Abstract
Cryptococcus neoformans can directly infect the vocal cords. Endoscopic findings were undistinctive from most infiltrative diseases. Tissue biopsy was essential for the diagnosis. Inhaled corticosteroids can predispose to the infection, and fluconazole 400 mg daily for at least 6 weeks appeared to be minimal to achieve a permanent cure.
Keywords: Cryptococcus, fluconazole, fungal infection, larynx, vocal cords
Infections caused by Cryptococcus neoformans occur mainly in immunocompromised hosts and most commonly present either as primary lower respiratory infections or as secondary disseminated processes [1]. Cryptococcus neoformans has been associated with exposure to pigeon excrements [2]. Viable airborne particles reach the respiratory tract and cause a primary focus of infection in the lungs. Cryptococcal pneumonia may remain confined to the lungs but may also disseminate to distant organs and tissues, most frequently the central nervous system (CNS). A deficit in host cellular immunity increases the risk of developing a cryptococcal infection [1]. Cryptococcus neoformans possesses certain virulence factors (such as the production of a large polysaccharide capsule and the accumulation of melanin pigment in the cell wall) to bypass host defenses.
Lesions associated with proven Cryptococcus infection of the larynx have been reported occasionally [3–16]. The nonspecific clinical findings can evoke a wide range of alternative diagnoses thus leading to a delay in appropriate treatment.
We describe the distinctive findings pertaining to laryngeal cryptococcal infection along with a well documented case of vocal cord infection and a review of the recent literature.
CASE REPORT
A 78-year-old woman was referred for persistent hoarseness evolving over the previous month. She was chronically treated with inhaled budesonide for mild asthma. Her medications included warfarin for chronic atrial fibrillation and pantoprazole for gastroesophageal reflux. She had no smoking history or risk factors for human immunodeficiency virus (HIV). No exposure to pigeon feces was reported, but the patient was an active gardener.
The first flexible laryngoscopy examination revealed bilateral whitish vocal cord lesions compatible with the diagnosis of fungal laryngitis. There was no clinical response to a 3-week course of oral fluconazole (100 mg daily). Subsequent endoscopic examination showed irregular vocal cords with erythroplakia—an erythematous area on the mucous membrane. A right vocal cord biopsy demonstrated nonspecific, chronic inflammation with hyperplasia and yeast-like structures beneath the hyperkeratosis. A second 3-week course of oral fluconazole (100 mg daily) was administered with a good clinical response, based on the clinical reassessment of the next follow-up visit.
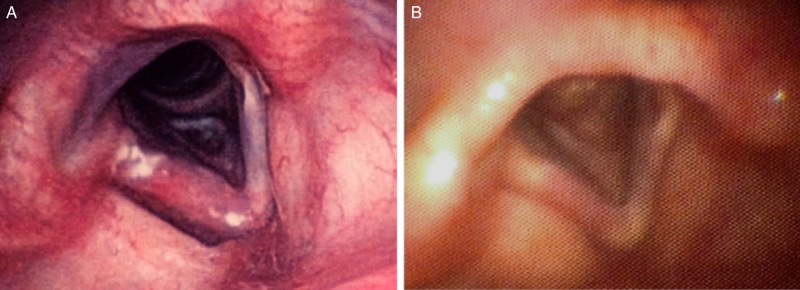
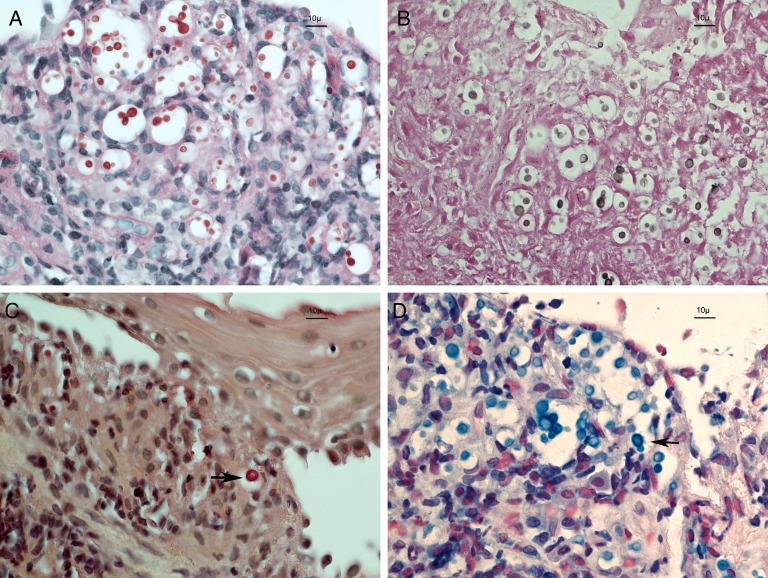
The patient presented with a similar alteration of her voice almost 1 year after the first episode. Marked edema and leukoerythroplakia of the right vocal cord was observed (Figure 1). A biopsy was performed and revealed the presence of numerous small budding yeast-like structures within a neutrophilic infiltrate (Figure 2). Positive alcian blue and mucicarmine staining revealed yeast-like structures enclosed in large capsular polysaccharides. Specific Fontana-Mason staining detected the presence of melanin in the yeast's cell walls. These combined findings were sufficient to confirm the diagnosis of C neoformans infection [17]. Furthermore, C neoformans nucleic acid was detected in the paraffin-embedded tissue biopsy using a polymerase chain reaction assay with pan-fungus primers that targeted ribosomal RNA genes and internal transcribed spacer sequences.
