Abstract
Inflammasomes are cytoplasmic, multiprotein complexes that trigger caspase-1 activation and IL-1β maturation in response to diverse stimuli. Although inflammasomes play important roles in host defense against microbial infection, overactive inflammasomes are deleterious and lead to various autoinflammatory diseases. In the current study, we demonstrated that genipin inhibits the induction of IL-1β production and caspase-1 activation by NLRP3 and NLRC4 inflammasomes. Furthermore, genipin specifically prevented NLRP3-mediated, but not NLRC4-mediated, ASC oligomerization. Notably, genipin inhibited autophagy, leading to NLRP3 and NLRC4 inflammasome inhibition. UCP2-ROS signaling may be involved in inflammasome suppression by genipin. In vivo, we showed that genipin inhibited NLRP3-dependent IL-1β production and neutrophil flux in LPS- and alum-induced murine peritonitis. Additionally, genipin provided protection against flagellin-induced lung inflammation by reducing IL-1β production and neutrophil recruitment. Collectively, our results revealed a novel role in inhibition of inflammatory diseases for genipin that has been used as therapeutics for centuries in herb medicine.
Inflammasomes are cytoplasmic, multiprotein complexes that control the production of pro-inflammatory cytokines, such as IL-1β and IL-18, in response to a diverse set of inflammation-inducing stimuli, including pathogen-derived, host-derived and environmental factors1. Members of both the NOD-like receptor (NLR) and the absent in melanoma 2 (AIM2)-like receptor (ALR) families act as sensor molecules in inflammasomes. Activation of inflammasome sensors leads to the recruitment of ASC, an adaptor molecule, and procaspase-1; this is followed by the autoproteolytic activation of procaspase-1. Activated caspase-1 then induces cleavage of the proforms of IL-1β and IL-18 and stimulates pyroptosis2, a type of cell death mediated by inflammasomes.
Although inflammasomes play critical roles in anti-bacterial, anti-viral, anti-fungal and anti-parasitic immune responses, inflammasome overactivation has emerged as a critical mechanism underlying various chronic inflammatory metabolic diseases, such as atherosclerosis3, type 2 diabetes (T2D)4,5, gout6, colitis7, and Alzheimer’s disease8. Thus, regulating inflammasome activation is a promising strategy for treating inflammasome-mediated disorders; however, effective therapies to control the detrimental effects of overactive inflammasomes are lacking.
Gardenis jasminoides Ellis fruits have been used for centuries in traditional Chinese medicine. Gardenis fruits protect the liver and gallbladder and exert anti-hypertensive, anti-bleeding, and anti-swelling properties, as well as other beneficial effects. Therefore, these fruits have been extensively used to treat various diseases, including icteric hepatitis, hypertension and diabetes. Genipin, a major component in Gardenis fruits, also exhibits anti-inflammatory and anti-angiogenic properties9,10. Thus, genipin may regulate inflammasome activation.
Here, we demonstrated that activation of the NLR family pyrin domain-containing protein 3 (NLRP3) and CARD domain-containing protein 4 (NLRC4) inflammasomes is inhibited by genipin. Consequently, genipin is a potential therapeutic for NLRP3- and NLRC4-driven diseases.
Results
Genipin suppresses NLRP3 and NLRC4 inflammasome-mediated IL-1β secretion and caspase-1 activation
To investigate the effect of genipin on NLRP3 inflammasome activation, lipopolysaccharide (LPS)-primed murine bone marrow-derived macrophages (BMDMs) were treated with genipin for 1 h before stimulation with ATP, a conventional NLRP3 inflammasome agonist. We found that genipin dramatically inhibited IL-1β secretion in a dose-dependent manner (Fig. 1A). In addition to ATP, a wide range of stimuli can activate the NLRP3 inflammasome. To examine whether genipin affects NLRP3 inflammasome activation via stimuli other than ATP, we treated LPS-primed BMDMs with nigericin, monosodium urate (MSU) crystals or Listeria monocytogenes (Listeria) in the presence of 200 μM genipin, a dose that did not inhibit bacterial growth or macrophage viability (Figure S1 and S3, Supplementary Information). Under these conditions, genipin also inhibited IL-1β secretion (Fig. 1B). NLRP3 inflammasome-dependent IL-1β secretion in response to nigericin or Listeria was confirmed using NLRP3-deficient macrophages (Figure S2A). Listeria-triggered IL-1β secretion was dose-dependently inhibited by genipin (Figure S2B).
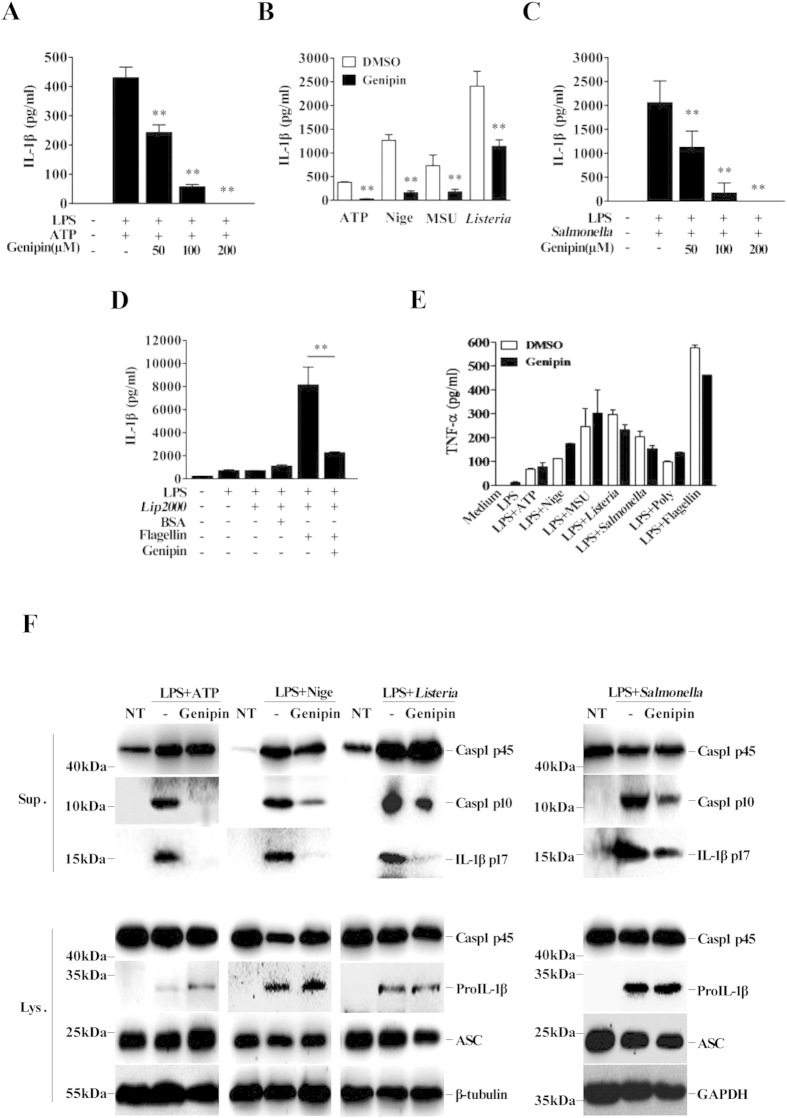
Figure 1. Genipin inhibits NLRP3 and NLRC4 inflammasome-mediated IL-1β secretion and caspase-1 activation in mouse macrophages.
