Abstract
In 2014 and 2015, the largest Ebola virus disease (EVD) outbreak in history affected large populations across West Africa. The goal of this report is to provide an update on the epidemic and review current progress in the development, evaluation and deployment of prevention and treatment strategies for EVD. Relevant information was identified through a comprehensive literature search using Medline, PubMed and CINAHL Complete and using the search terms Ebola, Ebola virus disease, Ebola hemorrhagic fever, West Africa outbreak, Ebola transmission, Ebola symptoms and signs, Ebola diagnosis, Ebola treatment, vaccines for Ebola and clinical trials on Ebola. Through 22 July 2015, a total of 27,741 EVD cases and 11,284 deaths were reported from all affected countries. Several therapeutic agents and novel vaccines for EVD have been developed and are now undergoing evaluation. Concurrent with active case investigation, contact tracing, surveillance and supportive care to patients and communities, there has been rapid progress in the development of new therapies and vaccines against EVD. Continued focus on strengthening clinical and public health infrastructure will have direct benefits in controlling the spread of EVD and will provide a strong foundation for deployment of new drugs and vaccines to affected countries when they become available. The unprecedented West Africa Ebola outbreak, response measures, and ensuing drug and vaccine development suggest that new tools for Ebola control may be available in the near future.
Electronic supplementary material
The online version of this article (doi:10.1007/s40121-015-0079-5) contains supplementary material, which is available to authorized users.
Keywords: Africa, Anti-viral, EBOV, EVD, Hemorrhagic fever, Immunization, Treatment, Vaccine
Introduction
Ebola virus (EBOV) derived its name from the Ebola River in Democratic Republic of Congo (DRC) (formerly Zaire) where the first Ebola virus disease (EVD) outbreak was identified in 1976 [1]. Historically, outbreaks of EVD have been confined to a single country and have been brought under control by domestic health agencies working in conjunction with international organizations such as the World Health Organization (WHO). However, since March 2014, West African countries, notably Guinea, Liberia and Sierra Leon, have experienced the largest EVD outbreak in their history [2]. Although the origins of EVD in the most recent outbreak remain under investigation, the spread of EBOV occurred rapidly because of a number of factors including funeral and burial practices for decedents [3, 4].
The scope and severity of the EVD outbreak underscore the urgent need for development and evaluation of affordable therapeutic and prophylactic agents that can be made available for at-risk populations across Africa. Over the past 17 months, the West Africa EVD outbreak has provided an important opportunity to consider use of and evaluate several therapeutic and prophylactic agents (e.g., vaccines) to determine their safety and efficacy [5, 6].
Review Methods
For this review, we considered published and unpublished reports related to EBOV and EVD. We reviewed reports from peer-reviewed literature published from 1993 through 2015 and cited in several electronic databases including Medline, PubMed and CINAHL Complete on “Ebola,” “Ebola virus disease,” “Ebola hemorrhagic fever,” “West Africa outbreak,” “Ebola transmission,” “Ebola symptoms and signs,” “Ebola diagnosis,” “Ebola treatment,” “vaccines for Ebola” and “clinical trials on Ebola” (Fig. 1). The gray literature, health organization websites, clinical trial registries and corporate websites were inspected and reviewed to identify up-to-date information relevant to Ebola. Studies were included in the proposed literature review if they (1) were published in the English language; (2) were full-text articles; (3) focused on Ebola virus virology, epidemiology, diagnosis, treatment and clinical trials on vaccines and treatment; (4) were published between 1993 and 2015; (5) were published in peer-reviewed journals. We excluded studies if they (1) were not full-text articles; (2) were published before 1993; (3) were published in non-peer-reviewed journals. We identified 156 studies for inclusion in this review. Seventy-one studies focused on epidemiology, public health issues, clinical syndrome of EVD and diagnostic tools. An additional 89 studies provided information on therapeutic and vaccine clinical trials that target EBOV. Data were abstracted from published and unpublished reports to describe disease patterns, burden of illness in past and present outbreaks as well as effects of investigational therapies and vaccines. This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Fig. 1.
Flowchart for the literature search
Virology
Filoviruses (family Filoviridae) are enveloped, linear, non-segmented, negative and single-stranded RNA viruses belonging to the order Mononegavirales. Ebolavirus and Marburgvirus are the two genera of filoviruses that have been identified to cause severe disease in humans [7, 8]. Within the genus Ebolavirus, five viruses are recognized (EBOV, Sudan virus, Reston virus, Taï Forest virus and Bundibugyo virus) with each representing a different virus species (Zaire ebolavirus, Sudan ebolavirus, Reston ebolavirus, Taï Forest ebolavirus and Bundibugyo ebolavirus). In contrast, the genus Marburgvirus contains a single virus species (Marburg marburgvirus), and two distinct viruses have been recognized, Marburg virus and Ravn virus [9–11]. In 2011, a novel third genus of filovirus named Cuevavirus was reported from post-mortem tissues of bats collected in 2002 in Northern Spain [12]. Cuevavirus has not been grown in cell culture, and its pathogenic potential for humans remains unknown. To date, a single species (Lloviu cuevavirus) has been approved by the International Committee on Taxonomy of Viruses (ICTV) [9].
The current West Africa outbreak is caused by Zaire ebolavirus, which shows 97% identity to EBOV strains from the DRC and Gabon [13]. The genome of EBOV contains seven genes named nucleoprotein (NP), virion protein (VP) 24, VP30, VP35, VP40, glycoprotein (GP) and L protein [14]. Each one of these genes encodes a corresponding structural protein. The main proteins targeted by experimental treatments are the NP, VP35, GP, VP24 and L protein. NP is the main component of the viral nucleocapsid and encapsulates the viral RNA. VP35 is also part of the nucleocapsid and, together with VP24, interferes with innate host immunity. The surface GP is responsible for the attachment to the cellular receptor and viral entry. L protein is the RNA-dependent RNA polymerase [15–20].
Early reports suggest that the EBOV variant of the 2014–2015 West Africa outbreak accumulated mutations that may have an impact on the performance of certain diagnostic tests or even on the efficacy of several experimental treatments. Gire et al. analyzed the genetic sequence of 99 EBOV genomes from 78 patients in the four most affected countries of the West African region [16]. They found significant rates of genomic variation in EBOV in the current outbreak when compared with the EBOV genomic sequence in the 2004 Ebola outbreak in the DRC. Although the impact of these mutations on the diagnostic tests and experimental therapeutics has not yet been proven, some mutations exist in viral genes that are targeted by primers of some reverse transcription-polymerase chain reaction (RT-PCR) protocols [21], as well as mutations in the binding sites of target proteins of some experimental treatments such as anti-GP monoclonal antibodies [22]. In 2015, Hoenen and colleagues studied full-length sequences of two clusters of EBOV imported from Mali and found that the gene sequence of EBOV has remained stable during the current Ebola outbreak [23].
Epidemiology and Outbreaks
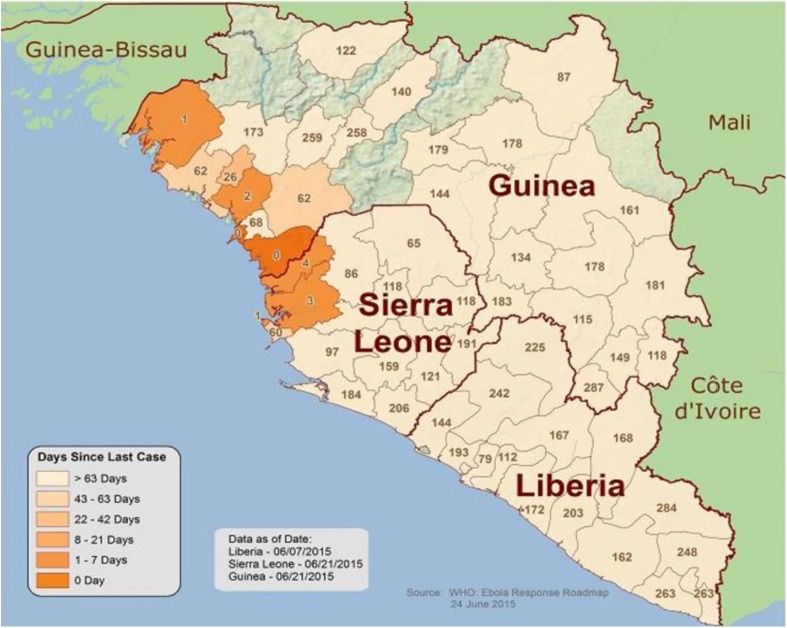
Ebola viruses have been responsible for 33 outbreaks in six African countries [2]. Historically, the outbreaks have affected hundreds of individuals where effective control of outbreaks was achieved primarily through isolation of cases and contact tracing. However, from 2000, EVD outbreaks have been recognized almost every year with substantial variation in morbidity and case-fatality rates ranging from 24% to 81% [24]. High case-fatality rates have been associated with the Zaire and Sudan subtypes [25]. In the ongoing Ebola outbreak, the overall case-fatality rate has been estimated to be approximately 41% for West Africa and other affected countries [26]. Most recently, although the number of cases has declined substantially (Fig. 2), EVD cases continue to be reported (please see the supplemental table for details) in Guinea, Liberia and Sierra Leone with WHO Ebola situation reports noting weekly cases in July 2015 [27]. In July 2015, situation report statistics from the WHO suggest that the greatest current burden of EVD is found in Guinea (n = 43) and Sierra Leone (n = 31). Liberia reported the lowest EVD case number (n = 3) in the week of 29 June through 5 July [27].
Fig. 2.
Map of the West African region showing the number of days passed since the last case reported to the World Health Organization. Last updated 24 June 2015. Reprinted from the Ebola Response Roadmap. Map of West Africa showing when the last cases of Ebola occurred. 24 June 2015. http://www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/distribution-map.html. Copyright 2015
The search for the natural reservoir host of EBOV has been a matter of investigation during the last decades. There is mounting evidence that a number of mammal species may harbor and transmit the virus. Several bat species (i.e., Epomops franqueti, Hypsignathus monstrosus and Myonycteris torquata) have been found to carry filoviruses [28–35]. In addition, EBOV viral RNA and/or antibodies have been found in these animals [30, 32, 34], and Marburg virus has been isolated from Egyptian fruit bats (Rousettus aegyptiacus) [29].
Transmission and Clinical Syndrome
Humans may acquire the disease by close contact with biological fluids from infected animals or patients. During the acute phase of illness, EBOV has been detected in a variety of body fluids including breast milk, saliva, semen, stool, sweat, tears and urine [36–40]. Nevertheless, the EBOV viral load by organ has not been extensively studied although EBOV has been detected in the semen of survivors up to 3 months following onset of symptoms [41, 42]. EBOV has been isolated from urine and from aqueous humor samples 9 days and 9 weeks, respectively, after the virus was cleared from plasma [37, 43].
