Abstract
The present review aims at summarizing the major therapeutic roles of resveratrol and omega-3 fatty acids (O3FAs) along with their related pathways. This article reviews some of the key studies involving the health benefits of resveratrol and O3FAs. Oxidative stress has been considered as one of the most important pathophysiological factors associated with various cardiovascular disease conditions. Resveratrol, with the potent antioxidant and free radical scavenging properties, has been proven to be a significantly protective compound in restoring the normal cardiac health. A plethora of research also demonstrated the reduction of the risk of coronary heart disease, hypertension, and stroke, and their complications by O3FAs derived from fish and fish oils. This review describes the potential cardioprotective role of resveratrol and O3FAs in ameliorating the endoplasmic reticulum stress.
Keywords: resveratrol, omega-3 fatty acid, oxidative stress, endoplasmic reticulum stress, cardiovascular diseases, coronary artery diseases
Introduction
Cardiovascular complications continue to be among the list of leading causes of death worldwide. According to centers for disease control and prevention (CDC) reports, more than half a million people in the USA die of heart disease every year, one in every four deaths every year (1). Increasing evidences suggest that metabolic syndrome constitutes a significant risk for cardiovascular diseases, which can also cause type 2 diabetes, obesity, and oxidative stress (2, 3). Effective tactics to reduce these conditions include immediate weight loss (4), and long-term dietary strategies enhancing health by maintaining the body weight (5). There are multiple non-pharmacological approaches, such as alternative medicine, that have the potential to reduce cardiovascular risks; resveratrol and omega-3 fatty acid (O3FA) are two well-known natural products that come under this category.
Resveratrol is chemically trans-3,5,4′-trihydroxystilbene. It is a polyphenol phytoalexin compound naturally abundant in a variety of plant species, including white hellebore (Veratrum grandiflorum O. Loes), Japanese knotweed (Polygonum cuspidatum), grapes, blueberries, peanuts, wine, and mulberries (6, 7). Resveratrol came into the limelight following the discovery of the cardioprotective nature of red wine (8). This has paved the way for new research activities involving clinical health benefits of resveratrol. A growing body of research therefore indicated that resveratrol could improve a wide variety of disease conditions, including cardiovascular diseases, cancer, ischemic disorders, and neurodegenerative disorders (9). It is also postulated that resveratrol can increase the stress resistance and also slow down the aging process to increase the life span in diverse organisms from microorganisms to vertebrates (6).
Resveratrol is one of the well-known natural compounds, which is well-studied for its free radical scavenger and antioxidant properties. It can modulate various intracellular signal transduction pathways and demonstrate therapeutic effects, including cell survival, modulation of apoptosis, and angiogenesis (10, 11). Growing evidences suggests the anti-cancer activity of resveratrol and its potential as a therapeutic as well as a chemo-preventive agent (12). Scientific outcomes demonstrated the potential use of resveratrol in various cancers (13–25). However, pre-clinical results were not well reflected in clinical studies in human. It may be due to the pharmacokinetic issues pertaining to resveratrol. It is lipophilic in nature and has a short half-life as well.
The use of O3FAs as a widespread alternative has been practiced globally by many societies. O3FAs are essential for normal growth and development of the nervous system. The benefits of O3FA have been documented in the past 35 years (26–28). All these research data strongly support the use of O3FAs in reducing the risk of cardiac diseases.
The purpose of this review is to summarize the major antioxidant role of resveratrol and O3FA along with their related pathways and highlight promising therapeutic approaches. The benefits of combinational therapy with these two natural compounds were also discussed.
Resveratrol and the Endoplasmic Reticulum Stress
The endoplasmic reticulum (ER) is a significant but complex intracellular organelle in the physiological system. It is a vital organelle involved in modification and synthesis of proteins, translational processes, and fabrication at the post-translational level. Physiologically, the integrity of ER is maintained for proper cellular functioning. Any stimuli from diverse origin can influence the ER environment and generate disturbance in terms of nutritional deficiency leading to a compromised pathological status. The stimuli can be of physiological and biochemical origin, which can produce moderate-to-severe abnormalities, including alterations in glycosylation, depletion of calcium stores, oxidative stress conditions, DNA damage, and other pathological conditions. These alternations generally produce a severe pathological stress condition in the ER known as ER stress or ER oxidative stress. This pathological stress is coexisted with the abnormal protein accumulations in the ER.
Many reports from animal and human studies have shown that oxygen deprivation in the heart causes ER stress of myocytes by accumulating unfolded and misfolded proteins, thereby triggering apoptosis (29–32). Also, the research data showed that ER stress may play a crucial role in the elevation of atherosclerotic plaque (33–35). Furthermore, recent evidences also suggest that a transcriptional factor, nuclear factor-E2-related factor (Nrf2), may play a central role to compensate ER stress conditions (36). This Nrf2 signaling has shown to upregulate the expression of proteasomal catalytic sub-units in several cell types, thereby contributing to the ER stress response by enhancing proteasomal-mediated ER-associated degradation (37). Other studies also suggest that interventions against ER stress and activation of Nrf2 significantly reduce myocardial infarct size in animals exposed to I/R injury and also reduce cardiac hypertrophy in heart failure animal models (38).
