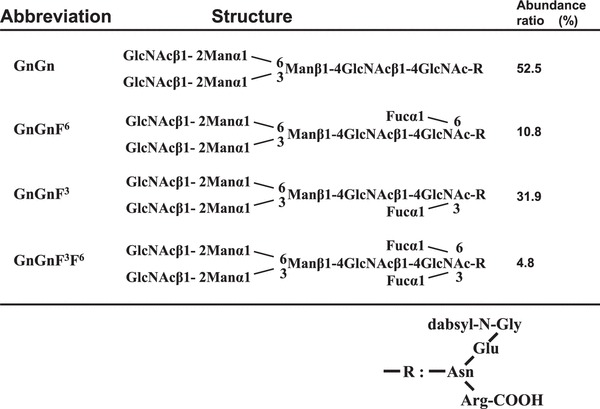 |
The abundance ratio of each products was shown as GnGnF6 (10.8%), GnGnF3 (31.9%), and GnGnF3F6 (4.8%) with CHO-Fuc-T C3 cell extract.
Gene Expression of Fuc-T C3
As shown in Fig. 7, Fuc-T C3 gene expression was detected in several organs and its expression level differed among them. The level of expression in MSG, fluid, and either of malpighian or genitalia tend to be higher than other organs. These expression profile were almost the same between two strains: Kinshu and w1-pnd.This result showed that the Fuc-T C3 gene was actually expressed in silkworm (Fig. 7).
Fig. 7.
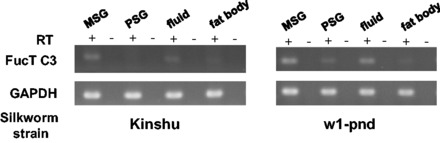
Gene expression pattern of the Fuc-T C3. RT-PCR of Fuc-T C3 gene was performed. MSG shows middle silk gland, and PSG shows posterior silk gland. GAPDH was used as internal controls.
Discussion
In this study, we isolated a candidate cDNA clone of silkworm Fuc-T C3 and confirmed the enzyme activity of its expression product in CHO cells. Deduced amino acid sequence of the gene showed the common structural features among 1,3-Fuc-T family including Fuc-T C3 from other origins: a single transmembrane domain in N-terminal region and 1,3-Fuc-T-specific motifs in C-terminal region (Breton et al. 1998, Oriol et al. 1999). The structural feature indicates that silkworm Fuc-T C3 is also classified into type II transmembrane proteins like most of the glycosyltransferases, the transmembrane domain of which crosses through Golgi membrane, and the catalytic domain is exposed to a luminal side of the Golgi membrane (Joziasse 1992).
Homology of the Fuc-T C3 C-terminal regions between silkworm (present work) and other organisms was 65% identities for fruit fly, 69% for honey bee, and 33% for mung bean (Vigna radiate). Homology of the C-terminal regions among these insect Fuc-T C3s were highly retained. HCA also showed a remarkable similarity between the regions of silkworm and fruit fly.
Silkworm Fuc-T C3 gene composed of seven exons and covered 9.7 kb region on the genome. This length is much longer than that of fruit fly 2.2 kb (four exons) but somehow close to that of honey bee 5.1 kb (seven exons). The exon-intron structure in the genes of silkworm and honey bee were revealed to be similar to each other, especially in their C-terminal regions where the consensus motifs of Fuc-T C3 exist. This suggests that the Fuc-T C3 genes of silkworm and honey bee have developed from the same original gene.
Fuc-T C3 genes have already been cloned in many organisms; however, enzyme activity of the gene expression products was confirmed only in several organisms (Leiter et al. 1999, Fabini et al. 2001, Wilson et al. 2001, Bondili et al. 2006). The silkworm Fuc-T C3 is the second successful case following fruit fly in Insecta.
To produce mammalian glycoproteins by insects without an attachment of core alpha 1,3-fucosyl residues on their N-glycans, Fuc-T C3 seems to be a key enzyme for its regulation. N-glycan profile of a fucTA knocked-down line of fruit fly was reported to be different from that of a normal line (Rendic et al. 2006): reduction of core alpha 1,3-fucosylated N-glycan content was observed. In silkworms, recombinant proteins produced by baculovirus expression system were reported to carry core alpha 1,3-fucosyl residues in their N-glycans (Shi and Jarvis 2007). In this system, Fuc-T C3 activity should be suppressed in any organs where the virus replicate.
In silkworm transgenic system, recombinant mouse IgG, which was biosynthesized at MSG and secreted into sericin layer of cocoons, carried no core alpha 1,3-fucosyl residues in its N-glycan (Iizuka et al. 2009). However, we clarified high-level expression of the Fuc-T C3 mRNA in MSG, which relates to be a potential risk for core alpha 1,3-fucosylation in this system.
