Abstract
Binding of the cardiac hormone atrial natriuretic peptide (ANP) to transmembrane guanylyl cyclase/natriuretic peptide receptor-A (GC-A/NPRA), produces the intracellular second messenger cGMP in target cells. To delineate the critical role of an endocytic signal in intracellular sorting of the receptor, we have identified a FQQI (Phe790, Gln791, Gln792, and Ile793) motif in the carboxyl-terminal region of NPRA. Mouse mesangial cells (MMCs) were transiently transfected with the enhanced green fluorescence protein (eGFP)-tagged wild-type (WT) and mutant constructs of eGFP-NPRA. The mutation FQQI/AAAA, in the eGFP-NPRA cDNA sequence, markedly attenuated the internalization of mutant receptors by almost 49% compared with the WT receptor. Interestingly, we show that the μ1B subunit of adaptor protein-1 binds directly to a phenylalanine-based FQQI motif in the cytoplasmic tail of the receptor. However, subcellular trafficking indicated that immunofluorescence colocalization of the mutated receptor with early endosome antigen-1 (EEA-1), lysosome-associated membrane protein-1 (LAMP-1), and Rab 11 marker was decreased by 57% in early endosomes, 48% in lysosomes, and 42% in recycling endosomes, respectively, compared with the WT receptor in MMCs. The receptor containing the mutated motif (FQQI/AAAA) also produced a significantly decreased level of intracellular cGMP during subcellular trafficking than the WT receptor. The coimmunoprecipitation assay confirmed a decreased level of colocalization of the mutant receptor with subcellular compartments during endocytic processes. The results suggest that the FQQI motif is essential for the internalization and subcellular trafficking of NPRA during the hormone signaling process in intact MMCs.
Keywords: guanylyl cyclase/natriuretic peptide receptor-A, immunofluorescence, receptor internalization, trafficking, mouse mesangial cells
atrial natriuretic peptide (ANP) is a member of the natriuretic peptide (NP) hormone family that consists of ANP, brain natriuretic peptide (BNP), and C-type natriuretic peptide (CNP), and each is derived from a separate gene (55). NPs are synthesized as preprohormones, which exhibit a number of vascular, renal, and endocrine effects, largely directed toward the reduction of blood pressure and extracellular fluid volume (10, 24, 36). There are three known NP receptors, namely, NP receptor-A, -B, and -C (NPRA, NPRB, and NPRC). Both NPRA and NPRB contain guanylyl cyclase (GC) activity and are also designated as GC-A, GC-B, while NPRC is known by default as the clearance receptor. Among three receptors, NPRA and NPRB contain an extracellular ligand-binding domain, a single transmembrane region, and intracellular protein kinase-like homology (KHD) and GC catalytic domains (21, 46). ANP and BNP activate NPRA, which produces second messenger cGMP in response to hormone binding. CNP activates NPRB that also generates cGMP; however, all three natriuretic peptides arbitrarily bind to NPRC, which lacks GC activity (2, 24, 32, 36, 37). The amount and duration of receptor signaling are strongly regulated by intracellular trafficking; however, receptors are removed from the cell surface by endocytosis, pass through the endosomal system, and are either recycled back to the plasma membrane or delivered to the lysosomes, thus resulting in receptor downregulation and degradation (50, 60). NPRA is considered to be the biological receptor of ANP and BNP because most of the physiological effects of these peptide hormones are activated by the production of second messenger cGMP (29, 36, 37, 58). Previously, it has been reported that cGMP interacts with three different types of intracellular effector proteins, which include cGMP-dependent protein kinases, cGMP-regulated ion channels, and cGMP-activated phosphodiesterases (26). It is possible that cGMP binding proteins transduce the cGMP signal to alter cellular function through various mechanisms, including stimulation of protein phosphorylation and/or by an inhibitory effect on this process (23, 57).
Upon ligand binding, NPRA dimerizes and the GC catalytic domain becomes activated (66). ANP binding also activates efficient internalization of NPRA in the cell interior; however, the trafficking itinerary of NPRA into the subcellular compartments has not been clearly established (45). Although considerable progress was made on the structure-function studies of NPRA, the issue of internalization remained controversial until recently. In the past, there has been debate over whether ANP/NPRA complexes were internalized at all or whether the cell utilized some other mechanisms to release ANP from its receptor. Indeed, controversy existed because it was reported by default that among the three NP receptors only NPRC was internalized with bound ligand (20). Those previous studies indicated that endogenous NPRA was not internalized in cultured renomedullary interstitial cells and suggested that a rapid dissociation of ligand-receptor complexes occurred upon ANP binding to NPRA at 37°C. However, it is difficult to contemplate the findings of those previous studies since the dissociation of ligand was carried out in a medium containing high concentrations of unlabeled ANP to preclude the rebinding of dissociated ligand to the receptor. Another study using the same binding protocols also suggested that intact 125I-ANP was released into medium in 293 T cells expressing NPRA (12). However, later these authors acknowledged that the absence of ANP-NPRA internalization in 293 T cells might have resulted from the slow rate of ligand degradation in a cell-specific manner (14). On the contrary, in several studies by us and others utilizing stoichiometric binding analyses, ANP/NPRA complexes have provided strong evidence that the bound ligand-receptor complexes are internalized, processed intracellularly, and degraded products are released into the culture medium in MA-10 and PC-12 cells containing endogenous receptors (40, 41, 52, 53) as well as in COS-7 and HEK-293 cells expressing recombinant receptors (42, 43, 59). Interestingly, NPRB has also been shown to be internalized and recycled back to the cell surface in cultured hippocampus neurons and C6 glioma cells in response to CNP binding (3). Thus the prevailing consensus is that in response to ligand binding both NPRA and NPRB are internalized into the cell interior. Now, it is important to visualize the cellular trafficking and redistribution of ANP/NPRA complexes in intact cells. The present work was undertaken to resolve this important issue and to elucidate unequivocally the ligand-dependent endocytosis and intracellular trafficking of NPRA by confocal immunofluorescence and coimmunoprecipitation of NPRA with organelle-specific marker proteins in enhanced green fluorescent protein (eGFP)/NPRA-transfected mouse mesangial cells (MMCs), a cell system closer to physiological relevance.
The short-sequence motifs located in the carboxyl-terminal domains of a large number of membrane receptors play an instrumental role in receptor internalization and redistribution in intracellular compartments (13, 38, 39). The region encoded in the carboxyl-terminal domain of NPRA contains one copy of the tetrapeptide sequence of FQQI. The objective of our present study was to determine whether the FQQI motif plays a role in ligand-mediated internalization and intracellular trafficking of NPRA in cultured primary MMCs. In the current study, we have identified the short-sequence motif FQQI as the critical structural determinant that controls the receptor activation, internalization, sorting, and subsequent intracellular signaling of NPRA in intact MMCs. The present findings provide direct evidence that the FQQI motif is an important motif, which is involved in ligand-mediated internalization, trafficking, and signaling of NPRA in the physiological context in MMCs.
MATERIALS AND METHODS
ANP (rat-28) and C-atrial natriuretic factor receptor ligand [C-ANF(4–18)] were purchased from Bachem Americas (Torrance, CA). 125I-ANP was purchased from PerkinElmer NEN (Shelton, CT). A QuickChange site-directed mutagenesis kit and β-X-galactosidase (β-X-gal) staining kit were from Stratagene (La Jolla, CA). Tissue culture supplies and 10% goat normal serum were purchased from Invitrogen/Life Technologies (Grand Island, NY). BSA was obtained from Polysciences (Warrington, PA). Plasma membrane marker anti-pan-cadherin, recycling endosome marker anti-Rab 11 antibodies, and normal donkey serum were from Abcam (Cambridge, MA). Texas Red anti-rabbit IgG (H+L) and 4′,6-diamidino-2-phenylindole (DAPI) were obtained from Vector Laboratories (Burlingame, CA). Rabbit polyclonal antibody early endosome antigen-1 (EEA-1), lysosome-associated membrane protein-1 (LAMP-1), goat polyclonal antibody adaptor-related protein complex 1, μ1B subunit (AP-1 μ1B), donkey anti-goat IgG, F(ab′)2-PE-Cy5 secondary antibody, and protein A/G PLUS-Agarose immunoprecipitation reagent were purchased from Santa Cruz Biotechnology (San Diego, CA). Protein-A agarose beads were purchased from Cell Signaling Technology (Danvers, MA). Mouse monoclonal eGFP antibody was purchased from Clontech (Mountain View, CA). Rabbit polyclonal cGMP antibody was purchased from Antibodies Online (Atlanta, GA), and cGMP complete-enzyme-linked immunosorbent assay (EIA) kit was obtained from Enzo Life Sciences (Plymouth Meeting, PA). Synthetic oligonucleotides primers were obtained from MWG Operon (Huntsville, AL). TransIT-LT1 transfection reagent was purchased from Mirus Bio (Madison, WI). Paraformaldehyde, saponin, and all other chemicals were reagent grade and obtained from Sigma (St. Louis, MO).
Construction of eGFP-NPRA chimeric vector and site-directed mutagenesis.
The fusion of C-terminus murine NPRA cDNA (46) to the N terminus of eGFP was done by subcloning it into a pcGFP-NT vector (Clontech, San Diego, CA) for its expression as a wild-type (WT) eGFP-NPRA fusion protein. We used site-directed mutagenesis to create a SmaI site to remove the stop codon in NPRA cDNA and bring it into the same reading frame as eGFP, using a Quick Change site-directed mutagenesis kit (Stratagene) according to our established methods (67). Mutagenesis was carried out using sense 5′-GCAGCTCTCGAGCCCGGGCTACTGCCCTGCTATTCC-3′ and antisense 5′-GGAATA GCAGGGCAGTAGCCCGGGCTCGAGAGCTGC-3′ primers. The mutated NPRA fragment was recloned into the mammalian expression vector peGFP-NT. The eGFP-NPRA cDNA construct was used for the mutation of the FQQI motif. The individual amino acids in the FQQI sequence along with two additional residues on each side of this motif were mutated as follows: single amino acids Pro788, Pro789, Phe790, Ile793, Arg794, and Leu795 and all four amino acids Phe790, Gln791, Gln792, and Ile793 were mutated to Ala residues. The oligonucleotide-mediated mutagenesis was performed using the Quick Change site-directed mutagenesis kit. The sequences of FQQI/AAAA mutagenic sense 5′-CAGGAGCGGCCACCCGCTGCTGCTGCTCGCCTGGCGCTGCGC-3′ and anti-sense 5′-GCGCAGCGCCAGGCGAGCAGCAGCAGCGGGTGGCCGCTCCTG-3′ primers were used, where the underlined nucleotides indicate mutations F790A (Phe790Ala), Q791A, Q792A, and I793A, respectively. The integrity of the mutated plasmid sequence was confirmed by double stranded DNA sequencing in the mutagenized region of NPRA. The nucleotide sequence was determined at the Center for Gene Therapy Sequencing Facility, Tulane University School of Medicine.
Cell culture and transient transfection of WT and mutant eGFP-NPRA.
For cell preparations, animals were used and handled under the protocols approved by the Institutional Animal Care and Use Committee at the Tulane University Health Sciences Center. MMCs were isolated from glomeruli and cultured in DMEM containing insulin-transferrin-sodium selenite (ITS) and 10% FBS as previously described (44). Cells were used between passages 5 and 15, and cultures were maintained at 37°C in an atmosphere of 5% CO2-95% O2. For eGFP-NPRA-WT and mutated overexpression experiments, 1 × 106 cells were transiently transfected with WT and mutant eGFP-NPRA cDNA constructs in cells using electroporation at 220 mV with a capacitance setting of 960 μF and seeded on a cover glass in six-well plates or 6-cm2 culture dishes. Cells were then synchronized by serum starvation by incubating in serum-free medium containing 0.1% BSA for 24 h. For hormonal treatment, cells were treated with the presence or absence of 100 nM ANP for different time periods (5, 10, 15, and 30 min). The reproducibility of transfection efficiency was examined using the β-X-gal staining kit. In the present experiments using electroporation, ∼70–75% transiently transfected cells were able to harbor recombinant eGFP-NPRA cDNA molecules.
Cell permeabilization and immunofluorescence staining.
For all immunofluorescence studies, the transfected cells were seeded on a cover glass and grown for 2 days. Cells were treated with ANP, fixed in 4% paraformaldehyde for 30 min, permeabilized in PBS containing 0.1% BSA and 0.2% saponin, then incubated for 10 min at room temperature. Cells were blocked with 1% normal goat serum or donkey serum, 0.1% saponin, and 1% BSA for 1 h at room temperature, then labeled with anti-EEA-1 (1:400), anti-LAMP-1 (1:200), anti-Rab 11 (1:500), or anti-AP-1 μ1B antibodies (1:200) in blocking buffer overnight at 4°C. Samples were incubated with secondary antibody anti-rabbit IgG (1:4,000) conjugated with Texas Red or anti-goat IgG (1:2,000) conjugated with Cy5 for 2 h at room temperature in the dark. The cells were washed three times for 15 min each in PBS. The cover glass was allowed to dry, then was mounted on a glass slide with DAPI containing Vectashield mounting media (Vector Laboratories, Burlingame, CA), and then sealed with Fixogum rubber cement.
