Abstract
Cysteine proteases continue to provide validated targets for treatment of human diseases. In neurodegenerative disorders, multiple cysteine proteases provide targets for enzyme inhibitors, notably caspases, calpains, and cathepsins. The reactive, active-site cysteine provides specificity for many inhibitor designs over other families of proteases, such as aspartate and serine; however, a) inhibitor strategies often use covalent enzyme modification, and b) obtaining selectivity within families of cysteine proteases and their isozymes is problematic. This review provides a general update on strategies for cysteine protease inhibitor design and a focus on cathepsin B and calpain 1 as drug targets for neurodegenerative disorders; the latter focus providing an interesting query for the contemporary assumptions that irreversible, covalent protein modification and low selectivity are anathema to therapeutic safety and efficacy.
Abbreviations: AD, Alzheimer׳s disease; Ala, alanine; ALS, amyotrophic lateral sclerosis; APP, amyloid precursor protein; APP/PS1, Aβ overexpressing mice APP (K670N/M671L) and PS1 (M146L) mutants; AppLon, London familial amyloid precursor protein mutation, APP (V717I); AppSwe, Swedish amyloid precursor protein mutation, APP (K670N/M671L); Arg, arginine; Aβ, amyloid β; Aβ1-42, amyloid β, 42 amino acid protein; BACE-1, β-amyloid cleaving enzyme; BBB, blood–brain barrier; CaMKII, Ca2+/calmodulin-dependent protein kinases II; CANP, calcium-activated neutral protease; Cdk5/p35, activator of cyclin-dependent kinase 5; CNS, central nervous system; CREB, cyclic adenosine monophosphate response element binding protein; DTT, dithioerythritol; EGFR, epidermal growth factor receptor; ERK1/2, extracellular signal-regulated kinase 1/2; Gln, glutamine; Glu, glutamic acid; Gly, glutamine; GSH, glutathione; Hsp70.1, heat shock protein 70.1; Ile, isoleucine; isoAsp, isoaspartate; KO, knockout; Leu, leucine; Lys, lysine; MAP-2, microtubule-associated protein 2; Met, methionine; MMP-9, matrix metalloproteinase 9; NFT, neurofibrilliary tangles; Nle, norleucine; PD, Parkinson׳s disease; pGlu, pyroglutamate; Phe, phenylalanine; PK, pharmacokinetic; PKC, protein kinase C; Pro, proline; PTP1B, protein-tyrosine phosphatase 1B; pyroGluAβ, pyroglutamate-amyloid β; SP, senile plaques; TBI, traumatic brain injury; Thr, threonine; TNF, tumor necrosis factor; Tyr, tyrosine; Val, valine; WRX, Trp-Arg containing epoxysuccinate cysteine protease inhibitor; WT, wildtype
Key words: Cysteine protease, Calpain, Cathepsin, Enzyme inhibitors, Neurodegeneration, Alzheimer׳s disease
Graphical abstract
This review provides a general update on strategies for cysteine protease inhibitor design and a focus on cathepsin B and calpain 1 as drug targets for neurodegenerative disorders. The selectivity requirements for the safe and efficacious treatment of neurodegenerative disorders are investigated.
1. Introduction
Proteases are enzymes that irreversibly hydrolyze a peptide bond in an amino acid sequence by nucleophilic attack and subsequent hydrolysis of a tetrahedral intermediate. Proteases are grouped according to the key catalytic group in the active site: serine (Ser), threonine (Thr), cysteine (Cys), aspartate (Asp), glutamate (Glu), or zinc in metalloproteases. Ser, Cys and Thr act directly as nucleophiles that attack an amide carbonyl C, whereas Asp, Glu and metalloproteases activate a water molecule that then acts as a nucleophile. The enzymes are also classified into exopeptidases and endopeptidases by the position of the peptide bond in a protein they cleave. Exopeptidases truncate one or several amino acids from either the N- or the C-terminus of a peptide, whereas endopeptidases cleave an internal peptide bond. The catalytic site of CA-clan papain-like cysteine proteases consists of Cys, histidine (His) and Asp residues and is highly conserved among members of the enzyme family1. This review will focus on approaches to inhibition of two families of protease enzymes, calpains and cathepsins, of interest in neurodegeneration and cancer therapy and the quixotic pursuit of selectivity.
2. Cysteine proteases
2.1. Cathepsins
Cathepsin inhibitors have been reviewed recently by Turk et al.2 and earlier by Hernandez and Roush3. A review specific to cathepsin B inhibitors has also been published by Frlan and Gobec4. Cathepsins are a group of protease enzymes originally discovered in the cell lysosome, with several members ubiquitous in the human body. They are not catalytically conserved: cathepsins A, G are serine proteases; cathepsins D, E are aspartate proteases; and the remainder are lysosomal cysteine proteases, including the human isoforms B, C, F, H, K, L, O, S, V, X and W2. Cathepsins B, F, H and L occur throughout the CNS, while C, S, V and X are expressed in specific cell types within the CNS. The pHmax for optimum cathepsin activity is slightly acidic, corresponding to the environment found in the lysosome. Although they have been traditionally viewed as enzymes involved in terminal protein degradation, knockout (KO) mice have revealed major roles in cell regulation, i.e. of cell proliferation and adhesion, apoptosis, lipid metabolism and immune response5, 6.
The crystal structure of a number of cathepsins has been determined, among them cathepsin B7. Cathepsin B is unique among the cathepsins in that it has an occluding loop, a peptide sequence which when closed can hinder access to the primed side of the substrate pocket. Thus cathepsin B can function as an endo- or exopeptidase depending on pH8. The occluding loop has been targeted for the design of non-electrophilic cathepsin B inhibitors9. The lysosomal cathepsin K occurs in osteoclasts and is a major factor in bone resorption and a target for treating osteoporosis. Several inhibitors are in development, with one, odanacatib, having reached phase III clinical trials10. Table 1 shows residue preference of cathepsin B in peptide substrates in each position11, 12. Fig. 1 shows primed and unprimed amino acid residues in protease substrates and inhibitors.
Table 1.
| Unprimeda | Preference | Primeda | Preference |
|---|---|---|---|
| P1 | Gly>Ala, Met, Gln | P1′ | Phe>Gly |
| P2 | Val>Phe, Tyr | P2′ | Val, Ile>Gly, Thr |
| P3 | Gly>Lys, Phe | P3′ | Gly |
See Fig. 1 for depiction of primed and unprimed sites. Ala, alanine; Gln, glutamine; Gly, glutamine; Ile, isoleucine; Lys, lysine; Met, methionine; Phe, phenylalanine; Thr, threonine; Tyr, tyrosine; Val, valine.
Figure 1.
Nomenclature of primed and unprimed amino acid residues in protease substrates and inhibitors.
2.2. Calpains
Calpains are neutral, cytosolic cysteine proteases with 15 isoforms reported, of which 11 have been identified in humans13, 14. The first reports characterizing members of the enzyme family emerged in 1964, naming the enzyme calcium-activated neutral protease (CANP)15, 16, 17. .The enzymes consist of a catalytic subunit (82 or 80 kDa for calpains 1 and 2, respectively) and a Ca2+ binding subunit (28 kDa)18. The enzymes are unique among cysteine proteases in that the cytosolic proenzyme is activated by Ca2+ ions, inducing a conformational change. This change drives spatial proximity of the catalytic triad to the regulatory subunit, domain I, and subsequent autocatalytic cleavage19. The two most widely researched isoforms of calpain are ubiquitous, these are termed calpains 1 and 2, or μ- and m-calpain, requiring 5–30 µmol/L or millimolar Ca2+ for activation, respectively18. The presence of phospholipids or phosphoinositides can decrease the Ca2+ concentration required for the activation of calpain 220, 21. The expression of calpains 1 and 2 can vary greatly depending on cell types and conditions. Other members of the calpain family are tissue-specific. The active sites and substrates of calpains 1 and 2 are very similar, and specific inhibitors have not been developed.
Calpains and cathepsins regulate the activity of other biomolecules through limited proteolytic cleavage at specific sites. The products of these enzyme catalyzed reactions are often functional proteins and therefore these cysteine proteases constitute important regulatory enzymes. Protease activation is a necessary cog in the cellular machine under physiological conditions. Constant overactivation of calpain and other proteolytic enzymes, however, causes excessive protein degradation and neuronal death22. Calpains play a key role in enzyme activation, platelet activation, cell proliferation and signal transduction.
Calpains have numerous protein substrates including G-proteins and cytoskeletal proteins such as spectrin, integrin and MAP-2. Calpain substrates that are protein kinases and further regulate the function and breakdown of cytoskeletal proteins are PKC, ERK1/2, CaMKII, Cdk-5/p35, Bid, and Bax23. Transcription factors (c-Jun, c-Fos24) and membrane receptors, e.g. EGFR, are also substrates of calpain. Calpain regulates the activity of a number of proteins that are part of processes influencing neuronal plasticity, cognition and neurodegeneration25. CREB is a key protein in synaptic plasticity, impaired activation of which is a key contributor to pathogenesis of Alzheimer׳s disease (AD)26, 27, 28, 29. CREB is a substrate of calpain, and thus inhibitors have been demonstrated to increase CREB phosphorylation, in turn restoring synaptic plasticity in the APP/PS1 transgenic mouse model of familial AD30. KO of calpain 1 in mice has been shown to influence degradation of erythrocyte membrane proteins and platelet aggregation, reportedly via action on the calpain substrate PTP1B31, 32.
The peptide sequence of the endogenous, specific inhibitor, calpastatin, is known and the inhibition mechanism has been elucidated33, 34. Calpastatin binds to both P and P′ sides of the active site, but does not occupy the active site, thus avoiding self-immolation. Calpastatin regulates the proteolytic activity of calpains35. Calpastatin is specific for the catalytically active form of calpain, bound to Ca2+, and consists of an N-terminal domain and four repeats of an inhibitory domain. The peptide sequence of the endogenous inhibitor has been truncated to generate calpain inhibitors36. Improved cell permeability has been attempted by conjugation of appropriate peptide sequences (i.e. penetratin), but their clinical use in CNS indications is limited by the usual bioavailability challenges of oligopeptide drugs37, 38. Nevertheless, upregulation or decreased degradation of calpastatin is a therapeutic target in AD39, 40. Table 2 shows residue preference of calpain in peptide substrates at each position41.
Table 2.
Calpain: residue preference in peptide substrates at each position41.
| Unprimed | Preference | Primed | Preference |
|---|---|---|---|
| P1 | Leu=Phe | P1′ | Met>Ala>Arg |
| P2 | Leu>Val | P2′ | Glu |
| P3 | Phe>Leu>Pro | P3′ | Arg>Lys |
| P4 | Phe | P4′ | No specificity |
| P5 | Pro | P5′ | No specificity |
Leu, leucine; Arg, arginine; Glu, glutamic acid; Leu, leucine; Pro, proline.
Abnormal activation or dysregulation of calpains has been linked to a number of pathological conditions. The increased intracellular Ca2+ levels in traumatic brain injury (TBI) and cerebral ischemia lead to increased calpain activation and secondary injury due to the degradation of cell membrane components. Calpain 1 is a target in chronic neurodegeneration occurring in AD, Parkinson׳s disease (PD)42, 43, Huntington׳s disease44, 45, multiple sclerosis46, 47, 48, and amyotrophic lateral sclerosis (ALS)49, 50. Calpain 10 and the gene encoding it have been linked to type 2 diabetes mellitus51, 52. A mutation causing loss of function of calpain 3 is believed to be responsible for limb-girdle muscular dystrophy53.
Cysteine protease inhibitors belong to two general classes: the most widely explored inhibitors use an electrophile to modify the active cysteine covalently and a recognition motif for binding to the active site; allosteric inhibitors have also been reported41.
3. Electrophilic warheads for Cys protease inhibitors
3.1. Irreversible covalent inhibitors
Electrophiles that alkylate, acylate, phosphonylate or sulfonylate the active site cysteine irreversibly, include simple non-selective alkylating or acylating agents such as iodoacetate, N-ethylmaleimide, and diisopropyl fluorophosphate. Examples of electrophilic warheads used in selective and potent cysteine protease inhibitors include epoxysuccinates, vinylsulfones, allyl sulfones54. vinyl sulfonates, diazomethyl ketones and fluoro- or chloromethyl ketones55, 56, 57. The latter were developed in the 1960s as inhibitors of trypsin and chymotrypsin and react with both serine and cysteine proteases. Halomethyl ketones can alkylate active site His residues and the activated ketone has been proposed to form a transition state analog at the active site57. Moderation of the reactivity of halomethyl ketones led to the development of acyloxymethyl ketones and other activated ketones such as aryloxymethyl, sulfonium methyl and ketoheterocycles. Other examples are vinyl ketones, vinyl esters and vinyl sulfones, which provide alternate Michael acceptor electrophiles58, 59. Diazomethylketones have been explored and selectivity among different cathepsins attempted by the use of an appropriate recognition group60, 61, 62.
