Abstract
Vineyards were surveyed for grapevine leafroll-associated viruses and their insect vectors in New York State’s Finger Lakes region in 2006–2008. Grape mealybug, Pseudococcus maritimus (Erhorn) (Hemiptera: Pseudococcidae), European Fruit Lecanium, Parthenolecanium corni (Bouche), and Cottony Maple Scale, Pulvinaria acericola (Walsh and Riley) (Hemiptera: Coccidae) were identified as vector species in this region. An increase in the incidence of Grapevine leafroll-associated virus 1 (GLRaV-1) and GLRaV-3 was observed in 8 of the 20 vineyards surveyed, which implies transmission by these insect vectors. Two of the vineyards for which a temporal increase in disease incidence was documented were then used to evaluate the efficacy of foliar applications of horticultural oil and two classes of insecticides for control of P. maritimus and for slowing virus spread over 2 years of vine protection. Delayed dormant applications of horticultural oil contributed to control of early season crawlers; however, this was not the case for control of summer populations. Applications of acetamiprid and spirotetramat achieved control in summer populations; however, spirotetramat outperformed acetamiprid in percent reduction of treated compared with control vines and in a side-by-side trial. Vines treated with spirotetramat had a lower percentage of new vines testing positive for GLRaV-1 than control vines after 2 years, while no other spray program altered the increase in incidence of GLRaV-1 or -3.
Keywords: grapevine leafroll-associated virus (GLRaV), grape mealybug, acetamiprid, spirotetramat
Grapevine leafroll disease negatively affects vineyard productivity and quality of wine and table grapes in all major grape-growing regions worldwide (Charles et al. 2006, Almeida et al. 2013, Rayapati et al. 2014). Disease symptoms such as downward rolling of leaf margins and interveinal discoloration are most likely to be observed late in the season and are more conspicuous on red varieties than on white varieties. Infection delays fruit maturity, decreases the productive life of the vineyard, and reduces fruit yield and quality by way of altered sugar content, acidity, pigmentation, and phenolic profiles (Goheen and Cook 1959, Over de Linden and Chamberlain 1970, Credi and Babini 1997, Martelli 2014, Rayapati et al. 2014).
Several phloem-limited filamentous viruses in the family Closteroviridae have been identified in leafroll-diseased vines as grapevine leafroll-associated viruses (GLRaVs) including GLRaV-1, -3, and -4 from the genus Ampelovirus, GLRaV-2 from the genus Closterovirus, and GLRaV-7 from the genus Velarivirus (Martelli 2014, Rayapati et al. 2014). Considered to be the main etiological agent contributing to the disease, GLRaV-3 is the most ubiquitous species worldwide (Maree et al. 2013) as well as the primary GLRaV species in New York vineyards (Fuchs et al. 2009b). Viruses are disseminated through propagation material, so infected planting material is often the initial source of infection in vineyards. However, ampeloviruses are also spread by phloem-feeding insects, such as soft scales and mealybugs (Coccidae and Pseudococcidae, respectively; Golino et al. 2002, Almeida et al. 2013). Infection is spread when insects acquire the pathogen from feeding on infected vines then walk to neighboring vines or are spread by vineyard equipment or wind dispersion (Habili et al. 1995, Habili and Nutter 1997, Cabaleiro et al. 2008, Grasswitz and James 2008, Daane et al. 2012). The primary vector species in North America is the grape mealybug, Pseudococcus maritimus (Erhorn) (Hemiptera: Pseudococcidae) (Daane et al. 2012). P. maritimus overwinters as first-instar nymphs or eggs on canes and trunks and emerges around bud swell to feed and develop to adulthood and their offspring hatch in mid-summer (Grimes and Cone 1985, Geiger and Daane 2001). Although all life stages are capable of transmitting GLRaVs, the first-instar “crawlers” are considered the most efficient vectors because of their high relative mobility compared with older stages, which are typically sessile upon reaching adulthood (Mahfoudhi et al. 2009, Tsai et al. 2010, Daane et al. 2012). There are two generations of P. maritimus in most regions, therefore two periods of high crawler activity which are the times of greatest concern for virus management (Daane et al. 2012).
Historically, foliar applications of organophosphate and carbamate insecticides were recommended during peaks of crawler activity, but many of these materials have lost their efficacy due to resistance or are no longer commercially available in the United States (Flaherty 1982, Grimes and Cone 1985, Bentley et al. 2006). Moreover, mealybugs can be particularly difficult to control with contact insecticides since they are often in protected areas under loose bark. Horticultural oils or broad-spectrum foliar insecticides are somewhat limited in their efficacy due to this behavior (Geiger and Daane 2001, Grasswitz and James 2008). Broad-spectrum insecticides also negatively affect nontarget insects, natural enemies, and pollinators (Walton and Pringle 1999). More recent work has investigated alternatives for control of mealybugs including biological control, mating disruption, insect growth regulators, and systemic insecticides (Daane et al. 2006, Franco et al. 2009).
Current management options of leafroll disease incur high costs in labor and materials, i.e., removing infected vines (roguing) and replacing with vines derived from certified virus-tested stock to reduce sources of inoculum (Atallah et al. 2012, Pereira-Crespo et al. 2012, Pietersen et al. 2013). Effective insecticidal control of mealybug vectors could slow the progression of the disease through affected vineyard blocks, thereby reducing the need for expensive vine removal and replacement initiatives, although this approach has not been rigorously tested. New York’s Finger Lakes region is an area of significant wine and grape juice production with a known history of grapevine leafroll disease (Fuchs et al. 2009b). Here we report on field surveys of more than 20 commercial vineyards that confirm P. maritimus as the primary mealybug species in New York’s Finger Lakes region. We also report on field trials evaluating the efficacy of horticultural oil and two classes of systemic insecticides for control of P. maritimus and the effect of P. maritimus control on the rate of GLRaV spread in two commercial vineyards.
