Abstract
A significant feature of the genomes of Lepidoptera, butterflies and moths, is the high conservation of chromosome organization. Recent remarkable progress in genome sequencing of Lepidoptera has revealed that syntenic gene order is extensively conserved across phylogenetically distant species. The ancestral karyotype of Lepidoptera is thought to be n=31; however, that of the most well-studied moth, Bombyx mori, is n=28, and diverse studies suggest that three chromosomal fusion events occurred in this lineage. To identify the boundaries between predicted ancient fusions involving B. mori chromosomes 11, 23 and 24, we constructed fluorescence in situ hybridization (FISH)-based chromosome maps of the European corn borer, Ostrinia nubilalis (n=31). We first determined a 511 Mb genomic sequence of the Asian corn borer, O. furnacalis, a congener of O. nubilalis, and isolated bacterial artificial chromosomes and fosmid clones that were expected to localize in candidate regions for the boundaries using these sequences. Combined with FISH and genetic analysis, we narrowed down the candidate regions to 40 kb–1.5 Mb, in strong agreement with a previous estimate based on the genome of a butterfly, Melitaea cinxia. The significant difference in the lengths of the candidate regions where no functional genes were observed may reflect the evolutionary time after fusion events.
Introduction
Evolutionary conservation of karyotypes and internal chromosome structure show high diversity among multicellular animals. A high degree of chromosome rearrangement has been reported in many organisms; yet, conservation of approximate haploid chromosome number and gene contents in parts of chromosomes (synteny blocks) makes it possible to trace the history of rearrangements in mammalian chromosomes (Kemkemer et al., 2009). In contrast, a slow rate of change in karyotype and interchromosomal rearrangements are observed for the vertebrate clade Sauropsida, which includes birds, lizards, snakes and turtles (Alföldi et al., 2011; Pokorná et al., 2012). Among insects, notably Diptera, flies and mosquitoes, comparative genomics reveals a high degree of gene-order shuffling which suggests frequently occurring chromosome rearrangements, although total chromosome numbers remain relatively low (Nene et al., 2007).
Accumulating evidence currently reveals that the gene order and content of each chromosome are highly conserved among lepidopteran species, butterflies and moths. As we first described syntenic gene order on four pairs of chromosomes between the silkworm, Bombyx mori, and a butterfly, Heliconius melpomene (Yasukochi et al., 2006), a number of reports have demonstrated extensive shared synteny between B. mori and other species based on genetic linkage studies (Pringle et al., 2007; Beldade et al., 2009; Baxter et al., 2011; Van't Hof et al., 2013) and chromosomal fluorescence in situ hybridization (FISH) analysis (Yasukochi et al., 2009; Yoshido et al., 2011; Sahara et al., 2013).
As n=31 is the most common chromosome number observed in distantly related families of Lepidoptera (Robinson, 1971), it is likely that n=31 was the karyotype of the common ancestor. Reports using distantly related species having 31 chromosomes consistently show that chromosomes 11, 23 and 24 of B. mori (n=28) were generated by ancestral chromosomal fusion events (Baxter et al., 2011; Sahara et al., 2013; Van't Hof et al., 2013). Genome sequencing with high resolution assignments to chromosomes enabled more direct and precise comparison between species (International Silkworm Genome Consortium 2008; Heliconius Genome Consortium, 2012; Ahola et al., 2014). The genomes of B. mori and H. melpomene (n=21) were revealed to be composed of 31 well-conserved chromosomal segments corresponding to 31 chromosomes of another butterfly, Melitaea cinxia (Heliconius Genome Consortium, 2012; Ahola et al., 2014). This hypothesis would be greatly strengthened if other species of n=31 karyotype that are distantly related to M. cinxia are shown to have similarly organized chromosomes.
The genus, Ostrinia, belonging to the superfamily Pyraloidea, which is distantly related to B. mori and butterflies (Regier et al., 2013), is widely utilized for analyzing evolution in female sex pheromone biosynthesis and male recognition systems during speciation (for review, see Lassance, 2010). Among them, the Asian and European corn borers, Ostrinia furnacalis (n=31) and O. nubilalis (n=31), are serious pests of maize. Although a linkage map was constructed for O. nubilalis based on amplified fragment length polymorphism and microsatellites, not many genes were mapped on it (Dopman et al., 2004; Streiff et al., 2014). Fine mapping of O. nubilalis genes is useful not only for characterization of the genetical basis of complex phenotypes but also for verification of conserved synteny. We previously succeeded in identifying three chromosomes of O. nubilalis orthologous to B. mori chromosomes 1(Z), 16 and 23 by FISH analysis (Yasukochi et al., 2011a).
Here, we describe construction of a cytogenetic map covering all 31 chromosomes of O. nubilalis using 122 bacterial artificial chromosomes (BACs) and eight fosmid clones as FISH probes. We then focused this study on the identification of boundaries between ancient chromosomes within B. mori chromosomes 11, 23 and 24. These regions have a highly distinctive feature of proximal and distal localization in B. mori and O. nubilalis chromosomes, which facilitated the detection of incorrectly isolated probes and improved accuracy of FISH analysis.
Materials and methods
Insects
The Z-race of O. nubilalis, originally collected in Darmstadt, Germany, was used in this study. O. furnacalis, O. scapulalis, O. latipennis, O. palustralis and Pleuroptya ruralis were from wild populations collected in the Kanto area, Japan. All species were fed an artificial diet, Insecta LFS (Nosan, Yokohama, Japan), in plastic cups. B. mori strain p50 was used and reared on mulberry leaves.
Genomic sequencing of O. furnacalis
Genomic DNA was prepared from an O. furnacalis male pupa that was a subcultured offspring of wild insects originally collected in Inawashiro, Fukushima, Japan, using a DNeasy Blood & Tissue Kit (Qiagen, Venlo, the Netherlands) according to the manufacturer's protocol. The genomic DNA was then submitted to Otogenetics Corporation (Norcross, GA, USA) for sequencing. An Illumina paired-end library with an average insert size of 196 bp was made from fragmented DNA using NEBNext reagents (New England Biolabs, Ipswich, MA, USA), and sequenced on an Illumina HiSeq 2000 which generated paired-end reads of 100 nucleotides.
Genome assembly
The raw paired-end reads were trimmed and filtered by Trimmomatic (Bolger et al., 2014) and reads of low quality and those contaminated with adapter sequences (approximately 15%) were removed. Then, genome assembly and scaffolding were carried out using SOAPdenovo2 (version r240) (Luo et al., 2012) with k-mer length 75.
