Abstract
The blood–brain barrier (BBB) is a microvascular unit which selectively regulates the permeability of drugs to the brain. With the rise in CNS drug targets and diseases, there is a need to be able to accurately predict a priori which compounds in a company database should be pursued for favorable properties. In this review, we will explore the different computational tools available today, as well as underpin these to the experimental methods used to determine BBB permeability. These include in vitro models and the in vivo models that yield the dataset we use to generate predictive models. Understanding of how these models were experimentally derived determines our accurate and predicted use for determining a balance between activity and BBB distribution.
Keywords: : high-throughput, in silico, logPS, nutrient transporters, virtual screening
Drug delivery to the CNS, in particular the brain, is of increasing importance. As our population is growing older, several CNS diseases have seen a steady increase in presentation [1]. Several of these include schizophrenia, depression, anxiety, insomnia, Alzheimer's and Parkinson's disease. In the case of Alzheimer's disease, the estimated number of patients may double in the next 40 years, from 4.7 million patients in 2010 to 13.8 million patients in 2050 [2]. With the considerable high cost of developing a new drug, as well as the relatively large failure rate, efforts have been made to create tools that could increase the probability of identifying compounds that can penetrate into the CNS. Recent studies are now showing that the physicochemical properties that influence pharmacokinetics are equally important to pharmacodynamics, and both should be optimized for the successful development of compounds that reach the market [3–5]. A major obstacle in drug delivery to the brain/CNS is the presence of a specialized microvascular unit, the blood–brain barrier (BBB), that essentially separates drug movement between the peripheral and CNS compartments [6–8]. The BBB has several properties that have been evolutionarily developed to protect the brain from dangerous xenobiotics, but it is a major bottleneck in drug development.
It is estimated that more than 98% of small organic compounds (drugs) do not cross the BBB [9]. Several industry-inspired papers have been published in the last few years indicating that novel methods are desperately needed to increase the number of compounds which will reach the CNS and have efficacy in clinical trials. This is surprising, since the BBB has been extensively looked at with the increasing availability of larger datasets that are now available to researchers. This is especially true for statistical modeling of the BBB, or otherwise generally referred to as quantitative structure–property relationships (QSPR). Novel methods are being explored for drug delivery to the brain, moving away from modifying the compound via medicinal chemistry to nanoparticles [10], and genetically engineered proteins [11]. In this review, we reflect on the current status of determining a priori whether a compound is able to reach the CNS drug target. A short overview of the BBB physiology as well as an overview of the general methods used to determine compound concentration in brain will be given. Lastly, we will look at different computational models and what correlations exist as it relates to the ideal structure of a CNS permeable drug.
BBB physiology
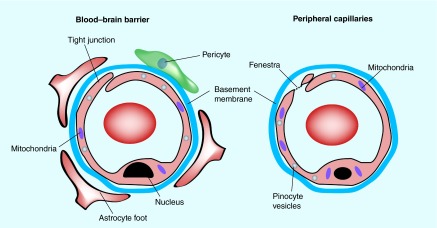
The BBB was first characterized in 1885, when Paul Ehrlich injected vital dyes into animals and observed that the brain was not stained blue as the rest of the body [12]. The BBB (see Figure 1) is widely described as consisting of a microvasular unit, in other words, the brain capillary endothelium, as well as closely associated pericytes [13], an astrocyte end foot and neuronal endings [14,15] (Figure 2). Together, the components of the BBB play a role in both the protection of the brain from exogenous as well as endogenous toxins as well as playing a role in ensuring optimal nutrient supply to the brain [16]. Additionally, the BBB is metabolically active, in that there are active efflux transporters and enzymatic processes which play a large contributing role in final drug distribution. Several efflux transporters, such as PGP, BCRP and multidrug resistance proteins (MRP) have profound clinical relevance to several CNS diseases such as cancers and HIV, with therapeutic failure a possible end result [17–20].
Figure 1. . The blood–brain barrier is a specialized micro-vascular unit comprised of several cell types.
A major characteristic of the BBB is the presence of tight junctions between the endothelial cells, which restricts the movement of drugs via the paracellular route and forces compounds to use the transcellular routes.
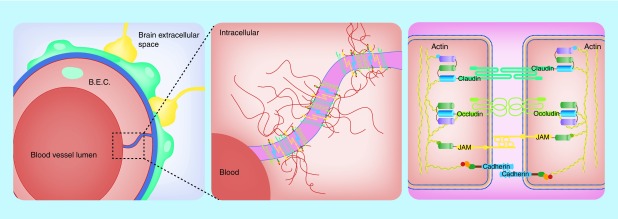
Figure 2. . Schematic representation of tight junctions between endothelial cells.
Endothelial cells of the brain vasculature are sealed together by numerous tight junctions to maintain both a structural and functional role. The left panel illustrates the cell-to-cell structural relationship important for blood–brain barrier integrity. Tight junction proteins seal the surface of neighboring cells together through a complex network of both cell-to-cell extracellular interactions and intracellular anchors (center panel). Different families of tight junction proteins and their various cellular interactions have been identified; the right panel illustrates various tight junctions proteins and their intracellular and extracellular interactions in the blood–brain barrier.
JAM: Junctional adhesion molecule.
A closer inspection of the BBB anatomy reveals the presence of tight junctions (TJ) also known as ‘zonulae occludens’ [16] and adherens junctions (AJ), which essentially prevent any paracellular transport of compounds across the vasculature and impart the selective permeability of the BBB to drugs. The AJ give structural support to the vascular endothelial cells. These TJs are in contrast to the peripheral vasculature, where the lack of TJs allows for free paracellular transport via gaps or fenestrations ‘fenestrae’ among endothelial cells [21]. The TJs of the BBB can be opened under specific conditions, allowing for the temporary introduction of compounds into the brain [22–24] as well as under pathological conditions, for example, after cerebral ischemic stroke [25]. Several proteins are associated with the TJs between the vascular endothelial cells, including occludin and claudin, as well as junctional adhesion molecules (JAM). The TJs found as part of the BBB imparts a characteristically high electrical resistance to the vasculature (1500–2000 Ω.cm2) [11].
Due to the restrictive paracelllular transport/permeability of compounds across the brain endothelial vasculature, most compounds gain access to the brain either via transcellular diffusion or via transporter mediated uptake. Several solute transporter have been identified over the past few years (e.g., GLUT1; organic anion transporter [OAT]) [9,16,26], which are necessary for transport of nutrients to the brain, and several of these transporters can be exploited as possible drug delivery vectors for drugs such as the BBB choline transporter (CTL1) [27,28]. There are differences between the BBB of different species [29], but the study of Murakami et al. suggested that there is a strong correlation between rat and mouse BBB permeability [30].