Resveratrol as a Cardioprotective Agent
Resveratrol has shown beneficial and protective effects against most degenerative and cardiovascular diseases, including ischemia/reperfusion injury, atherosclerosis, hypertension, and heart failure. The adaptive response through the preconditioning-like action of resveratrol is linked with the cardioprotective effects of the compound. With the expression of cardioprotective genes and proteins, such as heat shock and antioxidant proteins, resveratrol demonstrated an adaptive stress response as the preconditioning (39). These effects of resveratrol have been correlated with the modulation of mTOR-Rictor survival pathways (40). Results of several in vitro and in vivo studies of resveratrol in animal models suggested LDL oxidation inhibition, suppression of aggregation of platelets, and reduction of ischemia–reperfusion-induced myocardial injury, which in turn indicated the promising cardiovascular protective effects of resveratrol (41–43). Resveratrol also revealed anti-inflammatory activity, which was postulated to be linked with nitric oxide (NO) production. This study also demonstrated cardioprotective action of resveratrol by inhibiting toll-like receptor 4 (TLR4)/NF-κB signaling pathway in a rat model of myocardial ischemia and reperfusion (MI/R) (44). Another very common cardiovascular disease is stroke, which is one of the main causative factors of deaths throughout the world population. Scientific investigations suggest oxidative stress as the predominant pathophysiological modality associated with stroke (45, 46). Recent data also suggest that the ability of resveratrol in ameliorating the oxidative stress conditions might be due to the aggravation of NO production along with its anti-inflammatory activity (47). Studies also suggested several mechanisms of action for resveratrol to justify its cardioprotective actions (48, 49). Resveratrol was also found to be protective against the cardio toxicity induced by the potent anti-cancer agent doxorubicin (50). These authors showed that resveratrol restored the mitochondrial structure as evident from the electron microscope examinations. A recent pre-clinical study also suggested that resveratrol alleviate the cardio toxicity induced by doxorubicin (51).
Role of Omega-3 Fatty Acids as Antioxidant Agents in Cardiovascular Diseases
Two well-known fish/fish oil-derived O3FA are the eicosapentanoic acid (EPA) and docosahexanoic acid (DHA). DHA and EPA are vital nutrients required for brain and retina growth in order to have normal neuronal functioning and vision. These two in turn help humans develop their learning and mental abilities throughout life. These fatty acids are by-products derived from the metabolic conversion of the amino acid alanine (ALA) in the liver (52). Yet, human intake of O3FA is limited; they are not abundant in vegetables and grain food sources, and thus, they have to be supplemented directly. In addition, the metabolic change of alanine (ALA) to DHA/EPA in the body is limited; therefore, it is recommended to have sufficient amounts of fish, or O3FA directly as a dietary supplement.
The American Heart Association recommends 130 mg/day of DHA/EPA (combined), for people suffering from coronary heart disease. Various studies have indicated the benefits of fish consumption containing the O3FA, EPA/DHA, and lowering the risk of cardiovascular conditions, such as fatal heart attack, high cholesterol, and stroke (53–57). Yet, the signal transduction pathways and mechanisms associated with it and how they function have not fully been revealed.
Mechanisms Associated with O3FA
Omega-3 fatty acids seem to have versatile properties with no significant drug interactions. The suggested mechanisms associated with the benefits of O3FA are stabilization of plaque formation, improving lipid profile, normalizing blood pressure, and anti-inflammatory and anti-arrhythmic properties (58). Nevertheless, there is still a major controversy over O3FA having benefits against arrhythmias, which was suggested by the Italian GISSI-Prevenzione study (59). The exact mechanism of its action in reducing cardiovascular risk is yet to be understood.
O3FA and Oxidative Stress
One of the valuable properties of O3FAs is their antioxidative action. They seem to induce cardioprotection by ameliorating oxidative stress. Oxidative stress causes damage by the production of radicals that distress normal metabolic cell functioning by destroying lipids, proteins, and nucleic acids. Radical accumulation eventually causes structural cell membrane damage, vascular endothelial dysfunction, and cell death, often leading to cardiovascular diseases (60). A recent study in rats evaluated the combined effects of intermittent hypobaric hypoxia (IH) and O3FA on cardiac function, oxidative balance, and inflammatory state. Their findings showed that animals under IH have improved left ventricular function, reduced oxidative stress, and increased antioxidant enzyme expression when compared to the normobaric normoxia group. Supplementation of O3FA showed similar results to that of the IH group, suggesting that both conditions, IH and O3FA, induce functional improvement by antioxidant and anti-inflammatory mechanisms, thereby establishing cardioprotection (61).
