Abstract
BACKGROUND
The kidney, via its regulation of sodium excretion, which is modulated by humoral factors, including the dopamine and renin–angiotensin systems, keeps the blood pressure in the normal range. We have reported a negative interaction between dopamine D3 and AT1 receptors (D3R and AT1R) in renal proximal tubule (RPT) cells. Here, we studied the interaction between D3R and AT2R in vitro and in vivo.
METHODS AND RESULTS
Stimulation of either the D3R or AT2R, by the intrarenal arterial infusion of PD128907, a D3R agonist, or CGP42112A, an AT2R agonist, induced natriuresis and diuresis that were enhanced by the simultaneous infusion of PD128907 and CGP42112A in Wistar rats. The D3/AT2 receptor interaction was confirmed in in vitro, i.e., stimulation of either the D3R or AT2R inhibited Na+-K+-ATPase activity that was enhanced by the costimulation of these receptors. D3R and AT2R colocalized and coimmunoprecipitated in kidney and RPT cells (RPTCs). Stimulation of one receptor increased the localization of the other receptor at the plasma cell membrane. ERK1/2-MAPK is involved in the signaling pathway of D3R and AT2R interaction because costimulation of D3R and AT2R significantly increased ERK1/2-MAPK expression in RPTCs; inhibition of ERK1/2-MAPK abolished the inhibition of Na+-K+-ATPase activity that was enhanced by D3R and AT2R costimulation.
CONCLUSIONS
Our current study indicates that D3R, in combination with AT2R, enhances natriuresis and diuresis, via ERK1/2-MAPK pathway, that may be involved in the regulation of blood pressure.
Keywords: angiotensin II type 2 receptor, blood pressure, dopamine D3 receptor, hypertension, kidney, kidney tubules, proximal.
Hypertension, with its complications, is currently a big problem imperiling human health. The mechanisms of hypertension are complex but the kidney plays an important role in blood pressure control by regulating sodium excretion. The proximal tubule (PT) is the major site of salt and water reabsorption in the mammalian nephron, reabsorbing >65% of filtered sodium and water.1,2 Renal PT (RPT) function is under hormonal control, with angiotensin II stimulating sodium transport, in part, via activation of apical Na+-H+-exchanger-3 (NHE3) and basolateral Na+-K+-ATPase and dopamine inhibiting them.3
Dopamine exerts its action via 2 families of dopamine receptors: D1-like receptors (D1R and D5R) stimulate adenylyl cyclase activity and D2-like receptors (D2R, D3R, and D4R) inhibit adenylyl cyclase.4 Stimulation of dopamine receptors, especially the D1R and D3R, induces natriuresis and diuresis.5–7
The renin–angiotensin system is a major regulator of renal sodium transport and blood pressure. Angiotensin II is the primary peptide that mediates the effects of the renin–angiotensin system by binding to 2 receptors, AT1R and AT2R, which have opposing effects. The renal expression of AT1R is greater than AT2R which accounts for ~5% of total angiotensin II receptor binding in the RPT. The major effect of angiotensin II, through AT1R, increases sodium transport and aldosterone secretion.2,3,8,9 Under normal circumstances, the AT1R masks the renal effects of the AT2R, which can decrease renal sodium transport by inhibiting Na+-K+-ATPase and NHE3 activities in RPTs.2,10–12
The renin–angiotensin system and dopaminergic systems interact in the brain13 and kidney.3,14–18 For example, AT1R and D1R/D5R negatively interact to regulate renal sodium transport and blood pressure.14–16 By contrast, AT2R can mediate the natriuresis induced by D1R.17 In renal proximal tubule cells (RPTCs) from Wistar-Kyoto rats, D3R negatively regulates AT1R expression but this effect is impaired in spontaneously hypertensive rats.18 D3R, as with AT2R, is also expressed in RPT19 and inhibits sodium transport. Therefore, we tested the hypothesis that D3R and AT2R interact to regulate renal sodium transport in vivo and in vitro.
MATERIALS AND METHODS
Animals
The experiments, approved by the Experimental Animals Committee of Daping Hospital, were conducted in 280–300g Wistar rats. The rats, initially anesthetized by an intraperitoneal injection of pentobarbital (50mg/kg) and maintained by the intravenous infusion of pentobarbital (0.8mg/100g/hour), were placed on a heated blanket to maintain rectal temperature ~37 °C and tracheotomized (PE-240). The external jugular and femoral veins were catheterized (PE-50) for fluid administration while the left carotid artery was catheterized (PE-240) for the monitoring of blood pressure. The ureters, exposed via a laparotomy, were catheterized (PE-10) for urine collection. The right suprarenal artery, which originates from the right renal artery, was catheterized (PE-10, heat stretched to 180 µm) for intrarenal vehicle/drug administration (40 µl/hour). Fluid losses during surgery (~60 minutes) were replenished with 5% albumin in normal saline at 1% body weight over 30 minutes. After a 120-minute equilibration period, urine was collected from each ureter for 40 minutes for a total of 5 collection periods. Urinary sodium concentration was measured with an electrolyte analyzer (HC988; Histrong Medical, Shenzhen, China), using ion-selective electrode method.
Cell culture
Immortalized RPTCs from Wistar-Kyoto rats15,18,20,21 (passage 20–30) were maintained in Dulbecco’s Modified Eagle's medium/F12 medium supplemented with 5% fetal bovine serum, insulin, transferrin, selenium (5 µg/ml each), and epidermal growth factor (10ng/ml) (GIBCO, MD), at 37 °C in humidified 5% CO2 and 95% air. The cells were serum-starved for 2 hours, treated with vehicle, PD128907 (Tocris, Bristol, UK), CGP42112A, U99194A, PD123319, and PD98059 (Sigma Aldrich, MO), alone or in combination. The antagonist was added 10 minutes before the addition of agonist in the studies involving antagonists.
Immunoblotting
Immunoblotting was performed as reported.18,21 The cells were lysed in buffer containing 50mM Tris, 150mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, and protease inhibitors. After measuring the protein concentration with bicinchoninic acid kit (Pierce, IL), the protein samples in Laemmli buffer were subjected to 9% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred onto a nitrocellulose membrane, and probed with rabbit anti-phospho-ERK1/2 antibody or anti-ERK1/2 antibody (Cell Signaling Technology, MA), visualized with infrared IRDye antibodies (LI-COR, NE), and scanned by the Odyssey infrared imaging system. The band densities were quantified using the NIH Image J software.