The clinical presentation of the current West Africa outbreak is, in general, similar to that described in prior EBOV epidemics. The incubation period for person-to-person transmitted EVD typically ranges from 8 to 11 days, but cases have been reported with incubation periods as short as 2 and as long as 21 days. Shorter incubation periods may be observed following direct inoculation of virus through injection with contaminated needles [44, 45]. Patients often present to health care providers within 1 week of symptom onset [21, 46]. In the early clinical phase of EVD, patients manifest signs and symptoms that mimic common tropical illnesses (e.g., dengue, malaria, typhoid fever and other viral infections) [16, 47, 48]. The onset of the disease includes nonspecific clinical signs such as fever, headache, extreme asthenia, arthralgia, myalgia and back pain. Progressive gastrointestinal (GI) symptoms often develop within 3 to 5 days of symptom onset [44, 49–51]. GI manifestations include abdominal pain, anorexia, nausea, vomiting and diarrhea, which lead to profound electrolyte imbalance, intravascular volume depletion and shock. Conjunctival injection, rash, hiccups, respiratory and neurologic findings have been also reported. Bleeding is a late clinical sign that occurs only in less than 20% of patients with EVD [44]. However, if hemorrhage occurrs, a dismal outcome can be predicted. In 2015, Barry et al. studied the correlation between the occurrence of symptoms and death in 89 patients with EVD. These investigators found hemorrhage, shortness of breath and myalgia were independently associated with death [52]. Clinical deterioration may progress rapidly resulting in death within 7 to 10 days.
Vulnerable populations include children under the age of 5 years, pregnant women and the elderly [49]. EVD in these groups also include unspecific symptoms in the clinical presentation. Qin et al. did not find differences related to mortality between patients less than 10 years of age and others between 11 to 20 years old, but they found that patients aged <30 years had a much lower case fatality rate than those aged >30 years [22/38 (57.9%) and 20/23 (87.0%), respectively, with p = 0.0175] and that survivors attended Ebola Treatment Centers earlier after the onset of symptoms [53]. No evidence suggests that pregnant women are more susceptible to EBOV infection than the general population. However, they might be at increased risk of severe illness and fetal loss. Although no large series are available, the fetal outcome is generally fatal.
Immune suppression and a systemic inflammatory response due to the release of cytokines and other proinflammatory mediators lead to the impairment of vascular, coagulation and immune systems [54]. This can result in multiorgan failure and shock resembling a septic shock syndrome. Massive fluid losses due to intense vomiting and profuse diarrhea can result in dehydration and hypovolemic shock [49]. Severe lymphopenia as well as significant deterioration of renal and liver functions, which may be reflected in high blood urea nitrogen, serum creatinine and hepatic enzymes (i.e., aminotransferases and alkaline phosphatase), can occur [21, 55, 56].
Since EBOV is a contagious pathogen, the WHO and Centers for Disease Control (CDC) have issued recommendations for proper handling of biological specimens from suspected cases of EVD [57, 58]. Extreme caution should take place at all stages (i.e., specimen acquisition, transport, processing and testing) of specimen processing, and appropriate biosafety laboratory procedures must be used when handling biological specimens from patients with suspected EVD.
Diagnosis of Ebola Virus Disease
Rapid and reliable diagnosis of EVD is essential for appropriate and effective patient management, hospital or health center infection prevention and control, and optimization of use of healthcare resources [59]. Diagnosis of suspected cases is confirmed by EBOV-specific laboratory tests that detect the EBOV genome (e.g., RT-PCR) or measurement of the EBOV antigen or specific antibodies [42]. In the past 10 months, the West Africa EVD outbreak has stimulated the development of new diagnostic tests, including rapid antigen detection tests and nucleic acid detection (NAT) tests such as loop-mediated isothermal amplification (LAMP) assays [60, 61].
Enzyme-Linked Immunosorbent Assay
Antigen Detection
Prior to 2000, antigen detection methods [e.g., enzyme-linked immunosorbent assay (ELISA)] were the gold standard for EBOV detection in some outbreaks [62]. In the acute phase of EVD, ELISA has a relatively high sensitivity (93%), but EBOV antigen levels decline as disease progresses, rendering lower sensitivity for antigen detection 1–2 weeks following symptom onset [41, 63]. Several other antigen detections tests are currently under evaluation and may be deployed in the near future to complement RT-PCR testing [60]. ELISA testing has been largely replaced by RT-PCR, which permits more rapid detection and can now be deployed in mobile (portable) testing platforms in outbreak settings [64].
Antibody Detection
Detection of IgM antibodies against EBOV is performed by ELISA in the first week after the onset of symptoms with a peak of IgM levels occurring in the 2nd week of illness [41, 48, 62]. IgM antibodies are cleared at variable rates from 1 to 6 months after illness onset [41]. Data showed that serology can be highly specific for the EVD diagnosis but less sensitive in the intensive care unit setting. Hence, antibody testing may be less clinically useful in the diagnosis and management of critically ill EVD patients [49]. Although IgG antibodies appear soon after the IgM and may persist for years [41], a substantial number of EVD patients have died before they develop an IgG antibody response [48].
Reverse Transcription Polymerase Chain Reaction (RT-PCR)
Nucleic acid tests (NATs), particularly RT-PCR, are regarded as the gold standard for EVD diagnosis, in part because of their high sensitivity and specificity in detecting the Ebola viral genome. This is generally accomplished by international mobile teams deployed in institutions such as the European Mobile Laboratory or CDC. RT-PCR is a rapid and highly sensitive nucleic acid amplification test to detect EBOV nucleic acid [65]. The sensitivity and specificity of RT-PCR are approximately 100% and 97%, respectively [63]. Within the first 3 days of illness, molecular assays may not detect the viral genome, which may lead to false-negative results. Therefore, RT-PCR should be repeated in subsequent samples [49, 66]. To minimize false-negative results, proper sampling, collection, storage or transportation, and a proper RT-PCR technique have to be implemented to avoid cross-contamination [49, 54]. Quantitative RT-PCR has been developed and could possibly be used to monitor the viral load since data suggest high viremia might be associated with unfavorable outcomes and death [21, 46]. For those patients receiving experimental treatments, EBOV viral load monitoring could be useful to assess treatment response [48].
The WHO recommends that specimens tested by RT-PCR outside of the designated EBOV diagnostic laboratories should be sent to a WHO Collaborating Center for confirmatory testing. These collaborating centers include the Institut Pasteur de Lyon (France) and the Bernhard-Nocht Institute for Tropical Medicine (Germany), National Microbiology Laboratory Public Health Agency of Canada (Canada), Centre International de Recherches Médicales de Franceville (Gabon), Kenya Medical Research Institute (Kenya), Institut Pasteur de Dakar (Senegal), National Institute for Communicable Diseases (South Africa), Uganda Virus Research Institute (Uganda) and the Centers for Disease Control and Prevention (USA) [58].
Portable PCR techniques are currently under development and featured to be readily deployed in the field for rapid diagnosis (10–30 min). These techniques are anticipated to have minimum biosafety requirements and do not require laboratory infrastructure [67]. Portable PCR techniques can play a more effective role in disease surveillance and control including Ebola outbreaks and other infectious diseases [68].
Other Diagnostic Approaches
Next-generation sequencing (NGS) techniques provide powerful methods that allow screening for a wide number of pathogens and provide complete genome data. NGS methods may be useful to detect Ebola virus in situations where the clinical index of suspicion is not high or where there is low urgency for diagnostic information. However, once an Ebola outbreak is suspected, faster methods such as specific real-time RT-PCR protocols are preferred for screening of suspected patients. In this setting, NGS techniques are valuable tools for full-length genomic analysis, identification of viral variants and detection of possible emerging viral mutations.
The development of rapid point-of-care diagnostic tests has accelerated in the face of the West Africa outbreak. These tests may provide viable options for Ebola diagnosis particularly in field settings. The results of rapid Ebola test evaluations suggest that assays have reached high sensitivity (100%) and specificity (90%) [69–71]. Although rapid diagnostic tests are promising tools, they are not yet used in daily practice, and real-time RT-PCR remains the gold standard for EVD diagnosis.
Therapy
Supportive Care
The provision of clinical supportive care is now a cornerstone of EVD patient management, which includes rehydration, nutrition, analgesics and blood transfusion when appropriate, though no clear evidence proves their effectiveness [21]. A key aspect of supportive care is the maintenance of intravascular volume with oral rehydration solution (ORS) or intravenous fluids that provide appropriate electrolyte replacement. The use of antiemetics and antidiarrheal agents may also be important for patients with persistent vomiting and diarrhea [21, 49, 50]. Prophylactic antimicrobial agents with intravenous third-generation cephalosporins (e.g., ceftriaxone and cefotaxime) may be administered when secondary bacterial infections and septicemia are suspected [72]. Parasitic coinfections (e.g., malaria) can occur, and appropriate diagnosis and treatment for these diseases are recommended whenever feasible [73].
Targeted Antivirals Compounds/Drugs
The high case-fatality rate associated with advanced EVD highlights the need for therapeutic agents that reduce, inhibit or eliminate EBOV from infected tissues and organs. An available effective treatment would be necessary for outbreak management in order to improve the prognosis of infected patients as well as to reduce the viral load and therefore the risk of new infections. Among experimental antiviral treatments, several potential therapeutic agents have shown promise (Table 1), and their mechanisms of action are different [6].
Table 1.