Furthermore, another protective action of O3FA is to reinforce the body’s antioxidant defenses to improve cardiac function. Recent data suggest that DHA and EPA regulate cell membrane fluidity, organization, and permeability that affect signaling pathways and diffusion processes, with positive effects on key cardiovascular pathways (62). In addition, O3FAs manage to exert anti-inflammatory properties by controlling the synthesis of immune mediators (e.g., thromboxanes, prostaglandins, and leukotrienes) in murine cell lines and human primary monocyte-derived macrophages (63–65). These mediator molecules change the arachidonic acid metabolic pathway of prostaglandin E2 (PGE2), via the activation of cytosolic phospholipase A2 (cPLA2), cyclooxygenase 2 (COX-2), and the production of PGE2, as a cPLA2 inhibitor, and reduce other pro-inflammatory molecules in the heart (e.g., IL-1 and IL-6) (63). Also, a high O3FA enriched diet has shown benefits in a rat model with cardiac ischemia–reperfusion injury (66).
Furthermore, specific types of O3FAs can affect oxidative stress due to disparities in their chemical predisposition to oxidation. Experiments by Bellido et al. (67) showed an increase in activation of a redox-sensitive transcriptional factor, NF-κB, in peripheral blood mononuclear cells following a meal high in butter or walnuts but not olive oil (67). These experiments are interesting and require further expansion to determine specific fatty acid effects, and any other meals specifically enriched with O3FA.
Resveratrol and O3FA Combined Effect
Many in vivo and in vitro studies testing the combined effects of O3FA and resveratrol have shown them to have synergistic effects on inflammation. For example, a recent study by Schwager et al. (68) showed that the LPS-stimulated peripheral leukocytes and IL-1-activated human chondrocytes treated only with resveratrol significantly reduced levels of prostaglandin 2 (PGE2), CCL5/RANTES, and CXCL8/IL-8, but increased IL-1β, IL-6, and IL-10, whereas O3FA treatment increased the levels of PGE2 and CXCL8/IL-8. Surprisingly, their combined administration exerted joint effects on CCL5/RANTES and IL-6 or CXCL8/IL-8 (68). Thus, O3FA and resveratrol act together on inflammatory mediators when dealing with acute and chronic inflammation of chondrocytes, but not on acute inflammation of peripheral leukocytes. In diseases where chronic inflammation occurs, such as osteoarthritis, O3FA and resveratrol may be beneficial. In vitro studies of chronic inflammation implementing long-term treatment of these two have shown a reduction in the production of inflammatory mediators. For example, bovine chondrocytes and cartilage explants have identified that long-term in vitro treatment of chondrocytes with conjugated linoleic acids or EPA mitigated the production of PGE2 and nitric oxide (69). Also, other studies reported reduced mRNA levels of matrix metalloproteinaises (MMPs), a disintegrin and metalloproteinase with thrombospondin motifs 4 (ADAMTS-4), and interleukins (68, 70). Thus, resveratrol and O3FA are able to reduce the inflammatory processes during chronic inflammation. But, in instances of acute inflammation, resveratrol acts to reduce early inflammatory events, whereas O3FA has the opposite effect and increases inflammatory events.
In an attempt to identify an effective therapy for cardiovascular patients, Hobbs et al. (71) did a pilot study with a multi-ingredient supplement (MIS) featuring blend of red yeast rice, bioflavonoids, polycosanol, and resveratrol along with a high-potency omega-3 polyunsaturated fatty acid in 19 patients with hypercholesterolemia (71). They found that the MIS supplement offers a promising result for cholesterol management by decreasing total cholesterol and LDL significantly. A separate review by the World Health Organization (72) found that the Mediterranean diet (MeDi) is a promising strategy to prevent diseases such as cerebro- and cardiovascular diseases, cancer, respiratory diseases, diabetes, and neurodegenerative diseases, thereby improving life quality (72). The two major components in MeDi in this study are long-chain O3FAs derived from fish and resveratrol from plant phenols. The MeDi benefits have been studied in many human epidemiological studies suffering from cancer, cardiovascular conditions (myocardial infarction, stroke, angina pectoris, coronary bypass, and coronary angioplasty), and neurodegenerative diseases such as Alzheimer’s disease (AD) (73–76). Also, a recent long-term randomized clinical trial (RCT) in 334 participants with high cardiovascular risk at a mean age of 67 years (PREDIMED study) reported positive effects of MeDi (77, 78).
From these studies, it is evident that the combinational therapy with O3FAs and resveratrol is more beneficial, and therefore, more studies should be directed to understand more insight on their mechanism of action at a molecular level.