Coimmunoprecipitation
RPTCs were lysed and renal cortices were homogenized in ice-cold lysis buffer and centrifuged (1,000g for 10 minutes) to remove cellular debris. After measuring the protein concentration, 500 µg of cell or tissue lysates were mixed with 2 µg of rabbit anti-AT2R antibody (Santa Cruz Biotechnology, CA), rocking overnight at 4 °C. Normal rabbit IgG (Santa Cruz Biotechnology) was the negative control and rabbit anti-D3R antibody (Alpha Diagnostic International) was the positive control. Protein G-agarose beads (Santa Cruz Biotechnology) (30 µl/2 hours) were mixed with the lysates at room temperature. The immune complexes were eluted with 30 µl of 2× Laemmli buffer, boiled, subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and immunoblotted with rabbit anti-D3R antibody.21
Na+-K+-ATPase activity assay
Na+-K+-ATPase activity was measured as the rate of inorganic phosphate release in the presence or absence of ouabain.12,22 The cells was washed twice with chilled phosphate-free buffer (mM: NaCl 3.36, NaHCO3 0.54, KCl 0.4 and MgCl2 0.12) and centrifuged at 3,000g for 10 minutes. The cell pellets were lysed in buffer (mM: NaHCO3 1, CaCl2 2 and MgCl2 5) and centrifuged at 3,000g for 2 minutes. The supernatant was mixed in sodium iodide (1M) and centrifuged at 48,000g for 25 minutes to obtain membrane pellets. The pellets were washed and suspended in Tris–HCl (mM: Tris 10 and EDTA 1, pH 7.4). The protein concentrations were quantified with bicinchoninic acid assay. Hundred microliters of membrane suspension were mixed with 800 µl reaction mixture A (mM: NaCl 70, KCl 5, MgCl2 5, Na4EGTA 1, NaN3 6, imidazole 37.5, Tris–HCl 75; pH 7.4) for measurement of total ATPase activity and reaction mixture B (mM: MgCl2 5, Na4EGTA 1, NaN3 6, imidazole 37.5, Tris–HCl 75; pH 7.4) (Sigma Aldrich) with ouabain (Sigma Aldrich) (1mM) for measurement of ouabain-insensitive ATPase activity. Reactions were initiated by the addition of ATP (4mM), incubated at 37 °C/15 minutes, and terminated by the addition of trichloroacetate (50%). The tubes were placed on ice for 2 minutes. One milliliter of coloring reagent (5% FeSO4 in 1% ammonium molybdate in 1N sulfuric acid) was added into the reaction mixtures, mixed, and centrifuged at 3,000g for 10 minutes. The amount of inorganic phosphate in the supernatants was quantified spectrophotometrically at 740nm. A standard curve was constructed using KH2PO4. Na+-K+-ATPase activity, which was the difference between total and ouabain-insensitive ATPase activity, was normalized with protein concentration and activity expressed as nmol phosphate released per mg protein per minute.
Immunofluorescence and confocal microscopy
For immunofluorescence studies, kidney sections were deparaffinized, rehydrated, and subjected to antigen retrieval using citric acid buffer (10mM, pH 6.0). RPTCs on coverslips in 24-well plates were fixed with 4% paraformaldehyde. D3R was immunostained with goat anti-D3R antibody (Santa Cruz Biotechnology), followed by Cy3-labeled donkey anti-goat antibody (Beyotime Institute of Biotechnology, Haimen, China); AT2R was immunostained with rabbit anti-AT2R antibody, followed by Alexa fluor 488 goat anti-rabbit antibody (Molecular Probes, OR). Secondary antibodies from different species were used to avoid cross-reactivity; incubation of donkey anti-goat antibody was performed prior to the goat anti-rabbit antibody. The images were obtained using laser confocal microscopy.
Statistical analysis
Data are expressed as mean ± SEM. Significant differences within groups were determined by 1-way repeated measures ANOVA, followed by Holm-Sidak post hoc test. Significant differences among groups were determined by 1-way factorial ANOVA, followed by Holm-Sidak test. P < 0.05 was accepted as statistically significant.
RESULTS
Enhanced natriuretic and diuretic effect of renal D3R and AT2R costimulation in Wistar rats
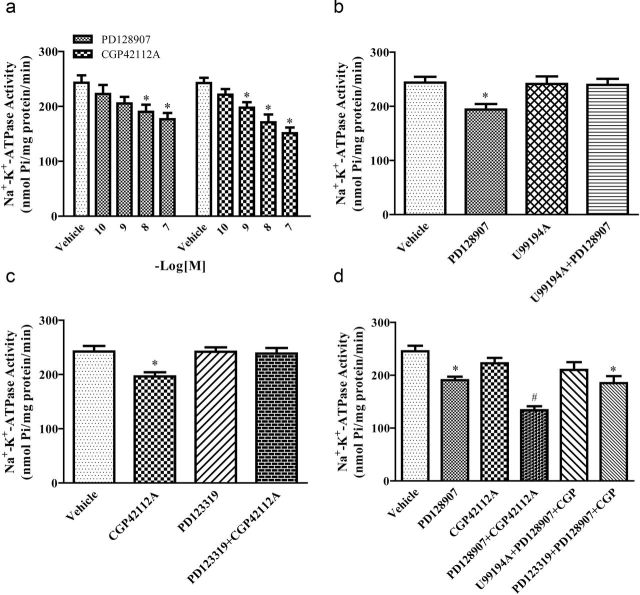
To determine the effect of D3R on sodium excretion, varying dosages of D3R agonist, PD128907 (0.5, 1.0, 5.0 µg/kg/minute × 40 minutes), were infused into the right renal artery in Wistar rats. The intrarenal arterial infusion of the vehicle into the right kidney had no effect on urine flow (V) and absolute sodium excretion (UNaV) (Figure 1a1,a2). However, the intrarenal arterial infusion of PD128907 increased V and UNaV, with significant effects first observed at 1.0 µg/kg/minute (Figure 1a1,a2). The specificity of PD128907 as a D3R agonist was verified by the coinfusion of a D3R antagonist, U99194A, at a dose that by itself had no effect on V or UNaV. In the presence of U99194A (5.0 µg/kg/minute), the PD128907 (1.0 µg/kg/minute)-induced diuresis and natriuresis were completely blocked (Figure 1b1,b2). The intrarenal arterial infusion of PD128907, U99194A, or their combination did not affect blood pressure (Supplementary Figure S1A,B).
Figure 1.
