Abstract
We have determined whole body protein kinetics, i.e., protein synthesis (PS), breakdown (PB), and net balance (NB) in human subjects in the fasted state and following ingestion of ∼40 g [moderate protein (MP)], which has been reported to maximize the protein synthetic response or ∼70 g [higher protein (HP)] protein, more representative of the amount of protein in the dinner of an average American diet. Twenty-three healthy young adults who had performed prior resistance exercise (X-MP or X-HP) or time-matched resting (R-MP or R-HP) were studied during a primed continuous infusion of l-[2H5]phenylalanine and l-[2H2]tyrosine. Subjects were randomly assigned into an exercise (X, n = 12) or resting (R, n = 11) group, and each group was studied at the two levels of dietary protein intake in random order. PS, PB, and NB were expressed as increases above the basal, fasting values (mg·kg lean body mass−1·min−1). Exercise did not significantly affect protein kinetics and blood chemistry. Feeding resulted in positive NB at both levels of protein intake: NB was greater in response to the meal containing HP vs. MP (P < 0.00001). The greater NB with HP was achieved primarily through a greater reduction in PB and to a lesser extent stimulation of protein synthesis (for all, P < 0.0001). HP resulted in greater plasma essential amino acid responses (P < 0.01) vs. MP, with no differences in insulin and glucose responses. In conclusion, whole body net protein balance improves with greater protein intake above that previously suggested to maximally stimulating muscle protein synthesis because of a simultaneous reduction in protein breakdown.
Keywords: essential amino acids, optimal protein intake, protein turnover, stable isotope tracers
the principal nutritional goal of a protein-rich meal is to induce an anabolic state in which protein synthesis exceeds breakdown. Several recent studies, including our own (25), indicate that the maximum acute stimulation of muscle protein synthesis (MPS) occurs with ingestion of ∼ 20–35 g of high-quality protein (20, 25, 31) or more specifically 0.24 g·kg body wt−1·meal−1 in healthy young adults (19). The maximal dose in terms of stimulation of MPS is less than that typically consumed with the dinner meal in the average American diet, which generates a hypothesis that distributing the amount of protein intake throughout the day can more effectively stimulate anabolic response. However, the assertion that there is limited effectiveness of the conventional protein intake with dinner is based on incomplete assessment of the metabolic response of muscle protein. Importantly, the extent of muscle protein anabolism (the anabolic response) is not simply the response of MPS but rather the net balance between the response of protein synthesis and protein breakdown. We recently demonstrated the potential importance of suppression of protein breakdown in response to dietary intake of meals containing two levels of protein totaling either 0.8 or 1.5 g protein·kg−1·day−1. We found that at both levels of dietary protein whole body net protein balance became positive in the fed state compared with the fasted state, mainly due to reductions in protein breakdown (18). Furthermore, the anabolic response was greater with the higher level of protein intake. Also, previous studies evaluated the anabolic response to protein or amino acids (AAs) in the circumstance of either the ingestion of only AAs/protein (34) or the ingestion of protein occurring in a particular food source (e.g., meat or milk) (20, 24, 25) but not in the context of a complete meal (18). Finally, previous studies have focused entirely on the response of muscle, but this approach may underestimate total anabolic response to feeding. Dietary protein supplies precursors for the synthesis of all proteins in the body. Furthermore, AAs taken up and incorporated into proteins in rapid turnover tissues such as the gut can be later released into systemic circulation and used for synthesis of protein in other tissues, including muscle (13, 14). A further consideration of potential importance is the nature and amount of prior physical activity before a meal. For example, it has been shown that resistance exercise stimulates MPS (28). Despite the stimulation of MPS, even resistance exercise may not result in a positive NB in the fasting state because of the simultaneous increase in muscle protein breakdown (MPB) (28). However, prior resistance exercise may amplify the normal stimulatory effect of protein/AA intake alone (28). Thus it is reasonable to examine whole body effects of exercise in the context of quantifying the anabolic response to different levels of dietary protein.
In the current study we have quantified protein kinetics [protein synthesis (PS), breakdown (PB), and net balance (NB)] at the whole body level before and throughout the response to two levels of protein intake in mixed meals with or without prior resistance exercise in healthy young adults. We hypothesized that 1) the whole body net anabolic response (NB) would be greater with intake of 70 g protein, compared with 40 g protein in mixed meals; and 2) the whole body net anabolic response to either level of dietary protein in mixed meals would be greater following resistance exercise.
SUBJECTS AND METHODS
Subjects.
Twenty-three healthy subjects [18–40 yr] were recruited from the Little Rock area using local newspaper advertisements and flyers posted around the University of Arkansas for Medical Sciences (UAMS) campus and the Little Rock area. Upon their first visit to the laboratory, a battery of medical tests was performed to determine subject eligibility, including medical history, blood count, plasma electrolytes, blood glucose concentration, and liver and renal function tests. Exclusion criteria ruled out subjects with diabetes, active malignancy within the past 6 mo, gastrointestinal bypass surgery, a chronic inflammatory disease, low hematocrit or hemoglobin concentration, low platelets, concomitant use of corticosteroids, any unstable medical conditions, and subjects who performed resistance exercise more than once per week. Eligible subjects were then randomly assigned to the resistance exercise group (X) or the resting group (R). Subjects assigned to the exercise group were then tested for determination of their one-repetition maximum (1RM, the heaviest weight lifted one time) of four different exercises (see Exercise protocol) at least 4 days apart from the start of the study. Written informed consent was obtained from all subjects, and the study was approved by the Institutional Review Board at the UAMS.
Experimental protocol.