LPS-primed BMDMs were incubated with genipin (200 μM unless otherwise indicated) or DMSO for 1 h, followed by treatment with various NLRP3 or NLRC4 inflammasome agonists. Culture supernatants were analyzed for IL-1β and TNF-α by ELISA. Precipitated cell supernatants (Sup) or cell extracts (Lysate) were immunoblotted using various antibodies. (A) IL-1β secretion in BMDMs stimulated with the indicated doses of genipin and ATP (5 mM, 1 h). (B) IL-1β secretion in BMDMs stimulated with genipin plus ATP, nigericin (Nige, 20 μM, 1 h), MSU (100 μg/ml, 4 h) or Listeria (MOI = 20, 4 h). (C) IL-1β secretion in BMDMs stimulated with the indicated doses of genipin and Salmonella (MOI = 20, 4 h). (D) IL-1β secretion in BMDMs transfected with 20 μg/ml flagellin or BSA for 8 h using Lipofectamine 2000. (E) TNF-α secretion in BMDMs stimulated with genipin plus ATP, nigericin, MSU, Listeria, Salmonella or flagellin. (F) Cell supernatants and cell extracts immunoblotted for caspase-1, IL-1β and ASC. GAPDH and β-tubulin served as loading controls. The data are representative of three independent experiments. **P < 0.01.
To determine whether genipin-mediated IL-1β inhibition is specific to the NLRP3 inflammasome, the effects of this compound on NLRC4 inflammasome activation were assessed. Salmonella typhimurium (Salmonella) triggers the secretion of IL-1β by activating the NLRC4 inflammasome, and this effect was dose-dependently inhibited by genipin (Fig. 1C). Cytosolic bacterial flagellin is the primary trigger that activates the NLRC4 inflammasome11. As shown in Fig. 1D, transfection with flagellin, but not with BSA, stimulated IL-1β secretion in LPS-primed BMDMs, and genipin pretreatment significantly inhibited this induction. In contrast, LPS-dependent TNF-α secretion was not impaired by genipin (Fig. 1E).
We next evaluated IL-1β production and caspase-1 cleavage in BMDMs stimulated with ATP, nigericin, Listeria or Salmonella using immunoblotting. Consistent with ELISA results, genipin inhibited the production of mature IL-1β in cell supernatants, suggesting that genipin inhibits IL-1β processing by NLRP3 and NLRC4 (Fig. 1F). Furthermore, caspase-1 p10 levels were reduced in supernatants collected from genipin-treated BMDMs, suggesting that genipin inhibits caspase-1 activation (Fig. 1F). The dependence of IL-1β release on caspase-1 activation was confirmed using the caspase inhibitor z-VAD-fmk (Figure S2C). Collectively, the above results indicate that genipin potently inhibits NLRP3 and NLRC4 inflammasome activation. Notably, genipin treatment did not affect the expression of pro-IL-1β, pro-caspase-1 or ASC proteins in cell lysates or supernatants.
Genipin inhibits NLRP3-mediated ASC oligomerization
We next examined ASC oligomerization, a common event associated with inflammasome activation12. The ASC pyroptosome, a complex of monomers, dimers and oligomers, can be cross-linked by applying disuccinimidyl suberate (DSS) to cytosolic fractions of cell lysates. Immunoblotting can then be used to detect this complex, the presence of which indicates ASC oligomerization. Alternatively, ASC oligomerization can be confirmed by the formation of ASC speckles via microscopy. In agreement with previous reports, stimulation with either LPS plus nigericin or Listeria induced the formation of ASC monomers, dimers and oligomers in the absence of genipin, whereas NLRP3 deficiency blocked nigericin-induced ASC pyroptosome formation (Fig. 2A,B). However, genipin dose-dependently inhibited the induction of ASC pyroptosome formation by nigericin or Listeria. Correspondingly, the quantity of active caspase-1 in cell supernatants was reduced by genipin. Additionally, immunostaining for endogenous ASC in macrophages showed that the induction of ASC speckles following treatment with either LPS plus nigericin or Listeria diminished in the presence of genipin (Fig. 2C,D). Thus, these data indicate that genipin inhibits NLRP3-dependent ASC oligomerization in addition to IL-1β secretion and caspase-1 activation.
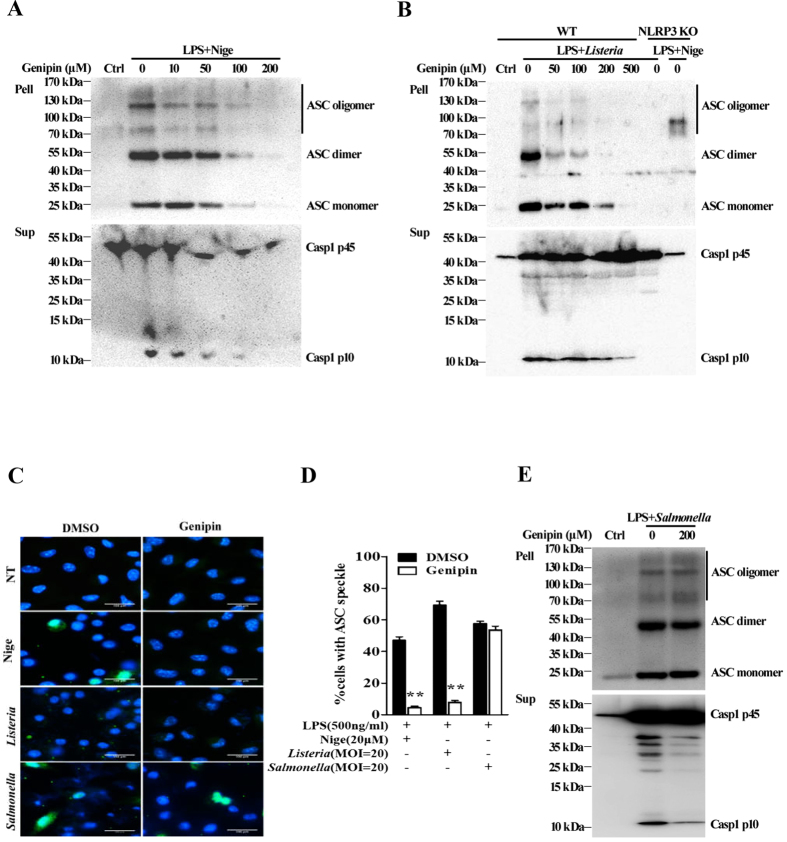
Figure 2. Genipin prevents NLRP3-mediated, but not NLRC4-mediated, ASC oligomerization.