Overview of Ebola virus therapeutics in development
| Agent | Manufacturer | Stage of evaluation | ClinicalTrials.gov identifier number |
|---|---|---|---|
| TKM-Ebola [74] | Tekmira (Burnaby, British Columbia, Canada) |
Phase I ongoing Phase I terminated |
|
| T-705 (Favipiravir) [93, 150, 151] | Institut National de la Santé Et de la Recherche Médicale, France | Phase II ongoing | NCT02329054 |
| CMX001 (Brincidofovir) [98] | Chimerix (Durham, NC) | Phase II (withdrawn prior recruitment) | NCT02271347 |
| JK-05 [152] |
Sihuan Pharmaceutical Holdings Group Ltd and Academy of Military Medical Sciences (Beijing, China) |
Animal studies completed; now considered for use in emergency situations for army only | N/A |
| BCX4430 [95] | BioCryst Pharmaceuticals Inc., Durham, NC | Phase I ongoing | NCT02319772 |
| AVI-6002 [80, 82] | Sarepta Therapeutics (Cambridge, MA) | Phase I completed | NCT01353027 |
| Anti-Ebola hyperimmune globulin [140, 153, 154] | None identified | Animal studies completed | N/A |
| ZMapp [132, 135, 155, 156] | National Institutes of Health Clinical Center (National Institute of Allergy and Infectious Diseases (NIAID) | Phase I/II ongoing | NCT02363322 |
FDA US Food and Drug Administration, N/A not applicable
Small Interfering RNA Agents
One formulation (i.e., TKM-Ebola) of small interfering RNAs (siRNAs) that target EBOV is encapsulated in lipid nanoparticles to facilitate cellular delivery. SiRNAs cause cleavage in the messenger RNAs, which subsequently prevent EBOV production of three key viral proteins. Early animal studies have demonstrated that TKM-Ebola prevents infection in animals challenged with a lethal dose of EBOV [74, 75]. TKM-Ebola was administered by intramuscular injection to two groups of macaques 30 min following receipt of a lethal dose of EBOV. One group was treated with TKM-Ebola on days 1, 3 and 5 post-exposure, and the other group was treated post-exposure every day for 6 consecutive days. The first regimen provided 66% protection, and the second gave 100% protection [74]. Although the drug was tested on quite a few patients with EVD in Europe and the US with most of them surviving the disease, but because these patients received other experimental therapies including hyperimmunoglobulin serum and proper supportive care in medically advanced facilities, clear evidence of effectiveness and safety in humans is lacking [76]. In 2014, TKM-Ebola entered phase I clinical trials to evaluate the safety and pharmacokinetics among volunteer participants. However, clinical manifestations of inflammatory mediator (cytokine) appeared in participants who were treated with TKM-Ebola [77, 78]. Given the observed adverse events, the US Food and Drug Administration (FDA) placed a partial clinical hold on the trial. Since dose modifications were introduced in the TKM-Ebola trial, the FDA has allowed continuation of the study for patients with EVD. Currently, one TKM-Ebola phase I trial is active and being undertaken in San Antonio, Texas, and another TKM-Ebola phase I trial has been terminated by Tekmira, Inc., aiming to reformulate the investigational therapeutic (Table 1). Additionally, Tekmira, Inc., started a phase II trial on TKM-Ebola in Guinea. However, on 19 June 2015, Tekmira, Inc., released a letter stating that the phase II trial closed enrollment prior to completion. Preliminary data from the incomplete phase II trial indicated no therapeutic benefit was achieved from the use of TKM-Ebola. A full report from this trial is pending [79].
Other siRNA-based agents are in development, including phosphorodiamidate morpholino oligomers [80–82]. These agents include AVI-6002 and AVI-6003, which are composed of multiple oligomers with positively charged piperazine residues located along the oligomeric backbone. In 2011, a phase I randomized, double-blind, placebo-controlled, single-dose, dose-escalation trial to assess the safety, tolerability and pharmacokinetics of AVI-6002 in healthy adult subjects was completed (ClinicalTrials.gov Identifier: NCT01353027). In a similar trial, the same group of investigators evaluated the use of AVI-6003 against Marburg virus as a post-exposure therapy (ClinicalTrials.gov Identifier: NCT01353040). In these trials, both AVI-6002 and AVI-6003 were well tolerated by healthy human volunteers [80].
Favipiravir (T-705)
Favipirarvir is a nucleotide analog and viral RNA polymerase inhibitor with a wide range of antiviral effects against numerous negative- or positive-strand RNA viruses [83–88]. Initially, favipiravir was developed to treat influenza viruses, and a phase III clinical trial was completed in which favipiravir was tested on several thousands of people and proven to be safe and effective [84]. Recently, favipiravir has also shown efficacy against EBOV in vitro and in vivo in a mouse model [89]. Two independent animal studies have demonstrated that treatment with favipiravir resulted in rapid viral clearance and led to survival of all animals infected with EBOV through intranasal inoculation [89, 90]. A phase II clinical trial to evaluate the efficacy of favipiravir against EBOV in 225 patients with an EVD trial has been completed in Guinea [91, 92]. The investigators of this trial are in the midst of data analysis with results forthcoming in the near future. The French drug safety agency approved compassionate use of favipiravir in patients with EVD [93], and the drug was used to treat a French nurse who contracted EBOV while a volunteer with Médecins Sans Frontières (MSF) in Liberia [94].
BCX4430
BCX4430 is a novel adenosine analog that inhibits viral RNA polymerase activity, which results in termination of RNA synthesis. This compound has shown promising results and confers protection to EBOV-challenged mice and monkeys, even when administered following challenge with filoviruses such as Marburg and Ravn viruses [95]. Furthermore, BCX4430 has demonstrated broad-spectrum antiviral activity against many viruses, including bunyaviruses, arenaviruses, paramyxoviruses, coronaviruses and flaviviruses [95]. From a safety perspective, it is worth noting that BCX4430 may have an acceptable side effect profile as it does not incorporate into human RNA or DNA. In vitro activity against EBOV has been shown for BCX4430, but no data in humans have been obtained to date. Currently, the timing of treatment for drugs such as BCX4430 has not been established, although early treatment in high-risk or potentially EBOV-exposed individuals may be an option [96]. Oral administration of BCX4430 may be feasible, although the pharmacokinetic data suggest that the intramuscular route may provide more favorable therapeutic levels [95].
Brincidofovir (CMX001)
Brincidofovir is a prodrug of cidofovir and a fairly recent oral nucleotide analog that prevents viral replication by inhibiting DNA polymerase [97]. Brincidofovir has shown broad- spectrum antiviral activity against DNA viruses such as herpes viruses and adenovirus and is currently in a phase III clinical trial against cytomegalovirus and adenovirus [98, 99]. Although the exact mechanism of action for brincidofovir in EVD is not yet well understood, brincidofovir may interfere with RNA polymerase of EBOV. The US FDA has put brincidofovir on fast-track approval for treatment of EVD based on in vitro data alone [99]. A phase II open-label multicenter study to assess the safety and efficacy of brincidofovir against EBOV in humans has been withdrawn prior recruitment (ClinicalTrials.gov Identifier: NCT02271347). As a result of the dramatic decline in the number of new cases in Liberia in the month of January 2015 where the trial was first initiated, Chimerix, Inc., decided to discontinue the trial with no further discussions [100].
Other Small Molecules and Compounds
There are a number of known agents and newly identified compounds that have shown anti-EBOV activity. For example, Compound 7 is a benzodiazepine derivative that also prevents EBOV entry into the host cells [101]. Preliminary analysis suggests that Compound 7 binds near a hydrophobic pocket near the EBOV GP1 and GP2 interface. Analysis of this compound suggests that it may have activity against several filoviruses including EBOV [101]. No animal or human trials have been reported to date.
NSC 62914 is a small molecule, which has an antioxidant property, and was found to inhibit many viruses, including EBOV, Lassa virus, Venezuelan equine encephalitis virus and Rift Valley fever virus [102]. This compound, a reactive oxygen species, has shown in vitro activity against EBOV as well as some evidence in the EBOV mouse model for protection against lethal EBOV infection at a treatment dose of 2 mg/kg/injection (higher doses did not improve survival in the mouse model). Further studies of this or related compounds in mouse and other animal models may be warranted to elucidate their role in the treatment of EBOV and other filovirus infections.
Of additional interest are compounds that have been proven to be effective in preventing the entry of filoviruses, including EBOV, into host cells [103]. LJ-001 binds to lipid membranes and prevents virus-cell fusion across a wide range of viruses. Additional research will be needed to understand how such compounds can be optimally formulated to maximize both the safety and pharmacologic activity in vivo. FGI-103, FGI-104, and FGI-106 are a group of broad-spectrum antiviral agents that inhibit viral replication in a dose-dependent manner among multiple and genetically distinct viruses including EBOV, bunyaviruses, dengue virus, HIV and hepatitis C virus [104]. Using a mouse model, Aman et al. found that FGI-106 yields a protection after a challenge with a lethal dose of EBOV. Aman and colleagues showed that protection was also found when FGI-106 was administered in a prophylactic fashion. In related studies, FGI-103 and FGI-104 are also small molecules that inhibit filovirus infection and are found to protect EBOV- or Marburg-infected mice, although their mechanism of actions are unclear [105, 106].
In 2011, a novel finding by Carette et al. showed that EBOV cell entry requires the endo/lysosomal Niemann-Pick C1 cholesterol transporter protein (NPC1) [107]. NPC1 is a fundamental component to facilitate EBOV glycoprotein host membrane fusion. Cells that are defective in the NPC1 are resistant to infection by EBOV. In addition, inhibition of NPC1 can also disrupt the release of EBOV from the intracellular vesicular compartment. Benzylpiperazine adamantane diamides are NPC1 inhibitors that have been found to interfere with EBOV binding to NPC1 on the host cells and subsequently inhibit EBOV entry and infection [108]. Clomiphene and toremifene, which are selective estrogen receptor modulators, have recently been discovered to have NPC1 inhibitor activity and act as potential inhibitors of EBOV [109, 110]. Some anti-arrhythmic therapeutics such as amiodarone and verapamil also have been identified as potent NPC1 inhibitors of the entry of the EBOV [109, 111]. Their effectiveness has been proven in cell culture, and some trial work on amiodarone is under preparation now in West Africa [112].
Immunotherapy
Convalescent Whole Blood and Plasma
Historically, convalescent whole blood (CWB) and plasma (sometimes referred to as convalescent sera) and hyperimmune serum (HS) have been employed for diseases that may be severe or result in serious consequences and for which there is no safe and effective drug or vaccine. In 1995, passive immunotherapy or convalescent immune plasma was used clinically to treat patients with EVD in the outbreak of Kikwit, DRC [113]. In this study, eight patients were given the blood of convalescent patients where 7/8 (87.5%) of patients survived the disease. To date, however, there are no definitive human clinical trial data showing that CWB or CP are effective in reducing either the severity or duration of EVD [114]. In animal studies reported in 2007, Jahrling et al. used whole blood from non-human primates (NHPs) surviving EVOV infection to treat other animals, which challenged with lethal dose of EBOV [115]. Researchers in this study concluded that there was no beneficial effect following the administration of convalescent-phase whole blood to EBOV-infected NHPs.
In contrast, Dye and colleagues used multiple doses of concentrated polyclonal IgG antibody from NHPs that had survived filovirus infection to treat Marburg virus-infected NHPs [116]. Results of this study demonstrated very effective post-exposure IgG treatment where none of the experimental NHPs showed signs of the disease or detectable viremia. Furthermore, surviving NHPs were re-challenged with Marburg virus, and all survived the re-challenge, indicating that they were able to produce Marburg virus-specific IgM antibodies to fight virus replication. Subsequent evaluations were conducted to demonstrate Marburg virus or EBOV-infected NHPs could survive the disease even if IgG treatment was delayed 2 days post infection. Successfully, both Marburg- and EBOV-infected animals survived the disease, though one out of three infected animals showed mild disease yet fully recovered afterwards. Promising results of these studies demonstrate the effectiveness of post-exposure antibody treatments and represent an option for EVD therapies for human use.
