Abstract
Background:
Salvinorin-A is a terpene with agonist properties at the kappa-opioid receptor, the binding site of endogenous dynorphins. Salvinorin-A is found in Salvia divinorum, a psychoactive plant traditionally used by the Mazatec people of Oaxaca, Mexico, for medicinal and spiritual purposes. Previous studies with the plant and salvinorin-A have reported psychedelic-like changes in perception, but also unusual changes in body awareness and detachment from external reality. Here we comprehensively studied the profiles of subjective effects of increasing doses of salvinorin-A in healthy volunteers, with a special emphasis on interoception.
Methods:
A placebo and three increasing doses of vaporized salvinorin-A (0.25, 0.50, and 1mg) were administered to eight healthy volunteers with previous experience in the use of psychedelics. Drug effects were assessed using a battery of questionnaires that included, among others, the Hallucinogen Rating Scale, the Altered States of Consciousness, and a new instrument that evaluates different aspects of body awareness: the Multidimensional Assessment for Interoceptive Awareness.
Results:
Salvinorin-A led to a disconnection from external reality, induced elaborate visions and auditory phenomena, and modified interoception. The lower doses increased somatic sensations, but the highest dose led to a sense of a complete loss of contact with the body.
Conclusions:
Salvinorin-A induced intense psychotropic effects characterized by a dose-dependent gating of external audio-visual information and an inverted-U dose-response effect on body awareness. These results suggest a prominent role for the kappa opioid receptor in the regulation of sensory perception, interoception, and the sense of body ownership in humans.
Keywords: Body ownership, dissociative effects, dynorphins, human, interoception, kappa opioid receptor, salvia divinorum., salvinorin-A
Introduction
Salvia divinorum (Labiatae) is a rare perennial herb endemic to the Sierra Madre Oriental of Oaxaca, Mexico. The Mazatec people who inhabit this area have been using the leaves of the plant for centuries in the treatment of headaches, rheumatism, gastrointestinal diseases, and as a general medicinal tonic (Valdés et al., 1983). The Mazatec also use S. divinorum leaves for spiritual purposes, divination, and as a catalyst for knowing the causes of illnesses and the adequate cures for their patients (Valdés et al., 1983; Ott, 1995).
During the last decade, interest in S. divinorum has increased in Europe and North America. Users of the plant in these areas have found new ways of administration that lead to stronger and more efficient effects, compared to the traditional intake routes used by the Mazatecs (González et al., 2006). These new administration routes include the sublingual route, and the smoking of dry leaves and fortified extracts (Siebert, 1994; Ott, 1995). Retrospective studies in non-traditional users indicate that S. divinorum is mainly consumed by smoking leaf extracts. Use is occasional and experimental. Subjects reported consuming the drug an average of only two times. This low frequency of use has been attributed to intense somatic-dysphoric effects (González et al., 2006).
Salvinorin-A, the main psychoactive constituent in S. divinorum, is a non-nitrogenous neoclerodane diterpene with an affinity for the kappa opioid receptor (KOR; Roth et al., 2002). Despite its intense perception-modifying effects in humans, in vitro studies have shown that salvinorin-A does not bind to 5HT2A receptors, the molecular target of classic psychedelics like lysergic acid diethylamide (LSD), mescaline, psilocybin, and dimethyltryptamine (Roth et al., 2002; Prisinzano, 2005). The combination of intense perceptual and somatic effects is characteristic of salvinorin-A. Older nitrogenated KOR agonists such as enadoline are known to induce unpleasant reactions, and modifications in perception usually appear at high doses (Walsh et al., 2001). The distinct effects of salvinorin-A are probably due to its high potency and selectivity at KOR (Roth et al., 2002).
Previous reports of subjective effects include prominent somatic modifications. These range from tactile effects to a marked loss of control over the body (González et al., 2006; Johnson et al., 2011; Addy et al., 2015). These modifications have been quantitatively demonstrated, for instance, as significant increases in scores on the Hallucinogen Rating Scale (HRS) somaesthesia subscale (González et al., 2006; MacLean et al., 2013; Addy et al., 2015), which measures tactile, visceral, and interoceptive effects (Strassman et al., 1994). These somatic and tactile effects may be related to altered interoception, but this has not been directly assessed. “Interoception” refers to the primary internal representation of the physiological condition of the body (Craig, 2009). This facet of awareness encompasses a series of processes that can range from somatic sensations to dissociation, and is related to the visceral state of the body and emotional experience. Altered interoceptive abilities have been implicated in addiction (Verdejo-Garcia et al., 2012), anxiety, and depression (Paulus and Stein, 2010), and their restoration is at the center of body-focused therapies (Mehling et al., 2012).