(A) LPS-primed BMDMs were pretreated with the indicated doses of genipin for 1 h and then stimulated with nigericin. The cells were extracted with 0.5% Triton X-100, and Triton-insoluble pellets were cross-linked with DSS. The cross-linked pellets were immunoblotted for ASC as described in the Materials and Methods section. The culture supernatants were immunoblotted for caspase-1. (B) LPS-primed wild-type (WT) or NLRP3 KO BMDMs were pretreated with the indicated doses of genipin, followed by Listeria or nigericin challenge. The cell pellets cross-linked with DSS were immunoblotted for ASC, and cell supernatants were immunoblotted for caspase-1. (C) LPS-primed BMDMs were stimulated with nigericin, Listeria or Salmonella in the absence or presence of genipin. The cells were fixed, permeabilized and stained for ASC (green). DAPI was used to label nuclei (blue). (D) The percentage of cells containing ASC speckles was quantified from three different view fields. (E) LPS-primed BMDMs were either not treated or pretreated with genipin, followed by Salmonella challenge. The cross-linked pellets were immunoblotted for ASC, and the culture supernatants were immunoblotted for caspase-1. The data are representative of three independent experiments. **P < 0.01.
Next, we examined the impact of genipin on NLRC4-mediated ASC oligomerization. As shown in Fig. 2C–E, stimulation with Salmonella induced the formation of ASC speckles and pyroptosomes in BMDMs. In contrast to its inhibitory effect on NLRP3-mediated ASC oligomerization, genipin did not affect NLRC4-mediated formation of ASC speckles or pyroptosomes, although NLRC4-mediated caspase-1 activation significantly diminished. These data indicate that the inhibitory effect of genipin on NLRC4 inflammasome activation is independent of ASC.
Genipin inhibits autophagy-mediated inflammasome activation
Inflammasome activation is often accompanied by caspase-1-mediated rapid cell death, which is known as pyroptosis. We examined the effect of genipin on cell death phenotype using an Annexin V/Propidium Iodide (Annexin V/PI) assay that has previously been applied to characterize pyroptosis13. Our results showed that cell death induction by either LPS plus nigericin or Salmonella was significantly inhibited by genipin (Fig. 3A and Figure S3). Furthermore, to exclude the effect of genipin on apoptotic cell death, we used immunoblotting to detect caspase-3 activation in response to various doses of genipin (Figure S3B). One limitation of the Annexin V/PI assay used above is its lack of specificity in detecting pyroptosis, as cells undergoing either autophagy or necroptosis also lose plasma membrane integrity14,15. Furthermore, the specific inhibitory effect of genipin on NLRP3-mediated, but not NLRC4-mediated, ASC oligomerization suggests that the compound may affect upstream signaling, such as the autophagy signaling pathway.
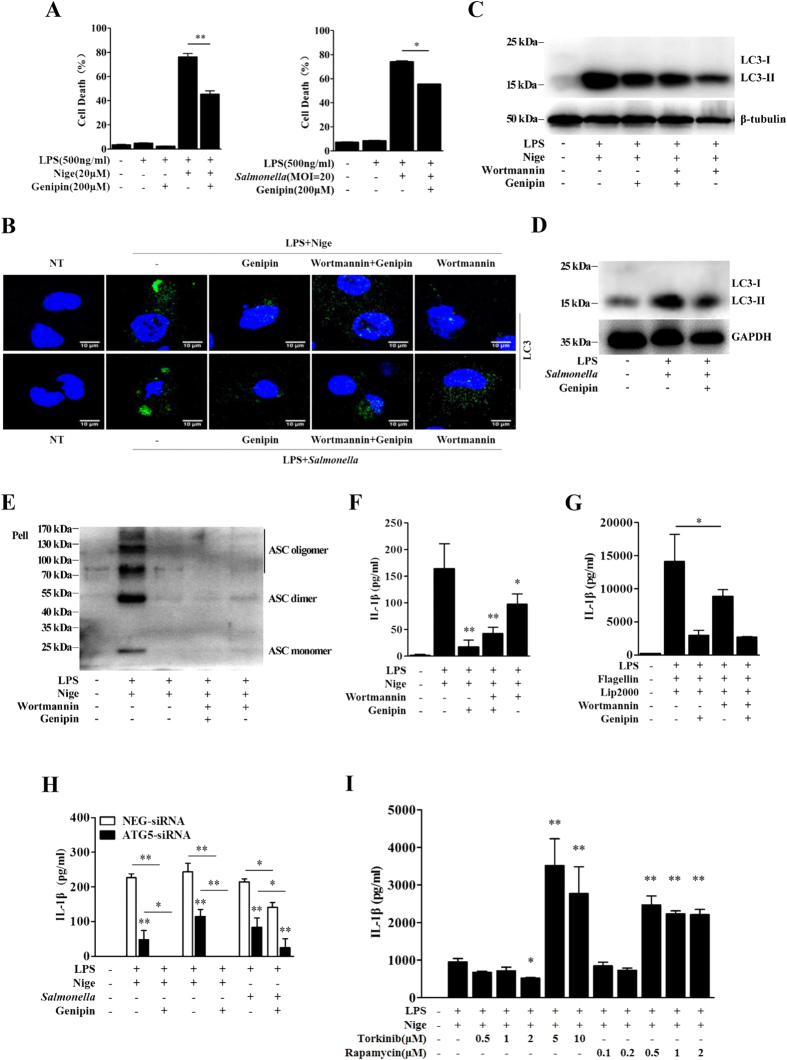
Figure 3. Genipin inhibits autophagy-dependent inflammasome activation.
(A) Analysis of the cell death phenotype by Annexin-V/PI staining. LPS-primed BMDMs were pretreated with genipin and then stimulated with nigericin or Salmonella. The cells were stained with Annexin-V and PI. The percentage of cells positive for both Annexin V and PI is shown. (B) LPS-primed BMDMs were incubated with genipin and/or 5 μM wortmannin for 1 h and then stimulated with nigericin or Salmonella. The cells were fixed, permeabilized and stained for LC3. LC3 is shown in green, and cell nuclei are shown in blue (DAPI). (C,D) LPS-primed BMDMs were incubated with genipin and/or wortmannin and then stimulated with nigericin or Salmonella. The cell lysates were immunoblotted for LC3, β-tubulin or GAPDH. (E) LPS-primed BMDMs were incubated with genipin and/or wortmannin and then stimulated with nigericin. The cross-linked pellets were immunoblotted for ASC. (F,G) LPS-primed BMDMs were incubated with genipin and/or wortmannin and then stimulated with nigericin or flagellin. IL-1β secretion was measured by ELISA. (H) BMDMs were transfected with either siRNA targeting ATG5 or scrambled siRNA (NEG). Forty-eight hours after transfection, the cells were primed with LPS for 4 h and then stimulated with genipin before nigericin or Salmonella challenge. IL-1β secretion was measured by ELISA. (I) LPS-primed BMDMs were incubated with the indicated doses of torkinib or rapamycin for 1 h and then stimulated with nigericin. IL-1β secretion was measured by ELISA. The data are from three independent experiments conducted in triplicate. *P < 0.05 and **P < 0.01 compared with controls.