Figure 1.
Left panel showed diffuse swelling on the right vocal cord and irregular patches of leukoerythroplakia. The right picture was taken 2 months after discontinuation of oral fluconazole. The lesions were markedly improved.
Figure 2.
Four different staining methods of the vocal cord biopsy defined that the infecting yeast was a Cryptococcus neoformans. Pink periodic acid-Schiff-positive yeast (A) take a brown color with the Fontana-Mason staining (B), indicating the presence of melanin. Polysaccharide capsules were stained in pink by the mucicarmine method (arrow, C) and light blue by the alcian blue method (arrow, D). Original magnification, 600×.
This unsuspected diagnosis lead to a complete medical investigation. Extensive physical and neurologic examinations were normal. Chest and brain computed tomography-scans were unremarkable for any sign of past or present infection. Complete blood count was normal. Serum cryptococcal antigen and HIV tests were negative. There was no indication for lumbar puncture in the absence of any neurologic manifestations. Oral daily fluconazole was increased to 400 mg. The dose of the inhaled corticosteroids was decreased. Her symptoms resolved progressively and fluconazole was stopped after 15 weeks of therapy. The patient remained asymptomatic on subsequent clinical evaluations. A follow-up laryngeal endoscopy was performed 9 weeks after the completion of the antifungal therapy. All of the previously described abnormalities had disappeared (Figure 1).
The first vocal cord biopsy taken 10 months earlier was retrospectively assessed. Similar histochemical characteristics were highlighted, suggesting that the yeast-like structures found at that time were indeed compatible with C neoformans.
Review of the Literature
We retrieved medical publications going back 20 years using “cryptococcal infection, vocal cord, larynx, laryngeal cryptococcosis, and Cryptoccoccus” in the MEDLINE/PubMed database. References from selected papers were systematically reviewed to find any supplementary cases.
Table 1 includes the 16 laryngeal infections previously reported in the literature along with the present case. There was a definite male predominance (71%; 12 of 17 cases) with a median age of 60 years. Only 2 patients had documented environmental exposure to chickens or pigeons. An underlying medical condition could have predisposed to the infection in 13 patients: 4 with HIV infections (3 with acquired immune deficiency syndrome [AIDS] criteria), 7 patients on glucocorticoid medication (including 5 inhaled topical steroids), and 3 diabetic patients. Furthermore, 5 patients were heavy smokers. The common initial complaint was the persistence of voice hoarseness (100%). It is remarkable that no concomitant systemic symptoms were reported for 14 of the 15 evaluable cases (93%). Furthermore, serum cryptococcal antigen was negative in 9 of the 11 evaluable patients. The titer of 1 antigen-positive patient was in the low positive range (1:8).
