Main Text
Traumatic brain injury is a nondegenerative, noncongenital insult to the brain caused by external mechanical forces. Recent studies suggest that even mild concussions—if repetitive—can trigger progressive neurological degeneration, a condition that is now widely known as “chronic traumatic encephalopathy” (1). Notably, chronic traumatic encephalopathy continues to progress even decades after the initial insult; the more severe the original injury and the longer the survival, the greater the severity of neurodegeneration. Progressive axonal damage and structural degradation are classic hallmarks of chronic traumatic encephalopathy. Strikingly, these symptoms appear to be shared by a number of other neurodegenerative diseases including Alzheimer’s disease and Parkinsonism. However, the molecular mechanisms of axonal failure remain poorly understood.
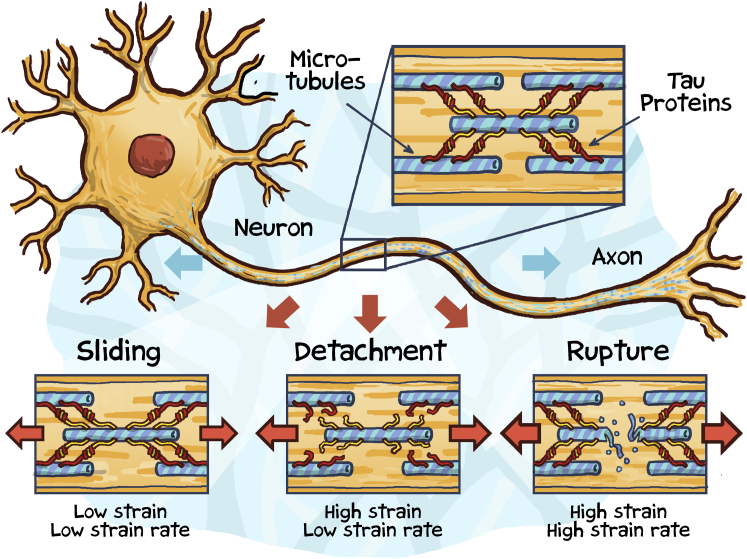
One of the most common pathological features of traumatic brain injury is diffuse axonal injury (2). To date, the only method to reliably diagnose diffuse axonal injury is post mortem histopathology, where it manifests itself through extensive damage in white-matter tissue. More than half a century ago, experiments with gelatin molds have established that pathological white-matter damage is a direct consequence of elevated mechanical strains and strain rates (3): At low strain rates, axons that make up white-matter tissue are highly compliant and ductile; they can easily deform and revert to their initial conformation. At high strain rates, axons stiffen and become brittle; they are vulnerable to mechanical failure (Fig. 1). Axonal failure manifests itself in two modes, primary axotomy, the immediate, complete mechanical rupture, and secondary axotomy, the progressive degradation and gradual failure (1). Although secondary axotomy is by far the more common failure mode, the precise sequence of events by which the axonal cytoskeleton degrades is unknown.
Figure 1.
Tau protein acts as a molecular switch between microtubule sliding, detachment, and rupture.
The axonal cytoskeleton is made up of microtubules, neurofilaments, and microfilaments. Neuronal microtubules are structurally similar to microtubules in all other cells of our body: Composed of heterodimers of α- and β-tubulin that form 13 laterally joined protofilaments, microtubules are hollow tubes with a diameter of 24 nm. With a high resistance to bending and a stiffness of 2.0 GPa, microtubules are undoubtedly the strongest cytoskeletal filaments in eukaryotic cells. As such, they play a unique role in a number of cellular processes, maintaining structural stability and providing highways for intracellular transport. While the microtubule ultrastructure is similar in all eukaryotic cells, microtubule organization in neurons differs significantly from nonneuronal cells (4): Axons can extend up to a meter in length and their microtubules never run continuously from the cell body to the distal end. Instead, they form bundles of microtubule segments with 10–20 microtubules in any given cross section. Neuronal microtubules are nucleated at the centrosome within the cell body, then rapidly released, and delivered into the axon via molecular motors. During transport, microtubules shorten to provide subunits for the elongation of other microtubules. This explains why the microtubule length can vary significantly along the axon, with up to 100 μm and longer toward the cell body and 2 μm and shorter toward the distal end (3). Individual microtubules are stabilized and cross-linked to form the axonal cytoskeleton via microtubule-associated proteins.