3.1.1. Epoxysuccinates
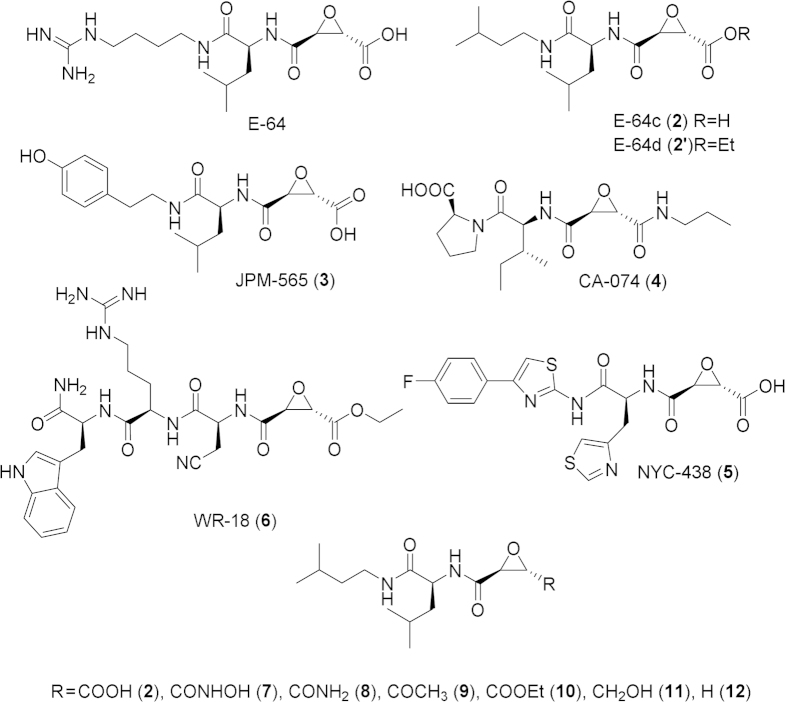
Epoxysuccinates occupy an important role as Cys protease inhibitors since the discovery of E-64 (1, Fig. 2) from Aspergillus japonicus in 197763. Total synthesis of E-64 soon followed64. A less hydrophilic derivative, E-64c (2), was designed later, targeted against muscular dystrophy, and its ethyl ester prodrug, E-64d (2′), developed to overcome the poor absorption of E-64c, progressed to phase III clinical trials65, 66. The epoxide irreversibly modifies the active site Cys, forming a thioether bond67, 68. Epoxysuccinates are selective towards Cys proteases due to the nucleophilicity of the active site cysteine. Peptidomimetic recognition groups are used to increase binding, selectivity, and potency. The amino acid preference of calpains has been investigated by the generation of positional scanning epoxide libraries by Cuerrier et al.69 The studies showed that for inhibition of calpains 1 and 2, the preferred residues in the P3 and P4 positions are Trp and Arg (Table 1) This led to the development of the WRX series of calpain inhibitors (e.g. 6, Fig. 2). Members of this compound library were reported to have 3 to 6-fold selectivity towards calpain 2 vs. calpain 1 and significant selectivity for calpains over cathepsins. However, changing the Leu or Val at the P2 position to Tyr switched the selectivity towards cathepsin B70. Calpain inhibitor reviews have appeared41, 71, 72, 73.
Figure 2.
Structures of epoxysuccinate cysteine protease inhibitors.
The chemical space around the P′ substituent of epoxide-containing peptidomimetics has been explored by Meara et al.74, 75. Carboxylic acid derivatives of E-64c were synthesized. The potency for inhibition of papain and cathepsin B was reported to increase by orders of magnitude in the following ranking of epoxide P′ substituents: CH2OH<COCH3<COOR<CONH2<H<CONHOH<COOH74 (Fig. 2). Assay of calpain inhibition by a series of ester and amide derivatives of E-64c in intact and lysed platelets revealed that a number of haloethyl esters were comparable in cell permeability and stability to E-64d, while amides of epoxysuccinic acids seemed to be low-potency inhibitors76. E-64c itself had too poor cell permeability to inhibit calpain in intact platelets76. Other amide derivatives of E-64c that extended into the P′ site were weak calpain inhibitors compared to the free acid76.
The first highly selective inhibitor of cathepsin B, CA-074 (4, Fig. 2) was reported to exploit the exopeptidase activity of cathepsin B, unique among the other members of the cathepsins. CA-074 and its analogs bind to the occluding loop at the P′ site. Its inactive methyl ester CA-074Me was designed to overcome poor cell permeability of the parent compound. CA-074 and CA-074Me were reported to undergo a loss of selectivity towards cathepsin B in the presence of GSH or dithioerythritol (DTT)77, 78. The selectivity and bioavailability of epoxysuccinates was improved by substituting heterocyclic analogs for His at the P2 recognition group position by Schiefer et al.79, resulting in the preclinical epoxysuccinate NYC-438 that reversed cognition deficits in the APP/PS1 AD mouse model and was devoid of toxicity even at 200 mg/kg.
3.1.2. Miscellaneous oxiranes and strained ring electrophiles
An arylsulfonyloxirane warhead was developed in 2013 as a cathepsin B, but the lack of a recognition group led to modest inhibition80. Cyclic sulfates have been developed that show selectivity for cathepsin B over calpain, presumably due to the steric hindrance in the calpain active site81, 82. Other Cys protease inhibitors containing oxiranes, thiiranes and aziridines were reviewed by Schirmeister et al.83 Vicik et al.84 explored a number of nitrogen-containing heterocycles in the P1 site of peptidomimetic cysteine protease inhibitors. Most compounds were micromolar inhibitors of cathepsin L, with selectivity against cathepsin B: the most potent compound had two electrophilic aziridines, with cathepsin L Ki=13 nmol/L and cathepsin B Ki=9.4 µmol/L (13, Fig. 3). This molecule conspicuously had an activity towards cathepsin L that exceeded that of all other inhibitors, hinting at a different binding mode.
Figure 3.
Structures of aziridine and β-lactone cysteine protease inhibitors.
Aziridines are inherently much more reactive to opportunistic biological nucleophiles than oxiranes; however, incorporation of N in an amide functionality attenuates this electrophilic reactivity. Miraziridine A (14, Fig. 3) is a natural product from a marine sponge, Theonella aff. mirabilis, with a reported cathepsin B IC50 of 2.1 µmol/L85, and both a reactive aziridine and less reactive Michael acceptor α,β-unsaturated carboxylate as terminal electrophiles.
β-Lactone and β-lactam electrophiles have been reported to acylate Ser and Thr residues at the active site of bacterial transpeptidases and have been used as antibiotics since the discovery of penicillin. With the appropriate recognition group, these four-membered rings (15, Fig. 3) also react with thiols in a Cys protease active site83. A series of 6-substituted oxapenams was developed with the more potent inhibitor having 4 nmol/L potency against cathepsin L and good selectivity versus cathepsin B86.
3.1.3. Michael acceptors
While fumaric acid derivatives were reported not to inhibit calpain and cathepsin B, similar Michael acceptors with an azapeptide recognition group (16, Fig. 4) have reported good activity against caspases87. Adducts of caspases with these Michael acceptors formed by 1,4-conjugate addition have been identified by X-ray crystallography87. The fumaric acid derivative of E-64c (DC-11, 17; Fig. 4) is 1000-fold less potent as an inhibitor of calpain 1 than E-64c88, 89, and is a weak irreversible inhibitor of cathepsins B and L88. A related, potent inhibitor of falcipain 2 (Ki=17 nmol/L, 18, Fig. 4) was developed as an antimalarial drug with good selectivity against cathepsins B and L (7.3 and 8.4 µmol/L)58.
Figure 4.
Structures of Michael acceptor warheads in cysteine protease inhibitors.
Vinyl sulfone containing peptidomimetics with varied P2 amino acids were explored as inhibitors of cathepsins K, L and S. The inhibitors had highest potency for cathepsin S, reaching 13 nmol/L in the case of P2=Leu90. LHVS (19, Fig. 4) has been used as a selective cathepsin S inhibitor in vivo and in vitro, to support a role for cathepsin S in TBI91; however, Ki=0.40, 3.4 and 4.7 µmol/L for cathepsins S, B, and L, respectively90. The vinyl sulfone group was successfully used in several falcipain 2 inhibitors (20, Fig. 4)92.
3.1.4. Halomethyl ketones
Developed as an His-selective alkylating agent, the chloromethyl ketone warhead is chemically reactive and alkylates reactive cysteine residues. The compound Z-Leu-Leu-Phe-CH2Cl (21, Fig. 5) was reported as a calpain inhibitor with moderate potency93. Fluoromethyl ketone inhibitors were developed after the synthetic methodology became available55. The direct analog of the aforementioned chloromethyl ketone (22, Fig. 5) is a potent inhibitor of human recombinant calpain 194. The chemical space around the “cap” group was explored, resulting in examples of inhibitors selective towards calpain 1 vs. cathepsin B. Fluoromethyl ketones are remarkably unreactive towards general thiol nucleophiles such as GSH95; however, the potential of fluoroacetate formation through metabolism has hindered clinical development96. Both fluoromethyl and chloromethyl ketones have been reported as potent inactivators of cathepsin B with the correct recognition group55, 97.
Figure 5.
Structures of diazomethyl, acyloxy and other ketone cysteine protease inhibitors.
3.1.5. Diazomethyl ketones
Pioneering work on this warhead by Shaw and others98, 99, 100 showed that diazomethyl ketones are irreversible inhibitors of papain. The inhibition of calpain and the cathepsins depends on the recognition peptide sequence used. Diazomethyl ketones are cell permeable and sufficiently stable in the presence of thiol-containing reducing agents such as DTT and mercaptoethanol101. Selectivity was targeted for cathepsin isozymes60, 61, 62. A diazomethyl ketone analog (Z-Leu-Leu-Tyr-CH2N2, 23; Fig. 5) of the aforementioned halomethyl ketones was reported with potency for calpain 1 inhibition intermediate between cathepsin L and cathepsin B.61
3.1.6. Acyloxymethyl and other activated ketones
Acyloxymethyl ketones are reported to have low reactivity towards GSH102. The cathepsin B inhibition of a series of acyloxymethyl ketones (Z-Phe-Ala-CH2OCO-R) was inversely correlated with the pKa of the carboxylate leaving group, with the 2,6-bis-trifluoromethylbenzoate (24, Fig. 5) having the highest potency103. The compounds also show potent inhibition of cathepsins L and S but not calpain 1. An alternative approach reported a sulfanylmethyl ketone (25, Fig. 5) that was a potent inhibitor of cruzain with good selectivity (cruzain Ki~0.9 nmol/L, cathepsin B~700 nmol/L, cathepsin L~28.8 nmol/L)104, 105. Unlike acyloxymethyl ketones, sulfonium methyl ketone peptidomimetics, e.g., Z-Leu-Leu-Phe-CH2S+(Me)2 (26, Fig. 5), inhibited calpain 1 with high potency.
Activated ketones provide a leaving group for displacement by active site thiol, presumably via a mechanism similar to that shown above for the diazomethyl ketones. The benzotriazol-1-yl leaving group has been successfully utilized to inhibit calpain potently; however, this activated ketone was unstable in aqueous solutions106. Tetrafluoro-phenoxymethyl ketones (e.g. 27) have been developed as potent cruzain inhibitors107.
The 1,2,4-thiadiazole based inhibitors do not easily fit into the classes of irreversible covalent enzyme inhibitors discussed above, but the use of this thiophilic warhead for inhibition of cathepsin B (28: Ki~2 µmol/L) deserves mention, providing selectivity over non-cysteine protease families108.
3.2. Reversible inhibitors
Reversible Cys protease inhibitors are compounds forming a non-covalent complex with the enzyme. These inhibitors can bind to the active site without substrate bound (transition state analogs, competitive inhibitors) or with substrate already bound (uncompetitive inhibitors). A third type of reversible inhibitor binds to an allosteric site (non-competitive inhibitors). Reversible inhibitors do not form covalent adducts with the enzyme and can be removed by dialysis if the non-covalent binding affinity is not too high.
3.2.1. Aldehydes and ketones
Since by definition, reversible covalent inhibitors do not yield a stable adducted enzyme, evidence for covalent mechanisms must be obtained from kinetic analysis, which has not always been carried out in sufficient detail for definitive conclusions. The most well studied examples are peptidomimetic aldehydes and trifluoromethyl ketones109, 110, 111. Peptide aldehydes were isolated from Streptomyces strains and were found to inhibit calpain and other proteases112. The physicochemical characteristics of the compounds were improved by substituting the terminal amino acid for a hydrophobic cap group such as benzyloxycarbonyl, resulting in calpeptin (Z-Leu-Nle(norleucine)-H, 29 (Fig. 6), a 40 nmol/L inhibitor of human platelet calpain 2113, 114. Another cell permeable aldehyde inhibitor relying on the same principles is Z-Val-Phe-H (MDL 28,170, 30)115. When a phenylbutyryl group was substituted for Z, the resulting compound inhibited calpains 1 and 2 with potency of 36 and 50 nmol/L, respectively, but the compound showed some inhibition of trypsin, chymotrypsin and cathepsin H, demonstrating the lower selectivity of the aldehyde warhead116. Using an acetyl cap group gave, Ac-Leu-Leu-Nle-H (31, Fig. 6) and Ac-Leu-Leu-Met-H (32, Fig. 6), which were named “calpain inhibitor I” and “calpain inhibitor II”, respectively.116 Calpain inhibitor I is a potent inhibitor of cathepsin L (0.5 nmol/L) and calpain inhibitor II, of cathepsin B (100 nmol/L). Peptidomimetic aldehydes can show high selectivity in biochemical assays; however, the chemical reactivity of aldehydes, leading to reversible Schiff base formation with proteins, metabolic oxidation and reduction, and pH-dependent hydrate formation, result in unsatisfactory stability and bioavailability, underlying a lack of progress to the clinic117. Nevertheless, these aldehydes are widely used as chemical probes for in vitro calpain inhibition.
Figure 6.
Structures of aldehyde and cyclopropenone inhibitors.
Cyclic hemiacetals provide a prodrug approach to aldehyde inhibitors designed to increase the biological half-life and enhance PK properties. The aldehyde SJA-6017 (33, Fig. 6), IC50=0.022 µmol/L (calpain 1) and IC50=0.049 µmol/L (calpain 2), was found to prevent cataract formation in rats. The hemiacetal prodrug itself (34, Fig. 6) is less active: IC50=0.88 µmol/L (calpain 1), IC50=2.6 µmol/L (calpain 2)118.
3.2.2. Cyclopropenones
Cyclopropenones have been reported as reversible covalent inhibitors of calpain119. The major example is BDA-410 (35, Fig. 6) with reported IC50 of 130 nmol/L and 630 nmol/L for calpains 1 and 2, respectively120. BDA-410 is orally bioavailable and has been reported to have a neuroprotective effect in AD mouse models121. Potential mechanisms of action include both 1,2- and 1,4- addition to the cyclopropenone ring and charge-transfer complex formation at the active site involving protonation of the cyclopropenone to a stabilized aromatic hydroxycyclopropenyl cation122.