It is known that some of the fucTA mutations induce lethal phenotype in fruit fly, because this gene has an important function in neural development (Yamamoto-Hino et al. 2010). So, in silkworm, inhibition of the Fuc-T C3 gene expression in neural tissues might also lead to abnormality or lethality as observed in fruit fly. To avoid such event, inhibition of Fuc-T C3 expression only in silk glands by means of certain technologies including RNA interference may be the best strategy for stable production of core alpha 1,3-fucose free recombinant proteins in the transgenic system. As a mean of production of safer recombinant glycoproteins with lower cost, silkworm system might be a promising candidate which comes next to mammalian cell culture system and the results of this study contributes to improvement of this system.
References Cited
- Bondili J. S., Castilho A., Mach L., Glossl J., Steinkellner H., Altmann F., Strasser R. 2006. Molecular cloning and heterologous expression of beta1,2-xylosyltransferase and core alpha1,3-fucosyltransferase from maize. Phytochemistry 67: 2215–2224. [DOI] [PubMed] [Google Scholar]
- Breton C., Oriol R., Imberty A. 1998. Conserved structural features in eukaryotic and prokaryotic fucosyltransferases. Glycobiology 8: 87–94. [DOI] [PubMed] [Google Scholar]
- Chen W. Y., Shen Q. X., Bahl O. P. 1991. Carbohydrate variant of the recombinant beta-subunit of human choriogonadotropin expressed in baculovirus expression system. J. Biol. Chem. 266: 4081–4087. [PubMed] [Google Scholar]
- Drugmand J. C., Schneider Y. J., Agathos S. N. 2012. Insect cells as factories for biomanufacturing. Biotechnol. Adv. 30:1140–1157. [DOI] [PubMed] [Google Scholar]
- Fabini G., Freilinger A., Altmann F., Wilson I. B. 2001. Identification of core alpha 1,3-fucosylated glycans and cloning of the requisite fucosyltransferase cDNA from Drosophila melanogaster. Potential basis of the neural anti-horseadish peroxidase epitope. J. Biol. Chem. 276: 28058–28067. [DOI] [PubMed] [Google Scholar]
- Gaboriaud C., Bissery V., Benchetrit T., Mornon J. P. 1987. Hydrophobic cluster analysis: an efficient new way to compare and analyse amino acid sequences. FEBS Lett. 224: 149–155. [DOI] [PubMed] [Google Scholar]
- Geisow M. J. 1992. Glycoprotein glycans—roles and controls. Trends Biotechnol. 10: 333–335. [DOI] [PubMed] [Google Scholar]
- Iizuka M., Ogawa S., Takeuchi A., Nakakita S., Kubo Y., Miyawaki Y., Hirabayashi J., Tomita M. 2009. Production of a recombinant mouse monoclonal antibody in transgenic silkworm cocoons. FEBS J. 276: 5806–5820. [DOI] [PubMed] [Google Scholar]
- Joziasse D. H. 1992. Mammalian glycosyltransferases: genomic organization and protein structure. Glycobiology 2: 271–277. [DOI] [PubMed] [Google Scholar]
- Kubelka V., Altmann F., Staudacher E., Tretter V., Marz L., Hard K., Kamerling J. P., Vliegenthart J. F. 1993. Primary structures of the N-linked carbohydrate chains from honeybee venom phospholipase A2. Eur. J. Biochem. 213: 1193–1204. [DOI] [PubMed] [Google Scholar]
- Kuroda K., Geyer H., Geyer R., Doerfler W., Klenk H. D. 1990. The oligosaccharides of influenza virus hemagglutinin expressed in insect cells by a baculovirus vector. Virology 174: 418–429. [DOI] [PubMed] [Google Scholar]
- Leiter H., Mucha J., Staudacher E., Grimm R., Glossl J., Altmann F. 1999. Purification, cDNA cloning, and expression of GDP-L-Fuc:Asn-linked GlcNAc alpha1,3-fucosyltransferase from mung beans. J. Biol. Chem. 274: 21830–21839. [DOI] [PubMed] [Google Scholar]
- Lerouge P., Cabanes-Macheteau M., Rayon C., Fischette-Laine A. C., Gomord V., Faye L. 1998. N-glycoprotein biosynthesis in plants: recent developments and future trends. Plant Mol. Biol. 38: 31–48. [PubMed] [Google Scholar]
- Longmore G. D., Schachter H. 1982. Product-identification and substrate-specificity studies of the GDP-L-fucose:2-acetamido-2-deoxy-beta-D-glucoside (FUC goes to Asn-linked GlcNAc) 6-alpha-L-fucosyltransferase in a Golgi-rich fraction from porcine liver. Carbohydr. Res. 100: 365–392. [DOI] [PubMed] [Google Scholar]