Dual-energy X-ray absorptiometry (QDR-4500A; Holologic, Waltham, MA) was performed for determination of body composition during the screening for subject eligibility (Table 1). Subjects were randomly assigned by a study coordinator to resistance exercise (X) or resting groups (R) and studied at two levels of protein intake in the form of 40 g [moderate protein (MP)] vs. 70 g [high protein (HP)] in mixed meals administered, in random order with >1-wk interval between the two levels of protein intake. The primary source of protein in each meal was beef patties. Subjects were instructed to abstain from strenuous physical activity for >72 h before the initiation of the metabolic study. Meals were provided in the 3-day run-in period before the metabolic study on day 4. All foods, including the interventional meals on day 4, were prepared by a study dietician in the Metabolic Kitchen at the Reynolds Institute on Aging (RIOA) (Table 2). The first 3 days served as dietary normalization period. Subjects obtained the 3-day meal allotments from our study coordinator and were also given a dietary record and point-and-shoot digital camera. Subjects were asked to record time of meal consumption and percentage of meal consumption and to photograph the meal before and after consumption. Subjects were instructed to return all unused or empty meal/supplement packaging and camera on the morning of the fourth day when they reported to the RIOA for the metabolic study. These data helped the research dietician ascertain caloric/protein intake as well as study compliance. Subjects who achieved a minimum compliance of 80% consumption of meals progressed to the metabolic study.
Table 1.
Subject characteristics
| Resting | Exercise | |
|---|---|---|
| Subjects (M/F) | 11 (4/7) | 12 (8/4) |
| Age, yr | 31.0 ± 1.6 | 29.3 ± 1.4 |
| Weight, kg | 82.7 ± 6.5 | 84.7 ± 4.6 |
| BMI, kg/m2 | 26.0 ± 1.7 | 27.6 ± 1.6 |
| LBM, kg | 55.0 ± 3.7 | 54.5 ± 2.5 |
| Fat mass, % | 27.7 ± 1.8 | 30.0 ± 2.1 |
Values are expressed as means ± SE. M/F, number of male and female subjects in the studies; BMI, body mass index; LBM, lean body mass. There were no significant statistical differences in any variables with respect to physical characteristics.
Table 2.
Macronutrients of 3-day run-in on day 1–3 and metabolic infusion study on day 4
| Group |
Macronutrients |
|||||||
|---|---|---|---|---|---|---|---|---|
|
Run-in foods on day 1–3 |
||||||||
| Protein |
Fat |
CHO |
||||||
| Activity/Protein levels | Energy intake, kcal | g | % | g | % | g | % | Fiber, g |
| X | ||||||||
| MP | 2,541 ± 103 | 84.5 ± 4.6 | 13.0 ± 0.5 | 100.3 ± 4.3 | 34.8 ± 0.3 | 337.5 ± 13.3 | 52.2 ± 0.3 | 29.7 ± 0.9 |
| HP | 2,575 ± 107 | 85.0 ± 4.7 | 12.9 ± 0.4 | 101.7 ± 4.1 | 34.9 ± 0.2 | 342.2 ± 14.4 | 52.2 ± 0.3 | 29.9 ± 1.0 |
| R | ||||||||
| MP | 2,549 ± 146 | 82.4 ± 6.2 | 12.6 ± 0.3 | 100.3 ± 5.4 | 34.8 ± 0.2 | 341.9 ± 19.0 | 52.6 ± 0.2 | 29.7 ± 1.2 |
| HP | 2,589 ± 83 | 83.3 ± 6.5 | 12.5 ± 0.3 | 102.0 ± 6.0 | 34.8 ± 0.1 | 347.3 ± 19.9 | 52.7 ± 0.2 | 29.9 ± 1.2 |
|
Interventional foods of metabolic infusion study on day 4 |
|||||||
|---|---|---|---|---|---|---|---|
| Meal Macronutrients, g |
Nonprotein Energy, % |
||||||
| Sex n (X/R)/Protein Levels | Meal, kcal | Beef protein, g | Protein | Fat | CHO | Fat | CHO |
| M 12 (8/4) | |||||||
| MP | 1,297 | 21.1 | 44.1 | 76.2 | 107.3 | 61.1 | 38.2 |
| HP | 1,301 | 52.7 | 70.0 | 68.9 | 95.3 | 60.8 | 37.3 |
| F 11 (4/7) | |||||||
| MP | 1,101 | 21.1 | 39.7 | 64.2 | 89.0 | 61.4 | 37.8 |
| HP | 1,103 | 52.7 | 65.7 | 56.9 | 77.1 | 60.9 | 36.7 |
Values are expressed as means ± SE. Each subject consumed his or her respective interventional foods based on their sex; n = number of subjects. X, exercise group; R, resting group; MP, low protein; HP, higher protein; M, male; F, female; CHO, carbohydrate.
Preparation of interventional meals.
We purchased 85% lean ground beef from a local grocery and formed them into patties weighing 113.4 g (4 oz) or 283.5 g (10 oz) of the beef (precooked/raw). The beef patties were cooked each in a skillet on a gas burning stove until fully cooked. The cooked beef patties were then individually packaged and were stored frozen at −18°C. The non-beef component of the meals was also prepared in advance in the Metabolic Kitchen and stored frozen at −18°C. The beef meals were thawed overnight under refrigeration at 4°C before being served. The meals were microwaved before service. The protein content was ∼19 g per 100 g raw beef.
Exercise protocol.
The resistance exercise bout consisted of 3 sets of 10 repetitions of bench press, lateralis pull-down, leg press, and leg extension each at 80% of 1 repetition maximum (1 RM, the maximum weight that can be lifted for 1 repetition). Each set was completed within 30 s. The rest interval between sets was <2 min, and the entire exercise bout was completed in ∼45–50 min.
Stable isotope tracer infusion protocol.