Conclusion
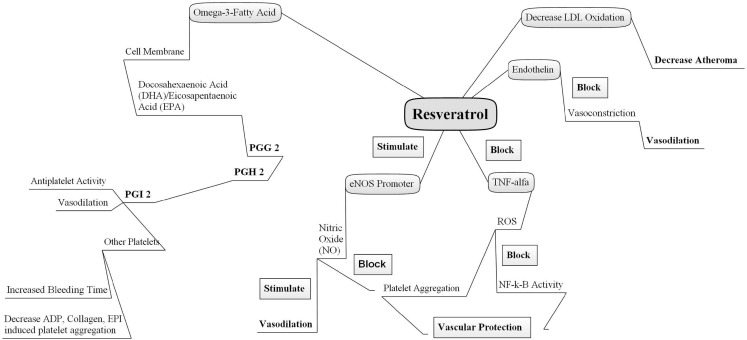
Based on the existing literature discussed in this review, it is evident that resveratrol and O3FAs are reported to be significantly protective and play crucial roles in the prevention of not only various cardiovascular diseases but also a variety of other diseases, including Alzheimer disease, cancer, diabetes, and inflammation (Figure 1). Resveratrol and O3FAs, with their unique antioxidant properties and cardioprotective nature, are demonstrated to be highly functional and beneficial ingredients in various functional foods, although the number of studies in terms of dosage regimens and pharmacokinetics profiles of these ingredients is scanty and newer studies should be initiated to profile the same. The growing body of scientific literatures indicated the potential health benefits of these functional ingredients. However, new mechanistic studies should be designed to evaluate and identify the mechanisms behind the observed fascinating beneficial outcomes of resveratrol and O3FAs along with their combinational benefits.
Figure 1.
Mechanisms of action of omega-3 fatty acid and resveratrol. Two natural cardioprotective compounds act independently via different pathways combination of both may offer beneficial effects. PGG2, prostaglandin G2; PGH2, prostaglandin H2; PGI2, prostaglandin I2 or prostacyclin; eNOS, endothelial nitric oxide synthase; NO, nitric oxide, TNF-alpha, tissue necrotic factor alpha; ROS, reactive oxygen species; LDL, low density lipoprotein; ADP, adenosine diphosphate; NF-κB, nuclear factor kappa B.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This work was supported by University of South Florida, College of Pharmacy startup funds (USF COP Startup funds) to Dr. Panguluri.
References
- 1.Centers for Disease Control and Prevention; National Center for Health Statistics. Underlying Cause of Death 1999-2013. Data Are from the Multiple Cause of Death Files, 1999-2013, as Compiled from Data Provided by the 57 Vital Statistics Jurisdictions Through the Vital Statistics Cooperative Program (2015). Available from: http://wonder.cdc.gov/ucd-icd10.html
- 2.Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R. Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation (2005) 111:1448–54. 10.1161/01.CIR.0000158483.13093.9D [DOI] [PubMed] [Google Scholar]
- 3.Peairs AD, Rankin JW, Lee YW. Effects of acute ingestion of different fats on oxidative stress and inflammation in overweight and obese adults. Nutr J (2011) 10:122. 10.1186/1475-2891-10-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basu A, Devaraj S, Jialal I. Dietary factors that promote or retard inflammation. Arterioscler Thromb Vasc Biol (2006) 26:995–1001. 10.1161/01.ATV.0000214295.86079.d1 [DOI] [PubMed] [Google Scholar]
- 5.Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr (2005) 82:222S–5S. [DOI] [PubMed] [Google Scholar]
- 6.Spanier G, Xu H, Xia N, Tobias S, Deng S, Wojnowski L, et al. Resveratrol reduces endothelial oxidative stress by modulating the gene expression of superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPX1) and Nadph oxidase subunit (NOX4). J Physiol Pharmacol (2009) 60:111–6. [PubMed] [Google Scholar]
- 7.Borrelli F, Capasso R, Izzo AA. Garlic (Allium sativum L.): adverse effects and drug interactions in humans. Mol Nutr Food Res (2007) 51:1386–97. 10.1002/mnfr.200700072 [DOI] [PubMed] [Google Scholar]
- 8.Vidavalur R, Otani H, Singal PK, Maulik N. Significance of wine and resveratrol in cardiovascular disease: French paradox revisited. Exp Clin Cardiol (2006) 11:217–25. [PMC free article] [PubMed] [Google Scholar]
- 9.Yang T, Wang L, Zhu M, Zhang L, Yan L. Properties and molecular mechanisms of resveratrol: a review. Pharmazie (2015) 70:501–6. 10.1691/ph.2015.5571 [DOI] [PubMed] [Google Scholar]
- 10.Nie S, Xing Y, Kim GJ, Simons JW. Nanotechnology applications in cancer. Annu Rev Biomed Eng (2007) 9:257–88. 10.1146/annurev.bioeng.9.060906.152025 [DOI] [PubMed] [Google Scholar]
- 11.Niemeyer CM. Semi-synthetic nucleic acid-protein conjugates: applications in life sciences and nanobiotechnology. J Biotechnol (2001) 82:47–66. 10.1016/S1389-0352(01)00027-7 [DOI] [PubMed] [Google Scholar]