To assess autophagy, LC3 puncta formation was assessed by detecting LC3-II, a key marker of autophagy, by immunofluorescence in GFP-LC3 plasmid-transfected cells or cell lysates. Both LPS plus nigericin and Salmonella induced LC3 puncta formation and increased LC3-II protein level (Fig. 3B–D). In addition to inflammasome suppression, genipin also prevented autophagosome induction by nigericin and Salmonella (Fig. 3B–D). Wortmannin, an inhibitor of phosphatidylinositol 3-kinase, which is involved in initial autophagosome formation, was used to inhibit autophagy. Our results showed that wortmannin indeed inhibited nigericin-induced LC3 puncta formation and LC3-II protein expression (Fig. 3B,C). Similar to the effects of genipin, the inhibition of autophagy by wortmannin reduced nigericin-triggered ASC oligomer formation and IL-1β secretion (Fig. 3E,F). Wortmannin also inhibited cytosolic flagellin-induced IL-1β production (Fig. 3G). Furthermore, ATG5 knockdown by siRNA significantly prevented the production of IL-1β in response to both LPS plus nigericin and Salmonella. This effect was potentiated by genipin (Fig. 3H). ATG5 knockdown was confirmed by Western blotting (Figure S4A). At low doses, pretreatment of BMDMs with rapamycin, an autophagy inducer, strongly enhanced nigericin-induced IL-1β release, caspase-1 cleavage and LC3-II protein up-regulation. Conversely, at high doses, rapamycin degraded pro-IL-1β and inhibited IL-1β release. Similarly, the induction of autophagy by torkinib, an mTOR inhibitor, also promoted nigericin-mediated IL-1β production, caspase-1 cleavage and LC3-II protein up-regulation (Fig. 3I and Figure S5 A,B). Collectively, these results indicate that genipin inhibits inflammasome activation by regulating autophagy.
Genipin inhibits UCP2 expression and promotes ROS production
Because genipin was previously categorized as an uncoupling protein 2 (UCP2) inhibitor16, we examined the expression of UCP2 in BMDMs stimulated with ATP or Salmonella in the absence or presence of genipin. By immunofluorescence and immunoblotting, we found that treatment of BMDMs with either LPS plus ATP or Salmonella induced UCP2 expression (Fig. 4A,B). UCP2 induction by Salmonella was also confirmed in human macrophage-like THP-1 cells and human cervical cancer cells (HeLa) (Figure S4B). Genipin treatment dramatically inhibited both ATP- and salmonella-stimulated UCP2 expression (Fig. 4A,B).
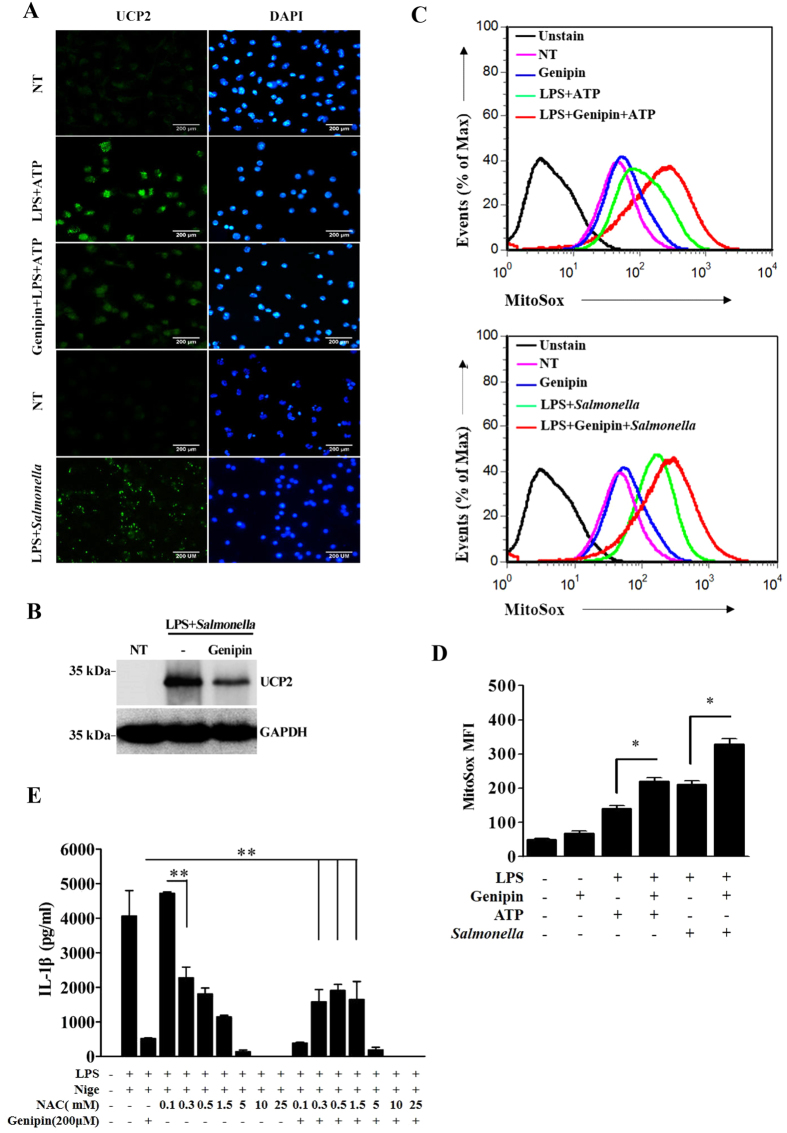
Figure 4. Genipin inhibits UCP2 expression and induces ROS accumulation.
(A) LPS-primed BMDMs were pretreated with genipin and then stimulated with ATP or Salmonella. The cells were fixed, permeabilized and stained for UCP2 (green). DAPI was used to label nuclei (blue). (B) LPS-primed BMDMs stimulated with genipin and/or Salmonella were lysed and blotted for UCP2. (C) LPS-primed BMDMs were stimulated with genipin and/or ATP and/or Salmonella. The cells were incubated with MitoSox Red for 10 min and analyzed using a FACSAria flow cytometer. (D) MitoSox MFI was analyzed using FCS express. (E) LPS-primed BMDMs were pretreated with genipin and/or NAC at the indicated doses and then stimulated with nigericin. IL-1β release in the cell supernatants was determined by ELISA. The data are representative of three independent experiments. **P < 0.01.