Construction of an O. furnacalis fosmid library
A fosmid library was constructed by Takara Bio (Kyoto, Japan). Genomic DNA was purified from whole male pupae of O. furnacalis by the cetyl trimethyl ammonium bromide method (Murray and Thompson, 1980). The DNA was fragmented by sonication and fractionated by pulse-field electrophoresis. Genomic fragments ranging from 33 to 48 kb were purified from agarose gels and cloned into a pCC1FOS vector (Epicentre, Madison, WI, USA). A total of 75 264 single colonies were arrayed into 196 384-well microplates.
Screening of BAC and fosmid libraries
An O. nubilalis BAC library, ON_Ba, was obtained from the Clemson University Genomics Institute (Clemson, SC, USA). Construction of a B. mori BAC library is described elsewhere (Wu et al., 1999). Detailed methods for PCR screening of large-insert libraries and FISH analysis are described in Yoshido et al. (2014). Briefly, the first screening was carried out against DNA pools representing each microplate containing 384 clones. A second screening was then carried out against DNA pools prepared from the mixture of cell cultures located in the same column or row of each positive microplate. This was followed by single colony isolation from frozen stocks of candidate clones and overnight cultures of the isolated clones for final PCR confirmation. PCR conditions were as follows: 3-min denaturation at 94 °C, followed by 45 cycles with a 1-min denaturation at 94 °C, 2-min annealing at 55 °C, and 3-min elongation at 72 °C, ending with a 5-min final extension at 72 °C. The sequences of primers used in this study are listed in Supplementary Table S1.
FISH analysis
Chromosome preparation was carried out following the method of Sahara et al. (1999) with slight modifications as described in Sahara et al. (2013). BAC- or fosmid-DNA extraction and probe labeling were carried out according to the descriptions in Yasukochi et al. (2009), Yoshido et al. (2011) and Sahara et al. (2013). A published reprobe technique for Lepidoptera (Shibata et al., 2009) was used to integrate results obtained by multiple hybridizations with different probes. All FISH and chromosome images were acquired with a DFC350FX CCD camera attached to a DM 6000B microscope (Leica Microsystems Japan, Tokyo, Japan) and processed with Adobe Photoshop ver 7.
Genotyping
The F2 progeny of B. mori and the BF1 progeny between O. nubilalis and O. scapulalis used in this study were identical to those described in previous reports (Yasukochi et al., 2006; Yasukochi et al., 2011a). Genomic DNAs were individually amplified in the same manner as library screens. The completed reactions were denatured at 95 °C for 5-min, annealed at 55 °C for 15-min to promote heteroduplex formation, and loaded on 0.67 × MDE gels (Cambrex, Rockland, ME, USA) in 0.5 × TBE to detect polymorphisms.
Search for telomere-associated sequences
B. mori genomic sequences (scaffold build2) position 5 400 000–5 800 000 of chromosome 11, 17 000 000–17 400 000 of chromosome 23, and 9 000 000–11 000 000 of chromosome 24, were obtained from a B. mori genome database, Kaikobase (Shimomura et al., 2009). The sequences were then searched for 1690 previously identified repeat sequences including 868 transposable elements (Osanai-Futahashi et al., 2008) using the repeat search program, RepeatMasker Open-4.0.5 (Smit et al., 2013).
Results and Discussion
FISH analysis using previously isolated O. nubilalis BAC clones
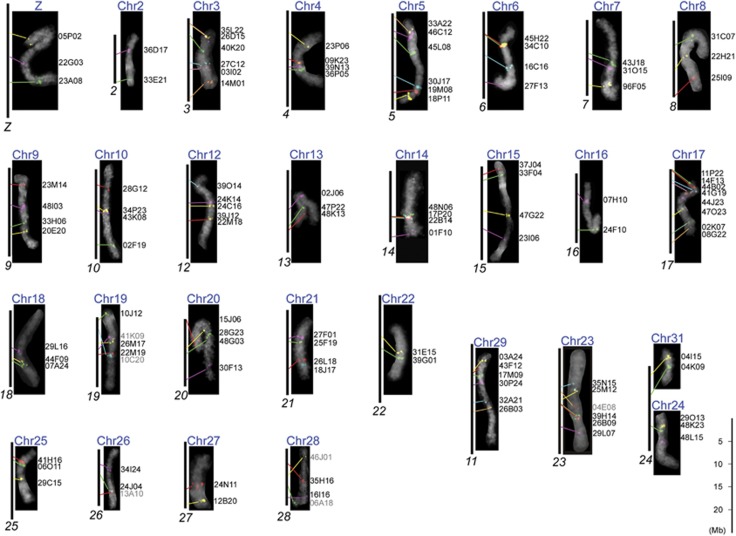
We had previously isolated 163 O. nubilalis BAC clones containing single-copy genes which could be used as anchors for comparing genomes (Yasukochi et al., 2011b). In addition, we isolated two BACs, 04I15 and 04K09, which contained O. nubilalis orthologues of B. mori genes located on the distal part of B. mori chromosome 24. Thus, we used these BACs as probes for FISH analysis of O. nubilalis. Subsequently, we obtained stable and reproducible signals from 112 BACs harboring 117 conserved genes between O. nubilalis and B. mori (Supplementary Table S2), which could identify 29 of 31 O. nubilalis chromosomes (Figure 1). The gene order on each chromosome was well conserved between O. nubilalis and B. mori with few exceptions (Figure 1). We designated the same chromosome numbers, 1–31 excluding 11 and 30, as those of orthologous chromosomes in previously characterized species of n=31 karyotype (Baxter et al., 2011; Sahara et al., 2013; Van't Hof et al., 2013).
Figure 1.
Comparison of orthologues between individual chromosomes of O. nubilalis and B. mori. 4',6-diamidino-2-phenylindole-stained images (gray pseudo color) of individual pachytene bivalents of O. nubilalis (blue chromosome numbers) show hybridization signals from O. nubilalis BAC probes. Black vertical bars represent orthologous B. mori chromosomes (italic black numbers) based on locations taken from Kaikobase (http://sgp.dna.affrc.go.jp/KAIKObase/). O. nubilalis BAC designations are shown on the right of each chromosome image. Gray letters indicate intrachromosomal rearrangement of localized genes and lack of conserved gene order between O. nubilalis and B. mori. Colored lines show correspondence of orthologues between B. mori and O. nubilalis based on the carrier BAC signals. Note that images of O. nubilalis chromosomes do not reflect true relative sizes because they were taken from different preparations.