Table 1.
Description of Reported Larynx Infection Caused by Cryptococcus Neoformans
| Age/Sex | Host Factors | Exposure | Systemic Symptoms | Endoscopic Findings (Site) | Cryptococcus Antigen | Treatment (Daily Dose/Duration) | Clinical Outcome | Control Endoscopy | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 53/M | Pigeons | No | Mass (unilateral) | No data | Fluco (400 mg/6 w) | Cure | Normal | [5] | |
| 60/M | Diabetes | Heavy smoking | No | Verrucous lesion | No data | Fluco ( /6 w) | Cure | [11] | |
| 73/M | Diabetes | Heavy smoking | No | Polyploid mass (left VC) | Negative | Lesion excision No antifungal therapy |
Lost on follow-up | Inflammation | [7] |
| 55/M | Inhaled corticosteroids | No | Leukoplakia/erythema (right VC) | Negative | Itra (400 mg/6 w) then fluco (400 mg/10 w) | Cure | Normal | [13] | |
| 58/M | Inhaled corticosteroids | No | Irregular red lesion (unilateral) | Negative | Fluco (400 mg/8 w) | No data | [12] | ||
| 64/M | Inhaled corticosteroids | No | Leukoplakia | No data | Fluco (400 mg/44 w) | No data | Normal | [8] | |
| 44/M | HIV infection | Heavy smoking | No | Irregular, thick, hyperemic lesion (bilateral) | No data | Fluco ( /12 w) | Cure | [8] | |
| 79/F | Inhaled corticosteroids | No | Thickening (bilateral) | Negative | Fluco (/26 w) | Cure | [8] | ||
| 87/M | Systemic corticosteroids | Heavy smoking | No | White lesion with edema and erythema (bilateral) | No data | Fluco (400 mg/8 w) | Cure | Normal | [9] |
| 68/F | Heavy smoking | No | Smooth spheroid cystic mass (right VC) | No data | Lesion excision No antifungal therapy |
Cure | [3] | ||
| 42/M | AIDS | No | Irregular lesion (bilateral) | Negative | Fluco (400 mg /8 w) | Cure | Normal | [6] | |
| 65/F | AIDS | Yes | Tumor-like lesion | Positive | Ampho B (3 w) followed by Fluco (400 mg /26 w) | Death | [16] | ||
| 47 ⁄ M | Chicken manure | No | White, raised lesions and edema | Negative | Ampho B (12 w) | Cure | [14] | ||
| 61/M | Systemic corticosteroid Diabetes | No | Exophytic mass | Positive (1:8) | Fluco (200 mg /6 w) | Persisting hoarseness | Normal | [10] | |
| 46⁄M | AIDS | No data | Erythema and edema | Negative | Ampho B + Fluco (unknown duration) | Cure | Normal | [4] | |
| 31⁄F | No data | Warty lesion (right VC) | Negative | No treatment | Cure | Swelling | [15] | ||
| 78/F | Inhaled corticosteroids | No | Leukoerythroplakia (right VC) | Negative | Fluco (400 mg /15 w) | Cure | Improvement |
Abbreviations: AIDS, acquired immune deficiency syndrome; Ampho B, amphotericin B; Fluco, fluconazole; HIV, human immunodeficiency virus; Itra, itraconazole; VC, vocal cord; w, weeks.
The spectrum of endoscopic findings ranged from limited local erythema (n = 6) with or without leucoplakia (n = 5) to a local swelling (n = 4) or verrucous to polypoid lesions (n = 12). Lesions were described as bilateral in 4 patients and unilateral in 7 cases. In all patients, a tissue biopsy was essential to obtain the appropriate diagnosis.
