Abstract
Introduction
New vaccines are being developed to improve the efficacy of seasonal influenza immunization in elderly persons aged ≥65 years. These products require clinical and economic evaluation to aid policy decisions.
Methods
To address this need, a two-part model has been developed, which we have applied to examine the potential clinical and economic impact of vaccinating elderly persons with adjuvanted trivalent inactivated influenza vaccine (aTIV) relative to conventional trivalent (TIV) and quadrivalent (QIV) vaccines. We compared outcomes in the US population for (1) aTIV in persons aged ≥65 years and QIV in all other age cohorts; (2) QIV in all cohorts; (3) TIV in all cohorts. Low, average, and high intensity seasons with low, average, and high vaccine match scenarios were compared. Probabilistic sensitivity analysis was conducted within each discrete scenario to explore the impact of variation in model inputs on potential outcomes.
Results
Assuming current vaccination coverage rates in the US population with (a) 25% better efficacy of adjuvanted versus non-adjuvanted vaccine against any strain and (b) 35% better efficacy of non-adjuvanted vaccine against matched B versus mismatched B strains, use of aTIV in persons aged ≥65 years and QIV in persons <65 years could reduce influenza cases by 11,166–1,329,200, hospitalizations by 1365–43,674, and deaths by 421–11,320 versus use of QIV in all cohorts. These outcomes are reflected in a corresponding increase in quality-adjusted life-years (QALYs) of 3003–94,084. If the prevalence of mismatched influenza B was >54.5% of all circulating strains, use of QIV in all cohorts would offset the clinical benefits of aTIV. Elderly aTIV or QIV vaccination was associated with improved outcomes over non-adjuvanted TIV in many of the scenarios, particularly in low match seasons of any intensity. Total cost savings (including direct and indirect healthcare costs plus productivity impacts) with aTIV in the elderly versus QIV in the whole population ranged from $27 million (low intensity, low match) to $934 million (high intensity, high match). Univariate sensitivity analysis of relative vaccine prices in the average intensity, average match scenario indicated that aTIV could be marginally cost saving relative to QIV at the currently published Medicare price for influenza vaccines offering enhanced efficacy in the elderly. Elderly vaccination with aTIV was associated with a higher overall cost compared with TIV in only two scenarios (low intensity with average or high match); the incremental cost/QALY relative to TIV was $9980 in the average match scenario and $28,800 in the high match scenario.
Conclusions
Vaccination of persons aged ≥65 years with aTIV has the potential to provide clinical and economic benefit relative to QIV and TIV. The new model allows the assessment of various alternative strategies for available influenza vaccines.
Funding
Novartis Vaccines.
Electronic supplementary material
The online version of this article (doi:10.1007/s40121-015-0076-8) contains supplementary material, which is available to authorized users.
Keywords: Influenza, Elderly, Vaccine, Trivalent, Quadrivalent, Adjuvant, Vaccination, Economic, Clinical, Outcome
Introduction
The clinical and economic burden of influenza is significant, particularly in vulnerable populations, such as children, persons with compromised immune function, and the elderly. Notably, the risk of influenza-related complications increases with age [1–3], influenza-associated hospitalizations are most common among elderly aged ≥65 years (especially in those with underlying medical conditions) [4–8], and up to 90% of influenza-attributable mortality is seen in this cohort [9–11].
Significant direct healthcare costs are linked to influenza in persons aged ≥65 years [6]. These costs are driven by substantial numbers of influenza-related hospitalizations, and are increasing as the overall population ages [9]. Moreover, influenza-related hospitalizations in the elderly are associated with significant disability and impairments in activities of daily living. These effects have societal implications as they can be long lasting, and catastrophic disability after influenza hospitalization is a significant problem in this age cohort [12, 13]. Influenza vaccination in the elderly provides substantial benefits [14], and past cost-benefit and cost-effectiveness studies from a number of countries have indicated that this intervention in this age cohort is always cost-effective and is frequently cost saving [15]. Influenza vaccination policy in most developed countries, therefore, targets people aged ≥65 years as part of the effort to reduce the mortality and disability burden in this population [16].
The efficacy of conventional inactivated influenza vaccine [trivalent (TIV) or quadrivalent (QIV)] decreases with advancing age because of reduced production of vaccine-specific antibodies [17]. Attempts to increase immunogenicity have been made with alternatives such as high-dose TIV (60 µg of hemagglutinin (HA)/strain) [18] and an intradermal vaccine [19]. Constant evolution of the influenza strains in circulation further challenges vaccine protection with approximately 35% reduction in efficacy against mismatched versus matched strains [20, 21]. As a result, QIV may offer benefit in seasons where the B lineage selected for TIVs does not match the dominant circulating strain [22]. Notably, however, TIV adjuvanted with the squalene-containing oil-in-water emulsion MF59 (aTIV) increases seroprotection [23, 24] and has been associated with a 25% reduction in risk of hospitalization for influenza or pneumonia versus TIV in the elderly population aged ≥65 years [25]. Moreover, in the elderly, aTIV has been reported to provide better cross-reactivity against mismatched strains than conventional vaccine [26].
As the economic burden of influenza is disproportionately concentrated in the elderly [27], improvements in vaccine immunogenicity and efficacy in this group have economic implications that warrant investigation. Economic modeling is an important and generally accepted method for estimating the economic effects of an intervention [28]. A two-part epidemiologic and economic model was, therefore, developed to assess the clinical and economic impact of vaccination with aTIV in persons aged ≥65 years and QIV in those aged <65 years versus QIV or TIV in all age cohorts.
Methods
Scenarios of Interest
This analysis compared clinical and economic outcomes over a 1-year period in the United States (US) population for three vaccination strategies: (1) aTIV in those ≥65 years (elderly) and QIV in all other age cohorts; (2) QIV in all cohorts; (3) TIV in all cohorts. We compared outcomes in nine discrete scenarios for low, average, and high intensity (i.e., transmissibility) seasons, factoring in low, average, and high vaccine match against circulating seasonal strains, in a 3 × 3 matrix. The analysis in this article is based on previously conducted studies, and does not involve any new studies of human or animal subjects performed by any of the authors.
The average match scenarios (based on the arithmetic means of strain circulation in the US from 1999–2000 through 2013–2014 [29–33]) were 48.3%, 30.4%, 11.0%, and 10.3% for A/H3N2, A/H1N1, B/Victoria, and B/Yamagata, respectively. The low and high match scenarios were based on the 2000/2001 season, during which there was a very high prevalence of influenza B/Yamagata (1.7%, 51.9%, 0%, and 46.4% for A/H3N2, A/H1N1, B/Victoria, and B/Yamagata, respectively).
The TIV and aTIV vaccines contain the dominant circulating B strain (B/Yamagata) in the high match scenarios, but not in the low match scenarios. We also investigated a breakeven scenario for vaccine match, modeled for a low intensity season in which the low attack rate minimizes the clinical impact of vaccine efficacy. We assumed (a) 25% better efficacy of adjuvanted versus non-adjuvanted vaccine against any strain and (b) 35% better efficacy of non-adjuvanted vaccine against matched B versus mismatched B strains.
The Two-Part Model
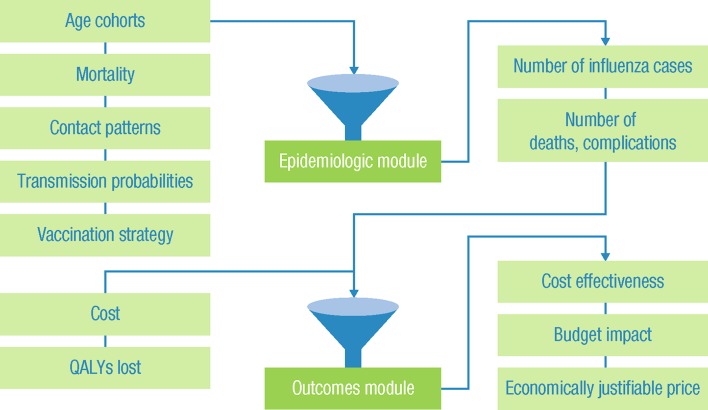
The model is programmed in Microsoft Excel, and consists of a compartmental, dynamic “epidemiologic module” to estimate the number of influenza cases and a tree-structured “outcomes module” to estimate complications, hospitalizations, deaths, life-years, quality-adjusted life-years (QALYs), and costs associated with influenza cases over a 1-year period (Fig. 1). The model assumes no adverse events of influenza vaccination or treatment, and mutual exclusivity of complications. It also does not consider the impact of strain mutation within a season, and recovered/protected patients remain immune for the duration of the simulation.
Fig. 1.