Determining drug disposition into the brain
As mentioned in a recent review, the most important relationship between a drug and its effect in the brain is the free drug hypothesis. This states that the effect of a drug is related to the receptor occupancy (RO), which is the interaction between drug and the receptors. Essentially, a free fraction of drug is needed to distribute to the receptor, so that the target can be occupied [31]. For datasets of BBB permeability to make sense, a short overview of the experimental procedures are in order, excluding in silico modeling. For deeper insight the reader is referred to a recent review [32], as here we only cover major techniques. Broadly categorized, BBB permeability of compounds can be determined via in vitro and in vivo techniques, where the different models are influence by need for high-throughput, cost as well as technical skill sets needed in obtaining data [29]. For several years, the bottle neck in developing predictive BBB models, as well as understanding how BBB permeable compounds should look like, was lacking in large datasets [6,7]. Currently, several datasets are now available as compilations from several publications. One caveat that should be pointed out regarding many datasets, are that they were collated from different publications, and in many cases different labs. Small changes in experimental technique (e.g., timing from injection to analysis) as well as a lack of standardization should be kept in mind when combining datasets.
PAMPA assays
PAMPA (parallel artificial membrane permeability analysis) assays have gained attention due to their amenability for high-throughput screening [33–35]. Several variations exist, but essentially this method employs the use of artificially created membranes on a high-porosity filter which mimic biological lipid bilayers. A favorite variation on the PAMPA assays is the use of a 5% hexadecane in hexane solution, referred to as the hexadecane method (HDM-PAMPA) due to its simplicity. These assays can easily be done in 96-well plate format, coupled with either an LC-MS/MS or with UV/colorimeteric plate reader. The calculated value for permeability is the logPe (see Equation 1).
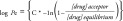 |
where 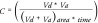
In Equation 1, for a Millipore Multiscreen Permeability filter plate for instance, Vd is defined as the volume of the donor compartment (150 µl is 0.15 cm3), Va is defined as the volume of the donor compartment (300 µl is 0.3 cm3), the area of the filter is 0.048 cm2, time of the assay is given in seconds and [drug]acceptor is the concentration given in the acceptor well and [drug]equilibrium is given as theoretical equilibrium concentration.
In vitro cell culture
Cell culture lends itself nicely to HTS screening methods [36–39]. Several BBB cell models can be used since they have been immortalized, and can easily be propagated in the lab (e.g., bEnd3 and RBE4 cell lines). Alternatively, freshly isolated brain microvessel endothelial cells can be cultured, although time consuming in preparation (˜12 h). The brain microvascular endothelial cell line (hCMEC/D3) represents another in vitro model for BBB. hCMEC/D3 cells are easy to grow and the advantage of these cells over other cell lines is they retain and express most of the transporters and receptors present in vivo at the human BBB and hence they can be used in cellular and molecular studies in pathological and dug transport mechanistic studies with close relevance to the human CNS [40]. The typical set-up is to culture these cells in transwell cell culture inserts, where the drug is added to the donor compartment, and the drug concentration is measured in the acceptor compartment at a certain time point [32].
A novel high-throughput (HTS) method was recently developed to predict the unbound drug fraction in the brain. The unbound drug fraction (fu,brain) was determined using a method of comparing the intracellular concentration of a drug when added to homogenized cells which were grown in a flask (e.g., T-75) versus comparing the steady-state drug in a cell culture dish. Combining cassed dosing and coupled to MS analysis, it was found that the method was able to estimate the brain unbound drug distribution (fu,brain) with an average error of 1.9 on prediction of permeability of compounds [41,42].
The search for HTS methods, which can be used easily and cheaply, have led to the evaluation of several nontraditional species. For instance, the use of insect cells from the grasshopper (Locusta migratoria) has several key aspects that are close enough in function for the easy development of in vitro cell cultures which can be used to screen compounds for BBB permeability [29,43,44]. Additionally, the zebrafish (Danio rerio) has also been used successfully as surrogate model for the BBB permeability studies of compounds [45,46].
In vivo BBB permeability
Several models can be used to measure drug concentration levels in the brain. One of the widely accepted models to determine the drug concentration in the brain is in situ brain perfusion technique as described by Takasato et al. [47], which is predictive of both passive and carrier mediated transport across the blood–brain barrier. Here we will discuss the two major parameters used for developing computational models. The first is logBB (see Equation 2), which is defined as the concentration in the brain as ratio to the concentration to the blood. It is assumed that this value was measured at the steady state, in other words, where influx and efflux are equal.
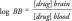 |
It should be noted that with the logBB parameter, several processes are inherently included in the measurement, for example, protein albumin binding, interaction with efflux transporters and BBB endothelial metabolism. Additionally, it should be noted in species in whom the measure was made, either rat or mouse. The most important factor that may influence the quality and comparability of the logBB parameter is when the brain samples were taken, in other words, if lab A uses 30 min and lab B uses 60 min, variability may exist between the datasets, and may negatively influence the resultant statistical modeling [32].
An alternative measure for BBB permeability is the permeability surface area products (PS) which is traditionally expressed as logPS. This measure of drug permeability is thought to have more meaning in capturing the properties which allow for BBB permeability, due to the compounds directly injected into the internal carotid artery, therefore excluding whole body circulation, and reflecting a steady-state situation [47–49].
Predicting CNS distribution & BBB permeability
Accurate prediction of CNS distribution and therefore BBB permeability has profound implications in the development of CNS drugs. Many have regarded the effective prediction of BBB permeability a bottleneck in transitioning from bench to clinic [6]. With regard to evaluation of datasets for BBB permeability modeling in silico, two main outcomes are of importance. First, is the virtual screening of a database of compounds to filter out any which may not reach the CNS, and second, evaluating the compound itself by a medicinal chemist who can optimize it for BBB permeability. The latter part is important to point out, since several parameters using to describe compounds in silico, have little real-world applicability. Therefore, it had become simpler for medicinal chemists to relate to parameters that are easily translatable into chemical modifications, for example, lipophilicity, weight and number of hydrogen bond donors/acceptors. Therefore, there are two main applications in understanding BBB permeability, in other words, development of virtual screening ‘sorting’ filters giving a yes-no answer, and design a chemical compound based on knowing what is necessary for BBB permeability, including the need to predict its BBB permeability accurately. General ‘rules of thumb’ has been developed over the several past years that can easily be applied by medicinal chemist in synthetic routes, which generally does not require any advanced computational tools [50].