In the current West Africa Ebola outbreak, convalescent-phase plasma administration for treatment of EVD is undergoing an open label, phase II/III nonrandomized trial among 130–200 patients in Guinea [117]. For use of CP or CWB, the WHO has provided guidance to improve the safety of product development as well as safety for patients who receive these products [118–120]. Convalescent sera-based therapy may cause some toxicity related problems, such as transmission of undetected pathogen(s) and transfusion reactions. A recent case report from treating a Spanish nurse who had contracted EBOV during her care to a patient with EVD in Spain shed some light on the issue of using experimental therapeutics including CP [121]. The infected nurse had received convalescent sera from two survivors of EVD, high-dose favipiravir and other supportive care treatment. On the 10th day of clinical disease, the nurse developed clinical signs and symptoms suggestive of post-transfusion acute lung injury, which was managed conservatively without the need of supportive mechanical ventilation. Although purified IgG can lower these risks, lot-to-lot variation remains a potential problem. Previous experience also highlights the risk of antibody-dependent enhancement of EBOV infection [122]. To address challenges with convalescent sera-based therapies, researchers have developed highly purified polyclonal antibodies or monoclonal antibodies that specifically target neutralizing glycoprotein epitopes of the EBOV envelope [123]. The long-term prospects for use of CP also require clinical laboratory infrastructure for the collection and testing of CWB or CP from recovered Ebola patients in order to ensure administration of safe blood products in the context of an EVD outbreak.
Monoclonal Antibodies (ZMapp)
Natural infection with EBOV induces antibodies directed against the EBOV envelope transmembrane glycoprotein, which is essential to virus attachment, fusion and entry into host cells. In the past several years, a number of studies have developed and characterized monoclonal antibodies directed against epitopes of EBOV antigens. Within the genus Ebolavirus, there are five antigenically distinct viruses that generate antibodies that may or may not cross-react with other Ebola species. In 2015, Hernandez and colleagues used a panel of mouse monoclonal antibodies to extensively study cross-reactivity, avidity and viral GP epitope binding [124]. This research team identified four monoclonal cross-reactive antibodies that bind to GPs of five Ebolavirus species. These findings suggest that monoclonal antibodies directed against the five species of Ebolavirus may be useful for both clinical diagnosis and therapy.
In earlier efforts, investigators developed a cocktail of antibodies from two groupings (MB-003 and ZMab), each containing three unique monoclonal antibodies. MB-003 was composed of three humanized chimeric monoclonal antibodies (c13C6, c6D8 and h13F6) [123], while ZMAb was composed of three other different monoclonal antibodies (m1H3, m2G4 and m4G7) [125]. By direct and specific reaction with EBOV GP, these antibodies are thought to provide passive immunity against the virus by directly reacting with the EBOV envelope glycoprotein [126–128]. In one study, ZMAb demonstrated 100% efficacy in NHPs where four of four cynomolgus macaques survived EBOV infection when given the first dose of ZMAb 24-h after exposure followed by two additional doses 3 days apart. However, when the first dose was delayed and given at 48 h after a lethal dose of EBOV exposure, 50% of the monkeys fully recovered [129]. A potent humoral and cell-mediated immune response was mounted in all animals recovered from the lethal challenge of EBOV, and all survived monkeys fully recovered from a subsequent EBOV reintroduction [130]. Recent research showed that treatment with ZMAb can be delayed as late as 72 h post EBOV exposure when ZMAb is given in combination with adenovirus vectored human interferon-α [131]. In 2012, another group of researchers demonstrated a high protection rate against EBOV infection among rhesus macaques when MB-003 monoclonal antibodies were first administered at 48 h post-exposure followed by two additional doses [125]. In this study, MB-003 monoclonal antibodies protected four of six animals (67%) against EBOV infection.
In an effort to optimize the monoclonal antibody cocktail composition to contain the most potent epitopes for neutralizing antibodies that may effectively inhibit EBOV replication and further prolong the post-exposure treatment window, a group of researchers conducted an animal study to test different combinations of monoclonal antibodies from MB-003 and ZMAb [132]. After several animal experimental trials, investigators of this study selected ZMapp as the best monoclonal antibody formulation with the best protection effect. ZMapp was composed of one monoclonal antibody from MB-003 (c13C6) and two monoclonal antibodies from ZMAb (2G4 and 4G7). With this selected combination of monoclonal antibodies, rhesus macaques were treated with ZMapp at 5, 8 and 11 days after challenge with EBOV at a lethal dose of 50 mg/kg per dose. Although animals of this study exhibited signs and symptoms of EVD and viral load was detected at 5 days post-challenge before treatment with ZMapp was initiated, all animals survived. A follow-up at 3 weeks post EBOV-exposure demonstrated an undetectable viral load in all animals [132].
This is strong evidence that ZMapp could be a potential therapeutic modality in humans even when signs and symptoms of EVD have manifested. In July 2014, two US healthcare workers were brought from Liberia to the USA after being diagnosed with EVD and were treated with aggressive fluid replacement and electrolyte correction. Both were given ZMapp and showed a decline in the level of Ebola plasma viral load in correlation with clinical improvement. Although they improved shortly after receiving ZMapp, their clinical course cannot be exclusively attributed to the monoclonal antibodies, as other measures of care could have and probably did play a major role to the clinical improvement [133].
Clinical use of ZMapp maybe practically challenging given the limited supplies of the drug since the three monoclonal antibodies of ZMapp are currently extracted from the plant Nicotiana benthamiana [132]. A new scalable transient expression technology (magnifection) could overcome future ZMapp shortages. Using magnifection, 0.5 g of ZMapp can be extracted and purified from 1 kg N. benthamiana leaf biomass [134]. While this product holds promise, a major hurdle in its future utility is to manufacture large quantities of each monoclonal antibody from plants in a way that ensures sustained high yield of monoclonal antibodies at reasonable cost [135]. To overcome potential rate-limiting steps in large-scale production, ZMapp can be manufactured using Chinese hamster ovary (CHO) cells.
In February 2015, a partnership between Liberia and the US National Institute of Allergy and Infectious Diseases (NIAID) initiated clinical trials to evaluate the safety and efficacy of ZMapp in the treatment of EVD. The original study is designed to have two-arm comparison with each arm including 100 people; one arm will receive the standard of care and the other arm the experimental ZMapp treatment [136]. While randomized controlled trials are planned, with declining EVD case loads, a modification of the original trial designs may be required to evaluate the safety and efficacy of ZMapp.
Nonspecific Agents
Many potential therapeutic agents have shown some post-exposure efficacy in alleviating symptoms of EVD with direct antiviral action. Despite the high safety profile compared with other newly discovered antivirals, these agents cannot be used alone because of their low efficacy, but they can be used in combination with other treatments available including supportive care measures.
Interferon: Since its discovery in 1950, interferon has not been widely used because of the associated adverse events [123]. The potential use of interferons in the treatment of EVD is rooted in the evidence that EBOV interferes with functions of type I interferons [137–139]. Previous pre-clinical research showed that exogenous interferon-α or interferon-β could delay the occurrence of viremia or prolong survival time, but could not save animals from death [140, 141].
Recombinant Nematode Anticoagulant Protein c2 (rNAPc2) and Recombinant Human Activated Protein c (rhAPC): EBOV infections cause coagulation diathesis as one of the important pathogenesis factors for the development of EVD [142]. The two originally licensed anticoagulant drugs available are rNAPc2 and rhAPC. These drugs have demonstrated in previous studies partial protection of NHPs from ZEBOV lethal challenge, with associated survival rates of 33% and 18%, respectively [143, 144]. In December 2014, the US FDA granted the manufacturer (ARCA Biopharma, Westminster, CO) orphan drug status for rNAPc2 as a potential post-exposure treatment for EVD [145].
Ebola Vaccine Candidates
Ebola vaccine development was initiated in the 1980s. A number of vaccine candidates have been tested in rodents and NHPs, including inactivated virus, DNA vaccines, virus-like particles (VLPs) and vaccines based on recombinant viral vectors [146]. Animal models have been developed to test the efficacy of vaccine candidates, including mice, guinea pigs and other NHPs. Rodent models do not always predict vaccine efficacy in NHPs and employ EBOV-adapted strains. NHPs (usually rhesus and cynomolgus macaque) can be infected with non-adapted strains and best mimic disease progression in humans, and therefore they are considered the “reference” animal model for vaccine studies [147]. Differences can also be found in the EBOV antigens included in the vaccines. While the main antigen for vaccine development has been the EBOV surface GP, some candidates such as the VLPs-based vaccine have also included the NP and the VP40 matrix protein. A novel approach includes a replication-deficient recombinant EBOV lacking the gene encoding for VP30, an essential transcription factor that plays a fundamental role in viral replication [148], and has been recently shown to protect NHPs against EBOV [149]. This vaccine differs from other EBOV advanced vaccine platforms in that all viral proteins and the viral RNA are presented to the immune system, which might contribute to protective immune responses.
With the global impact of the West Africa EVD outbreak, research and development for new Ebola vaccine candidates has been stimulated, though no licensed vaccine is currently available. In the past year, investment in vaccine candidates has led to initiation of phase I, II and III human clinical trials (Table 2). More advanced vaccine candidates are based on viral vectors such as adenoviruses and vesicular stomatitis virus modified to express the EVOB surface GP.
Table 2.
