Abstract
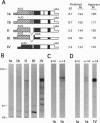
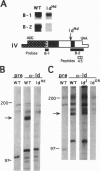
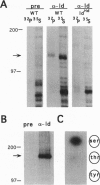
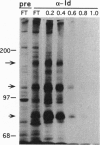
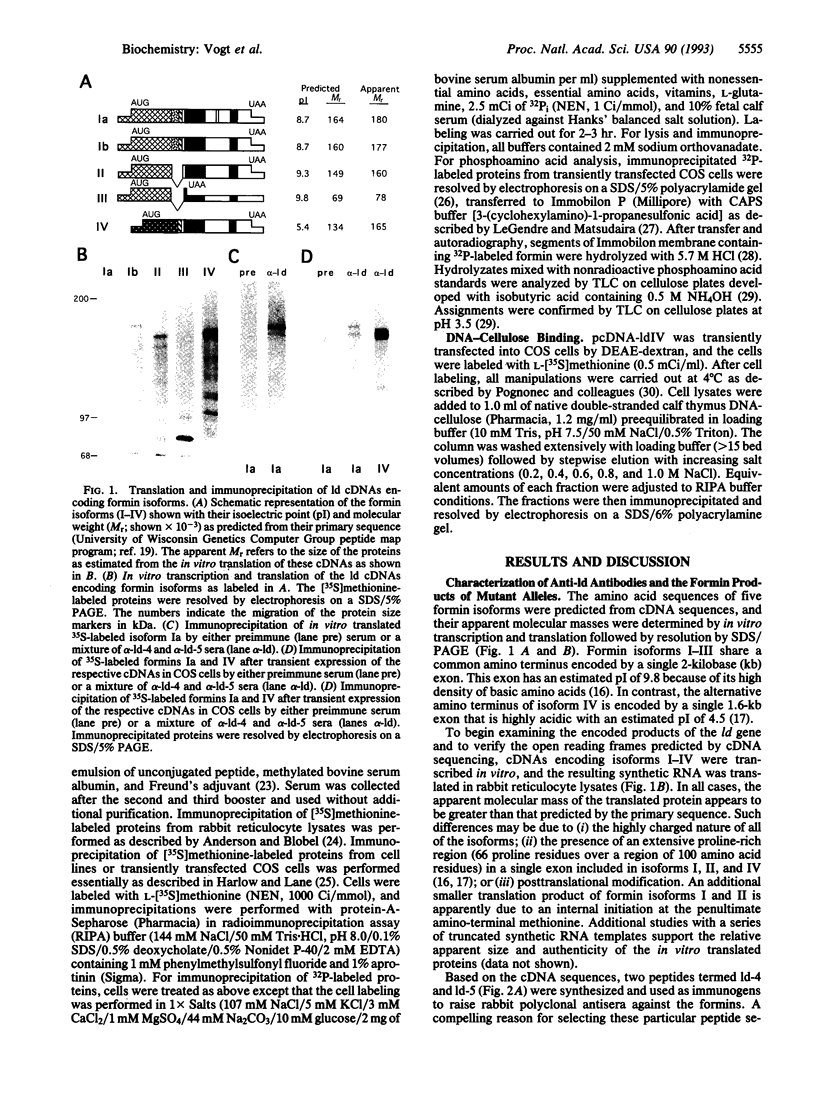
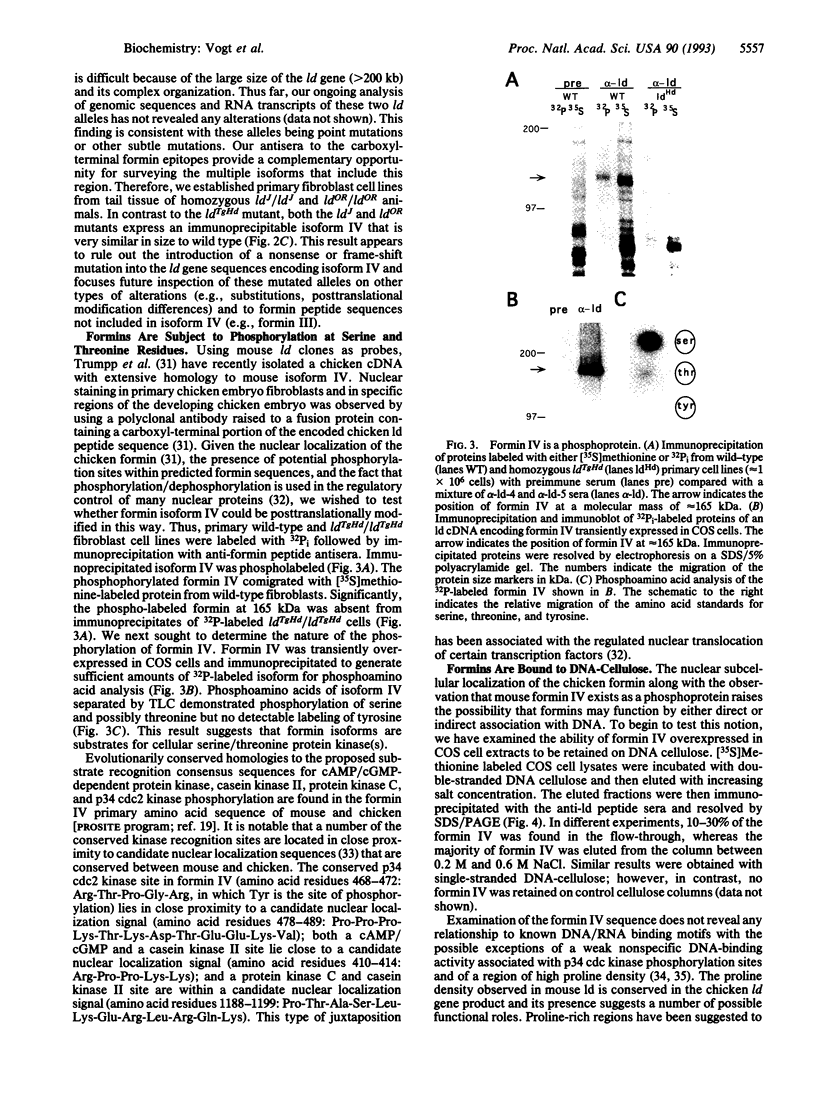
Mutations at the mouse limb deformity (ld) locus result in defects of growth and patterning of the limb and kidney during embryonic development. The gene responsible for this phenotype is large and complex, with the capacity to generate a number of alternatively spliced messenger RNA transcripts encoding nuclear protein isoforms called "formins." We have made polyclonal antibodies to specific formin peptides and have confirmed the authenticity of the antibodies' reactivity, using cell lines derived from mice with molecularly defined mutations at the ld locus. In addition, we have used these antibodies to detect and characterize polypeptides encoded by both wild-type and mutant ld alleles. In so doing, we show that a formin isoform (i) is modified by posttranslational phosphorylation at serine and threonine residues and (ii) when present in a crude nuclear extract, is retained by DNA-cellulose.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. J., Blobel G. Immunoprecipitation of proteins from cell-free translations. Methods Enzymol. 1983;96:111–120. doi: 10.1016/s0076-6879(83)96012-3. [DOI] [PubMed] [Google Scholar]
- Brockes J. P. Retinoids, homeobox genes, and limb morphogenesis. Neuron. 1989 Apr;2(4):1285–1294. doi: 10.1016/0896-6273(89)90066-4. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Churchill M. E., Suzuki M. 'SPKK' motifs prefer to bind to DNA at A/T-rich sites. EMBO J. 1989 Dec 20;8(13):4189–4195. doi: 10.1002/j.1460-2075.1989.tb08604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Coutu M. D., Craig S. W. cDNA-derived sequence of chicken embryo vinculin. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8535–8539. doi: 10.1073/pnas.85.22.8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Karin M. The regulation of transcription by phosphorylation. Cell. 1992 Aug 7;70(3):375–387. doi: 10.1016/0092-8674(92)90162-6. [DOI] [PubMed] [Google Scholar]