Confocal microscopy and image analysis.
Cells were examined and images acquired using a TCS SP2 confocal laser-scanning microscope (Leica Microsystems, Heidelberg, Germany). In all experiments, cells were visualized using the same confocal microscope settings (i.e., sequential scans with wavelengths set as follows: blue, 358–461; green, 488–510; red, 594–615; magenta, 649–670), using a ×63 Apo-oil immersion objective (NA 1.4) and 60-μm aperture using Leica Scan TCS-SP2 software (Leica Microsystems). The pinhole was adjusted to keep the same size of z-optical sections (1-μm z-axis) for all channels. In all the experiments, images of cells were acquired as single midcellular optical sections and averaged over eight scans/frame. To measure colocalization analysis, colocalization plugins in MetaMorph software (Molecular Devices, Downingtown, PA) were utilized.
Quantification of NPRA colocalization.
The MetaMorph colocalization application (Molecular Devices) plugins were used to quantify colocalization between eGFP-NPRA (green channels) and EEA-1, LAMP-1, Rab 11(red channels), or AP-1 μ1B (magenta channels) in individual cells. In brief, 50 cells or more (3 coverslips/experiment/condition) were scored (WT and mutated receptors) per condition using a confocal microscope (Leica) with a ×63/1.4 NA Plan-Apochromat oil immersion objective lens using the 488 green channel for eGFP-NPRA, 594 red channel for Texas Red, or 649 for far-red Cy5-conjugated secondary antibody. For quantification of colocalization, a minimum threshold of green and red or magenta channels was selected. The background (median) was subtracted from the original images (using process: Arithmetic). The actual images were analyzed (Choose Apps: measure colocalization), and the percentage of colocalization was calculated from the amount of colocalized areas from the total green or red or magenta area. Graph Prism software was used to generate all the bar graphs and statistical analysis of the data. The results are presented as means ± SE of four to five independent experiments.
Transfection of micro-RNA.
Cells expressing endogenous WT NPRA were grown in 6-cm2 culture dishes containing DMEM supplemented with 10% (vol/vol) FBS. Five micrograms of purified pcDNA-6.2-GW/GFP-miR expression vector containing Npr1-miRNA insert pCMVNpr1micro (mi)RNA-1595 and pCMV-Npr1miRNA-2931 were transfected in cells with the TransIT-LT1 reagent as previously described (59). After knockdown of the receptor through miRNA, WT and the mutated constructs of eGFP-NPRA cDNA were transiently transfected in cells using TransIT-LT1 transfected reagent according to the manufacturer's instructions.
Subcellular fractionation.
For subcellular fractionation, transiently transfected cells with WT and mutated receptors were lysed in a buffer containing 5 vol of 10 mM sodium phosphate (pH 7.4), 250 mM sucrose, 150 mM NaCl, 5 mM EDTA, 1 mM PMSF, 5 mM benzamidine, 10 μg/ml leupeptin, and 10 μg/ml aprotinin, essentially as previously described (19, 56) with minor modification. Briefly, the cells were homogenized in a Dounce homogenizer, and cellular debris was cleared by centrifugation at 1,000 g for 5 min at 4°C. The supernatant was collected, and the pellet was suspended in lysis buffer, homogenized, and centrifuged as above. Both supernatants were pooled and centrifuged at 100,000 g for 1 h at 4°C. The supernatant was collected, which represents the cytosolic fraction. The 100,000-g pellet was washed twice with lysis buffer and resuspended in 1 ml solubilization buffer containing 0.5% n-dodecyl β-d-maltoside (DDM), 75 mM Tris·HCl (pH 8.0), 2 mM EDTA, 5 mM MgCl2, 1 mM PMSF, 5 mM benzamidine, 10 μg/ml leupeptin, and 10 μg/ml aprotinin and then incubated overnight at 4°C on a rocker. The lysate was centrifuged at 60,000 g for 30 min to separate insoluble fractions from solubilized membranes. Proteins were quantified using the Bradford assay (Bio-Rad) and subjected to immunoprecipitation.
Coimmunoprecipitation of eGFP-NPRA-WT and mutated motif.
The subcellular fractions for the coimmunoprecipitation of WT or mutated eGFP-NPRA with plasma membranes, early endosomes, lysosomes, and recycling endosomes were prepared as described above under subcellular fractionation. The solubilized membrane and the cytosolic fraction resulting from 100,000-g centrifugation were subjected to protein quantification (Bio-Rad). Fifty micrograms of protein samples were used for immunoblot analysis representing the input before immunoprecipitation. In all cases, 500 μg of solubilized membranes or cytosolic fractions were incubated with 4 μg of the primary antibodies for 4 h at 4°C on a rocker. Next, a 50-μl agarose conjugate suspension (protein A or protein A/G agarose beads) was added and incubated overnight at 4°C on a rocker platform. After 18 h, the supernatant was removed by centrifugation at 3,000 rpm for 1 min at 4°C and the beads were washed three times with a buffer containing 1 mM Tris·HCl (pH 7.5), 1 mM EDTA, 150 mM NaCl, 0.1% Triton X-100, and 10% glycine and each time centrifuged at 3,600 g for 1 min at 4°C. The pellet was resuspended in 50 μl of 2× electrophoresis sample buffer boiled for 5 min and subjected to SDS-PAGE.
Western blot analysis.
For electrophoresis, 50 μg of protein samples were mixed with sample loading buffer, boiled, and resolved by 10% SDS-PAGE. Proteins were electrophoretically transferred onto a polyvinylidene fluoride (PVDF) membrane, which was then blocked with 5% fat-free milk solution in 1× Tris-buffered saline-Tween 20 (TBST) for 2 h at room temperature. The membrane was incubated with primary antibody of NPRA (1:1,000), eGFP (1:1,000), pan-cadherin (1:500), μ1B (1:200), EEA-1 (1:1,000), LAMP-1 (1:500), and Rab 11(1:1,000) overnight at 4°C in blocking solution and treated with secondary horseradish peroxidase (HRP)-conjugated antibody (1:5,000) for 2 h at room temperature. Protein bands were visualized using enhanced chemiluminescence (ECL) plus a detection system from Alpha-Innotech. The density of protein bands was determined using the Alpha Innotech Imaging System (San Leandro, CA).
Cell surface 125I-ANP binding assay.
Cells were transiently transfected with pcDNA-6.2-GW/GFP-miR expression vector containing Npr1-miRNA inserts pCMVNpr1miRNA-1595 and pCMV-Npr1miRNA-2931 to inhibit the endogenous expression of NPRA. After knockdown of the endogenous expression of NPRA, cells were transiently transfected with WT and mutated eGFP-NPRA. After transient transfection, confluent cells in 6-cm2 dishes were washed twice with 2 ml of assay medium (DMEM containing 0.1% BSA) pretreated with 100 nM C-ANF to block the NPRC, and then treated with 125I-ANP at 4°C for 1 h in the absence or presence of 100-fold excess unlabeled ANP. After completion of binding at 4°C, free ligand was removed from the dishes by four washes (2 ml each wash) with ice-cold assay medium. To determine the cell surface-associated radioactivity, the acid wash procedure was utilized as described previously (45). After binding was completed, each culture dish received 1 ml of ice-cold acetate buffer, pH 3.5, and cells were placed at 4°C for 2 min. The acid eluates from the dishes were collected, and each dish received another 1 ml of ice-cold acid buffer to wash the cells. Both solutions were combined to determine acid-sensitive radioactivity. Cells were then dissolved in 1 N NaOH, and acid-resistant radioactivity was determined. The acid-sensitive radioactivity was accounted as an index of cell surface-bound 125I-ANP, and the acid-resistant radioactivity was used as a measurement of the internalized ligand-receptor complexes.
Internalization of ligand-receptor complexes.
MMCs transiently expressing WT or mutant eGFP-NPRA were allowed to bind 125I-ANP by incubation at 4°C for 60 min in the presence of 100 nM C-ANF to block NPRC. The unbound 125I-ANP was removed by washing cells with ice-cold assay medium. The total cell-associated radioactivity was determined by dissolving cells in 1 N NaOH and counting the radioactivity in the cell lysate. This represented the initial zero time control value of 100%. To permit the internalization of ligand-receptor complexes, cells were warmed quickly to 37°C. At the indicated times, the culture dishes were removed from 37°C and placed on ice, and media were collected. The cell surface-associated radioactivity was removed by washing the cells with ice-cold acetate buffer (pH 3.5) at 4°C. After acid wash, the internalized 125I-ANP radioactivity was determined by dissolving cells in 1 N NaOH. To assess the internalization of ligand-receptor complexes at the indicated time intervals, culture dishes were removed from 37°C, the medium was collected, surface-associated radioactivity was removed by acetate buffer (pH 3.5), and cells were dissolved in 1 N NaOH. The radioactivity in the acid eluate, cell lysate, and culture medium was considered as cell surface-associated, internalized, and released into the medium, respectively. The quantitation of intact and degraded ligand released into the culture medium after internalization of ligand-receptor complexes was performed by precipitation of medium with 10% TCA containing 200 μg/ml BSA as a carrier. The recovered 125I-ANP in the TCA precipitate was considered intact 125I-ANP molecules, and those in the supernatant were regarded as degraded products as described previously (42).
Assay and immunofluorescence of cGMP.
Cells were transiently transfected with pcDNA-6.2-GW/GFP-miR expression vector containing Npr1-miRNA insert pCMV Npr1miRNA-1595 and pCMV-Npr1miRNA-2931 to inhibit the endogenous expression of NPRA. After knockdown of the endogenous expression of NPRA, cells were transiently transfected with WT and mutated eGFP-NPRA. Cells were treated with 100 nM ANP for different time courses (5, 10, 15, and 30 min) in the presence of 0.2 mM IBMX. Cells were washed three times with PBS and scraped in 0.1 M HCl. The cell suspension was subjected to five cycles of freeze and thaw and then centrifuged at 10,000 g for 15 min at 4°C. The supernatant was collected for cGMP assay using a direct cGMP complete-EIA kit (Enzo Life Sciences) according to the manufacturer's protocol. To visualize intracellular accumulations of cGMP, immunofluorescence staining was done as described previously (63, 69), with minor modification. Cells were treated with 100 nM ANP for 10 min in the presence of 0.2 mM IBMX. Cells were fixed in 4% paraformaldehyde for 10 min, permeabilized in PBS containing 0.1% BSA/0.2% saponin, and then incubated for 10 min at room temperature. Cells were blocked with 1% normal goat serum, 0.1% saponin, and 1% BSA in PBS for 1 h at room temperature, then labeled with anti-cGMP antibodies (1:1,000) in blocking buffer overnight at 4°C. Samples were incubated with secondary antibody anti-rabbit IgG (1:2,000) conjugated with Texas Red for 2 h at room temperature in the dark. The cells were washed three times for 15 min each in PBS. The cover glass was allowed to dry and mounted on glass slide with DAPI containing Vectashield mounting media (Vector Laboratories), then sealed with Fixogum rubber cement.
Statistical analysis.
Results are presented as means ± SE of the average responses in multiple experiments done with different cell preparations. Results were normalized relative to untreated controls. Statistical significance was assessed using ANOVA followed by Dunnett's multiple comparisons test. Student's unpaired t-test with two-tailed analysis was also performed for comparison between WT and the mutated receptor. The probability value of P < 0.05 was considered significant.
RESULTS
Internalization of eGFP-NPRA containing alanine mutations at different positions in the C-terminal FQQI motif.
The present results demonstrate that the carboxyl-terminal domain of NPRA contains one copy of the tetrapeptide sequence FQQI, which is important for receptor internalization and intracellular trafficking of NPRA from the cell surface into the subcellular compartments in cultured primary MMCs. We performed site-directed mutagenesis of the FQQI sequence in the eGFP-NPRA cDNA construct, which resulted in defective internalization and intracellular trafficking of the eGFP-NPRA fusion protein. A schematic diagram represents the extracellular, transmembrane, and GC catalytic domains of NPRA (Fig. 1A). The substituted amino acid residues with alanine in the FQQI motif are indicated in Fig. 1B. The expression of 162-kDa fusion proteins of WT and FQQI mutant receptors is indicated in Fig. 1C. A different combination of alanine was substituted for each of the amino acids in the conserved region from positions 788–795 in the C-terminal domain of eGFP-NPRA. A comparison was made between the cells expressing one or more of the eGFP-NPRA mutations with the cells expressing the WT receptor (Fig. 1D). Four of the alanine substitutions in the FQQI region decreased the internalization of the mutant receptor by almost 50% compared with the WT receptor. Two of the amino acids, including Phe790 and Ile793 in the FQQI motif, are important for receptor internalization. The substitutions of two amino acids on either side of the FQQI motif did not show any effect on internalization of eGFP-NPRA (Fig. 1D).