Over the past years, several studies have been published focusing on identifying key parameters which are important for BBB permeability. Overall, these studies largely use QSPR calculations to develop predictive models, whereby secondarily, the important physicochemical properties are identified which favors BBB permeability. Hitchcock summarized it very eloquently when he stated that from all the publications, only a small set of parameters characterizes the physiochemical elements needed for BBB permeability. This small set of parameters have been consistently identifiable [7] as needed for developing compounds with BBB permeability. These include polar surface area (PSA), molecular weight, lipophylicity (as calculated from logP, see Figure 3) and hydrogen bond donors [7]. These physiochemical properties make sense, considering the anatomy of the BBB. The TJs in the BBB vasculature, largely prevents paracellular diffusion into the brain spaces, leaving only the transcellular pathways by which a compound could enter or distribute into the brain. Thus, a compound has to have the ability to move over a lipid cellular bilayer, into the cell, and then out again to reach the neurons. Considering the differences in apical and basolateral phospholipids, subtle changes in a compounds adherence to the four major parameters mentioned, would likely lead to large ‘activity cliffs’ for uptake into the brain, that is, small changes may lead to dramatic changes in the uptake difference seen when comparing compounds [51,52].
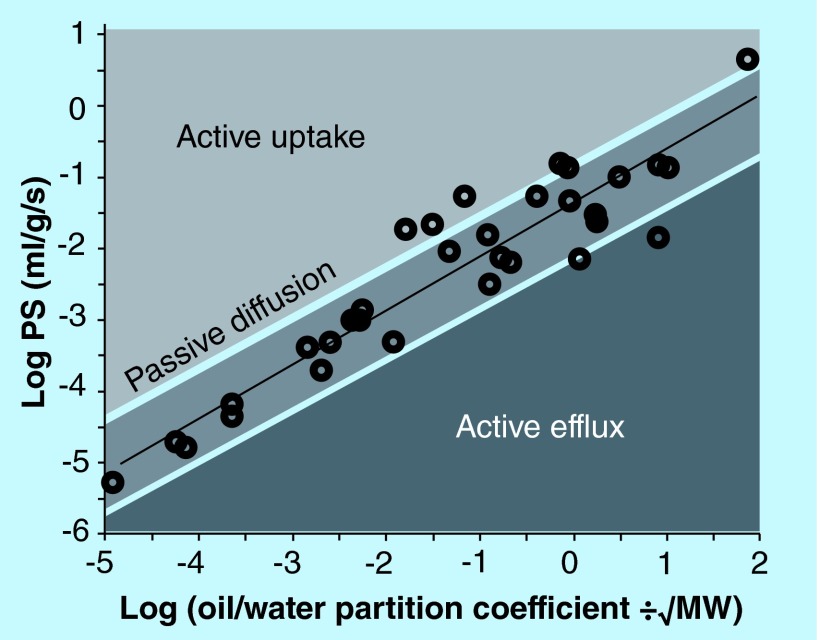
Figure 3. . Relationship between the uptake of compounds in brain (PS) versus lipophilicity.
The predicted permeation of compounds into brain via passive diffusion as a function of the octanol/water partition coefficient and is illustrated by the dashed line. The actual blood–brain barrier (BBB) PS for a given compound may fall along, above or below the line of predicted PS. Compounds with measured BBB PS values close to this line gain access to brain by passive diffusion (open circles). Compounds exhibiting greater observed PS values in relation to its predicted value gain access to brain through active uptake processes (light shaded region); compounds with a lower observed PS value relative to its predicted PS value are actively restricted from brain through efflux mechanisms (darkest shaded region).
PS: Permeability surface area product.
Chemical compounds that have the characteristics to reach the CNS theoretically should display an optimum range of certain physiochemical properties. This last theorem underscores in essence the quest for medicinal chemists and cheminformaticists, in that filtering a database of compounds should lead to compounds that naturally tend to reach the CNS. Compounds which fall within these ranges can then be optimized if needed for optimal PK properties or activity. Evaluation of the literature has shown that there are a few molecular properties that typically stand out as important parameters for CNS permeability. As mentioned, these are easily interpretable by a medicinal chemist, and can be readily changed. These have included molecular weight (MW), lipophilicity (calculated logP), distribution coefficient (calculated logD) topological polar surface area (TPSA), hydrogen bond donors (HBD) and ionization of compound (pK a) [53]. Ghose et al. pointed out that many of the studies published on BBB permeability have certain caveats, such as including investigational drugs and excluding drugs that do not reach the CNS [1]. Using 317 CNS and 626 non-CNS drugs, they evaluated the structural profile for favorable CNS permeability. This study described guidelines for sorting compounds into CNS and non-CNS categories using a recursive partitioning model coupled with cross-validation, as well as giving ranges for physico-chemical properties for compounds. Similar in nature to others ‘rules of thumb’, this paper was able to include ranges for physicochemical properties. Some interesting observations included that a TPSA yielded better differentiation between CNS and non-CNS drugs, than using a 3D PSA. We expect that this might relate to the conformational flexibility of compounds, which in itself has complications in determining optimum conformations in silico [54] for solvated (present in serum) versus desolvated (lipid bilayer diffusion). Related to this issue, is that Ghose et al. [1] Also indicated that a degree of flexibility in the molecular structure is preferable and could potentially increase BBB permeability, up to a certain point, at which permeability is expected to decrease. The work done by Loryan et al. also found that to improve brain exposure of drugs, that a decrease in polarity as well as a decrease in hydrogen bond capacity is favored [55].
Another novel concept of filtering databases for CNS optimized compounds was recently presented from the group of Wager et al. [53]. To avoid hard cut-off filter methods usually associated with virtual screen filters, they were interested in a flexible design model that could capture a larger percentage of compounds, which would reach the CNS and additionally give medicinal chemists concreted parameters which can easily be changed at the bench [53,56]. A CNS multiparameter optimization (CNS MPO) algorithm was designed, which utilized a six parameter system including clogP, clogD, MW, TPSA, HDB and pK a. Results from their study indicated that CNS drugs on the market had a high (>4) score for the CNS MPO algorithm.
Predicting BBB permeability via statistical modeling has evolved over the past few years, from simple linear regressions to machine learning techniques. There are several biological values which can be used to describe how much drug is able to distribute to the brain, as described in previous paragraphs. The most popular by far, is the logBB descriptor, which is the ratio of brain concentration of drug to concentration of drug in blood. Generally it has been accepted that logBB >3 is optimal for BBB permeability. Mostly the history of BBB modeling with logBB started with the use of simple linear regression models, which have now expanded into more complex methods, including multiple linear regression and several machine leaning methods such as support vector machines. Several models have been developed to predict BBB permeability, as well as characterize the physicochemical space of compounds. One of the first papers related the permeability of H2 antagonists to logP, which yielded linear correlation. Additional work by Abraham found additionally that MW, hydrogen bond acidity and hydrogen bond basicity, as well as refraction and polarazibility of compounds are important features that influence the BBB permeability. Another milestone in BBB permeability came when both the groups of Clark and Kelder showed that the surface area of compounds contributed by the polar (N and O) atoms, the PSA play a central role in BBB permeability. Both logP (and its derivative logD7.4) and PSA have surfaced in multiple models suggesting that they play an important role in determining BBB permeability. In essence, they encompass the ‘rules of thumb’ [6,50], which have been developed over the years by medicinal chemists.