3.2.3. α-Keto derivatives
A carbonyl group adjacent to an acyl group, usually a carboxylate ester/amide, or a heterocycle, provides an electrophile for reversible addition of a nucleophilic Cys, Ser, or Thr at an enzyme active site. α-Ketoacids, α-ketoesters, and α-ketoamides are not transition state analogs, but have the ability to form a tetrahedral transition state analog on addition of the enzyme nucleophile at the active site. Substitution of the aldehyde moiety of known calpain inhibitors with α-keto warheads has been a popular approach. In α-ketoacids, H-bonding with an active site His has also been proposed to contribute to inhibition to rationalize more potent inhibition compared to esters or amides: Z-Leu-Phe-COOH (36, Fig. 7) (calpain 1 Ki 8.7 nmol/L, calpain 2 Ki 5.7 nmol/L)123. The P1 and P2 peptide residues and hydrophobic cap group were explored extensively. In the α-ketoester series, selectivity up to 12-fold towards calpain 2 vs. calpain 1 was reported, with the best inhibitor (37, Fig. 7) providing calpain 1 Ki=100 nmol/L and calpain 2 Ki=200 nmol/L. This performance was bettered by the corresponding N-monosubstituted α-ketoamides: 20<Ki<200 nmol/L for calpains 1 and 2 and micromolar inhibition of cathepsin B. Calpain inhibitor, AK295 (38, Fig. 7), was reported to have neuroprotective effects in a rat model of cerebral focal ischemia124.
Figure 7.
Structures of ketoamide and ketoheterocycle cysteine protease inhibitors.
The chemical space around the P3 cap group of ketoamides was extensively explored, resulting in the creation of A-705239 (39) and A-705253 (40, Fig. 7), non-selective inhibitors of calpain (13.3 nmol/L, 27 nmol/L) and cathepsin B (27 nmol/L, 62 nmol/L), but with improved water solubility, cell permeability, and metabolic stability111. A-705239 rescued brain cells in a model of fluid percussive traumatic brain injury111. Inhibitors with a heterocyclic cap group, such as quinoline carboximides and chromenone derivatives were described, along with ring-opened 4-aryl-4-oxobutanoic acid derivatives125, 126. These calpain inhibitors have been developed by the cyclization and conformational restriction of the P3 amide and recognition group of conventional ketoamide inhibitors. Ketoamide inhibitors based on the structure of A-705239 and its analogs have been further developed by AbbVie127. The modifications explored included bioisosteric substitutions of the diarylalkene by substituted and fused pyrazoles and replacement of the central benzene ring with pyridine and oxopyrrole128, 129, 130, 131. ABT-957, currently in clinical trials for AD, represents the culmination of the ketoamide approach to calpain inhibition in neurodegenerative disorders. Two representative chemical structures published by Abbvie are shown (41, 42, Fig. 7).
α-Ketoheterocycles have been widely explored as inhibitors of many non-cysteine and cysteine proteases, with a number of examples of cathepsin inhibitors previously reviewed132. Early examples, were developed by optimization of P′, P2, and P3 interactions133. Cathepsin K inhibitors were described with Ki=1.7–54 nmol/L and reported selectivity over cathepsins B and L (e.g. 43: cathepsin K Ki=1.7 nmol/L, cathepsin S Ki=350 nmol/L, cathepsin L Ki=220 nmol/L, cathepsin B Ki=1 µmol/L; Fig. 7). Other examples showed high potency, but low selectivity: IC50=0.25–1 nmol/L for cathepsins B, L, K and S.134 Similar compounds with variations in the P2–P3 recognition motif were reported as inhibitors of cathepsin S, some of them (e.g. 44, Fig. 7): achieving a 100-fold selectivity vs. cathepsins B, K and L135.
Cyclic ketone inhibitors of cathepsin K have been described that have been developed by locking alkoxymethyl or alkylaminomethyl ketones into an aza- or oxacycle to induce conformational restraint. Such compounds are reported as noncompetitive reversible inhibitors of cathepsin Ki.136 SB-357114 (45, Fig. 7) was reported to inhibit human cathepsin K with a Ki of 0.16 nmol/L, and cathepsins L, S and B with Ki values of 2.2, 4.0, and 500 nmol/L, respectively, yielding a marked reduction in bone resorption in a nonhuman primate model of postmenopausal bone loss137.
3.2.4. Nitriles
Nitrile warheads have been traditionally targeted at cysteine proteases, although the increased reactivity of the related carbodiimide warhead has also been pursued for serine proteases. Several examples of nitriles inhibiting cathepsin K have been reported138. As introduced above, odanacatib (46, Fig. 8) has progressed to clinical trials for osteoporosis. Drug optimization incorporated an N-(1-cyanocyclopropyl)acetamide warhead to reduce the metabolic lability of less substituted nitrile warheads. Monoalkylated acetamides have been developed as potent inhibitors of cathepsin S (the most potent example is 47, Ki=15 nmol/L; see Fig. 8) with >1000-fold selectivity over cathepsins B, K and L.
Figure 8.
Structures of nitrile and carbodiimide inhibitors.
Selective cathepsin L inhibitors containing substituted and unsubstituted cyanomethylene warheads have been reported, the most potent (48, Fig. 8) having an IC50 of 1.26 nmol/L139, 140. The reactivity of carbodiimides towards nucleophilic addition is greater than that of nitriles. Examples of these, N-cyanopyrrolidines (Fig. 8, e.g. 49, IC50 cathepsin L 50 nmol/L, K 80 nmol/L, B 1.4 µmol/L) and N-cyanoazetidines (e.g. 50, IC50 cathepsin L 5 nmol/L, K 6 nmol/L, B 150 nmol/L), have been prepared as reversible inhibitors of cathepsins K, L, and B; expected to form a thiourea intermediate at the active site. The higher potency of the azetidine derivatives was attributed to the higher electrophilic reactivity. The formation of an isothiourea with the active site cysteine has been detected141. 2-Cyanopyrrolidines were described as selective cathepsin L inhibitors, the most potent compound (51, Ki=5.3 µmol/L, Fig. 8) was reported to be selective against cathepsin B142.
The 1,3,5-triazine-2-carbonitrile represents another approach to modulating the reactivity of the nitrile warhead, including potent inhibitors such as 52 (Fig. 8; Ki=9 nmol/L, rhodesain; 2 nmol/L, cathepsin L).143 Similarly, Merck have reported selective cathepsin S inhibiting purine-6-carbonitriles (e.g. 53, Fig. 8) that had been designed to exploit the differences between the active sites of cathepsins K and S, but aqueous stability was too poor for clinical progress144. Merck has reported other N-heterocycles as cathepsin inhibitors, such as 6-phenyl-1H-imidazo[4,5-c]pyridine-4-carbonitriles (54, Fig. 8: IC50 cathepsin S 6.9 nmol/L, cathepsin K 117 nmol/L)145. AstraZeneca has optimized pharmacokinetic properties of aminoacetonitrile inhibitors of cathepsin C working towards a clinical candidate, reporting an IC50 of 1 nmol/L and excellent selectivity for 55 (Fig. 8)146.
4. Non-covalent inhibitors
The endogenous inhibitor of calpain, calpastatin, interacts with both the unprimed and primed sites of the enzyme without extending into the active site. Truncated peptidic inhibitors were designed based on the structure. Although this review is focused on covalent inhibitors of cysteine proteases, examples of allosteric inhibitors deserve brief mention. Early research identified mercaptoacrylates as cell-permeable, selective, noncompetitive inhibitors of calpain (i.e. PD-150606, 56 and PD-151746, 57, Fig. 9), which were later reported to bind at an allosteric site on the Ca-binding domain VI147, 148. Neuroprotective activity was reported in models of ischemia and in electrophysiological studies149. More recently, biphenyl-containing high potency calpain inhibitors were reported by Montero et al.150, 151, 152 (e.g. IC50 58, 98 nmol/L, and 59, 24 nmol/L; Fig. 9). Although the authors proposed the chelation of Ca2+ as the mechanism of action, the variability of reported potency with the peptide sequence hints at an alternative allosteric mechanism. Macrocyclic compounds incorporating biphenyls were designed to improve physicochemical properties, at a significant cost in potency153.
Figure 9.
Allosteric inhibitors of calpain.
5. Covalent vs. non-covalent inhibition
Irreversible covalent enzyme inhibitors react with the target enzyme after binding to it, and thus enzyme inactivation is not an equilibrium process as with reversible inhibition, and requires re-expression of the enzyme to reverse drug action, which may occur after elimination of the drug from the body. There has been a tendency to avoid irreversible, covalent inhibitors in drug development to avoid the risk of: 1) unpredictable side effects such as the generation of allergenic modified proteins (haptens); 2) non-specific, irreversible modification of off-target proteins; and 3) the difficulty in tracking metabolites when covalently bound to proteins. The increased toxicity of “covalent drugs” has been widely perceived, despite studies suggesting otherwise, such as the lack of correlation of thiol conjugate formation with the in vivo toxicity of 50 approved drugs154. Aspirin is a textbook example of a covalent drug; however, it has taken the advent of covalent kinase inhibitors in cancer therapy to open the floodgates to such drugs155.
6. Therapeutic applications
6.1. Cathepsin inhibitors in cancer therapy
Inhibitors of cathepsins S, K, B and L have advanced to clinical trials in osteoporosis10 and cancer156. Cathepsins B and L are proposed biomarkers in cancer, with expression usually inversely correlated with outcome, for example, cathepsin B activity is increased in lung tumors and lymph node metastases157 ,and correlates with poor prognosis in metastatic non-small cell lung cancer157. Cathepsin B has been shown to have multiple roles in cancer, including tumor invasion, the formation of metastases and neovascularization, and is a pro-metastatic enzyme158. CA-074 inhibited the formation of bone metastases in breast cancer159; however, JPM-OEt, a prodrug of an inhibitor of cathepsins B and L, was ineffective in preventing metastases in breast cancer159. CA-074 and a cathepsin B antibody reduced lung metastases in mice after the injection of human melanoma cells160. In a further mouse breast cancer model, CA-074 but not JPM-OEt, was found to decrease tumor invasion, neovascularization, and bone metastases161. The bioavailability of cathepsin inhibitor drug generated by the prodrug, JPM-OEt, is problematic162.
6.2. Neurodegenerative diseases
Calpains and cathepsins play key roles in TBI, ischemic brain injury, and in normal proteolytic and regulatory pathways in the brain involved in signaling and synaptic and neuronal plasticity163. Increased proteolytic activity is observed in neurodegenerative diseases and numerous studies have been conducted to elucidate the role of not only calpains and cathepsins, but also caspases164, 165. Calpain 1 is highly expressed in neurons166 and calpain 2 in glial cells167. Elevated glutamate levels associated with excitotoxicity cause an influx of Ca2+ into neurons and consequent abnormal and extended hyperactivation of calpain. Chronic calpain activation, as opposed to transient activation, is associated with the breakdown of cell membranes, increased permeability of lysosomal membranes, and elevation of intracellular cathepsin levels168. Inhibition of both calpain and cathepsins has been reported to provide neuroprotection after cerebral ischemia169. A dose-dependent reduction in infarct volume by MDL 28,170, an aldehyde calpain inhibitor, was observed in a rat cerebral ischemia model169. Indeed, numerous studies have concluded that inhibition of cysteine proteases is neuroprotective in models of brain injury, and since these inhibitors often lack selectivity, the need for specific inhibitors needs examining91, 169, 170, 171, 172.
In TBI, secondary injury occurs after the initial insult as ion homeostasis is disturbed, excitatory mediators and reactive oxygen species are produced. As a consequence of cytosolic ion concentration change, calpains are activated and form part of a cascade of events leading to cell membrane breakdown, apoptotic and necrotic cell death165. The activity of cathepsin S was found to be increased in mice 2–4 h after TBI, indicating that cathepsin S is one of the enzymes causing secondary damage occurring after TBI. Neurological abnormalities were found to be decreased in mice that underwent TBI with prior intracerebral injection of LHVS, a vinyl sulfone cathepsin S inhibitor that does not penetrate the blood–brain barrier91. LHVS also inhibits other cathepsins with lower affinity90.
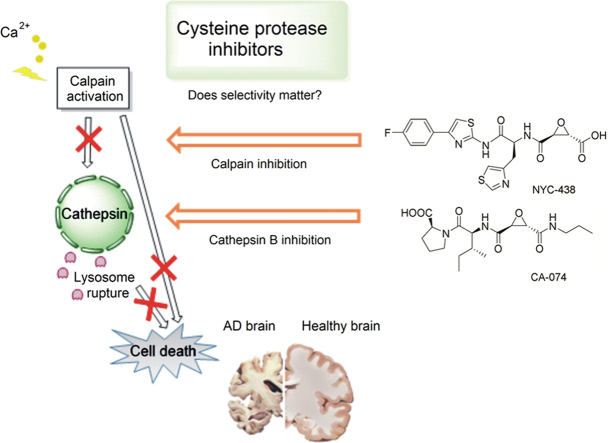
In AD, cathepsin B is found throughout the brain and also in neurites and dendrites, whereas in normal brains cathepsin B activity is localized in lysosomes173, 174. The localization of cystatins, the endogenous inhibitors of cathepsins, is also altered in neurodegenerative diseases173. Neurofibrillary tangles (NFTs) have been reported to contain increased amounts of calpain 2 and cathepsins175. Calpain 1 is known to be hyperactivated in brains of AD patients23. The level of calpastatin, the endogenous inhibitor of calpain, is also decreased176. A deuterated analog of E-64d, a pan-cysteine protease inhibitor known to inhibit calpains 1 and 2 as well as cathepsins B and L, is in clinical trials for AD therapy171, 177, 178.