Materials and Methods
Vector Surveys
Twenty-three commercial vineyards in New York’s Finger Lakes Region were scouted for soft scale insects and mealybugs from mid-June to mid-July 2006, and 17 vineyards were scouted in June 2007. A range of cultivars was sampled within V. vinifera, Vitis labrusca, and interspecific hybrids. All vineyards were in production and variable in age. Canopy management varied by cultivar and location. Sixteen to 20 sampling points were recorded following a systematic sampling scheme for each vineyard site in 2006. Based on the relatively low counts the previous year, in 2007 we increased the number of assessment points to 25–100 for a more comprehensive sampling effort. Sampling points were evaluated for 2 min by multiple observers who counted insects by continuously scanning under loose bark on trunks and canes. Observers used 10X optical glass binocular magnifiers (Opti Visor, Donegan Optical Company, Lenexa, KS) to aid in recognizing small instars. The presence and total number of soft scale insects and mealybugs were recorded, independent of lifestage. When found, samples of soft scale or mealybug specimens were collected and returned to the laboratory for identification (Kosztarab 1996). Specimens were also sent for confirmation to the USDA Systematic Identification Lab (Beltsville, MD).
Virus Surveys
Commercial vineyards in New York’s Finger Lakes Region were surveyed for GLRaV-1 and GLRaV-3 from late August to October in 2006–2008 using a 4 by 5 quadrat sampling approach where leaf samples are collected from vines at four equally spaced locations along a row (near start of row, 1/3 down the row, 2/3 down the row, and near end of row), and this was repeated every five rows for a maximum of 20 quadrats per vineyard block (Fuchs et al. 2009a). There was partial overlap between vineyards surveyed for vectors and for GLRaV-1 and GLRaV-3, but each survey included vineyard blocks not included in the other. Composite samples of three leaves per vine and five vines per quadrat were collected, making a total of 15 leaves per sample. Leaf samples were processed and tested by double-antibody sandwich (DAS) enzyme-linked immunosorbent assay (ELISA) using specific antibodies (Bioreba, Reinach, Switzerland) as previously described (Fuchs et al. 2009a). Substrate hydrolysis was recorded at 405 nm with an absorbance BioTek ELx808TM microplate reader (BioTek, Winooski, VT). Samples were considered positive if their optical density values at 405 nm were at least twice those of healthy controls. A total of 95 vineyard blocks were surveyed in 2006. Based on a low to moderate incidence (<20%) of GLRaV-1 and/or GLRaV-3 documented in 2006 (Fuchs et al. 2009b), 20 out of the 95 vineyards were further surveyed in 2007 and 2008. The number of quadrats with samples testing positive for GLRaV-1 or GLRaV-3 in DAS-ELISA was determined for every vineyard surveyed and 2007 data were compared with 2006 data; similarly, 2008 data were compared with 2007 data. An increase over time in the number of quadrats with infected samples was evidence of an increase in virus incidence in a given vineyard, likely as a result of vector-mediated virus spread.
Vector Management
Insecticide spray programs were evaluated for efficacy on P. maritimus at two commercial vineyards with a history of P. maritimus infestation and an increasing GLRaV incidence over time. The vineyards were managed by the cooperating growers for diseases following standard practices (Weigle and Muza 2013). Growers did not apply insecticides to blocks of grapes used in vector management experiments described below during the study period. An approximately 3 hectare plot of Vitis vinifera, “Chardonnay” at vineyard A (41° 41’ 5.21” N, 76° 44’ 36.64” W) was used to evaluate horticultural oil (PureSpray 10E, Petro-Canada, Mississauga, ON), acetamiprid (Assail 30SG, United Phosphorus Inc., King of Prussia, PA), and a combination of the two materials versus a water control during the 2009 and 2010 growing seasons. Vineyard A was cane-pruned using an umbrella kniffin training system (Reynolds and Vanden Heuvel 2009) and comprised older vines (>10 years in age) interspersed with less than 10% replacement vines of younger age. Treatments were assigned to plots using a randomized block experimental design (36 vines per plot = 4 rows by 9 vines/row), replicated four times per treatment, with horticultural oil applied to vines at delayed dormancy (late April) at a rate of 28 L/ha in 1,870 L/ha of water, and acetamiprid applied at a rate of 0.175 kg/ha in 935 liters/ha of water when the first new summer generation of crawlers was observed on vines (early July). Treatments included oil only, acetamiprid only, oil plus acetamiprid, and water control and were applied with a small plot sprayer and hand gun. Another approximately 3 hectare plot of V. vinifera “Chardonnay” at vineyard B (42o 28’ 22.80” N, 77o 10’ 56.1” W) was used to evaluate a newly labeled systemic insecticide, spirotetramat (Movento, Bayer CropScience, Research Triangle Park, NC) during the 2011 and 2012 growing seasons. Vineyard B was cane pruned using an umbrella kniffin training system and comprised older vines (>10 years in age) interspersed with less than 10% replacement vines of younger age. Treatments (spirotetramat or water control) were assigned to six plots using a randomized block experimental design (64 vines per plot = 4 rows by 16 vines/row), and spirotetramat was applied at a rate of 0.457 liter/ha in 935 liters/ha of water after bud break (early June) and again when the first new generation of crawlers was observed (early July) using a ATV-mounted sprayer with flat fan nozzles on a hand boom. An additional experiment was conducted in a section of vines at vineyard A in 2010 to assess efficacy of acetamiprid and spirotetramat using the same per ha rate of insecticide and amount of water per ha as used in larger plot experiments in a side-by-side comparison using a back pack sprayer with flat fan nozzle with six vine plots and five replicates per treatment. A nonionic surfactant (LI 700, Loveland Products, Greeley, CO) was included in all treatments at a rate of 0.25% by volume.