In the present study we wished to comprehensively assess the pattern of subjective effects of increasing doses of salvinorin-A, with a special emphasis on interoception. For safety reasons, we recruited a small number of participants who had extensive experience with psychedelics.
Materials and Methods
Ethics
The study was conducted in accordance with the Declarations of Helsinki and its updates concerning experimentation on humans, and was approved by the hospital’s ethics committee and the Spanish Ministry of Health. All participants gave their written informed consent prior to participation.
Participants
We contacted circles of individuals interested in the altered states of consciousness induced by psychoactive substances and explained to them the goals and methods of our study. We asked our contacts to pass the information to their acquaintances and we finally recruited a group of eight healthy volunteers (three males, five females). They all had a minimum experience with psychedelics of ten times and no history of adverse effects from their use. The volunteers were interviewed by the principal investigator (Dr Riba), who recorded their previous experience with perception-modifying drugs and explained the goals and methods of the present study. Exclusion criteria included a current or past history of psychiatric disorders, alcohol or other substance dependence, evidence of significant illness, and pregnancy. Participants underwent a complete physical examination that included a medical history, laboratory tests, ECG, and urinalysis. Participants received detailed information about the nature of salvinorin-A and the general psychological effects of psychedelics and their possible adverse effects as they are described in the psychiatric literature.
Drug
Three doses of pure (>99%) salvinorin-A of 0.25, 0.50, and 1 milligrams were administered via inhalation after vaporization. Using previously described procedures (Johnson et al., 2011), individual doses were prepared in vials that contained the salvinorin dose dissolved in 1ml of acetone vehicle. Placebo vials contained the 1ml acetone vehicle only. No other substance was added. Prior to administration, the content of the vial was placed in a round-bottom flask and the acetone allowed to evaporate. The residue was heated, vaporized, and administered to the participants as described below.
Study Design and Experimental Procedure
The study was carried out in a double-blind fashion. Volunteers were informed that they would receive three doses of salvinorin-A (0.25, 0.5, and 1.0mg) and placebo over four experimental days, with the sequence of drug conditions randomized. In order to control for expectancy, participants were told that administration would be made in a balanced fashion. For safety reasons, doses were actually administered in increasing order. The low dose of 0.25mg always preceded the medium 0.50mg dose and the medium dose always preceded the high 1mg dose. The position of the placebo was randomly determined for each volunteer. Experimenters were aware of the ascending order of the active doses but did not know the position of the placebo in the sequence. Two weeks before the beginning of the experimental sessions, volunteers were instructed to abstain from all medications and illicit drugs, and remain drug-free throughout the study. Urinalysis for illicit drug use was conducted before each experimental session; volunteers tested negative for cannabis, opiates, benzodiazepines, cocaine, and amphetamine. Additionally, volunteers were instructed to abstain from alcohol 24h before each experimental day, verified by breath analysis, and from tobacco and caffeinated drinks on the experimental day. Experimental days were 24h apart.
Upon arrival in the morning to the research unit, breath analysis for alcohol, urinalysis for illicit drug use, and a urine pregnancy test (for women only) were administered. Then salvinorin-A or placebo was administered by vaporization and inhalation. The administration method followed that previously developed and described in detail by Johnson and coworkers (Johnson et al., 2011; MacLean et al., 2013). The round-bottom flask was connected to a vacuum adapter, which was connected to a rubber tube to allow the inhalation of the vaporized salvinorin-A. The flask was kept from the participant’s view using an aluminum foil screen. The volunteer was seated in a recliner bed while performing a sustained inhalation for 30 seconds while the base of the flask was heated with a butane flame for 30 seconds. After the 30-second period, they were instructed to exhale. Immediately thereafter, the bed was reclined until the volunteer was in a recumbent position. The room was kept dimly lit but the eyes of the participants were not covered. No music was used during the sessions. During the acute salvinorin-A effects, at regular intervals between 0 and 45 minutes, the investigator asked the participant to rate the intensity of subjective effects. After 60 minutes, the participant left the room and answered the battery of subjective effects questionnaires and instruments (see below). If they had experienced any noticeable effects, they were also asked to write in their own words a report of their experience. Four hours after drug administration, they were discharged and allowed to leave the research unit.
Measurements
The psychological effects elicited by salvinorin-A were measured using a battery of subjective effects questionnaires and visual analog scales (VAS) administered in each experimental session.