- 12.Nishiyama N. Nanomedicine: nanocarriers shape up for long life. Nat Nanotechnol (2007) 2:203–4. 10.1038/nnano.2007.88 [DOI] [PubMed] [Google Scholar]
- 13.Shutava TG, Balkundi SS, Vangala P, Steffan JJ, Bigelow RL, Cardelli JA, et al. Layer-by-layer-coated gelatin nanoparticles as a vehicle for delivery of natural polyphenols. ACS Nano (2009) 3:1877–85. 10.1021/nn900451a [DOI] [PubMed] [Google Scholar]
- 14.Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer (2005) 5:161–71. 10.1038/nrc1566 [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Yang L, Chen ZG, Shin DM. Application of nanotechnology in cancer therapy and imaging. CA Cancer J Clin (2008) 58:97–110. 10.3322/CA.2007.0003 [DOI] [PubMed] [Google Scholar]
- 16.McNeil SE. Nanoparticle therapeutics: a personal perspective. Wiley Interdiscip Rev Nanomed Nanobiotechnol (2009) 1:264–71. 10.1002/wnan.6 [DOI] [PubMed] [Google Scholar]
- 17.Jagtap S, Meganathan K, Wagh V, Winkler J, Hescheler J, Sachinidis A. Chemoprotective mechanism of the natural compounds, epigallocatechin-3-O-gallate, quercetin and curcumin against cancer and cardiovascular diseases. Curr Med Chem (2009) 16:1451–62. 10.2174/092986709787909578 [DOI] [PubMed] [Google Scholar]
- 18.Lee KW, Bode AM, Dong Z. Molecular targets of phytochemicals for cancer prevention. Nat Rev Cancer (2011) 11:211–8. 10.1038/nrc3017 [DOI] [PubMed] [Google Scholar]
- 19.Nair HB, Sung B, Yadav VR, Kannappan R, Chaturvedi MM, Aggarwal BB. Delivery of antiinflammatory nutraceuticals by nanoparticles for the prevention and treatment of cancer. Biochem Pharmacol (2010) 80:1833–43. 10.1016/j.bcp.2010.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siddiqui IA, Adhami VM, Bharali DJ, Hafeez BB, Asim M, Khwaja SI, et al. Introducing nanochemoprevention as a novel approach for cancer control: proof of principle with green tea polyphenol epigallocatechin-3-gallate. Cancer Res (2009) 69:1712–6. 10.1158/0008-5472.CAN-08-3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barras A, Mezzetti A, Richard A, Lazzaroni S, Roux S, Melnyk P, et al. Formulation and characterization of polyphenol-loaded lipid nanocapsules. Int J Pharm (2009) 379:270–7. 10.1016/j.ijpharm.2009.05.054 [DOI] [PubMed] [Google Scholar]
- 22.Sanna V, Pintus G, Roggio AM, Punzoni S, Posadino AM, Arca A, et al. Targeted biocompatible nanoparticles for the delivery of (-)-epigallocatechin 3-gallate to prostate cancer cells. J Med Chem (2011) 54:1321–32. 10.1021/jm1013715 [DOI] [PubMed] [Google Scholar]
- 23.Yadav VR, Aggarwal BB. Curcumin: a component of the golden spice, targets multiple angiogenic pathways. Cancer Biol Ther (2011) 11:236–41. 10.4161/cbt.11.2.14405 [DOI] [PubMed] [Google Scholar]
- 24.Dube A, Nicolazzo JA, Larson I. Chitosan nanoparticles enhance the intestinal absorption of the green tea catechins (+)-catechin and (-)-epigallocatechingallate. Eur J Pharm Sci (2010) 41:219. 10.1016/j.ejps.2010.06.010 [DOI] [PubMed] [Google Scholar]
- 25.Carter LG, D’Orazio JA, Pearson KJ. Resveratrol and cancer: focus on in vivo evidence. Endocr Relat Cancer (2014) 21:R209–25. 10.1530/ERC-13-0171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buckley JD, Howe PR. Long-chain omega-3 polyunsaturated fatty acids may be beneficial for reducing obesity – a review. Nutrients (2010) 2:1212–30. 10.3390/nu2121212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bourre JM. Roles of unsaturated fatty acids (especially omega-3 fatty acids) in the brain at various ages and during ageing. J Nutr Health Aging (2004) 8:163–74. 10.1186/1476-511X-3-25 [DOI] [PubMed] [Google Scholar]
- 28.Holman RT. The slow discovery of the importance of omega 3 essential fatty acids in human health. J Nutr (1998) 128:427S–33S. [DOI] [PubMed] [Google Scholar]
- 29.Thuerauf DJ, Marcinko M, Gude N, Rubio M, Sussman MA, Glembotski CC. Activation of the unfolded protein response in infarcted mouse heart and hypoxic cultured cardiac myocytes. Circ Res (2006) 99:275–82. 10.1161/01.RES.0000233317.70421.03 [DOI] [PubMed] [Google Scholar]