6.3. The calpain–cathepsin hypothesis of neuronal loss
It is widely accepted that neuronal loss through neurodegeneration is a central event in the course of many acute and chronic disorders of the central nervous system such as cerebral ischemia, trauma and AD. The “calpain–cathepsin hypothesis” was formulated to provide a mechanism for neuronal death based upon experimental observations in the ischemic monkey paradigm. The hypothesis posits that calpain 1 hyperactivation compromises the lysosomal membranes and causes the release of cathepsins into the cytoplasm179. Calpain activation has been confirmed in the ischemic monkey brain180 and in brains of AD patients23, 181. Recent data also suggests a dual role for Hsp70 as a chaperone for damaged proteins and as an important factor in the maintenance of lysosomal integrity. Calpain-mediated cleavage of Hsp 70.1 that has been modified by oxidative stress may impair lysosomal autophagy182, 183.
Cathepsins released into the cytoplasm simultaneously damage the lysosomal membrane from outside and attack mitochondria, releasing cytochrome c and activating pro-apoptotic factors such as caspases-9 and caspase-3184, 185. Several gene KO studies and pharmacological inhibitor experiments support this hypothesis. For example, the cathepsin B inhibitor, CA-074Me, reduced biomarkers of apoptosis, such as Bax, and neuronal cell death, and reduced memory loss in a TBI model186. Moreover, cathepsin B has been shown to be critical to TNF-α-mediated apoptosis by experiments in a KO mouse.187 Interestingly, pro-apoptotic activation was profoundly suppressed by cysteine protease inhibitors leupeptin and E64.188 However, CA-074, an epoxysuccinate cathepsin B inhibitor, did not inhibit digitonin-mediated caspase activation188, indicating that not only lysosomal cathepsin B but also other lysosomal cysteine protease are involved in the cascade leading to neuronal death.189 In line with this postulate, the abnormally high concentrations of cathepsins D and cathepsin L in the cytosol, can activate Bid through proteolysis and cause the release of cytochrome c from mitochondria as well as the activation of caspase-9 and caspase-3184, 185.
6.4. Are specific inhibitors essential for clinical success?
In TBI, a substantial increase in cathepsin B brain levels and activity correlated with neuronal cell death and behavioral dysfunction186. E-64d treatment in a TBI mouse model led to similar improvements in WT compared to E-64d-treated cathepsin B KO mice, suggesting that E-64d, a non-selective cysteine protease inhibitor, functions primarily through cathepsin B inhibition in TBI170. However, at one day post-trauma, E-64d-treated cathepsin B KO mice showed faster recovery of the motor functions than was observed for untreated cathepsin B KO mice170, indicating a neuroprotective role for “off-target” inhibition of calpains, which are also validated drug targets in TBI164, 190. Indeed, brain calpain activity spikes within 24 h of trauma191, 192, and E-64d administration has been shown to reduce calpain activity and provide neuroprotection after trauma193, 194. Therefore, in TBI treatment, some additional benefits of E-64d may occur through inhibition of both cathepsin B and calpain 1, although other targets cannot be excluded. The benefits of E-64d treatment in a focal ischemia animal model were attributed to inhibition of cathepsin B, calpain 1, and matrix metallopeptidase-9 (MMP-9)195, a known contributor to TBI196, 197, although the mechanism of indirect inhibition of MMP-9 by E-64d is not known.
In AD, ischemic pathology was noted in the first description of disease neuropathology by Alois Alzheimer. Remarkably, the majority (~90%) of AD patients show a cerebral amyloid angiopathy that causes cerebral ischemia198, 199. It is therefore logical to propose that the calpain-cathepsin cascade, associated with ischemic neuronal death, contributes to AD pathogenesis200. In AD brains, calpain 1 activity is increased 7-fold compared to age-matched brains201. Amyloid precursor protein (APP) and amyloid β (Aβ) were also reported to induce calpain activation202, 203, and evidence exists for reciprocal processing by calpain1 of APP204 and tau proteins205, 206. Activated calpain was observed to occur in neurofibrillary tangles, senile plaques (SP), and dystrophic neuritis.23 Similarly, cathepsin D was observed to be localized extracellularly within senile plaques by immunoassay.207, 208 In line with this observation, an age-dependent significant increase of cathepsin D levels and activity was documented in AD human brains suggesting a possible relationship between cathepsin D activation and SP formation208.
In activated microglia, cathepsin B was claimed to be a key player in Aβ1-42 induced neuronal death209. Interestingly, this activated microglia-mediated neurotoxicity was corrected by cathepsin B gene knockdown as well as by the cathepsin B inhibitor CA-074. Accordingly, cathepsin B was proposed to mediate neuronal death initiated by inflammatory response to Aβ. Extra-lysosomal release of cathepsins has a major role in neuronal loss in AD. In this context, cathepsin B has been proposed to be an alternative executor of “β-secretase activity”, possessing excellent kinetic efficiency and specificity for cleaving wild-type APP at the β-secretase site in sporadic AD: cathepsin B may be key to amyloidogenesis in 99% of AD cases210. E-64d treatment rescued memory function, and decreased brain Aβ1-40/Aβ1-42 and amyloid plaque neuropathology in AD animal models expressing human APP containing the wild-type β- and London mutant γ-secretase site (APPLon) sequences171, 177. Cathepsin B inhibition had no effect on Aβ pathology in mice expressing APP containing the Swedish mutant β-secretase site sequence (APPSwe)171, 211. Nevertheless, BDA-410 and E-64 improved memory deficits in APPSwe mice30 in the absence of effects on Aβ, possibly by inhibition of calpain 1.
In contrast to cathepsin B, cathepsin D displays equivalent kinetic activity to BACE-1, cleaving the Swedish mutant β-secretase site more efficiently than the wild-type sequence212; importantly, relevant cathepsin D levels are about 280-fold greater than BACE-1212. In the APPSwe mutant, an asparagine residue replaces lysine in the wild-type protein. This P2 residue, is an important determinant of substrate specificity for proteases including cathepsins213. Therefore, it is possible that several cysteine proteases are involved in processing the different APP mutations in familial AD. Cathepsin B, but not BACE-1, efficiently cleaves the wild-type β-secretase site containing isoaspartate (isoAsp) post-translational modification that is abundant in AD brains, leading to further N-terminal truncated and modified, neurotoxic Aβ peptide species such as pyroGluAβ214. In turn, cathepsin B may be involved in the production of pGlu forms of Aβ214 that aggregate at accelerated rates215.
Given evidence for multiple roles for calpain and cathepsins in neurodegeneration, both independent of and associated with hallmark AD pathology (Aβ and tau), it is difficult to conclude that therapy will be unsuccessful without an entirely selective inhibitor of one specific calpain or cathepsin isoform. Although calpain inhibitors, theoretically may be effective in very, early presymptomatic AD, diagnosis of this disease stage is not currently possible. Therefore, the pharmacological inhibition of both calpain and “later” mediators of neuronal death (cathepsins B, L, and D) would seem a reasonable approach supported by results in animal models with agents such as E-64, E-64d, and NYC-438.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Lecaille F, Kaleta J, Brömme D. Human and parasitic papain-like cysteine proteases: their role in physiology and pathology and recent developments in inhibitor design. Chem Rev. 2002;102:4459–4488. doi: 10.1021/cr0101656. [DOI] [PubMed] [Google Scholar]
- 2.Turk V, Stoka V, Vasiljeva O, Renko M, Sun T, Turk B. Cysteine cathepsins: from structure, function and regulation to new frontiers. Biochim Biophys Acta. 2012;1824:68–88. doi: 10.1016/j.bbapap.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hernandez AA, Roush WR. Recent advances in the synthesis, design and selection of cysteine protease inhibitors. Curr Opin Chem Biol. 2002;6:459–465. doi: 10.1016/s1367-5931(02)00345-9. [DOI] [PubMed] [Google Scholar]
- 4.Gobec S, Frlan R. Inhibitors of cathepsin B. Curr Med Chem. 2006;13:2309–2327. doi: 10.2174/092986706777935122. [DOI] [PubMed] [Google Scholar]
- 5.Cheng XW, Huang Z, Kuzuya M, Okumura K, Murohara T. Cysteine protease cathepsins in atherosclerosis-based vascular disease and its complications. Hypertension. 2011;58:978–986. doi: 10.1161/HYPERTENSIONAHA.111.180935. [DOI] [PubMed] [Google Scholar]
- 6.Turk V, Turk B, Turk D. Lysosomal cysteine proteases: facts and opportunities. EMBO J. 2001;20:4629–4633. doi: 10.1093/emboj/20.17.4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Musil D, Zucic D, Turk D, Engh RA, Mayr I, Huber R. The refined 2.15 Å X-ray crystal structure of human liver cathepsin B: the structural basis for its specificity. EMBO J. 1991;10:2321–2330. doi: 10.1002/j.1460-2075.1991.tb07771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Illy C, Quraishi O, Wang J, Purisima E, Vernet T, Mort JS. Role of the occluding loop in cathepsin B activity. J Biol Chem. 1997;272:1197–1202. doi: 10.1074/jbc.272.2.1197. [DOI] [PubMed] [Google Scholar]
- 9.Schenker P, Alfarano P, Kolb P, Caflisch A, Baici A. A double-headed cathepsin B inhibitor devoid of warhead. Protein Sci. 2008;17:2145–2155. doi: 10.1110/ps.037341.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bone HG, Dempster DW, Eisman JA, Greenspan SL, McClung MR, Nakamura T. Odanacatib for the treatment of postmenopausal osteoporosis: development history and design and participant characteristics of LOFT, the long-term odanacatib fracture trial. Osteoporos Int. 2015;26:699–712. doi: 10.1007/s00198-014-2944-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cotrin SS, Puzer L, de Souza Judice WA, Juliano L, Carmona AK, Juliano MA. Positional-scanning combinatorial libraries of fluorescence resonance energy transfer peptides to define substrate specificity of carboxydipeptidases: assays with human cathepsin B. Anal Biochem. 2004;335:244–252. doi: 10.1016/j.ab.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Biniossek ML, Nägler DK, Becker-Pauly C, Schilling O. Proteomic identification of protease cleavage sites characterizes prime and non-prime specificity of cysteine cathepsins B, L, and S. J Proteome Res. 2011;10:5363–5373. doi: 10.1021/pr200621z. [DOI] [PubMed] [Google Scholar]
- 13.Huang YH, Wang KKW. The calpain family and human disease. Trends Mol Med. 2001;7:355–362. doi: 10.1016/s1471-4914(01)02049-4. [DOI] [PubMed] [Google Scholar]
- 14.Sorimachi H, Hata S, Ono Y. Impact of genetic insights into calpain biology. J Biochem. 2011;150:23–37. doi: 10.1093/jb/mvr070. [DOI] [PubMed] [Google Scholar]
- 15.Guroff G. A neutral, calcium-activated proteinase from the soluble fraction of rat brain. J Biol Chem. 1964;239:149–155. [PubMed] [Google Scholar]
- 16.Meyer WL, Fischer EH, Krebs EG. Activation of skeletal muscle phosphorylase B kinase by Ca2+ Biochemistry. 1964;3:1033–1039. doi: 10.1021/bi00896a004. [DOI] [PubMed] [Google Scholar]
- 17.Huston RB, Krebs EG. Activation of skeletal muscle phosphorylase kinase by calcium ions. II. Identification of the kinase activating factor as a proteolytic enzyme. Biochemistry. 1968;7:2116–2122. doi: 10.1021/bi00846a014. [DOI] [PubMed] [Google Scholar]
- 18.Goll DE, Thompson VF, Li HQ, Wei W, Cong JY. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 19.Moldoveanu T, Hosfield CM, Lim D, Elce JS, Jia ZC, Davies PL. A Ca2+ switch aligns the active site of calpain. Cell. 2002;108:649–660. doi: 10.1016/s0092-8674(02)00659-1. [DOI] [PubMed] [Google Scholar]
- 20.Arthur JSC, Crawford C. Investigation of the interaction of m-calpain with phospholipids: calpain-phospholipid interactions. Biochim Biophys Acta. 1996;1293:201–206. doi: 10.1016/0167-4838(95)00243-x. [DOI] [PubMed] [Google Scholar]
- 21.Shao HS, Chou J, Baty CJ, Burke NA, Watkins SC, Stolz DB. Spatial localization of m-calpain to the plasma membrane by phosphoinositide biphosphate binding during epidermal growth factor receptor-mediated activation. Mol Cell Biol. 2006;26:5481–5496. doi: 10.1128/MCB.02243-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reiser J, Adair B, Reinheckel T. Specialized roles for cysteine cathepsins in health and disease. J Clin Investig. 2010;120:3421–3431. doi: 10.1172/JCI42918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saito K, Elce JS, Hamos JE, Nixon RA. Widespread activation of calcium-activated neutral proteinase (calpain) in the brain in Alzheimer disease: a potential molecular basis for neuronal degeneration. Proc Natl Acad Sci USA. 1993;90:2628–2632. doi: 10.1073/pnas.90.7.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirai S, Kawasaki H, Yaniv M, Suzuki K. Degradation of transcription factors, c-Jun and c-Fos, by calpain. FEBS Lett. 1991;287:57–61. doi: 10.1016/0014-5793(91)80015-u. [DOI] [PubMed] [Google Scholar]