The two-part epidemiologic and economic model. QALY quality-adjusted life-year
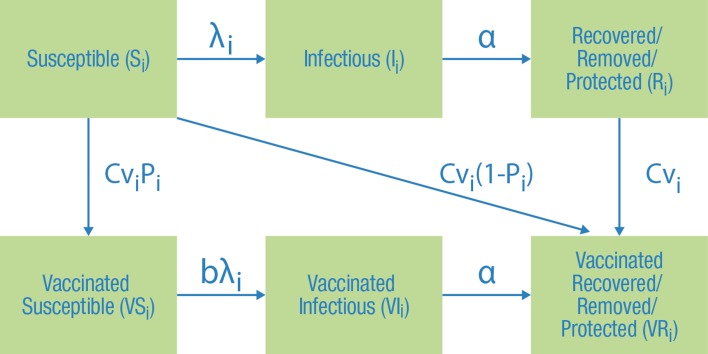
Epidemiologic Module
The “epidemiologic module” simulates the transmission of seasonal influenza with a susceptible-infectious-recovered/protected/removed (SIR) model consisting of 6 compartments (Fig. 2). It estimates the dynamic changes over one year with or without vaccination (three unique age-stratified strategies can be defined) in the uninfected, infected with influenza, and the recovered/protected/removed populations. The population size is constant and proportions in the different compartments (or health states) can vary by age cohort i (0–3 years, 4–6, 7–9, 10–19, 21–34, 35–49, 50–64, 65–69, 70+). No patients enter or leave the system over the modeled year, and proportions in each compartment sum to 1. Patients recovered/protected/removed from infection (including patients who die as a result of infection) are fully immune and, therefore, cannot be re-infected or infect others for the remainder of the simulation (effectively removed from the simulation).
Fig. 2.
The susceptible-infectious-recovered (SIR) compartment model for seasonal influenza. S i(t) = Fraction of susceptible individuals to influenza in age cohort i at time t. I i(t) = Fraction of infectious individuals in age cohort i at time t. R i(t) = Fraction of recovered/protected/removed from influenza in age cohort i at time t. VS i(t) = Fraction of vaccinated but still susceptible for influenza in age cohort i at time t. VI i(t) = Fraction of influenza cases despite being vaccinated in age cohort i at time t. VR i(t) = Fraction of vaccinated recovered/protected/removed in age cohort i at time t. Cv i = Vaccine coverage in age cohort i. P i = Probability of unsuccessful vaccination in age cohort i. λ i(t) = Force of infection in age cohort i at time t. bλ i(t) = Force of infection in vaccinated cohort in age cohort i at time t; α = Recovery rate
As illustrated in Fig. 2, in the proportion of the population that will be vaccinated (Cv i), some people who receive vaccination are susceptible (S i) and some are already protected by previous vaccination or influenza exposure (R i). Among those not yet protected (S i), vaccination can be successful (the individual enters compartment VR i) or unsuccessful (enters compartment VS i). Vaccine efficacy (1 − P i) determines distributions over the VS i and VR i compartments. All persons already protected before vaccination (R i) will move to compartment VR i.
The rate at which individuals in age cohort i transition from susceptible S i to infected/infectious I i is the force of infection, λ i. Patients who are not fully protected after vaccination (VS i) can be infected, but at a rate lower than or equal to unvaccinated individuals, represented by bλ i, where 0 < b ≤ 1. Upon infection with influenza, individuals recover according to rate α and move to R i (or VR i); they are immune to further influenza infection for the remainder of the year and, therefore, cannot infect others (i.e., effectively removed from the simulation).
Changes in the proportion of patients over the compartments are defined by the differential equations:
The force of infection λ i(t) is the rate at which susceptible individuals in age cohort i are infected at time t. It is the summation of the rates of infection at time t from all infected/infectious in age cohorts j:
where:
where:
σ is the transmissibility parameter.
γ ij is the average number of contacts per day between susceptible individuals in age cohort i with infectious individuals in age cohort j.
φ i is the susceptibility of a susceptible individual in age cohort i.
ζ j is the infectivity of an infective individual in age cohort j.
I j(t) is the fraction of infectious individuals in age cohort j at time t.
β ij is the rate of infection of susceptible individuals in age cohort i by infectious individuals in cohort j (referred to as the WAIFW or Who Acquired Infection from Whom matrix; see Electronic Supplementary Material (ESM) for further discussion of derivation and implications for transmission dynamics).
Inputs of the model include age-stratified population [34], an age-stratified contact matrix (γ) representing interactions between individuals in the population leading to spread of the disease ([35], adapted to fit this model’s age structure; Table S1), duration of infection (1/υ) (assumed gamma distribution with mean of 4 days and standard deviation of 1 day [36–38], strain circulation [29–33], vaccine coverage by age [39], and vaccination efficacy by strategy by level of vaccine match to circulating strains by age [20, 21 25, 40]. The susceptibility φ i, infectivity ζ j, and transmission σ parameters are derived through a calibration process described below and in the ESM. Full details of these inputs are shown in Tables S1–S8.
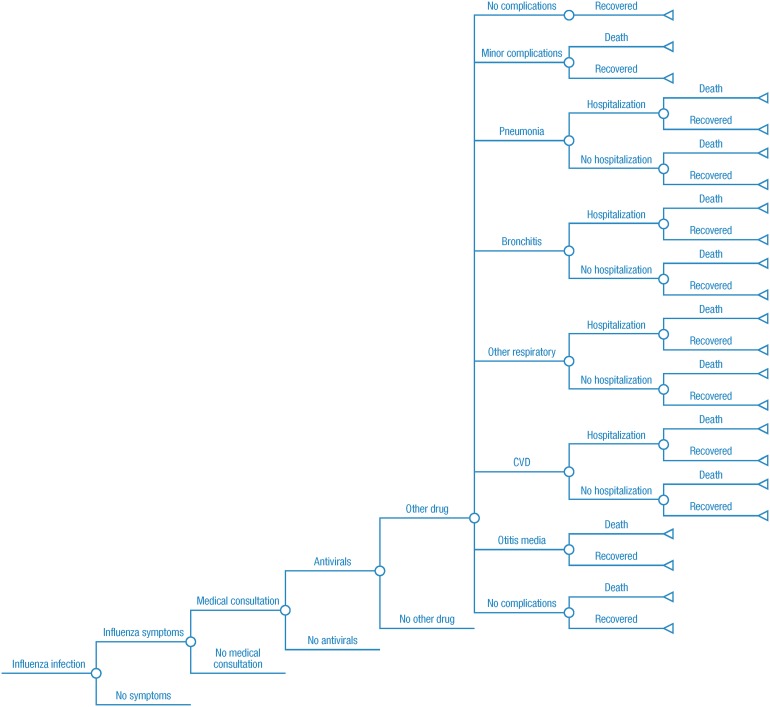
Outcomes Module
The “outcomes module” calculates the outcomes associated with influenza according to a tree structure (Fig. 3; Tables 1, 2, 3 [27, 39, 41–48]). Upon infection with influenza, a person can experience symptoms (first chance node and corresponding split path). If symptoms are present, a patient might seek medical consultation (second chance node). Given medical consultation, antivirals and/or other drugs might be prescribed (third and fourth chance nodes). The conditional probabilities of influenza-related complications can be influenced by the prescription of antivirals and other drugs. We assumed the risk of complications to be the same with and without vaccination (i.e., the risk of complications is entirely dependent on whether an individual is infected with influenza). The probability of hospitalization or death in the presence of a complication is the result of the calibration process described below. The conditional probabilities with their sources are shown in Table S9.
Fig. 3.
Outcomes associated with influenza. CVD cardiovascular disease
Table 1.
Input data: costs used in the model
| Variable | Estimate ($US) | Source |
|---|---|---|
| Vaccine acquisition | ||
| TIV | 9.45 | CDC [39] |
| aTIV | 13.65 | Assumption |
| QIV | 13.65 | Assumption |
| Vaccine administration | ||
| All ages | 21.00 | Prosser [41] |
| Antiviral cost | ||
| All ages | 92.35 | Talbird et al. [42] |
| Medical consultation | ||
| All ages | 98.79 | Talbird et al. [42] |
| Complications | ||
| Pneumonia without hospitalization | ||
| All ages | 206 | Talbird et al. [42] |
| Pneumonia with hospitalization | ||
| 0–19 | 5513 | Talbird et al. [42] |
| 20–64 | 14,828 | |
| ≥65 | 14,137 | |
| Bronchitis without hospitalization | ||
| All ages | 221 | Talbird et al. [42] |
| Bronchitis with hospitalization | ||
| 0–19 | 3906 | Talbird et al. [42] |
| 20–64 | 7449 | |
| ≥65 | 8834 | |
| Other respiratory without hospitalization | ||
| All ages | 221 | Talbird et al. [42] |
| Other respiratory with hospitalization | ||
| 0–19 | 3906 | Talbird et al. [42] |
| 20–64 | 7449 | |
| ≥65 | 8834 | |
| CVD without hospitalization | ||
| All ages | 2711 | American Heart Association [43] |
| CVD with hospitalization | ||
| All ages | 6017 | American Heart Association [43] |
| Otitis media | ||
| All ages | 224 | Talbird et al. [42] |
| Lost productivity costs | ||
| Without complications | ||
| 0–3 | 145 | Molinari et al. [27]; case not medically attended |
| 4–6 | 97 | |
| 7–19 | 73 | |
| 20–64 | 68 | |
| 65+ | 145 | |
| Extra loss due to minor complications | ||
| 0–3 | 183 | Molinari et al. [27]; weighted average of non-high-risk and high-risk outpatient visit |
| 4–6 | 209 | |
| 7–9 | 191 | |
| 10–19 | 186 | |
| 20–49 | 167 | |
| 50–64 | 386 | |
| 65+ | 832 | |
| Extra loss due to serious complications (pneumonia, bronchitis, CVD) | ||
| 0–3 | 1333 | Molinari et al. [27]; weighted average of non-high-risk and high-risk outpatient visit |
| 4–6 | 1576 | |
| 7–9 | 1520 | |
| 10–19 | 1603 | |
| 20–49 | 1934 | |
| 50–64 | 2411 | |
| 65+ | 2256 | |
| Otitis media | ||
| All ages | 0 | Assumed no additional days lost |
aTIV adjuvanted trivalent inactivated influenza vaccine, CDC Centers For Disease Control And Prevention, CVD cardiovascular disease, QIV Quadrivalent inactivated influenza vaccine, TIV trivalent inactivated influenza vaccine
Table 2.