UCP2 is an inner mitochondrial membrane protein that has been shown to directly prevent the generation of reactive oxygen species (ROS)17. Furthermore, emerging data suggest that mitochondrial ROS play a critical role in regulating inflammasome activation18,19,20. Therefore, we examined mitochondrial ROS production by measuring the mean fluorescence intensity (MFI) of MitoSox Red. After 4 h of LPS priming, treatment with ATP or Salmonella increased mitochondrial ROS production (Fig. 4C,D). In addition, genipin enhanced ATP- and Salmonella-induced mitochondrial ROS production. We then used the ROS scavenger NAC to determine whether the inhibitory effects of genipin require ROS. In agreement with previous reports21,22, we observed that various doses of NAC inhibited nigericin-induced IL-1β secretion in LPS-primed macrophages (Fig. 4E). Interestingly, pretreatment with NAC at low doses reversed the inhibitory effects of genipin on nigericin-induced IL-1β secretion. These results suggested that UCP2-mediated ROS induction triggered by genipin is involved in inflammasome suppression.
Genipin inhibits LPS- and alum-induced peritonitis and attenuates flagellin-induced lung inflammation
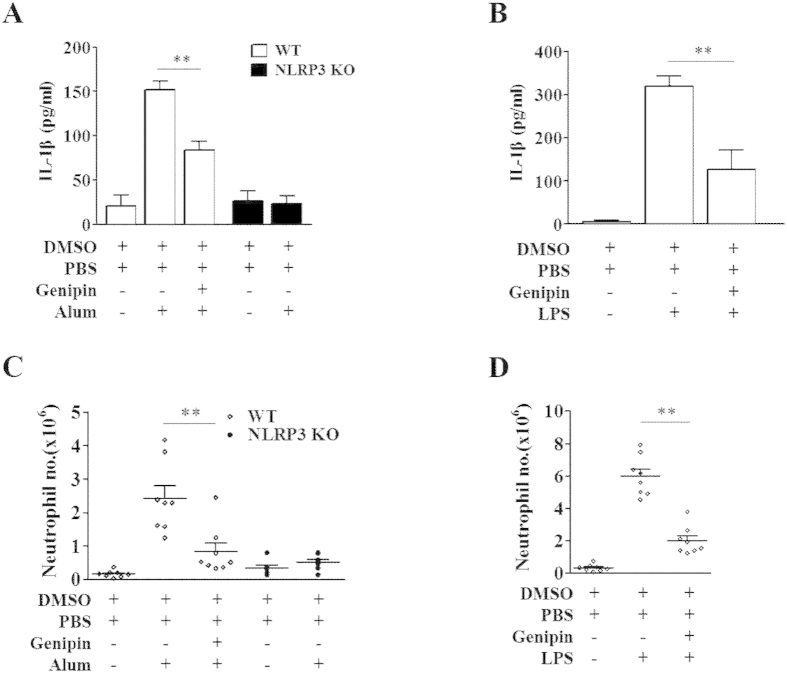
We next investigated the effects of genipin in vivo. IL-1β induction following intraperitoneal (i.p.) injection of either LPS or alum has been previously shown to be NLRP3 dependent23,24,25. Thus, we examined whether genipin can block this induction of IL-1β in vivo. Mice were pretreated with genipin 1 h before i.p. injection of LPS or alum and were assessed 6 h later (Fig. 5A–D and Figure S6A,B). IL-1β secretion and neutrophil recruitment in the peritoneum were significantly higher in mice treated with LPS or alum than in control mice, whereas NLRP3 deficiency abolished the induction by alum. Pretreatment with genipin significantly reduced IL-1β secretion and neutrophil flux. These results indicated that genipin attenuates disease development by inhibiting NLRP3 inflammasome activation in vivo.
Figure 5. Genipin inhibits inflammatory responses to alum and LPS in vivo.
C57BL/6J mice (n = 8 per group) and NLRP3−/− mice (n = 5 per group) were i.p. injected with genipin (2 mg per body weight, i.p.) or DMSO for 1 h, followed by LPS (35 mg/kg of body weight, i.p.) or alum (2 mg per body weight, i.p.) challenge for 6 h. (A,B) IL-1β concentration in the supernatant of peritoneal lavage fluid was determined. (C,D) The number of neutrophils that had migrated into the peritoneal cavities was quantified. **P < 0.01.
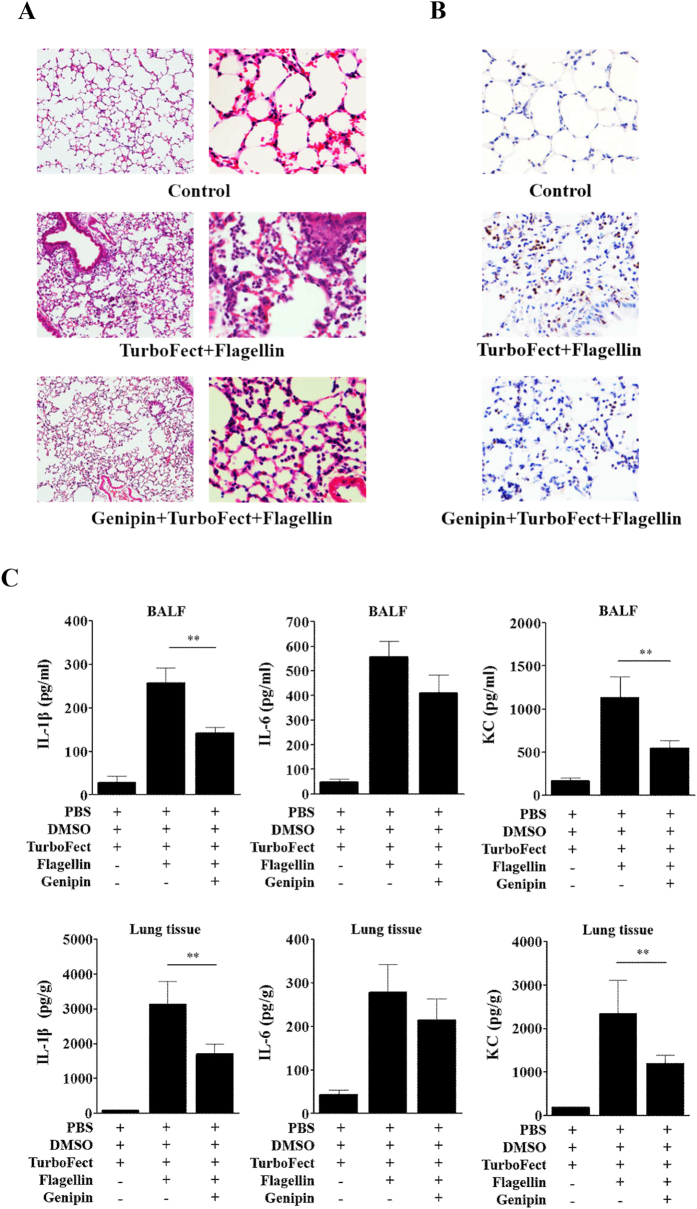
To evaluate the role of genipin on NLRC4 inflammasome activation in vivo, a mouse model of flagellin-induced lung inflammation was used. Analysis of histological sections stained with H&E showed severe pneumonia in flagellin-challenged mice, and pretreatment with genipin reduced this lung pathology (Fig. 6A). Consistent with pathological changes, neutrophil accumulation in the airspaces following flagellin challenge was strikingly attenuated by genipin (Fig. 6B). We further determined the expression levels of IL-1β, IL-6 (a known downstream target of IL-1β) and KC (a neutrophil chemoattractant chemokine) in bronchoalveolar lavage fluid (BALF) and lung homogenate. We found that genipin inhibited flagellin-induced IL-1β, IL-6 and KC expression (Fig. 6C). Collectively, these data suggest that genipin reduces flagellin-mediated inflammatory responses in vivo. In addition, these results supported that genipin inhibits inflammasome activation in macrophages.