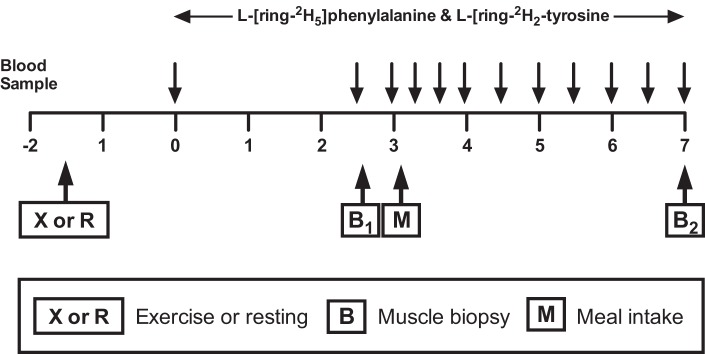
The 7-h tracer infusion protocol is presented in Fig. 1. On the fourth day, subjects reported to the RIOA after an overnight (after 2200) fast. Subjects in the exercise group performed resistance exercise as described above and then rested on a bed for 1 h. During this resting period (both groups), two polyethylene catheters were placed into each lower arm; one for the infusion of stable isotope tracers and the other for “arterialized” blood sampling via a heating box (1). Before the infusion of tracers, a baseline blood sample was collected to determine background isotopic enrichments. For determination of PS, PB, and NB at the whole body level, primed continuous infusions of l-[2H5]phenylalanine (prime, 5.04 μmol/kg; rate, 5.04 μmol·kg−1·h−1) and l-[2H2]tyrosine (prime, 2.16 μmol/kg; rate, 1.995 μmol·kg−1·h−1) were performed. To appropriately reach isotopic equilibrium of l-[ 2H4]tyrosine enrichment derived from l-[2H5]phenylalanine tracer infused, a priming dose of l-[2H4]tyrosine was also injected (prime: 5.04 μmol/kg). All isotope tracers were purchased from Cambridge Isotope Laboratories (Andover, MA). Blood samples were taken at 0, 150, and 180 min before a meal intake (the fasted blood samples) and at 200, 220, 240, 270, 300, 330, 360, 390, and 420 min to measure tracer enrichment and plasma responses of AAs, glucose, and insulin. A total of 12 blood samples were taken during the study (∼100 ml). Muscle samples were obtained before meal intake (at ∼180 min) and at the end of the metabolic study (at 420 min) from vastus lateralis muscles to determine muscle protein fractional synthetic responses to respective meal intake.
Fig. 1.
Tracer infusion protocol. X, exercise; R, resting.
Calculations.
Whole body protein kinetics were calculated based on the determinations of the rate of appearance (Ra) into the plasma of phenylalanine and tyrosine and the fractional Ra of endogenous tyrosine converted from phenylalanine as in the previous study (18). Briefly, area under the curve (AUC) of plasma enrichments of phenylalanine and tyrosine tracers (Fig. 2) was calculated using Graphpad Prism 5 for Mac (Graphpad Software, La Jolla, CA) to account for variations in postmeal tracer kinetics (18, 32). Whole body protein kinetics were calculated by dividing kinetic values of phenylalanine by its fractional contribution to protein (4%) (5). For the calculations for whole body protein breakdown rate, contribution from exogenous meal and tracers infused were subtracted from total Ra. The following equations were used for the calculations of whole body protein kinetics:
Enrichment (E) is expressed as tracer-to-tracee ratio (TTR) or mole percent excess (MPE), calculated as TTR/(TTR + 1). TTR was used for calculations of PB whereas MPE was used for calculations of PS. E is enrichment of respective tracers. F is the tracer infusion rate into a venous site: FPhe for phenylalanine tracer. ETyr M+4 and EPhe M+5 are plasma enrichments of tyrosine tracers at M+4 and M+5 relative to M+0, respectively. The correction factor of 25 is for conversion of value for phenylalanine to protein based on the assumption that contribution of phenylalanine to protein is 4% (100/4 = 25) (5). PRO is the amount of exogenous protein (g) that was the amount of AAs appearing in the circulation as a result of the exogenous protein digestion, accounting for splanchnic extraction (29%) of AAs in young adults (30). Phe hydroxylation rate is the rate of appearance of tyrosine derived from phenylalanine through process of hydroxylation. Calculation of MPS was performed as previously described (18). EBP1 and EBP2 are the enrichments of protein bound l-[ring-2H5]phenylalanine in the first and second muscle biopsies, respectively, and Em is the mean plasma enrichment (180 ∼ 420 min) of the l-[ring-2H5]phenylalanine. t I → is the duration of time in minutes elapsed between two muscle biopsies; 60 and 100 are factors used to express MPS in percent per hour.
Fig. 2.
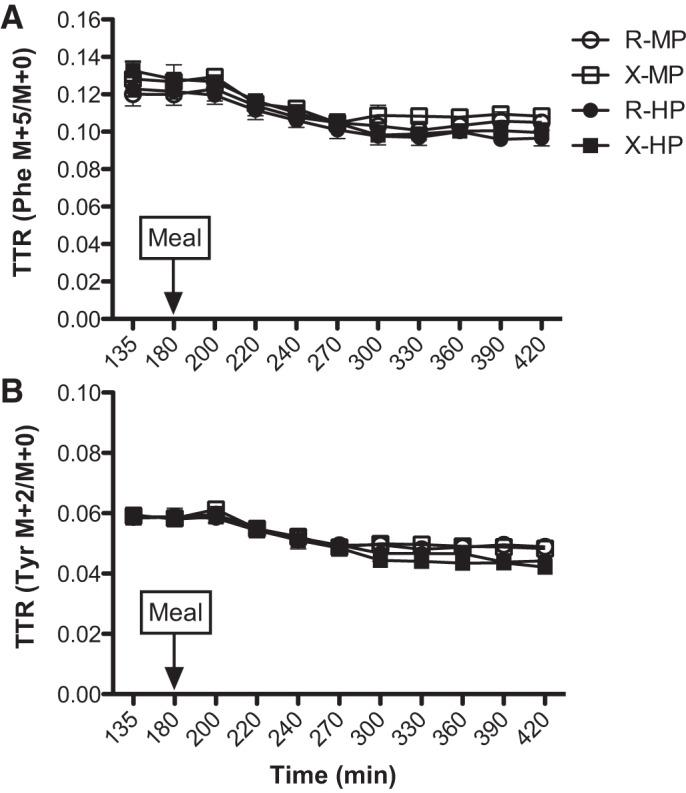
Plasma enrichments of infused tracers (A: Phe; B: Tyr) before and following a meal intake containing ∼40 g [moderate protein (MP)] or ∼70 g [high protein (HP)] of dietary protein with prior resistance exercise (X) or time-matched resting (R). Values are expressed as means ± SE. TTR, tracer-to-tracee ratio.