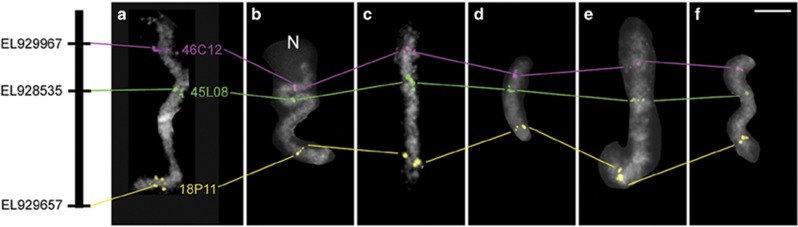
Mating experiments between species or populations belonging to the genus Ostrinia have been performed to map traits that might drive speciation by changes in behavior and pheromone composition (Dopman et al., 2005; Streiff et al., 2014; Wadsworth et al., 2015). However, genetic recombination in regions of interest was reported to be suppressed, presumably because of an inversion occurring on the Z chromosome of O. nubilalis (Wadsworth et al., 2015). Suppression of genetic recombination makes linkage analysis difficult; and therefore, we investigated the possibility of performing cross-species FISH analysis which is able to detect chromosome rearrangements peculiar to species or populations. We used four Ostrinia species (O. scapulalis, O. furnacalis, O. latipennis and O. palustralis), as well as P. ruralis, which belongs to the same subfamily, Pyraustinae. Of the four Ostrinia moths, O. scapulalis is most closely related and O. latipennis and O. palustralis are most distantly related to O. nubilalis (Kim et al., 1999). As shown in Figure 2, using three common O. nubilalis BAC probes previously located on chromosome 5, we obtained clear signals in the same order on a single chromosome of these five species.
Figure 2.
Conserved gene order on chromosome 5 of five Ostrinia species and a close relative based on cross-hybridization of three representative BACs from O. nubilalis. (a) O. nubilalis; (b) O. furnacalis; (c) O. scapulalis; (d) O. latipennis; (e) O. palustralis; and (f) P. ruralis. Signals from BAC probes 46C12, 45L08 and 18P11, which carry orthologues EL92996, EL928535 and EL929657, are pseudocolored with magenta, green and yellow, respectively. The black bar represents B. mori chromosome 5. N, nucleolus. Scale bar, 5 μm.
Sequencing of the O. furnacalis genome
As described above, we did not have any BAC probes ostensibly located on chromosomes 11 and 30. For BAC isolation, we had utilized sequences of known genes and expressed sequence tag in public databases or newly determined sequences of PCR fragments amplified by nested degenerate primers designed from conserved amino acid sequences (Yasukochi et al., 2011b). However, because genes expressed in limited stages and tissues are rarely included in expressed sequence tag, it was very difficult to find an adequate number of conserved genes on such narrow chromosomal regions having no markers. In addition, cDNA sequences lack sequences for introns, which makes it difficult to design PCR primers and to judge whether amplified fragments are genuine or non-specific.
Thus, we decided to obtain genome sequences of an Ostrinia species, which would greatly facilitate finding targeted genes and establishing genetic markers in targeted locations. Because O. nubilalis does not occur in Japan, strict escape prevention measures are required for rearing it. Consequently, we used a local congener, O. furnacalis, as a substitute. We sequenced a paired-end library (average insert size 196 bp) constructed from an O. furnacalis male pupa and obtained 511 Mb assembled sequences comprising 745 531 scaffolds and singleton contigs (N50=930 bp) (Supplementary Table S3).
Screening of O. furnacalis orthologues
Supplementary Table S4 lists lepidopteran genes that were previously found to be located near the regions orthologous to the boundaries between ancient chromosomes in B. mori when we embarked on the second-phase library screening aimed at these regions (Beldade et al., 2009; Yasukochi et al., 2009; Van't Hof et al., 2013; Sahara et al., 2013). The boundaries were predicted to be located at position 4 006 128–6 271 907 of chromosome 11, 17 071 002–17 410 092 of chromosome 23, and 8 719 098–11 527 820 of chromosome 24 in Kaikobase. Subsequently, we obtained full-length cDNAs, gene models and known genes that were predicted to localize in these regions from Kaikobase. In addition, we selected several B. mori sequences located at position 1–4 006 128 of chromosome 11 and position 17 410 092–23 133 604 of chromosome 23, which correspond to unidentified O. nubilalis chromosomes 11 and 30.
Candidate regions of ancient chromosomal fusions were located within single scaffolds, Bm_scaf59 and Bm_scaf12, of B. mori chromosomes 11 and 23. In contrast, the candidate region in B. mori chromosome 24 was separated into three scaffolds. Furthermore, we could not find any conserved genes in the middle scaffold, Bm_scaf147 (position 9 817 336–10 242 811), because no known genes or full-length cDNAs were located there, and gene models and transcripts assigned to the scaffold did not show significant similarities to available O. furnacalis genome sequences. Ultimately, we utilized 43 B. mori sequences as queries for a tblastn search against the O. furnacalis genome sequences. Thirty of the query sequences showed striking similarities to O. furnacalis scaffolds and contigs containing well-conserved single-copy genes (Table 1).
Table 1. Summary of the results onbtained by FISH and genetic analysis in this study.