The Hallucinogen Rating Scale (HRS) was designed to measure the effects of dimethyltryptamine (Strassman et al., 1994). It has previously shown sensitivity to inhaled salvinorin-A (Johnson et al., 2011) and to smoked Salvia divinorum (González et al., 2006; Addy et al., 2015). The HRS measures psychedelic-induced subjective effects on six subscales: somaesthesia, reflecting somatic effects; affect, showing sensitivity to emotional and affective responses; cognition, describing modifications in thought processes or content; perception, measuring visual, auditory, gustatory, and olfactory experiences; volition, indicating the volunteer’s capacity to willfully interact with his/her “self” and/or the environment; and intensity, which reflects the strength of the overall experience. The range of scores for all scales is 0–4. In the present study, a Spanish version of the questionnaire was administered (Riba et al., 2001a).
The Addiction Research Center Inventory (ARCI; Martin et al., 1971) consists of five scales or groups: the morphine-benzedrine group (MBG), measuring euphoria; the pentobarbital-chlorpromazine-alcohol group (PCAG), measuring sedation; the lysergic acid diethylamide scale (LSD), measuring somatic-dysphoric effects; the benzedrine group (BG), measuring subjectively experienced intellectual efficiency; and amphetamine (A), which is sensitive to stimulants. The range of scores is 0–16 for MBG, -4 to 11 for PCAG, -4 to 10 for LSD, -4 to 9 for BG, and 0–11 for A. A validated Spanish version was administered (Lamas et al., 1994).
The State-Trait Anxiety Inventory (STAI; Spielberger et al., 1970) consists of 20 self-rated-items on a four-point scale with the responses of not at all, somewhat, moderately, and very much. A validated Spanish version was administered (Seisdedos, 2002).
The Altered States of Consciousness questionnaire (Aussergewöhnliche Psychische Zustände, APZ; Dittrich, 1998) includes 72 true/false items distributed in three subscales: oceanic boundlessness (Ozeanische Selbst-entgrenzung, OSE), measuring changes in the sense of time, derealization, and depersonalization; dread of ego-dissolution (Angstvolle IchAuflösung, AIA), measuring thought disorder and decreased body and thought control associated with arousal and anxiety; and visionary restructuralization (Visionäre Umstrukturierung, VUS), referring to visual phenomena, such as illusions, hallucinations, and synesthesia, and to changes in the significance of objects. The range of scores is 0–13 for OSE, 0–22 for AIA, and 0–14 for VUS. A Spanish version of the questionnaire previously used in clinical studies involving psychedelic drugs was administered (Riba et al., 2002).
Self-administered Visual Analogue Scales (VAS) were used to retrospectively rate peak effects during the session. They were 100mm horizontal lines with which volunteers retrospectively indicated the intensity of the drug effects (from 0, no effects, to 100, extremely intense effects). There were ten labeled VAS lines. “Any effect” indicated any effect, either physical or psychological, that the volunteer attributed to the administered dosage. “Good effects” indicated any effect the volunteer assessed as good. “Bad effects” indicated any effect the volunteer assessed as bad. “Sudden start of the effects” indicated a rapid onset of the effects. “Fear” indicated apprehension or psychological discomfort. “Time” indicated modifications on the perception of time. “Changes in dimensionality” indicated alterations on the perception of dimensionality of the body. “Changes in external reality” indicated changes in perception, with eyes open, of external reality. “Loss of contact with external reality” indicated separation from the surroundings. “Visions” indicated visual modifications with eyes open or closed.
Experimenter-administered VAS were used during the acute effects. The experimenter verbally asked the participant to rate the overall intensity of the experienced effects on a scale that went from 0 (no effect) to 100 (extremely intense effects). This “intensity” VAS was administered immediately before drug inhalation (baseline) and at 1, 2, 5, 10, 15, 20, 30, and 45 minutes after administration. This allowed assessment of the time course of effects. In cases of unresponsiveness by the participant, the maximum score (100) was assigned to that time point.
The Multidimensional Assessment for Interoceptive Awareness (MAIA) is a 32-item, four-point Likert scale designed to measure interoceptive awareness. An exploratory factor analysis was conducted with 325 individuals trained and experienced in mind-body therapies such as yoga and Tai Chi (Mehling et al., 2012). This analysis revealed eight subscales: noticing (NT), awareness of uncomfortable, comfortable, and neutral body sensations; not distracting (ND), tendency not to ignore or distract oneself from sensations of pain or discomfort; not worrying (NW), tendency not to worry or feel emotional distress with sensations of pain or discomfort; attention regulation (AR), ability to sustain and control attention to body sensations; emotional awareness (EA), awareness of the connection between body sensations and emotional states; self-regulation (SR), ability to regulate psychological distress by attention to body sensations; body listening (BL), active listening to the body for insight; and trusting (TR), experiencing one’s body as safe and trustworthy (Mehling et al., 2013). The MAIA showed acceptable internal consistency (Cronbach’s alphas of 0.66 to 0.87), low intra-scale correlations (Person’s coefficients of 0.16 to 0.6), and good construct validity. A later confirmatory factor analysis with 435 patients with chronic low back pain (Mehling et al., 2013) confirmed the essential factor structure of the MAIA with similar internal consistency (0.48 to 0.9) and intra-scale correlations (0.16 to 0.7). In the current study, the MAIA was translated into Spanish (Ds Riba and Maqueda). Participants completed the written assessment at the end of each test day based on their acute experience in the laboratory.