P. maritimus abundance was recorded after treatments. Spring P. maritimus populations were measured a week after horticultural oil applications at vineyard A by four different observers who counted the number of crawlers present under or nearby the cracked bark of one vine stem at 20 locations per plot. We only sampled vines in the middle sections of the center two rows of each plot thereby avoiding edge vines. At this time of year, crawlers are in exposed sites out on canes and therefore more readily enumerated compared with less-exposed mid-summer crawlers. Summer P. maritimus abundance was measured at all sites in early August by four different observers who conducted 10 timed counts per plot at vineyards A and B where the number of adults and nymphs were counted anywhere on vines for 5 min (Geiger and Daane 2001). We only sampled vines in the middle sections of the center two rows of each plot thereby avoiding edge vines. All observers conducted timed counts in every replicate plot. Observers used optivisors when necessary to confirm presence of small instars.
Effect of Vector Management on Virus Spread
All vines were tested for GLRaV-1 and GLRaV-3 at vineyard A and for GLRaV-1 in vineyard B by DAS-ELISA in September of each year using composite samples of four to six lower canopy leaves per vine. Samples were processed and tested as previously described (Fuchs et al. 2009a). Spirotetramat has been reported to control scale insects for up to 18 months (McKenna et al. 2013). Although no insecticide treatments were applied in the third year at vineyard B, these vines were tested for GLRaV, using the same methods as previously described, to evaluate this spray program for residual effects on disease spread.
Statistical Analysis
All statistical analyses were conducted using JMP (SAS Institute, Cary, NC). Count data from observations of P. maritimus were highly skewed and did not respond to transformation, so effect of year and spray program were evaluated in a generalized log-linear model, assuming a Poisson distribution (O’Hara 2009). There were no statistical differences among observers in the number of P. maritimus recorded at any date, so counts were summed across observer in spring counts (n = 80), summer counts (n = 40), and counts from the side-by-side trial (n = 15). Effects found to be significant were followed by pairwise contrast analysis, using a Bonferroni corrected P value to determine significant difference between means. An analysis of variance followed by Tukey’s HSD means separation was used to determine significant difference in the proportion of newly infected vines between treatments in year 2 at vineyard A (four replicates per treatment). A Student’s t-test was used to determine significant difference in the proportion of newly infected vines between treatments in year 2 at vineyard B (6 replicates per treatment). Newly infected vines were those that tested positive for GLRaV in year 2 (or three) but were not positive for the virus in year 1.
Results
Only one species of mealybug, confirmed as P. maritimus, was observed in vineyards in the 2006 and 2007 surveys. Mealybugs were observed at 6 out of the 23 sites surveyed in 2006 and at 5 out of the 17 sites surveyed in 2007. Mealybug abundance ranged from 0.10 to 0.60 and 0.02 to 0.23 mealybugs per observation in 2006 and 2007, respectively. Two species of soft scale were identified, European Fruit Lecanium, Parthenolecanium corni (Bouche), and Cottony Maple Scale, Pulvinaria acericola (Walsh and Riley) (Hemiptera: Coccidae). Scale insects were observed at 15 of the 23 sites surveyed in 2006, and 10 of the 17 sites surveyed in 2007. Scale abundance ranged from 0.10 to 2.00 and 0.01 to 0.18 scale insects per observation in 2006 and 2007, respectively.
Virus surveys indicated a temporal increase of GLRaV-1 and GLRaV-3 in seven and 2 of the 20 vineyards surveyed, respectively (Table 1). No increase in virus incidence was documented in 12 of the 20 vineyards surveyed from 2006 to 2008. Vineyards A and B were selected for further mealybug management studies because virus spread and the presence of P. maritimus were documented, and virus incidence was moderate to high (25–50%) in 2008. Vines in vineyard A were coinfected with GLRaV-1 and GLRaV-3, while vines in vineyard B were infected with GLRaV-1 (Table 1).
Table 1.