Effect of the intrarenal arterial infusion of D3R and AT2R agonist and/or antagonist on urine flow and sodium excretion in Wistar rats. There were 4 series of studies. The effects of the D3R agonist PD128907 and AT2R agonist CGP42112A were studied in the first series (a1: urine flow [V] and a2: sodium excretion [UNaV]). The effects of the D3R agonist PD128907 and D3R antagonist U99194A were studied in the second series (b1 and b2). The effects of the AT2R agonist CGP42112A and AT2R antagonist PD123319 were studied in the third series (c1 and c2). The effects of the D3R agonist PD128907, D3R antagonist U99194A, AT2R agonist CGP42112A, AT2R antagonist PD123319, and their combinations were studied in the fourth series. There were 5 urine collection periods, each period lasting for 40 minutes. During the control period, vehicle (normal saline) was infused followed by vehicle (Vehicle Group) or drug infusion periods and a recovery period in which only vehicle (normal saline) was infused in all groups. Figures a1 and a2 show the effect of D3R agonist PD128907 or AT2R agonist CGP42112A on V and UNaV. PD128907 or CGP42112A was infused at 0.5, 1.0, and 5.0 µg/kg/minute. Data are expressed as mean ± SEM, *P < 0.05 vs. each Control (repeated measures analysis of variance [ANOVA], Holm-Sidak test). # P < 0.05 vs. Vehicle, n = 6 (1-way factorial ANOVA, Holm-Sidak test). Figures b1 and b2 show the effect of D3R antagonist U99194A on the D3R agonist PD128907-mediated effect on V and UNaV. During drug infusion period 1 (P1), D3R antagonist U99194A (5 µg/kg/minute) was infused into U99194A and PD128907 + U99194A groups. The drug infusion (P2 and P3) groups consisted of the D3R agonist PD128907 (1.0 µg/kg/minute), D3R antagonist U99194A (5 µg/kg/minute), or their combination (PD128907 + U99194A). Data are expressed as mean ± SEM, *P < 0.05 vs. Control (repeated measures ANOVA, Holm-Sidak test). # P < 0.05 vs. other groups, n = 5–6 (1-way factorial ANOVA, Holm-Sidak test). Figures c1 and c2 show the effect of AT2R antagonist PD123319 on the AT2R agonist CGP42112A-mediated effect on V and UNaV. During drug infusion period 1 (P1), AT2R antagonist PD123319 (5 µg/kg/minute) was infused into PD123319 and CGP42112A + PD123319 groups. The drug infusion (P2 and P3) groups consisted of the AT2R agonist CGP42112A (1.0 µg/kg/minute), AT2R antagonist PD123319 (5 µg/kg/minute), or their combination (CGP42112A + PD123319). Data are expressed as mean ± SEM, *P < 0.05 vs. Control (repeated measures ANOVA, Holm-Sidak test). # P < 0.05 vs. other groups, n = 5–6 (1-way factorial ANOVA, Holm-Sidak test). Figures d1 and d2 show the effect of D3R agonist (PD128907), D3R antagonist (U99194A), AT2R agonist (CGP42112A), AT2R antagonist (PD123319), or their combinations on V and UNaV. During drug infusion period 1 (P1), D3R antagonist U99194A (5 µg/kg/minute) and AT2R antagonist PD123319 (5 µg/kg/minute) were infused into PD128907 + CGP42112A + U99194A and PD128907 + CGP42112A + PD123319 group, respectively. The drug infusion (P2 and P3) groups consisted of PD128907 (0.5 µg/kg/minute), CGP42112A (0.5 µg/kg/minute), PD128907 + CGP42112A, PD128907 + CGP42112A + U99194A, and PD128907 + CGP42112A + PD123319. Data are expressed as mean ± SEM, *P < 0.05 vs. Control (repeated measures ANOVA, Holm-Sidak test). # P < 0.05 vs. other groups, n = 5–6 (1-way factorial ANOVA, Holm-Sidak test).
We next investigated the effect of AT2R on V and UNaV. CGP42112A, an AT2R agonist, infused at 0.5, 1.0, 5.0 µg/kg/minute × 40 minute), induced a diuresis and natriuresis in a dose-dependent manner with significant effects first observed at a dose of 1.0 µg/kg/minute (Figure 1a1,a2). In the presence of PD123319 (5.0 µg/kg/minute), an AT2R antagonist, the CGP42112A (1.0 µg/kg/minute)-induced diuresis and natriuresis were completely blocked (Figure 1c1,c2). The intrarenal arterial infusion of CGP42112A, PD123319, or the combination of CGP42112A and PD123319 did not affect blood pressure (Supplementary Figure S1A,C).
Consistent with the studies shown in Figure 1a,b, the intrarenal infusion of 0.5 µg/kg/minute of either PD128907 or CGP42112A did not significantly induce diuresis or natriuresis (Figure 1d1,d2; Tables 1 and 2). However, the simultaneous infusion of 0.5 µg/kg/minute of both PD128907 and CGP42112A produced a greater than an additive increase in V and UNaV in periods 2, 3, and recovery. The enhanced increase in V and UNaV was blocked by either D3R or AT2R antagonist. The intrarenal arterial infusion of D3R and AT2R agonist, antagonist, or their combination did not affect blood pressure (Supplementary Figure S1D).
Table 1.
Effect of D3R agonist or antagonist, AT2R agonist or antagonist, alone or in combination on urine flow in Wistar rats
| Control | P1 | P2 | P3 | Recovery | |
|---|---|---|---|---|---|
| Vehicle | 4.11±0.60 | 4.0±0.62 | 3.91±0.30 | 3.89±0.59 | 3.80±0.44 |
| PD128907 (PD) | 3.95±0.47 | 4.11±0.36 | 5.55±0.52 | 6.81±0.58a,c | 5.68±0.40 |
| CGP42112A (CGP) | 4.16±0.65 | 4.18±0.35 | 4.87±0.79 | 5.45±0.71 | 5.18±0.49 |
| PD128907 + CGP42112A | 3.91±0.25 | 4.11±0.33 | 7.90±0.45a,b | 8.93±0.51a,b | 7.46±0.62a,b |
| U99194A + PD128907 + CGP | 4.06±0.58 | 4.17±0.35 | 5.10±0.48 | 5.69±0.55 | 5.41±0.30 |
| PD123319 + PD128907 + CGP | 3.99±0.46 | 4.05±0.49 | 5.58±0.56 | 6.43±0.62a,c | 5.60±0.54 |
Wistar rats were treated as described in Figure 1d.
a P < 0.05 vs. Control (repeated measures analysis of variance [ANOVA], Holm-Sidak test). b P < 0.05 vs. others, c P < 0.05 vs. Vehicle, n = 5–6 (1-way factorial ANOVA, Holm-Sidak test).
Table 2.
Effect of D3R agonist or antagonist, AT2R agonist or antagonist, alone or in combination on sodium excretion in Wistar rats
| Control | P1 | P2 | P3 | Recovery | |
|---|---|---|---|---|---|
| Vehicle | 0.187±0.0195 | 0.196±0.0285 | 0.204±0.0158 | 0.205±0.0202 | 0.197±0.0236 |
| PD128907 (PD) | 0.197±0.0109 | 0.193±0.0094 | 0.251±0.0165 | 0.291±0.0199a,c | 0.245±0.0207 |
| CGP42112A (CGP) | 0.202±0.0256 | 0.207±0.0293 | 0.233±0.0355 | 0.268±0.0354 | 0.242±0.0198 |
| PD128907 + CGP42112A | 0.204±0.0265 | 0.211±0.0373 | 0.450±0.0667a,b | 0.671±0.0871a,b | 0.482±0.0585a,b |
| U99194A + PD128907 + CGP | 0.205±0.0351 | 0.199±0.0421 | 0.274±0.0450 | 0.322±0.050 | 0.261±0.0455 |
| PD123319 + PD128907 + CGP | 0.201±0.0441 | 0.205±0.0280 | 0.280±0.0240 | 0.356±0.0292a,c | 0.296±0.0314 |
Wistar rats were treated as described in Figure 1d.
a P < 0.05 vs. Control (repeated measures analysis of variance [ANOVA], Holm-Sidak test). b P < 0.05 vs. others, c P < 0.05 vs. Vehicle, n = 5–6 (1-way factorial ANOVA, Holm-Sidak test).