Personal narratives
Participants were requested to write in their own words a description of the effects they had experienced, if any had been noticed.
Statistical Analyses
The statistical analysis was conducted using the SPSS software. Descriptive and inferential statistics were used on all instruments administered, except on the free narratives. Scores on each questionnaire subscale and VAS item were calculated for each participant and dosing condition. For the experimenter-administered VAS, the maximum score obtained in each session was used for comparisons between doses. Means and standard errors of the mean were used in the figures. However, given the small sample size, the obtained scores were analyzed by means of non-parametric statistics. First, we used Friedman tests for related samples with dose (placebo, 0.25mg, 0.5mg, and 1.0mg) as a factor. When a significant effect was found, we performed pair-wise comparisons between doses using Wilcoxon signed-rank tests. In all tests performed, differences were considered statistically significant for p values lower than 0.05.
Results
Participants
All participants were Spanish nationals who were interested in psychedelics for self-knowledge purposes. They had experience with synthetic and semi-synthetic mind-altering compounds, but also with natural psychedelics. The context of use had been among friends, and in the case of ayahuasca they had attended European adaptations of Brazilian ayahuasca ceremonies. Volunteers had a mean age of 31 years (21–38). They had extensive previous experience with psychedelic drugs, all having taken LSD and Psilocybe mushrooms. Four participants had smoked S. divinorum. Seven had experience with ketamine, five with 2C-B, five with smoked dimethyltryptamine, four with ayahuasca, and three with mescaline-containing San Pedro cacti. Five were not using cannabis at the time of the study and three were smoking one or two cannabis cigarettes per day.
Psychological Effects
HRS
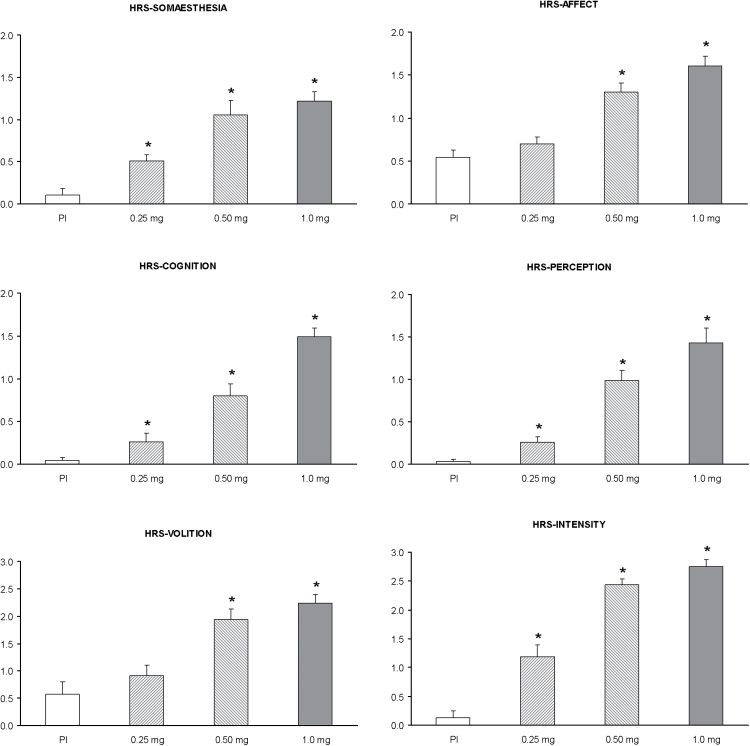
Mean scores on all subscales of the HRS at the different doses administered are shown in Figure 1, and the results of the statistical analyses in Table 1. A significant effect of dose was observed in all subscales of the HRS (df = 3): somaesthesia (χ2 = 18.04, p < 0.001), affect (χ2 = 19.65, p < 0.001), perception (χ2 = 22.67, p < 0.001), cognition (χ2 = 23.42, p < 0.001), volition (χ2 = 17.13, p = 0.001), and intensity (χ2 = 23.26, p < 0.001). Post hoc comparisons revealed orderly dose-response relationships for all subscales.
Figure 1.
Mean scores on the six Hallucinogen Rating Scale (HRS) subscales after administration of placebo, 0.25mg, 0.50mg, and 1mg salvinorin-A. Error bars denote 1 standard error of the mean (n=8). The asterisk indicates significant differences with placebo at p<0.05.
Table 1.