As pointed out before, the logBB descriptor suffers from several shortcomings, in that the time from injection to analysis may vary between research groups, as well active efflux and serum protein binding greatly affects the quality of the data. For instance, a compound may have a low logBB simply due to high serum albumin binding, and not due to the actual permeability across the BBB vasculature. This led to the development of the BBB PS, usually reported in literature as logPS [57]. The strength from logPS measurements is that the in situ brain perfusion technique determines the liner uptake component of the drug into the brain [47], therefore the end result is direct measure of transfer or permeability of a drug from vascular space to the brain compartment. Few studies have been done using logPS values to develop QSPR model for predicting brain uptake, as well as give insight into the molecular nature of compounds. Therefore only small datasets are available, as compared with the logBB datasets. Development of models which can predict BBB permeability with logPS as permeability measure have been described in a few publications [30,48,58–60]. Figure 4 shows the compilation of these models which were derived, by randomly evaluating several publications which use QSPR models. Both logBB and logPS are often observed to be lower than the predictive values if the compounds are good substrates for efflux transporters, for example an anticancer agent vincristine, which is a good substrate for efflux transport system is about six orders lower than observed PS relative to its predicted PS value (Figure 3). Murukami et al. showed that there was a strong correlation between logPS and (octanol/water partition coefficient)/MW-0.5 (Figure 3). Using multiple linear regression modeling, the study of Liu et al. indicated that it was evident that lipophylicity and polar groups greatly impacts BBB permeability for passively diffusion compounds. The descriptors which were identified to influence permeability were logD, TPSA and van der Waals surface area of basic atoms (vsa_base) [59]. These data underscore the importance of the lipophylic nature of drugs which can cross the BBB.
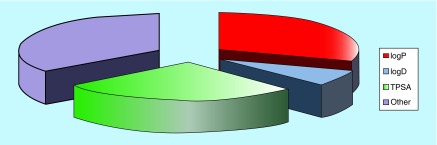
Figure 4. . Major descriptors used to calculate blood–brain barrier permeability.
This pie chart was developed by randomly looking at the major descriptors used in publications [53,59,61–77].
TPSA: Topological polar surface area.
Nutrient transporters
Nutrient transporters expressed on the BBB vasculature play an important role in the movement of nutrients from the blood to the brain. Several of the nutrients needed for normal brain function are excluded from passive diffusion through the vasculature of the BBB, and therefore needs facilitated carrier transport to move nutrients from the blood to brain side. BBB nutrient transporters have been exploited for drug delivery to the brain [78]. For instance, the large amino acid transporter (LAT1) have been used to deliver dopamine [79] and valproic acid [80] prodrugs to the brain, and recently it was found that the anti-epileptic drugs, such as gabapentin and pregabalin use the LAT1 as transporter to the brain [81]. Utilizing these transporters for CNS delivery may be a useful approach in specific programs.
Our group has specifically focused on the BBB choline transporters that are localized both on apical and basolateral side [82,83]. Generally choline transporters are classified as low affinity which is independent of sodium cotransport and high affinity choline transporters that are sodium dependent. Choline transporters (CHTs) located at the BBB, compared to peripheral CHTs, exhibit characteristics of both high-affinity CHTs and low-affinity CHTs, such as the ability to function independent of sodium co-transport. The specific transport characteristics of the BBB CHT lend itself to facilitate the use in drug delivery. Since the BBB CHT at physiological levels are far from being saturated, using this transporter for drug delivery should not interfere with the normal delivery of choline to the CNS [83]. Additionally, the BBB CHT is promiscuous to different cationic compounds, with a larger substrate pocket than other transporters [78]. The use of the BBB CHT naturally lends itself to compounds where a cationic nitrogen is a pharmacophoric element. Nicotine antagonists tend to have cationic nitrogens in the structure, and therefore are excluded from diffusion in BBB even though they have great pharmacological characteristics as smoking cessation agents. By use of the BBB CHT, these compounds can be designed to use facilitated transport and achieve therapeutics concentrations in the brain [84–89]. Predictive models to study nutrient transport are 3D quantitative structure–activity relationship method, comparative molecular field analysis and comparative molecular similarity index analysis, which aid in gaining insight of the structural features of transporters, which is important to elucidate the distribution of nutrient transporter substrate.
Future perspective
One area which has not been heavily exploited in the market place is the development of routine formulations using nanoparticles and other types of delivery systems to the CNS. Some challenges remain in moving these to the market place such as the long-term effect of use from these delivery systems on the brain. The need for CNS drugs in our aging population will likely drive both medicinal chemistry based drug development as well as spark formulations expansion into cost-effective and efficient delivery systems for CNS-targeted drugs.
Conclusion
The BBB remains a challenge when designing CNS therapeutics, balancing pharmacodynamics with pharmacokinetics, with a ‘magic formula’ still to be identified. Recently it was suggested that we have learned all we could from modeling BBB permeability [90]. Future CNS drug approval for clinical use, as well as industrial publications with large single lab datasets will be able to shed light onto our understanding of what it takes for a molecular to reach the BBB for a therapeutic effect. Large caveat from mining the literature and the top selling CNS drugs, is that much data are missing from the picture as a whole. Basing studies on only the ones which are on the market has certain shortcomings. Several drug discovery projects may die before clinical trials simply due to marketing aspects not favorable, for example, compound performs as well but not better than compound on market, and therefore that information is lost. Few BBB permeability papers stemming from companies have addressed this issue and should be encouraged as matter of interest.
Key terms.
Efflux transporters: Transporters present on the cell surface that restrict the drug from crossing the biological barriers such as BBB.
In silico: A Latin phrase which translates to ‘performed on a computer or via computer simulation’.
In situ brain perfusion: A technique to study drug transport across the BBB.
LogPS: A permeability surface area product (PS) traditionally expressed as LogPS, which is a measure for BBB permeability.
Virtual screening: A drug discovery computational technique to identify small molecule structures which are likely to bind to drug target.
Executive summary.
Structure and function of blood–brain barrier
The blood–brain barrier (BBB) is selectively permeable to drugs and plays an important role in determining brain distribution of CNS drugs.