Overview of EVD developing vaccines
| Vaccine candidate names | Sponsor or manufacturer | Location | Stage of evaluation | ClinicalTrials.gov identifier number |
|---|---|---|---|---|
|
VRC-EBODNA023-00-VP [157] (Ebola DNA Plasmid Vaccine) VRC-MARDNA025-00-VP [157] (Marburg DNA Plasmid Vaccine) |
National Institute of Allergy and Infectious Diseases (NIAID) | Uganda | Phase IB completed | NCT00997607 |
|
VRC-EBODNA023-00-VP [158] (Ebola DNA Plasmid Vaccine) VRC-MARDNA025-00-VP [158] (Marburg DNA Plasmid Vaccine) |
NIAID | US | Phase I completed | NCT00605514 |
| Ad5-EBOV (Ebola Adenovirus Vector Vaccine) | Jiangsu Province CDC with Beijing Institute of Biotechnology and Tianjin Cansino Biotechnology, Inc. | China | Phase I ongoing | NCT02326194 |
| rVSVΔG-ZEBOV (recombinant vesicular stomatitis virus expressing the envelope glycoprotein of Ebola virus Zaire) | US Centers for Disease Control and Prevention (CDC) | Sierra Leone | Phase II/III ongoing | NCT02378753 |
| cAd3-EBOZ with MVA-BN® FILO (Prime-Boost regimen) | University of Maryland with Wellcome Trust, NIAID and Leidos, Inc. | Mali | Phase IB ongoing | NCT02267109 |
| (Bivalent) VRC-EBOAdc069-00-vp (cAd3-EBO) | University of Maryland | Mali | Phase I ongoing | NCT02368119 |
|
VRC-EBOADC069-00-VP (cAd3-EBO), Ebola Chimpanzee Adenovirus Vector Vaccine VRC-EBOADC076-00-VP (cAd3-EBOZ), Ebola Chimpanzee Adenovirus Vector Vaccines |
NIAID | US | Phase I ongoing | NCT02231866 |
| VRC-EBOMVA079-00-VP (MVA-EbolaZ) (Ebola Modified Vaccinia Virus Ankara Vaccine) with and without boost to VRC-EBOADC069-00-VP (cAd3-EBO) | NIAID | US | Phase I/IB ongoing | NCT02408913 |
|
VRC-EBOADC069-00-VP (cAd3-EBO) (Ebola Chimpanzee Adenovirus Vector Vaccine) VRC-EBOADC076-00-VP (cAd3-EBOZ), Ebola Chimpanzee Adenovirus Vector Vaccines |
NIAID | Uganda | Phase IB ongoing | NCT02354404 |
| rVSVΔ-ZEBOV GP (BPSC1001) | Universitätsklinikum Hamburg-Eppendorf | Germany | Phase I ongoing | NCT02283099 |
| rVSVΔ-ZEBOV GP (BPSC1001) | Dalhousie University, Canadian Institutes of Health Research, NewLink Genetics Corp. | Canada | Phase I ongoing | NCT02374385 |
| Monovalent Zaire Ebola Viral Vector Candidate Vaccine (cAd3-EBO Z) with MVA-BN® Filo (Prime-boost regimen) | University of Oxford | UK | Phase IA ongoing | NCT02240875 |
| MVA-EBO Z alone and cAd3-EBO Z with MVA-EBOZ (prime-boost regimen) | University of Oxford | UK | Phase I ongoing | NCT02451891 |
| EBOV GP Vaccine (Ebola Virus Glycoprotein) with or without Matrix-M® adjuvant | Novavax | Australia | Phase I ongoing | NCT02370589 |
| VRC-EBOADV018-00-VP (Recombinant Ebola Adenoviral Vector Vaccine) | NIAID | US | Phase I completed | NCT00374309 |
| VSVG-ZEBOV (Vesicular stomatitis virus-Ebola Zaire) and ChAd3-EBO Z (chimpanzee adenovirus 3-Ebola Zaire) | NIAID | Liberia | Phase II ongoing | NCT02344407 |
| VSV-ZEBOV | University of Oxford, WHO, Wellcome Trust and others | Kenya | Phase I ongoing | NCT02296983 |
| Ad5-EBOV (Adenovirus type 5 vector-based EBOV) | First Affiliated Hospital of Zhejiang University, Beijing Institute of Bioengineering, Academy of Military Medical Sciences, Tianjin Cansino Biotechnology Inc. | China | Phase I ongoing | NCT02401373 |
| BPSC-1001 (VSV ΔG-ZEBOV) (recombinant vesicular stomatitis virus expressing envelope glycoprotein of Ebola virus Zaire | NewLink Genetics Corp. | US | Phase I ongoing | NCT02314923, NCT02269423, NCT02280408a |
| MVA-BN-Filo and Ad26.ZEBOV (human adenovirus serotype 26 expressing the Ebola virus Mayinga variant glycoprotein) | Crucell Holland BV |
UK Ghana/Kenya Tanzania/Uganda US |
Phase I ongoing | NCT02313077, NCT02376426, NCT02376400, NCT02325050 |
| MVA-BN-Filo and Ad26.ZEBOV | Crucell Holland BV | UK | Phase II ongoing | NCT02416453 |
| BPSC-1001 (VSV G-ZEBOV) | University Hospital, Geneva, WHO, Wellcome Trust and others | Switzerland | Phase I/II ongoing | NCT02287480 |
| cAd3-EBOZ (Monovalent Zaire Ebola Chimpanzee Adenovirus Vector Candidate Vaccine) | University of Lausanne Hospitals | Switzerland | Phase I/II ongoing | NCT02289027 |
| VRC-EBODNA012-00-VP (Multiple Strain Ebola DNA Plasmid Vaccine) | NIAID | US | Phase I completed | NCT00072605 |
| EBOVΔVP30 (EBOV) vaccine based on a replication-defective EBOV |
Regional Center of Excellence and by Health and Labour Sciences Research Grants, Japan NIAID |
Japan and US | Animal study completed | [149] |
aTrial evaluating BPSC-1001 (VSV ΔG-ZEBOV in a prime-boost regimen)
Future Directions
Evidence identified through the cumulative experience across multiple EVD outbreaks since the 1970s strongly suggests that EVD can be considered a zoonotic disease that has emerged and spread as human contact with wild animal species has increased. Additional ecological, epidemiologic and clinical disease surveillance will continue to be important throughout Ebola-endemic countries and to investigate possible triggering factors and predictors of new future outbreaks. In 2014 and 2015, the rapid evolution of the West Africa outbreak highlighted the need for additional research into systems and technologies that accelerate local, national and international health organization responsiveness to containment of EBOV transmission and epidemics. Multi-disciplinary team-based research will be extremely important particularly given the socio-cultural factors that have fueled and sustained EVD outbreaks. While new diagnostic tests look promising in providing more timely and accurate EBOV detection, there will be a need for additional research to study optimal strategies for deploying these diagnostics to locations where testing is most needed. In the near future, there is a substantial likelihood that one or more drugs and vaccines will be approved for use in the treatment and prevention of EVD. The availability of new agents for prevention, acute therapy and post-exposure prophylaxis will require additional research to identify and reduce barriers to their access, distribution and administration.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
No funding or sponsorship was received for this study or publication of this article. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole and have given final approval to the version to be published.
Conflict of interest
Miguel Martínez, Abdulbaset Salim, Juan Hurtado and Paul Kilgore declare that they have no conflict of interest.
Compliance with ethics guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
References
- 1.Chowell G, Nishiura H. Characterizing the transmission dynamics and control of Ebola virus disease. PLoS Biol. 2015;13(1):e1002057. doi: 10.1371/journal.pbio.1002057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Ebola (Ebola virus disease), outbreaks chronology: Ebola virus disease. 2015. http://www.cdc.gov/vhf/ebola/outbreaks/history/chronology.html. Accessed 07 May 2015.
- 3.Hewlett BS, Amola RP. Cultural contexts of Ebola in northern Uganda. Emerg Infect Dis. 2003;9(10):1242. doi: 10.3201/eid0910.020493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pandey A, et al. Strategies for containing Ebola in West Africa. Science. 2014;346(6212):991–995. doi: 10.1126/science.1260612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kilgore PE, et al. Treatment of Ebola virus disease. Pharmacother J Hum Pharmacol Drug Ther. 2015;35(1):43–53. doi: 10.1002/phar.1545. [DOI] [PubMed] [Google Scholar]
- 6.Yazdanpanah Y, Arribas JR, Malvy D. Treatment of Ebola virus disease. Intensive Care Med. 2015;41(1):115–117. doi: 10.1007/s00134-014-3529-8. [DOI] [PubMed] [Google Scholar]
- 7.Weik M, et al. The Ebola virus genomic replication promoter is bipartite and follows the rule of six. J Virol. 2005;79(16):10660–10671. doi: 10.1128/JVI.79.16.10660-10671.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fauquet CM, et al. Virus taxonomy: VIIIth report of the International Committee on Taxonomy of Viruses. Academic Press; 2005.
- 9.Kuhn JH, et al. Proposal for a revised taxonomy of the family Filoviridae: classification, names of taxa and viruses, and virus abbreviations. Arch Virol. 2010;155(12):2083–2103. doi: 10.1007/s00705-010-0814-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuhn JH, et al. Virus nomenclature below the species level: a standardized nomenclature for laboratory animal-adapted strains and variants of viruses assigned to the family Filoviridae. Arch Virol. 2013;158(6):1425–1432. doi: 10.1007/s00705-012-1594-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuhn JH, et al. Virus nomenclature below the species level: a standardized nomenclature for filovirus strains and variants rescued from cDNA. Arch Virol. 2014;159(5):1229–1237. doi: 10.1007/s00705-013-1877-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Negredo A, et al. Discovery of an Ebolavirus-like filovirus in Europe. PLoS Pathog. 2011;7(10):e1002304. doi: 10.1371/journal.ppat.1002304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baize S, et al. Emergence of Zaire Ebola virus disease in Guinea. N Engl J Med. 2014;371(15):1418–1425. doi: 10.1056/NEJMoa1404505. [DOI] [PubMed] [Google Scholar]
- 14.Feldmann H, Klenk HD, Sanchez A. Molecular biology and evolution of filoviruses. Arch Virol Suppl. 1993;7:81–100. doi: 10.1007/978-3-7091-9300-6_8. [DOI] [PubMed] [Google Scholar]
- 15.Sanchez A. Ebola viruses. 2003. Available from: http://onlinelibrary.wiley.com/doi/10.1038/npg.els.0001019/full. Accessed 23 July 2015.
- 16.Gire SK, et al. Genomic surveillance elucidates Ebola virus origin and transmission during the 2014 outbreak. Science. 2014;345(6202):1369–1372. doi: 10.1126/science.1259657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haeussler M, et al. The UCSC Ebola genome portal. PLoS Curr. 2014;6. doi:10.1371/currents.outbreaks.386ab0964ab4d6c8cb550bfb6071d822. [DOI] [PMC free article] [PubMed]
- 18.Bahcall O. Ebola genomes track virus evolution in real-time epidemic. Nat Genet. 2014;46(10):1050-1050. doi: 10.1038/ng.3108. [DOI] [Google Scholar]
- 19.Becquart P, et al. Identification of continuous human B-cell epitopes in the VP35, VP40, nucleoprotein and glycoprotein of Ebola virus. PLoS One. 2014;9(6):e96360. doi: 10.1371/journal.pone.0096360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watt A, et al. A novel life cycle modeling system for Ebola virus shows a genome length-dependent role of VP24 in virus infectivity. J Virol. 2014;88(18):10511–10524. doi: 10.1128/JVI.01272-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schieffelin JS, et al. Clinical illness and outcomes in patients with Ebola in Sierra Leone. N Engl J Med. 2014;371(22):2092–2100. doi: 10.1056/NEJMoa1411680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kugelman JR, et al. Evaluation of the potential impact of Ebola virus genomic drift on the efficacy of sequence-based candidate therapeutics. MBio. 2015;6(1):e2227–14. doi: 10.1128/mBio.02227-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoenen T, et al. Virology. Mutation rate and genotype variation of Ebola virus from Mali case sequences. Science. 2015;348(6230):117–119. doi: 10.1126/science.aaa5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. Communicable Disease Surveillance and Response-Global Alert and Response (GAR). 2015. http://www.who.int/csr/don/en/. Accessed 15 June 2015.