- Jackson-Grusby L., Kuo A., Leder P. A variant limb deformity transcript expressed in the embryonic mouse limb defines a novel formin. Genes Dev. 1992 Jan;6(1):29–37. doi: 10.1101/gad.6.1.29. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Sefton B. M. Acid and base hydrolysis of phosphoproteins bound to immobilon facilitates analysis of phosphoamino acids in gel-fractionated proteins. Anal Biochem. 1989 Jan;176(1):22–27. doi: 10.1016/0003-2697(89)90266-2. [DOI] [PubMed] [Google Scholar]
- Kleinebrecht J., Selow J., Winkler W. The mouse mutant limb-deformity (ld). Anat Anz. 1982;152(4):313–324. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maini P. K., Solursh M. Cellular mechanisms of pattern formation in the developing limb. Int Rev Cytol. 1991;129:91–133. doi: 10.1016/s0074-7696(08)60510-0. [DOI] [PubMed] [Google Scholar]
- Mermod N., O'Neill E. A., Kelly T. J., Tjian R. The proline-rich transcriptional activator of CTF/NF-I is distinct from the replication and DNA binding domain. Cell. 1989 Aug 25;58(4):741–753. doi: 10.1016/0092-8674(89)90108-6. [DOI] [PubMed] [Google Scholar]
- Pognonec P., Boulukos K. E., Ghysdael J. The c-ets-1 protein is chromatin associated and binds to DNA in vitro. Oncogene. 1989 Jun;4(6):691–697. [PubMed] [Google Scholar]
- Reith A. D., Bernstein A. Molecular basis of mouse developmental mutants. Genes Dev. 1991 Jul;5(7):1115–1123. doi: 10.1101/gad.5.7.1115. [DOI] [PubMed] [Google Scholar]
- Ren R., Mayer B. J., Cicchetti P., Baltimore D. Identification of a ten-amino acid proline-rich SH3 binding site. Science. 1993 Feb 19;259(5098):1157–1161. doi: 10.1126/science.8438166. [DOI] [PubMed] [Google Scholar]
- Seed B., Aruffo A. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc Natl Acad Sci U S A. 1987 May;84(10):3365–3369. doi: 10.1073/pnas.84.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth A., Alvarez E., Gupta S., Davis R. J. A phosphorylation site located in the NH2-terminal domain of c-Myc increases transactivation of gene expression. J Biol Chem. 1991 Dec 15;266(35):23521–23524. [PubMed] [Google Scholar]
- Silver P. A. How proteins enter the nucleus. Cell. 1991 Feb 8;64(3):489–497. doi: 10.1016/0092-8674(91)90233-o. [DOI] [PubMed] [Google Scholar]