The most prominent microtubule-associated protein, tau, was discovered more than four decades ago (5). A decade later, tau took center stage as the major component of neurofibrillary tangles, which are now widely known as the primary markers of Alzheimer’s disease (6). Tau is primarily a neuronal protein. In the adult human brain, alternative mRNA splicing generates six major isoforms of tau varying from 352 to 441 amino acids in length. All isoforms share three common domains: a microtubule-binding domain composed of repeats of an evolutionarily conserved tubulin binding motif, a positively charged proline-rich region, and a negatively charged N-terminus (7). The six isoforms differ by the number of microtubule-binding repeats, either three or four, and by the presence or absence of one or two N-terminal inserts. Tau is natively disordered. By binding to microtubules, tau becomes more organized and contributes directly or indirectly to key structural and regulatory cellular function. Within individual microtubules, tau modulates microtubule polymerization, controls microtubule structure, and regulates axonal transport; the tubulin-binding repeats of tau bind to hydrophobic pockets between the α- and β-tubulin heterodimers of a microtubule to stabilize its straight protofilament conformation (8). Within the axonal cytoskeleton, tau promotes the assembly of individual microtubules into well-organized, evenly spaced bundles; two tau proteins of neighboring microtubules form an electrostatic zipper interface via their positively and negatively charged domains to assemble a dimer that cross-links adjacent microtubules (9).
Phosphorylation, the site-specific addition of a phosphate group, is the primary mechanism to regulate tau function (7). In the normal adult brain, phosphorylated tau promotes association with tubulin and stabilizes microtubule structure. Pathological hyperphosphorylation reduces tau’s affinity to bind to microtubules, destabilizes microtubule structure, and makes tau more fibrillogenic. Eventually, these processes disrupt intracellular transport and result in synapse loss, cell death, disrupted neural circuits, and, ultimately, in cognitive decline and impaired motor function. Pathologies that share these common neurodegenerative pathways are now collectively known as “tauopathies”.
To understand the biophysics of traumatic brain injury, it is critical to map how neurodegeneration progresses across the scales, from tau protein unfolding and microtubule rupture via diffuse axonal injury toward the forces acting on the whole brain. Computationally integrating the scales can help us understand the biophysics of traumatic brain injury and elucidate the common molecular mechanisms of tauopathies (10). Existing mechanical models universally acknowledge the viscoelastic nature of brain tissue during injury, but almost exclusively model the brain phenomenologically at the macroscopic scale (11). As such, they are incapable of capturing molecular failure mechanisms, and their parameters typically lack a clear biophysical interpretation. In a comprehensive mechanistic study in this issue of Biophysical Journal, Ahmadzadeh et al. (12) now show, for the first time, how elevated macroscopic strains can trigger microscopic tau protein unfolding and microtubule failure. Collectively, their findings suggest that tau protein acts as a molecular switch between microtubule sliding, detachment, and rupture (Fig. 1).
The new model of axonal failure is a significant advancement of the original model, the first mechanistic approach to explore the competition between microtubule sliding and stretching (3). The core of the model is a representative unit cell of an axon that consists of two adjacent microtubules, represented by elastic springs, connected via tau proteins, represented by the parallel arrangement of an elastic spring and a viscous damper. The spring stiffness follows from a freely jointed chain model, and the viscous damping scales linearly with the number of intramolecular bonds governed by the Bell equation of bond rupture (13). In addition, the model accounts for the transient binding and unbinding of the dimer interface between two cross-linking tau proteins (9). Simulations with the new model substantiate that diffuse axonal injury is highly sensitive to the rate of mechanical loading: At low strain rates, recurring binding and unbinding of tau dimers enables microtubules to slide along one another, to allow axons to stretch up to twice their initial length without notable signs of damage. At high strain rates, tau proteins stiffen and microtubules become the weakest link and rupture. The model predicts that tau-tau unbinding is homogeneous at low strain rates and progresses heterogeneously from the free ends toward the center at high strain rates. The model also explains why longer microtubules are more likely to rupture, whereas shorter microtubules are more likely to detach from the microtubule bundle (12). Taken together, these findings strongly support the central role of tau protein in mediating the elastic-viscoelastic transition between physiological protein binding and unbinding and pathological microtubule breakdown.
In retrospect, including the mechanisms of binding and unbinding between two cross-bridging tau proteins and within the tau protein itself was a logical extension of the existing model (3). While the new model is more conclusive and successfully explains a wide variety of common pathologies associated with traumatic brain injury, it has some limitations that require attention. For example, the molecular mechanisms by which microtubules rupture, remain unaddressed. The model attributes microtubule detachment exclusively to failure of the tau-tau interface and assumes that the tau-microtubule interface remains intact at all times. However, recent studies have shown that the tau-microtubule interface is highly dynamic (8); in fact, frequent cycles of tau-microtubule binding and unbinding reportedly play an important role in effective axonal transport (7). Microtubule depolymerization—caused by a sudden loss of the stabilizing tau-microtubule interface—could be a major mechanism of microtubule breakdown, which is neglected by the current model (8). Another limitation is that the model attributes its viscoelasticity to the untangling of tau observed during single-molecule experiments on disordered tau monomers. When dimerized and bound to microtubules, tau adopts an ordered structure with short linkers between dimer and microtubule, which could make viscoelastic untangling less relevant than assumed here (9). While viscoelasticity could also result from unzipping the dimer interface, this mechanism remains unaddressed. These important questions could be resolved with carefully designed experiments to validate the model assumptions and calibrate its rate constants.