Fig. 1.
Schematic representation of the intracellular trafficking motif, expression, and amino acid residue requirements (AAAA, AQQA, AQQI, and FQQA) for internalization of wild-type (WT) and mutant (natriuretic peptide receptor-A; NPRA) in intact mouse mesangial cells (MMCs). A: topology indicating the extracellular, transmembrane (TM), and cytoplasmic regions of NPRA. The internalization motif analyzed corresponds to amino acids 790–793. eGFP, enhanced green fluorescent protein. B: amino acid sequence of the WT internalization motif in the carboxyl-terminal domain of NPRA as indicated by the single letter code. Δ790–793 indicates the internal substitution of residues Phe790, Gln791, Gln792, and Ile793 with alanine. C: lanes a, b, c, d, and e represents the expressed WT and mutant receptor (AAAA, AQQA, AQQI, and FQQA) bands in MMCs, respectively. The arrow indicates the position of 162-kDa eGFP-NPRA fusion protein band. D: alanine substitutions at amino acid positions 788–795 and in different combinations in the FQQI motif. Confluent MMCs in 6-cm2 dishes transiently transfected with WT and mutant receptors were pretreated with 100 nM C-atrial natriuretic factor receptor ligand (C-ANF) to block the NPRC, and then treated with 125I-labeled atrial natriuretic peptide (ANP) at 4°C for 1 h in the absence or presence of unlabeled ANP. After 15 min of incubation, the internalization of ligand-receptor complexes was quantified as described in materials and methods. Values are means ± SE of 6 separate experiments in triplicate. **P < 0.01, ***P < 0.001 relative to WT receptor.
Kinetics of internalization and intracellular sequestration of WT and mutant eGFP-NPRA in MMCs.
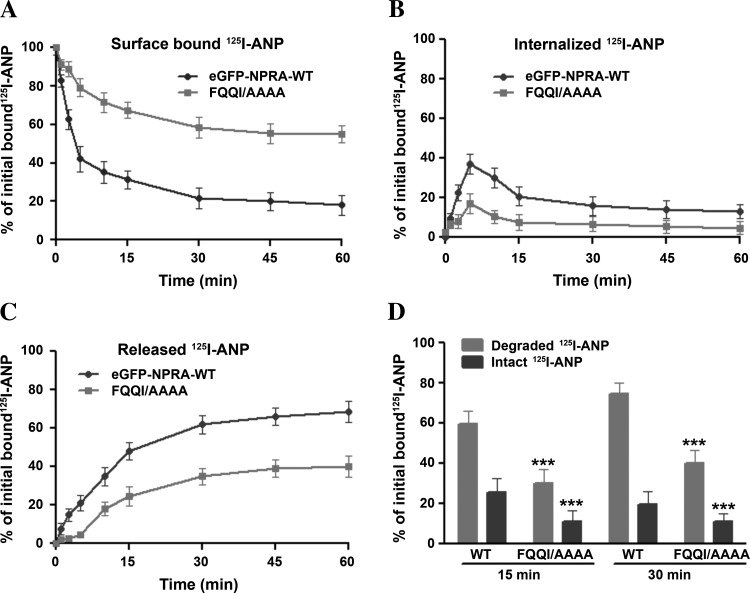
We determined the cell surface-associated, internalized, and released 125I-ANP radioactivity in MMCs expressing either the WT or FQQI/AAAA mutant receptor utilizing the acid wash procedure. MMCs expressing WT eGFP-NPRA showed a rapid internalization of ligand-receptor complexes, with almost 60- 65% of the receptors internalized after 5 min at 37°C. On the contrary, only 30–35% of the receptors were internalized in cells expressing the FQQI/AAAA mutant receptor at the same incubation time periods (Fig. 2A). The amount of internalized receptor decreased rapidly after 5 min at 37°C, indicating that almost 80% WT eGFP-NPRA was released into the medium compared with only 40% of mutant eGFP-NPRA. Approximately 40% of 125I-ANP was bound to WT eGFP-NPRA in the intracellular compartments, which was resistant to removal by acid wash. In contrast, only 20% of the 125I-ANP-bound ligand-receptor complexes were localized in the intracellular compartments in MMCs expressing FQQI/AAAA mutant eGFP-NPRA (Fig. 2B). We observed that a large proportion of ligand-receptor complexes were not internalized and remained on the plasma membrane in cells expressing the FQQI/AAAA mutant receptor. The release of 125I-ANP into the culture medium of cells expressing WT eGFP-NPRA increased progressively, reaching 70–80% after 30 min incubation at 37°C. However, only 40–45% of 125I-ANP was released into the culture medium of cells expressing FQQI/AAAA mutant eGFP-NPRA (Fig. 2C). A quantitative assessment of the intact and degraded ligand was performed by measuring the solubility of 125I-ANP products in 10% TCA solution. The TCA precipitates (containing intact ligand) and supernatants (containing degraded ligand products) were separated by centrifugation of TCA-soluble extracts. Higher amounts of both intact and degraded ligand products were found in the culture medium of cells expressing eGFP-NPRA-WT compared with the culture medium of cells expressing the FQQI/AAAA mutant receptor. The total released 125I-ANP was composed of ∼50–55% of degraded ligand products and 20–25% of intact ligand in the culture medium of cells expressing the WT receptor (Fig. 2D). On the other hand, the total 125I-ANP released into the culture medium of cells expressing the mutant receptor was composed of 30–35% of degraded products and only 10–15% of intact ligand. The degraded and intact ligand released into the culture medium were quantitatively determined on the basis of the amount of total initial 125I-ANP bound on the cell surface.
Fig. 2.
Kinetics of cell surface-associated, internalized, and released 125I-ANP radioactivity in MMCs transiently expressing WT or FQQI/AAAA mutant receptors. Cells were transiently transfected with pcDNA-6.2-GW/GFP-microRNA (miR) expression vector containing Npr1-miRNA inserts pCMVNpr1miRNA-1595 and pCMV-Npr1miRNA-2931 to inhibit the endogenous expression of NPRA. After knockdown of the endogenous expression of NPRA, cells were transiently transfected with WT and mutated eGFP-NPRA. After transient transfection, confluent cells in 6-cm2 dishes were pretreated with 100 nM C-ANF to block the NPRC, then labeled with 125I-ANP at 4°C for 1 h in the absence or presence of unlabeled ANP. Cells were washed 4 times with assay medium and incubated in 2 ml of fresh medium at 37°C. At the indicated time points, the cell surface-associated (acid-sensitive) radioactivity was eluted with acetate buffer (pH 3.5), and cells were dissolved in 1 N NaOH to measure the internalized (acid-resistant) radioactivity. Cell surface-associated (A), internalized (B), and released (C) 125I-ANP radioactivity was determined in the acid eluate, cell extract, and culture medium, respectively, as described in materials and methods. In the culture medium, the intact and degraded ligand (D) products were quantified as described in materials and methods. Values are means ± SE of 4 independent experiments in triplicate dishes. :***P < 0.001 relative to WT receptor.
Internalization and colocalization of eGFP-NPRA with AP-1 μ1B.
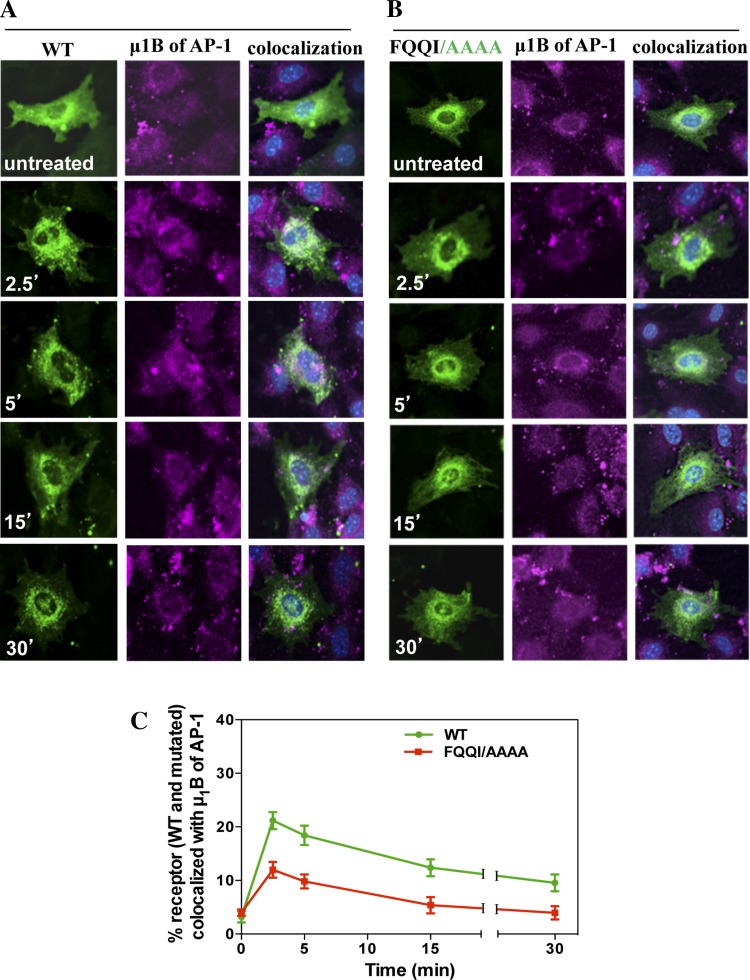
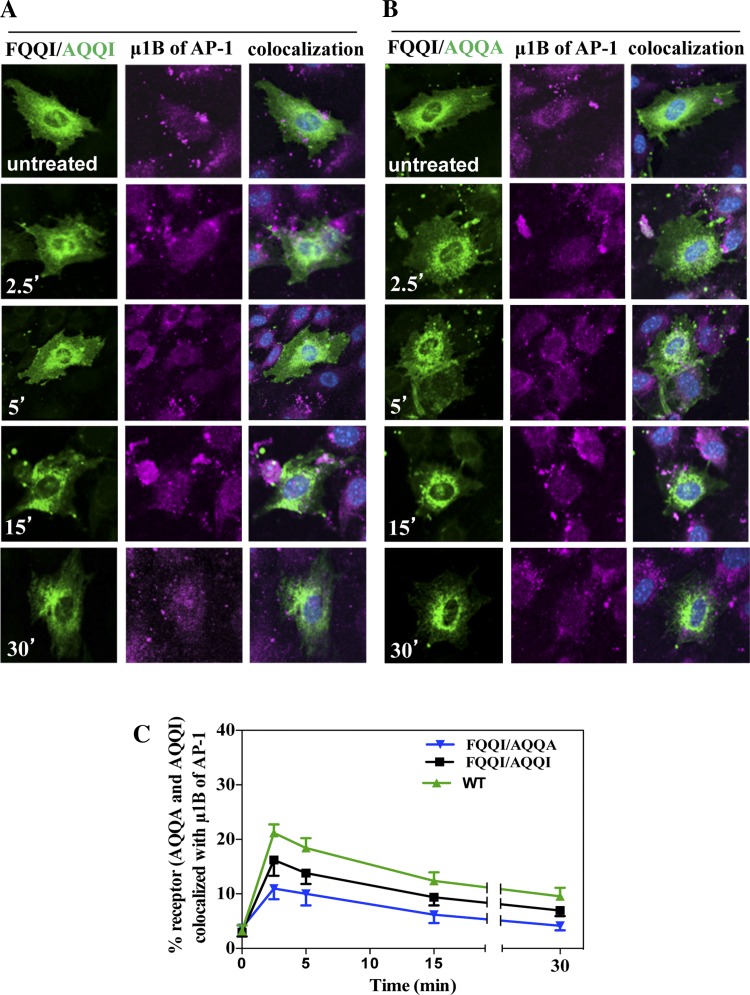
To investigate whether the FQQI motif interacts with AP-1 μ1B, we utilized the immunofluorescence staining of AP-1 μ1B using its specific antibodies. To examine the colocalization of eGFP-NPRA-WT with AP-1 μ1B, cells were treated with 100 nM ANP for different times (2.5, 5, 15, and 30 min). In the untreated cells, receptors were present in the plasma membrane. The colocalization of eGFP-NPRA-WT was observed with AP-1 μ1B. During the internalization, the receptor formed the complex with clathrin-coated adaptor protein. The colocalization of eGFP-NPRA with AP-1 μ1B was analyzed as 21% at 2.5 min, but gradually decreased to 18% at 5 min, 12% at 15 min, and 9% at 30 min. Colocalization analysis showed that localization of eGFP-NPRA-WT with AP-1 μ1B was maximum at 2.5 min and almost completed at 5 min, then gradually decreased from 15 to 30 min (Fig. 3A). Colocalization of eGFP-NPRA mutated motif FQQI/AAAA with AP-1 μ1B was analyzed as 12% at 2.5 min, 10% at 5 min, 5% at 15 min, and 4% at 30 min, respectively. Quantitative analysis of the colocalization measurement revealed that the density of eGFP-NPRA mutated motif FQQI/AAAA with AP-1 μ1B progressively decreased compared with the WT receptor (Fig. 3B). Colocalization of eGFP-NPRA mutated motif FQQI/AAAA with AP-1 μ1B was decreased by 50% (Fig. 3C). However, colocalization of eGFP-NPRA mutated motif FQQI/AQQI (Fig. 4A) was decreased by 23% and FQQI/AQQA (Fig. 4B) was decreased by 45% compared with the WT receptor. Quantitative analysis of the colocalization showed that the density of eGFP-NPRA with the mutated motif gradually decreased with AP-1 μ1B compared with the WT receptor (Fig. 4C).