Analytic methods.
Plasma samples were processed as previously described before determination of enrichment by gas chromatography-mass spectrometry (GCMS: Models 7890A/5975; Agilent Technologies, Santa Clara CA) (18). Briefly, 125 ul of 10% sulfosalicylic acid were added to plasma samples to precipitate protein. Plasma free AAs were then extracted from 300 ul supernatant fluid by cation exchange chromatography (Strata-X-C; Phenomenex, Torrance, CA) and dried under Speed Vac (Savant Instruments, Farmingdale, NY). Enrichments of phenylalanine and tyrosine were measured on the tert-butyldimethylsilyl derivative with the use of GCMS (18, 29). Ions of mass to charge ratios of 234, 235, and 239 for phenylalanine and of 466, 467, 468, and 470 for tyrosine were monitored with electron impact ionization and selected ion monitoring. Plasma glucose concentrations were measured spectrophotometrically on a Cobas c 111 analyzer (Roche, F. Hoffman-La Roche, Basel, Switzerland). Plasma insulin concentrations were measured by using commercially available human insulin ELISA kit (Alpco Diagnostics, Salem, MA). Plasma AA concentrations were determined by using liquid chromatography-mass spectrometry (QTrap 5500 MS; AB Sciex) using internal standard method (12). Preparations of muscle tissue samples obtained from the vastus lateralis muscles at 180 and 420 min were performed as previously described (18). Phenylalanine tracer enrichment from muscle bound protein was determined as in plasma analyses.
Statistical analysis.
Mixed effects ANOVA models were used to analyze protein kinetics (NB, PS, and PB), MPS, and concentrations of AAs, insulin, and glucose in the plasma. Each model included fixed effects for protein amount (MP and HP) and physical activity (R and X). Because this was a cross-over study, factors representing sequence order and period were included in the model to allow for the assessment of possible carryover effects. The analyses of the AA, insulin, and glucose data also included sampling time, which was modeled as a continuous variable. P < 0.05 were considered to be statistically significant. In cases where multiple testing was necessary, Sime's method was used to adjust the P values. This analysis was performed using SAS (version 9.4 SAS Institute, Cary, NC).
RESULTS
Protein kinetics at whole body and muscle levels.
Whole body protein kinetics (NB, PS, and PB) were calculated as changes from the fasted to the fed states to account for any variability in the fasting kinetic values (Fig. 3). Statistical comparisons for kinetics between fasted and fed states were not made, since the focus of this paper was the response to dietary intake of protein. There was no sequence (MP and HP) effect on any kinetics (for all, P > 0.39). In contrast to the hypothesis, we did not find an effect of exercise on protein kinetics (P > 0.490). However, we found there were significant effects of protein amount on the protein kinetics (P < 0.00005). NB was increased in both MP and HP groups, with the increase in response to HP significantly greater than the response to MP (for all, P < 0.00001). PS was stimulated in response to both levels of protein intake, and the magnitude was greater with HP. Similarly, PB was decreased in both MP and HP groups, with the reduction in PB markedly greater in the HP group (for all, P < 0.00001). Although the difference in NB between MP and HP groups was due to differences in the magnitude of changes in both PS (P < 0.00004) and PB, the predominant factor in the difference in NB was the greater reduction in PB in the HP group. MPS responses following meal intake are presented in Fig. 4. There were no significant effects with respect to interaction (group-by-amount), protein amount, and groups (R vs. X) for MPS (for all, P > 0.25).
Fig. 3.
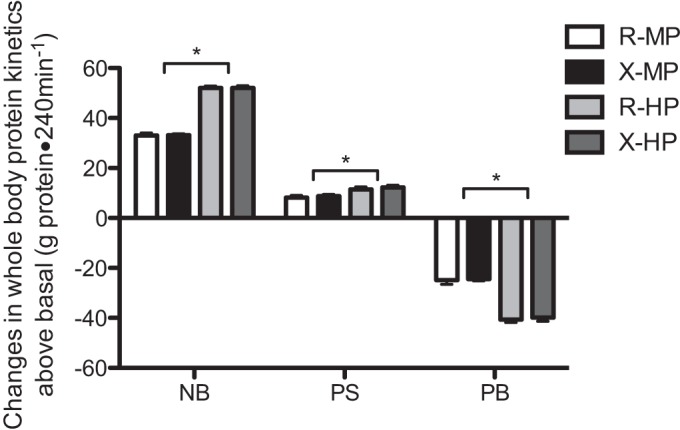
Changes in rates of whole body protein net balance (NB), synthesis (PS), and breakdown (PB) from the fasted state in response to meal containing ∼40 g (MP) or ∼70 g (HP) of dietary protein with prior resistance exercise (X) or time-matched resting (R). *Significantly different from MP within the same activity group (P < 0.0001). Values are expressed as means ± SE.
Fig. 4.
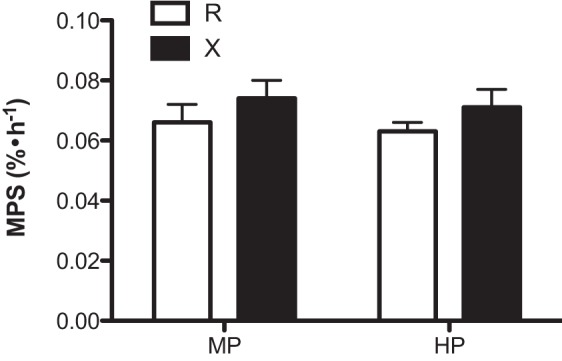
Muscle protein fractional synthesis rate (MPS; %/h) following a meal intake containing ∼40 g (MP) or ∼70 g (HP) of dietary protein with prior resistance exercise (X) or time-matched resting (R). Values are expressed as means ± SE.
Plasma concentrations.