- 25.Centers for Disease Control and Prevention. Ebola (Ebola virus disease): Ebola outbreaks 2000–2014. 2015. http://www.cdc.gov/vhf/ebola/outbreaks/history/summaries.html. Accessed 08 June 2015.
- 26.Centers for Disease Control and Prevention. Ebola (Ebola virus disease), 2014 Ebola outbreak in West Africa—case counts. 2015. http://www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/case-counts.html. Accessed 17 June 2015.
- 27.World Health Organization. Ebola situation report—8 July 2015. 2015. http://apps.who.int/ebola/current-situation/ebola-situation-report-8-july-2015. Accessed 21 July 2015.
- 28.Towner JS, et al. Isolation of genetically diverse Marburg viruses from Egyptian fruit bats. PLoS Pathog. 2009;5(7):e1000536. doi: 10.1371/journal.ppat.1000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Towner JS, et al. Marburg virus infection detected in a common African bat. PLoS One. 2007;2(8):e764. doi: 10.1371/journal.pone.0000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pourrut X, et al. Large serological survey showing cocirculation of Ebola and Marburg viruses in Gabonese bat populations, and a high seroprevalence of both viruses in Rousettus aegyptiacus. BMC Infect Dis. 2009;9:159. doi: 10.1186/1471-2334-9-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leroy EM, et al. Human Ebola outbreak resulting from direct exposure to fruit bats in Luebo, Democratic Republic of Congo, 2007. Vector Borne Zoonotic Dis. 2009;9(6):723–728. doi: 10.1089/vbz.2008.0167. [DOI] [PubMed] [Google Scholar]
- 32.Leroy EM, et al. Fruit bats as reservoirs of Ebola virus. Nature. 2005;438(7068):575–576. doi: 10.1038/438575a. [DOI] [PubMed] [Google Scholar]
- 33.Kuzmin IV, et al. Marburg virus in fruit bat, Kenya. Emerg Infect Dis. 2010;16(2):352–354. doi: 10.3201/eid1602.091269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayman DT, et al. Ebola virus antibodies in fruit bats, Ghana, West Africa. Emerg Infect Dis. 2012;18(7):1207–1209. doi: 10.3201/eid1807.111654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pourrut X, et al. Spatial and temporal patterns of Zaire ebolavirus antibody prevalence in the possible reservoir bat species. J Infect Dis. 2007;196(Suppl 2):S176–S183. doi: 10.1086/520541. [DOI] [PubMed] [Google Scholar]
- 36.Bausch DG, et al. Assessment of the risk of Ebola virus transmission from bodily fluids and fomites. J Infect Dis. 2007;196(Suppl 2):S142–S147. doi: 10.1086/520545. [DOI] [PubMed] [Google Scholar]
- 37.Kreuels B, et al. A case of severe Ebola virus infection complicated by gram-negative septicemia. N Engl J Med. 2014;371(25):2394–2401. doi: 10.1056/NEJMoa1411677. [DOI] [PubMed] [Google Scholar]
- 38.Rogstad KE, Tunbridge A. Ebola virus as a sexually transmitted infection. Curr Opin Infect Dis. 2015;28(1):83–85. doi: 10.1097/QCO.0000000000000135. [DOI] [PubMed] [Google Scholar]
- 39.Ergonul O, Battal I. Potential sexual transmission of Crimean-Congo hemorrhagic fever infection. Jpn J Infect Dis. 2014;67(2):137–138. doi: 10.7883/yoken.67.137. [DOI] [PubMed] [Google Scholar]
- 40.Moreau M, et al. Lactating mothers infected with Ebola virus: EBOV RT-PCR of blood only may be insufficient. Euro Surveill. 2015;20(3). http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=21017 [DOI] [PubMed]
- 41.Rowe AK, et al. Clinical, virologic, and immunologic follow-up of convalescent Ebola hemorrhagic fever patients and their household contacts, Kikwit, Democratic Republic of the Congo. Commission de Lutte contre les Epidemies a Kikwit. J Infect Dis. 1999;179(Suppl 1):S28–S35. doi: 10.1086/514318. [DOI] [PubMed] [Google Scholar]
- 42.Shaw M, Palese P. Fields Virology. Philadelphia, PA: Lippincott Williams & Wilkins; 2013. p. 1151–1185
- 43.Varkey JB, et al. Persistence of Ebola virus in ocular fluid during convalescence. N Engl J Med. 2015;372(25):2423–2427. [DOI] [PMC free article] [PubMed]
- 44.World Health Organization Ebola Response Team Ebola virus disease in West Africa—the first 9 months of the epidemic and forward projections. N Engl J Med. 2014;371(16):1481–1495. doi: 10.1056/NEJMoa1411100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Breman JG, Johnson KM. Ebola then and now. N Engl J Med. 2014;371(18):1663–1666. doi: 10.1056/NEJMp1410540. [DOI] [PubMed] [Google Scholar]
- 46.Bah EI, et al. Clinical presentation of patients with Ebola virus disease in Conakry, Guinea. N Engl J Med. 2015;372(1):40–47. doi: 10.1056/NEJMoa1411249. [DOI] [PubMed] [Google Scholar]
- 47.Tattevin P, Durante-Mangoni E, Massaquoi M. Does this patient have Ebola virus disease? Intensive Care Med. 2014;40(11):1738–1741. doi: 10.1007/s00134-014-3473-7. [DOI] [PubMed] [Google Scholar]
- 48.Kortepeter MG, Bausch DG, Bray M. Basic clinical and laboratory features of filoviral hemorrhagic fever. J Infect Dis. 2011;204(Suppl 3):S810–S816. doi: 10.1093/infdis/jir299. [DOI] [PubMed] [Google Scholar]
- 49.Chertow DS, et al. Ebola virus disease in West Africa—clinical manifestations and management. N Engl J Med. 2014;371(22):2054–2057. doi: 10.1056/NEJMp1413084. [DOI] [PubMed] [Google Scholar]
- 50.Fowler RA, et al. Caring for critically ill patients with Ebola virus disease. Perspectives from West Africa. Am J Respir Crit Care Med. 2014;190(7):733–737. doi: 10.1164/rccm.201408-1514CP. [DOI] [PubMed] [Google Scholar]
- 51.Bwaka MA, et al. Ebola hemorrhagic fever in Kikwit, Democratic Republic of the Congo: clinical observations in 103 patients. J Infect Dis. 1999;179(Suppl 1):S1–S7. doi: 10.1086/514308. [DOI] [PubMed] [Google Scholar]
- 52.Barry M, et al. Clinical predictors of mortality in patients with Ebola virus disease. Clin Infect Dis. 2015;60(12):1821–1824. [DOI] [PubMed]
- 53.Qin E, et al. Clinical features of patients with Ebola virus disease in Sierra Leone. Clin Infect Dis. 2015;61(4):491–495. doi:10.1093/cid/civ319 [DOI] [PubMed]
- 54.Feldmann H, Geisbert TW. Ebola haemorrhagic fever. Lancet. 2011;377(9768):849–862. doi: 10.1016/S0140-6736(10)60667-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jaax NK, et al. Lethal experimental infection of rhesus monkeys with Ebola-Zaire (Mayinga) virus by the oral and conjunctival route of exposure. Arch Pathol Lab Med. 1996;120(2):140–155. [PubMed] [Google Scholar]
- 56.Rollin PE, Bausch DG, Sanchez A. Blood chemistry measurements and D-Dimer levels associated with fatal and nonfatal outcomes in humans infected with Sudan Ebola virus. J Infect Dis. 2007;196(Suppl 2):S364–S371. doi: 10.1086/520613. [DOI] [PubMed] [Google Scholar]
- 57.Centers for Disease Control and Prevention. Ebola (Ebola virus disease), guidance for collection, transport and submission of specimens for Ebola virus testing. 2015. http://www.cdc.gov/vhf/ebola/healthcare-us/laboratories/specimens.html. Accessed 14 May 2015.
- 58.World Health Organization. Laboratory diagnosis of Ebola virus disease. 2014. http://apps.who.int/iris/bitstream/10665/134009/1/WHO_EVD_GUIDANCE_LAB_14.1_eng.pdf?ua=1. Accessed 14 May 2015.
- 59.Martin P, et al. Laboratory diagnosis of Ebola virus disease. Intensive Care Med. 2015;41(5):895–898. doi: 10.1007/s00134-015-3671-y. [DOI] [PubMed] [Google Scholar]
- 60.Vogel G. Infectious diseases. Testing new Ebola tests. Science. 2014;345(6204):1549–1550. doi: 10.1126/science.345.6204.1549. [DOI] [PubMed] [Google Scholar]
- 61.Perkins MD, Kessel M. What Ebola tells us about outbreak diagnostic readiness. Nat Biotechnol. 2015;33(5):464–469. doi: 10.1038/nbt.3215. [DOI] [PubMed] [Google Scholar]
- 62.Ksiazek TG, et al. Clinical virology of Ebola hemorrhagic fever (EHF): virus, virus antigen, and IgG and IgM antibody findings among EHF patients in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179(Suppl 1):S177–S187. doi: 10.1086/514321. [DOI] [PubMed] [Google Scholar]
- 63.Leroy EM, et al. Diagnosis of Ebola haemorrhagic fever by RT-PCR in an epidemic setting. J Med Virol. 2000;60(4):463–467. doi: 10.1002/(SICI)1096-9071(200004)60:4<463::AID-JMV15>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 64.Towner JS, et al. Rapid diagnosis of Ebola hemorrhagic fever by reverse transcription-PCR in an outbreak setting and assessment of patient viral load as a predictor of outcome. J Virol. 2004;78(8):4330–4341. doi: 10.1128/JVI.78.8.4330-4341.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grolla A, et al. Laboratory diagnosis of Ebola and Marburg hemorrhagic fever. Bull Soc Pathol Exot. 2005;98(3):205–209. [PubMed] [Google Scholar]
- 66.Centers for Disease Control and Prevention. Guidance for collection, transport, and submission of specimens for Ebola virus testing in the United States. 2015. http://www.cdc.gov/vhf/ebola/pdf/ebola-lab-guidance.pdf. Accessed 27 Feb 2015.