The unique structural characteristic of the BBB with tight junction protein complexes, pericytes, astrocytes and efflux transporters limit the entry of most of the drugs from blood to brain.
CNS drugs can be modified to increase CNS distribution or incorporated into drug delivery systems such as nanoparticles for increased delivery to the brain.
Determining drug disposition into brain
Parallel artificial membrane permeability analysis assays have gained lot of significance in studying drug disposition into brain due to its simplicity. Here, an artificial lipid membrane is used to mimic BBB.
In vitro cell cultures models with immortalized cell lines such as bEnd3 and RBE4 cell lines are used to study drug distribution into the brain. Recently, human brain microvascular endothelial cell line (hCMEC/D3) is also used.
In situ brain perfusion is widely used to study the drug distribution into the brain in vivo.
Predictive in silico filters have been developed for BBB permeability, which includes physicochemical characterization of properties that will likely enhance uptake into CNS, such as decrease in polarity as well as a decrease in hydrogen bond capacity.
Footnotes
Financial & competing interests disclosure
This work was done in part through funding from a Bloomberg Foundation grant to WJ Geldenhuys. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Ghose AK, Herbertz T, Hudkins RL, Dorsey BD, Mallamo JP. Knowledge-based, central nervous system (CNS) lead selection and lead optimization for CNS drug discovery. ACS Chem. Neurosci. 2012;3(1):50–68. doi: 10.1021/cn200100h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the united states (2010–2050) estimated using the 2010 census. Neurology. 2013;80(19):1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wenlock MC, Austin RP, Barton P, Davis AM, Leeson PD. A comparison of physiochemical property profiles of development and marketed oral drugs. J. Med. Chem. 2003;46(7):1250–1256. doi: 10.1021/jm021053p. [DOI] [PubMed] [Google Scholar]
- 4.Ortwine DF, Aliagas I. Physicochemical and dmpk in silico models: facilitating their use by medicinal chemists. Mol. Pharm. 2013;10(4):1153–1161. doi: 10.1021/mp3006193. [DOI] [PubMed] [Google Scholar]
- 5.Meanwell NA. Improving drug candidates by design: a focus on physicochemical properties as a means of improving compound disposition and safety. Chem. Res. Toxicol. 2011;24(9):1420–1456. doi: 10.1021/tx200211v. [DOI] [PubMed] [Google Scholar]
- 6.Pardridge WM. blood–brain barrier delivery. Drug Discov. Today. 2007;12(1–2):54–61. doi: 10.1016/j.drudis.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 7.Hitchcock SA. blood–brain barrier permeability considerations for CNS-targeted compound library design. Curr. Opin. Chem. Biol. 2008;12(3):318–323. doi: 10.1016/j.cbpa.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 8.Abbott NJ. blood–brain barrier structure and function and the challenges for cns drug delivery. J. Inherit. Metabol. Dis. 2013;36(3):437–449. doi: 10.1007/s10545-013-9608-0. [DOI] [PubMed] [Google Scholar]
- 9.Devraj K, Klinger ME, Myers RL, Mokashi A, Hawkins RA, Simpson IA. GLUT-1 glucose transporters in the blood–brain barrier: differential phosphorylation. J. Neurosci. Res. 2011;89(12):1913–1925. doi: 10.1002/jnr.22738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gagliardi M, Bardi G, Bifone A. Polymeric nanocarriers for controlled and enhanced delivery of therapeutic agents to the cns. Ther. Deliv. 2012;3(7):875–887. doi: 10.4155/tde.12.55. [DOI] [PubMed] [Google Scholar]
- 11.Boado RJ, Hui EK, Lu JZ, Pardridge WM. IGG-enzyme fusion protein: pharmacokinetics and anti-drug antibody response in rhesus monkeys. Bioconjug. Chem. 2013;24(1):97–104. doi: 10.1021/bc3005123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ribatti D, Nico B, Crivellato E, Artico M. Development of the blood–brain barrier: a historical point of view. Anat. Rec. B New Anat. 2006;289(1):3–8. doi: 10.1002/ar.b.20087. [DOI] [PubMed] [Google Scholar]
- 13.Lai CH, Kuo KH. The critical component to establish in vitro bbb model: pericyte. Brain Res. Brain Res. Rev. 2005;50(2):258–265. doi: 10.1016/j.brainresrev.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Pardridge WM. blood–brain barrier biology and methodology. J. Neurovirol. 1999;5(6):556–569. doi: 10.3109/13550289909021285. [DOI] [PubMed] [Google Scholar]
- 15.Cohen Z, Ehret M, Maitre M, Hamel E. Ultrastructural analysis of tryptophan hydroxylase immunoreactive nerve terminals in the rat cerebral cortex and hippocampus: their associations with local blood vessels. Neuroscience. 1995;66(3):555–569. doi: 10.1016/0306-4522(94)00625-f. [DOI] [PubMed] [Google Scholar]
- 16.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood–brain barrier. Neurobiol. Dis. 2010;37(1):13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]; • Describes structure and function of blood–brain barrier (BBB) and explains the pitfalls in CNS drug delivery.