Input data: QALY loss with influenza and impact of events
| Variable | Estimate | Low | High | Distribution | Source/comments |
|---|---|---|---|---|---|
| Minor complications | 0.00000 | 0.00000 | 0.00000 | Gamma | Assumption |
| Pneumonia | 0.01041 | 0.00674 | 0.01487 | Gamma | Lee et al. [44] |
| Bronchitis | 0.00904 | 0.00585 | 0.01291 | Gamma | Lee et al. [44] |
| Other respiratory | 0.00904 | 0.00585 | 0.01291 | Gamma | Lee et al. [44] |
| CVD | 0.10000 | 0.06471 | 0.14284 | Gamma | Dyer et al. [45] |
| Otitis media | 0.01382 | 0.00894 | 0.01974 | Gamma | Prosser et al. [46] |
CVD cardiovascular disease, QALY quality-adjusted life-year
Table 3.
Input data: life expectancy and QALY loss due to death
| Age | Discounted life-years lost when dying | Discounted QALYs lost when dying | Source/comments | |||
|---|---|---|---|---|---|---|
| Estimate | Estimate | Low | High | Distribution | ||
| 0–3 | 30.8 | 27.7 | 25.0 | 30.5 | Normal | EQ-5D assumed 0.9 over rest of life |
| 4–6 | 30.5 | 27.5 | 24.8 | 30.2 | Normal | EQ-5D assumed 0.9 over rest of life |
| 7–9 | 30.2 | 27.2 | 24.5 | 29.8 | Normal | EQ-5D assumed 0.9 over rest of life |
| 10–19 | 29.3 | 26.4 | 23.8 | 29.0 | Normal | EQ-5D assumed 0.9 over rest of life |
| 20–34 | 27.2 | 24.5 | 22.1 | 26.9 | Normal | EQ-5D assumed 0.9 over rest of life |
| 35–49 | 23.5 | 21.1 | 19.1 | 23.2 | Normal | EQ-5D assumed 0.9 over rest of life |
| 50–64 | 18.3 | 15.8 | 14.2 | 17.3 | Normal | Fryback et al. [47] adjusted average for remaining life-years |
| 65–69 | 14.2 | 12.0 | 10.9 | 13.2 | Normal | Fryback et al. [47] adjusted average for remaining life-years |
| 70+ | 7.1 | 5.9 | 5.4 | 6.5 | Normal | Fryback et al. [47] adjusted average for remaining life-years |
Source: life expectancy: [48]
CDC Centers for Disease Control and Prevention, QALY Quality-adjusted life-year
Direct costs (US$) for medical care, including medical consultation, prescriptions for antivirals and other drugs [42], and costs of complications with and without hospitalization [42, 43, 49], were included (Table 1). Indirect costs covered productivity losses based on the severity of complications [27]. QALY reductions for the duration of symptoms of influenza and its complications (Table 2) and QALY losses due to life-years lost due to fatal complications were also included (Table 3). Life-years and QALYs lost were discounted at 3% per annum.
Expected outcomes and costs for each new influenza case were calculated by multiplying the probability of each event as reflected in the final branches of the tree with the corresponding outcomes and costs. In combination with the incidence of influenza over the course of a 1-year period as estimated with the “epidemiologic module”, the expected outcomes associated with influenza over this period were obtained.
Calibration of the Model
The model was calibrated to the age-stratified number of cases based on age-stratified estimated gross attack rates [27] and estimates of influenza incidence [27, 29, 50] by adjusting a factor for transmissibility (σ) and age-specific factors for susceptibility (φ i). Conditional probabilities for hospitalization and death by complication according to age were then calibrated to literature-based estimates [27] (see the ESM for further detail).
Estimation of Model Outcomes
For each scenario and vaccination strategy of interest, we conducted a probabilistic analysis (second-order Monte Carlo simulation) by which variation in the model input parameters (summarized with probability density functions) was propagated through the model to obtain distributions for the following outcomes of interest: number of influenza cases, complications, deaths, life-years lost, QALYs lost, and costs. The distributions for these outcomes were summarized with the mean (“probabilistic mean”), low (2.5th percentile), and high (97.5th percentile) estimates.
The model output was further used to estimate budget impacts and cost-effectiveness of the competing interventions.
Results
The model outputs for the low, average, and high intensity modeled seasons with low, average, and high vaccine match are summarized in Tables 4, 5, 6 and Figs. 4 and 5. Assuming (a) 25% better efficacy of adjuvanted versus non-adjuvanted vaccine against any strain and (b) 35% better efficacy of non-adjuvanted vaccine against matched B versus mismatched B strains, clinical benefits of aTIV vaccination in the population ≥65 years and QIV in the population <65 years suggested by the model (versus vaccination of the whole population with QIV) included reductions in influenza cases of 11,166–1,329,200 (low estimate reflects probabilistic mean in low intensity, low match scenario; high estimate reflects probabilistic mean in high intensity, high match scenario), 1365–43,674 fewer hospitalizations, and 421–11,320 fewer deaths (Table 4). These clinical outcomes were reflected in gains of 3003–94,084 QALYs. As illustrated in Fig. 4, elderly vaccination with aTIV or QIV was associated with improved outcomes over non-adjuvanted TIV in many of the scenarios, particularly in low match seasons of any intensity. Moreover, in all scenarios, elderly vaccination with aTIV was shown by the model to be at least as effective as QIV in reducing cases and other clinical outcome rates (Fig. 4; Tables 4, 5). The number of influenza cases (95% Credible Interval [CrI]) when vaccinating all age cohorts with TIV ranged from 5.6 million (0.0–35.7) in the low intensity, high match scenario to 45.9 million (1.4–71.4) in the high intensity, low match scenario. Total costs (95% CrI) when vaccinating all age cohorts with TIV ranged from $6.2 billion (3.8–16.4) in the low intensity, high match scenario to $19.8 billion (4.9–29.4) in the high intensity, low match scenario.
Table 4.
Probabilistic means of clinical model outputs for all scenarios, together with absolute and percentage differences between the aTIV ≥ 65 QIV < 65 and QIV groups
| Probabilistic means (95% CrI) | |||||
|---|---|---|---|---|---|
| Low intensity | Average intensity | ||||
| Low match | Average match | High match | Low match | Average match | |
| No. of influenza cases | |||||
| aTIV ≥ 65 QIV < 65 | 6,027,660 (78,909; 36,311,558) | 5,777,353 (75,423; 38,843,395) | 5,261,063 (75,878; 33,685,356) | 21,578,258 (123,112; 59,447,194) | 19,316,966 (126,949; 55,496,404) |
| QIV | 6,038,826 (78,925; 36,766,189) | 6,157,580 (76,309; 39,373,704) | 5,623,605 (76,992; 36,418,579) | 21,741,326 (122,355; 58,978,203) | 19,986,583 (127,646; 57,253,746) |
| TIV | 9,223,607 (84,893; 45,308,781) | 6,646,200 (76,373; 40,819,092) | 5,567,761 (78,157; 35,703,665) | 28,003,833 (147,513; 65,918,137) | 21,369,092 (129,967; 58,358,976) |
| Differencea | −11,166 (−15; −454,631) | −380,227 (−886; −530,310) | −362,542 (−1114; −2,733,223) | −163,069 (757; 468,991) | −669,617 (−697; −1,757,342) |
| % difference | −0.2% (0%; −1.2%) | −6.2% (−1.2%; −1.3%) | −6.4% (−1.4%; −7.5%) | −0.8% (0.6%; 0.8%) | −3.4% (−0.5%; −3.1%) |
| No. of hospitalized cases | |||||