- 67.Kumar VS, Webster M. Extreme PCR: a breakthrough innovation for outbreaks? Clin Chem. 2015;61(4):674–676. doi: 10.1373/clinchem.2014.236950. [DOI] [PubMed] [Google Scholar]
- 68.Inglis T. Developed nations must not fear sending Ebola help. Nature. 2014;514(7524):537. doi: 10.1038/514537a. [DOI] [PubMed] [Google Scholar]
- 69.Kaasik-Aaslav K, Coulombier D. The tail of the epidemic and the challenge of tracing the very last Ebola case. Euro surveill. 2015;20(12). [DOI] [PubMed]
- 70.Walker NF, et al. Evaluation of a point-of-care blood test for identification of Ebola virus disease at Ebola holding units, Western Area, Sierra Leone, January to February 2015. Euro Surveill. 2015;20(12). http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=21073. [DOI] [PubMed]
- 71.Broadhurst MJ, et al. ReEBOV Antigen Rapid Test kit for point-of-care and laboratory-based testing for Ebola virus disease: a field validation study. Lancet, 2015. doi:10.1016/S0140-6736(15)61042-X. [DOI] [PubMed]
- 72.Plachouras D, Monnet DL, Catchpole M. Severe Ebola virus infection complicated by gram-negative septicemia. N Engl J Med. 2015;372(14):1376–1377. doi: 10.1056/NEJMc1500455. [DOI] [PubMed] [Google Scholar]
- 73.O’Shea MK, Clay KA, Craig DG, Matthews SW, Kao RL, Fletcher TE, et al. Diagnosis of febrile illnesses other than Ebola virus disease at an Ebola treatment unit in Sierra Leone. Clin Infect Dis. 2015;61(5):795–8. doi:10.1093/cid/civ399. [DOI] [PMC free article] [PubMed]
- 74.Geisbert TW, et al. Postexposure protection of non-human primates against a lethal Ebola virus challenge with RNA interference: a proof-of-concept study. Lancet. 2010;375(9729):1896–1905. doi: 10.1016/S0140-6736(10)60357-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thi EP, et al. Lipid nanoparticle siRNA treatment of Ebola-virus-Makona-infected nonhuman primates. Nature. 2015;521(7552):362–365. doi: 10.1038/nature14442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Geisbert TW. Emergency treatment for exposure to Ebola virus: the need to fast-track promising vaccines. JAMA. 2015;313(12):1221–1222. doi: 10.1001/jama.2015.2057. [DOI] [PubMed] [Google Scholar]
- 77.McCarthy M. FDA allows second experimental drug to be tested in Ebola patients. BMJ. 2014;349:g5103. doi: 10.1136/bmj.g5103. [DOI] [PubMed] [Google Scholar]
- 78.Maurice J. WHO meeting chooses untried interventions to defeat Ebola. Lancet. 2014;384(9948):e45–e46. doi: 10.1016/S0140-6736(14)61411-2. [DOI] [PubMed] [Google Scholar]
- 79.Tekmira Pharmaceuticals Corporation. Tekmira provides update on TKM-Ebola-Guinea. 2015. http://investor.tekmirapharm.com/releasedetail.cfm?ReleaseID=918694. Accessed 23 July 2015.
- 80.Heald AE, et al. Safety and pharmacokinetic profiles of phosphorodiamidate morpholino oligomers with activity against Ebola virus and Marburg virus: results of two single-ascending-dose studies. Antimicrob Agents Chemother. 2014;58(11):6639–6647. doi: 10.1128/AAC.03442-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.De Clercq E. Ebola virus (EBOV) infection: therapeutic strategies. Biochem Pharmacol. 2015;93(1):1–10. doi: 10.1016/j.bcp.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Iversen PL, et al. Discovery and early development of AVI-7537 and AVI-7288 for the treatment of Ebola virus and Marburg virus infections. Viruses. 2012;4(11):2806–2830. doi: 10.3390/v4112806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Arias A, Thorne L, Goodfellow I. Favipiravir elicits antiviral mutagenesis during virus replication in vivo. Elife. 2014;3:e03679. doi: 10.7554/eLife.03679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Furuta Y, et al. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antivir Res. 2013;100(2):446–454. doi: 10.1016/j.antiviral.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gowen BB, et al. In vitro and in vivo activities of T-705 against arenavirus and bunyavirus infections. Antimicrob Agents Chemother. 2007;51(9):3168–3176. doi: 10.1128/AAC.00356-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Safronetz D, et al. Antiviral efficacy of favipiravir against two prominent etiological agents of hantavirus pulmonary syndrome. Antimicrob Agents Chemother. 2013;57(10):4673–4680. doi: 10.1128/AAC.00886-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rocha-Pereira J, et al. Favipiravir (T-705) inhibits in vitro norovirus replication. Biochem Biophys Res Commun. 2012;424(4):777–780. doi: 10.1016/j.bbrc.2012.07.034. [DOI] [PubMed] [Google Scholar]
- 88.Morrey JD, et al. Efficacy of orally administered T-705 pyrazine analog on lethal West Nile virus infection in rodents. Antivir Res. 2008;80(3):377–379. doi: 10.1016/j.antiviral.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Oestereich L, et al. Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model. Antiviral Res. 2014;105:17–21. doi: 10.1016/j.antiviral.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 90.Smither SJ, et al. Post-exposure efficacy of oral T-705 (Favipiravir) against inhalational Ebola virus infection in a mouse model. Antivir Res. 2014;104:153–155. doi: 10.1016/j.antiviral.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 91.ClinicalTrials.gov. Efficacy of Favipiravir against Ebola (JIKI). 2015. https://clinicaltrials.gov/ct2/show/NCT02329054?term=favipiravir&rank=5. Accessed 27 May 2015.
- 92.World Health Organization. International Clinical Trials Registry Platform. 2015. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=NCT02329054. Accessed 24 July 2015.
- 93.Mentre F, et al. Dose regimen of favipiravir for Ebola virus disease. Lancet Infect Dis. 2015;15(2):150–151. doi: 10.1016/S1473-3099(14)71047-3. [DOI] [PubMed] [Google Scholar]
- 94.FRANCE 24. French MSF nurse ‘cured’ of Ebola, health minister says. 2014. http://www.france24.com/en/20141004-french-nurse-cured-ebola-health-minister-msf-touraine. Accessed 09 June 2015.
- 95.Warren TK, et al. Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue BCX4430. Nature. 2014;508(7496):402–405. doi: 10.1038/nature13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Falzarano D, Feldmann H. Possible leap ahead in filovirus therapeutics. Cell Res. 2014;24(6):647–648. doi: 10.1038/cr.2014.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Price NB, Prichard MN. Progress in the development of new therapies for herpesvirus infections. Curr Opin Virol. 2011;1(6):548–554. doi: 10.1016/j.coviro.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Florescu DF, Keck MA. Development of CMX001 (Brincidofovir) for the treatment of serious diseases or conditions caused by dsDNA viruses. Expert Rev Anti Infect Ther. 2014;12(10):1171–1178. doi: 10.1586/14787210.2014.948847. [DOI] [PubMed] [Google Scholar]
- 99.Chimerix I. Brincidofovir for Ebola. 2015. http://www.chimerix.com/discovery-clinical-trials/brincidofovir/brincidofovir-for-ebola/. Accessed 03 Jan 2015.
- 100.Forbes. Chimerix ends Brincidofovir Ebola trials to focus on Adenovirus and CMV. 2015. http://www.forbes.com/sites/davidkroll/2015/01/31/chimerix-ends-brincidofovir-ebola-trials-to-focus-on-adenovirus-and-cmv/. Accessed 23 July 2015.
- 101.Basu A, et al. Identification of a small-molecule entry inhibitor for filoviruses. J Virol. 2011;85(7):3106–3119. doi: 10.1128/JVI.01456-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Panchal RG, et al. Identification of an antioxidant small-molecule with broad-spectrum antiviral activity. Antiviral Res. 2012;93(1):23–29. doi: 10.1016/j.antiviral.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 103.Wolf MC, et al. A broad-spectrum antiviral targeting entry of enveloped viruses. Proc Natl Acad Sci USA. 2010;107(7):3157–3162. doi: 10.1073/pnas.0909587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Aman MJ, et al. Development of a broad-spectrum antiviral with activity against Ebola virus. Antiviral Res. 2009;83(3):245–251. doi: 10.1016/j.antiviral.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 105.Warren TK, et al. Antiviral activity of a small-molecule inhibitor of filovirus infection. Antimicrob Agents Chemother. 2010;54(5):2152–2159. doi: 10.1128/AAC.01315-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kinch MS, et al. FGI-104: a broad-spectrum small molecule inhibitor of viral infection. Am J Transl Res. 2009;1(1):87–98. [PMC free article] [PubMed] [Google Scholar]
- 107.Carette JE, et al. Ebola virus entry requires the cholesterol transporter Niemann-Pick C1. Nature. 2011;477(7364):340–343. doi: 10.1038/nature10348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cote M, et al. Small molecule inhibitors reveal Niemann-Pick C1 is essential for Ebola virus infection. Nature. 2011;477(7364):344–348. doi: 10.1038/nature10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shoemaker CJ, et al. Multiple cationic amphiphiles induce a Niemann-Pick C phenotype and inhibit Ebola virus entry and infection. Plos one. 2013;8(2):e56265. doi:10.1371/journal.pone.0056265. [DOI] [PMC free article] [PubMed]
- 110.Johansen LM, et al. FDA-approved selective estrogen receptor modulators inhibit Ebola virus infection. Science Transl Med. 2013;5(190):190ra79-190ra79. doi: 10.1126/scitranslmed.3005471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gehring G, et al. The clinically approved drugs amiodarone, dronedarone and verapamil inhibit filovirus cell entry. J Antimicrob Chemother. 2014;69(8):2123–2131. doi: 10.1093/jac/dku091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Clinicaltrials.gov. Clinical study to assess efficacy and safety of Amiodarone in treating patients with Ebola. Virus disease (EVD) in Sierra Leone. EASE (EMERGENCY Amiodarone Study Against Ebola). 2015. https://clinicaltrials.gov/ct2/results?term=NCT02307591+&Search=Search. Accessed 13 June 2015.