- Luckow V. A., Summers M. D. 1989. High level expression of nonfused foreign genes with Autographa californica nuclear polyhedrosis virus expression vectors. Virology 170: 31–39. [DOI] [PubMed] [Google Scholar]
- Ludwig H., Sundal E., Pecherstorfer M., Leitgeb C., Bauernhofer T., Beinhauer A., Samonigg H., Kappeler A. W., Fritz E. 1995. Recombinant human erythropoietin for the correction of cancer associated anemia with and without concomitant cytotoxic chemotherapy. Cancer 76: 2319–2329. [DOI] [PubMed] [Google Scholar]
- Oriol R., Mollicone R., Cailleau A., Balanzino L., Breton C. 1999. Divergent evolution of fucosyltransferase genes from vertebrates, invertebrates, and bacteria. Glycobiology 9: 323–334. [DOI] [PubMed] [Google Scholar]
- Rendic D., Linder A., Paschinger K., Borth N., Wilson I. B., Fabini G. 2006. Modulation of neural carbohydrate epitope expression in Drosophila melanogaster cells. J. Biol. Chem. 281: 3343–3353. [DOI] [PubMed] [Google Scholar]
- Rendic D., Klaudiny J., Stemmer U., Schmidt J., Paschinger K., Wilson I. B. 2007. Towards abolition of immunogenic structures in insect cells: characterization of a honey-bee (Apis mellifera) multi-gene family reveals both an allergy-related core alpha1,3-fucosyltransferase and the first insect Lewis-histo-blood-group-related antigen-synthesizing enzyme. Biochem. J. 402: 105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X., Jarvis D. L. 2007. Protein N-glycosylation in the baculovirus-insect cell system. Curr. Drug Targets 10: 1116–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D., Fraser M. J. 1983. Production of human beta interferon in insect cells infected with a baculovirus expression vector. Mol. Cell. Biol. 3: 2156–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudacher E., Altmann F., Marz L., Hard K., Kamerling J. P., Vliegenthart J. F. 1992. Alpha 1-6(alpha 1-3)-difucosylation of the asparagine-bound N-acetylglucosamine in honeybee venom phospholipase A2. Glycoconj. J. 9: 82–85. [DOI] [PubMed] [Google Scholar]
- Tomita M., Munetsuna H., Sato T., Adachi T., Hino R., Hayashi M., Shimizu K., Nakamura N., Tamura T., Yoshizato K. 2003. Transgenic silkworms produce recombinant human type III procollagen in cocoons. Nat. Biotechnol. 21: 52–56. [DOI] [PubMed] [Google Scholar]
- Tomita M., Hino R., Ogawa S., Iizuka M., Adachi T., Shimizu K., Sotoshiro H., Yoshizato K. 2007. A germline transgenic silkworm that secretes recombinant proteins in the sericin layer of cocoon. Transgenic Res. 16: 449–465. [DOI] [PubMed] [Google Scholar]
- Tretter V., Altmann F., Kubelka V., Marz L., Becker W. M. 1993. Fucose alpha 1,3-linked to the core region of glycoprotein N-glycans creates an important epitope for IgE from honeybee venom allergic individuals. Int. Arch. Allergy Immunol. 102: 259–266. [DOI] [PubMed] [Google Scholar]
- Williams P. J., Wormald M. R., Dwek R. A., Rademacher T. W., Parker G. F., Roberts D. R. 1991. Characterisation of oligosaccharides from Drosophila melanogaster glycoproteins. Biochim. Biophys. Acta 1075: 146–153. [DOI] [PubMed] [Google Scholar]
- Wilson I. B., Rendic D., Freilinger A., Dumic J., Altmann F., Mucha J., Muller S., Hauser M. T. 2001. Cloning and expression of cDNAs encoding alpha1,3-fucosyltransferase homologues from Arabidopsis thaliana. Biochim. Biophys. Acta 1527: 88–96. [DOI] [PubMed] [Google Scholar]
- Yagi H., Takahashi N., Yamaguchi Y., Kimura N., Uchimura K., Kannagi R., Kato K. 2005. Development of structural analysis of sulfated N-glycans by multidimensional high performance liquid chromatography mapping methods. Glycobiology 15: 1051–1060. [DOI] [PubMed] [Google Scholar]
- Yamamoto-Hino M., Kanie Y., Awano W., Aoki-Kinoshita K. F., Yano H., Nishihara S., Okano H., Ueda R., Kanie O., Goto S. 2010. Identification of genes required for neural-specific glycosylation using functional genomics. PLoS Genet. 6: e1001254. [DOI] [PMC free article] [PubMed] [Google Scholar]