Excision of the lesion without antifungal treatment appeared to be effective in 2 cases where the mass was polypoid or well circumscribed. One patient received no treatment, and 14 patients were treated with systemic antifungal drugs. Three patients were initially treated with amphotericin-B, one of whom was a severely ill AIDS patients with CNS infection and systemic symptoms and subsequently died.
Eleven patients received a triazole antifungal drug as the only treatment. Fluconazole was the preferred drug for 10 of them. The usual daily fluconazole dose was 400 mg, and the treatment duration ranged from 6 to 44 weeks (median 8 weeks). In the 14 evaluable patients, only 1 had persistent symptoms after treatment completion. Ten patients had a posttreatment endoscopic examination. Seven patients were completely cured, 1 was improved, and 2 had persistent inflammation or swelling.
DISCUSSION
Cryptococcus infection of the larynx has been observed sporadically [3–16]. In the reported cases, both the nonspecific local symptoms and the lack of obvious exposure to an environmental source delayed the correct diagnosis. Because the endoscopic findings were diverse and nonspecific, a biopsy was critical to making the diagnosis. The presence of yeast-like structures within the deep inflammatory reaction proved that the C neoformans was the cause of the chronic local infection. The fact that many patients had no evidence of respiratory or systemic infection along with the negative serum antigen suggested that the larynx could have been the first and only site of the Cryptococcus infection.
Only 4 of the 17 reported patients were HIV infected. The most commonly predisposing factor for Cryptococcus infection in non-AIDS patients was the use of high doses of adrenal corticosteroids [18]. In this series, 7 patients were exposed to glucocorticoid medication, 5 of them with inhaled steroids as their only risk factor. We suspected that the inhaled corticosteroids could impair the local defenses and predispose to the development of the Cryptococcus infection at the level of the vocal cords.
Current guidelines for the management of Cryptococcus infections do not address specific treatment for infections involving the vocal cords [19]. In the present case report, multiple courses of low-dose fluconazole failed to eradicate the infection. Another case had persistence of hoarseness after 6 weeks of fluconazole 200 mg daily. A higher dose of fluconazole appeared to be important to achieve the permanent eradication of the infection. This is supported by epidemiological data showing that C neoformans isolates usually express a reduced susceptibility to fluconazole compared with Candida isolates [20]. All infections treated for at least 6 weeks with a dose of fluconazole of 400 mg daily resolved.
Current clinical practice guidelines for the management of cryptococcal disease recommend lowering systemic corticosteroid doses in the immunosuppressed hosts [19]. It seem reasonable to extrapolate to consider lowering or discontinuing inhaled steroids as part of the management of cryptococcal infections of the vocal cords.
CONCLUSIONS
Despite of the small number and the retrospective nature of the reported cases, we were able to describe the most relevant clinical data pertaining to C neoformans infection of the vocal cords. This entity could represent a unique primary local infection that was promoted by the use of inhaled corticosteroids. Tissue biopsy was essential to making the diagnosis. Complete local excision or intensive antifungal therapy were necessary to achieve a cure. Clinicians should consider a C neoformans infection of the vocal cords in the differential diagnosis of patients presenting with chronic hoarseness, especially amongst patients using steroids.