Incidence of GLRaV-1 and GLRaV-3 in 20 vineyards surveyed from 2006 to 2008 in the Finger Lakes region of New York
| Vineyard | Cultivar | GLRaV-1 |
GLRaV-3 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 2006 | 2007 | 2008 | Increase | 2006 | 2007 | 2008 | Increase | ||
| A | Chardonnay | 2/20 | 5/20 | 9/20 | + | 3/20 | 7/20 | 10/10 | + |
| B | Chardonnay | 2/20 | 3/20 | 7/20 | + | 0/20 | 0/20 | 0/20 | − |
| C | Cabernet Sauvignon | 1/20 | 1/20 | 1/20 | − | 2/20 | 2/20 | 2/20 | − |
| D | Pinot noir | 2/20 | 2/20 | 2/20 | − | 3/20 | 3/20 | 3/20 | − |
| E | Pinot noir | 2/20 | 2/20 | 2/20 | − | 2/10 | 2/10 | 2/10 | − |
| F | Pinot noir | 1/10 | 1/10 | 1/10 | − | 2/10 | 2/10 | 2/20 | − |
| G | Cabernet Sauvignon | 1/10 | 1/10 | 1/10 | − | 1/10 | 1/10 | 1/10 | − |
| H | Caberet Franc | 0/20 | 1/10 | 1/10 | + | 1/10 | 1/10 | 1/10 | − |
| I | Caberet Franc | 0/20 | 0/20 | 0/20 | − | 7/20 | 7/20 | 7/20 | − |
| J | Merlot | 0/20 | 0/20 | 0/20 | − | 9/20 | 9/20 | 9/20 | − |
| K | Lemberger | 0/20 | 0/20 | 2/20 | + | 4/20 | 4/20 | 4/20 | −− |
| L | Chardonnay | 0/20 | 3/20 | 3/20 | + | 3/20 | 3/20 | 3/20 | − |
| M | Pinot noir | 2/20 | 2/20 | 2/20 | − | 2/20 | 2/20 | 2/20 | − |
| N | Lemberger | 3/20 | 3/20 | 3/20 | − | 3/20 | 3/20 | 3/20 | − |
| O | Lemberger | 7/20 | 7/20 | 14/20 | + | 0/20 | 0/20 | 0/20 | − |
| P | Lemberger | 2/30 | 2/30 | 2/30 | − | 1/20 | 1/20 | 1/20 | − |
| Q | Riesling | 0/20 | 0/20 | 0/20 | − | 5/20 | 9/20 | 14/20 | + |
| R | Riesling | 0/20 | 0/20 | 0/20 | − | 3/20 | 3/20 | 3/20 | − |
| S | Lemberger | 2/15 | 10/15 | 10/15 | + | 0/15 | 0/15 | 0/15 | − |
| T | Seyval | 1/10 | 1/10 | 1/10 | − | 2/20 | 2/20 | 2/20 | − |
Data represent the number of quadrats with infected vine samples, as shown by DAS-ELISA, over the number of quadrats analyzed per vineyard. An increase in virus incidence is indicated by (+).
The interaction between year and spray program was found to have a significant effect on the number of P. maritimus observed in the spring at vineyard A (χ2 = 37.70, df = 3, P < 0.0001). Subsequent pairwise contrast tests found no differences between P. maritimus abundance on controls between the two experimental years, but acetamiprid-treated vines had fewer P. maritimus in the second year of the experiment, while there was an increase in the number of P. maritimus observed on vines treated with horticultural oil only. Fewer P. maritimus were observed on vines treated with any spray program than controls in each year, and fewer early season P. maritimus were observed on vines treated with oil and acetamiprid than vines of any other treatment in year 2 (Table 2).
Table 2.
Springtime P. maritimus abundance in vineyard A (mean ± SE mealybugs/observation) for year 1 (2009) and year 2 (2010)
| Spray program | Year 1 | Year 2 |
|---|---|---|
| Control (surfactant only) | 1.31 ± 0.32a | 1.18 ± 0.26A |
| Horticultural oil | 0.30 ± 0.10a | 0.96 ± 0.23B* |
| Acetamiprid | 0.86 ± 0.23b | 0.43 ± 0.16B* |
| Oil + acetamiprid | 0.46 ± 0.19b | 0.13 ± 0.05C* |
Mean values followed by the same letter indicate significant difference between treatments and asterisks indicate difference between years according to pairwise contrast test (α = 0.05).
The interaction between year and spray program was found to have a significant effect on the number of P. maritimus observed in the summer at vineyard A (χ2 = 39.10, df = 3, P < 0.0001) and vineyard B (χ2 = 39.81, df = 1, P < 0.0001). Spray program had a significant effect on the number of P. maritimus observed in the side-by-side trial (χ2 = 81.50, df = 2, P < 0.0001). Subsequent pairwise contrast tests showed more P. maritimus on control vines and acetamiprid-treated vines in the second experimental year compared with year 1 at vineyard A, while there was no difference between counts on vines treated with horticultural oil in years 1 and 2. There was no difference in the number of P. maritimus observed on vines treated with horticultural oil compared with control vines in the first year, while fewer P. maritimus were observed on vines treated with acetamiprid or a combination of oil and acetamiprid (66% and 45% decrease, respectively). Fewer P. maritimus were observed on vines treated with any spray program than control vines in the second year at vineyard A. There were no differences between P. maritimus abundances on controls between the two experimental years at vineyard B, while there was a decrease on spirotetramat-treated vines between experimental years. Fewer P. maritimus were observed on spirotetramat-treated vines than control vines at vineyard B in each year (68% and 100% decrease, respectively), as well as a 92% reduction for spirotetramat-treated vines relative to control vines in the side-by-side trial (Table 3).
Table 3.
Summertime P. maritimus abundance (mean ± SE mealybugs per observation) in vineyard A for year 1 (2009) and year 2 (2010), in the side-by-side trial (2010) and in vineyard B for year 1 (2011) and year 2 (2012), and percent change in P. maritimus abundance compared with the mean number of mealybugs observed on control vines
| Spray program | Year 1 |
Year 2 |
||
|---|---|---|---|---|
| Mealybugs/obs. | % change | Mealybugs/obs. | % change | |
| Vineyard A | ||||
| Control (surfactant only) | 4.85 ± 0.79a | 9.10 ± 0.93a | ||
| Horticultural oil | 6.28 ± 0.82ab | +29 | 6.83 ± 0.83ab | −25 |
| Oil + acetamiprid | 2.65 ± 0.57bc | −45 | 4.15 ± 0.54b | −54 |
| Acetamiprid | 1.65 ± 0.41c | −66 | 4.63 ± 0.71b | −49 |
| Side-by-side | ||||
| Control (surfactant only) | 5.60 + 0.71a | |||
| Acetamiprid | 4.33 + 0.64a | −22 | ||
| Spirotetramat | 0.47 + 0.19b | −92 | ||
| Vineyard B | ||||
| Control (surfactant only) | 3.77 ± 0.54a | 2.80 ± 0.72a | ||
| Spirotetramat | 1.22 ± 0.35b | −68 | 0.00b | −100 |
Mean values for each experiment and each year followed by the same letter are not different according to pairwise contrasts (α = 0.05).