Fig. 3.
Colocalization of internalized eGFP-NPRA-WT and mutated motif FQQI/AAAA with μ1B subunit of adaptor protein-1 (AP-1) in MMCs. Colocalization experiments were performed 48 h after transfection of cells with eGFP-NPRA-WT and mutated motif FQQI/AAAA. Cells were treated with 100 nM ANP for different times. A: colocalization of eGFP-NPRA-WT with μ1B of AP-1 was observed after 2.5- and 5-min treatment with ANP. B: colocalization of eGFP-NPRA mutated motif FQQI/AAAA with μ1B of AP-1 decreased by 50% compared with WT receptor. C: quantification of the percent colocalization of eGFP-NPRA-WT and mutated motif FQQI/AAAA with μ1B of AP-1. The images shown are typical of 5 independent experiments. Values are means ± SE.
Fig. 4.
Colocalization of internalized eGFP-NPRA mutated motif FQQI/AQQI or FQQI/AQQA with μ1B of AP-1 in cultured MMCs. Colocalization experiments were performed 48 h after transfection of cells with eGFP-NPRA mutated motifs FQQI/AQQI and FQQI/AQQA. Cells were treated with 100 nM ANP for different times. The mutation FQQI/AQQA decreased the receptor interaction with the adapter protein. Colocalization of eGFP-NPRA mutated motif FQQI/AQQI with μ1B of AP-1 was decreased by 23% (A) and FQQI/AQQA was decreased by 45% (B) compared with the WT receptor. C: quantification of the percent colocalization of eGFP-NPRA-WT, mutated motif FQQI/AQQI, and FQQI/AQQA with μ1B of AP-1. The images shown are typical of 5 independent experiments. Values are means ± SE.
Colocalization of internalized eGFP-NPRA with early endosomal marker EEA-1.
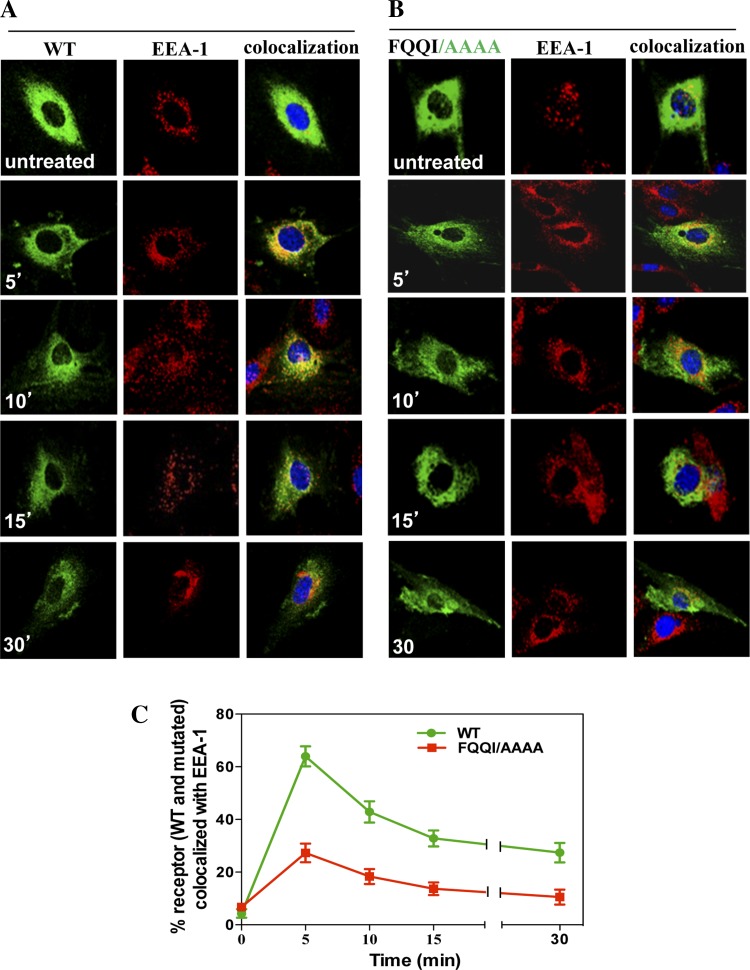
To examine the effect of the FQQI motif in the endosomal trafficking of receptors, we utilized specific antibodies to the endosomal marker protein. After internalization, the colocalization of eGFP-NPRA-WT was observed with the EEA-1 marker in early endosomes. The colocalization of eGFP-NPRA with EEA-1 was analyzed as 64% at 5 min, but gradually decreased to 42% at 10 min, 32% at 15 min, and 25% at 30 min, respectively. Colocalization analysis showed that the localization of eGFP-NPRA-WT with EEA-1 was maximum at 5 min, was almost completed at 10 min, and then gradually declined from 15 to 30 min (Fig. 5A). Colocalization of eGFP-NPRA mutated motif FQQI/AAAA with EEA-1 was analyzed as 27% at 5 min, 18% at 10 min, 14% at 15 min, and 10% at 30 min, respectively. Quantitative analysis of the colocalization measurement values indicated that the density of eGFP-NPRA with EEA-1 gradually decreased compared with the WT receptor (Fig. 5B). Colocalization of eGFP-NPRA mutated motif FQQI/AAAA with EEA-1 was decreased by 57% (Fig. 5C). However, colocalization of eGFP-NPRA mutated motif FQQI/AQQI (Fig. 6A) was decreased by 31%, and FQQI/AQQA (Fig. 6B) was decreased by 55% compared with the WT receptor. Quantitative analysis of the colocalization measurement revealed that the density of eGFP-NPRA with the mutated motif gradually decreased with EEA-1 compared with the WT receptor (Fig. 6C).
Fig. 5.
Colocalization of internalized eGFP-NPRA-WT and mutated motif FQQI/AAAA with early endosome antigen-1 (EEA-1) in MMCs. Colocalization experiments were performed 48 h after transfection of cells with eGFP-NPRA-WT and mutated motif FQQI/AAAA. Cells were treated with 100 nM ANP for different times. A: colocalization of eGFP-NPRA-WT and EEA-1 marker was observed after 5 and 10 min of treatment with ANP, after which the fluorescence intensity gradually decreased from 15 to 30 min. B: colocalization of mutant receptor FQQI/AAAA into endosomes. Colocalization of mutated eGFP-NPRA and EEA-1 marker was observed with a marked decrease by 57% compared with the WT receptor. C: quantification of the percent colocalization of eGFP-NPRA-WT and mutated motif FQQI/AAAA with EEA-1. The images shown are typical of the images obtained in 5 independent experiments. Values are means ± SE.
Fig. 6.
Colocalization of internalized eGFP-NPRA mutated motif FQQI/AQQI or FQQI/AQQA with EEA-1 in cultured MMCs. Colocalization experiments were performed 48 h after transfection of cells with eGFP-NPRA mutated motifs FQQI/AQQI and FQQI/AQQA. Cells were treated with 100 nM ANP for different times. The mutation of FQQI/AQQA significantly decreased the receptor colocalization into endosomes. Colocalization of eGFP-NPRA mutated motif FQQI/AQQI with EEA-1 was decreased by 31% (A) and FQQI/AQQA was decreased by 55% (B) compared with the WT receptor. C: quantification of percent colocalization of eGFP-NPRA-WT, mutated motif FQQI/AQQI, and FQQI/AQQA with EEA-1. The images shown are typical of 4–5 independent experiments. Values are means ± SE.
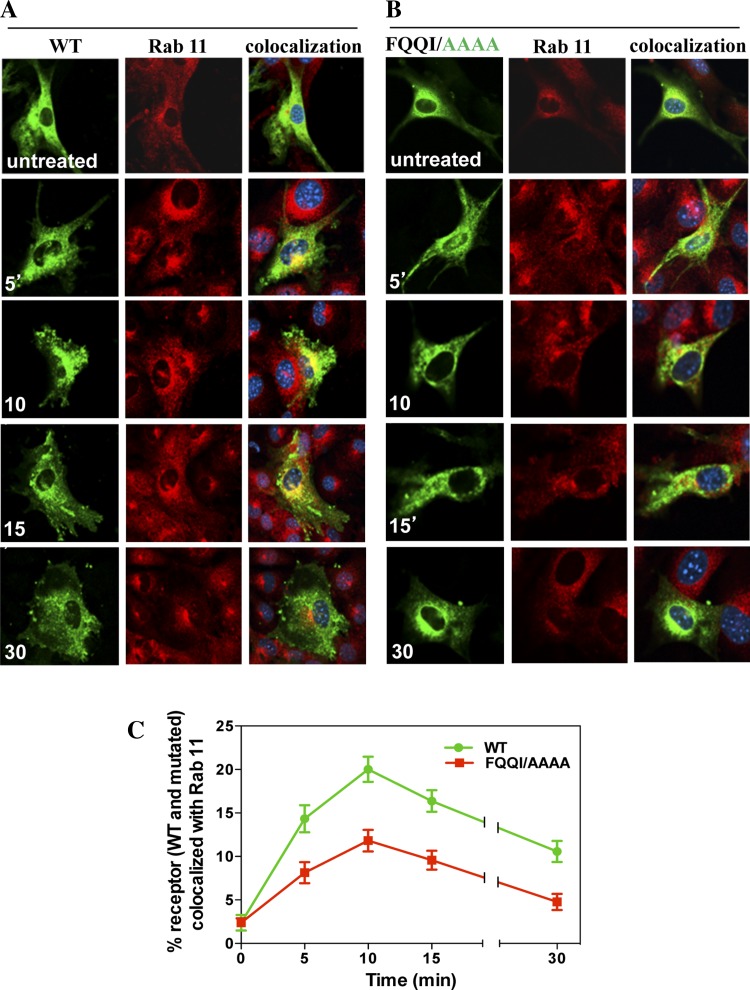
Trafficking and colocalization of eGFP-NPRA with recycling endosome marker Rab 11.
After subcellular trafficking of receptors into early endosomes, we also examined the effect of the FQQI motif in the subcellular trafficking of receptors into recycling endosomes by using the specific antibodies for Rab 11. Colocalization of eGFP-NPRA-WT with Rab 11 showed that NPRA recycled back to the plasma membrane through recycling endosomes. The percent colocalization value of eGFP-NPRA-WT with Rab 11 showed that receptor recycling accounted at 5 (14%), 10 (20%), 15 (16%), and 30 min (10%), respectively (Fig. 7A). On the other hand, mutated motif FQQI/AAAA (Fig. 7B), FQQI/AQQI, or FQQI/AQQA (data not shown) significantly inhibited the colocalization of the receptor with Rab 11 by 42 (Fig. 7C), 19, and 37% (data not shown), respectively.
Fig. 7.
Colocalization of internalized eGFP-NPRA-WT and mutated motif FQQI/AAAA with Rab 11 in MMCs. Colocalization experiments were performed 48 h after transfection of cells with eGFP-NPRA-WT and mutated motif FQQI/AAAA. Cells were treated with 100 nM ANP for different times. A: colocalization of eGFP-NPRA-WT with Rab 11 was observed after 5- and 15-min treatment with ANP. B: colocalization of eGFP-NPRA mutated motif FQQI/AAAA with Rab 11 marker decreased by 40%. C: quantification of percent colocalization of eGFP-NPRA-WT and mutated motif FQQI/AAAA with Rab 11. The images shown are typical of 5 independent experiments. Values are means ± SE.