| B. mori |
Accession No. of O. furnacalis orthologues | O. nubilalis Chr. |
O. nubilalis BAC | O. furnacalis fosmid | |||||
|---|---|---|---|---|---|---|---|---|---|
| Gene | Chr. | Bm_scaf | Location in Kaikobase | BAC | Genotyping | FISH | |||
| NM_001043856.1 | 11 | 35 | 2426352-2420617 | LC002981 | 11 | 11 | 32O01 | ||
| BGIBMGA001828 | 11 | 35 | 4003867-4006125 | LC002982 | 11 | 49B19 | |||
| AK383118 | 11 | 59 | 4988171-4987961 | LC002983 | 23 | 23 | 02B23 | ||
| AK385284 | 11 | 59 | 5050845-5051092 | LC002984 | 11 | ||||
| AK378226 | 11 | 59 | 5110274-5114967 | LC002985 | 11 | 11 | 08O23 | ||
| NM_001173138.1 | 11 | 59 | 5302237-5342471 | LC002986 | 11 | 03G23 | |||
| AK385912 | 11 | 59 | 5436505-5445770 | LC002987 | 11 | 13G13 | |||
| AK378774 | 11 | 59 | 5455029-5460410 | LC002988 | 11 | ||||
| BGIBMGA011986 | 11 | 59 | 5474195-5467027 | LC002989 | 11 | 07M24 | |||
| BGIBMGA011911 | 11 | 59 | 5565779-5572726 | LC002990 | 29 | ||||
| AK378190 | 11 | 59 | 5748073-5732931 | LC002991 | 29 | 29 | 02G09 | ||
| AK384105 | 11 | 59 | 5778613-5759910 | LC002992 | 29 | ||||
| AK381196 | 11 | 59 | 6133070-6140746 | LC002993 | 29 | 32C13 | |||
| NM_001043886.1 | 23 | 12 | 17064070-17064217 | LC002994 | 23 | 23 | 03N16 | 35A13 | |
| AK378672 | 23 | 12 | 17155881-17150565 | LC002995 | 23 | ||||
| AK383286 | 23 | 12 | 17195618-17248107 | LC002996 | 30 | 76F08 | |||
| AK385089 | 23 | 12 | 17315309-17309972 | LC002997 | 30 | 09P08 | |||
| AK378640 | 23 | 12 | 17328996-17334405 | LC002998 | 30 | ||||
| AK384547 | 23 | 12 | 17408923-17419988 | LC002999 | 63L08 | ||||
| AK386411 | 23 | 12 | 17506663-17510276 | LC003000 | 30 | 39I14 | |||
| NM_001043790.1 | 23 | 12 | 21055045-21060438 | LC003001 | 30 | 14J14 | |||
| NM_001135885.1 | 24 | 43 | 7589049-7590339 | LC003002 | 31 | 01J24 | |||
| NM_001173167.1 | 24 | 43 | 9082682-9097385 | LC003003 | 31 | 04D21 | |||
| AK378694 | 24 | 43 | 9124973-9128620 | LC003004 | 31 | 26P11 | |||
| AK386872 | 24 | 43 | 9232080-9237493 | 17J12F | LC003005 | 31 | 06H23 | ||
| AK385865 | 24 | 103 | 10769257-10780986 | 19D05C | LC003006 | 24 | 24 | 36F10 | |
| AK377444 | 24 | 103 | 10967433-10954881 | LC003007 | 24 | 24 | 09N16 | ||
| AK380262 | 24 | 103 | 11063900-11060287 | LC003008 | 24 | ||||
| NM_001046732.1 | 24 | 103 | 11454798-11469070 | LC003009 | 24 | 04O02 | |||
| AK385042 | 24 | 208 | 12462114-12471476 | LC003010 | 24 | 18O24 | |||
| NM_001043588.1 | 24 | not mapped in Kaikobase | 04L10D | ||||||
| BP125410 | 24 | 75 | 17956279-17955584 | 04A07H | |||||
Abbreviations: BAC, bacterial artificial chromosome; FISH, fluorescence in situ hybridization.
Screening of O. nubilalis BAC and O. furnacalis fosmid libraries
We then designed 30 pairs of PCR primers to isolate genomic clones containing candidate genes (Supplementary Table S1). In general, BACs are preferable as FISH probes compared with fosmids, presumably because longer inserts can label longer chromosomal regions more stably. In addition, the chromosome preparations used for this analysis were derived from O. nubilalis, which might require longer regions to form stable hybridization signals with the heterologous O. furnacalis probes.
Thus, we first screened the O. nubilalis BAC library, followed by screening of a newly constructed fosmid library from O. furnacalis pupae from which we obtained 75 264 colonies (average insert size, 33.1 kb; total estimate size, 2491 Mb). Consequently, we isolated 10 BACs (Table 1) from the O. nubilalis BAC library, and then isolated 14 fosmids (Table 1) by screening the O. furnacalis fosmid library with markers that had failed to isolate positive O. nubilalis BACs.
FISH and genetic analysis
We performed FISH analysis against O. nubilalis chromosomes using the 24 BAC and fosmid probes described above. In parallel with the FISH analysis, we performed genetic analysis of the candidate genes using 24 BF1 progeny between O. nubilalis and O. scapulalis to validate and complement the FISH results, because we could not isolate BACs or fosmids for six genes (Table 1).
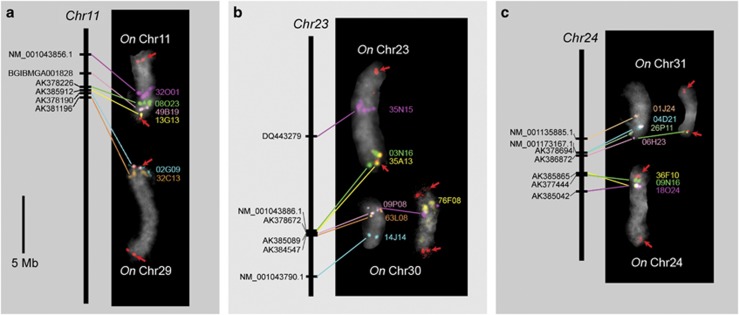
We obtained clear signals from five BACs and two fosmids containing Ostrinia orthologues of B. mori genes located on chromosome 11. Two BACs, 02G09 and 32C13, which had been assigned to previously identified O. nubilalis chromosome 29, and signals from two BACs (08O23, 32O01) and two fosmids (13G13, 49B19), were mapped onto the same chromosome (Figure 3a). Thus, we designated this newly identified chromosome as O. nubilalis chromosome 11. In addition, we obtained polymorphic PCR products from nine orthologues that were mapped onto O. nubilalis chromosomes 11 or 29, as expected (Table 1, Supplementary Table S5). Taking all these data together, the boundary between ancestral chromosomes 11 and 29 on B. mori chromosome 11 was narrowed to the region between gene models BGIBMGA011986 and BGIBMGA011911 (Table 1,Figure 4a).
Figure 3.
FISH detection of O. nubilalis chromosomes 11 and 29 (a), 23 and 30 (b) and 24 and 31 (c). Signals on the O. nubilalis chromosome (white numbers) are pseudocolored and probe names are shown to the right of the FISH images. See Table 1 for details. Arrows point to (TTAGG)n signals pseudocolored red. Note that left images of chromosomes 30 and 31 were not hybridized with (TTAGG)n probes. Left-side bars represent orthologous B. mori chromosomes (italic black numbers). Locations of B. mori orthologues are taken from Kaikobase. FISH images are from different individuals and/or preparations. Hence, O. nubilalis bivalent lengths vary depending on the pachytene stage.