| aTIV ≥ 65 QIV < 65 | 74,540 (732; 478,659) | 68,658 (731; 488,150) | 61,649 (717; 422,060) | 270,480 (1400; 887,920) | 234,462 (1386; 783,668) |
| QIV | 75,905 (737; 504,229) | 76,027 (753; 529,411) | 70,303 (729; 477,838) | 275,925 (1417; 892,722) | 254,636 (1456; 861,123) |
| TIV | 119,250 (838; 638,887) | 83,191 (761; 553,680) | 69,182 (737; 474,702) | 364,768 (1746; 1,065,454) | 272,990 (1512; 870,456) |
| Differencea | −1365 (−5; −25,570) | −7369 (−22; −41,261) | −8654 (−12; −55,778) | −5445 (−17; −4802) | −20,174 (−70; −77,455) |
| % difference | −1.8% (−0.6%; −5.1%) | −9.7% (−2.9%; −7.8%) | −12.3% (−1.7%; −11.7%) | −2.0% (−1.2%; −0.5%) | −7.9% (−4.8%; 9.0%) |
| No. of deaths | |||||
| aTIV ≥ 65 QIV < 65 | 11,111 (128; 69,130) | 9972 (123; 68,219) | 8612 (117; 58,637) | 41,558 (207; 125,706) | 34,556 (218; 108,141 |
| QIV | 11,532 (134; 70,644) | 11,661 (129; 79,266) | 10,638 (123; 70,694) | 43,098 (210; 127,822) | 39,650 (232; 125,079) |
| TIV | 18,554 (145; 93,987) | 12,898 (129; 81,952) | 10,403 (122; 71,827) | 58,292 (258; 153,658) | 42,631 (238; 125,801) |
| Differencea | −421 (−5; −1515) | −1689 (−6; −11,047) | −2026 (−5; −12,057) | −1540 (−3; −2116) | −5094 (−14; −16,939) |
| % difference | −3.7% (−4.0%; −2.1%) | −14.5% (−4.7%; −13.9%) | −19.0% (−4.3%; −17.1%) | −3.6% (−1.3%; −1.7%) | −12.8% (−6.1%; −13.5%) |
| Probabilistic means (95% CrI) | ||||
|---|---|---|---|---|
| Average intensity | High intensity | |||
| High match | Low match | Average match | High match | |
| No. of influenza cases | ||||
| aTIV ≥ 65 QIV < 65 | 21,745,069 (135,227; 57,835,457) | 38,432,664 (456,246; 64,711,422) | 38,509,481 (342,987; 68,646,356) | 37,884,145 (485,622; 66,618,131) |
| QIV | 22,616,695 (136,917; 59,223,921) | 38,792,998 (458,714; 65,017,135) | 39,367,544 (329,147; 70,314,941) | 39,213,345 (591,599; 68,087,578) |
| TIV | 22,528,976 (137,585; 57,974,422) | 45,945,792 (1,428,394; 71,443,604) | 41,066,246 (397,398; 71,657,334) | 39,161,286 (457,098; 67,290,103) |
| Differencea | −871,626 (−1,60; −1,388,463) | −360,335 (−2468; −305,713) | −858,063 (13,839; −1,668,585) | −1,329,200 (−105,978; −1,469,448) |
| % difference | −3.9% (−1.2%; −2.3%) | −0.9% (−0.5%; −0.5%) | −2.2% (4.2%; −2.4%) | −3.4% (−17.9%; −2.2%) |
| No. of hospitalized cases | ||||
| aTIV ≥ 65 QIV < 65 | 265,663 (1394; 843,838) | 484,446 (6399; 1,016,720) | 467,724 (3770; 1,067,724) | 457,235 (5820; 1,030,868) |
| QIV | 291,576 (1496; 925,050) | 496,117 (6064; 1,044,831) | 500,958 (4026; 1,146,636) | 500,909 (6419; 1,100,235) |
| TIV | 290,282 (1467; 910,043) | 601,994 (17,940; 1,177,357) | 526,056 (4632; 1,197,190) | 499,814 (5722; 1,122,835) |
| Differencea | −25,914 (−102; −81,212) | −11,671 (335; −28,111) | −33,234 (−256; −78,912) | −43,674 (−599; −69,367) |
| % difference | −8.9% (−6.8%; −8.8%) | −2.4% (5.5%; −2.7%) | −6.6% (−6.4%; −6.9%) | −8.7% (−9.3%; −6.3%) |
| No. of deaths | ||||
| aTIV ≥ 65 QIV < 65 | 39,103 (195; 118,692) | 75,847 (834; 142,897) | 70,199 (512; 144,230) | 67,877 (821; 139,586) |
| QIV | 45,781 (222; 136,438) | 78,792 (854; 148,743) | 79,315 (603; 157,884) | 79,197 (949; 159,363) |
| TIV | 45,515 (223; 134,019) | 97,605 (2707; 172,222) | 83,680 (641; 168,366) | 78,954 (856; 161,318) |
| Differencea | −6678 (−27; −17,747) | −2945 (−19; −5847) | −9116 (−92; −13,654) | −11,320 (−128; −19,777) |
| % difference | −14.6% (−12.1%; −13.0%) | −3.7% (−2.3%; −3.9%) | −11.5% (−15.2%; −8.6%) | −14.3% (−13.5%; −12.4%) |
aTIV adjuvanted TIV, CrI Credible interval, QIV quadrivalent inactivated seasonal influenza vaccine, TIV trivalent inactivated seasonal influenza vaccine
aaTIV ≥ 65 QIV < 65 vs. QIV
Table 5.
Probabilistic means of life-year model outputs for all scenarios, together with absolute and percentage differences between the aTIV ≥ 65 QIV < 65 and QIV groups
| Probabilistic means (95% CrI) | |||||
|---|---|---|---|---|---|
| Low intensity | Average intensity | ||||
| Low match | Average match | High match | Low match | Average match | |
| Life-years lost | |||||
| aTIV ≥ 65 QIV < 65 | 119,855 (1453; 754,342) | 108,682 (1399; 736,809) | 94,467 (1319; 657,418) | 449,394 (2386; 1,340,734) | 377,962 (2440; 1,148,208) |
| QIV | 123,419 (1498; 763,164) | 125,035 (1411; 852,997) | 113,796 (1395; 776,478) | 463,373 (2398; 1,383,146) | 426,823 (2518; 1,309,509) |
| TIV | 196,872 (1616; 1,004,312) | 137,566 (1452; 909,825) | 111,621 (1396; 771,007) | 621,855 (2877; 1,630,082) | 458,629 (2601; 1,342,590) |
| Differencea | −3564 (−45; −8822) | −16,354 (−11; −116,189) | −19,329 (−76; −119,060) | −13,979 (−13; −42,412) | −48,860 (−78; −161,301) |
| % difference | −2.9% (−3.0%; −1.2%) | −13.1% (−0.8%; −13.6%) | −17.0% (−5.4%; −15.3%) | −3.0% (−0.5%; −3.1%) | −11.45% (−3.1%; −12.3%) |
| QALYs lost | |||||
| aTIV ≥ 65 QIV < 65 | 117,725 (1468; 750,989) | 107,623 (1373; 733,587) | 94,487 (1322; 647,505) | 438,909 (2388; 1,285,147) | 372,051 (2412; 1,132,941) |
| QIV | 120,729 (1485; 743,748) | 122,300 (1436; 845,124) | 111,623 (1385; 763,855) | 450,997 (2369; 1,323,062) | 414,739 (2498; 1,225,306) |
| TIV | 191,326 (1594; 979,054) | 134,239 (1420; 848,383) | 109,669 (1391; 742,570) | 601,487 (2832; 1,571,490) | 445,309 (2621; 1,267,852) |
| Differencea | −3,003 (−17; 7241) | −14,676 (−63; −111,537) | −17,136 (−62; −116,350) | −12,088 (19; −37,916) | −42,688 (−86; −92,365) |
| % difference | −2.5% (−1.1%; 1.0%) | −12.0% (−4.4%; −13.2%) | −15.4% (−4.5%; −15.2%) | −2.7% (−0.8%; −2.9%) | −10.3% (−3.5%; −7.5%) |
| Probabilistic means (95% CrI) | ||||
|---|---|---|---|---|
| Average intensity | High intensity | |||
| High match | Low match | Average match | High match | |
| Life-years lost | ||||
| aTIV ≥ 65 QIV < 65 | 430,921 (2180; 1,270,924) | 820,806 (8712; 1,539,347) | 769,512 (5592; 1,504,797) | 746,076 (8396; 1,478,850) |
| QIV | 494,200 (2303; 1,373,854) | 849,283 (9001; 1,585,870) | 855,562 (6176; 1,693,696) | 854,594 (9581; 1,656,636) |
| TIV | 491,324 (2400; 1,387,800) | 1,044,395 (28,369; 1,807,352) | 900,522 (6859; 1,731,406) | 852,086 (8770; 1,674,012) |
| Differencea | −63,279 (−123; −102,930) | −28,477 (−288; −46,523) | −86,050 (−583; −188,899) | −108,518 (−1185; −177,786) |
| % difference | −12.8% (−5.3%; −7.5%) | −3.4% (−3.2%; −2.9%) | −10.1% (−9.4%; −11.2%) | −12.7% (−12.4%; −10.7%) |
| QALYs lost | ||||
| aTIV ≥ 65 QIV < 65 | 425,351 (2139; 1,244,395) | 799,416 (9050; 1,514,181) | 756,731 (5738; 1,481,470) | 735,083 (8837; 1,411,542) |
| QIV | 480,757 (2305; 1,376,679) | 824,106 (9078; 1,526,880) | 830,698 (6341; 1,630,491) | 829,167 (9502; 1,578,863) |
| TIV | 478,110 (2371; 1,343,693) | 1,008,153 (28,582; 1,729,806) | 872,887 (7000; 1,661,897) | 826,870 (8940; 1,572,709) |
| Differencea | −55,406 (−165; −132,284) | −24,690 (−28; −12,699) | −73,966 (−603; −149,021) | −94,084 (−665; −167,320) |
| % difference | −11.5% (−7.2%; −9.6%) | −3.0% (−0.3%; −0.8%) | −8.9% (−9.5%; −9.1%) | −11.3% (−7.0%; −10.6%) |
aTIV adjuvanted TIV, CrI credible interval, QALY quality-adjusted life-year, QIV quadrivalent inactivated seasonal influenza vaccine, TIV trivalent inactivated seasonal influenza vaccine
aaTIV ≥ 65 QIV < 65 vs. QIV
Table 6.