Plasma responses of insulin, glucose, and total essential amino acids (EAA) and nonessential amino acid (NEAA) are expressed as area under the curves in Figs. 5 and 6. There was no significant exercise effect on any concentration value (P > 0.150). For the EAA, there was a protein-by-time interaction (P < 0.00001), which suggests the effect of protein amount depends on the time elapsed since ingestion. Furthermore, there was a significant effect of protein amount (P < 0.001): EAA was significantly higher with HP compared with MP. For NEAA, there was a significant protein effect (P < 0.0001) that was not dependent on time: NEAA was significantly higher with HP compared with MP. Both insulin and glucose were elevated upon meal intake (P < 0.0001; Fig. 6). However, there was no protein effect for insulin (P = 0.4053) or for glucose (P = 0.0866).
Fig. 5.
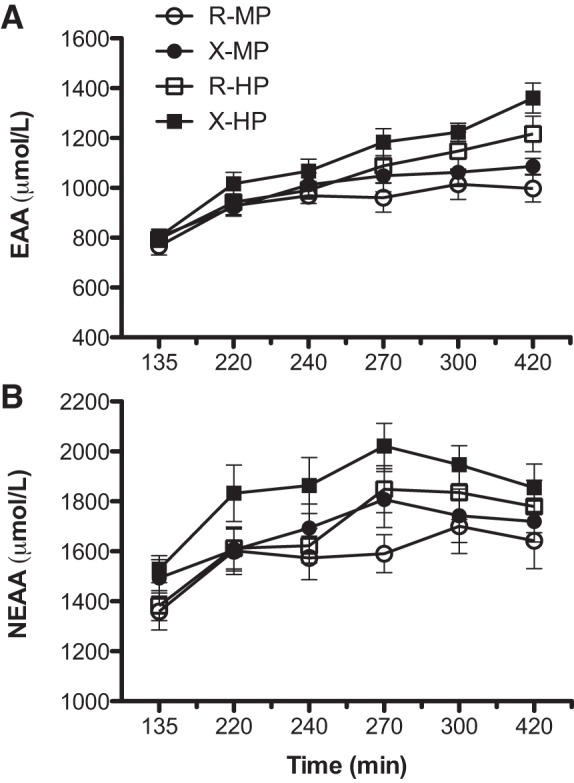
Plasma concentrations of total essential (EAA; A) and nonessential amino acids (NEAA; B) in the fasted states (time at 135 min) and following meal intake containing ∼40 g (MP) or ∼70 g (HP) of dietary protein with prior resistance exercise (X) or time-matched resting (R). For the EAA, there were a protein-by-time interaction (P < 0.00001) and a protein amount effect (P < 0.001): HP was significantly higher than MP. For NEAA, there was only a protein amount effect (P < 0.0001): HP was significantly higher than MP. Values are expressed as means ± SE.
Fig. 6.
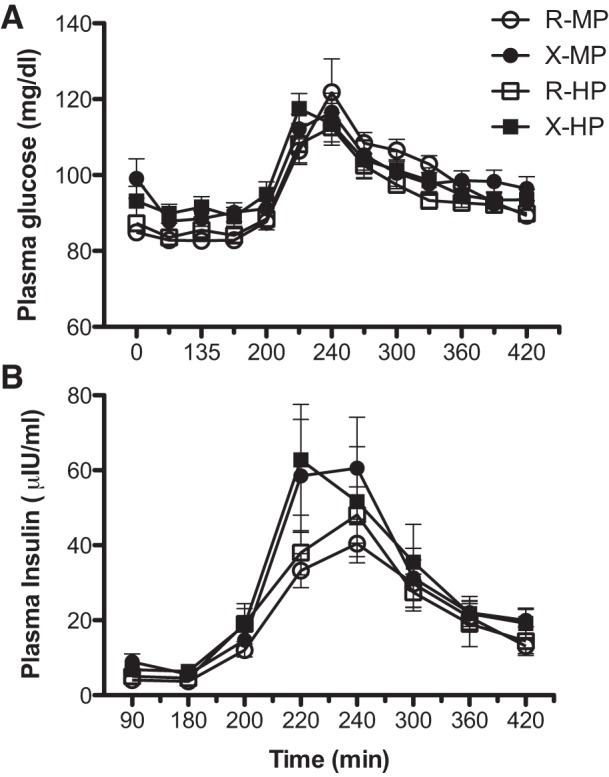
Plasma concentrations of glucose (A) and insulin (B) in the fasted states (times at 90 and 180 min) and following intake of meals containing ∼40 g (MP) or ∼70 g (HP) of dietary protein with prior resistance exercise (X) or time-matched resting (R). Both insulin and glucose were elevated upon meal intake (P < 0.0001). However, there was no protein effect for insulin (P = 0.4053) and for glucose (P = 0.0866). Values are expressed as means ± SE.
DISCUSSION
In this study we assessed the response to ingestion of isocaloric meals that contained either the amount of protein (i.e., MP) that others have considered to be optimal based on the effect on stimulation of MPS or twice the moderate amount of protein (i.e., HP). We found that both levels of protein intake resulted in a positive whole body NB (i.e., anabolic response) and that the higher level of protein intake resulted in a significantly greater anabolic response than the previously described “optimal” amount of protein (20, 25). The greater NB with the higher protein intake was achieved largely through a greater reduction in PB and, to a lesser extent, through a greater increase in PS than with the lower protein intake.