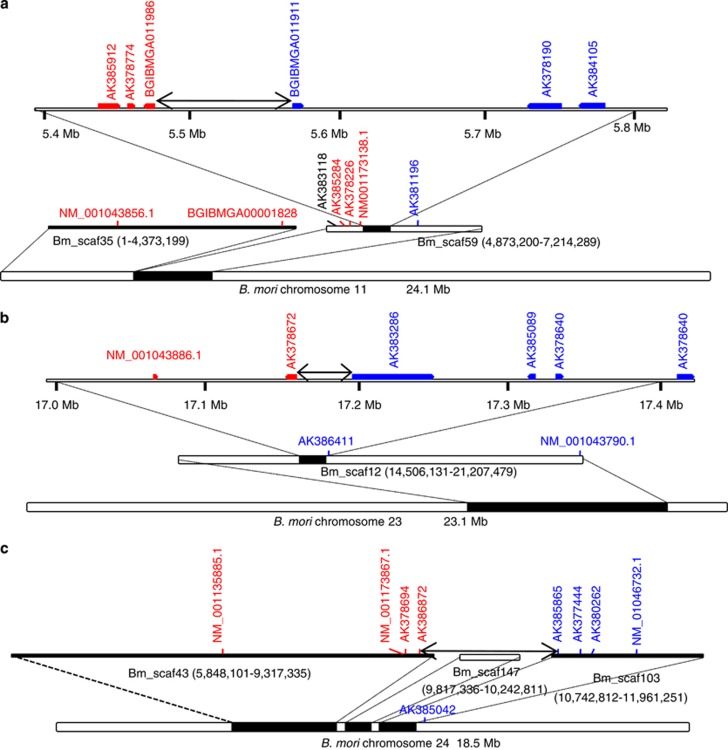
Figure 4.
Candidate regions of ancient chromosomal fusions in B. mori chromosomes 11 (a), 23 (b) and 24 (c). Candidate regions are shown as two-headed arrows. Vertical letters indicate B. mori genes for which orthologues are located on O. nubilalis chromosomes 11 (red letters, a), 23 (red letters, b), 24 (red letters, c), 29 (blue letters, a), 30 (blue letters, b) and 31 (blue letters, c). Letters in parentheses indicate locations of B. mori scaffolds in Kaikobase.
Unexpectedly, BAC 02B23, which contained an O. nubilalis orthologue (LC002983) of a B. mori full-length cDNA, AK383118, was localized on another chromosome, excluding it as belonging to O. nubilalis chromosome 11 or 29. Genetic analysis revealed that this O. nubilalis orthologue was located on O. nubilalis chromosome 23 (Table 1, Supplementary Table S5). However, there remained the possibility that AK383118 was incorrectly assembled into Bm_scaf59. Thus, we performed linkage mapping of AK383118 in B. mori and confirmed its location on B. mori chromosome 11 (data not shown). This was the only example for which we found evidence suggesting an ancient translocation.
Similarly, FISH analysis identified missing O. nubilalis chromosome 30 (Figure 3b) on B. mori chromosome 23, where the boundary between ancestral chromosomes 23 and 30 was estimated to be localized between full-length cDNAs AK378672 and AK383286 (Table 1, Figure 4b). Finally, O. nubilalis orthologues of B. mori genes on Bm_scaf43 and Bm_scaf103 were assigned to O. nubilalis chromosomes 31 and 24, respectively (Figures 3c and 4c).
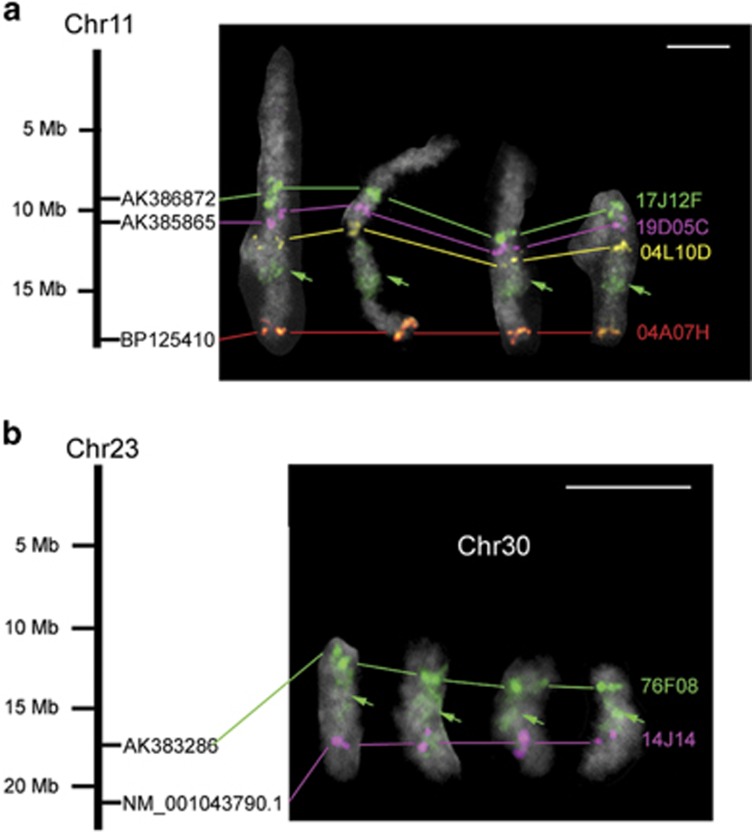
Intervals between B. mori scaffolds in Kaikobase are not experimentally confirmed but are designated arbitrarily as 500 kb (http://sgp.dna.affrc.go.jp/KAIKObase/). Hence, we suspected that the distance between Bm_scaf43 and Bm_scaf103 was much shorter than the estimated length of 1.5 Mb from the genome database. Conversely, assigning unmapped contigs located in this gap might enlarge the actual distance. Thus, we isolated B. mori BACs, 17J12F and 19D5C, containing the most distal full-length cDNAs of the scaffolds, AK386872 and AK385865, respectively (Table 1). FISH probing with these clones suggested that the interval between Bm_scaf43 and Bm_scaf103 was longer than 1 Mb, although the estimate varied depending on the state of chromosome condensation (Figure 5).
Figure 5.