Probabilistic means of cost model outputs for all scenarios, together with absolute and percentage differences between the aTIV ≥ 65 QIV < 65 and QIV groups
| Probabilistic means (95% CrI) | |||||
|---|---|---|---|---|---|
| Low intensity | Average intensity | ||||
| Low match | Average match | High match | Low match | Average match | |
| Total direct cost ($) | |||||
| aTIV ≥ 65 QIV < 65 | 5,862,744,268 (4,979,922,003; 10,166,329,974) | 5,796,738,194 (4,994,456,377; 10,204,589,456) | 5,698,162,740 (4,292,767,662; 9,643,329,431) | 8,042,255,362 (5,017,513,611; 13,684,333,296) | 7,637,145,164 (5,030,839,595; 12,801,170,782) |
| QIV | 5,878,055,661 (4,982,789,561; 10,145,399,899) | 5,878,094,283 (5,001,678,875; 10,603,198,208) | 5,822,014,487 (4,294,639,371; 10,273,676,447) | 8,101,747,787 (5,015,458,077; 13,751,296,307) | 7,859,208,792 (5,025,722,591; 13,373,063,888) |
| TIV | 5,739,239,448 (4,378,561,262; 10,856,173,435) | 5,342,177,037 (4,391,573,217; 10,286,682,937) | 5,175,843,264 (3,709,083,469; 9,818,803,927) | 8,460,800,122 (4,421,920,239; 14,362,290,693) | 7,448,134,136 (4,420,810,998; 12,886,597,651) |
| Differencea | −15,311,393 (−2,867,558; 20,930,076) | −81,356,090 (−7,222,498; −398,608,752) | −123,851,747 (−1,871,709; −630,347,016) | −59,492,425 (2,055,534; −66,963,011) | −222,063,629 (5,117,004; −571,893,106) |
| % difference | −0.3% (−0.1%; 0.2%) | −1.4% (−0.1%; −3.8%) | −2.1% (0%; −6.1%) | −0.7% (0%; −0.5%) | −2.8% (−0.5%; −3.1%) |
| Indirect cost ($) | |||||
| aTIV ≥ 65 QIV < 65 | 1,110,302,032 (13,854,187; 6,784,356,482) | 1,032,491,013 (13,489,616; 6,797,683,492) | 935,607,459 (13,392,215; 6,020,317,629) | 3,970,902,930 (23,200,992; 11,022,832,351) | 3,493,632,292 (21,642,149; 10,008,866,236) |
| QIV | 1,121,879,432 (13,857,192; 6,894,131,583) | 1,122,205,497 (13,412,778; 7,266,508,478) | 1,034,308,658 (13,623,912; 6,824,717,033) | 4,029,749,632 (23,517,142; 11,121,157,288) | 3,710,329,636 (22,568,815; 10,539,753,244) |
| TIV | 1,742,858,530 (14,947,014; 8,509,014,244) | 1,221,436,489 (13,965,662; 7,659,444,819) | 1,021,275,371 (13,674,913; 6,658,587,239) | 5,264,980,704 (27,958,457; 12,760,578,793) | 3,972,405,432 (22,813,591; 10,773,920,531) |
| Differencea | −11,577,400 (−3005; −109,775,101) | −89,714,484 (76,838; −468,824,986 | −98,701,199 (−231,697; −804,399,404) | −58,846,702 (−316,150; −98,324,937) | −216,697,344 (−926,666; −530,887,008) |
| % difference | −1.0% (0; −1.6%) | −8.0% (0.6%; −6.5%) | −9.5% (−1.7%; −11.8%) | −1.5% (−1.3%; −0.9%) | −5.8% (−4.1%; −5.0%) |
| Total direct + indirect cost ($) | |||||
| aTIV ≥ 65 QIV < 65 | 6,973,046,301 (5,003,286,410; 16,935,685,977) | 6,829,229,207 (5,014,149,292; 17,041,186,131) | 6,633,770,199 (4,335,127,694; 15,573,397,118) | 12,013,158,292 (5,051,828,358; 24,754,660,792) | 11,130,777,456 5,059,015,169; 22,495,970,672) |
| QIV | 6,999,935,094 (5,012,016,015; 16,969,916,361) | 7,000,299,780 (5,025,257,724; 17,873,288,303) | 6,856,323,145 (4,335,505,451; 16,786,551,891) | 12,131,497,418 (5,051,677,478; 24,808,699,146) | 11,569,538,428 (5,059,573,085; 23,847,488,759) |
| TIV | 7,482,097,978 (4,413,863,390; 19,288,387,147) | 6,563,613,526 (4,415,528,049; 17,916,981,003) | 6,197,118,635 (3,781,220,870; 16,424,107,679) | 13,725,780,826 (4,457,920,325; 26,960,720,664) | 11,420,539,567 (4,454,804,797; 23,556,900,364) |
| Differencea | −26,888,793 (−8,729,605; −34,230,384) | −171,070,573 (−11,108,432; −832,102,172) | −222,552,946 (−377,757; −1,213,154,773) | −118,339,127 (150,880; −54,038,354) | −438,760,972 |
| % difference | −0.4% (−0.2%; −0.2%) | −2.4% (−0.2%; −4.7%) | −3.2% (0%; −7.2%) | −1.0% (0%; −0.2%) | −3.8% (0%; −5.7%) |
| Probabilistic means (95% CrI) | ||||
|---|---|---|---|---|
| Average intensity | High intensity | |||
| High match | Low match | Average match | High match | |
| Total direct cost ($) | ||||
| aTIV ≥ 65 QIV < 65 | 7,986,717,334 (4,477,887,289; 13,311,825,736) | 10,451,882,571 (5,131,545,727; 14,743,859,812) | 10,280,324,161 (5,090,903,332; 15,151,961,976) | 10,166,916,234 (5,094,458,505; 14,819,582,203) |
| QIV | 8,265,153,875 (4,568,855,041; 14,250,701,310) | 10,577,579,955 (5,114,923,273; 14,940,387,210) | 10,643,914,304 (5,087,375,982; 15,671,318,987) | 10,642,258,998 (5,124,313,065; 15,497,524,847) |
| TIV | 7,621,849,879 (3,829,515,363; 13,262,097,930) | 11,129,946,026 (4,624,382,452; 15,498,063,020) | 10,304,309,619, (4,490,331,834; 15,587,278,564) | 10,018,298,331 (4,499,448,132; 14,906,251,468) |
| Differencea | −278,436,541 (−90,967,751; −938,875,574) | −125,697,384 (16,622,455; −196,527,398) | −363,590,142 (3,527,350; −519,357,010) | −475,342,764 (−29,854,560; −677,942,645) |
| % difference | −3.4% (−2.0%; −6.6%) | −1.2% (0.3%; −1.3%) | −3.4% (0.1%; −3.3%) | −4.5% (−0.6%; −4.4%) |
| Indirect cost ($) | ||||
| aTIV ≥ 65 QIV < 65 | 3,973,558,988 (22,931,245; 10,755,438,916) | 7,084,181,963 (86,711,992; 12,413,064,115) | 6,977,144,522 (64,687,791; 13,166,770,234) | 6,847,245,982 (96,087,031; 12,647,790,170) |
| QIV | 4,251,961,937 (23,716,365; 11,446,478,381) | 7,206,819,673 (91,200,807; 12,682,056,752) | 7,314,457,858 (56,299,005; 13,684,890,330) | 7,306,436,705 (94,599,232; 13,393,293,148) |
| TIV | 4,235,281,989 (23,738,955; 11,275,778,442) | 8,652,279,149 (261,563,517; 13,974,858,650) | 7,656,940,347 (71,689,988; 14,130,577,268) | 7,290,869,681 (85,840,012; 13,337,119,616) |
| Differencea | −278,402,949 (−785,449; −691,039,465) | −122,637,710 (−4,488,815; −268,992,637) | −337,313,335 (8,388,786; −518,120,096) | −459,190,723 (1,487,799; −745,502,978) |
| % difference | −6.5% (−3.3%; −6.0%) | −1.7% (−4.9%; −2.1%) | −4.6% (14.9%; −3.8%) | −6.3% (1.6%; −5.6%) |
| Total direct + indirect cost ($) | ||||
| aTIV ≥ 65 QIV < 65 | 11,960,276,322 (4,576,925,878; 24,229,988,647) | 17,536,064,533 (5,226,804,809; 27,034,351,861) | 17,257,468,684 (5,156,471,377; 28,168,163,497) | 17,014,162,216 (5,187,077,012; 27,323,581,109) |
| QIV | 12,517,115,812 (4,670,390,664; 25,768,606,570) | 17,784,399,628 (5,200,635,357; 27,440,685,930) | 17,958,372,161 (5,138,305,995; 29,275,883,763) | 17,948,695,702 (5,232,732,541; 28,800,572,607) |
| TIV | 11,857,131,868 (3,928,358,136; 24,373,203,580) | 19,782,225,175 (4,885,909,124; 29,392,587,997) | 17,961,249,966 (4,545,947,757; 29,714,206,125) | 17,309,168,012 (4,570,594,151; 28,079,994,755) |
| Differencea | −556,839,490 (−93,464,786; −1,538,617,923) | −248,335,094 (26,169,452; −406,334,069) | −700,903,477 (18,165,382; −1,107,720,266) | −934,533,487 (−45,655,530; −1,476,991,498) |
| % difference | −4.4% (−2.0%; −6.0%) | −1.4% (0.5%; −1.5%) | −3.9% (0.4%; −3.8%) | −5.2% (−0.9%; −5.1%) |
aTIV adjuvanted TIV, CrI credible interval, QIV quadrivalent inactivated seasonal influenza vaccine, TIV trivalent inactivated seasonal influenza vaccine
aaTIV ≥ 65 QIV < 65 vs. QIV
Fig. 4.