At vineyard A, the mean percentage of untreated vines that tested positive for GLRaV-1 increased from 29% in 2009 to 42% in 2010 and untreated vines testing positive for GLRaV-3 increased from 69% in 2009 to 90% in 2010. At vineyard B, the mean percent of untreated vines testing positive for GLRaV-1 increased from 26% in 2011 to 35% in 2012. There was no difference among treatments in the increase of newly GLRaV-infected vines in the second year of screening at vineyard A. Spirotetramat treatments slowed the spread of the virus at vineyard B as treated vines had a lower percentage of new vines testing positive for GLRaV-1 than control vines (t = 3.40, df = 5, P = 0.0030; Fig. 1). There was no difference among treated blocks in the percent increase of GLRaV-infected vines in the third year of screening (no insecticide application) at vineyard B.
Fig. 1.
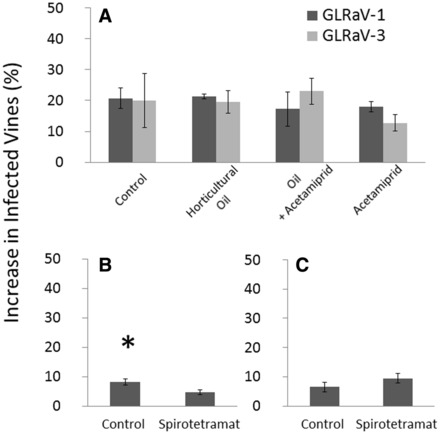
Percent mean (±SE) increase in GLRaV-infected vines after insecticidal control of P. maritimus for (A) year 2 at vineyard A, (B) year 2 at vineyard B, and (C) year 3 at vineyard B. No insecticidal control was applied in year 3 at vineyard B. Asterisk indicates significant difference according to a t-test (α = 0.05).
Discussion
All materials (horticultural oil and two classes of insecticides) tested showed some efficacy against P. maritimus but varied in the level of control. Summertime P. maritimus populations were reduced by 45–66% in vines treated with acetamiprid, and the treatments may have even contributed to reductions in springtime populations in year 2 at vineyard A, before that year’s application (Tables 2 and 3). Neonicotinoids are reported to provide multi-year control of sucking insects in woody hosts when used as a soil drench (Szczepaniec and Raupp 2000); however, the long-term residual life of acetamiprid when applied as a foliar is not known. Although horticultural oil did not contribute to the control of summertime P. maritimus populations, this treatment provided some control in the spring of the first year at vineyard A, and combination of horticultural oil followed by acetamiprid was the most successful in reducing early season P. maritimus populations after 2 years of application (Table 2).
Vines treated with spirotetramat had a 68–100% reduction in summer P. maritimus populations, and spirotetramat treatments were the only spray program found to slow virus spread (Fig. 1). Spirotetramat outperformed acetamiprid in efficacy against P. maritimus in the side-by-side trial, and both spirotetramat- and acetamiprid-treated vines had numerically higher percent P. maritimus reduction than those treated with horticultural oil alone (Tables 2 and 3). Similar control has been reported for the vine mealybug, Plannococcus ficus (Signoret), where both acetamiprid and spirotetramat in combination provided significant reductions in pest population, but spirotetramat generally outperformed the neonicotinoid (Haviland et al. 2010, 2011; Sial et al. 2012). Both classes of insecticides have translaminar systemic activity, which increases the probability of phytophagous insect exposure to a foliar insecticide application (Horowitz et al. 1998, Bucholz and Nauen 2002, Brück et al. 2009, Gaskin et al. 2010). However, the translaminar transport of acetamiprid does not likely match that of spirotetramat, as spirotetramat is the only product labeled on grapes that is fully systemic when applied to foliage while acetamiprid is generally considered most effective as a systemic insecticide in soil drench applications. Spirotetramat is also reported to reduce fecundity in sublethal doses and was found to provide control of scale for 18 months (Brück et al. 2009, McKenna et al. 2013). However, in our experiment, any extended control from this material did not extend beyond the season of application as indicated by no differences in virus spread at Vineyard B in the third year of testing, a year after spirotetramat was applied.
We found that low vector abundance can result in spread of GLRaVs. The mean percent infected vines increased 13% in controls at vineyard A, where 5–10 P. maritimus were observed per 5 min observation. Vineyard B saw a 21% increase in untreated vines, where 3–4 P. maritimus were observed per 5-min observation. Similar rates of spread were seen by Golino et al. (2008) in a Napa Valley, CA. “Cabernet Sauvignon” vineyard where P. maritimus were found at low levels and the number of infected vines increased by an average of 10% per year. Even with very good control of vectors within a vineyard block, virus spread may occur due to migration from outside areas if the population size of vectors and level of disease in surrounding vineyards are high. These results are consistent with insecticidal control of the glassy-winged sharpshooter to limit spread of Xylella fastidiosa in California vineyards (Daugherty et al. 2015). In the case of our experiments, vector populations are relatively low, and there is little evidence of vector movement of virus from vineyard block to vineyard block based on high nucleotide sequence similarity of virus in the vector and the vine from which it is collected (Fuchs et al. 2009a).