- 25.Teich AF, Arancio O. Is the amyloid hypothesis of Alzheimer’s disease therapeutically relevant? Biochem J. 2012;446:165–177. doi: 10.1042/BJ20120653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang A, Bibb JA. Is CREB the angry bird that releases memory in Alzheimer׳s? Neuropsychopharmacology. 2011;36:2153–2154. doi: 10.1038/npp.2011.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saura CA, Valero J. The role of CREB signaling in Alzheimer׳s disease and other cognitive disorders. Rev Neurosci. 2011;22:153–169. doi: 10.1515/RNS.2011.018. [DOI] [PubMed] [Google Scholar]
- 28.Pugazhenthi S, Wang MR, Pham S, Sze CI, Eckman CB. Downregulation of CREB expression in Alzheimer׳s brain and in Aβ-treated rat hippocampal neurons. Mol Neurodegener. 2011;6:60. doi: 10.1186/1750-1326-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Satoh J, Tabunoki H, Arima K. Molecular network analysis suggests aberrant CREB-mediated gene regulation in the Alzheimer disease hippocampus. Dis Markers. 2009;27:239–252. doi: 10.3233/DMA-2009-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trinchese F, Fa MR, Liu SM, Zhang H, Hidalgo A, Schmidt SD. Inhibition of calpains improves memory and synaptic transmission in a mouse model of Alzheimer disease. J Clin Investig. 2008;118:2796–2807. doi: 10.1172/JCI34254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wieschhaus A, Khan A, Zaidi A, Rogalin H, Hanada T, Liu F. Calpain-1 knockout reveals broad effects on erythrocyte deformability and physiology. Biochem J. 2012;448:141–152. doi: 10.1042/BJ20121008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuchay SM, Kim N, Grunz EA, Fay WP, Chishti AH. Double knockouts reveal that protein tyrosine phosphatase 1B is a physiological target of calpain-1 in platelets. Mol Cell Biol. 2007;27:6038–6052. doi: 10.1128/MCB.00522-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanna RA, Campbell RL, Davies PL. Calcium-bound structure of calpain and its mechanism of inhibition by calpastatin. Nature. 2008;456:409–412. doi: 10.1038/nature07451. [DOI] [PubMed] [Google Scholar]
- 34.Uemori T, Shimojo T, Asada K, Asano T, Kimizuka F, Kato I. Characterization of a functional domain of human calpastatin. Biochem Biophys Res Commun. 1990;166:1485–1493. doi: 10.1016/0006-291x(90)91035-q. [DOI] [PubMed] [Google Scholar]
- 35.Vaisid T, Kosower NS, Katzav A, Chapman J, Barnoy S. Calpastatin levels affect calpain activation and calpain proteolytic activity in APP transgenic mouse model of Alzheimer׳s disease. Neurochem Int. 2007;51:391–397. doi: 10.1016/j.neuint.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Sasaki M, Taniguchi K, Suzuki K, Imahori K. Human plasma alpha 1- and alpha 2-thiol proteinase inhibitors strongly inhibit Ca-activated neutral protease from muscle. Biochem Biophys Res Commun. 1983;110:256–261. doi: 10.1016/0006-291x(83)91288-3. [DOI] [PubMed] [Google Scholar]
- 37.Fiorino F, Gil-Parrado S, Assfalg-Machleidt I, Machleidt W, Moroder L. A new cell-permeable calpain inhibitor. J Pept Sci. 2007;13:70–73. doi: 10.1002/psc.790. [DOI] [PubMed] [Google Scholar]
- 38.Gil-Parrado S, Assfalg-Machleidt I, Fiorino F, Deluca D, Pfeiler D, Schaschke N. Calpastatin exon 1B-derived peptide, a selective inhibitor of calpain: enhancing cell permeability by conjugation with penetratin. Biol Chem. 2003;384:395–402. doi: 10.1515/BC.2003.045. [DOI] [PubMed] [Google Scholar]
- 39.Rao MV, McBrayer MK, Campbell J, Kumar A, Hashim A, Sershen H. Specific calpain inhibition by calpastatin prevents tauopathy and neurodegeneration and restores normal lifespan in tau P301L mice. J Neurosci. 2014;34:9222–9234. doi: 10.1523/JNEUROSCI.1132-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rao MV, Mohan PS, Peterhoff CM, Yang DS, Schmidt SD, Stavrides PH. Marked calpastatin (CAST) depletion in Alzheimer׳s disease accelerates cytoskeleton disruption and neurodegeneration: neuroprotection by CAST overexpression. J Neurosci. 2008;28:12241–12254. doi: 10.1523/JNEUROSCI.4119-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Donkor IO. Calpain inhibitors: a survey of compounds reported in the patent and scientific literature. Expert Opin Ther Pat. 2011;21:601–636. doi: 10.1517/13543776.2011.568480. [DOI] [PubMed] [Google Scholar]
- 42.Samantaray S, Knaryan VH, Shields DC, Banik NL. Critical role of calpain in spinal cord degeneration in Parkinson׳s disease. J Neurochem. 2013;127:880–890. doi: 10.1111/jnc.12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Samantaray S, Ray SK, Banik NL. Calpain as a potential therapeutic target in Parkinson׳s disease. CNS Neurol Disord Drug Targets. 2008;7:305–312. doi: 10.2174/187152708784936680. [DOI] [PubMed] [Google Scholar]
- 44.Gafni J, Ellerby LM. Calpain activation in Huntington׳s disease. J Neurosci. 2002;22:4842–4849. doi: 10.1523/JNEUROSCI.22-12-04842.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim M, Roh JK, Yoon BW, Kang LM, Kim YJ, Aronin N. Huntingtin is degraded to small fragments by calpain after ischemic injury. Exp Neurol. 2003;183:109–115. doi: 10.1016/s0014-4886(03)00132-8. [DOI] [PubMed] [Google Scholar]
- 46.Rosenberger TA. Targeting calpain-mediated proteolysis and peptide signaling as a strategy to reduce injury in multiple sclerosis. J Neurochem. 2014;130:161–164. doi: 10.1111/jnc.12732. [DOI] [PubMed] [Google Scholar]
- 47.Trager N, Butler JT, Haque A, Ray SK, Beeson C, Banik NL. The involvement of calpain in CD4+ T helper cell bias in multple sclerosis. J Clin Cell Immunol. 2013;4:1000153. doi: 10.4172/2155-9899.1000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trager N, Smith A, Wallace G, IV, Azuma M, Inoue J, Beeson C. Effects of a novel orally administered calpain inhibitor SNJ-1945 on immunomodulation and neurodegeneration in a murine model of multiple sclerosis. J Neurochem. 2014;130:268–279. doi: 10.1111/jnc.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Camins A, Verdaguer E, Folch J, Pallàs M. Involvement of calpain activation in neurodegenerative processes. CNS Drug Rev. 2006;12:135–148. doi: 10.1111/j.1527-3458.2006.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vosler PS, Brennan CS, Chen J. Calpain-mediated signaling mechanisms in neuronal injury and neurodegeneration. Mol Neurobiol. 2008;38:78–100. doi: 10.1007/s12035-008-8036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harris F, Biswas S, Singh J, Dennison S, Phoenix DA. Calpains and their multiple roles in diabetes mellitus. Ann N Y Acad Sci. 2006;1084:452–480. doi: 10.1196/annals.1372.011. [DOI] [PubMed] [Google Scholar]
- 52.Sreenan SK, Zhou YP, Otani K, Hansen PA, Currie KP, Pan CY. Calpains play a role in insulin secretion and action. Diabetes. 2001;50:2013–2020. doi: 10.2337/diabetes.50.9.2013. [DOI] [PubMed] [Google Scholar]
- 53.Richard I, Broux O, Allamand V, Fougerousse F, Chiannilkulchai N, Bourg N. Mutations in the proteolytic enzyme calpain 3 cause limb-girdle muscular dystrophy type 2A. Cell. 1995;81:27–40. doi: 10.1016/0092-8674(95)90368-2. [DOI] [PubMed] [Google Scholar]
- 54.Fennell BD, Warren JM, Chung KK, Main HL, Arend AB, Tochowicz A. Optimization of peptidyl allyl sulfones as clan CA cysteine protease inhibitors. J Enzyme Inhib Med Chem. 2013;28:468–478. doi: 10.3109/14756366.2011.651466. [DOI] [PubMed] [Google Scholar]
- 55.Rasnick D. Synthesis of peptide fluoromethyl ketones and the inhibition of human cathepsin B. Anal Biochem. 1985;149:461–465. doi: 10.1016/0003-2697(85)90598-6. [DOI] [PubMed] [Google Scholar]
- 56.Rauber P, Angliker H, Walker B, Shaw E. The synthesis of peptidylfluoromethanes and their properties as inhibitors of serine proteinases and cysteine proteinases. Biochem J. 1986;239:633–640. doi: 10.1042/bj2390633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Powers JC, Asgian JL, Ekici ÖD, James KE. Irreversible inhibitors of serine, cysteine, and threonine proteases. Chem Rev. 2002;102:4639–4750. doi: 10.1021/cr010182v. [DOI] [PubMed] [Google Scholar]
- 58.Ettari R, Micale N, Schirmeister T, Gelhaus C, Leippe M, Nizi E. Novel peptidomimetics containing a vinyl ester moiety as highly potent and selective falcipain-2 inhibitors. J Med Chem. 2009;52:2157–2160. doi: 10.1021/jm900047j. [DOI] [PubMed] [Google Scholar]
- 59.Dunny E, Doherty W, Evans P, Malthouse JPG, Nolan D, Knox AJS. Vinyl sulfone-based peptidomimetics as anti-trypanosomal agents: design, synthesis, biological and computational evaluation. J Med Chem. 2013;56:6638–6650. doi: 10.1021/jm400294w. [DOI] [PubMed] [Google Scholar]
- 60.Angliker H, Wikstrom P, Kirschke H, Shaw E. The inactivation of the cysteinyl exopeptidases cathepsin H and C by affinity-labelling reagents. Biochem J. 1989;262:63–68. doi: 10.1042/bj2620063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Crawford C, Mason RW, Wikstrom P, Shaw E. The design of peptidyldiazomethane inhibitors to distinguish between the cysteine proteinases calpain II, cathepsin L and cathepsin B. Biochem J. 1988;253:751–758. doi: 10.1042/bj2530751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shaw E, Mohanty S, Colic A, Stoka V, Turk V. The affinity-labelling of cathepsin S with peptidyl diazomethyl ketones: comparison with the inhibition of cathepsin L and calpain. FEBS Lett. 1993;334:340–342. doi: 10.1016/0014-5793(93)80707-2. [DOI] [PubMed] [Google Scholar]
- 63.Hanada K, Tamai M, Yamagishi M, Ohmura S, Sawada J, Tanaka I. Isolation and characterization of E-64, a new thiol protease inhibitor. Agric Biol Chem. 1978;42:523–528. [Google Scholar]
- 64.Hanada K, Tamai M, Ohmura S, Sawada J, Seki T, Tanaka I. Structure and synthesis of E-64, a new thiol protease inhibitor. Agric Biol Chem. 1978;42:529–536. [Google Scholar]
- 65.Satoyoshi E. Therapeutic trials on progressive muscular dystrophy. Int Med. 1992;31:841–846. doi: 10.2169/internalmedicine.31.841. [DOI] [PubMed] [Google Scholar]
- 66.Tamai M, Matsumoto K, Omura S, Koyama I, Ozawa Y, Hanada K. In vitro and in vivo inhibition of cysteine proteinases by EST, a new analog of E-64. J Pharmacobiodyn. 1986;9:672–677. doi: 10.1248/bpb1978.9.672. [DOI] [PubMed] [Google Scholar]
- 67.Kerr ID, Lee JH, Pandey KC, Harrison A, Sajid M, Rosenthal PJ. Structures of falcipain-2 and falcipain-3 bound to small molecule inhibitors: implications for substrate specificity. J Med Chem. 2009;52:852–857. doi: 10.1021/jm8013663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moldoveanu T, Campbell RL, Cuerrier D, Davies PL. Crystal structures of calpain-E64 and -leupeptin inhibitor complexes reveal mobile loops gating the active site. J Mol Biol. 2004;343:1313–1326. doi: 10.1016/j.jmb.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 69.Cuerrier D, Moldoveanu T, Campbell RL, Kelly J, Yoruk B, Verhelst SHL. Development of calpain-specific inactivators by screening of positional scanning epoxide libraries. J Biol Chem. 2007;282:9600–9611. doi: 10.1074/jbc.M610372200. [DOI] [PubMed] [Google Scholar]
- 70.Giordano C, Calabretta R, Gallina C, Consalvi V, Scandurra R, Noya FC. Iodo and diiodotyrosine epoxysuccinyl derivatives as selective inhibitors of cathepsin B. Eur J Med Chem. 1993;28:917–926. [Google Scholar]
- 71.Donkor IO. A survey of calpain inhibitors. Curr Med Chem. 2000;7:1171–1188. doi: 10.2174/0929867003374129. [DOI] [PubMed] [Google Scholar]
- 72.Donkor IO. An updated patent review of calpain inhibitors (2012-2014) Expert Opin Ther Pat. 2015;25:17–31. doi: 10.1517/13543776.2014.982534. [DOI] [PubMed] [Google Scholar]
- 73.Pietsch M, Chua KCH, Abell AD. Calpains: attractive targets for the development of synthetic inhibitors. Curr Top Med Chem. 2010;10:270–293. doi: 10.2174/156802610790725489. [DOI] [PubMed] [Google Scholar]
- 74.Meara JP, Rich DH. Mechanistic studies on the inactivation of papain by epoxysuccinyl inhibitors. J Med Chem. 1996;39:3357–3366. doi: 10.1021/jm950445b. [DOI] [PubMed] [Google Scholar]
- 75.Bihovsky R, Powers JC, Kam CM, Walton R, Loewi RC. Further evidence for the importance of free carboxylate in epoxysuccinate inhibitors of thiol proteases. J Enzyme Inhib Med Chem. 1993;7:15–25. doi: 10.3109/14756369309020184. [DOI] [PubMed] [Google Scholar]
- 76.Huang ZY, McGowan EB, Detwiler TC. Ester and amide derivatives of E64c as inhibitors of platelet calpains. J Med Chem. 1992;35:2048–2054. doi: 10.1021/jm00089a015. [DOI] [PubMed] [Google Scholar]
- 77.Steverding D. The cathepsin B-selective inhibitors CA-074 and CA-074Me inactivate cathepsin L under reducing conditions. Open Enzyme Inhib J. 2011;4:11–16. [Google Scholar]
- 78.Montaser M, Lalmanach G, Mach L. CA-074 but not its methyl ester CA-074Me, is a selective inhibitor of cathepsin B within living cells. Biol Chem. 2002;383:1305–1308. doi: 10.1515/BC.2002.147. [DOI] [PubMed] [Google Scholar]
- 79.Schiefer IT, Tapadar S, Litosh V, Siklos M, Scism R, Wijewickrama GT. Design, synthesis, and optimization of novel epoxide incorporating peptidomimetics as selective calpain inhibitors. J Med Chem. 2013;56:6054–6068. doi: 10.1021/jm4006719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dana D, Davalos AR, De S, Rathod P, Gamage RK, Huestis J. Development of cell-active non-peptidyl inhibitors of cysteine cathepsins. Bioorg Med Chem. 2013;21:2975–2987. doi: 10.1016/j.bmc.2013.03.062. [DOI] [PubMed] [Google Scholar]
- 81.Hoye TR, Crawford KB. Enolate and other carbon nucleophile alkylation reactions using 1,2-cyclic sulfates as terminal epoxide equivalents. J Org Chem. 1994;59:520–522. [Google Scholar]
- 82.Haruta J, Tanaka M, Uchida I, Ohta A, Hara S, inventors; Japan Tobacco Inc, assignee. 1,3,2-dioxathiolane oxide derivative. European Patent Application EP 0460239; 1991 Dec 11.