Change in protein mass of the body is not determined solely by PS but by the balance between PS and PB. However, the important role of changes in PB in amplifying the anabolic response to increased dietary protein in the context of mixed meals has been largely ignored. The previously suggested “optimal dose” of protein intake in a meal (20, 25, 31) was recommended entirely based on the response of PS at the muscle level. The determination of the true optimal dose of protein in a meal should also account for the other side of the protein balance equation, i.e., PB. There is a complex relationship between protein synthesis and breakdown in a variety of circumstances. In general, PS is closely linked to PB, as AAs derived from PB serve as the major source of precursors for the synthesis of new proteins (32). For example, in clinical circumstances such as in type I diabetes, NB may be negative, indicating a net catabolic state. Nonetheless, PS is likely to be normal (26) or paradoxically increased (21). The maintenance of a normal or elevated rate of PS in the catabolic circumstance of a negative NB can be attributed to the increased availability of intracellular AAs resulting from accelerated PB. Since some oxidation as well as outward transport of AAs released from PB occurs continuously, PS generally cannot keep pace with accelerated PB in a catabolic state. If only PS is measured in this circumstance, it would appear that the individual is not in a catabolic state, whereas in fact an (unmeasured) elevated rate of PB is responsible for the negative NB characteristic of a catabolic state. Alternatively, in an anabolic state in which stimulation of PS is the principal response driving the improvement in NB, PB may be either unchanged or increased, but to a lesser extent than PS (17, 27, 29). In contrast to the link between PS and PB in a variety of circumstances, our results indicate that PS and PB change in the opposite direction following a meal containing protein. This is not entirely unexpected, since there are separate regulatory factors affecting PS and PB, and these factors appear to function at the same time following a meal.
Our observation that whole body NB was increased to a greater extent with HP compared with MP, is at odds with the previous observation that the maximal MPS is achieved with protein dose of 20–35 g of high-quality protein (20, 25, 31). However, the previous assertion that an optimal dose (i.e., 20–35 g) can be based on the MPS response alone is incorrect. First, the optimal dose is based on an incomplete assessment of the anabolic response by measuring only PS. The rationale for using only PS to estimate the total anabolic response is supported by the results of many previous studies in which increased NB following intake of protein/AA alone was achieved through increases in PS without apparent changes in PB (17, 27, 29). However, in the context of mixed meals, the main contributing factor to achieving positive NB appears to be reductions in PB, not in increases in PS, as shown in our previous findings (18). In contrast to the previous finding at one recommended dietary allowance (1RDA 0.8 g·kg−1·day−1), both levels of protein intake in the present study not only prevented plasma AA levels from declining but actually increased plasma AA levels well above the fasted levels. The magnitude of increases in plasma EAA levels in the present study was comparable to that observed when the protein was given by itself (e.g., 40 g of protein intake in the exercise groups in both studies) (20). These findings are consistent with the notion that plasma EAA levels are the main determinant of protein synthesis (10). In the present study, NB increased 58% more above the fasted state with HP compared with MP, which closely paralleled the 62% difference in the amount of protein dose between MP and HP. These results are consistent with our previous observation that whole-body NB increases linearly with increasing amounts of dietary protein (18). Furthermore, the linear relationship between the increase in dietary protein in the meal and the increase in NB is consistent with our previous observation in older adults (18) over levels of protein intake ranging from 6.4 to ∼91.7 g protein. This suggests that the gain in NB following a meal is not maximized at 70 g.
In the present study, we did not find a synergistic response between prior resistance exercise and the intake of protein on NB, PS, and PB. The absence of an effect of prior exercise is in contrast to the initial hypothesis that prior resistance exercise would amplify the increase in NB observed when meals were consumed at rest. This original hypothesis was based on previous findings demonstrating that prior resistance exercise synergistically increased the rate of MPS following ingestion of either protein or AAs. Results were consistent throughout tracer methodologies, whether utilizing the precursor product method (20, 31) or the arteriovenous (a-v) balance method (7). Although prior experiments involved measurements at the muscle level, an exercise effect would be expected at the whole body level as well. Several studies have shown that changes in protein turnover in muscle are also reflected at the whole body level (14, 15). There are several potential explanations for the lack of an exercise effect on the response to dietary protein in the current study. It is likely that a maximal increase in MPS was already achieved in the MP. In support, we observed no significance increases in MPS with HP compared with MP in both exercise and resting groups. However, it is also possible that in our study a stimulation of MPS after exercise was diminished over the 7-h experimental time period (22) as it has been shown that MPS response generally peaks within ∼2 h (2, 11). An effect of the meal on protein breakdown is also a possible explanation for the lack of an effect of prior exercise. MPB increases in response to resistance exercise (6, 8, 23). On the other hand, carbohydrate feeding suppresses MPB via insulin (3, 9). However, insulin has differential effects on MPB at resting or following exercise. For example, Biolo and Wolfe (8) have shown that combined infusions of insulin and glucose abolished postexercise stimulation of MPB but not in resting state in healthy male adults (8). Glynn et al. (16) have recently shown a reduction in MPB following combined ingestion of 20 g EAA and carbohydrate (30 or 90 g). Thus a suppression of PB by insulin may have contributed to the absence of an exercise effect on NB in the present study.
The mechanisms responsible for the greater reduction in PB with HP compared with MP are unclear. Plasma insulin responses were similar between HP and MP, which was to be expected since the meals contained similar amounts of carbohydrate. Thus differences in plasma AA response between groups cannot be attributed to insulin alone. We have previously postulated that intracellular EAAs play a role in regulating protein breakdown. When the rate of EAA influx into the intracellular compartment exceeds the capacity of the cell to incorporate EAAs into protein, intracellular EAA concentrations will increase. Thus, at low rates of entry of EAAs, such as occurs after a small dose of dietary protein, the rate of protein synthesis will be stimulated sufficiently to maintain constant levels of EAAs. However, at higher rates of entry of EAAs the corresponding intracellular concentrations will rise, and this may inhibit protein breakdown. Consistent with this notion, Bohé et al. (10) demonstrated that intracellular EAA concentrations remained constant during the infusion of an AA mixture at increasing rates up to 87 mg·kg−1·h−1. MPS increased proportionately to the increases in AA infusion rate. When the AA infusion rate was increased to 261 mg·kg−1·h−1, there was no further stimulation of MPS, indicating that the maximal capacity of the cell to dispose of free EAAs via incorporation to protein had been exceeded. At that point the intracellular concentrations of EAAs increased significantly. The amounts of AA infused over 4 h in the study by Bohé et al. (10) were ∼25g (87 mg·kg−1·h−1) and 50 g (261 mg·kg−1·h−1), respectively, which are comparable to MP (29 g) and HP (50 g) in the present study, assuming that 71% of the ingested protein was absorbed and appeared into systemic circulation (30). In accordance with the notion, we observed that MPS did not increase with HP, compared with MP, regardless of groups. Furthermore, in contrast to the present study, in our previous study differences in NB between 1RDA and ∼2RDA were solely due to differences in PS. This suggests that the amount of protein intake in our previous study (average protein intake/meal <40 g even in the higher protein group) might not reach the threshold level above which PB is reduced. It also must be considered that some aspect of the reductions in PB may be due to a limitation of the methodology. Total protein breakdown is the sum of Ra AAs into plasma and rate of AAs that are released from protein breakdown but directly reincorporated into protein without appearing in plasma (33). The tracer methodology used in the present study only determines Ra AA using a representative EAA (i.e., phenylalanine), which thus could underestimate total PB if reincorporation of AA released from PB to proteins is elevated due to feeding (via insulin) (4). Although total PB might be underestimated due to the methodological limitation, this does not explain differences in PB between MP and HP. In addition, the magnitude of reductions in PB in both MP and HP is far greater than those of whole body PS. Thus it is unlikely that the difference in PB is due to a methodological limitation. Taken together, we found PB was reduced to a greater extent with HP compared with MP despite similar plasma insulin responses. This suggests the existence of factor(s) affecting MPB, other than the insulin response, and intracellular EAAs are good candidates.