Enhanced inhibition of Na+-K+-ATPase activity by D3R and AT2R costimulation in RPTCs
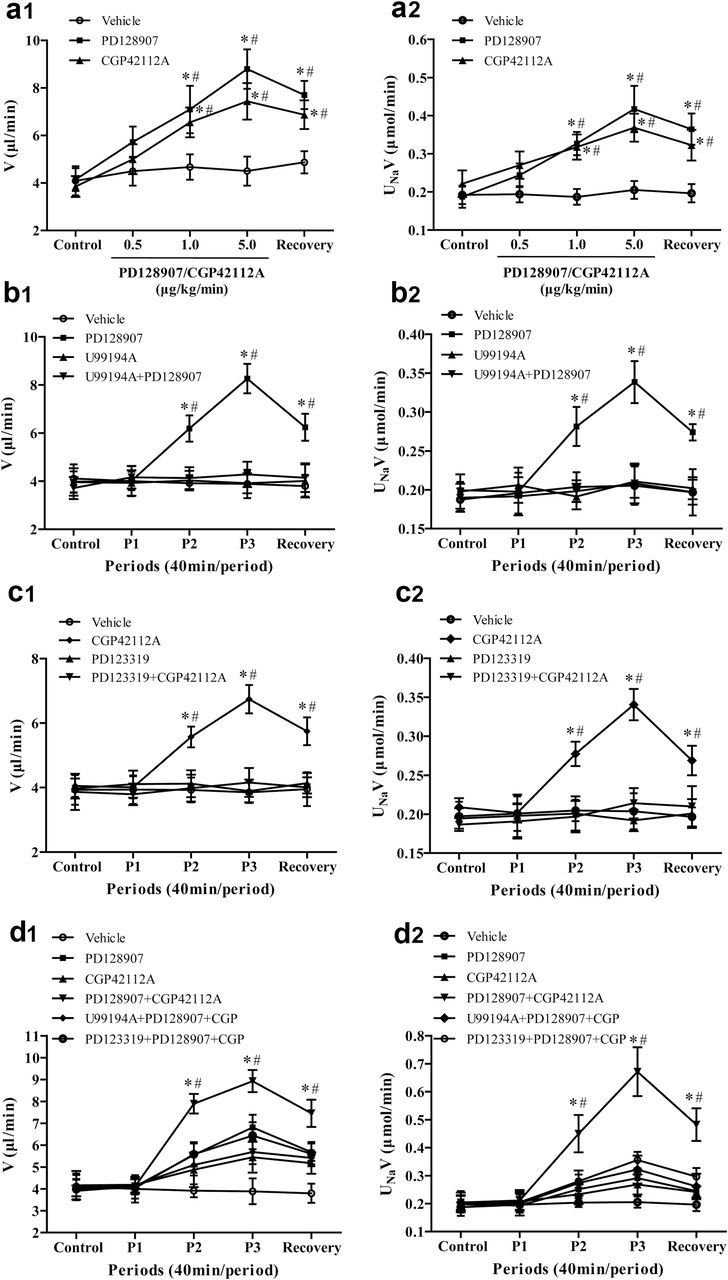
Consistent with our previous report,23 the stimulation of D3R with PD128907 (10−10–10−7 M, 15 minutes) inhibited Na+-K+-ATPase activity in a concentration-dependent manner, with a significant effect first observed at 10−8 M, in immortalized RPTCs (Figure 2a). The inhibitory effect of PD128907 (10−8 M) was specific to the D3R because its effect was blocked by the D3R antagonist U99194A (10−5 M) (Figure 2b). CGP42112A (10−10–10−7 M, 15 minutes) also inhibited Na+-K+-ATPase activity in a concentration-dependent manner, with a significant effect first observed at 10−9 M, in immortalized RPTCs (Figure 2a). The inhibitory effect of CGP42112A (10−9 M) was specific to the AT2R because its effect was blocked by the AT2R antagonist PD123319 (10−6 M) (Figure 2c). The D3R antagonist U99194A and AT2R antagonist PD123319, by themselves, had no effect on basal Na+-K+-ATPase activity.
Figure 2.
Effect of D3R and AT2R costimulation on Na+-K+-ATPase activity in renal proximal tubule cells (RPTCs). (a) Effect of the D3R agonist PD128907 or the AT2R agonist CGP42112A on Na+-K+-ATPase activity. RPTCs were treated with varying concentrations of PD128907 or CGP42112A (10−10–10−7 M) for 15 minutes. Protein concentration was used to normalize Na+-K+-ATPase activity. Data are expressed as mean ± SEM. *P < 0.05 vs. respective Vehicle, n = 6 (1-way analysis of variance [ANOVA], Holm-Sidak test). (b) Effect of the D3R agonist PD128907, D3R antagonist U99194A, alone or in combination on Na+-K+-ATPase activity in RPTCs. RPTCs were pretreated with vehicle or U99194A (10−5 M) for 10 minutes, then treated with PD128907 (10−8 M) for 15 minutes. Protein concentration was used to normalize the results. Data are expressed as mean ± SEM. *P < 0.05 vs. others, n = 6 (1-way ANOVA, Holm-Sidak test). (c) Effect of the AT2R agonist CGP42112A, AT2R antagonist PD123319, alone or in combination on Na+-K+-ATPase activity in RPTCs. RPTCs were pretreated with vehicle or PD123319 (10−6 M) for 10 minutes, then treated with CGP42112A (10−9 M) for 15 minutes. Protein concentration was used to normalize the results. Data are expressed as mean ± SEM. *P < 0.05 vs. others, n = 7 (1-way ANOVA, Holm-Sidak test). (d) Effect of D3R and AT2R agonists and antagonists on Na+-K+-ATPase activity. The effect of the D3R agonist PD128907 (10−8 M), D3R antagonist U99194A (10−5 M), AT2R agonist CGP42112A (10−10 M), AT2R antagonist PD123319 (10−6 M), alone or in combination (PD128907 + CGP42112A; PD128907 + CGP42112A + U99194A; PD128907 + CGP42112A + PD123319) on Na+-K+-ATPase activity. RPTCs were pretreated with vehicle, U99194A, or PD123319 for 10 minutes, then treated with PD128907 and CGP42112A, alone or in combination for 15 minutes. Drugs were mixed immediately before treatment; protein concentration was used to normalize the results. Data are expressed as mean ± SEM. *P < 0.05 vs. Vehicle. # P < 0.05 vs. others, n = 6–8 (1-way ANOVA, Holm-Sidak test).
The costimulation of D3R, with the lowest concentration of PD128907 (10−8 M) that inhibited Na+-K+-ATPase activity and a concentration of CGP42112A (10−10 M) that did not affect Na+-K+-ATPase activity, inhibited Na+-K+-ATPase activity to a greater extent than that caused by PD128907 (10−8 M), alone, in RPTCs (Figure 2d; Table 3), indicating an enhanced rather than additive effect, which is similar to that observed with the increase in V and UNaV observed in vivo. The presence of either their respective antagonist (U99194A, D3R, or PD123319, AT2R) blocked the enhanced inhibition of Na+-K+-ATPase activity by D3R and AT2R costimulation (Figure 2d).
Table 3.
Effect of D3R and AT2R agonist, antagonist, alone or in combination on Na+-K+-ATPase activity in RPTCs
| Treatment | Na+-K+-ATPase activity |
|---|---|
| Vehicle | 245.27±10.70 |
| PD128907(PD) | 190.60±6.87a |
| CGP42112A (CGP) | 222.61±10.26 |
| PD128907 + CGP42112A | 133.87±7.50b |
| U99194A + PD128907 + CGP42112A | 210.13±14.81 |
| PD123319 + PD128907 + CGP42112A | 185.22±13.06a |
RPTCs were treated as described in Figure 2d.