- 83.Schirmeister T, Klockow A. Cysteine protease inhibitors containing small rings. Mini Rev Med Chem. 2003;3:585–596. doi: 10.2174/1389557033487935. [DOI] [PubMed] [Google Scholar]
- 84.Vicik R, Busemann M, Gelhaus C, Stiefl N, Scheiber J, Schmitz W. Aziridide-based inhibitors of cathepsin L: synthesis, inhibition activity, and docking studies. ChemMedChem. 2006;1:1126–1141. doi: 10.1002/cmdc.200600106. [DOI] [PubMed] [Google Scholar]
- 85.Nakao Y, Fujita M, Warabi K, Matsunaga S, Fusetani N. Miraziridine A a novel cysteine protease inhibitor from the marine sponge theonella aff. mirabilis. J Am Chem Soc. 2000;122:10462–10463. [Google Scholar]
- 86.Singh R, Zhou NE, Guo DQ, Kaleta J, Cameron A, Purisima E, et al., inventors; Naeja Pharmaceutical Inc., National Research Council of Canada, assignee. 6-substituted amino-4-oxa-1-azabicyclo[3,2,0] heptan-7-one derivatives as cysteine protease inhibitors. European patent EP 0904284; 1997 Apr 9.
- 87.Ekici ÖD, Li ZZ, Campbell AJ, James KE, Asgian JL, Mikolajczyk J. Design, synthesis, and evaluation of aza-peptide Michael acceptors as selective and potent inhibitors of caspases-2, -3, -6, -7, -8, -9, and -10. J Med Chem. 2006;49:5728–5749. doi: 10.1021/jm0601405. [DOI] [PubMed] [Google Scholar]
- 88.Barrett AJ, Kembhavi AA, Brown MA, Kirschke H, Knight CG, Tamai M. l-Trans-epoxysuccinyl-leucylamido(4-guanidino)butane (E-64) and its analogues as inhibitors of cysteine proteinases including cathepsins B, H and L. Biochem J. 1982;201:189–198. doi: 10.1042/bj2010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Parkes C, Kembhavi AA, Barrett AJ. Calpain inhibition by peptide epoxides. Biochem J. 1985;230:509–516. doi: 10.1042/bj2300509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brömme D, Klaus JL, Okamoto K, Rasnick D, Palmer JT. Peptidyl vinyl sulphones: a new class of potent and selective cysteine protease inhibitors: S2P2 specificity of human cathepsin O2 in comparison with cathepsins S and L. Biochem J. 1996;315(Pt 1):85–89. doi: 10.1042/bj3150085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu JG, Wang HD, Ding K, Lu XY, Li T, Wang JW. Inhibition of cathepsin S produces neuroprotective effects after traumatic brain injury in mice. Mediat Inflamm. 2013;2013:187873. doi: 10.1155/2013/187873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ettari R, Nizi E, di Francesco ME, Dude M-A, Pradel G, Vičik R. Development of peptidomimetics with a vinyl sulfone warhead as irreversible falcipain-2 inhibitors. J Med Chem. 2008;51:988–996. doi: 10.1021/jm701141u. [DOI] [PubMed] [Google Scholar]
- 93.Sasaki T, Kikuchi T, Fukui I, Murachi T. Inactivation of calpain I and calpain II by specificity-oriented tripeptidyl chloromethyl ketones. J Biochem. 1986;99:173–179. doi: 10.1093/oxfordjournals.jbchem.a135457. [DOI] [PubMed] [Google Scholar]
- 94.Chatterjee S, Ator MA, Bozyczko-Coyne D, Josef K, Wells G, Tripathy R. Synthesis and biological activity of a series of potent fluoromethyl ketone inhibitors of recombinant human calpain I. J Med Chem. 1997;40:3820–3828. doi: 10.1021/jm970197e. [DOI] [PubMed] [Google Scholar]
- 95.Angliker H, Wikstrom P, Rauber P, Shaw E. The synthesis of lysylfluoromethanes and their properties as inhibitors of trypsin, plasmin and cathepsin B. Biochem J. 1987;241:871–875. doi: 10.1042/bj2410871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Eichhold TH, Hookfin EB, Taiwo YO, De B, Wehmeyer KR. Isolation and quantification of fluoroacetate in rat tissues, following dosing of Z-Phe-Ala-CH2-F, a peptidyl fluoromethyl ketone protease inhibitor. J Pharm Biomed Anal. 1997;16:459–467. doi: 10.1016/s0731-7085(97)00102-7. [DOI] [PubMed] [Google Scholar]
- 97.Angliker H, Wikstrom P, Rauber P, Stone S, Shaw E. Synthesis and properties of peptidyl derivatives of arginylfluoromethanes. Biochem J. 1988;256:481–486. doi: 10.1042/bj2560481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brocklehurst K, Malthouse JP. Mechanism of the reaction of papain with substrate-derived diazomethyl ketones. Implications for the difference in site specificity of halomethyl ketones for serine proteinases and cysteine proteinases and for stereoelectronic requirements in the papain catalytic mechanism. Biochem J. 1978;175:761–764. doi: 10.1042/bj1750761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Leary R, Larsen D, Watanabe H, Shaw E. Diazomethyl ketone substrate derivatives as active-site-directed inhibitors of thiol proteases. Papain. Biochemistry. 1977;16:5857–5861. doi: 10.1021/bi00645a033. [DOI] [PubMed] [Google Scholar]
- 100.Leary R, Shaw E. Inactivation of cathepsin B1 by diazomethyl ketones. Biochem Biophys Res Commun. 1977;79:926–931. doi: 10.1016/0006-291x(77)91199-8. [DOI] [PubMed] [Google Scholar]
- 101.Shaw E. Peptidyl diazomethanes as inhibitors of cysteine and serine proteinases. Methods Enzymol. 1994;244:649–656. doi: 10.1016/0076-6879(94)44048-4. [DOI] [PubMed] [Google Scholar]
- 102.Krantz A, Copp LJ, Coles PJ, Smith RA, Heard SB. Peptidyl (acyloxy)methyl ketones and the quiescent affinity label concept: the departing group as a variable structural element in the design of inactivators of cysteine proteinases. Biochemistry. 1991;30:4678–4687. doi: 10.1021/bi00233a007. [DOI] [PubMed] [Google Scholar]
- 103.Krantz A. Peptidyl (acyloxy)methanes as quiescent affinity labels for cysteine proteinases. Methods Enzymol. 1994;244:656–671. doi: 10.1016/0076-6879(94)44049-2. [DOI] [PubMed] [Google Scholar]
- 104.Lee A, Ellman JA. Parallel solution-phase synthesis of mechanism-based cysteine protease inhibitors. Org Lett. 2001;3:3707–3709. doi: 10.1021/ol0166496. [DOI] [PubMed] [Google Scholar]
- 105.Maly DJ, Huang LL, Ellman JA. Combinatorial strategies for targeting protein families: application to the proteases. ChemBioChem. 2002;3:17–37. doi: 10.1002/1439-7633(20020104)3:1<16::AID-CBIC16>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 106.Tripathy R, Ator MA, Mallamo JP. Calpain inhibitors based on the quiescent affinity label concept: high rates of calpain inactivation with leaving groups derived from N-hydroxy peptide coupling reagents. Bioorg Med Chem Lett. 2000;10:2315–2319. doi: 10.1016/s0960-894x(00)00451-0. [DOI] [PubMed] [Google Scholar]
- 107.Brak K, Doyle PS, McKerrow JH, Ellman JA. Identification of a new class of nonpeptidic inhibitors of cruzain. J Am Chem Soc. 2008;130:6404–6410. doi: 10.1021/ja710254m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Leung-Toung R, Wodzinska J, Li WR, Lowrie J, Kukreja R, Desilets D. 1,2,4-thiadiazole: a novel cathepsin B inhibitor. Bioorg Med Chem. 2003;11:5529–5537. doi: 10.1016/j.bmc.2003.09.040. [DOI] [PubMed] [Google Scholar]
- 109.Yoon SY, Choi JE, Ham JH, Choe H, Lee HS, Kim DH. zVLL-CHO at low concentrations acts as a calpain inhibitor to protect neurons against okadaic acid-induced neurodegeneration. Neurosci Lett. 2012;509:33–38. doi: 10.1016/j.neulet.2011.12.042. [DOI] [PubMed] [Google Scholar]
- 110.Nikkel AL, Martino B, Markosyan S, Brederson JD, Medeiros R, Moeller A. The novel calpain inhibitor A-705253 prevents stress-induced tau hyperphosphorylation in vitro and in vivo. Neuropharmacology. 2012;63:606–612. doi: 10.1016/j.neuropharm.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 111.Lubisch W, Beckenbach E, Bopp S, Hofmann HP, Kartal A, Kastel C. Benzoylalanine-derived ketoamides carrying vinylbenzyl amino residues: discovery of potent water-soluble calpain inhibitors with oral bioavailability. J Med Chem. 2003;46:2404–2412. doi: 10.1021/jm0210717. [DOI] [PubMed] [Google Scholar]
- 112.Sasaki T, Kikuchi T, Yumoto N, Yoshimura N, Murachi T. Comparative specificity and kinetic studies on porcine calpain I and calpain II with naturally occurring peptides and synthetic fluorogenic substrates. J Biol Chem. 1984;259:12489–12494. [PubMed] [Google Scholar]
- 113.Tsujinaka T, Kajiwara Y, Kambayashi J, Sakon M, Higuchi N, Tanaka T. Synthesis of a new cell penetrating calpain inhibitor (calpeptin) Biochem Biophys Res Commun. 1988;153:1201–1208. doi: 10.1016/s0006-291x(88)81355-x. [DOI] [PubMed] [Google Scholar]
- 114.Yano Y, Shiba E, Kambayashi J, Sakon M, Kawasaki T, Fujitani K. The effects of calpeptin (a calpain specific inhibitor) on agonist induced microparticle formation from the platelet plasma membrane. Thromb Res. 1993;71:385–396. doi: 10.1016/0049-3848(93)90163-i. [DOI] [PubMed] [Google Scholar]
- 115.Mehdi S, Angelastro MR, Wiseman JS, Bey P. Inhibition of the proteolysis of rat erythrocyte membrane proteins by a synthetic inhibitor of calpain. Biochem Biophys Res Commun. 1988;157:1117–1123. doi: 10.1016/s0006-291x(88)80989-6. [DOI] [PubMed] [Google Scholar]
- 116.Sasaki T, Kishi M, Saito M, Tanaka T, Higuchi N, Kominami E. Inhibitory effect of di- and tripeptidyl aldehydes on calpains and cathepsins. J Enzyme Inhib Med Chem. 1990;3:195–201. doi: 10.3109/14756369009035837. [DOI] [PubMed] [Google Scholar]
- 117.Shaw E. Cysteinyl proteinases and their selective inactivation. Adv Enzymol Relat Areas Mol Biol. 1990;63:271–347. doi: 10.1002/9780470123096.ch5. [DOI] [PubMed] [Google Scholar]
- 118.Nakamura M, Yamaguchi M, Sakai O, Inoue J. Exploration of cornea permeable calpain inhibitors as anticataract agents. Bioorg Med Chem. 2003;11:1371–1379. doi: 10.1016/s0968-0896(02)00612-0. [DOI] [PubMed] [Google Scholar]
- 119.Ando R, Sakaki T, Morinaka Y, Takahashi C, Tamao Y, Yoshii N. Cyclopropenone-containing cysteine proteinase inhibitors. Synthesis and enzyme inhibitory activities. Bioorg Med Chem. 1999;7:571–579. doi: 10.1016/s0968-0896(99)00007-3. [DOI] [PubMed] [Google Scholar]
- 120.Ando R, Morinaka Y, Tokuyama H, Isaka M, Nakamura E. A new class of proteinase-inhibitor. Cyclopropenone-containing inhibitor of papain. J Am Chem Soc. 1993;115:1174–1175. [Google Scholar]
- 121.Battaglia F, Trinchese F, Liu SM, Walter S, Nixon RA, Arancio O. Calpain inhibitors, a treatment for alzheimer׳s disease. J Mol Neurosci. 2003;20:357–362. doi: 10.1385/JMN:20:3:357. [DOI] [PubMed] [Google Scholar]
- 122.Cohen M, Bretler U, Albeck A. Peptidyl cyclopropenones: reversible inhibitors, irreversible inhibitors, or substrates of cysteine proteases? Protein Sci. 2013;22:788–799. doi: 10.1002/pro.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li ZZ, Patil GS, Golubski ZE, Hori H, Tehrani K, Foreman JE. Peptide alpha-keto ester, alpha-keto amide, and alpha-keto acid inhibitors of calpains and other cysteine proteases. J Med Chem. 1993;36:3472–3480. doi: 10.1021/jm00074a031. [DOI] [PubMed] [Google Scholar]
- 124.Bartus RT, Hayward NJ, Elliott PJ, Sawyer SD, Baker KL, Dean RL. Calpain inhibitor AK295 protects neurons from focal brain ischemia. Effects of postocclusion intra-arterial administration. Stroke. 1994;25:2265–2270. doi: 10.1161/01.str.25.11.2265. [DOI] [PubMed] [Google Scholar]
- 125.Lee KS, Seo SH, Lee YH, Kim HD, Son MH, Chung BY. Synthesis and biological evaluation of chromone carboxamides as calpain inhibitors. Bioorg Med Chem Lett. 2005;15:2857–2860. doi: 10.1016/j.bmcl.2005.03.095. [DOI] [PubMed] [Google Scholar]
- 126.Zhang Y, Jung SY, Jin C, Kim ND, Gong P, Lee YS. Design and synthesis of 4-aryl-4-oxobutanoic acid amides as calpain inhibitors. Bioorg Med Chem Lett. 2009;19:502–507. doi: 10.1016/j.bmcl.2008.11.030. [DOI] [PubMed] [Google Scholar]
- 127.Kling A, Hornberger W, Mack H, Moeller A, Nimmrich V, Seemann D, Lubisch W, inventors; N-(3-Amino-1-benzyl-2,3-dioxopropyl)-2-{3-[3-(trifluoromethyl)phenyl]-1H-pyrazol-1-yl}nicotinamide; neurodegenerative disorders, chronic brain supply deficit, ischemia (stroke) obrain trauma, Alzheimer׳s disease, Parkinson׳s disease, amyotrophic lateral sclerosis, Huntington׳s disease. United States Patent 7728012; 2010 Jun 1.