Graphic summary of the main clinical and life-year outputs of the model. All values are probabilistic means (see Tables 4 and 5 for 95% credible intervals for probabilistically modeled values). aTIV adjuvanted TIV, QALY quality-adjusted life-year, QIV quadrivalent inactivated seasonal influenza vaccine, TIV trivalent inactivated seasonal influenza vaccine
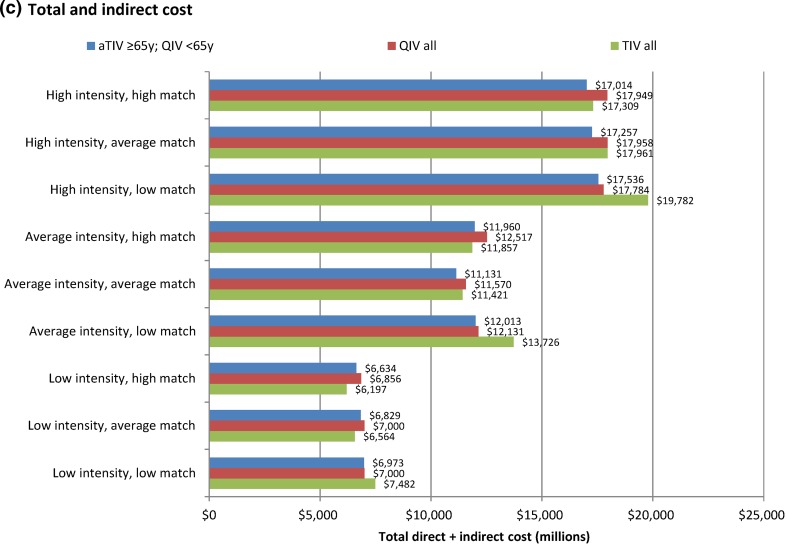
Fig. 5.
Graphic summary of the main cost outputs of the model. All values are probabilistic means (see Table 6 for 95% credible intervals for probabilistically modeled values). aTIV adjuvanted TIV, QIV quadrivalent inactivated seasonal influenza vaccine, TIV trivalent inactivated seasonal influenza vaccine
The breakeven analysis, which modeled a low intensity season in which the difference between elderly aTIV and QIV in number of cases was as close to zero as possible, showed that the prevalence of circulating mismatched strains would have to exceed 54.5% for QIV to offset the benefits of aTIV.
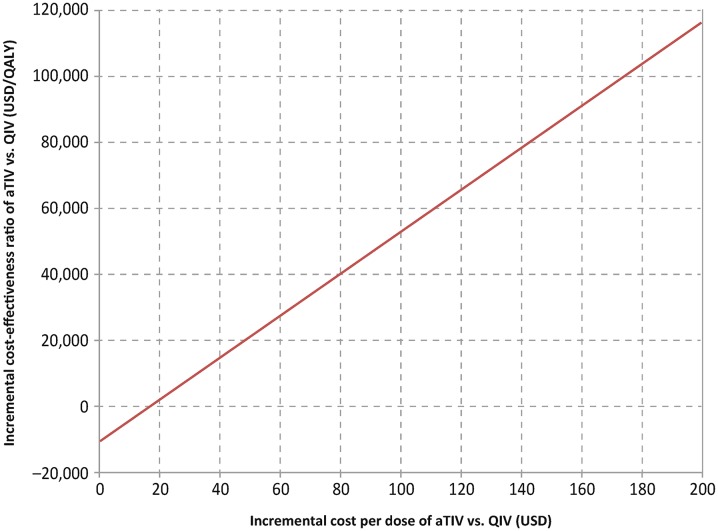
The potential impact of aTIV versus QIV vaccination in the elderly on costs was split evenly between direct savings ranging from $15 million (low intensity, low match) to $475 million (high intensity, high match) and indirect savings from $12 million to $459 million (Fig. 5; Table 6). Elderly aTIV vaccination was associated with increased overall cost over TIV in only two scenarios (low intensity with average or high match) but still represented good value for money with an incremental cost/QALY relative to TIV of $9980 in the average match scenario and $28,800 in the high match scenario. In both these scenarios, however, elderly aTIV dominated QIV with lower cost (−$171,071; −$222,553) and higher QALYs (14,676; 17,136). Univariate sensitivity analysis of relative vaccine prices in the average intensity, average match scenario indicated that aTIV could be marginally cost saving relative to QIV at current Medicare prices for influenza vaccines (Fig. 6 [51–53]).
Fig. 6.
Univariate sensitivity analysis of relative vaccine prices in the average intensity, average match scenario. aTIV adjuvanted trivalent inactivated influenza vaccine, QALY quality-adjusted life-year, QIV quadrivalent inactivated influenza vaccine
Inspection of variability of results across scenarios showed that the outputs were most sensitive to vaccine match, followed by season intensity. The probabilistic nature of the model was illustrated by the probabilistic mean, low, and high estimates for each outcome within each discrete scenario, reflecting variation of inputs in observational studies and surveillance data.
Discussion
We demonstrate that vaccination of persons aged ≥65 years with aTIV has the potential to provide clinical and economic benefit relative to QIV and TIV. The dynamic model described herein can be used to inform policy decisions regarding seasonal influenza vaccination. Similar dynamic compartmental models have been described previously [54–57]. Importantly, they enable herd immunity benefits of vaccination, which are not adequately addressed by static models, to be captured. The use of dynamic models such as that described here to inform policy making in persons aged ≥65 years is particularly pertinent because significant healthcare costs are linked to influenza in this population [6]. Note that the present analysis was based on the results of previous studies and other information from the literature, and did not involve the collection of any new data from human or animal subjects.
In the US, a very broad routine annual vaccination recommendation is in place (all persons aged ≥6 months with no contraindications) [58]. Moreover, the US Centers for Disease Control and Prevention (CDC) does not currently recommend any one influenza vaccine product over another in the elderly (i.e., no preference is expressed at present for QIV, high-dose, or adjuvanted vaccine over conventional TIV) [58]. As a result of recent studies indicating increased vaccine effectiveness of high-dose influenza vaccine in the over-65 population [59, 60], there is sentiment towards using the high-dose vaccine in that population, despite the current lack of a preferential recommendation for its use. Additionally, as QIV supplies continue to increase within the US, there appears to be increasing support for its use in the general population, again despite the lack of a preferential recommendation for such use by the CDC. Both the QIV and high-dose TIV vaccines are covered by insurance in the US. Of note, the high-dose TIV is covered by the Medicare Part B program despite its higher cost; this is significant, as the majority of the over-65 population in the US is covered by this public payer [61].
The effectiveness of current vaccination recommendations in the elderly is under debate, as conventional vaccines are reported to provide suboptimal protection in this group [7]. High-dose TIV uptake in the elderly is expected to increase in the US and other countries in the near future, but there remains a need for additional vaccines offering enhanced immunogenicity in this population [62].
Adjuvantation of inactivated vaccines (e.g., with oil-in water adjuvants) has the potential to address unmet influenza vaccination needs in the elderly [16, 62], and has been reported to provide better cross-reactivity against mismatched strains than conventional vaccines in the elderly in several studies [26, 63–65]. The potential for improved outcomes with adjuvanted vaccine was apparent from the model, which showed probabilistic mean reductions in clinical outputs and overall costs relative to QIV even in modeled low match seasons. The breakeven analysis carried out after the main simulations suggested that QIV would only offer incremental benefit over aTIV in the elderly if the proportion of mismatched strains in circulation was as high as 54.5% or above, a level which has not been observed in the US over the last 15 years.
Of note, high-dose TIV has been associated with a 24.2% increase in efficacy against laboratory-confirmed influenza [59] and a 22% increase in effectiveness for preventing probable influenza infections and hospitalizations in persons aged ≥65 years [60]. This suggests that economic comparisons of high-dose TIV with other inactivated influenza vaccine options using the model would be of considerable interest and could be the subject of a future analysis. For now, and on the basis of the data and assumptions built into the present model and the above observations with high-dose vaccine, we would expect such an analysis to yield results comparable to those obtained here with aTIV in subjects aged ≥65 years. Such a hypothesis would require future confirmation, however.
As reported above, the potential impact of aTIV versus QIV on costs was split evenly between direct and indirect savings. Direct cost savings were derived from reduced hospitalizations among the elderly, who experience increased serious influenza complication rates over younger populations [27]. Indirect cost savings accrued mainly from the impact on younger working relatives/associates, who are likely to experience work interruptions due to the need for visitation and care giving, with accompanying losses of productivity [27]. Sensitivity analysis of vaccine prices in the average intensity, average match scenario indicated that aTIV could be marginally cost saving relative to QIV at current Medicare prices.