Statistical analyses of subjective effects measures (HRS, ARCI, and STAI) after placebo, and 0.25, 0.5 and 1.0mg salvinorin-A (n=8).
| HRS | Friedman test(a) | Pairwise comparisons (Wilcoxon test)a | |||||
|---|---|---|---|---|---|---|---|
| Pla: LD | Pla: MD | Pla: HD | LD:MD | LD:HD | MD:HD | ||
| Somaesthesia | *** | * | * | * | * | * | NS |
| Affect | *** | NS | * | * | * | * | * |
| Cognition | *** | * | * | * | * | * | * |
| Perception | *** | * | * | * | * | * | * |
| Volition | *** | NS | * | * | * | * | NS |
| Intensity | *** | * | * | * | * | * | * |
| ARCI | |||||||
| MBG | NS | NS | NS | NS | NS | NS | NS |
| PCAG | NS | NS | NS | NS | NS | NS | NS |
| LSD | ** | * | * | * | NS | NS | NS |
| BG | NS | NS | * | * | NS | NS | NS |
| A | NS | NS | NS | NS | NS | NS | NS |
| STAI | * | * | * | NS | NS | NS | NS |
Pla: placebo, LD: low dose (0.25mg), MD: medium dose (0.50mg), HD: high dose (1.0mg). ARCI – A: Amphetamine scale; BG: Benzedrine-Group; MBG: Morphine-Benzedrine-Group; PCAG: Pentobarbital-Chlorpromazine-Alcohol-Group; LSD: Lysergic acid diethylamide scale. (a) p values. NS: not significant; * p < 0.05, ** p < 0.01, *** p < 0.001.
ARCI
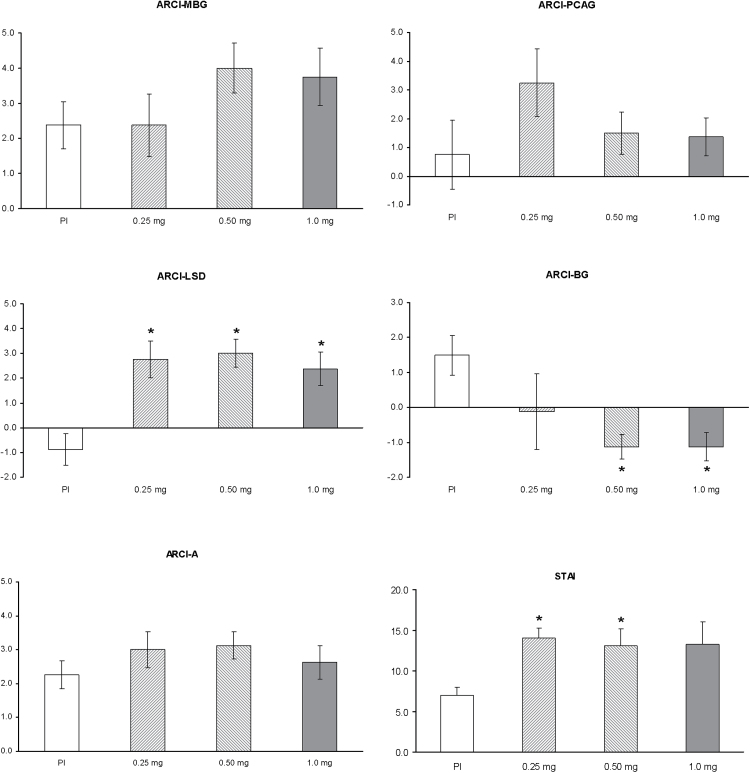
Mean scores on all subscales of the ARCI are shown in Figure 2, and the results of the statistical analyses in Table 1. A significant effect of dose was observed in the LSD subscale only (df = 3; χ2 = 14.92, p = 0.002). Post-hoc comparisons revealed significant differences between placebo and the low, medium, and high doses in the LSD subscale, but no differences between active doses. Despite the lack of an overall effect of dose, the post hoc comparisons showed statistically significant decreases for the BG subscale at the medium and high doses compared to placebo.
Figure 2.
Mean scores on the five Addiction Research Center Inventory (ARCI) subscales, and the State-Trait Anxiety Inventory (STAI) questionnaire after administration of placebo, 0.25mg, 0.50mg, and 1mg salvinorin-A. Error bars denote ±1 standard error of mean (n=8) in all panels except the STAI panel where it denotes 1 standard error of mean. A: Amphetamine scale; BG: Benzedrine-Group; LSD: Lysergic acid diethylamide scale; MBG: Morphine-Benzedrine-Group; PCAG: Pentobarbital-Chlorpromazine-Alcohol-Group. The asterisk indicates significant differences with placebo at p<0.05.