Figure 6. Genipin inhibits pneumonia induced by the mucosal administration of flagellin.
C57BL/6J mice (n = 8 per group) were i.p. injected with genipin (2 mg per body weight, i.p.) 1 h before being intranasally instilled with a mixture of TurboFect in vivo transfection reagent and flagellin in a total volume of 50 μl. Lung tissue and BALF were collected 12 h after flagellin instillation. Control mice received an equivalent dose of DMSO and the mixture of PBS and TurboFect. (A) Lung sections were prepared and stained with H&E for histological analysis (magnification, 10/40×). (B) Neutrophil infiltration was detected by immunohistochemical staining for Gr-1 (magnification, 40×). (C) BALF and lung homogenate were collected for the analysis of IL-1β, IL-6 and KC production by ELISA. **P < 0.01.
Discussion
Gardenia jasminoides Ellis fruits have been used for centuries as an herbal medicine to relieve the symptoms of various diseases. Genipin, the major active component in Gardenia fruits, has been reported to exhibit anti-inflammatory activities. In the current study, we presented a new application of genipin as an anti-inflammatory agent. Our work suggests that the anti-inflammatory property of genipin may result from its ability to suppress inflammasome activation, which consequently inhibits IL-1β secretion and caspase-1 cleavage. The lack of effect of genipin on TNF-α, pro-IL-1β, pro-caspase-1 and ASC levels demonstrates that it specifically inhibits inflammasome activation.
ASC oligomerization is a common event associated with NLRP3 inflammasome activation26. Although NLRC4 can directly interact with pro-caspase-1 via CARD-CARD interactions, efficient pro-IL-1β processing in macrophages by the NLRC4 inflammasome still depends on ASC recruitment27,28,29. In the current study, genipin prevented NLRP3 agonist-induced ASC-complex formation, but it did not affect ASC-complex formation induced by stimuli that activate NLRC4.
The specific inhibition of NLRP3-mediated, but not NLRC4-mediated, ASC oligomerization suggests that genipin may affect upstream signaling, such as the autophagy signaling pathway. NLRP3 and NLRC4 inflammasome agonists significantly triggered autophagy in macrophages. Moreover, autophagy induction potentiated inflammasome-mediated IL-1β secretion and ASC oligomerization, whereas autophagy suppression by chemical or gene knockdown prevented inflammasome activation. These findings indicate that genipin inhibits inflammasome activation by suppressing autophagy in macrophages. Paradoxically, autophagy has previously been shown to both promote30 and inhibit NLRP3 inflammasome-mediated IL-1β secretion31,32. Our results confirmed that autophagy contributes to inflammasome activation. However, the underlying mechanism leading to this discrepancy remains elusive.
Mitochondrial ROS are critical to inflammasome activation18,33,34. Moreover, the UCP2 gene, a known target of genipin, encodes an inner mitochondrial membrane protein that directly prevents ROS generation17,35. Thus, we examined whether the UCP2-ROS signaling pathway is involved in the inhibitory effects of genipin. We found that agonists of NLRP3 and NLRC4 inflammasomes induced UCP2 expression and ROS production. Furthermore, genipin inhibited UCP2 expression and enhanced ROS production. The increases in ROS triggered by genipin appear to contradict its inhibitory effects on the inflammasome. We further used the ROS scavenger NAC to determine whether ROS affect the inhibitory effects of genipin on inflammasome activation. Consistent with previous reports, our results showed that NAC pretreatment inhibited nigericin-induced IL-1β secretion18,33,34. However, low doses of NAC reversed the inhibitory effects of genipin on nigericin-induced IL-1β secretion. In fact, there is some evidence that ROS are not absolutely required for inflammasome activation22. For example, the ketone metabolite β-hydroxybutyrate mediates NLRP3 inflammasome inhibition in an ROS-independent manner36. Furthermore, ROS induction by As2O3 is required to inhibit inflammasome activation37; this phenomenon is very similar to that observed with genipin. Thus, the above results indicate that UCP2-ROS signaling is involved in genipin-mediated inflammasome suppression. In addition, in THP-1 cells, genipin suppresses ATP-induced, but not nigericin-induced, NLRP3 inflammasome activation via UCP238. However, our results show that genipin inhibits ATP-, nigericin-, MSU- and Listeria-induced NLRP3 inflammasome activation in BMDMs, as well as Salmonella- and flagellin-induced NLRC4 inflammasome activation. The current study did not elucidate how UCP2-ROS signaling regulates the inflammasome or autophagy; consequently, this relationship requires further investigation.
Because overactive inflammasomes play critical roles in various chronic inflammatory diseases, the identification of anti-inflammasome compounds is of clinical importance37,39. Inflammasome activation can be inhibited by several small molecules, including MCC95025, glyburide40, 3,4-methylenedioxy-β-nitrostyrene41, arsenic37 and NO24. We demonstrated that genipin ameliorated the severity of LPS- and alum-induced peritonitis and flagellin-induced pneumonia in vivo; both conditions are well-known inflammasome-mediated inflammatory diseases. Thus, genipin and other structurally related compounds may be useful anti-inflammatory agents for the treatment of inflammasome-related disorders.
In conclusion, our results demonstrated that genipin plays a prominent role in inhibiting NLRP3 and NLRC4 inflammasome activation in vitro and NLRP3- and NLRC4-dependent inflammation in vivo. Thus, genipin is a potential therapeutic for NLRP3- and NLRC4-associated syndromes and a valid reagent for further studies examining the roles of NLRP3 and NLRC4 inflammasomes in human health.
Methods
Mice and cells
NLRP3−/− (knockout) and C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). All animal studies were conducted according to experimental practices and standards approved by the Animal Welfare and Research Ethics Committee at Jilin University. BMDMs were prepared and cultured as previously described42. In brief, BMDMs were isolated from the femurs of 8- to 10-week-old mice and cultured in RPMI-1640 medium containing 10% heat-inactivated FBS, 25% L929 cell-conditioned medium, 100 U/ml penicillin, and 100 U/ml streptomycin at 37 °C in a humidified atmosphere containing 5% CO2. The cells were harvested for assays after 7 days of differentiation.
Bacteria
S. typhimurium SL1344 was a gift from Dr. Xiang-Chao Cheng (Henan University of Science and Technology, Luoyang, China). L. monocytogenes 10403S was a gift from Dr. Wei-Huan Fang (Microbiology Institute of Preventive Veterinary Medicine, Zhejiang University, Hangzhou, China).