Abbreviation: RPTC, renal proximal tubule cells.
a P < 0.05 vs. Vehicle, b P < 0.05 vs. others, n = 6–8 (1-way ANOVA, Holm-Sidak test).
Colocalization of D3R and AT2R in the kidney of Wistar rats
D3R and AT2R were both expressed in RPTs of Wistar rats, in agreement with previous reports.11,19 There was colocalization of D3R and AT2R in RPTs (Figure 3a). There was also a physical interaction between D3R and AT2R; renal cortex homogenates immunoprecipitated with anti-AT2R antibody and immunoblotted with anti-D3R antibody, revealed a band that corresponded with the D3R (Figure 3b). The D3R and AT2R also colocalized and coimmunoprecipitated in immortalized RPTCs. Stimulation of either D3R or AT2R minimally increased the D3R and AT2R colocalization. However, the stimulation of RPTCs with the lowest concentration of PD128907 (10−8 M) that inhibited Na+-K+-ATPase activity and a concentration of CGP42112A (10−10 M) that did not inhibit Na+-K+-ATPase activity increased their colocalization in RPTC membranes and cytoplasm (Figure 3c). Under basal conditions, endogenously expressed D3R and AT2R were both membrane-bound and scattered in the cytoplasm, with slight intracellular colocalization. Activation of either D3R or AT2R resulted in granular staining at the membrane and cytoplasm but simultaneous stimulation of D3R and AT2R led to a strong granular staining of both receptors at the plasma membrane and cytoplasm, subjacent to the plasma membrane, and increased their colocalization. There are some differences in the cellular localization of the D3R in the current report from our previous report in which D3R stimulation resulted in its internalization with the endothelin B (ETB) receptor in the same RPTCs.7 These differences may relate to a shorter duration of incubation and lower concentration of the D3R agonist (PD128907 10−8 M, 15 minute vs. PD128907 10−6 M, 30 minute) and different receptors studied that interact with the D3R (AT2R vs. ETB receptor), different sources (Alpha Diagnostic International vs. Zymed) and types (polyclonal vs. monoclonal) of the D3R antibodies. A coimmunoprecipitation study confirmed the interaction. Stimulation of RPTCs with the lowest concentration of PD128907 (10−8 M) that inhibited Na+-K+-ATPase increased the coimmunoprecipitation of D3R and AT2R. The concentration of CGP42112A (10−10 M) that did not inhibit Na+-K+-ATPase activity did not increase the coimmunoprecipitation of D3R and AT2R but their combination increased their coimmunoprecipitation to a greater extent than that caused by PD128907 (10−8 M), alone, in RPTCs (Figure 3d).
Figure 3.
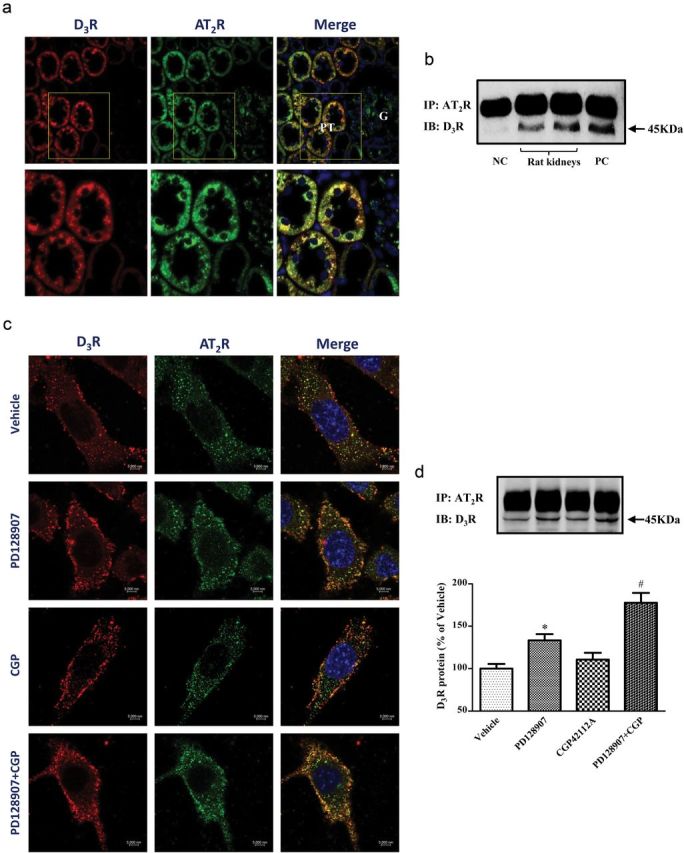
Colocalization and coimmunoprecipitation of D3R and AT2R in Wistar rat kidney and renal proximal tubule cells (RPTCs). (a) Cellular distribution and colocalization of D3R and AT2R in rat kidney cortex. Formalin-fixed, paraffin-embedded sections of rat renal cortex were stained with anti-D3R and anti-AT2R antibodies. Colocalization was determined by confocal microscopy. D3R was pseudocolored red and AT2R was pseudocolored green; the yellow areas show their colocalization in the merged images. The nuclei are stained blue; PT, proximal tubule; G, glomerulus. Images are representative of 3 experiments using different kidney sections. (b) D3R and AT2R physically interact in the rat kidney. Renal cortex lysates were immunoprecipitated with anti-AT2R antibody and immunoblotted with anti-D3R antibody. Normal rabbit IgG served as a negative control, anti-D3R antibody served as positive control. (c) Cellular distribution and colocalization of D3R and AT2R in RPTCs. The cells were treated with vehicle, and PD128907 (10−8 M) and CGP42112A (10−10 M), or their combination for 15 minutes. D3R was pseudocolored red and AT2R was pseudocolored green; the yellow areas show their colocalization in the merged images. The nuclei are stained blue. Images are representative of 3 experiments using different cell preparations. (d) Coimmunoprecipitation of D3R and AT2R in RPTCs. The cells were treated with vehicle, and PD128907 (10−8 M) and CGP42112A (10−10 M), or their combination for 15 minutes. The cell lysates were immunoprecipitated with anti-AT2R antibody and immunoblotted with anti-D3R antibody. Data are expressed as mean ± SEM. *P < 0.05 vs. Vehicle, # P < 0.05 vs. others, n = 4 (1-way analysis of variance, Holm-Sidak test).
MAPK is involved in the enhanced effect of D3R and AT2R costimulation in RPTCs
MAPK and extracellular signal-regulated kinase (ERK) are involved in the signaling pathway of both D3R and AT2R.10,24 Therefore, we determined if D3R and AT2R affect ERK phosphorylation (p-ERK). Similar to the experiments in Figure 2d, we used the lowest concentration of PD128907 (10−8 M) that inhibited Na+-K+-ATPase activity and a concentration of CGP42112A (10−10 M) that did not inhibit Na+-K+-ATPase activity. We found that PD128907 (10−8 M) increased p-ERK while CGP42112A (10−10 M) did not affect p-ERK. The addition of the nonstimulatory effect (p-ERK) of CGP42112A (10−10 M) almost doubled the ability of PD128907 (10−8 M) to increase p-ERK expression (Figure 4a). The presence of a MAPK inhibitor, PD98059 (10−5 M), reduced the enhanced effect of D3R and AT2R costimulation on Na+-K+-ATPase activity (Figure 4b). The presence of the MAPK inhibitor PD98059 (10−5 M) also decreased the colocalization and coimmunoprecipitation of D3R and AT2R induced by D3R (PD128907, 10−8 M) and AT2R (CGP42112A, 10−10 M) agonists in RPTCs (Figures 4c,d). The enhanced colocalization of the D3R and AT2R at the membrane and cytoplasm, subjacent to the membrane with the combination of PD128907 and CGP42112A, was partially abolished by the MAPK inhibitor PD98059.