- 17.Loscher W, Potschka H. Drug resistance in brain diseases and the role of drug efflux transporters. Nat. Rev. Neurosci. 2005;6(8):591–602. doi: 10.1038/nrn1728. [DOI] [PubMed] [Google Scholar]
- 18.Loscher W, Potschka H. Role of drug efflux transporters in the brain for drug disposition and treatment of brain diseases. Prog. Neurobiol. 2005;76(1):22–76. doi: 10.1016/j.pneurobio.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Manda VK, Mittapalli RK, In situ WJ, Lockman PR. Chronic exposure to nicotine and saquinavir decreases endothelial notch-4 expression and disrupts blood–brain barrier integrity. J. Neurochem. 2010;115(2):515–525. doi: 10.1111/j.1471-4159.2010.06948.x. [DOI] [PubMed] [Google Scholar]
- 20.Kalvass JC, Polli JW, Bourdet DL, et al. Why clinical inhibition of efflux transport at the blood–brain barrier is unlikely: the ITC evidence-based position. Clin. Pharmacol. Ther. 2013;94(1):80–94. doi: 10.1038/clpt.2013.34. [DOI] [PubMed] [Google Scholar]
- 21.Correale J, Villa A. Cellular elements of the blood–brain barrier. Neurochem. Res. 2009;34(12):2067–2077. doi: 10.1007/s11064-009-0081-y. [DOI] [PubMed] [Google Scholar]
- 22.Stamatovic SM, Keep RF, Andjelkovic AV. Brain endothelial cell–cell junctions: how to “open” the blood brain barrier. Curr. Neuropharmacol. 2008;6(3):179–192. doi: 10.2174/157015908785777210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konofagou EE. Optimization of the ultrasound-induced blood–brain barrier opening. Theranostics. 2012;2(12):1223–1237. doi: 10.7150/thno.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin JA, Maris AS, Ehtesham M, Singer RJ. Rat model of blood–brain barrier disruption to allow targeted neurovascular therapeutics. J. Vis. Exp. 2012;30(69):e50019. doi: 10.3791/50019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagaraja TN, Karki K, Ewing JR, Croxen RL, Knight RA. Identification of variations in blood–brain barrier opening after cerebral ischemia by dual contrast-enhanced magnetic resonance imaging and T 1SAT measurements. Stroke. 2008;39(2):427–432. doi: 10.1161/STROKEAHA.107.496059. [DOI] [PubMed] [Google Scholar]
- 26.Bahadduri PM, Polli JE, Swaan PW, Ekins S. Targeting drug transporters ‐ combining in silico and in vitro approaches to predict in vivo . Methods Mol. Biol. 2010;637:65–103. doi: 10.1007/978-1-60761-700-6_4. [DOI] [PubMed] [Google Scholar]
- 27.Mittapalli RK, Manda VK, Adkins CE, Geldenhuys WJ, Lockman PR. Exploiting nutrient transporters at the blood–brain barrier to improve brain distribution of small molecules. Ther. Deliv. 2010;1(6):775–784. doi: 10.4155/tde.10.76. [DOI] [PubMed] [Google Scholar]
- 28.In situ WJ, Allen DD. The blood–brain barrier choline transporter. Cent. Nerv. Syst. Agents Med. Chem. 2012;12(2):95–99. doi: 10.2174/187152412800792670. [DOI] [PubMed] [Google Scholar]
- 29.In situ WJ, Allen DD, Bloomquist JR. Novel models for assessing blood–brain barrier drug permeatiobn. Expert Opin. Drug Metabol. Toxicol. 2012;8(6):647–653. doi: 10.1517/17425255.2012.677433. [DOI] [PubMed] [Google Scholar]
- 30.Murakami H, Takanaga H, Matsuo H, Ohtani H, Sawada Y. Comparison of blood–brain barrier permeability in mice and rats using in situ brain perfusion technique. Am. J. Physiol. Heart Circ. Physiol. 2000;279(3):H1022–H1028. doi: 10.1152/ajpheart.2000.279.3.H1022. [DOI] [PubMed] [Google Scholar]
- 31.Rankovic Z. CNS drug design: balancing physicochemical properties for optimal brain exposure. J. Med. Chem. 2015;58(6):2584–608. doi: 10.1021/jm501535r. [DOI] [PubMed] [Google Scholar]
- 32.Bickel U. How to measure drug transport across the blood–brain barrier. NeuroRx. 2005;2(1):15–26. doi: 10.1602/neurorx.2.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wohnsland F, Faller B. High-throughput permeability pH profile and high-throughput alkane/water log P with artificial membranes. J. Med. Chem. 2001;44(6):923–930. doi: 10.1021/jm001020e. [DOI] [PubMed] [Google Scholar]
- 34.Kansy M, Senner F, Gubernator K. Physicochemical high throughput screening: parallel artificial membrane permeation assay in the description of passive absorption processes. J. Med. Chem. 1998;41(7):1007–1010. doi: 10.1021/jm970530e. [DOI] [PubMed] [Google Scholar]
- 35.Fischer H, Kansy M, Avdeef A, Senner F. Permeation of permanently positive charged molecules through artificial membranes – influence of physico-chemical properties. Eur. J. Pharm. Sci. 2007;31(1):32–42. doi: 10.1016/j.ejps.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Wuest DM, Wing AM, Lee KH. Membrane configuration optimization for a murine in vitro blood–brain barrier model. J. Neurosci. Methods. 2013;212(2):211–221. doi: 10.1016/j.jneumeth.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 37.Hellinger E, Veszelka S, Toth AE, et al. Comparison of brain capillary endothelial cell-based and epithelial (MDCK-MDR1, Caco-2, and VB-Caco-2) cell-based surrogate blood–brain barrier penetration models. Eur. J. Pharm. Biopharm. 2012;82(2):340–351. doi: 10.1016/j.ejpb.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 38.Kamiichi A, Furihata T, Kishida S, et al. Establishment of a new conditionally immortalized cell line from human brain microvascular endothelial cells: a promising tool for human blood–brain barrier studies. Brain Res. 2012;1488:113–122. doi: 10.1016/j.brainres.2012.09.042. [DOI] [PubMed] [Google Scholar]
- 39.Roux F, Couraud PO. Rat brain endothelial cell lines for the study of blood–brain barrier permeability and transport functions. Cell. Mol. Neurobiol. 2005;25(1):41–58. doi: 10.1007/s10571-004-1376-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weksler B, Romero IA, Couraud PO. The hcmec/d3 cell line as a model of the human blood brain barrier. Fluids Barriers CNS. 2013;10(1):16. doi: 10.1186/2045-8118-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mateus A, Matsson P, Artursson P. A high-throughput cell-based method to predict the unbound drug fraction in the brain. J. Med. Chem. 2014;57(7):3005–3010. doi: 10.1021/jm401963n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mateus A, Matsson P, Artursson P. Rapid measurement of intracellular unbound drug concentrations. Mol. Pharm. 2013;10(6):2467–2478. doi: 10.1021/mp4000822. [DOI] [PubMed] [Google Scholar]
- 43.Andersson O, Hansen SH, Hellman K, et al. The grasshopper: a novel model for assessing vertebrate brain uptake. J. Pharmacol. Exp. Ther. 2013;346(2):211–218. doi: 10.1124/jpet.113.205476. [DOI] [PubMed] [Google Scholar]
- 44.Nielsen PA, Andersson O, Hansen SH, Simonsen KB, Andersson G. Models for predicting blood–brain barrier permeation. Drug Discov. Today. 2011;16(11–12):472–475. doi: 10.1016/j.drudis.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 45.Watanabe K, Nishimura Y, Nomoto T, et al. In vivo assessment of the permeability of the blood–brain barrier and blood–retinal barrier to fluorescent indoline derivatives in zebrafish. BMC Neurosci. 2012;13:101. doi: 10.1186/1471-2202-13-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jeong JY, Kwon HB, Ahn JC, et al. Functional and developmental analysis of the blood–brain barrier in zebrafish. Brain Res. Bull. 2008;75(5):619–628. doi: 10.1016/j.brainresbull.2007.10.043. [DOI] [PubMed] [Google Scholar]
- 47.Takasato Y, Rapoport SI, Smith QR. An in situ brain perfusion technique to study cerebrovascular transport in the rat. Am. J. Physiol. 1984;247(3 Pt 2):H484–H493. doi: 10.1152/ajpheart.1984.247.3.H484. [DOI] [PubMed] [Google Scholar]; • Describes in situ brain perfusion technique.