- 128.Mack H, Kling A, Jantos K, Moeller A, Hornberger W, Hutchins CW, inventors; Abbott & Co. KG, Abbott Laboratories, assignee. Carboxamide compounds and their use as calpain inhibitors. United States Patent 8236798; 2012 Aug 7.
- 129.Hornberger W, Mack H, Kling A, Moeller A, Vogg B, Delzer J, et al., inventors; AbbVie Inc., AbbVie Deutschl and GmbH & Co. KG, assignees. Carboxamide compounds and their use as calpain inhibitors. United States Patent 8906941; 2009 Dec 9.
- 130.Mack H, Kling A, Jantos K, Moeller A, Hornberger W, Hutchins CW, inventors; Abbott Laboratories, Abbott & Co. KG, assignee. Carboxamide compounds and their use as calpain inhibitors. United States Patent 8283363; 2012 Oct 9.
- 131.Kling A, Jantos K, Mack H, Moeller A, Hornberger W, Lao YB, et al., inventors; AbbVie, AbbVie Deutschland & Co. KG, assignee. Carboxamide compounds and their use as calpain inhibitors IV. United States Patent 8598211; 2013 Dec 3.
- 132.Maryanoff BE, Costanzo MJ. Inhibitors of proteases and amide hydrolases that employ an α-ketoheterocycle as a key enabling functionality. Bioorg Med Chem. 2008;16:1562–1595. doi: 10.1016/j.bmc.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 133.Tao M, Bihovsky R, Kauer JC. Inhibition of calpain by peptidyl heterocycles. Bioorg Med Chem Lett. 1996;6:3009–3012. [Google Scholar]
- 134.Palmer JT, Hirschbein BL, Cheung H, McCarter J, Janc JW, Yu ZW. Keto-1,3,4-oxadiazoles as cathepsin K inhibitors. Bioorg Med Chem Lett. 2006;16:2909–2914. doi: 10.1016/j.bmcl.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 135.Liu H, Tully DC, Chatterjee A, Alper PB, Woodmansee DH, Mutnick D, inventors. IRM LLC, assignee. Compounds and compositions as cathepsin S inhibitors. United States Patent 6977256; 2005 Dec 20.
- 136.Marquis RW, Ru Y, Zeng J, Trout REL, LoCastro SM, Gribble AD. Cyclic ketone inhibitors of the cysteine protease cathepsin K. J Med Chem. 2001;44:725–736. doi: 10.1021/jm000320t. [DOI] [PubMed] [Google Scholar]
- 137.Stroup GB, Lark MW, Veber DF, Bhattacharyya A, Blake S, Dare LC. Potent and selective inhibition of human cathepsin K leads to inhibition of bone resorption in vivo in a nonhuman primate. J Bone Miner Res. 2001;16:1739–1746. doi: 10.1359/jbmr.2001.16.10.1739. [DOI] [PubMed] [Google Scholar]
- 138.Wijkmans J, Gossen J. Inhibitors of cathepsin K: a patent review (2004-2010) Expert Opin Ther Pat. 2011;21:1611–1629. doi: 10.1517/13543776.2011.616283. [DOI] [PubMed] [Google Scholar]
- 139.Asaad N, Bethel PA, Coulson MD, Dawson JE, Ford SJ, Gerhardt S. Dipeptidyl nitrile inhibitors of cathepsin L. Bioorg Med Chem Lett. 2009;19:4280–4283. doi: 10.1016/j.bmcl.2009.05.071. [DOI] [PubMed] [Google Scholar]
- 140.Bethel PA, Gerhardt S, Jones EV, Kenny PW, Karoutchi GI, Morley AD. Design of selective cathepsin inhibitors. Bioorg Med Chem Lett. 2009;19:4622–4625. doi: 10.1016/j.bmcl.2009.06.090. [DOI] [PubMed] [Google Scholar]
- 141.Falgueyret JP, Oballa RM, Okamoto O, Wesolowski G, Aubin Y, Rydzewski RM. Novel, nonpeptidic cyanamides as potent and reversible inhibitors of human cathepsins K and L. J Med Chem. 2001;44:94–104. doi: 10.1021/jm0003440. [DOI] [PubMed] [Google Scholar]
- 142.Yadav MR, Shinde AK, Chouhan BS, Giridhar R, Menard R. Peptidomimetic 2-cyanopyrrolidines as potent selective cathepsin L inhibitors. J Enzyme Inhib Med Chem. 2008;23:190–197. doi: 10.1080/14756360701504842. [DOI] [PubMed] [Google Scholar]
- 143.Ehmke V, Quinsaat JEQ, Rivera-Fuentes P, Heindl C, Freymond C, Rottmann M. Tuning and predicting biological affinity: aryl nitriles as cysteine protease inhibitors. Org Biomol Chem. 2012;10:5764–5768. doi: 10.1039/c2ob00034b. [DOI] [PubMed] [Google Scholar]
- 144.Cai JQ, Bennett DJ, Rankovic Z, Dempster M, Fradera X, Gillespie J. 2-Phenyl-9H-purine-6-carbonitrile derivatives as selective cathepsin S inhibitors. Bioorg Med Chem Lett. 2010;20:4447–4450. doi: 10.1016/j.bmcl.2010.06.049. [DOI] [PubMed] [Google Scholar]
- 145.Cai JQ, Baugh M, Black D, Long C, Jonathan Bennett D, Dempster M. 6-Phenyl-1H-imidazo[4,5-c]pyridine-4-carbonitrile as cathepsin S inhibitors. Bioorg Med Chem Lett. 2010;20:4350–4354. doi: 10.1016/j.bmcl.2010.06.072. [DOI] [PubMed] [Google Scholar]
- 146.Furber M, Tiden AK, Gardiner P, Mete A, Ford R, Millichip I. Cathepsin C inhibitors: property optimization and identification of a clinical candidate. J Med Chem. 2014;57:2357–2367. doi: 10.1021/jm401705g. [DOI] [PubMed] [Google Scholar]
- 147.Wang KK, Nath R, Posner A, Raser KJ, Buroker-Kilgore M, Hajimohammadreza I. An alpha-mercaptoacrylic acid derivative is a selective nonpeptide cell-permeable calpain inhibitor and is neuroprotective. Proc Natl Acad Sci USA. 1996;93:6687–6692. doi: 10.1073/pnas.93.13.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lin GD, Chattopadhyay D, Maki M, Wang KK, Carson M, Jin L. Crystal structure of calcium bound domain VI of calpain at 1.9 Å resolution and its role in enzyme assembly, regulation, and inhibitor binding. Nat Struct Biol. 1997;4:539–547. doi: 10.1038/nsb0797-539. [DOI] [PubMed] [Google Scholar]
- 149.Farkas B, Tantos A, Schlett K, Világi I, Friedrich P. Ischemia-induced increase in long-term potentiation is warded off by specific calpain inhibitor PD150606. Brain Res. 2004;1024:150–158. doi: 10.1016/j.brainres.2004.07.059. [DOI] [PubMed] [Google Scholar]
- 150.Montero A, Alonso M, Benito E, Chana A, Mann E, Navas JM. Studies on aromatic compounds: inhibition of calpain I by biphenyl derivatives and peptide-biphenyl hybrids. Bioorg Med Chem Lett. 2004;14:2753–2757. doi: 10.1016/j.bmcl.2004.03.071. [DOI] [PubMed] [Google Scholar]
- 151.Montero A, Albericio F, Royo M, Herradón B. Solid-phase combinatorial synthesis of peptide-biphenyl hybrids as calpain inhibitors. Org Lett. 2004;6:4089–4092. doi: 10.1021/ol048216j. [DOI] [PubMed] [Google Scholar]
- 152.Herradon, B., Alonso, M., Benito, E., Chana, A., Montero, A. Biphenyl Derived Thiamides as Calpain Inhibitors. United States Patent 7476754; Jan 13 2009.
- 153.Montero A, Albericio F, Royo M, Herradón B. Synthesis of a 24-membered cyclic peptide-biphenyl hybrid. Eur J Organ Chem. 2007;2007:1301–1308. [Google Scholar]
- 154.Gan JP, Ruan Q, He B, Zhu MS, Shyu WC, Humphreys WG. In vitro screening of 50 highly prescribed drugs for thiol adduct formation-comparison of potential for drug-induced toxicity and extent of adduct formation. Chem Res Toxicol. 2009;22:690–698. doi: 10.1021/tx800368n. [DOI] [PubMed] [Google Scholar]
- 155.Singh J, Petter RC, Kluge AF. Targeted covalent drugs of the kinase family. Curr Opin Chem Biol. 2010;14:475–480. doi: 10.1016/j.cbpa.2010.06.168. [DOI] [PubMed] [Google Scholar]
- 156.Turk B. Targeting proteases: successes, failures and future prospects. Nat Rev Drug Discov. 2006;5:785–799. doi: 10.1038/nrd2092. [DOI] [PubMed] [Google Scholar]
- 157.Werle B, Kraft C, Lah TT, Kos J, Schanzenbächer U, Kayser K. Cathepsin B in infiltrated lymph nodes is of prognostic significance for patients with nonsmall cell lung carcinoma. Cancer. 2000;89:2282–2291. [PubMed] [Google Scholar]
- 158.Aggarwal N, Sloane BF., Cathepsin B. multiple roles in cancer. Proteomics Clin Appl. 2014;8:427–437. doi: 10.1002/prca.201300105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Withana NP, Blum G, Sameni M, Slaney C, Anbalagan A, Olive MB. Cathepsin B inhibition limits bone metastasis in breast cancer. Cancer Res. 2012;72:1199–1209. doi: 10.1158/0008-5472.CAN-11-2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Matarrese P, Ascione B, Ciarlo L, Vona R, Leonetti C, Scarsella M. Cathepsin B inhibition interferes with metastatic potential of human melanoma: an in vitro and in vivo study. Mol Cancer. 2010;9:207. doi: 10.1186/1476-4598-9-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Schurigt U, Sevenich L, Vannier C, Gajda M, Schwinde A, Werner F. Trial of the cysteine cathepsin inhibitor JPM-OEt on early and advanced mammary cancer stages in the MMTV-PyMT-transgenic mouse model. Biol Chem. 2008;389:1067–1074. doi: 10.1515/BC.2008.115. [DOI] [PubMed] [Google Scholar]
- 162.Sadaghiani AM, Verhelst SHL, Gocheva V, Hill K, Majerova E, Stinson S. Design, synthesis, and evaluation of in vivo potency and selectivity of epoxysuccinyl-based inhibitors of papain-family cysteine proteases. Chem Biol. 2007;14:499–511. doi: 10.1016/j.chembiol.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 163.Pišlar A, Kos J. Cysteine cathepsins in neurological disorders. Mol Neurobiol. 2014;49:1017–1030. doi: 10.1007/s12035-013-8576-6. [DOI] [PubMed] [Google Scholar]
- 164.Saatman KE, Creed J, Raghupathi R. Calpain as a therapeutic target in traumatic brain injury. Neurotherapeutics. 2010;7:31–42. doi: 10.1016/j.nurt.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Liu S, Yin F, Zhang JN, Qian YM. The role of calpains in traumatic brain injury. Brain Inj. 2014;28:133–137. doi: 10.3109/02699052.2013.860479. [DOI] [PubMed] [Google Scholar]
- 166.Sribnick EA, Matzelle DD, Banik NL, Ray SK. Direct evidence for calpain involvement in apoptotic death of neurons in spinal cord injury in rats and neuroprotection with calpain inhibitor. Neurochem Res. 2007;32:2210–2216. doi: 10.1007/s11064-007-9433-7. [DOI] [PubMed] [Google Scholar]
- 167.Adamec E, Mohan P, Vonsattel JP, Nixon RA. Calpain activation in neurodegenerative diseases: confocal immunofluorescence study with antibodies specifically recognizing the active form of calpain 2. Acta Neuropathol. 2002;104:92–104. doi: 10.1007/s00401-002-0528-6. [DOI] [PubMed] [Google Scholar]
- 168.Nakanishi H. Neuronal and microglial cathepsins in aging and age-related diseases. Ageing Res Rev. 2003;2:367–381. doi: 10.1016/s1568-1637(03)00027-8. [DOI] [PubMed] [Google Scholar]
- 169.Markgraf CG, Velayo NL, Johnson MP, McCarty DR, Medhi S, Koehl JR. Six-hour window of opportunity for calpain inhibition in focal cerebral ischemia in rats. Stroke. 1998;29:152–158. doi: 10.1161/01.str.29.1.152. [DOI] [PubMed] [Google Scholar]
- 170.Hook GR, Yu J, Sipes N, Pierschbacher MD, Hook V, Kindy MS. The cysteine protease cathepsin B is a key drug target and cysteine protease inhibitors are potential therapeutics for traumatic brain injury. J Neurotrauma. 2014;31:515–529. doi: 10.1089/neu.2013.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hook VYH, Kindy M, Hook G. Inhibitors of cathepsin B improve memory and reduce β-amyloid in transgenic Alzheimer disease mice expressing the wild-type, but not the Swedish mutant, β-secretase site of the amyloid precursor protein. J Biol Chem. 2008;283:7745–7753. doi: 10.1074/jbc.M708362200. [DOI] [PubMed] [Google Scholar]
- 172.Wan HI, Jacobsen JS, Rutkowski JL, Feuerstein GZ. Translational medicine lessons from flurizan׳s failure in Alzheimer׳s disease (AD) trial: implication for future drug discovery and development for AD. Clin Transl Sci. 2009;2:242–247. doi: 10.1111/j.1752-8062.2009.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ii K, Ito H, Kominami E, Hirano A. Abnormal distribution of cathepsin proteinases and endogenous inhibitors (cystatins) in the hippocampus of patients with Alzheimer׳s disease, parkinsonism-dementia complex on Guam, and senile dementia and in the aged. Virchows Arch A Pathol Anat Histopathol. 1993;423:185–194. doi: 10.1007/BF01614769. [DOI] [PubMed] [Google Scholar]