(a) A sequence gap between Bm_scaf43 and Bm_scaf 103 of B. mori chromosome 11 revealed by BAC-FISH. Signals from 17J12F (green), 19D05C (magenta) 04L10D (yellow) and 04A07H (red) represent AK386872, AK385865, NM_001043588.1 and BP125410, respectively. Note that the green signals from 17J12F faintly stain the heterochromatic region (refer to Figure 2 in Yoshido et al. 2005) of B. mori chromosome 11 (green arrows). (b) Ectopic signals from an O. furnacalis fosmid (76F08) located near the end of chromosome 30 appear in O. nubilalis chromosome 30 (Chr30 shown in white letters). Two fosmids, 76F08 and 14J14, carrying representative single genes, LC002996 and LC003001, are orthologues of B. mori AK383286 and NM_001043790.1, respectively (see Table 1). Black bars represent B. mori chromosomes 11 and 23 (Chr11 and Chr23). Scale bars, 5 μm.
Intriguingly, the 17J12F probe also painted a segment of chromosome 24 (Figure 5a) which was positionally consistent with a previously described 4',6-diamidino-2-phenylindole-positive and presumably heterochromatic segment (Yoshido et al., 2005). Similar ectopic painting was also observed for Ostrinia BACs and fosmids putatively located near the ends of chromosomes (Figure 5b). A possible explanation is that these clones contain subtelomeric heterochromatin and tend to label heterochromatin scattered around chromosomes. If so, 17J12F may contain subtelomeric heterochromatin that originated from an ancient chromosomal end.
Comparison with the M. cinxia genome data
Judging from the results described above, we estimated the positions of boundaries between ancient chromosomes in B. mori chromosomes 11, 23 and 24 as 5 474 195–5 565 779, 17 155 881–17 195 618 and 9 237 493–10 769 257, respectively. In the proceeding work comparing B. mori and M. cinxia genomes, these boundaries were estimated to be 5 410 160–5 798 000 in chromosome 11, 17 180 240–17 247 200 in chromosome 23, and 9 953 070–10 742 570 in chromosome 24 (Ahola et al., 2014). Our estimates based on FISH and genetic analysis of O. nubilalis conserved genes is in good accordance with those based on M. cinxia genome sequences in spite of the distant phylogenetic relationships between the two species. Taken together, these data provide strong evidence to support the hypothesis that species having 31 chromosomes per haploid genome basically retain the ancestral karyotype of Lepidoptera.
Search for telomere-associated sequences
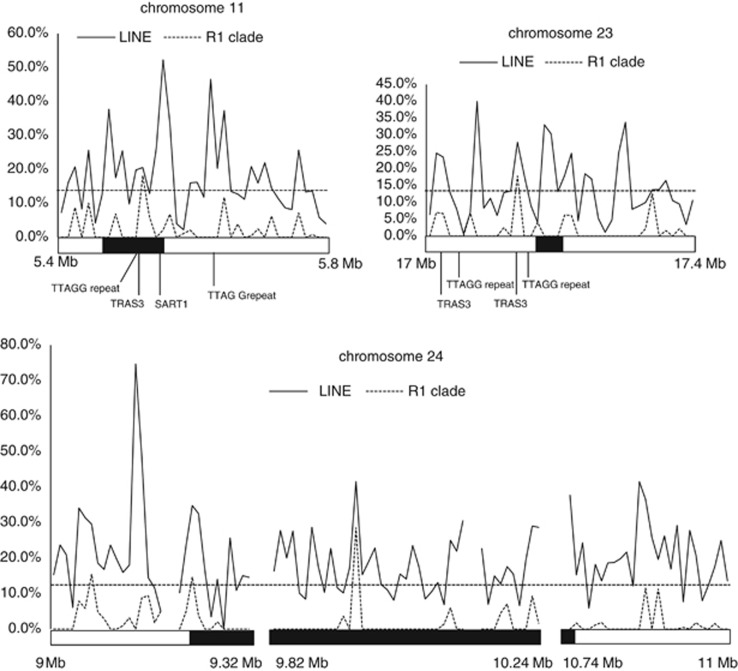
The telomeres of B. mori are composed of pentanucleotide telomeric repeats, (TTAGG)n, and several families of long interspersed elements (Okazaki et al., 1993). The TTAGG telomeric repeats are conserved among all arthropod groups, although lacking in spiders and some insect orders (Okazaki et al., 1993; Sahara et al., 1999; Vítková et al., 2005). Thus, we searched five B. mori scaffolds located on the boundaries of putative chromosomal fusions for TTAGG repeats. Many of the repeat-like sequences we found were short and incomplete (Supplementary Table S6), but a complete 28-mer repeat was located adjacent to a 117 bp-gap on Bm_scaf59 (chr. 11: position 5 933 800–5 933 773), raising the possibility that this repeat might be longer (Supplementary Table S6).
We also searched for long interspersed elements, especially the R1 clade which includes telomeric repeat-specific TRAS family and SART1 retrotransposons (Kubo et al., 2001; Osanai-Futahashi et al., 2008). The proportion of long interspersed elements at the boundary region in B. mori chromosome 11 was much higher than the estimated average of the whole genome, 13.8% (Osanai-Futahashi et al., 2008). In addition, we found retrotransposon-derived sequences similar to TRAS3 and SART1, as well as clear peaks in the proportion of R1 clade non-LTRs in this region (Figure 6). Intriguingly, a TTAGG repeat was neighboring the TRAS3-like sequence (Figure 6), which might be another vestigial feature of former subtelomeric heterochromatin.
Figure 6.
Distribution of long interspersed elements (LINEs) and telomeric repeats around the candidate regions of ancient chromosomal fusions. Genomic sequences with gaps were divided into 10-kb windows, and the ratio of the length of all (solid lines) or R1-clade (dotted lines) LINEs in total genomic sequence without gaps was calculated for each window. Filled squares indicate candidate regions. Horizontal dotted lines indicate 13.8%, average of LINE content in the B. mori genome (Osanai-Futahashi et al., 2008). Vertical lines indicate insertion sites of a TTAGG repeat and truncated copies of TTAGG repeat-specific retrotransposons, TRAS3 and SART1.
TTAGG repeats, TRAS3-like sequences and accumulation of the R1 clade long interspersed elements were detected not in the candidate region but in the surrounding region of the boundary in B. mori chromosome 23 (Figure 6). The region between the signals of telomeric repeats and 76F08 on O. nubilalis chromosome 23 was relatively long compared with other chromosomal ends (Figures 3b and 5b), which might mean that internal deletion and translocation of the boundary region occurred after the fusion event generating B. mori chromosome 23. Taken together, we speculate that the fusion event generating B. mori chromosome 23 occurred before those generating chromosome 11.