STAI
Mean scores on STAI are shown in Figure 2, and the results of the statistical analyses in Table 1. A significant effect of dose was observed in the STAI (df =3; χ2 = 8.47, p = 0.037). Post hoc comparisons revealed significant increases between placebo and the low and medium doses, but no differences between active doses or between placebo and the high dose.
APZ
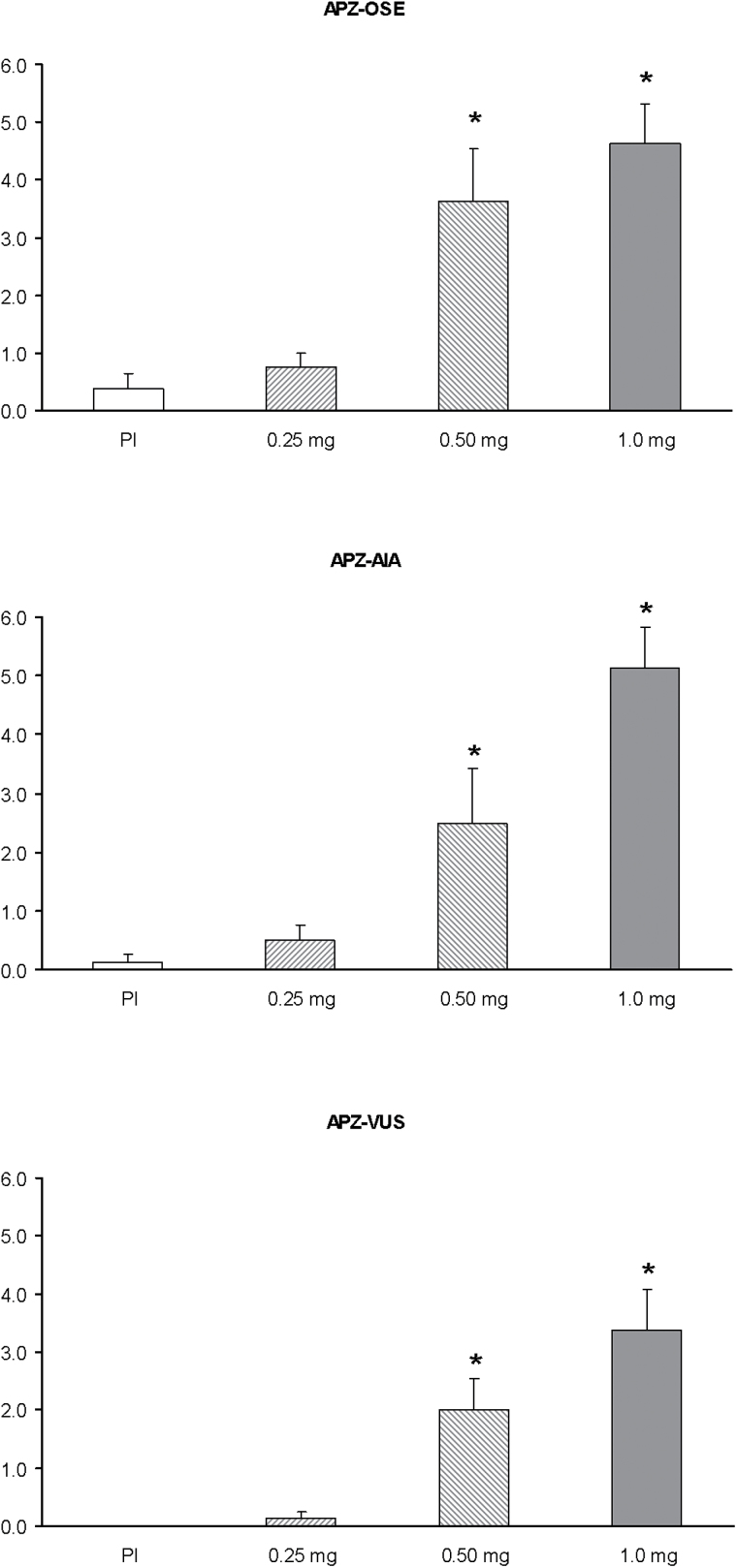
Mean scores on all subscales of the APZ are shown in Figure 3, and the results of the statistical analyses in Table 2. A significant effect of dose was observed in all subscales of the APZ (df =3): OSE (χ2 = 17.88, p < 0.001), AIA (χ2 = 18.29, p < 0.001), and VUS (χ2 = 21.17, p < 0.001). Post hoc comparisons revealed orderly dose-response increases. Significant differences were seen between the medium and high doses and the placebo for all subscales. The medium and high doses were statistically different from the low dose and the AIA subscale differentiated between the medium and the high dose.
Figure 3.