P. maritimus was the focus vector species in this study because mealybugs are considered the primary vector in most GLRaV-affected regions; however, each region will have different challenges depending on the vector complex. Although potential vectors were found in the majority of vineyards surveyed in our study, P. maritimus populations were generally low. Moreover, a majority of vineyards in this region (12 out of 20) did not show increases in the incidence of GLRaV infection over three consecutive years (Table 1), suggesting at many sites, vector abundance was below some critical level that promotes measurable disease spread. Improved knowledge of this critical vector population size will be important in assessing the potential of using chemical control of vector populations to prevent spread of leafroll disease. Although P. maritimus rarely reaches economically damaging levels as a direct pest in any region, mealybug species in other regions, which complete more generations in a year, will often reach levels exceeding economic damage to grape clusters (Charles 1982, Daane et al. 2006). Higher rates of spread of grapevine leafroll disease are reported in some areas where the vector complexes are made up of these species (i.e., Planaococcus ficus, P. longispinus, and Pl. citri), which further differentiates the Finger Lakes region from other grapevine leafroll disease-affected areas (Engelbrecht and Kasdorf 1990, Jordan 1993, Cabaleiro and Segura 1997, Habili and Nutter 1997). Additionally, our survey found scale more frequently than mealybug in Finger Lake vineyards; more than half of the surveyed sites in both years compared with roughly a quarter where mealybugs were observed. The residual efficacy of the materials used in these experiments should provide control of related vector species as the life histories of these groups would indicate some temporal overlap of crawler periods (Kosztarab 1996, Geiger and Daane 2001), but the control of all species should be considered in each region.
An economic analysis of grapevine leafroll disease management strategies reported by Atallah et al. (2012) suggested greater than the 25% infection as the economic cut-off point for complete vineyard replacement when factoring in the costs of roguing against the cost of removing the entire vineyard and replacing vines with certified virus-free stock. Although this economic analysis did not include costs or benefits of insecticidal control of vectors, the benefit of using insecticidal control in combination with roguing for control of grapevine leafroll disease has been indicated in other studies. Control of vine mealybug (Pl. ficus) using insecticides and roguing with herbicide to kill remnant roots resulted in very good control of grapevine leafroll disease in South African vineyards; however, roguing alone may not remove 100% of infected plant material from blocks as virus may remain in root tissue for several years (Pietersen et al. 2013).
Insecticide use may play a critical role in protecting new grapevines in or near grapevine leafroll disease-affected blocks, and acetamiprid or spirotetramat applications would also provide control of other key pests of grapevine in the Finger Lakes, such as Japanese beetle and other hemipteran pests like grape phylloxera and leafhoppers (Weigle and Muza 2013). These materials are also relatively safer than broad- spectrum insecticides for nontarget insects like predators and parasitoids, particularly spirotetramat which could be an integrated pest management compatible insecticide (Broughton et al. 2013, Garcera et al. 2013, Beers and Schmidt 2014).
In conclusion, this study found that insecticidal control of vector insects can slow the spread of GLRaVs when vector abundance was relatively low. However, clearly more research is required to determine under what situations chemical control of vectors, on its own or in combination with other measures such as roguing, should be recommended for managing grape leafroll disease. Early intervention in the life of the vineyard is likely a critical factor, further elevating the importance of using planting material derived from certified virus-tested stock and monitoring to maintain healthy and productive vineyards.
Acknowledgments
We thank Bill Wilsey, Rosemary Cox, Yen Mei Cheung, Pat-Marsella Herrick, and Dave MacUmber for sample collection and processing. Michael Colizzi, Tim Robideau, Rachael Tucker, Sara Villani, Arianna Waheed, Allison Wentworth, Bill Wilsey, and Jessica Worden assisted with surveys of mealybug and soft scale. We thank Erika Mudrak for her assistance with statistical analysis. We thank the owners and managers of participating vineyards. The manuscript benefited from comments from two anonymous reviewers for which we are appreciative. This study was partially supported by USDA-CSREES Viticulture Consortium-East, New York State Wine and Grape Foundation, Lake Erie Regional Grape Program, and a Specialty Crop Block Grant through the New York State Department of Agriculture and Markets.