In contrast, neither telomeric repeat-specific retrotransposons nor TTAGG repeats were found within or around the boundary in B. mori chromosome 24 (Figure 6). As described above, the maximum estimate of the boundary between ancient chromosomes is much longer in B. mori chromosome 24 (1532 kb) compared with the estimates for chromosomes 11 (91.6 kb) and 23 (39.7 kb) (Figure 4). As many long gaps in the sequence assembly still remain, including two inter-scaffold ones in this region, we were unable to exclude the possibility that sequences harboring telomeric features are located in these gaps.
Further analysis is needed to understand sequential changes that have occurred in the former chromosomal ends after fusion events. Significant stability of lepidopteran chromosomes enabled us to distinguish which portion fused chromosomes were derived from. Diverged clades with a reduced and variable chromosome number are adequate for this purpose because many boundaries at different temporal stages are available and the chronological order of fusion events can be determined to some extent by detection of shared fused chromosomes among closely or distantly related species. For instance, we previously compared subspecies of a wild silkmoth, Samia cynthia (2n=25–28), with B. mori, and identified more than 15 boundaries between ancectral chromosomes excluding 24 and 30 (Yoshido et al., 2011).
Current knowledge about shared gene order in Lepidoptera is limited to the clade Ditrysia, which constitutes 98% of lepidopteran species (Regier et al., 2013), and little is known about karyotype evolution in more primitive groups. Additionally, Trichoptera is not only most closely related with Lepidoptera among extant insect orders (Wiegmann et al., 2009), but also shares unique features such as a sex determination system with female heterogamety and holocentric chromosomes. Analysis of basal non-ditrysian species and Trichoptera is critical to reveal the origin of the n=31 karyotype and assess the stability of lepidopteran chromosomes.
Conclusion
To establish new markers for a FISH-based chromosome map of O. nubilalis by efficient screening of Ostrinia orthologues of B. mori genes located on targeted chromosomal regions lacking markers, we determined and assembled 511 Mb genome sequence and constructed a fosmid library of O. furnacalis. Consequently, we constructed a cytogenetic map of O. nubilalis consisting of 122 BAC and eight fosmid probes covering all 31 chromosomes. This map can be applied to other Ostrinia moths and related species, and will be useful for the detection of local chromosomal rearrangements that have occurred uniquely in particular species where genetic recombination is suppressed. The targeted selection of BAC and fosmid clones reported here is especially effective for rapid determination of extensive genomic sequences or genome-wide characterization of multiple-copy genes in organisms lacking genome reference sequences.
We estimated the boundaries between ancient chromosomes on B. mori chromosomes 11, 23 and 24 by mapping O. nubilalis orthologues, which agreed well with a previous estimation based on sequence comparison between B. mori and M. cinxia. These findings strongly suggest that lepidopteran species having 31 chromosomes per haploid genome basically retain the ancestral karyotype of Lepidoptera.
Data archiving
Sequence data listed in Table 1 have been submitted to the DDBJ/EMBL/GenBank databases under the accession numbers LC002981–LC003010. Sequence scaffolds and singleton contigs used in this study are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.q2k15.
Acknowledgments
We are grateful to Dr M. R. Goldsmith for critical reading of the manuscript. We thank Aya Kawai and Noriko Fujikawa-Kojima for their technical assistance in FISH analysis. A part of this study is financially supported by the Program for Promotion of Basic Research Activities for Innovative Biosciences (PROBRAIN) and grants 22380040, 23248008, 23380030, 25292204 of Japan Society for the Promotion of Science (JSPS).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Heredity website (http://www.nature.com/hdy)
Supplementary Material
References
- Ahola V, Lehtonen R, Somervuo P, Salmela L, Koskinen P, Rastas P et al. (2014). The Glanville fritillary genome retains an ancient karyotype and reveals selective chromosomal fusions in Lepidoptera. Nat Commun 5: 4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alföldi J, Di Palma F, Grabherr M, Williams C, Kong L, Russell EP et al. (2011). The genome of the green anole lizard and a comparative analysis with birds and mammals. Nature 477: 587–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter SW, Davey JW, Johnston JS, Shelton AM, Heckel DG, Jiggins CD et al. (2011). Linkage mapping and comparative genomics using next-generation RAD sequencing of a non-model organism. PLoS ONE 6: e19315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beldade P, Saenko SV, Pul N, Long AD. (2009). A gene-based linkage map for Bicyclus anynana butterflies allows for a comprehensive analysis of synteny with the lepidopteran reference genome. PLoS Genet 5: e1000366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B. (2014). Trimmomatic: A flexible trimmer for Illumina Sequence Data. Bioinformatics 30: 2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopman EB, Bogdanowicz SM, Harrison RG. (2004). Genetic mapping of sexual isolation between E and Z pheromone strains of the European corn borer (Ostrinia nubilalis. Genetics 167: 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopman EB, Pérez L, Bogdanowicz SM, Harrison RG. (2005). Consequences of reproductive barriers for genealogical discordance in the European corn borer. Proc Natl Acad Sci USA 102: 14706–14711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heliconius Genome Consortium. (2012). Butterfly genome reveals promiscuous exchange of mimicry adaptations among species. Nature 487: 94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Silkworm Genome Consortium. (2008). The genome of a lepidopteran model insect, the silkworm Bombyx mori. Insect Biochem Mol Biol 38: 1036–1045. [DOI] [PubMed] [Google Scholar]
- Kemkemer C, Kohn M, Cooper DN, Froenicke L, Högel J, Hameister H et al. (2009). Gene synteny comparisons between different vertebrates provide new insights into breakage and fusion events during mammalian karyotype evolution. BMC Evol Biol 9: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CG, Hoshizaki S, Huang YP, Tatsuki S, Ishikawa Y. (1999). Usefulness of mitochondrial COII gene sequences in examining phylogenetic relationships in the Asian corn borer, Ostrinia furnacalis, and allied species (Lepidoptera: Pyralidae). Appl Entomol Zool 34: 405–412. [Google Scholar]