The importance of indirect protection of older persons through herd immunity effects is illustrated by experience of influenza outbreaks in residential institutions [5]. As mentioned earlier, the structure of the SIR model on which the analysis was based accounts for herd immunity effects, through which the elderly benefit from decreased transmission of influenza among the broader population as a result of vaccination [16].
The data used to populate the model were subject to a number of limitations. Mannino et al.’s study [25] demonstrating the 25% reduction in risk of hospitalization for influenza or pneumonia versus TIV in adults aged ≥65 years was observational only, did not assess aTIV’s impact on the reduction of influenza cases, and did not compare aTIV directly with QIV [25]. There was no stratification other than age in the model, with no account taken of chronic disease status or residential/contact status. The model does not account for adverse effects of influenza vaccination. In addition, costs were treated deterministically in the model even though significant variation occurs in clinical practice. Despite these limitations, the findings are in line with what would be expected for a vaccine offering enhanced immunogenicity that has a potentially positive effect on clinical outcomes and costs relative to conventional vaccines, given the known economic burden of influenza [66] and the public health and economic benefits of vaccination [66, 67].
Conclusion
We have developed a dynamic model to assist vaccination policy decisions directing choices between different formulations of seasonal influenza vaccine. The US scenario modeled suggests that vaccination of persons aged ≥65 years with aTIV has the potential to provide clinical and economic benefit relative to QIV and TIV. We recommend further investigation of the clinical and economic impact of aTIV relative to other vaccine formulations in the elderly.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. Medical Writing support for the development of this manuscript was provided by Christopher Dunn and Scott Malkin at Gardiner-Caldwell Communications, Macclesfield, UK. Funding for this support was provided by Novartis Vaccines. Modeling support for the analyses described in this manuscript was provided by MAPI Values, Boston, MA, USA. Funding for this support was provided by Novartis Vaccines.
Conflict of interest
Mark Mullikin, Norbert Farkas, and Eckhardt Petri declare that they are current or former employees of Novartis Vaccines. Jeroen P. Jansen and Marc Van Ranst have no conflict of interest. Litjen Tan has served on scientific advisory boards for Pfizer, Merck, Novartis, Temptime Corp., and TruMedSystems within the past year. All honoraria for these services were donated directly to the Immunization Action Coalition.
Compliance with ethics guidelines
The analysis in this article is based on previously conducted studies, and does not involve any new studies of human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
References
- 1.Meier CR, Napalkov PN, Wegmüller Y, Jefferson T, Jick H. Population-based study on incidence, risk factors, clinical complications and drug utilisation associated with influenza in the United Kingdom. Eur J Clin Microbiol Infect Dis. 2000;19:834–842. doi: 10.1007/s100960000376. [DOI] [PubMed] [Google Scholar]
- 2.Meyers DG. Myocardial infarction, stroke, and sudden cardiac death may be prevented by influenza vaccination. Curr Atheroscler Rep. 2003;2003(5):146–149. doi: 10.1007/s11883-003-0087-x. [DOI] [PubMed] [Google Scholar]
- 3.Nicholson KG, Kent J, Hammersley V, Cancio E. Acute viral infections of upper respiratory tract in elderly people living in the community: comparative, prospective, population based study of disease burden. BMJ. 1997;315:1060–1064. doi: 10.1136/bmj.315.7115.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. J Am Med Assoc. 2004;292:1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 5.Menec VH, MacWilliam L, Aoki FY. Hospitalizations and deaths due to respiratory illnesses during influenza seasons: a comparison of community residents, senior housing residents, and nursing home residents. J Gerontol A Biol Sci Med Sci. 2002;57:M629–M635. doi: 10.1093/gerona/57.10.M629. [DOI] [PubMed] [Google Scholar]
- 6.Menec VH, Black C, MacWilliam L, Aoki FY. The impact of influenza-associated respiratory illnesses on hospitalizations, physician visits, emergency room visits, and mortality. Can J Public Health Rev Can Santé Publique. 2003;94:59–63. doi: 10.1007/BF03405054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simonsen L, Fukuda K, Schonberger LB, Cox NJ. The impact of influenza epidemics on hospitalizations. J Infect Dis. 2000;181:831–837. doi: 10.1086/315320. [DOI] [PubMed] [Google Scholar]
- 8.Mullooly JP, Bridges CB, Thompson WW, on behalf of the Vaccine Safety Datalink Adult Working Group et al. Influenza- and RSV-associated hospitalizations among adults. Vaccine. 2007;25:846–855. doi: 10.1016/j.vaccine.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 9.Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. J Am Med Assoc. 2003;289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 10.Zamparo E, Little D. Immunogenicity and effectiveness of virosomal adjuvanted vaccines against influenza: a brief review of their utility in the elderly population. J Prev Med Hyg. 2011;52:116–119. [PubMed] [Google Scholar]
- 11.Viboud C, Miller M. Health benefits of universal influenza vaccination strategy. PLoS Med. 2008;5:e216. doi: 10.1371/journal.pmed.0050216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McElhaney JE, Zhou X, Talbot HK, et al. The unmet need in the elderly: how immunosenescence, CMV infection, co-morbidities and frailty are a challenge for the development of more effective influenza vaccines. Vaccine. 2012;30:2060–2067. doi: 10.1016/j.vaccine.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monto AS, Ansaldi F, Aspinall R, et al. Influenza control in the 21st century: optimizing protection of older adults. Vaccine. 2009;27:5043–5053. doi: 10.1016/j.vaccine.2009.06.032. [DOI] [PubMed] [Google Scholar]
- 14.Nichol KL. Efficacy and effectiveness of influenza vaccination. Vaccine. 2008;26(Suppl. 4):D17–D22. doi: 10.1016/j.vaccine.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 15.Nichol KL. The efficacy, effectiveness and cost-effectiveness of inactivated influenza virus vaccines. Vaccine. 2003;21:1769–1775. doi: 10.1016/S0264-410X(03)00070-7. [DOI] [PubMed] [Google Scholar]
- 16.Simonsen L, Taylor RJ, Viboud C, Miller MA, Jackson LA. Mortality benefits of influenza vaccination in elderly people: an ongoing controversy. Lancet Infect Dis. 2007;7:658–666. doi: 10.1016/S1473-3099(07)70236-0. [DOI] [PubMed] [Google Scholar]
- 17.Sasaki S, Sullivan M, Narvaez CF, et al. Limited efficacy of inactivated influenza vaccine in elderly individuals is associated with decreased production of vaccine-specific antibodies. J Clin Invest. 2011;121:3109–3119. doi: 10.1172/JCI57834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis. 2009;200:172–180. doi: 10.1086/599790. [DOI] [PubMed] [Google Scholar]
- 19.Coudeville L, Andre P, Bailleux F, Weber F, Plotkin S. A new approach to estimate vaccine efficacy based on immunogenicity data applied to influenza vaccines administered by the intradermal or intramuscular routes. Hum Vaccin. 2010;6:841–848. doi: 10.4161/hv.6.10.12636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tricco AC, Chit A, Soobiah C, et al. Comparing influenza vaccine efficacy against mismatched and matched strains: a systematic review and meta-analysis. BMC Med. 2013;11:153. doi: 10.1186/1741-7015-11-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DiazGranados CA, Denis M, Plotkin S. Seasonal influenza vaccine efficacy and its determinants in children and non-elderly adults: a systematic review with meta-analyses of controlled trials. Vaccine. 2012;31:49–57. doi: 10.1016/j.vaccine.2012.10.084. [DOI] [PubMed] [Google Scholar]
- 22.McKeage K. Inactivated quadrivalent split-virus seasonal influenza vaccine (Fluarix® quadrivalent): a review of its use in the prevention of disease caused by influenza A and B. Drugs. 2013;73:1587–1594. doi: 10.1007/s40265-013-0114-3. [DOI] [PubMed] [Google Scholar]
- 23.De Donato S, Granoff D, Minutello M, et al. Safety and immunogenicity of MF59-adjuvanted influenza vaccine in the elderly. Vaccine. 1999;17:3094–3101. doi: 10.1016/S0264-410X(99)00138-3. [DOI] [PubMed] [Google Scholar]
- 24.Squarcione S, Sgricia S, Biasio LR, Perinetti E. Comparison of the reactogenicity and immunogenicity of a split and a subunit-adjuvanted influenza vaccine in elderly subjects. Vaccine. 2003;21:1268–1274. doi: 10.1016/S0264-410X(02)00401-2. [DOI] [PubMed] [Google Scholar]
- 25.Mannino S, Villa M, Apolone G, et al. Effectiveness of adjuvanted influenza vaccination in elderly subjects in northern Italy. Am J Epidemiol. 2012;176:527–533. doi: 10.1093/aje/kws313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Durando P, Icardi G, Ansaldi F. MF59-adjuvanted vaccine: a safe and useful tool to enhance and broaden protection against seasonal influenza viruses in subjects at risk. Expert Opin Biol Ther. 2010;10:639–651. doi: 10.1517/14712591003724662. [DOI] [PubMed] [Google Scholar]
- 27.Molinari N-AM, Ortega-Sanchez IR, Messonnier ML, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25:5086–5096. doi: 10.1016/j.vaccine.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 28.Annemans L. Methods for economic evaluations. In: Health economics for non-economists. Gent: Academia Press; 2008. p. 29–79.