Mean scores on the Altered States of Consciousness (Aussergewöhnliche Psychische Zustände, APZ) questionnaire after administration of placebo, 0.25mg, 0.50mg, and 1mg salvinorin-A. Error bars denote 1 standard error of mean (n=8). AIA: Dread of Ego-Dissolution (“Angstvolle IchAuflösung”); OSE: Oceanic Boundlessness (“Ozeanische Selbstentgrenzung”); VUS: Visionary Restructuralization (“Visionäre Umstrukturierung”). The asterisk indicates significant differences with placebo at p<0.05.
Table 2.
Statistical analyses of subjective effects measures (APZ, VAS, and MAIA) after placebo, and 0.25, 0.5 and 1.0mg salvinorin-A (n=8).
| APZ | Friedman test(a) | Pairwise comparisons (Wilcoxon test)a | |||||
|---|---|---|---|---|---|---|---|
| Pla: LD | Pla: MD | Pla: HD | LD:MD | LD:HD | MD:HD | ||
| OSE | *** | NS | * | * | * | * | NS |
| AIA | *** | NS | * | * | * | * | * |
| VUS | *** | NS | * | * | * | * | NS |
| VAS Self-administered | |||||||
| AE | *** | * | * | * | * | * | * |
| GE | *** | * | * | * | NS | * | NS |
| BE | NS | NS | NS | * | NS | NS | NS |
| SE | *** | * | * | * | * | * | NS |
| FE | * | NS | NS | * | NS | NS | * |
| TI | *** | NS | * | * | NS | * | * |
| DI | ** | NS | * | * | * | NS | NS |
| RE | ** | * | * | * | * | * | NS |
| CO | *** | NS | * | * | * | * | NS |
| VI | *** | NS | * | * | * | * | NS |
| VAS Experimenter-administered | |||||||
| Intensity | *** | * | * | * | * | * | * |
| MAIA | |||||||
| NT | NS | NS | NS | NS | NS | NS | NS |
| ND | NS | NS | NS | NS | NS | NS | NS |
| NW | NS | NS | NS | NS | NS | NS | NS |
| AR | ** | NS | * | * | * | * | NS |
| EA | NS | NS | NS | * | NS | NS | NS |
| SR | NS | NS | NS | NS | NS | NS | NS |
| BL | NS | NS | NS | NS | NS | NS | NS |
| TR | ** | * | * | NS | NS | * | * |
Pla: placebo, LD: low dose (0.25mg), MD: medium dose (0.50), HD: high dose (1.0mg) APZ – OSE: Oceanic Boundlessness (“Ozeanische Selbstentgrenzung”); AIA: Dread of Ego-Dissolution (“Angstvolle IchAuflösung”); VUS: Visionary Restructuralization (“Visionäre Umstrukturierung”). VAS, Visual analogue scales – AE: any effect; GE: good effects; BE: bad effects; SE: sudden start of effects; FE: fear; TI: time; DI: changes in dimensionality; RE: changes in external reality; CO: loss of contact with external reality; VI: visions. MAIA – NT: noticing; ND: not distracting; NW: not worrying; AR: attention regulation; EA: emotional awareness; SR: self-regulation; BL: body listening; TR: trusting. (a) p values. NS: not significant; * p < 0.05, ** p < 0.01, *** p < 0.001.