Chemicals and antibodies
Genipin, wortmannin, rapamycin, and torkinib were purchased from Selleck (Shanghai, China). Lipofectamine 2000 and Opti-MEM were purchased from Invitrogen (Carlsbad, CA, USA). LPS from E. coli 0111:B4, ATP, nigericin, MSU, and z-VAD-fmk were purchased from InvivoGen (San Diego, CA, USA). Alum, flagellin and N-acetylcysteine (NAC) were purchased from Sigma (Shanghai, China). Caspase-1, ASC and IL-1β antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Rabbit anti-UCP2 polyclonal antibody and GAPDH were purchased from Proteintech (Wuhan, China). Rabbit anti-LC3 polyclonal antibody was obtained from Abcam (Cambridge, MA, USA). Rabbit anti-cleaved caspase-3 polyclonal antibody was purchased from Cell Signaling (Beverly, MA). Mouse anti-β-tubulin monoclonal antibody was purchased from Sungene Biotech (Tianjin, China).
Inflammasome activation
BMDMs were primed with LPS (500 ng/ml) for 4 h in serum-free RPMI-1640 medium and then washed twice with RPMI-1640 medium. The cells were then incubated with genipin or inhibitors for 1 h before being pulsed with ATP (5 mM, 1 h), nigericin (20 μM, 1 h), MSU (100 μg/ml, 4 h), flagellin (20 μg/ml, 8 h), Salmonella (MOI = 20, 4 h) or Listeria (MOI = 20, 4 h). Flagellin was introduced into macrophages using Lipofectamine 2000 reagent. BMDMs were treated with live Salmonella and Listeria for 1 h and then washed and incubated in RPMI-1640 medium containing a cocktail of antibiotics (200 U/ml penicillin, 200 mg/ml streptomycin and 100 μg/ml gentamicin) for 3 h. After treatment, cell supernatants and cell pellets were collected for ELISA and Western blotting.
ASC speckle detection and UCP2 staining
ASC speckles and UCP2 were detected using an indirect immunofluorescence method as previously described43. After stimulation, BMDMs plated on 24-well chamber slides were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100. After blocking with 5% goat serum for 1 h at room temperature, the cells were incubated with anti-ASC (1:100) and Alexa Fluor® 488-conjugated anti-rabbit IgG (1:1000, Invitrogen). DAPI (1 μg/ml) was used to stain nuclei. After sequential excitation, images of the same cell were saved with cellSens Dimension software and analyzed using ImageJ software.
ASC oligomer cross-linking
For ASC oligomer cross-linking, cells were dissolved with lysis buffer (1% NP-40, 150 mM KCl and 20 mM HEPES-KOH, pH 7.5) supplemented with protease inhibitors (0.1 mM PMSF, 1 μg/ml leupeptin, 11.5 μg/ml aprotinin and 1 mM sodium orthovanadate). The lysates were centrifuged at 6000 rpm for 15 min at 4 °C. The pellets were washed twice with PBS and then suspended in 500 μl of PBS. The pellets were then cross-linked at room temperature for 30 min by adding disuccinimidyl substrate (2 mM). The cross-linked pellets were centrifuged at 13,000 rpm for 15 min and then dissolved in SDS sample buffer.
Flow cytometric determination of cell death
Cell death was analyzed using an Annexin V apoptosis detection kit (eBioscience) as previously described43. Briefly, both floating and adherent BMDMs were collected and resuspended in 100 μl of Annexin V-binding buffer. After staining with 5 μl of FITC-conjugated Annexin V for 15 min at room temperature in the dark, the cells were washed and incubated with propidium iodide (PI). All cell samples were assayed using a FACSAria flow cytometer (BD Biosciences), and the acquired data were further analyzed using FCS Express (De Novo Software).
ROS assay
To evaluate mitochondrial superoxide production, BMDMs were incubated with MitoSox Red (M36008, Invitrogen, UK) for 10 min at 37 °C in the dark, followed by inflammasome activation treatment as previously described. The cells were then washed with HBSS and analyzed using a FACSAria flow cytometer. In addition, MitoSox MFI was analyzed using FCS Express.
Western blotting
Cell culture supernatants were precipitated by the addition of an equal volume of methanol and 0.25 volumes of chloroform and then vortexed for 10 seconds44. After standing for 5 minutes at room temperature, the mixtures were centrifuged at 13,000 × g for 10 min. The upper phase was discarded, and 200 μl of methanol was added to the interphase. The mixture was centrifuged for 10 min at 13,000 × g, and the protein pellets were dried at 55 °C, resuspended and boiled for 5 min at 99 °C. The samples were separated by SDS-PAGE and immunoblotted.
Cytokine and chemokine production
The concentrations of IL-1β, TNF-α, Il-6 and KC in cell-free supernatants, lung tissues and BALF were measured by ELISA using antibody pairs from R&D Systems according to the manufacturer’s manual. The assays were independently performed three times in triplicate.
RNA-mediated interference
For RNA-mediated interference, 7-day-old BMDMs were transfected with 30 nM validated mouse ATG-5 esiRNA (EMU038061) or negative esiRNA (both from Sigma-Aldrich) using Lipofectamine 2000 transfection reagent. Forty-eight hours after transfection, the cells were used for experiments.
Peritonitis
Mice were i.p. injected with genipin (2 mg per body weight, i.p.), followed by LPS (35 mg/kg of body weight, i.p.) or alum (2 mg per body weight, i.p.) challenge 1 h later. Control mice received equivalent amounts of DMSO/PBS. The mice were killed 6 h later, and their peritoneal cavities were lavaged with 5 ml of PBS. The peritoneal fluids were centrifuged at 2000 rpm for 10 min, and the supernatants were analyzed for cytokine production by ELISA. The pelleted cells were stained using the May-Grünwald-Giemsa method, and the neutrophils that had migrated into the peritoneal cavities were counted. Neutrophils were also counted using another method: the pelleted cells were stained for the neutrophil surface marker Gr-1 (anti-mouse Ly-6G/Ly-6c or anti-rat IgG2b, BioLegend), followed by staining with anti-rat IgG FITC, and signals were detected using flow cytometry. The number of neutrophils was calculated by multiplying the total number of cells by the percentage of Gr-1-positive cells45.
Flagellin-induced pneumonia
To induce pneumonia, mice were intranasally instilled with a mixture of flagellin and TurboFect in vivo transfection reagent (Thermo Scientific, China) in a total volume of 50 μl. After 12 h, BALF was obtained by lavaging the lung with 3 × 1 ml PBS containing soybean trypsin inhibitor (100 μg/ml). Cytokines and chemokines in the BALF and homogenized lung tissues were detected by ELISA. Neutrophil infiltration was detected by immunohistochemical staining, and pathological changes in the lung were detected by H&E staining.
Additional Information
How to cite this article: Yu, S.-X. et al. Genipin inhibits NLRP3 and NLRC4 inflammasome activation via autophagy suppression. Sci. Rep. 5, 17935; doi: 10.1038/srep17935 (2015).