Figure 4.
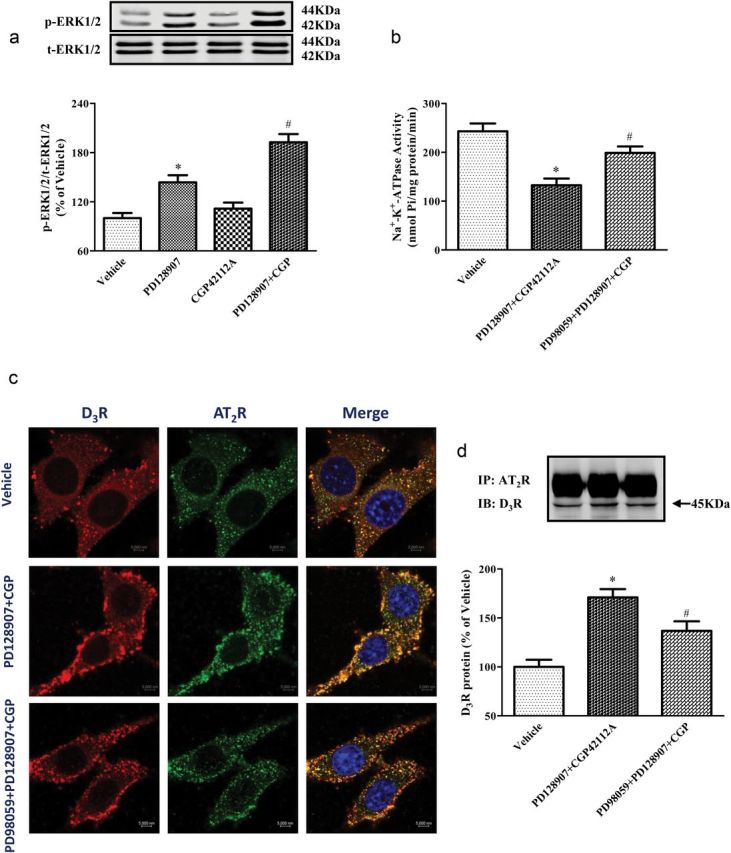
Effect of MAPK inhibitor on D3R and AT2R agonist-mediated effects on phospho-ERK, Na+-K+-ATPase activity, and D3R and AT2R colocalization and coimmunoprecipitation in renal proximal tubule cells (RPTCs). (a) Effect of D3R and AT2R agonists on phospho-ERK1/2 (p-ERK1/2) protein expression in RPTCs. RPTCs were treated with vehicle, and the D3R agonist, PD128907 (10−8 M), and AT2R agonist CGP42112A (10−10 M), or their combination. The cell lysates were immunoblotted with anti-phospho-ERK antibody and anti-total (t) ERK antibody. P-ERK expression was corrected by t-ERK protein expression. Data are expressed as mean ± SEM. *P < 0.05 vs. Vehicle, # P < 0.05 vs. others, n = 5 (1-way analysis of variance [ANOVA], Holm-Sidak test). (b) Effect of D3R and AT2R agonists on the Na+-K+-ATPase activity of RPTCs in the absence or presence of MAPK inhibitor PD98059. RPTCs were pretreated with vehicle or PD98059 (10−5 M) for 10 minutes, then treated with PD128907 (10−8 M) in combination with CGP42112A (10−10 M) for 15 minutes. Protein concentration was used to normalize the results. Data are expressed as mean ± SEM. *P < 0.05 vs. Vehicle, # P < 0.05 vs. others, n = 6 (1-way ANOVA, Holm-Sidak test). (c) Effect of D3R and AT2R agonists on the cellular distribution and colocalization of D3R and AT2R in RPTCs in the absence and presence of MAPK inhibitor PD98059. RPTCs were pretreated with PD98059 (10−5 M) for 10 minutes, then treated with PD128907 (10−8 M) in combination with CGP42112A (CGP, 10−10 M) for 15 minutes. Colocalization of D3R (red) and AT2R (green) was observed as discrete yellow areas in merged images. Images are representative of 3 experiments using different cell preparations. (d) Effect of D3R and AT2R agonists on the coimmunoprecipitation of D3R and AT2R in RPTCs in the absence and presence of MAPK inhibitor PD98059. RPTCs were pretreated with vehicle or PD98059 (10−5 M) for 10 minutes, then treated with PD128907 (10−8 M) in combination with CGP42112A (CGP, 10−10 M) for 15 minutes. Data are expressed as mean ± SEM. *P < 0.05 vs. Vehicle, # P < 0.05 vs. others, n = 6 (1-way ANOVA, Holm-Sidak test).
DISCUSSION
Interactions among G protein-coupled receptors are important not only in their normal function but also in the pathogenesis of disease.7,18,25,26 Heteromerization of receptors leads to modulation of signaling and activating properties of individual G protein-coupled receptors. For example, the D1R and D3R interact to decrease sodium transport in RPTCs and relax vascular smooth muscle cells.21,27,28
There are many reports on the interaction of the dopaminergic and renin–angiotensin system in the regulation of renal sodium excretion and blood pressure.3 All the dopamine receptor subtypes can negatively interact with AT1R.14–16,18,29–31 The AT2R can mediate the natriuresis induced by D1-like receptors.17 However, an interaction between D2-like receptors and the AT2R has not been reported. We now report an enhanced effect of D3R and AT2R costimulation in decreasing Na+-K+-ATPase activity and increasing sodium excretion. The D3R and AT2R probably regulate each other by physical interaction. We show that these receptors colocalize and coimmunoprecipitate in renal cortex homogenates and RPTCs. This physical interaction between D3R and AT2R is important in the inhibition of Na+-K+-ATPase activity that is mediated by the MAPK and ERK pathway. As indicated earlier, G protein-coupled receptor signaling and function can be affected by heteromerization.21,26–28 Based on those reports, we presume that D3R and AT2Rs may also heterodimerize and enhance receptor crosstalk and downstream signal transduction, leading to amplification of their effects and inhibition of sodium transport, in this instance.25
We used CGP42112A, a peptide AT2R agonist; a nonpeptide AT2R agonist, Compound 21,32 as with CGP42112A, has a high affinity for AT2R.33 CGP42112A is easily degraded whereas Compound 21 may not.34 The current experiments were performed in the short term to avoid possible pharmacokinetic problems and used a low concentration (10−10 M) of CGP42112A that acts as a full agonist without antagonist effect.35 The natriuretic effect of AT2R stimulation may be due to renal tubular rather than a hemodynamic effect.36,37 We have reported that the intrarenal infusion of D3R agonist did not affect glomerular filtration rate,7 suggesting that the natriuretic effect of D3R stimulation could be also attributed to tubular rather than hemodynamic effect. However, it should be noted that the natriuresis observed in the current study (~0.5 µmol/minute) is not large and could be accounted for by a small increase in glomerular filtration rate (<0.3%). The proximal tubules, which is the major site of sodium reabsorption in the nephron,1,2 also express the D3R and AT2R.8,19 However, nephron segments beyond the proximal tubule could also be involved in the natriuresis due to D3R and AT2R interaction.7,38 We also studied only the short-term effect of the intrarenal arterial infusion of drugs to avoid the confounding influence of alterations in systemic hemodynamic or hormones, such as aldosterone. However, urinary potassium was not monitored in the current study. Our results show that the antagonists of D3R and AT2R, by themselves, do not affect Na+-K+-ATPase activity or sodium excretion, suggesting absence of constitutive activity, at least in the kidney.38 Another D3R antagonist, GR103691, did not affect renal sodium excretion in Wistar-Kyoto rats on high-salt diet,7 in agreement with our results.