- Tabin C. J. Retinoids, homeoboxes, and growth factors: toward molecular models for limb development. Cell. 1991 Jul 26;66(2):199–217. doi: 10.1016/0092-8674(91)90612-3. [DOI] [PubMed] [Google Scholar]
- Tickle C. Retinoic acid and chick limb bud development. Dev Suppl. 1991;1:113–121. [PubMed] [Google Scholar]
- Trumpp A., Blundell P. A., de la Pompa J. L., Zeller R. The chicken limb deformity gene encodes nuclear proteins expressed in specific cell types during morphogenesis. Genes Dev. 1992 Jan;6(1):14–28. doi: 10.1101/gad.6.1.14. [DOI] [PubMed] [Google Scholar]
- Vogt T. F., Jackson-Grusby L., Wynshaw-Boris A. J., Chan D. C., Leder P. The same genomic region is disrupted in two transgene-induced limb deformity alleles. Mamm Genome. 1992;3(8):431–437. doi: 10.1007/BF00356152. [DOI] [PubMed] [Google Scholar]
- Woychik R. P., Generoso W. M., Russell L. B., Cain K. T., Cacheiro N. L., Bultman S. J., Selby P. B., Dickinson M. E., Hogan B. L., Rutledge J. C. Molecular and genetic characterization of a radiation-induced structural rearrangement in mouse chromosome 2 causing mutations at the limb deformity and agouti loci. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2588–2592. doi: 10.1073/pnas.87.7.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woychik R. P., Maas R. L., Zeller R., Vogt T. F., Leder P. 'Formins': proteins deduced from the alternative transcripts of the limb deformity gene. Nature. 1990 Aug 30;346(6287):850–853. doi: 10.1038/346850a0. [DOI] [PubMed] [Google Scholar]
- Woychik R. P., Maas R. L., Zeller R., Vogt T. F., Leder P. 'Formins': proteins deduced from the alternative transcripts of the limb deformity gene. Nature. 1990 Aug 30;346(6287):850–853. doi: 10.1038/346850a0. [DOI] [PubMed] [Google Scholar]
- Woychik R. P., Stewart T. A., Davis L. G., D'Eustachio P., Leder P. An inherited limb deformity created by insertional mutagenesis in a transgenic mouse. Nature. 1985 Nov 7;318(6041):36–40. doi: 10.1038/318036a0. [DOI] [PubMed] [Google Scholar]
- Zeller R., Jackson-Grusby L., Leder P. The limb deformity gene is required for apical ectodermal ridge differentiation and anteroposterior limb pattern formation. Genes Dev. 1989 Oct;3(10):1481–1492. doi: 10.1101/gad.3.10.1481. [DOI] [PubMed] [Google Scholar]