Combining the entropic elasticity of biomolecules with the binding kinetics of intra- and intermolecular protein bonds allows us to computationally probe the landscape and timeline of intracellular events. Interestingly, with only minor modifications, the current model can predict selected effects of tau hyperphosphorylation (6). Through a concentration-dependent rate constant associated with the kinetics of the tau-tau binding, the model should be capable of predicting the detachment of microtubules, even in the absence of significant strain. This could provide fundamental insight into the common molecular mechanisms shared by chronic traumatic encephalopathy and other tauopathies including Alzheimer’s disease, progressive supranuclear palsy, frontal dementia, and Parkinsonism (7). Yet, rather than modeling the binding kinetics phenomenologically using the simplifying Bell equation (13), the model could be made more mechanistic by using molecular-dynamics simulations or time-dependent experiments (14) to elucidate the precise sequence of intracellular events that compromise cytoskeletal integrity. Ultimately, a better mechanistic understanding of the tau-microtubule complex during progressive neurodegeneration could help identify potential drug targets and design inhibitors for their degeneration pathways (10).
Undeniably, the dynamic tau-microtubule model by Ahmadzadeh et al. (12) has the potential to become the core of a broader multiscale model both in time and space. Temporally, expanding the simulation window from seconds to years could explain the mechanisms by which several tauopathies worsen progressively in time. This could help identify common early markers of neurodegenerative disease and facilitate early treatment. Spatially, bridging the scales from the molecular level to the whole brain could explain how macroscopic forces during traumatic brain injury translate into cellular and subcellular failure. This could guide the design of novel strategies to slow down, block, or reverse neurodegeneration (10). We are only beginning to understand the complex interplay between protein structure, protein dynamics, and mechanical forces. The simple and elegant model by Ahmadzadeh et al. (12) is a powerful first step with excellent potential for expansion.
Acknowledgments
This work was supported by National Institutes of Health grant U01-HL119578.
Editor: Jennifer Ross.
References
- 1.McKee A.C., Cantu R.C., Stern R.A. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J. Neuropathol. Exp. Neurol. 2009;68:709–735. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson V.E., Stewart W., Smith D.H. Axonal pathology in traumatic brain injury. Exp. Neurol. 2013;246:35–43. doi: 10.1016/j.expneurol.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmadzadeh H., Smith D.H., Shenoy V.B. Viscoelasticity of tau proteins leads to strain rate-dependent breaking of microtubules during axonal stretch injury: predictions from a mathematical model. Biophys. J. 2014;106:1123–1133. doi: 10.1016/j.bpj.2014.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conde C., Cáceres A. Microtubule assembly, organization and dynamics in axons and dendrites. Nat. Rev. Neurosci. 2009;10:319–332. doi: 10.1038/nrn2631. [DOI] [PubMed] [Google Scholar]
- 5.Weingarten M.D., Lockwood A.H., Kirschner M.W. A protein factor essential for microtubule assembly. Proc. Natl. Acad. Sci. USA. 1975;72:1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spires-Jones T.L., Stoothoff W.H., Hyman B.T. Tau pathophysiology in neurodegeneration: a tangled issue. Trends Neurosci. 2009;32:150–159. doi: 10.1016/j.tins.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Ballatore C., Lee V.M., Trojanowski J.Q. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat. Rev. Neurosci. 2007;8:663–672. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- 8.Kadavath H., Hofele R.V., Zweckstetter M. Tau stabilizes microtubules by binding at the interface between tubulin heterodimers. Proc. Natl. Acad. Sci. USA. 2015;112:7501–7506. doi: 10.1073/pnas.1504081112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenberg K.J., Ross J.L., Israelachvili J. Complementary dimerization of microtubule-associated tau protein: Implications for microtubule bundling and tau-mediated pathogenesis. Proc. Natl. Acad. Sci. USA. 2008;105:7445–7450. doi: 10.1073/pnas.0802036105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goriely A., Geers M.G.D., Kuhl E. Mechanics of the brain: perspectives, challenges, and opportunities. Biomech. Model. Mechanobiol. 2015;14:931–965. doi: 10.1007/s10237-015-0662-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Budday S., Nay R., Kuhl E. Mechanical properties of gray and white matter brain tissue by indentation. J. Mech. Behav. Biomed. Mater. 2015;46:318–330. doi: 10.1016/j.jmbbm.2015.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmadzadeh H., Smith D.H., Shenoy V.B. Mechanical effects of dynamic binding between tau proteins on axonal microtubules during traumatic brain injury: predictions from a computational model. Biophys. J. 2015;109:2328–2337. doi: 10.1016/j.bpj.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bell G.I. Models for the specific adhesion of cells to cells. Science. 1978;200:618–627. doi: 10.1126/science.347575. [DOI] [PubMed] [Google Scholar]
- 14.van den Bedem H., Fraser J.S. Integrative, dynamic structural biology at atomic resolution-It's about time. Nat. Meth. 2015;12:307–318. doi: 10.1038/nmeth.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]