- 113.Mupapa K, et al. Treatment of Ebola hemorrhagic fever with blood transfusions from convalescent patients. International Scientific and Technical Committee. J Infect Dis. 1999;179(Suppl 1):S18–S23. doi: 10.1086/514298. [DOI] [PubMed] [Google Scholar]
- 114.Colebunders RL, Cannon RO. Large-scale convalescent blood and plasma transfusion therapy for Ebola virus disease. J Infect Dis. 2015;211(8):1208–1210. doi: 10.1093/infdis/jiv043. [DOI] [PubMed] [Google Scholar]
- 115.Jahrling PB, et al. Ebola hemorrhagic fever: evaluation of passive immunotherapy in nonhuman primates. J Infect Dis. 2007;196(Suppl 2):S400–S403. doi: 10.1086/520587. [DOI] [PubMed] [Google Scholar]
- 116.Dye JM, et al. Postexposure antibody prophylaxis protects nonhuman primates from filovirus disease. Proc Natl Acad Sci USA. 2012;109(13):5034–5039. doi: 10.1073/pnas.1200409109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Clinicaltrials.gov. Emergency evaluation of convalescent plasma for Ebola viral disease (EVD) in Guinea (Ebola-Tx). 2015. https://clinicaltrials.gov/ct2/show/NCT02342171?term=NCT02342171&rank=1. Accessed 13 June 2015.
- 118.World Health Organization. Use of convalescent whole blood or plasma collected from patients recovered from Ebola virus disease for transfusion, as an emprical treatment during outbreaks. 2014. http://www.searo.who.int/entity/emerging_diseases/ebola/who_his_sds_2014.8_eng.pdf. Accessed 01 June 2015.
- 119.Gulland A. First Ebola treatment is approved by WHO. BMJ. 2014;349:g5539. doi: 10.1136/bmj.g5539. [DOI] [PubMed] [Google Scholar]
- 120.WHO Blood Regulators Network (BRN). Position paper on collection and use of convalescent plasma or serum as an element in filovirus outbreak response. Geneva: WHO; 2014.
- 121.Mora-Rillo M, et al. Acute respiratory distress syndrome after convalescent plasma use: treatment of a patient with Ebola virus disease contracted in Madrid, Spain. Lancet Respir Med. 2015. doi:10.1016/S2213-2600(15)00180-0 [DOI] [PubMed]
- 122.Takada A, et al. Antibody-dependent enhancement of Ebola virus infection. J Virol. 2003;77(13):7539–7544. doi: 10.1128/JVI.77.13.7539-7544.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li H, et al. Development of therapeutics for treatment of Ebola virus infection. Microbes Infect. 2015;17(2):109–117. doi: 10.1016/j.micinf.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 124.Hernandez H, et al. Development and characterization of broadly cross-reactive monoclonal antibodies against all known Ebolavirus species. J Infect Dis. 2015. doi:10.1093/infdis/jiv209. [DOI] [PMC free article] [PubMed]
- 125.Olinger GG, Jr, et al. Delayed treatment of Ebola virus infection with plant-derived monoclonal antibodies provides protection in rhesus macaques. Proc Natl Acad Sci USA. 2012;109(44):18030–18035. doi: 10.1073/pnas.1213709109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bishop BM. Potential and emerging treatment options for Ebola virus disease. Ann Pharmacother. 2015;49(2):196–206. doi: 10.1177/1060028014561227. [DOI] [PubMed] [Google Scholar]
- 127.Yu JS, et al. Anti-Ebola MAb 17A3 reacts with bovine and human alpha-2-macroglobulin proteins. J Virol Methods. 2010;168(1–2):248–250. doi: 10.1016/j.jviromet.2010.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Audet J, et al. Molecular characterization of the monoclonal antibodies composing ZMAb: a protective cocktail against Ebola virus. Sci Rep. 2014;4:6881. doi: 10.1038/srep06881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Qiu X, et al. Successful treatment of ebola virus-infected cynomolgus macaques with monoclonal antibodies. Sci Transl Med. 2012;4(138):138ra81. doi: 10.1126/scitranslmed.3003876. [DOI] [PubMed] [Google Scholar]
- 130.Qiu X, et al. Sustained protection against Ebola virus infection following treatment of infected nonhuman primates with ZMAb. Sci Rep. 2013;3:3365. doi: 10.1038/srep03365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Qiu X, et al. mAbs and Ad-vectored IFN-alpha therapy rescue Ebola-infected nonhuman primates when administered after the detection of viremia and symptoms. Sci Transl Med. 2013;5(207):207ra143. doi: 10.1126/scitranslmed.3006605. [DOI] [PubMed] [Google Scholar]
- 132.Qiu X, et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature. 2014;514(7520):47–53. doi: 10.1038/514016a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lyon GM, et al. Clinical care of two patients with Ebola virus disease in the United States. N Engl J Med. 2014;371(25):2402–2409. doi: 10.1056/NEJMoa1409838. [DOI] [PubMed] [Google Scholar]
- 134.Giritch A, et al. Rapid high-yield expression of full-size IgG antibodies in plants coinfected with noncompeting viral vectors. Proc Natl Acad Sci USA. 2006;103(40):14701–14706. doi: 10.1073/pnas.0606631103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang Y, et al. Fighting Ebola with ZMapp: spotlight on plant-made antibody. Sci China Life Sci. 2014;57(10):987–988. doi: 10.1007/s11427-014-4746-7. [DOI] [PubMed] [Google Scholar]
- 136.ClinicalTrials.gov. Putative investigational therapeutics in the treatment of patients with known Ebola infection. 2015. https://clinicaltrials.gov/ct2/show/NCT02363322?term=zmapp&rank=1. Accessed 27 May 2015.
- 137.Wong G, Kobinger GP, Qiu X. Characterization of host immune responses in Ebola virus infections. Expert Rev Clin Immunol. 2014;10(6):781–790. doi: 10.1586/1744666X.2014.908705. [DOI] [PubMed] [Google Scholar]
- 138.Cardenas WB. Evasion of the interferon-mediated antiviral response by filoviruses. Viruses. 2010;2(1):262–282. doi: 10.3390/v2010262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kuhl A, Pohlmann S. How Ebola virus counters the interferon system. Zoonoses Public Health. 2012;59(Suppl 2):116–131. doi: 10.1111/j.1863-2378.2012.01454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jahrling PB, et al. Evaluation of immune globulin and recombinant interferon-alpha2b for treatment of experimental Ebola virus infections. J Infect Dis. 1999;179(Suppl 1):S224–S234. doi: 10.1086/514310. [DOI] [PubMed] [Google Scholar]
- 141.Smith LM, et al. Interferon-beta therapy prolongs survival in rhesus macaque models of Ebola and Marburg hemorrhagic fever. J Infect Dis. 2013;208(2):310–318. doi: 10.1093/infdis/jis921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ansari AA. Clinical features and pathobiology of Ebolavirus infection. J Autoimmun. 2014;55:1–9. doi: 10.1016/j.jaut.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 143.Geisbert TW, et al. Treatment of Ebola virus infection with a recombinant inhibitor of factor VIIa/tissue factor: a study in rhesus monkeys. Lancet. 2003;362(9400):1953–1958. doi: 10.1016/S0140-6736(03)15012-X. [DOI] [PubMed] [Google Scholar]
- 144.Hensley LE, et al. Recombinant human activated protein C for the postexposure treatment of Ebola hemorrhagic fever. J Infect Dis. 2007;196(Suppl 2):S390–S399. doi: 10.1086/520598. [DOI] [PubMed] [Google Scholar]
- 145.Business Wire. ARCA biopharma receives FDA orphan drug designation for rNAPc2 as a potential treatment for Ebola. 2014. http://www.businesswire.com/news/home/20141210005243/en/ARCA-biopharma-Receives-FDA-Orphan-Drug-Designation#.VYIkWmOuTzI. Accessed 17 June 2015.
- 146.Marzi A, Feldmann H. Ebola virus vaccines: an overview of current approaches. Expert Rev Vaccines. 2014;13(4):521–531. doi: 10.1586/14760584.2014.885841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Geisbert TW, et al. Evaluation in nonhuman primates of vaccines against Ebola virus. Emerg Infect Dis. 2002;8(5):503–507. doi: 10.3201/eid0805.010284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Martinez MJ, et al. Role of VP30 phosphorylation in the Ebola virus replication cycle. J Infect Dis. 2011;204(Suppl 3):S934–S940. doi: 10.1093/infdis/jir320. [DOI] [PubMed] [Google Scholar]
- 149.Marzi A, et al. Vaccines. An Ebola whole-virus vaccine is protective in nonhuman primates. Science. 2015;348(6233):439–442. doi: 10.1126/science.aaa4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang AP, et al. The ebolavirus VP24 interferon antagonist: know your enemy. Virulence. 2012;3(5):440–445. doi: 10.4161/viru.21302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang AP, et al. The ebola virus interferon antagonist VP24 directly binds STAT1 and has a novel, pyramidal fold. PLoS Pathog. 2012;8(2):e1002550. doi: 10.1371/journal.ppat.1002550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gao J, Yin L. Drug development for controlling Ebola epidemic—a race against time. Drug Discov Ther. 2014;8(5):229–231. doi: 10.5582/ddt.2014.01040. [DOI] [PubMed] [Google Scholar]
- 153.Kudoyarova-Zubavichene NM, et al. Preparation and use of hyperimmune serum for prophylaxis and therapy of Ebola virus infections. J Infect Dis. 1999;179(Suppl 1):S218–S223. doi: 10.1086/514294. [DOI] [PubMed] [Google Scholar]
- 154.Jahrling PB, et al. State-of-the-art workshops on medical countermeasures potentially available for human use following accidental exposures to Ebola virus. J Infect Dis. 2015. doi:10.1093/infdis/jiv115 [DOI] [PMC free article] [PubMed]
- 155.Pettitt J, et al. Therapeutic intervention of Ebola virus infection in rhesus macaques with the MB-003 monoclonal antibody cocktail. Sci Transl Med. 2013;5(199):199ra113. doi: 10.1126/scitranslmed.3006608. [DOI] [PubMed] [Google Scholar]
- 156.Murin CD, et al. Structures of protective antibodies reveal sites of vulnerability on Ebola virus. Proc Natl Acad Sci USA. 2014;111(48):17182–17187. doi: 10.1073/pnas.1414164111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kibuuka H, et al. Safety and immunogenicity of Ebola virus and Marburg virus glycoprotein DNA vaccines assessed separately and concomitantly in healthy Ugandan adults: a phase 1b, randomised, double-blind, placebo-controlled clinical trial. Lancet. 2015;385(9977):1545–1554. doi: 10.1016/S0140-6736(14)62385-0. [DOI] [PubMed] [Google Scholar]
- 158.Sarwar UN, et al. Safety and immunogenicity of DNA vaccines encoding Ebolavirus and Marburgvirus wild-type glycoproteins in a phase I clinical trial. J Infect Dis. 2015;211(4):549–557. doi: 10.1093/infdis/jiu511. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.