In the present study, we observed increases in PS with both levels of protein intake above the fasted values. It has been shown that stimulation of MPS is mainly affected by extracellular (plasma and interstitial) AA availability (10), and systemic insulin response following intake of mixed meals leads to hypoaminoacedemia via stimulating AA uptake and perhaps inhibiting protein breakdown (4). Thus, to stimulate MPS, it is important to prevent plasma AA concentration from declining by giving a sufficient amount of protein. A decline in plasma AAs can occur following relatively small amounts of protein intake in the context of mixed meals, as shown in our previous study (18), leading to reductions in PS from the fasted state despite the intake of protein. In the present study, both levels of protein intake that resulted in progressive increases in plasma EAA (also NEAA) levels were accompanied by increases in PS above the fasted states, confirming the importance of plasma AA availability for stimulation of PS (10). Although all of our meals contained more than the dose (35 g) of protein promoted as optimal, we observed further increases in PS with HP (∼70 g) compared with MP (∼40 g). It is likely that increases in whole body PS likely reflected increases in PS in tissues other than muscle, such as gut (14) as we did not find increases in MPS with HP compared with MP. Consistent with this notion, it was reported that feeding and concomitant insulin response increase gut tissue net protein synthesis (13). Stimulation of gut protein synthesis is potentially beneficial, particularly in a situation where MPS has been already maximized. Proteins retained in the gut can be released into the circulation as a consequence of gut protein turnover and then be used for MPS (14). This mechanism could be particularly important overnight, in which the fasting state is predominated by PB, with resultant negative NB.
In conclusion, in the context of a mixed meal, whole body net protein balance increases in healthy young individuals with protein intake above the amount of protein that was previously shown to maximally increase muscle protein synthesis. The increase in net balance was primarily the result of reductions in protein breakdown, and to a lesser extent, increases in protein synthesis. Prior exercise did not influence this response in the setting of our experiment.
GRANTS
The project was financially supported by a grant from National Cattlemen's Beef Association. The project was partially supported by Pepper Center Grant AG-028718 and National Center for Advancing Translational Sciences (NCATS) Awards UL1TR000039 and KL2TR000063.
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official view of the NCATS or the National Institutes of Health.
DISCLOSURES
R. R. Wolfe has received research grants and honoraria from the National Cattleman's Beef Checkoff Program. Other authors have no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Author contributions: I.-Y.K. and R.R.W. conception and design of research; I.-Y.K., S.S., and G.A. performed experiments; I.-Y.K., A.S., H.J.S., A.A.F., and R.R.W. analyzed data; I.-Y.K., A.A.F., and R.R.W. interpreted results of experiments; I.-Y.K. prepared figures; I.-Y.K. drafted manuscript; I.-Y.K., A.A.F., and R.R.W. edited and revised manuscript; I.-Y.K., S.S., A.S., H.J.S., G.A., A.A.F., and R.R.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the research subjects for participation in the study. We also thank the research staffs/associates for support in conducting isotope tracer infusion protocols and sample analyses; Cosby J. Lasely, Orlov Alexandra, and Josh Spore for coordinating study subjects and helping conducting protocols of the resistance exercise and the isotope infusion study; and Josh Spore and Rick Williams for liquid or gas chromatography-mass spectrometry analysis and determination of blood chemistry. Lastly, we thank the study dietician Amanda M. Dawson for preparing study foods for subjects.