- 30.Pan YX, Lin L, Ren AJ, Pan XJ, Chen H, Tang CS, et al. HSP70 and GRP78 induced by endothelin-1 pretreatment enhance tolerance to hypoxia in cultured neonatal rat cardiomyocytes. J Cardiovasc Pharmacol (2004) 44(Suppl 1):S117–20. 10.1097/01.fjc.0000166234.11336.a9 [DOI] [PubMed] [Google Scholar]
- 31.Vitadello M, Penzo D, Petronilli V, Michieli G, Gomirato S, Menabo R, et al. Overexpression of the stress protein Grp94 reduces cardiomyocyte necrosis due to calcium overload and simulated ischemia. FASEB J (2003) 17:923–5. 10.1096/fj.02-0644fje [DOI] [PubMed] [Google Scholar]
- 32.Severino A, Campioni M, Straino S, Salloum FN, Schmidt N, Herbrand U, et al. Identification of protein disulfide isomerase as a cardiomyocyte survival factor in ischemic cardiomyopathy. J Am Coll Cardiol (2007) 50:1029–37. 10.1016/j.jacc.2007.06.006 [DOI] [PubMed] [Google Scholar]
- 33.Feng B, Yao PM, Li Y, Devlin CM, Zhang D, Harding HP, et al. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol (2003) 5:781–92. 10.1038/ncb1035 [DOI] [PubMed] [Google Scholar]
- 34.Zhou J, Lhoták Š, Hilditch BA, Austin RC. Activation of the unfolded protein response occurs at all stages of atherosclerotic lesion development in apolipoprotein E-deficient mice. Circulation (2005) 111:1814–21. 10.1161/01.CIR.0000160864.31351.C1 [DOI] [PubMed] [Google Scholar]
- 35.Myoishi M, Hao H, Minamino T, Watanabe K, Nishihira K, Hatakeyama K, et al. Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation (2007) 116:1226–33. 10.1161/CIRCULATIONAHA.106.682054 [DOI] [PubMed] [Google Scholar]
- 36.Digaleh H, Kiaei M, Khodagholi F. Nrf2 and Nrf1 signaling and ER stress crosstalk: implication for proteasomal degradation and autophagy. Cell Mol Life Sci (2013) 70:4681–94. 10.1007/s00018-013-1409-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee S, Hur EG, Ryoo IG, Jung KA, Kwak J, Kwak MK. Involvement of the Nrf2-proteasome pathway in the endoplasmic reticulum stress response in pancreatic beta-cells. Toxicol Appl Pharmacol (2012) 264:431–8. 10.1016/j.taap.2012.08.021 [DOI] [PubMed] [Google Scholar]
- 38.Cominacini L, Mozzini C, Garbin U, Pasini A, Stranieri C, Solani E, et al. Endoplasmic reticulum stress and Nrf2 signaling in cardiovascular diseases. Free Radic Biol Med (2015) 88(Pt B):233–42. 10.1016/j.freeradbiomed.2015.05.027 [DOI] [PubMed] [Google Scholar]
- 39.Petrovski G, Gurusamy N, Das DK. Resveratrol in cardiovascular health and disease. Ann N Y Acad Sci (2011) 1215:22–33. 10.1111/j.1749-6632.2010.05843.x [DOI] [PubMed] [Google Scholar]
- 40.Gurusamy N, Lekli I, Mukherjee S, Ray D, Ahsan MK, Gherghiceanu M, et al. Cardioprotection by resveratrol: a novel mechanism via autophagy involving the mTORC2 pathway. Cardiovasc Res (2010) 86:103–12. 10.1093/cvr/cvp384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bradamante S, Barenghi L, Villa A. Cardiovascular protective effects of resveratrol. Cardiovasc Drug Rev (2004) 22:169–88. 10.1111/j.1527-3466.2004.tb00139.x [DOI] [PubMed] [Google Scholar]
- 42.Wu JM, Wang ZR, Hsieh TC, Bruder JL, Zou JG, Huang YZ. Mechanism of cardioprotection by resveratrol, a phenolic antioxidant present in red wine (review). Int J Mol Med (2001) 8:3–17. 10.3892/ijmm.8.1.3 [DOI] [PubMed] [Google Scholar]
- 43.Tang PC, NG YF, HO S, Gyda M, Chan SW. Resveratrol and cardiovascular health – promising therapeutic or hopeless illusion? Pharmacol Res (2014) 90:88–115. 10.1016/j.phrs.2014.08.001 [DOI] [PubMed] [Google Scholar]
- 44.Li J, Xie C, Zhuang J, Li H, Yao Y, Shao C, et al. Resveratrol attenuates inflammation in the rat heart subjected to ischemia-reperfusion: role of the TLR4/NF-kappaB signaling pathway. Mol Med Rep (2015) 11(2):1120–6. 10.3892/mmr.2014.2955 [DOI] [PubMed] [Google Scholar]
- 45.Weaver J, Liu KJ. Does normobaric hyperoxia increase oxidative stress in acute ischemic stroke? A critical review of the literature. Med Gas Res (2015) 5:015–0032. 10.1186/s13618-015-0032-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saita E, Kondo K, Momiyama Y. Anti-inflammatory diet for atherosclerosis and coronary artery disease: antioxidant foods. Clin Med Insights Cardiol (2015) 8:61–5. 10.4137/CMC.S17071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nabavi SF, Li H, Daglia M, Nabavi SM. Resveratrol and stroke: from chemistry to medicine. Curr Neurovasc Res (2014) 11:390–7. 10.2174/1567202611666140912114833 [DOI] [PubMed] [Google Scholar]