Supplementary Material
Acknowledgments
This work was supported by National Natural Science Foundation of China (grant numbers 31172298, 31201908), Changjiang Scholars and Innovative Research Team in University (grant numbers IRT1248), National Key Basic Research Program of China (grant numbers 2011CB944404).
Footnotes
Author Contributions Y.S.X., D.C.T. and Y.Y.J. designed experiments. Y.S.X., D.C.T., C.W., L.N., L.Q.Q., Q.S., Z.X.J., H.G.Q., D.X.M. and H.W.Y. performed the experiments and analysed the data. Y.Y.J. wrote the manuscript. All authors read and approved the final manuscript.
References
- Strowig T., Henao-Mejia J., Elinav E. & Flavell R. Inflammasomes in health and disease. Nature 481, 278–286 (2012). [DOI] [PubMed] [Google Scholar]
- Rathinam V. A., Vanaja S. K. & Fitzgerald K. A. Regulation of inflammasome signaling. Nat Immunol 13, 333–332 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duewell P. et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464, 1357–1361 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandanmagsar B. et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med 17, 179–188 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen H. et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol 12, 408–415 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon F., Petrilli V., Mayor A., Tardivel A. & Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 (2006). [DOI] [PubMed] [Google Scholar]
- Zaki M. H. et al. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity 32, 379–391 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halle A. et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol 9, 857–865 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo H. J., Lim K. H., Jung H. J. & Park E. H. Anti-inflammatory evaluation of gardenia extract, geniposide and genipin. Journal of ethnopharmacology 103, 496–500 (2006). [DOI] [PubMed] [Google Scholar]
- Nam K. N. et al. Genipin inhibits the inflammatory response of rat brain microglial cells. Int Immunopharmacol 10, 493–499 (2010). [DOI] [PubMed] [Google Scholar]
- Cai S., Batra S., Wakamatsu N., Pacher P. & Jeyaseelan S. NLRC4 inflammasome-mediated production of IL-1beta modulates mucosal immunity in the lung against gram-negative bacterial infection. J Immunol 188, 5623–5635 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Planillo R. et al. K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38, 1142–1153 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagulenko V. et al. AIM2 and NLRP3 inflammasomes activate both apoptotic and pyroptotic death pathways via ASC. Cell Death Differ 20, 1149–1160 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovski G. et al. Clearance of dying autophagic cells of different origin by professional and non-professional phagocytes. Cell Death Differ 14, 1117–1128 (2007). [DOI] [PubMed] [Google Scholar]
- Salazar M. et al. Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. J Clin Invest 119, 1359–1372 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C. Y. et al. Genipin inhibits UCP2-mediated proton leak and acutely reverses obesity- and high glucose-induced beta cell dysfunction in isolated pancreatic islets. Cell Metab 3, 417–427 (2006). [DOI] [PubMed] [Google Scholar]
- Arsenijevic D. et al. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat Genet 26, 435–439 (2000). [DOI] [PubMed] [Google Scholar]
- Zhou R., Yazdi A. S., Menu P. & Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225 (2011). [DOI] [PubMed] [Google Scholar]
- Martinon F. Signaling by ROS drives inflammasome activation. Eur J Immunol 40, 616–619 (2010). [DOI] [PubMed] [Google Scholar]
- Lupfer C. R. et al. Reactive oxygen species regulate caspase-11 expression and activation of the non-canonical NLRP3 inflammasome during enteric pathogen infection. PLoS Pathog 10, e1004410 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrilli V. et al. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ 14, 1583–1589 (2007). [DOI] [PubMed] [Google Scholar]
- Iyer S. S. et al. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity 39, 311–323 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbarth S. C., Colegio O. R., O’Connor W., Sutterwala F. S. & Flavell R. A. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature 453, 1122–1126 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao K. et al. Nitric oxide suppresses NLRP3 inflammasome activation and protects against LPS-induced septic shock. Cell Res 23, 201–212 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll R. C. et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med 21, 248–255 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes-Alnemri T. et al. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell death and differentiation 14, 1590–1604 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broz P. et al. Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. J Exp Med 207, 1745–1755 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K. W. et al. The neutrophil NLRC4 inflammasome selectively promotes IL-1beta maturation without pyroptosis during acute Salmonella challenge. Cell Rep 8, 570–582 (2014). [DOI] [PubMed] [Google Scholar]
- Yu J. W. et al. Cryopyrin and pyrin activate caspase-1, but not NF-kappaB, via ASC oligomerization. Cell death and differentiation 13, 236–249 (2006). [DOI] [PubMed] [Google Scholar]
- Dupont N. et al. Autophagy-based unconventional secretory pathway for extracellular delivery of IL-1beta. The EMBO journal 30, 4701–4711 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. et al. Autophagy controls IL-1beta secretion by targeting pro-IL-1beta for degradation. The Journal of biological chemistry 286, 9587–9597 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahira K. et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol 12, 222–230 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorbara M. T. & Girardin S. E. Mitochondrial ROS fuel the inflammasome. Cell Research 21, 558–560 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostert C. et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320, 674–677 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousset S. et al. UCP2 is a mitochondrial transporter with an unusual very short half-life. FEBS Lett 581, 479–482 (2007). [DOI] [PubMed] [Google Scholar]
- Youm Y. H. et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med 21, 263–269 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier N. K., Crown D., Liu J., Leppla S. H. & Moayeri M. Arsenic trioxide and other arsenical compounds inhibit the NLRP1, NLRP3, and NAIP5/NLRC4 inflammasomes. J Immunol 192, 763–770 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajanbabu V. et al. Genipin suppresses NLRP3 inflammasome activation through uncoupling protein-2. Cellular immunology 297, 40–45 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Castejon G. & Pelegrin P. Current status of inflammasome blockers as anti-inflammatory drugs. Expert opinion on investigational drugs 21, 995–1007 (2012). [DOI] [PubMed] [Google Scholar]
- Lamkanfi M. et al. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J Cell Biol 187, 61–70 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y. et al. 3,4-methylenedioxy-beta-nitrostyrene inhibits NLRP3 inflammasome activation by blocking assembly of the inflammasome. J Biol Chem 289, 1142–1150 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi L., Eigenbrod T. & Nunez G. Cutting edge: TNF-alpha mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J Immunol 183, 792–796 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu X. et al. Cytosolic double-stranded DNA induces nonnecroptotic programmed cell death in trophoblasts via IFI16. J Infect Dis 210, 1476–1486 (2014). [DOI] [PubMed] [Google Scholar]
- Jin J. et al. LRRFIP2 negatively regulates NLRP3 inflammasome activation in macrophages by promoting Flightless-I-mediated caspase-1 inhibition. Nat Commun 4, 2075 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y. et al. Tripartite-motif protein 30 negatively regulates NLRP3 inflammasome activation by modulating reactive oxygen species production. Journal of immunology 185, 7699–7705 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.