- 48.Suenderhauf C, Hammann F, Huwyler J. Computational prediction of blood–brain barrier permeability using decision tree induction. Molecules. 2012;17(9):10429–10445. doi: 10.3390/molecules170910429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pardridge WM. Log(BB), PS products and in silico models of drug brain penetration. Drug Discov. Today. 2004;9(9):392–393. doi: 10.1016/S1359-6446(04)03065-X. [DOI] [PubMed] [Google Scholar]; • Describes log BBB and logPS.
- 50.Clark DE. In silico prediction of blood–brain barrier permeation. Drug Discov. Today. 2003;8(20):927–933. doi: 10.1016/s1359-6446(03)02827-7. [DOI] [PubMed] [Google Scholar]
- 51.Tewes BJ, Galla HJ. Lipid polarity in brain capillary endothelial cells. Endothelium. 2001;8(3):207–220. doi: 10.1080/10623320109051566. [DOI] [PubMed] [Google Scholar]
- 52.Nitz T, Eisenblatter T, Psathaki K, Galla HJ. Serum-derived factors weaken the barrier properties of cultured porcine brain capillary endothelial cells in vitro . Brain Res. 2003;981(1–2):30–40. doi: 10.1016/s0006-8993(03)02834-8. [DOI] [PubMed] [Google Scholar]; • Discusses in vitro BBB model utilizing porcine brain capillary endothelial cells.
- 53.Wager TT, Hou X, Verhoest PR, Villalobos A. Moving beyond rules: the development of a central nervous system multiparameter optimization (CNS MPO) approach to enable alignment of druglike properties. ACS Chem. Neurosci. 2010;1(6):435–449. doi: 10.1021/cn100008c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hawkins PC, Nicholls A. Conformer generation with omega: learning from the data set and the analysis of failures. J. Chem. Inform. Model. 2012;52(11):2919–2936. doi: 10.1021/ci300314k. [DOI] [PubMed] [Google Scholar]
- 55.Loryan I, Sinha V, Mackie C, et al. Molecular properties determining unbound intracellular and extracellular brain exposure of CNS drug candidates. Mol. Pharm. 2014;12(2):520–532. doi: 10.1021/mp5005965. [DOI] [PubMed] [Google Scholar]
- 56.Wager TT, Chandrasekaran RY, Hou X, et al. Defining desirable central nervous system drug space through the alignment of molecular properties, in vitro ADME, and safety attributes. ACS Chem. Neurosci. 2010;1(6):420–434. doi: 10.1021/cn100007x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Allen DD, Smith QR. Characterization of the blood–brain barrier choline transporter using the in situ rat brain perfusion technique. J. Neurochem. 2001;76(4):1032–1041. doi: 10.1046/j.1471-4159.2001.00093.x. [DOI] [PubMed] [Google Scholar]
- 58.Gratton JA, Abraham MH, Bradbury MW, Chadha HS. Molecular factors influencing drug transfer across the blood–brain barrier. J. Pharm. Pharmaol. 1997;49(12):1211–1216. doi: 10.1111/j.2042-7158.1997.tb06072.x. [DOI] [PubMed] [Google Scholar]
- 59.Liu X, Tu M, Kelly RS, Chen C, Smith BJ. Development of a computational approach to predict blood–brain barrier permeability. Drug Metab. Dispos. 2004;32(1):132–139. doi: 10.1124/dmd.32.1.132. [DOI] [PubMed] [Google Scholar]
- 60.Dagenais C, Avdeef A, Tsinman O, Dudley A, Beliveau R. P-glycoprotein deficient mouse in situ blood–brain barrier permeability and its prediction using an in combo pampa model. Eur. J. Pharm. Sci. 2009;38(2):121–137. doi: 10.1016/j.ejps.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ooms F, Weber P, Carrupt PA, Testa B. A simple model to predict blood–brain barrier permeation from 3D molecular fields. Biochim. Biophys. Acta. 2002;1587(2–3):118–125. doi: 10.1016/s0925-4439(02)00074-1. [DOI] [PubMed] [Google Scholar]
- 62.Crivori P, Cruciani G, Carrupt PA, Testa B. Predicting blood–brain barrier permeation from three-dimensional molecular structure. J. Med. Chem. 2000;43(11):2204–2216. doi: 10.1021/jm990968+. [DOI] [PubMed] [Google Scholar]
- 63.Luco JM. Prediction of the brain–blood distribution of a large set of drugs from structurally derived descriptors using partial least-squares (PLS) modeling. J. Chem. Inform. Comput. Sci. 1999;39(2):396–404. doi: 10.1021/ci980411n. [DOI] [PubMed] [Google Scholar]
- 64.Hutter MC. Prediction of blood–brain barrier permeation using quantum chemically derived information. J. Comput. Aid. Mol. Dis. 2003;17(7):415–433. doi: 10.1023/a:1027359714663. [DOI] [PubMed] [Google Scholar]
- 65.Keseru GM, Molnar L. High-throughput prediction of blood–brain partitioning: a thermodynamic approach. J. Chem. Inform. Comput. Sci. 2001;41(1):120–128. doi: 10.1021/ci000043z. [DOI] [PubMed] [Google Scholar]
- 66.Lobell M, Molnar L, Keseru GM. Recent advances in the prediction of blood–brain partitioning from molecular structure. J. Pharm. Sci. 2003;92(2):360–370. doi: 10.1002/jps.10282. [DOI] [PubMed] [Google Scholar]
- 67.Ma XL, Chen C, Yang J. Predictive model of blood–brain barrier penetration of organic compounds. Acta Pharmacol. Sin. 2005;26(4):500–512. doi: 10.1111/j.1745-7254.2005.00068.x. [DOI] [PubMed] [Google Scholar]
- 68.Clark DE. Rapid calculation of polar molecular surface area and its application to the prediction of transport phenomena. 2. Prediction of blood–brain barrier penetration. J. Pharm. Sci. 1999;88(8):815–821. doi: 10.1021/js980402t. [DOI] [PubMed] [Google Scholar]
- 69.Platts JA, Abraham MH, Zhao YH, Hersey A, Ijaz L, Butina D. Correlation and prediction of a large blood–brain distribution data set – an lfer study. Eur. J. Med. Chem. 2001;36(9):719–730. doi: 10.1016/s0223-5234(01)01269-7. [DOI] [PubMed] [Google Scholar]
- 70.Garg P, Verma J. In silico prediction of blood brain barrier permeability: an artificial neural network model. J. Chem. Inform. Model. 2006;46(1):289–297. doi: 10.1021/ci050303i. [DOI] [PubMed] [Google Scholar]