- 174.Cataldo AM, Hamilton DJ, Nixon RA. Lysosomal abnormalities in degenerating neurons link neuronal compromise to senile plaque development in Alzheimer disease. Brain Res. 1994;640:68–80. doi: 10.1016/0006-8993(94)91858-9. [DOI] [PubMed] [Google Scholar]
- 175.Grynspan F, Griffin WR, Cataldo A, Katayama S, Nixon RA. Active site-directed antibodies identify calpain II as an early-appearing and pervasive component of neurofibrillary pathology in Alzheimer׳s disease. Brain Res. 1997;763:145–158. doi: 10.1016/s0006-8993(97)00384-3. [DOI] [PubMed] [Google Scholar]
- 176.Nixon RA. The calpains in aging and aging-related diseases. Ageing Res Rev. 2003;2:407–418. doi: 10.1016/s1568-1637(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 177.Hook G, Hook V, Kindy M. The cysteine protease inhibitor, E64d, reduces brain amyloid-β and improves memory deficits in Alzheimer׳s disease animal models by inhibiting cathepsin B, but not BACE1, β-secretase activity. J Alzheimers Dis. 2011;26:387–408. doi: 10.3233/JAD-2011-110101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hook V, Kindy M, Hook G. Cysteine protease inhibitors effectively reduce in vivo levels of brain β-amyloid related to Alzheimer׳s disease. Biol Chem. 2007;388:247–252. doi: 10.1515/BC.2007.027. [DOI] [PubMed] [Google Scholar]
- 179.Yamashima T, Kohda Y, Tsuchiya K, Ueno T, Yamashita J, Yoshioka T. Inhibition of ischaemic hippocampal neuronal death in primates with cathepsin B inhibitor CA-074: a novel strategy for neuroprotection based on ‘calpain-cathepsin hypothesis’. Eur J Neurosci. 1998;10:1723–1733. doi: 10.1046/j.1460-9568.1998.00184.x. [DOI] [PubMed] [Google Scholar]
- 180.Sahara S, Yamashima T. Calpain-mediated Hsp70.1 cleavage in hippocampal CA1 neuronal death. Biochem Biophys Res Commun. 2010;393:806–811. doi: 10.1016/j.bbrc.2010.02.087. [DOI] [PubMed] [Google Scholar]
- 181.Sultana R, Perluigi M, Newman SF, Pierce WM, Cini C, Coccia R. Redox proteomic analysis of carbonylated brain proteins in mild cognitive impairment and early Alzheimer׳s disease. Antioxid Redox Signal. 2010;12:327–336. doi: 10.1089/ars.2009.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yamashima T. Hsp70.1 and related lysosomal factors for necrotic neuronal death. J Neurochem. 2012;120:477–494. doi: 10.1111/j.1471-4159.2011.07596.x. [DOI] [PubMed] [Google Scholar]
- 183.Zhu H, Yoshimoto T, Yamashima T. Heat shock protein 70.1 (Hsp70.1) affects neuronal cell fate by regulating lysosomal acid sphingomyelinase. J Biol Chem. 2014;289:27432–27443. doi: 10.1074/jbc.M114.560334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Stoka V, Turk B, Schendel SL, Kim TH, Cirman T, Snipas SJ. Lysosomal protease pathways to apoptosis cleavage of bid, not pro-caspases, is the most likely route. J Biol Chem. 2001;276:3149–3157. doi: 10.1074/jbc.M008944200. [DOI] [PubMed] [Google Scholar]
- 185.Heinrich M, Neumeyer J, Jakob M, Hallas C, Tchikov V, Winoto-Morbach S. Cathepsin D links TNF-induced acid sphingomyelinase to Bid-mediated caspase-9 and -3 activation. Cell Death Differ. 2004;11:550–563. doi: 10.1038/sj.cdd.4401382. [DOI] [PubMed] [Google Scholar]
- 186.Luo CL, Chen XP, Yang R, Sun YX, Li QQ, Bao HJ. Cathepsin B contributes to traumatic brain injury-induced cell death through a mitochondria-mediated apoptotic pathway. J Neurosci Res. 2010;88:2847–2858. doi: 10.1002/jnr.22453. [DOI] [PubMed] [Google Scholar]
- 187.Guicciardi ME, Deussing J, Miyoshi H, Bronk SF, Svingen PA, Peters C. Cathepsin B contributes to TNF-α-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J Clin Investig. 2000;106:1127–1137. doi: 10.1172/JCI9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ishisaka R, Utsumi T, Yabuki M, Kanno T, Furuno T, Inoue M. Activation of caspase-3-like protease by digitonin-treated lysosomes. FEBS Lett. 1998;435:233–236. doi: 10.1016/s0014-5793(98)01080-1. [DOI] [PubMed] [Google Scholar]
- 189.Ishisaka R, Utsumi T, Kanno T, Arita K, Katunuma N, Akiyama J. Participation of a cathepsin L-type protease in the activation of caspase-3. Cell Struct Funct. 1999;24:465–470. doi: 10.1247/csf.24.465. [DOI] [PubMed] [Google Scholar]
- 190.Yamada KH, Kozlowski DA, Seidl SE, Lance S, Wieschhaus AJ, Sundivakkam P. Targeted gene inactivation of calpain-1 suppresses cortical degeneration due to traumatic brain injury and neuronal apoptosis induced by oxidative stress. J Biol Chem. 2012;287:13182–13193. doi: 10.1074/jbc.M111.302612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Posmantur R, Kampfl A, Siman R, Liu SJ, Zhao X, Clifton GL. A calpain inhibitor attenuates cortical cytoskeletal protein loss after experimental traumatic brain injury in the rat. Neuroscience. 1997;77:875–888. doi: 10.1016/s0306-4522(96)00483-6. [DOI] [PubMed] [Google Scholar]
- 192.Deng Y, Thompson BM, Gao X, Hall ED. Temporal relationship of peroxynitrite-induced oxidative damage, calpain-mediated cytoskeletal degradation and neurodegeneration after traumatic brain injury. Exp Neurol. 2007;205:154–165. doi: 10.1016/j.expneurol.2007.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Saatman KE, Murai H, Bartus RT, Smith DH, Hayward NJ, Perri BR. Calpain inhibitor AK295 attenuates motor and cognitive deficits following experimental brain injury in the rat. Proc Natl Acad Sci USA. 1996;93:3428–3433. doi: 10.1073/pnas.93.8.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Çolak A, Karaog˘lan A, Kaya M, Sag˘manligil A, Akdemir O, Şahan E. Calpain inhibitor AK 295 inhibits calpain-induced apoptosis and improves neurologic function after traumatic spinal cord injury in rats. Neurocirugía. 2009;20:245–254. [PubMed] [Google Scholar]
- 195.Tsubokawa T, Solaroglu I, Yatsushige H, Cahill J, Yata K, Zhang JH. Cathepsin and calpain inhibitor E64d attenuates matrix metalloproteinase-9 activity after focal cerebral ischemia in rats. Stroke. 2006;37:1888–1894. doi: 10.1161/01.STR.0000227259.15506.24. [DOI] [PubMed] [Google Scholar]
- 196.Wang X, Jung J, Asahi M, Chwang W, Russo L, Moskowitz MA. Effects of matrix metalloproteinase-9 gene knock-out on morphological and motor outcomes after traumatic brain injury. J Neurosci. 2000;20:7037–7042. doi: 10.1523/JNEUROSCI.20-18-07037.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yanamandra N, Gumidyala KV, Waldron KG, Gujrati M, Olivero WC, Dinh DH. Blockade of cathepsin B expression in human glioblastoma cells is associated with suppression of angiogenesis. Oncogene. 2004;23:2224–2230. doi: 10.1038/sj.onc.1207338. [DOI] [PubMed] [Google Scholar]
- 198.Farkas E, Luiten PGM. Cerebral microvascular pathology in aging and Alzheimer׳s disease. Prog Neurobiol. 2001;64:575–611. doi: 10.1016/s0301-0082(00)00068-x. [DOI] [PubMed] [Google Scholar]
- 199.Hardy J, Cullen K. Amyloid at the blood vessel wall. Nat Med. 2006;12:756–757. doi: 10.1038/nm0706-756. [DOI] [PubMed] [Google Scholar]
- 200.Yamashima T. Reconsider Alzheimer׳s disease by the ‘calpain-cathepsin hypothesis’–a perspective review. Prog Neurobiol. 2013;105:1–23. doi: 10.1016/j.pneurobio.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 201.Taniguchi S, Fujita Y, Hayashi S, Kakita A, Takahashi H, Murayama S. Calpain-mediated degradation of p35 to p25 in postmortem human and rat brains. FEBS Lett. 2001;489:46–50. doi: 10.1016/s0014-5793(00)02431-5. [DOI] [PubMed] [Google Scholar]
- 202.Kuwako K, Nishimura I, Uetsuki T, Saido TC, Yoshikawa K. Activation of calpain in cultured neurons overexpressing Alzheimer amyloid precursor protein. Mol Brain Res. 2002;107:166–175. doi: 10.1016/s0169-328x(02)00489-8. [DOI] [PubMed] [Google Scholar]
- 203.Reifert J, Hartung-Cranston D, Feinstein SC. Amyloid β-mediated cell death of cultured hippocampal neurons reveals extensive Tau fragmentation without increased full-length tau phosphorylation. J Biol Chem. 2011;286:20797–20811. doi: 10.1074/jbc.M111.234674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Siman R, Card JP, Davis LG. Proteolytic processing of β-amyloid precursor by calpain I. J Neurosci. 1990;10:2400–2411. doi: 10.1523/JNEUROSCI.10-07-02400.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Mercken M, Grynspan F, Nixon RA. Differential sensitivity to proteolysis by brain calpain of adult human tau, fetal human tau and PHF-tau. FEBS Lett. 1995;368:10–14. doi: 10.1016/0014-5793(95)00590-6. [DOI] [PubMed] [Google Scholar]
- 206.Yang LS, Ksiezak-Reding H. Calpain-induced proteolysis of normal human tau and tau associated with paired helical filaments. Eur J Biochem. 1995;233:9–17. doi: 10.1111/j.1432-1033.1995.009_1.x. [DOI] [PubMed] [Google Scholar]
- 207.Cataldo AM, Thayer CY, Bird ED, Wheelock TR, Nixon RA. Lysosomal proteinase antigens are prominently localized within senile plaques of Alzheimer׳s disease: evidence for a neuronal origin. Brain Res. 1990;513:181–192. doi: 10.1016/0006-8993(90)90456-l. [DOI] [PubMed] [Google Scholar]
- 208.Haas U, Sparks DL. Cortical cathepsin D activity and immunolocalization in Alzheimer disease, critical coronary artery disease, and aging. Mol Chem Neuropathol. 1996;29:1–14. doi: 10.1007/BF02815189. [DOI] [PubMed] [Google Scholar]
- 209.Gan L, Ye SM, Chu AL, Anton K, Yi SL, Vincent VA. Identification of cathepsin B as a mediator of neuronal death induced by Aβ-activated microglial cells using a functional genomics approach. J Biol Chem. 2004;279:5565–5572. doi: 10.1074/jbc.M306183200. [DOI] [PubMed] [Google Scholar]
- 210.Hook V, Toneff T, Bogyo M, Greenbaum D, Medzihradszky KF, Neveu J. Inhibition of cathepsin B reduces beta-amyloid production in regulated secretory vesicles of neuronal chromaffin cells: evidence for cathepsin B as a candidate β-secretase of Alzheimer׳s disease. Biol Chem. 2005;386:931–940. doi: 10.1515/BC.2005.108. [DOI] [PubMed] [Google Scholar]
- 211.Mueller-Steiner S, Zhou YG, Arai H, Roberson ED, Sun BG, Chen J. Antiamyloidogenic and neuroprotective functions of cathepsin B: implications for Alzheimer׳s disease. Neuron. 2006;51:703–714. doi: 10.1016/j.neuron.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 212.Schechter I, Ziv E. Kinetic properties of cathepsin D and BACE 1 indicate the need to search for additional beta-secretase candidate(s) Biol Chem. 2008;389:313–320. doi: 10.1515/BC.2008.025. [DOI] [PubMed] [Google Scholar]
- 213.Rawlings ND, Barrett AJ. Chapter 404-introduction: the clans and families of cysteine peptidases. In: Salvesen NDR, editor. Handbook of proteolytic enzymes. 3rd ed. Academic Press; London: 2013. pp. 1743–1773. [Google Scholar]
- 214.Cynis H, Scheel E, Saido TC, Schilling S, Demuth H-U. Amyloidogenic processing of amyloid precursor protein: evidence of a pivotal role of glutaminyl cyclase in generation of pyroglutamate-modified amyloid-β. Biochemistry. 2008;47:7405–7413. doi: 10.1021/bi800250p. [DOI] [PubMed] [Google Scholar]
- 215.Schilling S, Lauber T, Schaupp M, Manhart S, Scheel E, Bohm G. On the seeding and oligomerization of pGlu-amyloid peptides (in vitro) Biochemistry. 2006;45:12393–12399. doi: 10.1021/bi0612667. [DOI] [PubMed] [Google Scholar]