REFERENCES
- 1.Abumrad NN, Rabin D, Diamond MP, Lacy WW. Use of a heated superficial hand vein as an alternative site for the measurement of amino acid concentrations and for the study of glucose and alanine kinetics in man. Metab Clin Exp 30: 936–940, 1981. [DOI] [PubMed] [Google Scholar]
- 2.Atherton PJ, Etheridge T, Watt PW, Wilkinson D, Selby A, Rankin D, Smith K, Rennie MJ. Muscle full effect after oral protein: time-dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am J Clin Nutr 92: 1080–1088, 2010. [DOI] [PubMed] [Google Scholar]
- 3.Atherton PJ, Smith K. Muscle protein synthesis in response to nutrition and exercise. J Physiol 590: 1049–1057, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biolo G, Declan Fleming RY, Wolfe RR. Physiologic hyperinsulinemia stimulates protein synthesis and enhances transport of selected amino acids in human skeletal muscle. J Clin Invest 95: 811–819, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biolo G, Fleming RY, Maggi SP, Wolfe RR. Transmembrane transport and intracellular kinetics of amino acids in human skeletal muscle. Am J Physiol Endocrinol Metab 268: E75–E84, 1995. [DOI] [PubMed] [Google Scholar]
- 6.Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol Endocrinol Metab 268: E514–E520, 1995. [DOI] [PubMed] [Google Scholar]
- 7.Biolo G, Tipton KD, Klein S, Wolfe RR. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol Endocrinol Metab 273: E122–E129, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Biolo G, Williams BD, Fleming RY, Wolfe RR. Insulin action on muscle protein kinetics and amino acid transport during recovery after resistance exercise. Diabetes 48: 949–957, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Biolo G, Wolfe RR. Insulin action on protein metabolism. Baillieres Clin Endocrinol Metab 7: 989–1005, 1993. [DOI] [PubMed] [Google Scholar]
- 10.Bohé J, Low A, Wolfe RR, Rennie MJ. Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: a dose-response study. J Physiol 552: 315–324, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bohé J, Low JF, Wolfe RR, Rennie MJ. Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J Physiol 532: 575–579, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Betue CT, Joosten KF, Deutz NE, Vreugdenhil AC, van Waardenburg DA. Arginine appearance and nitric oxide synthesis in critically ill infants can be increased with a protein-energy-enriched enteral formula. Am J Clin Nutr 98: 907–916, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deutz NE, Have Ten GA, Soeters PB, Moughan PJ. Increased intestinal amino-acid retention from the addition of carbohydrates to a meal. Clin Nutr 14: 354–364, 1995. [DOI] [PubMed] [Google Scholar]
- 14.Deutz NE, Wolfe RR. Is there a maximal anabolic response to protein intake with a meal? Clin Nutr 32: 309–313, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fouillet H, Gaudichon C, Mariotti F, Bos C, Huneau JF, Tomé D. Energy nutrients modulate the splanchnic sequestration of dietary nitrogen in humans: a compartmental analysis. Am J Physiol Endocrinol Metab 281: E248–E260, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Glynn EL, Fry CS, Drummond MJ, Dreyer HC, Dhanani S, Volpi E, Rasmussen BB. Muscle protein breakdown has a minor role in the protein anabolic response to essential amino acid and carbohydrate intake following resistance exercise. Am J Physiol Regul Integr Comp Physiol 299: R533–R540, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr 82: 1065–1073, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Kim IY, Schutzler S, Schrader A, Spencer H, Kortebein P, Deutz NEP, Wolfe RR, Ferrando AA. Quantity of dietary protein intake, but not pattern of intake, affects net protein balance primarily through differences in protein synthesis in older adults. Am J Physiol Endocrinol Metab 308: E21–E28, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore DR, Churchward-Venne TA, Witard O, Breen L, Burd NA, Tipton KD, Phillips SM. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontol A Biol Sci Med Sci 70: 57–62, 2015. [DOI] [PubMed] [Google Scholar]
- 20.Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, Prior T, Tarnopolsky MA, Phillips SM. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr 89: 161–168, 2009. [DOI] [PubMed] [Google Scholar]
- 21.Nair KS, Garrow JS, Ford C, Mahler RF, Halliday D. Effect of poor diabetic control and obesity on whole body protein metabolism in man. Diabetologia 25: 400–403, 1983. [DOI] [PubMed] [Google Scholar]
- 22.Pennings B, Groen BB, van Dijk JW, de Lange A, Kiskini A, Kuklinski M, Senden JM, van Loon LJ. Minced beef is more rapidly digested and absorbed than beef steak, resulting in greater postprandial protein retention in older men. Am J Clin Nutr 98: 121–128, 2013. [DOI] [PubMed] [Google Scholar]
- 23.Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol Endocrinol Metab 273: E99–E107, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Robinson MJ, Burd NA, Breen L, Rerecich T, Yang Y, Hector AJ, Baker SK, Phillips SM. Dose-dependent responses of myofibrillar protein synthesis with beef ingestion are enhanced with resistance exercise in middle-aged men. Appl Physiol Nutr Metab 38: 120–125, 2013. [DOI] [PubMed] [Google Scholar]
- 25.Symons TB, Sheffield-Moore M, Wolfe RR, Paddon-Jones D. A moderate serving of high-quality protein maximally stimulates skeletal muscle protein synthesis in young and elderly subjects. J Am Diet Assoc 109: 1582–1586, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tessari P, Nosadini R, Trevisan R, De Kreutzenberg SV, Inchiostro S, Duner E, Biolo G, Marescotti MC, Tiengo A, Crepaldi G. Defective suppression by insulin of leucine-carbon appearance and oxidation in type 1, insulin-dependent diabetes mellitus. Evidence for insulin resistance involving glucose and amino acid metabolism. J Clin Invest 77: 1797–1804, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tipton KD, Borsheim E, Wolf SE, Sanford AP, Wolfe RR. Acute response of net muscle protein balance reflects 24-h balance after exercise and amino acid ingestion. Am J Physiol Endocrinol Metab 284: E76–E89, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Tipton KD, Ferrando AA, Phillips SM, Doyle D, Wolfe RR. Postexercise net protein synthesis in human muscle from orally administered amino acids. Am J Physiol Endocrinol Metab 276: E628–E634, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Volpi E, Ferrando AA, Yeckel CW, Tipton KD, Wolfe RR. Exogenous amino acids stimulate net muscle protein synthesis in the elderly. J Clin Invest 101: 2000–2007, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Volpi E, Mittendorfer B, Wolf SE, Wolfe RR. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am J Physiol Endocrinol Metab 277: E513–E520, 1999. [DOI] [PubMed] [Google Scholar]
- 31.Witard OC, Jackman SR, Breen L, Smith K, Selby A, Tipton KD. Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Am J Clin Nutr 99: 86–95, 2014. [DOI] [PubMed] [Google Scholar]
- 32.Wolfe RR, Chinkes DL. Isotope Tracers in Metabolic Research (2nd ed). Hoboken, NJ: John Wiley & Sons, 2005. [Google Scholar]
- 33.Wolfe RR. Effects of insulin on muscle tissue. Curr Opin Clin Nutr Metab Care 3: 67–71, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Yang Y, Breen L, Burd NA, Hector AJ, Churchward-Venne TA, Josse AR, Tarnopolsky MA, Phillips SM. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br J Nutr 108: 1780–1788, 2012. [DOI] [PubMed] [Google Scholar]