Owing to the low expression of AT2R in adult kidney,8,11 renal endogenous AT2R effect on sodium transport is masked by AT1R.9,39 However, AT2R can directly interact with AT1R in immortalized RPTCs from Wistar-Kyoto rats; AT2R stimulation inhibited AT1R expression as early as 8 hours, lasting for at least 30 hours. In these RPTCs treated with AT2R agonist for 24 hours, the decrease in AT1R protein was associated with a decrease in angiotensin II-mediated stimulation of Na+-K+-ATPase activity.22 In these RPTCs, we also reported that D3R stimulation decreased AT1R protein as early as 2 hours, lasting for at least 24 hours.18 In the current study, short-term (40 minutes) D3R stimulation did not change AT1R expression. Therefore, the enhancement of sodium and water excretion by the costimulation of the D3R and AT2R is probably caused by an increase in their physical interaction rather than a change in the amount of receptor expression. This may be the reason why the functional interaction between the D3R and AT2R did not last for a long time. Our results show that MAPK and ERK participate in the enhancement of D3R and AT2R-induced natriuresis and diuresis. NO/cGMP signaling cascade is also involved in AT2R-mediated natriuresis,39 however, NO does not participate in D3R signaling.40 The role of the heterodimerization of D3R, AT2R, and AT1R18,22 in their interaction needs to be studied.
In summary, stimulation of D3R or AT2R induces diuresis and natriuresis; costimulation of D3R and AT2R produces an enhanced effect, which may be mediated by their physical interaction. MAPK and ERK are involved in the signaling pathway of the D3R and AT2R receptor-enhanced effect.
SUPPLEMENTARY MATERIAL
Supplementary materials are available at American Journal of Hypertension (http://ajh.oxfordjournals.org).
DISCLOSURE
The authors declare no conflict of interest.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by grants from the National Natural Science Foundation of China (31130029), National Basic Research Program of China (2013CB531104 and 2012CB517801), Program for Changjiang Scholars and Innovative Research Team in University (IRT1216), and USA NIH5P01 HL074940.
REFERENCES
- 1. Coffman TM. The inextricable role of the kidney in hypertension. J Clin Invest 2014; 124:2341–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhuo JL, Li XC. Proximal nephron. Compr Physiol 2013; 3:1079–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carey RM. The intrarenal renin-angiotensin and dopaminergic systems: control of renal sodium excretion and blood pressure. Hypertension 2013; 61:673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 2011; 63:182–217. [DOI] [PubMed] [Google Scholar]
- 5. Asghar M, Tayebati SK, Lokhandwala MF, Hussain T. Potential dopamine-1 receptor stimulation in hypertension management. Curr Hypertens Rep 2011; 13:294–302. [DOI] [PubMed] [Google Scholar]
- 6. Luippold G, Küster E, Joos TO, Mühlbauer B. Dopamine D3 receptor activation modulates renal function in anesthetized rats. Naunyn Schmiedebergs Arch Pharmacol 1998; 358:690–693. [DOI] [PubMed] [Google Scholar]
- 7. Zeng C, Asico LD, Yu C, Villar VA, Shi W, Luo Y, Wang Z, He D, Liu Y, Huang L, Yang C, Wang X, Hopfer U, Eisner GM, Jose PA. Renal D3 dopamine receptor stimulation induces natriuresis by endothelin B receptor interactions. Kidney Int 2008; 74:750–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhuo J, Song K, Harris PJ, Mendelsohn FA. In vitro autoradiography reveals predominantly AT1 angiotensin II receptors in rat kidney. Ren Physiol Biochem 1992; 15:231–239. [DOI] [PubMed] [Google Scholar]
- 9. Danyel LA, Schmerler P, Paulis L, Unger T, Steckelings UM. Impact of AT2-receptor stimulation on vascular biology, kidney function, and blood pressure. Integr Blood Press Control 2013; 6:153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kemp BA, Howell NL, Gildea JJ, Keller SR, Padia SH, Carey RM. AT2 receptor activation induces natriuresis and lowers blood pressure. Circ Res 2014; 115:388–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ozono R, Wang ZQ, Moore AF, Inagami T, Siragy HM, Carey RM. Expression of the subtype 2 angiotensin (AT2) receptor protein in rat kidney. Hypertension 1997; 30:1238–1246. [DOI] [PubMed] [Google Scholar]
- 12. Hakam AC, Hussain T. Angiotensin II AT2 receptors inhibit proximal tubular Na+-K+-ATPase activity via a NO/cGMP-dependent pathway. Am J Physiol Renal Physiol 2006; 290:F1430–F1436. [DOI] [PubMed] [Google Scholar]
- 13. Villar-Cheda B, Dominguez-Meijide A, Valenzuela R, Granado N, Moratalla R, Labandeira-Garcia JL. Aging-related dysregulation of dopamine and angiotensin receptor interaction. Neurobiol Aging 2014; 35:1726–1738. [DOI] [PubMed] [Google Scholar]
- 14. Li D, Scott L, Crambert S, Zelenin S, Eklöf AC, Di Ciano L, Ibarra F, Aperia A. Binding of losartan to angiotensin AT1 receptors increases dopamine D1 receptor activation. J Am Soc Nephrol 2012; 23:421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zeng C, Yang Z, Wang Z, Jones J, Wang X, Altea J, Mangrum AJ, Hopfer U, Sibley DR, Eisner GM, Felder RA, Jose PA. Interaction of angiotensin II type 1 and D5 dopamine receptors in renal proximal tubule cells. Hypertension 2005; 45:804–810. [DOI] [PubMed] [Google Scholar]
- 16. Efendiev R, Budu CE, Cinelli AR, Bertorello AM, Pedemonte CH. Intracellular Na+ regulates dopamine and angiotensin II receptors availability at the plasma membrane and their cellular responses in renal epithelia. J Biol Chem 2003; 278:28719–28726. [DOI] [PubMed] [Google Scholar]
- 17. Salomone LJ, Howell NL, McGrath HE, Kemp BA, Keller SR, Gildea JJ, Felder RA, Carey RM. Intrarenal dopamine D1-like receptor stimulation induces natriuresis via an angiotensin type-2 receptor mechanism. Hypertension 2007; 49:155–161. [DOI] [PubMed] [Google Scholar]
- 18. Zeng C, Liu Y, Wang Z, He D, Huang L, Yu P, Zheng S, Jones JE, Asico LD, Hopfer U, Eisner GM, Felder RA, Jose PA. Activation of D3 dopamine receptor decreases angiotensin II type 1 receptor expression in rat renal proximal tubule cells. Circ Res 2006; 99:494–500. [DOI] [PubMed] [Google Scholar]