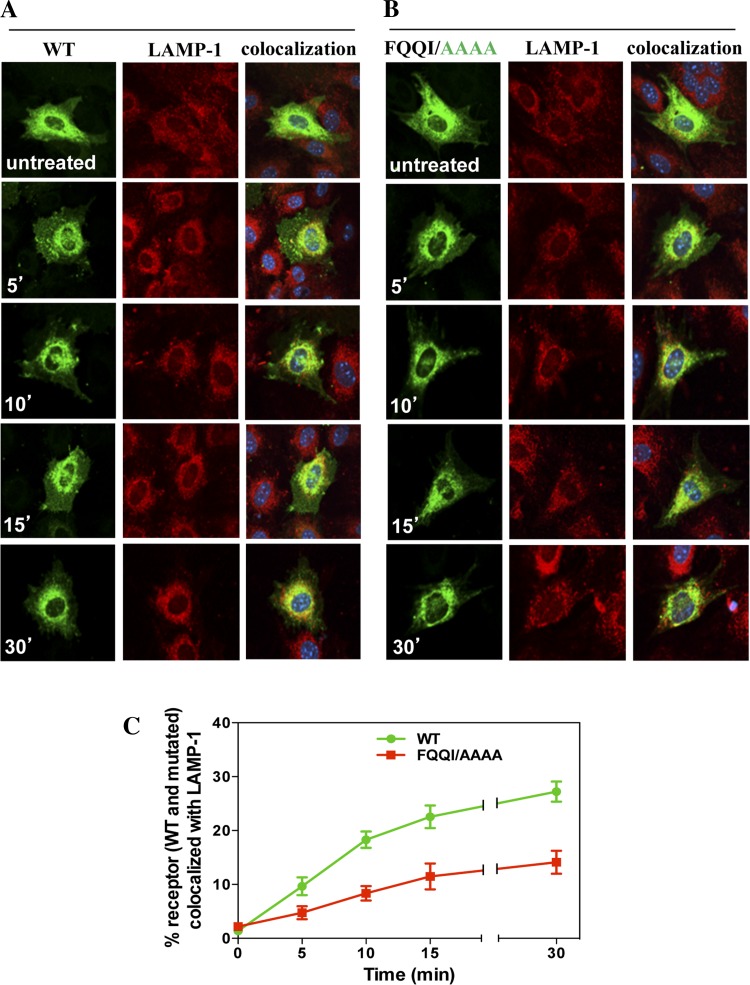
Colocalization of internalized eGFP-NPRA with lysosomal marker LAMP-1.
After internalization and subcellular trafficking of receptors into endosomes, a large population of receptors travelled into the lysosomes for degradation. We analyzed the effect of the FQQI motif in the subcellular trafficking of receptors into the lysosomes by using specific antibodies for LAMP-1. The subcellular trafficking of receptors was tracked by colocalization of eGFP-NPRA-WT with the lysosomal marker LAMP-1, which identifies the lysosomes. The colocalization of receptors with LAMP-1 increased at 5 (10%), 10 (18%), 15 (22%), and 30 min (27%), respectively. The colocalization measurement value demonstrated that accumulation of eGFP-NPRA-WT and LAMP-1 gradually increased after 10 min and was almost complete at 30 min (Fig. 8A). Colocalization of eGFP-NPRA mutated motif FQQI/AAAA with LAMP-1 was analyzed as 4% at 5 min, 8% at 10 min, 11% at 15 min, and 14% at 30 min, respectively. Quantitative analysis of the colocalization measurement values showed that the density of eGFP-NPRA with LAMP-1 gradually decreased compared with the WT receptor (Fig. 8B). However, colocalization of eGFP-NPRA mutated motif FQQI/AAAA with LAMP-1 was decreased by 48% (Fig. 8C). Colocalization of eGFP-NPRA mutated motif FQQI/AQQI was decreased by 30% and FQQI/AQQA was decreased by 44% compared with the WT receptor (data not shown). Quantitative analysis of the colocalization measurement values showed that the density of eGFP-NPRA with a mutated motif gradually decreased with LAMP-1 compared with the WT receptor.
Fig. 8.
Colocalization of internalized eGFP-NPRA-WT and mutated motif FQQI/AAAA with lysosome-associated membrane protein-1 (LAMP-1) in MMCs. Cells were treated with 100 nM ANP for different times. A: colocalization of eGFP-NPRA-WT with LAMP-1 marker was gradually increased from 5 to 30 min after ANP treatment. B: colocalization of eGFP-NPRA mutated motif FQQI/AAAA with LAMP-1 marker was decreased by 31%. C: quantification of the percent colocalization of eGFP-NPRA-WT and mutated motif FQQI/AAAA with LAMP-1. The images shown are typical of the images obtained in 4 independent experiments. Values are means ± SE.
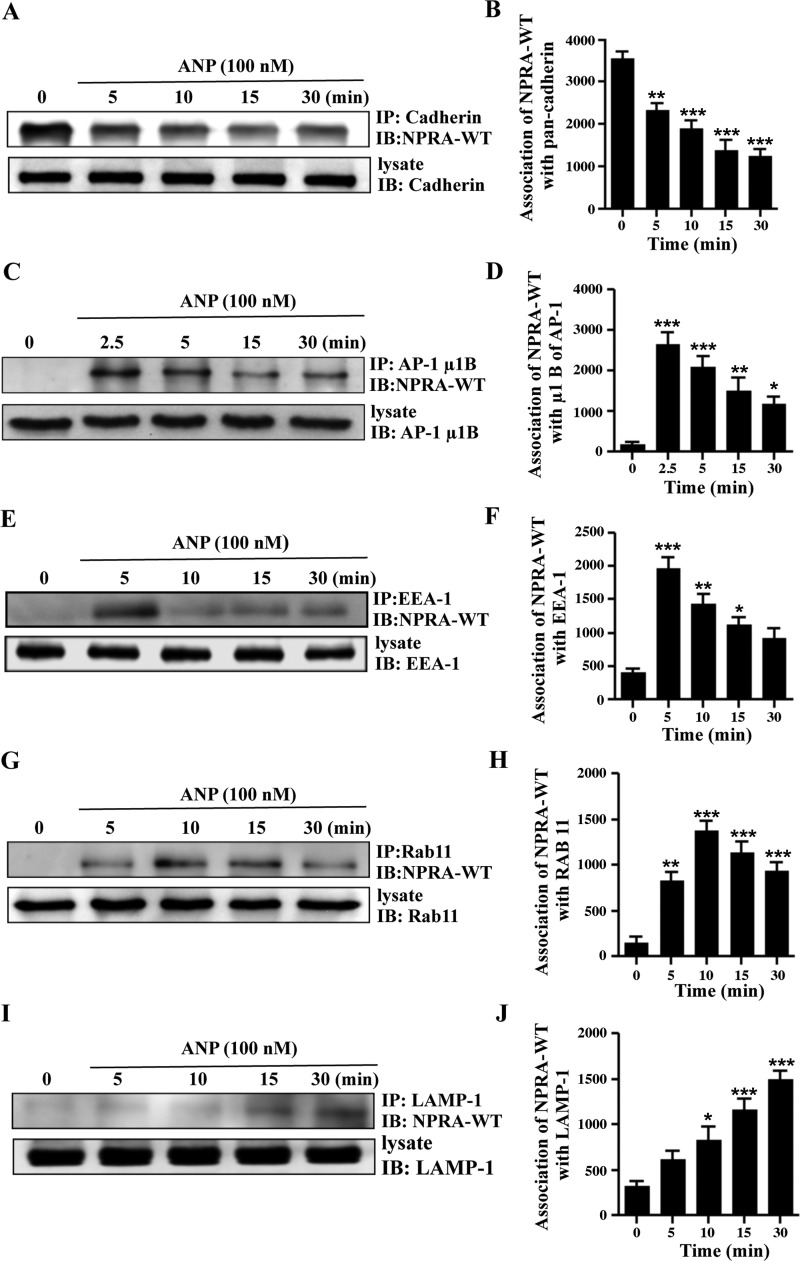
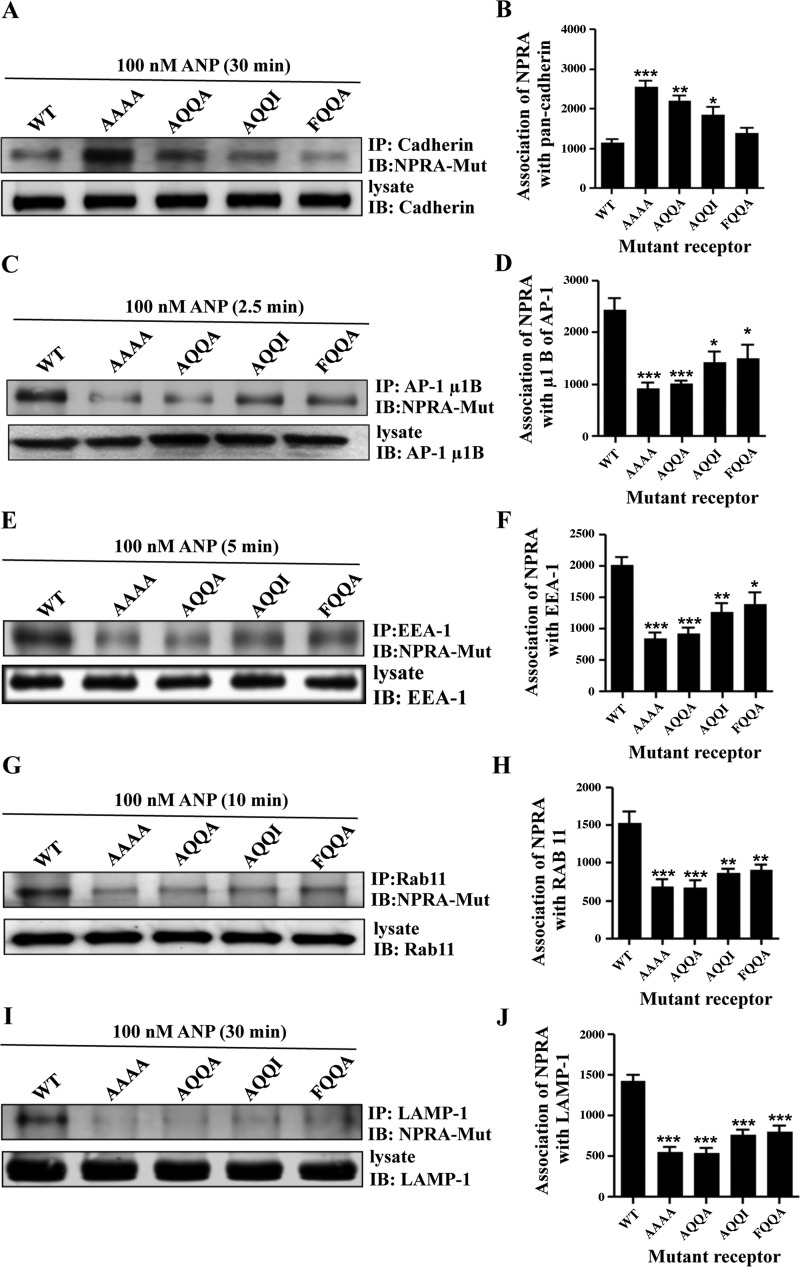
Coimmunoprecipitation of eGFP-NPRA with pan-cadherin, μ1B, EEA-1, Rab 11, and LAMP-1.
Biochemical assays were performed to confirm the results from the immunofluorescence analyses. Coimmunoprecipitation results indicated that strong associations of WT eGFP-NPRA occurred with pan-cadherin, μ1B, EEA-1, Rab 11, and LAMP-1 compartments during endocytic events after treatments of MMCs with 100 nM ANP. A decreased association of receptors with the plasma membrane (NPRA-pan-cadherin complex) was examined after increasing time courses (Fig. 9, A and B). Association of eGFP-NPRA with AP-1 (NPRA-μ1B complex) was maximum at 2.5 min, after which it gradually decreased (Fig. 9, C and D). Association of eGFP-NPRA with early endosomes (NPRA-EEA-1 complex) was maximum at 5 min, after which it gradually decreased (Fig. 9, E and F). The highest association of receptors with recycling endosomes (NPRA-Rab 11 complex) occurred at 10 min (Fig. 9, G and H). The association of NPRA with lysosomes (NPRA-LAMP-1 complex) was gradually increased after 5 min and reached a maximum level at 30 min (Fig. 9, I and J). Coimmunoprecipitation was also performed to test the association of mutant eGFP-NPRA with subcellular compartments. Coimmunoprecipitation results indicated that strong association of mutant eGFP-NPRA occurred with pan-cadherin; however, it was decreased with EEA-1, μ1B, Rab 11, and LAMP-1 compartments during colocalization and endocytic events. An increased association of mutant receptors (FQQI/AAAA and FQQI/AQQA) occurred with the plasma membrane (NPRA-pan-cadherin complex). However, mutated motifs FQQI/AQQI and FQQI/FQQA showed a weak association with the plasma membrane (Fig. 10, A and B). Association of mutant receptors (FQQI/AAAA<FQQI/AQQA< FQQI/AQQI ≤FQQI/FQQA) with AP-1 (NPRA-μ1B complex) was weaker than for the WT receptor after 2.5 min of ANP treatment (Fig. 10, C and D). Similarly, association of mutant receptors (FQQI/AAAA, FQQI/AQQA, FQQI/AQQI, and FQQI/FQQA) with early endosomes (NPRA-EEA-1 complex) was also weaker than for the WT receptor after 5 min of ANP treatment (Fig. 10, E and F). The association of mutant receptors (FQQI/AAAA, FQQI/AQQA, FQQI/AQQI, and FQQI/FQQA) with recycling endosomes (NPRA-Rab 11 complex) was also decreased compared with the WT receptor even at 10 min (Fig. 10, G and H). The association of mutant receptors (FQQI/AAAA, FQQI/AQQA, FQQI/AQQI, and FQQI/FQQA) with lysosomes (NPRA-LAMP-1 complex) was greatly decreased compared with the WT receptor at 30 min (Fig. 10, I and J).