- 29.Reed C, Meltzer MI, Finelli L, Fiore A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine. 2012;30:1993–1998. doi: 10.1016/j.vaccine.2011.12.098. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention (CDC) Update: influenza activity—United States, 2010–11 season, and composition of the 2011–12 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2011;60:705–712. [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention (CDC) Update: influenza activity—United States, 2011–12 season and composition of the 2012–13 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2012;61:414–420. [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention (CDC) Influenza activity—United States, 2012–13 season and composition of the 2013–14 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2013;62:473–479. [PMC free article] [PubMed] [Google Scholar]
- 33.Epperson S, Blanton L, Kniss K, on behalf of the Influenza Division NCfI, Respiratory Diseases CDC et al. Influenza activity—United States, 2013–14 season and composition of the 2014–15 influenza vaccines. MMWR Morb Mortal Wkly Rep. 2014;63:483–490. [PMC free article] [PubMed] [Google Scholar]
- 34.US Department of Commerce. Current Population Survey (CPS)—a joint effort between the Bureau of Labor Statistics and the Census Bureau—People and Households—US Census Bureau [online document]. US Census Bureau. 2012. http://www.census.gov/cps/. Accessed 27 Oct 2014.
- 35.Del Valle SY, Hyman JM, Hethcote HW, Eubank SG. Mixing patterns between age groups in social networks. Soc Netw. 2007;29:539–554. doi: 10.1016/j.socnet.2007.04.005. [DOI] [Google Scholar]
- 36.Biggerstaff M, Cauchemez S, Reed C, Gambhir M, Finelli L. Estimates of the reproduction number for seasonal, pandemic, and zoonotic influenza: a systematic review of the literature. BMC Infect Dis. 2014;14:480. doi: 10.1186/1471-2334-14-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cowling BJ, Fang VJ, Riley S, Peiris JSM, Leung GM. Estimation of the serial interval of influenza. Epidemiology. 2009;20:344–347. doi: 10.1097/EDE.0b013e31819d1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fraser C, Donnelly CA, Cauchemez S, on behalf of the WHO Rapid Pandemic Assessment Collaboration et al. Pandemic potential of a strain of influenza A (H1N1): early findings. Science. 2009;324:1557–1561. doi: 10.1126/science.1176062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention (CDC). Flu vaccination coverage, United States, 2013–14 Influenza Season/FluVaxView/Seasonal Influenza (Flu)/CDC [online document]. 2014. http://www.cdc.gov/flu/fluvaxview/coverage-1314estimates.htm. Accessed 27 Oct 2014).
- 40.Blyth CC, Jacoby P, Effler PV, on behalf of the WAIVE Study Team et al. Effectiveness of trivalent flu vaccine in healthy young children. Pediatrics. 2014;133:1218–1225. doi: 10.1542/peds.2013-3707. [DOI] [PubMed] [Google Scholar]
- 41.Prosser LA. Cost-effectiveness of Influenza Vaccine [online document]. NIVS, May 16–17, 2012, Atlanta, GA, USA. National Adult Influenza Immun. Summit. http://www.izsummitpartners.org/summit/archive/2012-nivs/. Accessed 28 Oct 2014.
- 42.Talbird SE, Brogan AJ, Winiarski AP, Sander B. Cost-effectiveness of treating influenza-like illness with oseltamivir in the United States. Am J Health Syst Pharm. 2009;66:469–480. doi: 10.2146/ajhp080296. [DOI] [PubMed] [Google Scholar]
- 43.Lloyd-Jones D, Adams RJ, Brown TM, on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee et al. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 44.Lee GM, Salomon JA, LeBaron CW, Lieu TA. Health-state valuations for pertussis: methods for valuing short-term health states. Health Qual Life Outcomes. 2005;3:17. doi: 10.1186/1477-7525-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dyer MTD, Goldsmith KA, Sharples LS, Buxton MJ. A review of health utilities using the EQ-5D in studies of cardiovascular disease. Health Qual Life Outcomes. 2010;8:13. doi: 10.1186/1477-7525-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prosser LA, Ray GT, O’Brien M, Kleinman K, Santoli J, Lieu TA. Preferences and willingness to pay for health states prevented by pneumococcal conjugate vaccine. Pediatrics. 2004;113:283–290. doi: 10.1542/peds.113.2.283. [DOI] [PubMed] [Google Scholar]
- 47.Fryback DG, Dunham NC, Palta M, et al. US norms for six generic health-related quality-of-life indexes from the National Health Measurement study. Med Care. 2007;45:1162–1170. doi: 10.1097/MLR.0b013e31814848f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.ftp.cdc.gov-/pub/health_Statistics/NCHS/Publications/NVSR/56_09/ [online document] [no date]. http://ftp.cdc.gov/pub/health_Statistics/NCHS/Publications/NVSR/56_09/. Accessed 15 June 2015.
- 49.Fisman DN, Tuite AR. Estimation of the health impact and cost-effectiveness of influenza vaccination with enhanced effectiveness in Canada. PLoS One. 2011;6:e27420. doi: 10.1371/journal.pone.0027420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seasonal Flu [online document]. 2015. Flu.gov. http://www.flu.gov/about_the_flu/seasonal/index.html. Accessed 12 June 2015.
- 51.Centers for Medicare and Medicaid Services. Physician fee schedule. 2014. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/index.html?redirect=/PhysicianFeeSched/. Accessed 22 July 2015.
- 52.Centers for Medicare and Medicaid Services. Clinical laboratory fee schedule. 2014. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/clinlab.html. Accessed 22 July 2015.
- 53.Centers for Medicare and Medicaid Services. Average sales price (ASP) drug pricing files. 2014. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/2014ASPFiles.html. Accessed 22 July 2015.
- 54.Nichol KL, Tummers K, Hoyer-Leitzel A, Marsh J, Moynihan M, McKelvey S. Modeling seasonal influenza outbreak in a closed college campus: impact of pre-season vaccination, in-season vaccination and holidays/breaks. PLoS One. 2010;5:e9548. doi: 10.1371/journal.pone.0009548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coburn BJ, Wagner BG, Blower S. Modeling influenza epidemics and pandemics: insights into the future of swine flu (H1N1) BMC Med. 2009;7:30. doi: 10.1186/1741-7015-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Knipl DH, Röst G. Modelling the strategies for age specific vaccination scheduling during influenza pandemic outbreaks. Math Biosci Eng. 2011;8:123–139. doi: 10.3934/mbe.2011.8.123. [DOI] [PubMed] [Google Scholar]
- 57.Damm O, Eichner M, Rose MA, et al. Public health impact and cost-effectiveness of intranasal live attenuated influenza vaccination of children in Germany. Eur J Health Econ. 2015;16:471–488. doi: 10.1007/s10198-014-0586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Centers for Disease Control and Prevention (CDC). Prevention and Control of Seasonal Influenza with Vaccines/Health Professionals/Seasonal Influenza (Flu) [online document]. 2014. http://www.cdc.gov/flu/professionals/acip/index.htm. Accessed 30 Oct 2014).
- 59.DiazGranados CA, Dunning AJ, Kimmel M, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med. 2014;371:635–645. doi: 10.1056/NEJMoa1315727. [DOI] [PubMed] [Google Scholar]
- 60.Izurieta HS, Thadani N, Shay DK, et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccines in US residents aged 65 years and older from 2012 to 2013 using Medicare data: a retrospective cohort analysis. Lancet Infect Dis. 2015;15:293–300. doi: 10.1016/S1473-3099(14)71087-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Centers for Medicare and Medicaid Services. Medicare Enrollment—National Trends 1966–2013. Medicare aged and disabled enrollees by type of coverage. All areas, as of July 1, 1966–2013. Hospital and/or Supplementary Medical Insurance, Medicare Enrollment Reports. Baltimore: Centers for Medicare and Medicaid Services; 2014.
- 62.Datamonitor Healthcare. Influenza vaccines forecast (No. DMKC0107117). London: Informa UK Ltd; 2014.
- 63.Baldo V, Baldovin T, Pellegrini M, et al. Immunogenicity of three different influenza vaccines against homologous and heterologous strains in nursing home elderly residents. Clin Dev Immunol. 2010;2010:517198. doi: 10.1155/2010/517198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ansaldi F, Zancolli M, Durando P, et al. Antibody response against heterogeneous circulating influenza virus strains elicited by MF59- and non-adjuvanted vaccines during seasons with good or partial matching between vaccine strain and clinical isolates. Vaccine. 2010;28:4123–4129. doi: 10.1016/j.vaccine.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 65.Orsi A, Ansaldi F, de Florentiis D, et al. Cross-protection against drifted influenza viruses: options offered by adjuvanted and intradermal vaccines. Hum Vaccines Immunother. 2013;9:582–590. doi: 10.4161/hv.23239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xue Y, Kristiansen IS, de Blasio BF. Modeling the cost of influenza: the impact of missing costs of unreported complications and sick leave. BMC Public Health. 2010;10:724. doi: 10.1186/1471-2458-10-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Preaud E, Durand L, Macabeo B, on behalf of the Vaccines Europe Influenza Working Group et al. Annual public health and economic benefits of seasonal influenza vaccination: a European estimate. BMC Public Health. 2014;14:813. doi: 10.1186/1471-2458-14-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.