- 19. O’Connell DP, Vaughan CJ, Aherne AM, Botkin SJ, Wang ZQ, Felder RA, Carey RM. Expression of the dopamine D3 receptor protein in the rat kidney. Hypertension 1998; 32:886–895. [DOI] [PubMed] [Google Scholar]
- 20. Woost PG, Orosz DE, Jin W, Frisa PS, Jacobberger JW, Douglas JG, Hopfer U. Immortalization and characterization of proximal tubule cells derived from kidneys of spontaneously hypertensive and normotensive rats. Kidney Int 1996; 50:125–134. [DOI] [PubMed] [Google Scholar]
- 21. Zeng C, Wang Z, Li H, Yu P, Zheng S, Wu L, Asico LD, Hopfer U, Eisner GM, Felder RA, Jose PA. D3 dopamine receptor directly interacts with D1 dopamine receptor in immortalized renal proximal tubule cells. Hypertension 2006; 47:573–579. [DOI] [PubMed] [Google Scholar]
- 22. Yang J, Chen C, Ren H, Han Y, He D, Zhou L, Hopfer U, Jose PA, Zeng C. Angiotensin II AT2 receptor decreases AT1 receptor expression and function via nitric oxide/cGMP/Sp1 in renal proximal tubule cells from Wistar-Kyoto rats. J Hypertens 2012; 30:1176–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang Y, Fu C, Ren H, He D, Wang X, Asico LD, Jose PA, Zeng C. Impaired stimulatory effect of ETB receptor on D3 receptor in immortalized renal proximal tubule cells of spontaneously hypertensive rats. Kidney Blood Press Res 2011; 34:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guitart X, Navarro G, Moreno E, Yano H, Cai NS, Sanchez M, Kumar-Barodia S, Naidu Y, Mallol J, Cortes A, Lluis C, Canela EI, Casado V, McCormick PJ, Ferre S. Functional selectivity of allosteric interactions within GPCR oligomers: the dopamine D1-D3 Receptor heterotetramer. Mol Pharmacol 2014; 86:417–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gomes I, Jordan BA, Gupta A, Rios C, Trapaidze N, Devi LA. G protein coupled receptor dimerization: implications in modulating receptor function. J Mol Med (Berl) 2001; 79:226–242. [DOI] [PubMed] [Google Scholar]
- 26. Marcellino D, Ferré S, Casadó V, Cortés A, Le Foll B, Mazzola C, Drago F, Saur O, Stark H, Soriano A, Barnes C, Goldberg SR, Lluis C, Fuxe K, Franco R. Identification of dopamine D1–D3 receptor heteromers. Indications for a role of synergistic D1–D3 receptor interactions in the striatum. J Biol Chem 2008; 283:26016–26025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li Z, Yu C, Han Y, Ren H, Shi W, Fu C, He D, Huang L, Yang C, Wang X, Zhou L, Asico LD, Zeng C, Jose PA. Inhibitory effect of D1-like and D3 dopamine receptors on norepinephrine-induced proliferation in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 2008; 294:H2761–H2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jose PA, Asico LD, Eisner GM, Pocchiari F, Semeraro C, Felder RA. Effects of costimulation of dopamine D1- and D2-like receptors on renal function. Am J Physiol 1998; 275:R986–R994. [DOI] [PubMed] [Google Scholar]
- 29. Chen C, Lokhandwala MF. Potentiation by enalaprilat of fenoldopam-evoked natriuresis is due to blockade of intrarenal production of angiotensin-II in rats. Naunyn Schmiedebergs Arch Pharmacol 1995; 352:194–200. [DOI] [PubMed] [Google Scholar]
- 30. Bek MJ, Wang X, Asico LD, Jones JE, Zheng S, Li X, Eisner GM, Grandy DK, Carey RM, Soares-da-Silva P, Jose PA. Angiotensin-II type 1 receptor-mediated hypertension in D4 dopamine receptor-deficient mice. Hypertension 2006; 47:288–295. [DOI] [PubMed] [Google Scholar]
- 31. Jenkins TA, Chai SY, Mendelsohn FA. Upregulation of angiotensin II AT1 receptors in the mouse nucleus accumbens by chronic haloperidol treatment. Brain Res 1997; 748:137–142. [DOI] [PubMed] [Google Scholar]
- 32. Wan Y, Wallinder C, Johansson B, Holm M, Mahalingam AK, Wu X, Botros M, Karlén A, Pettersson A, Nyberg F, Fändriks L, Hallberg A, Alterman M. First reported nonpeptide AT1 receptor agonist (L-162,313) acts as an AT2 receptor agonist in vivo. J Med Chem 2004; 47:1536–1546. [DOI] [PubMed] [Google Scholar]
- 33. Bosnyak S, Jones ES, Christopoulos A, Aguilar MI, Thomas WG, Widdop RE. Relative affinity of angiotensin peptides and novel ligands at AT1 and AT2 receptors. Clin Sci (Lond) 2011; 121:297–303. [DOI] [PubMed] [Google Scholar]
- 34. Steckelings UM, Larhed M, Hallberg A, Widdop RE, Jones ES, Wallinder C, Namsolleck P, Dahlöf B, Unger T. Non-peptide AT2-receptor agonists. Curr Opin Pharmacol 2011; 11:187–192. [DOI] [PubMed] [Google Scholar]
- 35. Brechler V, Jones PW, Levens NR, de Gasparo M, Bottari SP. Agonistic and antagonistic properties of angiotensin analogs at the AT2 receptor in PC12W cells. Regul Pept 1993; 44:207–213. [DOI] [PubMed] [Google Scholar]
- 36. Brouwers S, Smolders I, Massie A, Dupont AG. Angiotensin II type 2 receptor-mediated and nitric oxide-dependent renal vasodilator response to compound 21 unmasked by angiotensin-converting enzyme inhibition in spontaneously hypertensive rats in vivo. Hypertension 2013; 62:920–926. [DOI] [PubMed] [Google Scholar]
- 37. Hilliard LM, Jones ES, Steckelings UM, Unger T, Widdop RE, Denton KM. Sex-specific influence of angiotensin type 2 receptor stimulation on renal function: a novel therapeutic target for hypertension. Hypertension 2012; 59:409–414. [DOI] [PubMed] [Google Scholar]
- 38. Ali Q, Hussain T. AT2 receptor non-peptide agonist C21 promotes natriuresis in obese Zucker rats. Hypertens Res 2012; 35:654–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kemp BA, Bell JF, Rottkamp DM, Howell NL, Shao W, Navar LG, Padia SH, Carey RM. Intrarenal angiotensin III is the predominant agonist for proximal tubule angiotensin type 2 receptors. Hypertension 2012; 60:387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pyne-Geithman GJ, Caudell DN, Cooper M, Clark JF, Shutter LA. Dopamine D2-receptor-mediated increase in vascular and endothelial NOS activity ameliorates cerebral vasospasm after subarachnoid hemorrhage in vitro . Neurocrit Care 2009; 10:225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.