Fig. 9.
Coimmunoprecipitation of eGFP-NPRA-WT with pan-cadherin, μ1B, EEA-1, Rab 11, and LAMP-1 in MMCs. To determine the association of NPRA with pan-cadherin, μ1B, EEA-1, Rab 11, and LAMP-1, cells were stimulated with 100 nM ANP for different time points. Anti-eGFP antibody was utilized for immunoblotting (IB) of eGFP-NPRA fusion protein. A: immunoblot of NPRA after immunoprecipitation (IP) of pan-cadherin showed a decreased association with increasing time points. B: densitometric Western blot quantification of NPRA association with pan-cadherin relative to untreated cells. C: coimmunoprecipitation of NPRA with μ1B showed maximum association at 2.5 min, and after that it gradually decreased. D: densitometric Western blot quantification of NPRA with μ1B relative to untreated cells. E: coimmunoprecipitation of NPRA with EEA-1 showed maximum association at 5 min, and after that it gradually decreased. F: densitometric Western blot quantification of NPRA with EEA-1 relative to untreated cells. G: strong association of receptor and recycling endosomes was observed at 10 min, and after that it gradually decreased. H: densitometric Western blot quantification of NPRA with Rab 11 relative to untreated cells. I: association of NPRA with lysosomes gradually increased after 5 min, and it was maximum at 30 min. J: densitometric Western blot quantification of NPRA with LAMP-1 relative to untreated cells. Values are means ± SE of 4 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 relative to untreated cells.
Fig. 10.
Coimmunoprecipitation of mutant eGFP-NPRA with pan-cadherin, μ1B, EEA-1, Rab 11 and LAMP-1 in MMCs. Anti-eGFP antibody was utilized for immunoblotting of eGFP-NPRA fusion protein. A: immunoblot of WT and mutant eGFP-NPRA after immunoprecipitation of pan-cadherin showed an increased association with mutated motifs FQQI/AAAA and FQQI/AQQA at 30 min and decreased association with other mutated residue of FQQI/AQQI and FQQI/FQQA. B: densitometric Western blot quantification of mutant eGFP-NPRA association with pan-cadherin relative to the WT receptor. C: coimmunoprecipitation of mutant eGFP-NPRA (mutated motifs FQQI/AAAA and FQQI/AQQA) with μ1B showed decreased association at 2.5 min and increased association with mutated motifs FQQI/AQQI and FQQI/FQQA. D: densitometric Western blot quantification of mutant eGFP-NPRA with μ1B relative to the WT receptor. E: coimmunoprecipitation of mutant eGFP-NPRA (mutated motifs FQQI/AAAA and FQQI/AQQA) with EEA-1 showed decreased association at 5 min and increased association with mutated motifs FQQI/AQQI and FQQI/FQQA. F: densitometric Western blot quantification of mutant eGFP-NPRA with EEA-1 relative to the WT receptor. G: association of the WT receptor and recycling endosomes was observed at 10 min. However, mutant receptors (mutated motifs FQQI/AAAA and FQQI/AQQA) showed a decreased association with mutated motifs FQQI/AQQI and FQQI/FQQA. H: densitometric Western blot quantification of mutant eGFP-NPRA with Rab 11 relative to the WT receptor. I: association of mutant eGFP-NPRA (mutated motifs FQQI/AAAA and FQQI/AQQA) with lysosomes showed decreased association at 30 min; however, it was increased with FQQI/AQQI and FQQI/FQQA motifs. J: densitometric Western blot quantification of mutant eGFP-NPRA with LAMP-1 relative to the WT receptor. Values are means ± SE of 5 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 relative to WT receptor.
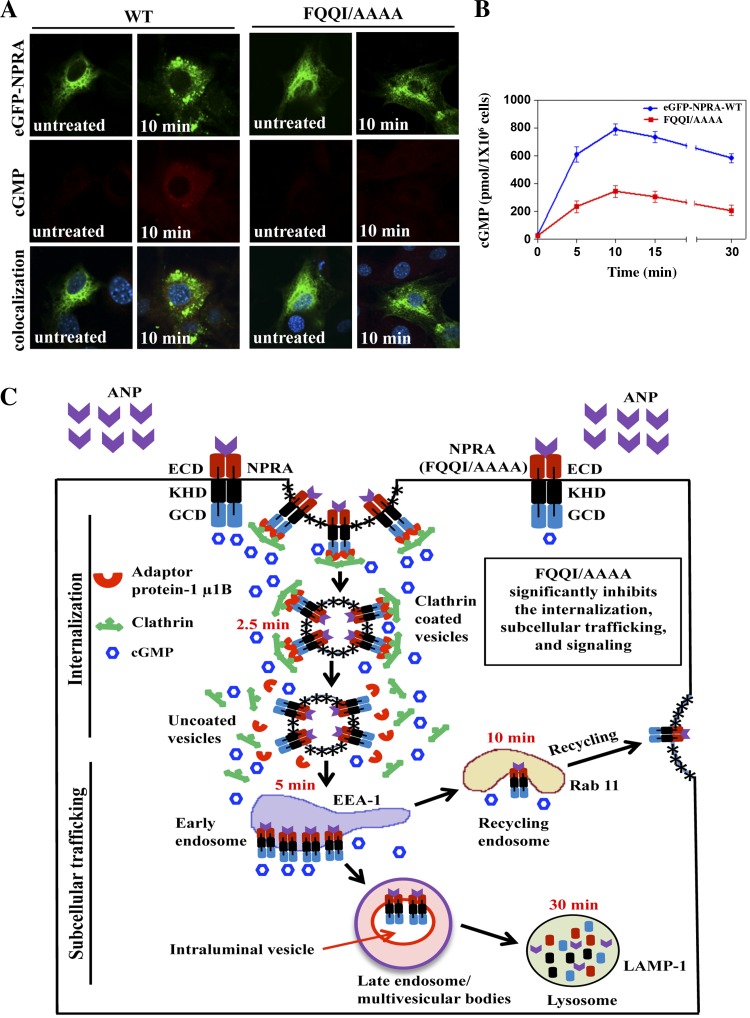
The mutated motif FQQI/AAAA inhibits the intracellular accumulation of cGMP.
The FQQI/AAAA mutant receptor showed a dose-response curve for 125I-ANP binding at 4°C similar to that seen for the WT receptor, exhibiting similar Kd values of 2.1 × 10−10 in MMCs. The binding parameters also indicated that transiently transfected MMCs expressed both WT and mutant receptors at a similar density of ∼0.75 × 106 sites/cell. Intracellular accumulation of cGMP was visualized using fluorescence intensity by measuring the cGMP immunofluorescence in transiently transfected WT and the mutated motif FQQI/AAAA in intact MMCs treated with 100 nM ANP for 10 min. To inhibit the endogenous expression of NPRA, cells were transiently transfected with pcDNA-6.2-GW/GFP-miR expression vector containing Npr1-miRNA insert pCMVNpr1miRNA-1595 and pCMV-Npr1miRNA-2931. The diffused staining was observed inside the cell with significant enhancement of cGMP fluorescence intensity at 10 min for the WT receptor (∼3-fold) than the mutant receptor (Fig. 11A). ANP stimulated the intracellular accumulation of cGMP in a time-dependent manner at 5, 10, 15, and 30 min with maximum levels at 10 min, which then declined up to 30 min in cells transfected with the WT receptor. However, the mutated motif FQQI/AAAA significantly decreased intracellular accumulation of cGMP by almost 65% (Fig. 11B).
Fig. 11.
Immunofluorescence localization of cGMP and schematic representation of internalization, intracellular trafficking, and signaling pathway of NPRA in MMCs. Immunofluorescence localization of cGMP was performed 48 h after transfection of cells with eGFP-NPRA-WT and mutated motif FQQI/AAAA. To inhibit the endogenous expression of NPRA, previously cells were transiently transfected with pcDNA-6.2-GW/GFP-miR expression vector containing Npr1-miRNA insert pCMVNpr1miRNA-1595 and pCMV-Npr1miRNA-2931. The cells were treated with 100 nM ANP for 10 min at 37°C in the presence of IBMX, as described in materials and methods. A: after treatment with ANP for 10 min, eGFP-NPRA-WT showed significantly more diffused fluorescence intensities in the cytoplasm than mutated motif FQQI/AAAA. B: to assay the stimulation of intracellular accumulation of cGMP, cells were transiently transfected with eGFP-NPRA-WT and mutated motif FQQI/AAAA. Intracellular accumulation of cGMP through eGFP-NPRA-WT and mutated motif FQQI/AAAA was quantitated by ELISA. The images shown are typical of 4 independent experiments. Values are means ± SE. C: scheme depicting the sequential events of internalization, trafficking, recycling, and degradation of ligand-receptor complexes in the intracellular compartments. After binding of ANP to NPRA, ligand-receptor complexes enter the cell via clathrin-coated pits, and the ligand-bound receptor complex traffics intracellularly through the endosomes, lysosomes, and a population of receptor recycles back to the plasma membrane through the recycling endosomes, with concurrent generation of intracellular cGMP. Sorting of the bound ANP-NPRA complex occurs by endosomal dissociation metabolic and lysosomal degradative pathways. On the other hand, the mutation of FQQI residues to AAAA (FQQI/AAAA) significantly inhibited the receptor internalization and intracellular trafficking. Note that the multivesicular body formation likely places the receptor in the lumen of the lysosomes. ECD, extracellular domain; KHD, kinase homology domain; GCD, guanylyl cyclase domain.
DISCUSSION
The results presented herein demonstrate a critical role for the aromatic residue FQQI motif in the internalization, subcellular trafficking, and signaling of chimeric eGFP-NPRA in primary MMCs. To delineate the critical role of endocytic signals in the intracellular sorting and signaling of NPRA, we utilized immunofluorescence staining and coimmunoprecipitation of eGFP-NPRA with the plasma membrane, AP-1, early endosomes, recycling endosomes, and lysosome markers to follow intracellular trafficking and concurrent signaling of mutated and WT receptors by confocal immunofluorescence microscopy and immunoblotting in MMCs. We have utilized eGFP-NPRA to capture the immunofluorescence images generated only by this receptor protein during the endocytosis and trafficking process, without any interference from either NPRB or NPRC. Furthermore, since, ANP binds to both NPRA and NPRC, we have utilized C-ANF to block the ANP binding to NPRC in the 125I-ANP binding assay (Figs. 1D and 2). C-ANF binds only to NPRC but neither to NPRA nor NPRB; thus all the binding parameters presented here are accounted for due to NPRA (25). The functional compartments denote the physical basis for the association and turnover of signaling complexes, which in turn can define particular endolysosomal signaling platforms. The expression of fully functional NPRA tagged with eGFP permitted a detailed analyses of the consequences of the itinerary of receptor endocytosis and its selective removal from the plasma membrane, followed by sorting into intraluminal vesicles of late endosomes or multivesicular bodies (MVBs), and subsequent delivery into the lysosomal lumen for degradation. The proposed schematic representation demonstrates the itinerary of NPRA internalization, intracellular trafficking, recycling, and degradation of ligand receptor complexes from the cell surface to the cell interior and back to the plasma membrane (Fig. 11C).