- Kubo Y, Okazaki S, Anzai T, Fujiwara H. (2001). Structural and phylogenetic analysis of TRAS, telomeric repeat-specific retrotransposon family in lepidopteran insects. Mol Biol Evol 18: 848–857. [DOI] [PubMed] [Google Scholar]
- Lassance JM. (2010). Journey in the Ostrinia world: From pest to model in chemical ecology. J Chem Ecol 36: 1155–1169. [DOI] [PubMed] [Google Scholar]
- Luo R, Liu B, Xie Y, Li Z, Huang W, Yuan J et al. (2012). SOAPdenovo2: an empirically improved memory-efficient short-read de novo assembler. Gigascience 1: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray MG, Thompson WF. (1980). Rapid isolation of high molecular weight plant DNA. Nucl Acids Res 8: 4321–4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nene V, Wortman JR, Lawson D, Haas B, Kodira C, Tu ZJ et al. (2007). Genome sequence of Aedes aegypti, a major arbovirus vector. Science 316: 1718–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki S, Ishikawa H, Fujiwara H. (1995). Structural analysis of TRAS1, a novel family of telomeric repeat-associated retroposons in the silkworm Bombyx mori. Mol Cell Biol 15: 4545–4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki S, Tsuchida K, Maekawa H, Ishikawa H, Fujiwara H. (1993). Ideintfication of a pentanucleotide telomeric sequence, (TTAGG)n, in the silkworm, Bombyx mori and other insects. Mol Cell Biol 13: 1424–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osanai-Futahashi M, Suetsugu Y, Mita K, Fujiwara H. (2008). Genome-wide screening and characterization of transposable elements and their distribution analysis in the silkworm Bombyx mori. Insect Biochem Mol Biol 38: 1046–1057. [DOI] [PubMed] [Google Scholar]
- Pokorná M, Giovannotti M, Kratochvíl L, Caputo V, Olmo E, Ferguson-Smith MA et al. (2012). Conservation of chromosomes syntenic with avian autosomes in squamate reptiles revealed by comparative chromosome painting. Chromosoma 121: 409–418. [DOI] [PubMed] [Google Scholar]
- Pringle EG, Baxter SW, Webster CL, Papanicolaou A, Lee SF, Jiggins CD. (2007). Synteny and chromosome evolution in the Lepidoptera: Evidence from mapping in Heliconius melpomene. Genetics 177: 417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier JC, Mitter C, Zwick A, Bazinet AL, Cummings MP, Kawahara AY et al. (2013). A large-scale, higher-level, molecular phylogenetic study of the insect order Lepidoptera (moths and butterflies). PLoS ONE 8: e58568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson R. (1971) Lepidoptera Genetics. Pergamon: Oxford. [Google Scholar]
- Sahara K, Marec F, Traut W. (1999). TTAGG telomeric repeats in chromosomes of some insects and other arthropods. Chromosome Res 7: 449–460. [DOI] [PubMed] [Google Scholar]
- Sahara K, Yoshido A, Shibata F, Fujikawa N, Okabe T, Tanaka-Okuyama M et al. (2013). FISH identification of Helicoverpa armigera and Mamestra brassicae chromosomes by BAC and fosmid probes. Insect Biochem Mol Biol 43: 644–653. [DOI] [PubMed] [Google Scholar]
- Shibata F, Sahara K, Naito Y, Yasukochi Y. (2009). Reprobing of multicolour FISH in preparations of lepidopteran chromosomes. Zool Sci 26: 187–190. [DOI] [PubMed] [Google Scholar]
- Shimomura M, Minami H, Suetsugu Y, Ohyanagi H, Satoh C, Antonio B et al. (2009). KAIKObase: an integrated silkworm genome database and data mining tool. BMC Genomics 10: 486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit AFA, Hubley R, Green P. (2013–2015) . RepeatMasker Open-4.0.5 http://www.repeatmasker.org.
- Streiff R, Courtois B, Meusnier S, Bourguet D. (2014). Genetic mapping of two components of reproductive isolation between two sibling species of moths, Ostrinia nubilalis and O. scapulalis. Heredity 112: 370–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van't Hof AE, Nguyen P, Dalíková M, Edmonds N, Marec F, Saccheri IJ. (2013). Linkage map of the peppered moth, Biston betularia (Lepidoptera, Geometridae): a model of industrial melanism. Heredity 110: 283–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vítková M, Král J, Traut W, Zrzavý J, Marec F. (2005). The evolutionary origin of insect telomeric repeats. (TTAGG)n. Chromosome Res 13: 145–156. [DOI] [PubMed] [Google Scholar]
- Wadsworth CB, Li X, Dopman EB. (2015). A recombination suppressor contributes to ecological speciation in OSTRINIA moths. Heredity 114: 593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegmann BM, Trautwein MD, Kim JW, Cassel BK, Bertone MA, Winterton SL et al. (2009). Single-copy nuclear genes resolve the phylogeny of the holometabolous insects. BMC Biol 7: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Asakawa S, Shimizu N, Kawasaki S, Yasukochi Y. (1999). Construction and characterization of bacterial artificial chromosome libraries from the silkworm Bombyx mori. Mol Gen Genet 261: 698–706. [DOI] [PubMed] [Google Scholar]
- Yasukochi Y, Ashakumary L, Baba K, Yoshido A, Sahara K. (2006). A second generation integrated map of the silkworm reveals synteny and conserved gene order between lepidopteran insects. Genetics 173: 1319–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasukochi Y, Miura N, Nakano R, Sahara K, Ishikawa Y. (2011. a). Sex-linked pheromone receptor genes of the European corn borer, Ostrinia nubilalis, are in tandem arrays. PLoS ONE 6: e18843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasukochi Y, Tanaka-Okuyama M, Kamimura M, Nakano R, Naito Y, Ishikawa Y et al. (2011. b). Isolation of BAC clones containing conserved genes from libraries of three distantly related moths: a useful resource for comparative genomics of Lepidoptera. J Biomed Biotechnol 2011: 165894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasukochi Y, Tanaka-Okuyama M, Shibata F, Yoshido A, Marec F, Wu C et al. (2009). Extensive conserved synteny of genes between the karyotypes of Manduca sexta and Bombyx mori revealed by BAC-FISH mapping. PLoS ONE 4: e7465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshido A, Bando H, Yasukochi Y, Sahara K. (2005). The Bombyx mori karyotype and the assignment of linkage groups. Genetics 170: 675–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshido A, Sahara K, Yasukochi Y. (2014) Silk Moths (Lepidoptera). In: Sharakhov IV (ed)Protocols for Cytogenetic Mapping of Arthropod Genomes. CRC Press:: Boca Ranton, FL, USA, pp 219–256. [Google Scholar]
- Yoshido A, Yasukochi Y, Sahara K. (2011). Samia cynthia versus Bombyx mori: comparative gene mapping between a species with a low-number karyotype and the model species of Lepidoptera. Insect Biochem Mol Biol 41: 370–377. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.