- 48.Lippi G, Franchini M, Favaloro EJ, Targher G. Moderate red wine consumption and cardiovascular disease risk: beyond the “French paradox”. Semin Thromb Hemost (2010) 36:59–70. 10.1055/s-0030-1248725 [DOI] [PubMed] [Google Scholar]
- 49.Fagherazzi G, Vilier A, Lajous M, Boutron-Ruault MC, Balkau B, Clavel-Chapelon F, et al. Wine consumption throughout life is inversely associated with type 2 diabetes risk, but only in overweight individuals: results from a large female French cohort study. Eur J Epidemiol (2014) 29:831–9. 10.1007/s10654-014-9955-7 [DOI] [PubMed] [Google Scholar]
- 50.Al-Harthi SE, Alarabi OM, Ramadan WS, Alaama MN, Al-Kreathy HM, Damanhouri ZA, et al. Amelioration of doxorubicin-induced cardiotoxicity by resveratrol. Mol Med Rep (2014) 10:1455–60. 10.3892/mmr.2014.2384 [DOI] [PubMed] [Google Scholar]
- 51.Arafa MH, Mohammad NS, Atteia HH, Abd-Elaziz HR. Protective effect of resveratrol against doxorubicin-induced cardiac toxicity and fibrosis in male experimental rats. J Physiol Biochem (2014) 70:701–11. 10.1007/s13105-014-0339-y [DOI] [PubMed] [Google Scholar]
- 52.Lamptey MS, Walker BL. A possible essential role for dietary linolenic acid in the development of the young rat. J Nutr (1976) 106:86–93. [DOI] [PubMed] [Google Scholar]
- 53.Bacarin CC, De Sa-Nakanishi AB, Bracht A, Matsushita M, Previdelli IS, Mori MA, et al. Fish oil prevents oxidative stress and exerts sustained antiamnesic effect after global cerebral ischemia. CNS Neurol Disord Drug Targets (2015) 14(3):400–10. 10.2174/1871527314666150225125232 [DOI] [PubMed] [Google Scholar]
- 54.Darghosian L, Free M, Li J, Gebretsadik T, Bian A, Shintani A, et al. Effect of omega-three polyunsaturated fatty acids on inflammation, oxidative stress, and recurrence of atrial fibrillation. Am J Cardiol (2015) 115:196–201. 10.1016/j.amjcard.2014.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jain AP, Aggarwal KK, Zhang PY. Omega-3 fatty acids and cardiovascular disease. Eur Rev Med Pharmacol Sci (2015) 19:441–5. [PubMed] [Google Scholar]
- 56.McEwen BJ, Morel-Kopp MC, Tofler GH, Ward CM. The effect of omega-3 polyunsaturated fatty acids on fibrin and thrombin generation in healthy subjects and subjects with cardiovascular disease. Semin Thromb Hemost (2015) 41(3):315–22. 10.1055/s-0034-1395352 [DOI] [PubMed] [Google Scholar]
- 57.Nigam A, Talajic M, Roy D, Nattel S, Lambert J, Nozza A, et al. Fish oil for the reduction of atrial fibrillation recurrence, inflammation, and oxidative stress. J Am Coll Cardiol (2014) 64:1441–8. 10.1016/j.jacc.2014.07.956 [DOI] [PubMed] [Google Scholar]
- 58.Delgado-Lista J, Perez-Martinez P, Lopez-Miranda J, Perez-Jimenez F. Long chain omega-3 fatty acids and cardiovascular disease: a systematic review. Br J Nutr (2012) 107(Suppl 2):S201–13. 10.1017/S0007114512001596 [DOI] [PubMed] [Google Scholar]
- 59.GISSI. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. (Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico). Lancet (1999) 354:447–55. 10.1016/S0140-6736(99)07072-5 [DOI] [PubMed] [Google Scholar]
- 60.Da Cunha NV, Pinge-Filho P, Panis C, Silva BR, Pernomian L, Grando MD, et al. Decreased endothelial nitric oxide, systemic oxidative stress, and increased sympathetic modulation contribute to hypertension in obese rats. Am J Physiol Heart Circ Physiol (2014) 306:H1472–80. 10.1152/ajpheart.00520.2013 [DOI] [PubMed] [Google Scholar]
- 61.Herrera EA, Farias JG, Gonzalez-Candia A, Short SE, Carrasco-Pozo C, Castillo RL. Omega3 supplementation and intermittent hypobaric hypoxia induce cardioprotection enhancing antioxidant mechanisms in adult rats. Mar Drugs (2015) 13:838–60. 10.3390/md13020838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gorjao R, Azevedo-Martins AK, Rodrigues HG, Abdulkader F, Arcisio-Miranda M, Procopio J, et al. Comparative effects of DHA and EPA on cell function. Pharmacol Ther (2009) 122:56–64. 10.1016/j.pharmthera.2009.01.004 [DOI] [PubMed] [Google Scholar]