- 71.Wu ZY, Pan J, Yuan Y, Hui AL, Yang Y, Zhou A. Comparison of prediction models for blood brain barrier permeability and analysis of the molecular descriptors. Pharmazie. 2012;67(7):628–634. [PubMed] [Google Scholar]
- 72.Lanevskij K, Japertas P, Didziapetris R. Improving the prediction of drug disposition in the brain. Expert Opin. Drug Metabol. Toxicol. 2013;9(4):473–486. doi: 10.1517/17425255.2013.754423. [DOI] [PubMed] [Google Scholar]
- 73.Fan Y, Unwalla R, Denny RA, et al. Insights for predicting blood–brain barrier penetration of CNS targeted molecules using QSPR approaches. J. Chem. Inform. Model. 2010;50(6):1123–1133. doi: 10.1021/ci900384c. [DOI] [PubMed] [Google Scholar]
- 74.Liu R, Sun H, So SS. Development of quantitative structure-property relationship models for early ADME evaluation in drug discovery. 2. blood–brain barrier penetration. J. Chem. Inform. Comput. Sci. 2001;41(6):1623–1632. doi: 10.1021/ci010290i. [DOI] [PubMed] [Google Scholar]
- 75.Klon AE, Lowrie JF, Diller DJ. Improved naive bayesian modeling of numerical data for absorption, distribution, metabolism and excretion (ADME) property prediction. J. Chem. Inform. Model. 2006;46(5):1945–1956. doi: 10.1021/ci0601315. [DOI] [PubMed] [Google Scholar]
- 76.Li H, Yap CW, Ung CY, Xue Y, Cao ZW, Chen YZ. Effect of selection of molecular descriptors on the prediction of blood–brain barrier penetrating and nonpenetrating agents by statistical learning methods. J. Chem. Inform. Model. 2005;45(5):1376–1384. doi: 10.1021/ci050135u. [DOI] [PubMed] [Google Scholar]
- 77.Rishton GM, Labonte K, Williams AJ, Kassam K, Kolovanov E. Computational approaches to the prediction of blood–brain barrier permeability: a comparative analysis of central nervous system drugs versus secretase inhibitors for Alzheimer's disease. Curr. Opin. Drug Discov. Dev. 2006;9(3):303–313. [PubMed] [Google Scholar]; • Descibes virtual screening of drugs by predicting their BBB permeability.
- 78.Mittapalli RK, Manda VK, Adkins CE, Geldenhuys WJ, Lockman PR. Exploiting nutrient transproters at the blood–brain barrier to improve brain distribution of small molecules. Ther. Deliv. 2011;1(6):775–784. doi: 10.4155/tde.10.76. [DOI] [PubMed] [Google Scholar]; •• Describes the role of nutrient transporters in brain distribution of drugs.
- 79.Peura L, Malmioja K, Huttunen K, et al. Design, synthesis and brain uptake of lat1-targeted amino acid prodrugs of dopamine. Pharm. Res. 2013;30(10):2523–2537. doi: 10.1007/s11095-012-0966-3. [DOI] [PubMed] [Google Scholar]
- 80.Peura L, Malmioja K, Laine K, et al. Large amino acid transporter 1 (LAT1) prodrugs of valproic acid: new prodrug design ideas for central nervous system delivery. Mol. Pharm. 2011;8(5):1857–1866. doi: 10.1021/mp2001878. [DOI] [PubMed] [Google Scholar]
- 81.Dickens D, Webb SD, Antonyuk S, et al. Transport of gabapentin by lat1 (slc7a5) Biochem. Pharmacol. 2013;85(11):1672–1683. doi: 10.1016/j.bcp.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 82.Koziara JM, Lockman PR, Allen DD, Mumper RJ. The blood–brain barrier and brain drug delivery. J. Nanosci. Nanotechnol. 2006;6(9–10):2712–2735. doi: 10.1166/jnn.2006.441. [DOI] [PubMed] [Google Scholar]
- 83.Allen DD, Lockman PR. The blood–brain barrier choline transporter as a brain drug delivery vector. Life Sci. 2003;73(13):1609–1615. doi: 10.1016/s0024-3205(03)00504-6. [DOI] [PubMed] [Google Scholar]
- 84.Zheng G, Zhang Z, Lockman PR, et al. Bis-azaaromatic quaternary ammonium salts as ligands for the blood–brain barrier choline transporter. Bioorg. Med. Chem. Lett. 2010;20(11):3208–3210. doi: 10.1016/j.bmcl.2010.04.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.In situ WJ, Manda VK, Mittapalli RK, et al. Predictive screening model for potential vector-mediated transport of cationic substrates at the blood–brain barrier choline transporter. Bioorg. Med. Chem. Lett. 2010;20(3):870–877. doi: 10.1016/j.bmcl.2009.12.079. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Describes predictive screening models for choline transporters.
- 86.Zhang Z, Lockman PR, Mittapalli RK, Allen DD, Dwoskin LP, Crooks PA. Bis-pyridinium cyclophanes: novel ligands with high affinity for the blood–brain barrier choline transporter. Bioorg. Med. Chem. Lett. 2008;18(20):5622–5625. doi: 10.1016/j.bmcl.2008.08.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lockman PR, Manda VK, Geldenhuys WJ, et al. Carrier-mediated transport of the quaternary ammonium neuronal nicotinic receptor antagonist n, n’-dodecylbispicolinium dibromide at the blood–brain barrier. J. Pharmacol. Exp. Ther. 2008;324(1):244–250. doi: 10.1124/jpet.107.130906. [DOI] [PubMed] [Google Scholar]
- 88.Geldenhuys WJ, Lockman PR, Nguyen TH, et al. 3D-QSAR study of bis-azaaromatic quaternary ammonium analogs at the blood–brain barrier choline transporter. Bioorg. Med. Chem. 2005;13(13):4253–4261. doi: 10.1016/j.bmc.2005.04.020. [DOI] [PubMed] [Google Scholar]; •• Discusses various predictive models for transport assisted by transporter.
- 89.Geldenhuys WJ, Lockman PR, Mcafee JH, Fitzpatrick KT, Van Der Schyf CJ, Allen DD. Molecular modeling studies on the active binding site of the blood–brain barrier choline transporter. Bioorg. Med. Chem. Lett. 2004;14(12):3085–3092. doi: 10.1016/j.bmcl.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 90.Ekins S, Tropsha A. A turning point for blood–brain barrier modeling. Pharm. Res. 2009;26(5):1283–1284. doi: 10.1007/s11095-009-9832-3. [DOI] [PubMed] [Google Scholar]