Acknowledgments
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Chayakulkeeree M, Perfect JR.. Cryptococcosis. Infect Dis Clin North Am 2006; 20:507–44, v-vi. [DOI] [PubMed] [Google Scholar]
- 2.Delgado AC, Taguchi H, Mikami Y et al. . Human cryptococcosis: relationship of environmental and clinical strains of Cryptococcus neoformans var. neoformans from urban and rural areas. Mycopathologia 2005; 159:7–11. [DOI] [PubMed] [Google Scholar]
- 3.Bamba H, Tatemoto K, Inoue M et al. . A case of vocal cord cyst with cryptococcal infection. Otolaryngol Head Neck Surg 2005; 133:150–2. [DOI] [PubMed] [Google Scholar]
- 4.Browning DG, Schwartz DA, Jurado RL. Cryptococcosis of the larynx in a patient with AIDS: an unusual cause of fungal laryngitis. South Med J 1992; 85:762–4. [DOI] [PubMed] [Google Scholar]
- 5.Chang YL, Hung SH, Liu CH et al. . Cryptococcal infection of the vocal folds. Southeast Asian J Trop Med Public Health 2013; 44:1043–6. [PubMed] [Google Scholar]
- 6.Chongkolwatana C, Suwanagool P, Suwanagool S et al. . Primary cryptococcal infection of the larynx in a patient with AIDS: a case report. J Med Assoc Thai 1998; 81:462–7. [PubMed] [Google Scholar]
- 7.Frisch M, Gnepp DR. Primary cryptococcal infection of the larynx: report of a case. Otolaryngol Head Neck Surg 1995; 113:477–80. [DOI] [PubMed] [Google Scholar]
- 8.Gordon DH, Stow NW, Yapa HM et al. . Laryngeal cryptococcosis: clinical presentation and treatment of a rare cause of hoarseness. Otolaryngol Head Neck Surg 2010; 142:S7–9. [DOI] [PubMed] [Google Scholar]
- 9.Isaacson JE, Frable MA. Cryptococcosis of the larynx. Otolaryngol Head Neck Surg 1996; 114:106–9. [DOI] [PubMed] [Google Scholar]
- 10.Kerschner JE, Ridley MB, Greene JN. Laryngeal Cryptococcus. Treatment with oral fluconazole. Arch Otolaryngol Head Neck Surg 1995; 121:1193–5. [DOI] [PubMed] [Google Scholar]
- 11.McGregor DK, Citron D, Shahab I. Cryptococcal infection of the larynx simulating laryngeal carcinoma. South Med J 2003; 96:74–7. [DOI] [PubMed] [Google Scholar]
- 12.Mittal N, Collignon P, Pham T, Robbie M. Cryptococcal infection of the larynx: case report. J Laryngol Otol 2013; 127:S54–6. [DOI] [PubMed] [Google Scholar]
- 13.Nadrous HF, Ryu JH, Lewis JE, Sabri AN. Cryptococcal laryngitis: case report and review of the literature. Ann Otol Rhinol Laryngol 2004; 113:121–3. [DOI] [PubMed] [Google Scholar]
- 14.Reese MC, Colclasure JB. Cryptococcosis of the larynx. Arch Otolaryngol 1975; 101:698–701. [DOI] [PubMed] [Google Scholar]
- 15.Smallman LA, Stores OP, Watson MG, Proops DW. Cryptococcosis of the larynx. J Laryngol Otol 1989; 103:214–5. [DOI] [PubMed] [Google Scholar]
- 16.Zeglaoui I, Belcadhi M, Mani R et al. . Laryngeal cryptococcosis revealing AIDS: a case report. Rev Laryngol Otol Rhinol (Bord) 2009; 130:307–11. [PubMed] [Google Scholar]
- 17.Lazcano O, Speights VO Jr, Strickler JG et al. . Combined histochemical stains in the differential diagnosis of Cryptococcus neoformans. Mod Pathol 1993; 6:80–4. [PubMed] [Google Scholar]
- 18.Diamond RD, Bennett JE. Prognostic factors in cryptococcal meningitis. A study in 111 cases. Ann Intern Med 1974; 80:176–81. [DOI] [PubMed] [Google Scholar]
- 19.Perfect JR, Dismukes WE, Dromer F et al. . Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 2010; 50:291–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfaller MA, Castanheira M, Diekema DJ et al. . Wild-type MIC distributions and epidemiologic cutoff values for fluconazole, posaconazole, and voriconazole when testing Cryptococcus neoformans as determined by the CLSI broth microdilution method. Diagn Microbiol Infect Dis 2011; 71:252–9. [DOI] [PubMed] [Google Scholar]