References Cited
- Almeida R.P.P., Daane K. M., Bell V. A., Blaisdell G. K., Cooper M. L., Herrbach E., Pietersen G. 2013. Ecology and management of grapevine leafroll disease. Front. Microbiol. 4: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atallah S. S., Gomez M. I., Fuchs M. F., Martinson T. E. 2012. Economic impact of grapevine leafroll disease on Vitis vinifera cv. Cabernet franc in Finger Lakes vineyards of New York. Am. J. Enol. Vitic. 63: 73–79. [Google Scholar]
- Beers E. H., Schmidt R. A. 2014. Impacts of orchard pesticides on Galendromus occidentalis: lethal and sublethal effects. Crop Prot. 56: 16–24. [Google Scholar]
- Bentley W. J., Varela L. G., Zalom F. G., Smith R. J., Purcell A. H., Philips P. A., Haviland D. R., Daane K. M., Battany M. C., Granett J. 2006. Grape mealybugs (Pseudococcus). UC IPM Pest Management Guidelines: Grape. UC ANR Pub. 3448. Oakland, CA. [Google Scholar]
- Broughton S., Harrison J., Rahman T. 2013. Effect of new and old pesticides on Orius armatus (Gross)—an Australian predator of western flower thrips, Frankliniella occidentalis (Pergande). Pest Manage. Sci. 70: 389–397. [DOI] [PubMed] [Google Scholar]
- Brück E., Elbert A., Fischer R., Kreuger S., Kühnhold J., Kleuken A. M., Nauen R., Niebes J. F., Reckmann U., Schnorback H. J., et al. 2009. Movento®, an innovative ambimobile insecticide for sucking insect pest control in agriculture: biological profile and field performance. Crop Prot. 28: 838–844. [Google Scholar]
- Bucholz A., Nauen R. 2002. Translocation and translaminar bioavailability of two neonicotinoid insecticides after foliar application to cabbage and cotton. Pest Manage. Sci. 58: 10–16. [DOI] [PubMed] [Google Scholar]
- Cabaleiro C., Segura A. 1997. Some characteristics of the transmission of grapevine leafroll associated virus 3 by Planococcus citri Risso. Eur. J. Plant Pathol. 103: 373–378. [DOI] [PubMed] [Google Scholar]
- Cabaleiro C., Couceiro C., Pereira S., Cid M., Barrasa M., Segura A. 2008. Spatial analysis of epidemics of Grapevine leafroll associated virus-3. Eur. J. Plant Pathol. 121: 121–130. [Google Scholar]
- Charles J. G. 1982. Economic damage and preliminary economic threshold for mealybugs (Pseudococcus longispinus T-T.) in Auckland vineyards. N. Z. J. Agric. Res. 25: 415–420. [Google Scholar]
- Charles J. G., Cohen D., Walker J.T.S., Forgie S. A., Bell V. A., Breen K. C. 2006. A review of the ecology of grapevine leafroll associated virus type 3 (GLRaV-3). N. Z. Plant Protect. 59: 330–337. [Google Scholar]
- Credi R., Babini A. R. 1997. Effects of virus and virus-like infections on growth, yield, and fruit quality of Albana and Trebbiano Romagnolo grapevines. Am. J. Enol. Vitic. 48: 7–12. [Google Scholar]
- Daane K. M., Walton V. M., Malakar-Kuenen R., Millar J. G., Ingels C. A., Weber E. A., Gispert C. 2006. New controls investigated for vine mealybug. Calif. Agric. 60: 31–38. [Google Scholar]
- Daane K. M., Almeida R.P.P., Bell V. A., Walker J.T.S., Botton M., Fallahzadeh M., Mani M., Miano J. L., Sforza R., Walton V. M., et al. 2012. Biology and management of mealybugs in vineyards, pp. 271–307. In Bostanian N. J., Vincent C., Isaacs R. (eds.), Arthropod management in vineyards: pests, approaches, and future directions. Springer, Netherlands. [Google Scholar]
- Daugherty M. P., O’Neill S., Byrne F., Zeilinger A. 2015. Is vector control sufficient to limit pathogen spread in vineyards? Environ. Entomol. 44: 789–797. [DOI] [PubMed] [Google Scholar]
- Engelbrecht D. J., Kasdorf G.G.F. 1990. Transmission of grapevine leafroll disease and associated Closteroviruses by the vine mealybug Planococcus ficus. Phytophylactica 22: 341–346. [Google Scholar]
- Flaherty D. L. 1982. Chemicals losing effect against grape mealybug. Calif. Agric. 36: 15–16. [Google Scholar]
- Franco J. C., Zada A., Mendel Z. 2009. Novel approaches for the management of mealybug pests, pp. 233–278. In Ishaaya I., Horowitz A. R. (eds.), Biorational control of arthropod pests: application and resistance management. Springer, New York. [Google Scholar]
- Fuchs M., Marsella-Herrick P., Loeb G. M., Martinson T. E., Hoch H. C. 2009a. Diversity of Ampeloviruses in mealybug and softscale vectors and in grapevine hosts from leafroll-affected vineyards. Phytopathology 99: 1177–1184. [DOI] [PubMed] [Google Scholar]
- Fuchs M., Martinson T. E., Loeb G. M., Hoch H. C. 2009b. Survey for the three major leafroll disease-associated viruses in Finger Lakes vineyards in New York. Plant Dis. 93: 395–401. [DOI] [PubMed] [Google Scholar]
- Garcerá C., Ouyang Y., Scott S. J., Moltó E., Grafton-Cardwell E. E. 2013. Effects of spirotetramat on Aonidiella aurantii (Homoptera: Diaspididae) and its parasitoid, Aphytis melinus (Hymenoptera: Aphelinidae). J. Econ. Entomol. 106; 2126–2134. [DOI] [PubMed] [Google Scholar]
- Gaskin R. E., Horgan D. B., Leeuwen R. M. van, Manktelow D. W. 2010. Adjuvant effects on the retention and uptake of spirotetramat insecticide sprays on kiwifruit. N. Z. Plant Prot. 63: 60–65. [Google Scholar]
- Geiger C. A., Daane K. M. 2001. Seasonal movement and distribution of the grape mealybug (Homoptera: Pseudococcidae): developing a sampling program for San Joaquin Valley vineyards. J. Econ. Entomol. 94: 291–301. [DOI] [PubMed] [Google Scholar]