- 63.Liu Y, Chen LY, Sokolowska M, Eberlein M, Alsaaty S, Martinez-Anton A, et al. The fish oil ingredient, docosahexaenoic acid, activates cytosolic phospholipase A(2) via GPR120 receptor to produce prostaglandin E(2) and plays an anti-inflammatory role in macrophages. Immunology (2014) 143:81–95. 10.1111/imm.12296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yates CM, Calder PC, Ed Rainger G. Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacol Ther (2014) 141:272–82. 10.1016/j.pharmthera.2013.10.010 [DOI] [PubMed] [Google Scholar]
- 65.Balakumar P, Taneja G. Fish oil and vascular endothelial protection: bench to bedside. Free Radic Biol Med (2012) 53:271–9. 10.1016/j.freeradbiomed.2012.05.005 [DOI] [PubMed] [Google Scholar]
- 66.Al-Khalifa A, Maddaford TG, Chahine MN, Austria JA, Edel AL, Richard MN, et al. Effect of dietary hempseed intake on cardiac ischemia-reperfusion injury. Am J Physiol Regul Integr Comp Physiol (2007) 292:R1198–203. 10.1152/ajpregu.00661.2006 [DOI] [PubMed] [Google Scholar]
- 67.Bellido C, Lopez-Miranda J, Blanco-Colio LM, Perez-Martinez P, Muriana FJ, Martin-Ventura JL, et al. Butter and walnuts, but not olive oil, elicit postprandial activation of nuclear transcription factor kappaB in peripheral blood mononuclear cells from healthy men. Am J Clin Nutr (2004) 80:1487–91. [DOI] [PubMed] [Google Scholar]
- 68.Schwager J, Richard N, Riegger C, Salem N., Jr Omega-3 PUFAs and resveratrol differently modulate acute and chronic inflammatory processes. Biomed Res Int (2015) 2015:535189. 10.1155/2015/535189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schwager J, Richard N, Schoop R, Wolfram S. A novel rose hip preparation with enhanced anti-inflammatory and chondroprotective effects. Mediators Inflamm (2014) 2014:105710. 10.1155/2014/105710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shen CL, Dunn DM, Henry JH, Li Y, Watkins BA. Decreased production of inflammatory mediators in human osteoarthritic chondrocytes by conjugated linoleic acids. Lipids (2004) 39:161–6. 10.1007/s11745-004-1214-6 [DOI] [PubMed] [Google Scholar]
- 71.Hobbs T, Caso R, McMahon D, Nymark M. A novel, multi-ingredient supplement to manage elevated blood lipids in patients with no evidence of cardiovascular disease: a pilot study. Altern Ther Health Med (2014) 20:18–23. [PubMed] [Google Scholar]
- 72.WHO. Interventions on Diet and Physical Activity: What Works: Summary Report. Geneva: World Health Organization; (2009). [PubMed] [Google Scholar]
- 73.Huhn S, Kharabian Masouleh S, Stumvoll M, Villringer A, Witte AV. Components of a Mediterranean diet and their impact on cognitive functions in aging. Front Aging Neurosci (2015) 7:132. 10.3389/fnagi.2015.00132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Couto E, Boffetta P, Lagiou P, Ferrari P, Buckland G, Overvad K, et al. Mediterranean dietary pattern and cancer risk in the EPIC cohort. Br J Cancer (2011) 104:1493–9. 10.1038/bjc.2011.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lopez-Garcia E, Rodriguez-Artalejo F, Li TY, Fung TT, Li S, Willett WC, et al. The Mediterranean-style dietary pattern and mortality among men and women with cardiovascular disease. Am J Clin Nutr (2014) 99:172–80. 10.3945/ajcn.113.068106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lourida I, Soni M, Thompson-Coon J, Purandare N, Lang IA, Ukoumunne OC, et al. Mediterranean diet, cognitive function, and dementia: a systematic review. Epidemiology (2013) 24:479–89. 10.1097/EDE.0b013e3182944410 [DOI] [PubMed] [Google Scholar]
- 77.Valls-Pedret C, Ros E. Commentary: Mediterranean diet and cognitive outcomes: epidemiological evidence suggestive, randomized trials needed. Epidemiology (2013) 24:503–6. 10.1097/EDE.0b013e318296bf8e [DOI] [PubMed] [Google Scholar]
- 78.Valls-Pedret C, Sala-Vila A, Serra-Mir M, Corella D, DE LA, Torre R. Mediterranean diet and age-related cognitive decline: a randomized clinical trial. JAMA Intern Med (2015) 175:1094–103. 10.1001/jamainternmed.2015.1668 [DOI] [PubMed] [Google Scholar]