It has recently been reported that the endocytic processes need not be distinguished to designate the correct subset of plasma membrane proteins for sorting but be regulated at several steps along the endocytic pathways (49). Results of the present study provide new insights into the structure-function relationship of NPRA by the demonstration that the FQQI motif in the carboxyl-terminal region plays a significant role in the receptor internalization into the cell interior, trafficking into endosomes to lysosomes, and subsequent recycling of the internalized receptor back to the plasma membrane. The mutations FQQI/AAAA, F790/A, or Ile793/A in the eGFP-NPRA cDNA sequence markedly attenuated the internalization of mutant receptors by almost 49% compared with the WT receptor. Similarly, after inhibition of internalization, subcellular trafficking of receptors is also affected because of impaired internalization. However, in certain instances, the localization of mutant receptors seems to be denser than the expected level of internalization, which might be due to defective recycling or degradation pathway of mutant receptors. Subcellular trafficking indicated that immunofluorescence colocalization of mutated receptors with μ1B, EEA-1, LAMP-1, and Rab 11 markers was decreased by 50% for the subunit of AP-1, 57% into early endosomes, 48% in lysosomes, and 42% in recycling endosomes, respectively, compared with the WT receptor in MMCs. The present results suggest that the regulation of receptor internalization and subcellular trafficking relies largely on Phe790 and Ile793 residues in the C-terminal domain of NPRA. The role of the cytoplasmic tail of NPRA, therefore, is important and comparable with the thyrotropin-stimulating hormone receptor (35). Our previous deletion studies have provided evidence that the C-terminal region of NPRA is critical for internalization and trafficking of the receptor (42). However, the immunofluorescence demonstration of the internalization and intracellular trafficking process through the mutated FQQI motif was not previously determined. In the present study, we have extended the results of our previous findings by showing that point mutations within the C-terminal region of NPRA have major effects on the internalization, trafficking, and recycling of this receptor protein. The present series of experiments utilized site-directed mutagenesis to replace selected Phe790, Glu791, Glu792, and Ile793 residues with alanine in the tetrameric sequence F790QQI793 located within the cytoplasmic tail of NPRA. The mutant receptor was internalized at a significantly lower rate compared with the WT receptor. Similarly, the consensus sequence NPXY in the cytoplasmic domain of the LDL receptor has been shown to be necessary for receptor internalization (6, 15).
Previous studies have suggested that the FQQI motif contributes in the initial endocytosis of glucose transporter 4 (GLUT4) from the plasma membrane into the cell interior (4). However, once internalized, the FQQI motif was not responsible for intracellular recycling of GLUT4 in to the specialized compartments. Together, those previous studies have demonstrated that the FQQI motif within the amino terminus of GLUT4 is essential for GLUT4 endocytosis and AS160-dependent intracellular retention but not for the GGA-dependent sorting of GLUT4 into the insulin-responsive storage compartments (4). The present study provides the evidence that the FQQI motif is required for internalization, subcellular trafficking, and signaling of NPRA. Similarly, the GDAY motif in the carboxyl-terminal domain of NPRA revealed that endocytosis of the mutant receptor in HEK-293 cells was decreased by almost 50% and downregulation was decreased by 35% compared with WT NPRA (43). Other motifs such as di-leucine within the tail of glycosylated mannose-6-phosphate receptors are also required for sorting events of the receptor in the endosomes and deleting of the carboxyl terminus of CXCR7, impairment of the constitutive internalization of the receptor, and reduction of the activation of ERK1/2 by CXCL12-CXCR7 motifs (54, 64). Interestingly, the earlier studies have suggested that glycosylation sites in the guanylyl cyclase-coupled receptors seem to be important for proper folding and stability and might be essential for ligand binding of NPRA and NPRB (18, 22, 27). On the contrary, one study has suggested that glycosylation is not essential for ligand binding of NPRA (33). Indeed, more experimentation is needed to confirm the functional role of glycosylation in the ligand binding and trafficking of NPRA. Transforming growth factor (TGF)-family proteins form heteromeric complexes with transmembrane serine/threonine kinases and are referred to as type I and type II receptors. The receptor of the type II TGF-β receptor was dependent upon the clathrin adaptor protein Dab2. Loss of Dab2 was not sufficient to inhibit uptake of clathrin-dependent or -independent cargo, and it has an obligatory role in constitutive TGF-β receptor recycling because it is required for receptor trafficking from an EEA1-positive compartment to the Rab11-positive recycling compartment (48).
To examine the receptor recycling on the plasma membrane, we utilized Rab 11, a marker for endocytic recycling compartments. Immunofluorescence and coimmunoprecipitation data demonstrated that ∼20% of receptors recycled back to the plasma membrane. Interestingly, the trafficking of mutant receptors was significantly impaired through recycling endosomes. Rab11 is one of the widely studied Rab GTPases, and it primarily associates with recycling endosomes and regulates the recycling process of endocytosed proteins (16, 17, 61). It has been recently reported that Rab11 is involved in the regulation of the final exocytic event of recycling carriers at the plasma membrane, although it is well known to associate with perinuclear recycling endosomes (62). In another study, Rab11 has been shown to regulate the transit of cargo from early endosomes to the trans-Golgi network (TGN) and delivery from the TGN back to the plasma membrane (5, 68). Rab 11 was colocalized with the internalized transferrin receptor in the pericentriolar recycling compartment, which was detected by confocal immunofluorescence and electron microscopy (65). The present study demonstrates that Rab 11 interacts with NPRA, while the mutated receptor was significantly decreased in the recycling endosomes. Interestingly, NPRB is also internalized and recycled back to the plasma membrane in hippocampus neurons and C6 glioma cells (3). Those previous findings suggested that the trafficking of NPRB occurs in response to ligand binding and stimulation of receptor endocytosis. Generally, the recycling of internalized receptors back to the plasma membrane occurs simultaneously with the processes leading to degradation of the majority of ligand-receptor complexes into lysosomes as suggested for epidermal growth factor receptors (7, 34). The heptahelical G protein-coupled receptors (GPCRs) belong to the largest family of cell surface signaling transmembrane receptors, which signal in response to diverse extracellular stimuli (9, 70). Furthermore, a small linear peptide sequence including tyrosine- and di-leucine-based motifs, and PDZ ligands that are recognized by different endocytic adaptor proteins also mediate internalization and endosomal sorting of GPCRs (31). The coimmunoprecipitation studies revealed that after internalization, eGFP-NPRA shared the subcellular compartments during the intracellular trafficking. On the other hand, mutation of FQQI/AAAA significantly impaired the internalization, subcellular trafficking, and signaling of NPRA.
The fusion protein eGFP-NPRA generated optimum levels of intracellular accumulation of cGMP in intact cells, indicating that the eGFP moiety did not sterically hinder the biological activity of NPRA. The expression of fully functional eGFP-NPRA permitted detailed analyses of the consequences of WT and mutant receptor internalization, trafficking, and signaling in MMCs. Our present results have provided strong evidence for the association of NPRA with the subcellular organelles and showed the functional role of the FQQI motif in the endocytic processes and cGMP signaling of this receptor protein. In this regard, other studies have also suggested that the FQQI motif plays an essential role in the endocytosis of plasma membrane proteins (1, 8, 28, 47). The association of other receptors with the subcellular organelles has also been reported through coimmunoprecipitation analysis, which has shown the interaction of p97 ATPase with EEA1 in early endosomes (51). Similarly, the association of arrestin with GPCR has also been observed in the endolysosomal compartments, including the coimmunoprecipitation of the human AT1 receptor with Rab 11 in the recycling endosomes (11, 30). We have utilized the 125I-ANP binding assay to support the immunofluorescence-based internalization kinetics and cellular trafficking of the WT and mutated eGFP-NPRA in intact MMCs. The homeostatic regulation of native NPRA and cellular sensitivity of ANP depends on a dynamic equilibrium and reutilization of the ligand-receptor complexes from the cell surface to the cell interior. The results presented herein demonstrate that 125I-ANP binds to cell surface eGFP-NPRA, enters through the process of receptor-mediated endocytosis, and is delivered to the intracellular compartments until it reaches a steady-state level after 30 min. The rates of internalization, degradation, and release of 125I-ANP were markedly decreased in the mutant receptor, which suggested that the FQQI motif plays an important role in the internalization and subcellular trafficking events of eGFP-NPRA in intact cells.
In conclusion, the present results depict that both Phe and Ile residues play a critical role in the internalization and subcellular trafficking of NPRA in primary MMCs. Our results showed that after ligand binding, WT receptor eGFP-NPRA is rapidly internalized and trafficked from the cell surface to the subcellular compartment. However, the mutant motif FQQI/AAAA significantly inhibited the internalization, subcellular trafficking, and signaling of NPRA. These results demonstrate an important role of FQQI in sustaining the receptor signaling in MMCs and help in an understanding of the molecular mechanisms of vesicular transport, subcellular trafficking, and signaling of NPRA. Understanding the molecular determinants mediating the association of NPRA with subcellular organelles during intracellular signaling pathways will provide the basis for new molecular studies with possible association of single nucleotide polymorphisms of the Npr1 gene (coding for NPRA) in translational studies.
GRANTS
This work was supported by National Institutes of Health Grants R01HL057531 and R01HL062147.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: I.M. and K.N.P. provided conception and design of research; I.M., R.G., and K.N.P. performed experiments; I.M. and K.N.P. analyzed data; I.M. and K.N.P. interpreted results of experiments; I.M. and K.N.P. prepared figures; I.M. and K.N.P. drafted manuscript; I.M. and K.N.P. edited and revised manuscript; I.M., R.G., and K.N.P. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Meaghan Bloodworth, Alice Yeh, and Whitney Nolan for excellent technical assistance and Kamala Pandey for assistance during the preparation of this manuscript. We also acknowledge Courtney A. Lopreore for help during confocal immunofluorescence imaging.