- Goheen A. C., Cook J. A. 1959. Leafroll (red-leaf or rougeau) and its effects on vine growth, fruit quality, and yields. Am. J. Enol. Vitic. 10:173–181. [Google Scholar]
- Golino D. A., Sim S. T., Gill R., Rowhani A. 2002. California mealybugs can spread grapevine leafroll disease. Calif. Agric. 56: 196–201. [Google Scholar]
- Golino D. A., Weber E., Sim S. 2008. Leafroll disease is spreading rapidly in a Napa Valley vineyard. Calif. Agric. 62: 156–160. [Google Scholar]
- Grasswitz T. R., James D. G. 2008. Movement of grape mealybug, Pseudococcus maritimus, on and between host plants. Entomol. Exp. Appl. 129: 268–275. [Google Scholar]
- Grimes E. W., Cone W. W. 1985. Life history, sex attraction, mating, and natural enemies of the grape mealybug, Pseudococcus maritimus (Homoptera: Pseudococcidae). Ann. Entomol. Soc. Am. 78: 554–558. [Google Scholar]
- Habili N., Nutter F. W. 1997. Temporal and spatial analysis of grapevine leafroll-associated virus 3 in Pinot Noir grapevines in Australia. Plant Dis. 81: 625–628. [DOI] [PubMed] [Google Scholar]
- Habili N., Fazeli C. F., Ewart A., Hamilton R., Cirami R., Saldarelli P., Minafra A., Rezaian M. A. 1995. Natural spread and molecular analysis of grapevine leafroll-associated virus-3 in Australia. Phytopathology 85: 1418–1422. [Google Scholar]
- Haviland D. R., Hashim-Buckey J., Rill S. M. 2010. In-season control of vine mealybug in ‘Flame Seedless’ table grapes in Kern County, 2008. Arthropod Manage. Tests 35: C13. [Google Scholar]
- Haviland D. R., Hashim-Buckey J., Rill S. M. 2011. In-season control of vine mealybug in ‘Red Globe’ table grapes in Kern County, 2010. Arthropod Manage. Tests 36: C12. [Google Scholar]
- Horowitz A. R., Mendelson Z., Weintraub P. G., Ishaaya I. 1998. Comparative toxicity of foliar and systemic applications of acetamiprid and imidacloprid against the cotton whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae). Bull. Entomol. Res. 88: 437–442. [Google Scholar]
- Jordan D. 1993. Leafroll spread in New Zealand vineyards. Aust. N. Z. Wine Indust. J. 8: 322–324. [Google Scholar]
- Kosztarab M. 1996. Scale insects of Northeastern North America, number 3, 650 pp. Virginia Museum of Natural History Special Publication. [Google Scholar]
- Mahfoudhi N., Digiaro M., Dhouibi M. H. 2009. Transmission of grapevine leafroll viruses by Planococcus ficus (Hemiptera: Pseudococcidae) and Ceroplastes rusci (Hemiptera: Coccidae). Plant Dis. 93: 999–1002. [DOI] [PubMed] [Google Scholar]
- Maree H. J., Almeida R.P.P., Bester R., Chooi K. M., Cohen D., Dolja V. V., Fuchs M. F., Golino D. A., Jooste A.E.C., Martelli G. P., et al. 2013. Grapevine leafroll-associated virus 3. Front. Microbiol. 4: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelli G. P. 2014. Directory of virus and virus like diseases of the grapevine and their agents. J. Plant Pathol. 96: 51–70. [Google Scholar]
- McKenna C., Gaskin R., Horgan D., Dobson S., Jia Y. 2013. Efficacy of a postharvest spirotetramat spray against armoured scale insects on kiwifruit vines. N. Z. J. Crop Hortic. Sci. 41: 105–116. [Google Scholar]
- O’Hara R. B. 2009. How to make models add up—a primer on GLMMs. Ann. Zool. Fennici 46: 124–137. [Google Scholar]
- Over de Linden A. J., Chamberlain E. E. 1970. Effect of grapevine leafroll virus on vine growth and fruit yield and quality. New Zeal. J. Agric. Res. 13: 689–698. [Google Scholar]
- Pereira-Crespo S., Segura A., Garcia-Berrios J., Cabaleiro C. 2012. Partial defoliation improves must quality of cv. Albariño infected by Grapevine leafroll associated virus 3. Phytopathol. Mediterr. 51: 383–389. [Google Scholar]
- Pietersen G., Spreeth N., Oosthuizen T., Andre van Rensburg, van Rensburg M., Lottering D., Rossouw N., Tooth D. 2013. Control of grapevine leafroll disease spread at a commercial wine estate in South Africa: a case study. Am. J. Enol. Vitic. 64: 296–305. [Google Scholar]
- Rayapati N., Rowhani A., Fuchs M., Golino D., Martelli G. P. 2014. Grapevine leafroll: a complex viral disease affecting high-value fruit crop. Plant Dis. 98: 1172–1185. [DOI] [PubMed] [Google Scholar]
- Reynolds A. G., Vanden Heuvel J. E. 2009. Influence of grapevine training systems on vine growth and fruit composition: a review. Am. J. Enol. Vitic.60: 251–268. [Google Scholar]
- Sial A., Hutchins J. T., Daane K. M. 2012. In-season movement of vine mealybug using single application of reduce risk insecticides, 2011. Arthropod Manage. Tests 37: C16. [Google Scholar]
- Szczepaniec A., Raupp M. J. 2000. Residual toxicity of imidacloprid to hawthorn lace bug, Corythuca cydoniae, feeding on cotoneasters in landscapes and containers. J. Environ. Hortic. 25: 43–46. [Google Scholar]
- Tsai C.-W., Rowhani A., Golino D. A., Daane K. M., Almeida R.P.P. 2010. Mealybug transmission of grapevine leafroll viruses: an analysis of virus-vector specificity. Phytopathology 100: 830–834. [DOI] [PubMed] [Google Scholar]
- Walton V. M., Pringle K. L. 1999. Effects of pesticides used on table grapes on the mealybug parasitoid Coccidocenoides peregrinus (Timberlake) (Hymenoptera: Encyrtidae). A. Afr. J. Enol. Vitic. 20: 31–34. [Google Scholar]
- Weigle T. H., Muza A. J. 2013. New York and Pennsylvania pest management recommendations for grapes. A Cornell and Penn State Cooperative Extension Publication, Ithaca, NY. [Google Scholar]