REFERENCES
- 1.Ahn KH, Bertalovitz AC, Mierke DF, Kendall DA. Dual role of the second extracellular loop of the cannabinoid receptor 1: ligand binding and receptor localization. Mol Pharmacol 76: 833–842, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anand-Srivastava MB. Natriuretic peptide receptor-C signaling and regulation. Peptides 26: 1044–1059, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Brackmann M, Schuchmann S, Anand R, Braunewell KH. Neuronal Ca2+ sensor protein VILIP-1 affects cGMP signalling of guanylyl cyclase B by regulating clathrin-dependent receptor recycling in hippocampal neurons. J Cell Sci 118: 2495–2505, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Capilla E, Suzuki N, Pessin JE, Hou JC. The glucose transporter 4 FQQI motif is necessary for Akt substrate of 160-kilodalton-dependent plasma membrane translocation but not Golgi-localized (gamma)-ear-containing Arf-binding protein-dependent entry into the insulin-responsive storage compartment. Mol Endocrinol 21: 3087–3099, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Chen W, Feng Y, Chen D, Wandinger-Ness A. Rab11 is required for trans-Golgi network-to-plasma membrane transport and a preferential target for GDP dissociation inhibitor. Mol Biol Cell 9: 3241–3257, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen WJ, Goldstein JL, Brown MS. NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. J Biol Chem 265: 3116–3123, 1990. [PubMed] [Google Scholar]
- 7.Chi S, Cao H, Wang Y, McNiven MA. Recycling of the epidermal growth factor receptor is mediated by a novel form of the clathrin adaptor protein Eps15. J Biol Chem 286: 35196–35208, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark KD, Volkman BF, Thoetkiattikul H, Hayakawa Y, Strand MR. N-terminal residues of plasmatocyte-spreading peptide possess specific determinants required for biological activity. J Biol Chem 276: 37431–37435, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Conti F, Sertic S, Reversi A, Chini B. Intracellular trafficking of the human oxytocin receptor: evidence of receptor recycling via a Rab4/Rab5 “short cycle.” Am J Physiol Endocrinol Metab 296: E532–E542, 2009. [DOI] [PubMed] [Google Scholar]
- 10.de Bold AJ. Atrial natriuretic factor: a hormone produced by the heart. Science 230: 767–770, 1985. [DOI] [PubMed] [Google Scholar]
- 11.Esseltine JL, Dale LB, Ferguson SS. Rab GTPases bind at a common site within the angiotensin II type I receptor carboxyl-terminal tail: evidence that Rab4 regulates receptor phosphorylation, desensitization, and resensitization. Mol Pharmacol 79: 175–184, 2011. [DOI] [PubMed] [Google Scholar]
- 12.Fan D, Bryan PM, Antos LK, Potthast RJ, Potter LR. Down-regulation does not mediate natriuretic peptide-dependent desensitization of natriuretic peptide receptor (NPR)-A or NPR-B: guanylyl cyclase-linked natriuretic peptide receptors do not internalize. Mol Pharmacol 67: 174–183, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Favre N, Fanelli F, Missotten M, Nichols A, Wilson J, di Tiani M, Rommel C, Scheer A. The DRY motif as a molecular switch of the human oxytocin receptor. Biochemistry 44: 9990–10008, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Flora DR, Potter LR. Prolonged atrial natriuretic peptide exposure stimulates guanylyl cyclase-a degradation. Endocrinology 151: 2769–2776, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstein JL, Brown MS, Stone NJ. Genetics of the LDL receptor: evidence that the mutations affecting binding and internalization are allelic. Cell 12: 629–641, 1977. [DOI] [PubMed] [Google Scholar]
- 16.Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol 10: 597–608, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci USA 103: 11821–11827, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heim JM, Singh S, Gerzer R. Effect of glycosylation on cloned ANF-sensitive guanylyl cyclase. Life Sci 59: PL61–PL68, 1996. [DOI] [PubMed] [Google Scholar]
- 19.Khurana ML, Pandey KN. Catalytic activation of guanylate cyclase/atrial natriuretic factor receptor by combined effects of ANF and GTP gamma S in plasma membranes of Leydig tumor cells: involvement of G-proteins. Arch Biochem Biophys 316: 392–398, 1995. [DOI] [PubMed] [Google Scholar]
- 20.Koh GY, Nussenzveig DR, Okolicany J, Price DA, Maack T. Dynamics of atrial natriuretic factor-guanylate cyclase receptors and receptor-ligand complexes in cultured glomerular mesangial and renomedullary interstitial cells. J Biol Chem 267: 11987–11994, 1992. [PubMed] [Google Scholar]
- 21.Koller KJ, de Sauvage FJ, Lowe DG, Goeddel DV. Conservation of the kinase like regulatory domain is essential for activation of the natriuretic peptide receptor guanylyl cyclases. Mol Cell Biol 12: 2581–2590, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koller KJ, Lipari MT, Goeddel DV. Proper glycosylation and phosphorylation of the type A natriuretic peptide receptor are required for hormone-stimulated guanylyl cyclase activity. J Biol Chem 268: 5997–6003, 1993. [PubMed] [Google Scholar]
- 23.Kumar R, Cartledge WA, Lincoln TM, Pandey KN. Expression of guanylyl cyclase-A/atrial natriuretic peptide receptor blocks the activation of protein kinase C in vascular smooth muscle cells. Role of cGMP and cGMP-dependent protein kinase. Hypertension 29: 414–421, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med 339: 321–328, 1998. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Madiraju P, Anand-Srivastava MB. Knockdown of natriuretic peptide receptor-A enhances receptor C expression and signalling in vascular smooth muscle cells. Cardiovasc Res 93: 350–359, 2012. [DOI] [PubMed] [Google Scholar]
- 26.Lincoln TM, Cornwell TL. Intracellular cyclic GMP receptor proteins. FASEB J 7: 328–338, 1993. [DOI] [PubMed] [Google Scholar]
- 27.Lowe DG, Fendly BM. Human natriuretic peptide receptor-A guanylyl cyclase. Hormone cross-linking and antibody reactivity distinguish receptor glycoforms. J Biol Chem 267: 21691–21697, 1992. [PubMed] [Google Scholar]
- 28.Lu JC, Scott P, Strous GJ, Schuler LA. Multiple internalization motifs differentially used by prolactin receptor isoforms mediate similar endocytic pathways. Mol Endocrinol 16: 2515–2527, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Lucas KA, Pitari GM, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S, Chepenik KP, Waldman SA. Guanylyl cyclases and signaling by cyclic GMP. Pharmacol Rev 52: 375–414, 2000. [PubMed] [Google Scholar]
- 30.Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, Lin F, Kawakatsu H, Owada K, Luttrell DK, Caron MG, Lefkowitz RJ. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science 283: 655–661, 1999. [DOI] [PubMed] [Google Scholar]
- 31.Marchese A, Paing MM, Temple BR, Trejo J. G protein-coupled receptor sorting to endosomes and lysosomes. Annu Rev Pharmacol Toxicol 48: 601–629, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Misono KS, Philo JS, Arakawa T, Ogata CM, Qiu Y, Ogawa H, Young HS. Structure, signaling mechanism and regulation of the natriuretic peptide receptor guanylate cyclase. FEBS J 278: 1818–1829, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyagi M, Zhang X, Misono KS. Glycosylation sites in the atrial natriuretic peptide receptor: oligosaccharide structures are not required for hormone binding. Eur J Biochem 267: 5758–5768, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Mulkearns EE, Cooper JA. FCH domain only-2 organizes clathrin-coated structures and interacts with Disabled-2 for low-density lipoprotein receptor endocytosis. Mol Biol Cell 23: 1330–1342, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nussenzveig DR, Heinflink M, Gershengorn MC. Agonist-stimulated internalization of the thyrotropin-releasing hormone receptor is dependent on two domains in the receptor carboxyl terminus. J Biol Chem 268: 2389–2392, 1993. [PubMed] [Google Scholar]
- 36.Pandey KN. Biology of natriuretic peptides and their receptors. Peptides 26: 901–932, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Pandey KN. The functional genomics of guanylyl cyclase/natriuretic peptide receptor-A: perspectives and paradigms. FEBS J 278: 1792–1807, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pandey KN. Functional roles of short sequence motifs in the endocytosis of membrane receptors. Front Biosci 14: 5339–5360, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pandey KN. Small peptide recognition sequence for intracellular sorting. Curr Opin Biotechnol 21: 611–620, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pandey KN. Stoichiometric analysis of internalization, recycling, and redistribution of photoaffinity-labeled guanylate cyclase/atrial natriuretic factor receptors in cultured murine Leydig tumor cells. J Biol Chem 268: 4382–4390, 1993. [PubMed] [Google Scholar]
- 41.Pandey KN, Inagami T, Misono KS. Atrial natriuretic factor receptor on cultured Leydig tumor cells: ligand binding and photoaffinity labeling. Biochemistry 25: 8467–8472, 1986. [DOI] [PubMed] [Google Scholar]
- 42.Pandey KN, Kumar R, Li M, Nguyen H. Functional domains and expression of truncated atrial natriuretic peptide receptor-A: the carboxyl-terminal regions direct the receptor internalization and sequestration in COS-7 cells. Mol Pharmacol 57: 259–267, 2000. [PubMed] [Google Scholar]
- 43.Pandey KN, Nguyen HT, Garg R, Khurana ML, Fink J. Internalization and trafficking of guanylyl (guanylate) cyclase/natriuretic peptide receptor A is regulated by an acidic tyrosine-based cytoplasmic motif GDAY. Biochem J 388: 103–113, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pandey KN, Nguyen HT, Li M, Boyle JW. Natriuretic peptide receptor-A negatively regulates mitogen-activated protein kinase and proliferation of mesangial cells: role of cGMP-dependent protein kinase. Biochem Biophys Res Commun 271: 374–379, 2000. [DOI] [PubMed] [Google Scholar]
- 45.Pandey KN, Nguyen HT, Sharma GD, Shi SJ, Kriegel AM. Ligand-regulated internalization, trafficking, and down-regulation of guanylyl cyclase/atrial natriuretic peptide receptor-A in human embryonic kidney 293 cells. J Biol Chem 277: 4618–4627, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Pandey KN, Singh S. Molecular cloning and expression of murine guanylate cyclase/atrial natriuretic factor receptor cDNA. J Biol Chem 265: 12342–12348, 1990. [PubMed] [Google Scholar]
- 47.Pauwels PJ, Gouble A, Wurch T. Activation of constitutive 5-hydroxytryptamine (1B) receptor by a series of mutations in the BBXXB motif: positioning of the third intracellular loop distal junction and its G(o)alpha protein interactions. Biochem J 343: 435–442, 1999. [PMC free article] [PubMed] [Google Scholar]
- 48.Penheiter SG, Singh RD, Repellin CE, Wilkes MC, Edens M, Howe PH, Pagano RE, Leof EB. Type II transforming growth factor-beta receptor recycling is dependent upon the clathrin adaptor protein Dab2. Mol Biol Cell 21: 4009–4019, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Piper RC, Lehner PJ. Endosomal transport via ubiquitination. Trends Cell Biol 21: 647–655, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Platta HW, Stenmark H. Endocytosis and signaling. Curr Opin Cell Biol 23: 393–403, 2011. [DOI] [PubMed] [Google Scholar]
- 51.Ramanathan HN, Ye Y. The p97 ATPase associates with EEA1 to regulate the size of early endosomes. Cell Res 22: 346–359, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rathinavelu A, Isom GE. Differential internalization and processing of atrial-natriuretic-factor B and C receptor in PC12 cells. Biochem J 276: 493–497, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rathinavelu A, Isom GE. Lysosomal delivery of ANP receptors following internalization in PC12 cell. Life Sci 53: 1007–1014, 1993. [DOI] [PubMed] [Google Scholar]
- 54.Ray P, Mihalko LA, Coggins NL, Moudgil P, Ehrlich A, Luker KE, Luker GD. Carboxy-terminus of CXCR7 regulates receptor localization and function. Int J Biochem Cell Biol 44: 669–678, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosenzweig A, Seidman CE. Atrial natriuretic factor and related peptide hormones. Annu Rev Biochem 60: 229–255, 1991. [DOI] [PubMed] [Google Scholar]
- 56.Roy SJ, Glazkova I, Frechette L, Iorio-Morin C, Binda C, Petrin D, Trieu P, Robitaille M, Angers S, Hebert TE, Parent JL. Novel, gel-free proteomics approach identifies RNF5 and JAMP as modulators of GPCR stability. Mol Endocrinol 27: 1245–1266, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sauro MD, Fitzpatrick DF. Atrial natriuretic peptides inhibit protein kinase C activation in rat aortic smooth muscle. Pept Res 3: 138–141, 1990. [PubMed] [Google Scholar]
- 58.Sharma RK, Duda T. ROS-GC subfamily membrane guanylate cyclase-linked transduction systems: taste, pineal gland and hippocampus. Mol Cell Biochem 334: 199–206, 2010. [DOI] [PubMed] [Google Scholar]
- 59.Somanna NK, Pandey AC, Arise KK, Nguyen V, Pandey KN. Functional silencing of guanylyl cyclase/natriuretic peptide receptor-A by microRNA interference: analysis of receptor endocytosis. Int J Biochem Mol Biol 4: 41–53, 2013. [PMC free article] [PubMed] [Google Scholar]
- 60.Sorkin A, von Zastrow M. Endocytosis and signalling: intertwining molecular networks. Nat Rev Mol Cell Biol 10: 609–622, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 10: 513–525, 2009. [DOI] [PubMed] [Google Scholar]
- 62.Takahashi S, Kubo K, Waguri S, Yabashi A, Shin HW, Katoh Y, Nakayama K. Rab11 regulates exocytosis of recycling vesicles at the plasma membrane. J Cell Sci 125: 4049–4057, 2012. [DOI] [PubMed] [Google Scholar]
- 63.Tanabe K, Lanaspa MA, Kitagawa W, Rivard CJ, Miyazaki M, Klawitter J, Schreiner GF, Saleem MA, Mathieson PW, Makino H, Johnson RJ, Nakagawa T. Nicorandil as a novel therapy for advanced diabetic nephropathy in the eNOS-deficient mouse. Am J Physiol Renal Physiol 302: F1151–F1160, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tikkanen R, Obermuller S, Denzer K, Pungitore R, Geuze HJ, von Figura K, Honing S. The dileucine motif within the tail of MPR46 is required for sorting of the receptor in endosomes. Traffic 1: 631–640, 2000. [DOI] [PubMed] [Google Scholar]
- 65.Ullrich O, Reinsch S, Urbe S, Zerial M, Parton RG. Rab11 regulates recycling through the pericentriolar recycling endosome. J Cell Biol 135: 913–924, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van den Akker F, Zhang X, Miyagi M, Huo X, Misono KS, Yee VC. Structure of the dimerized hormone-binding domain of a guanylyl-cyclase-coupled receptor. Nature 406: 101–104, 2000. [DOI] [PubMed] [Google Scholar]
- 67.Vellaichamy E, Khurana ML, Fink J, Pandey KN. Involvement of the NF-kappa B/matrix metalloproteinase pathway in cardiac fibrosis of mice lacking guanylyl cyclase/natriuretic peptide receptor A. J Biol Chem 280: 19230–19242, 2005. [DOI] [PubMed] [Google Scholar]
- 68.Wilcke M, Johannes L, Galli T, Mayau V, Goud B, Salamero J. Rab11 regulates the compartmentalization of early endosomes required for efficient transport from early endosomes to the trans-Golgi network. J Cell Biol 151: 1207–1220, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yin D, Wang X, Konda BM, Ong JM, Hu J, Sacapano MR, Ko MK, Espinoza AJ, Irvin DK, Shu Y, Black KL. Increase in brain tumor permeability in glioma-bearing rats with nitric oxide donors. Clin Cancer Res 14: 4002–4009, 2008. [DOI] [PubMed] [Google Scholar]
- 70.Zhuo JL, Imig JD, Hammond TG, Orengo S, Benes E, Navar LG. Ang II accumulation in rat renal endosomes during Ang II-induced hypertension: role of AT1 receptor. Hypertension 39: 116–121, 2002. [DOI] [PubMed] [Google Scholar]