Self-Administered VAS
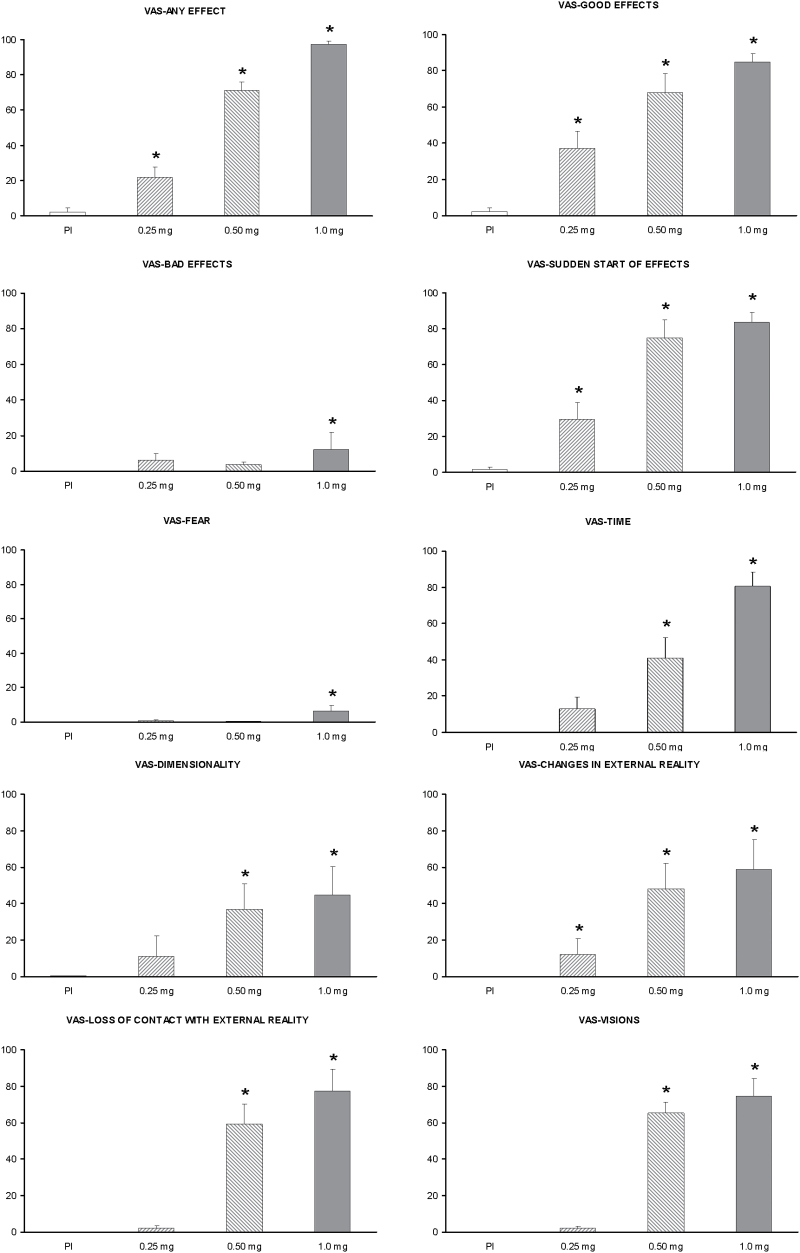
Mean scores on all self-administered VAS items are shown in Figure 4, and the results of the statistical analyses in Table 2. A significant effect of dose was observed in all but one of the VAS items (df =3): any effect (χ2 = 22.95, p < 0.001), good effects (χ2 = 19.86, p < 0.001), sudden start of effects (χ2 = 19.77, p < 0.001), fear (χ2 = 11.14, p = 0.011), altered time perception (χ2 = 19.77, p < 0.001), altered body dimensionality (χ2 = 12.13, p = 0.007), altered external reality (χ2 = 14.67, p = 0.002), lost contact with external reality (χ2 = 17.29, p = 0.001), and visual effects (χ2 = 21.00, p < 0.001). The item “bad effects” did not show any effects of dose. Post hoc comparisons revealed orderly dose-response relationships. Four VAS items were different from placebo at the low dose, eight at the medium dose, and ten at the high dose, including the “bad effects” item.
Figure 4.
Mean scores on the self-administered visual analogue scales (VAS) items after administration of placebo, 0.25mg, 0.50mg, and 1mg salvinorin-A. Error bars denote 1 standard error of mean (n=8). The asterisk indicates significant differences with placebo at p<0.05.
Experimenter-Administered VAS
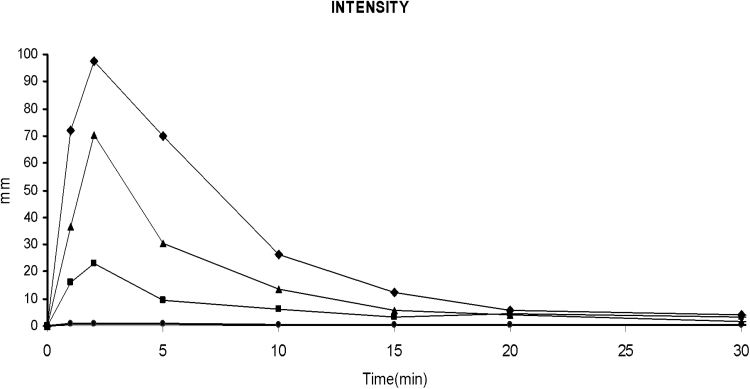
As shown in Figure 5, the onset of salvinorin-A effects was very rapid, reaching its maximum at 2min post-administration and gradually decreasing to baseline levels after 20 minutes. The analysis of the peak values showed a significant effect of dose (χ2 = 23.26, df =3, p < 0.001). Post hoc comparisons showed significant effects relative to placebo and orderly dose-response relationships for the three active treatments (see Table 2).
Figure 5.
Mean (n=8) scores on the Experimenter-administered “intensity” visual analog scale (VAS) at the different measurement time points. The plots show data following placebo (circle), 0.25mg (square), 0.50mg (triangle), and 1mg (diamond) salvinorin-A.
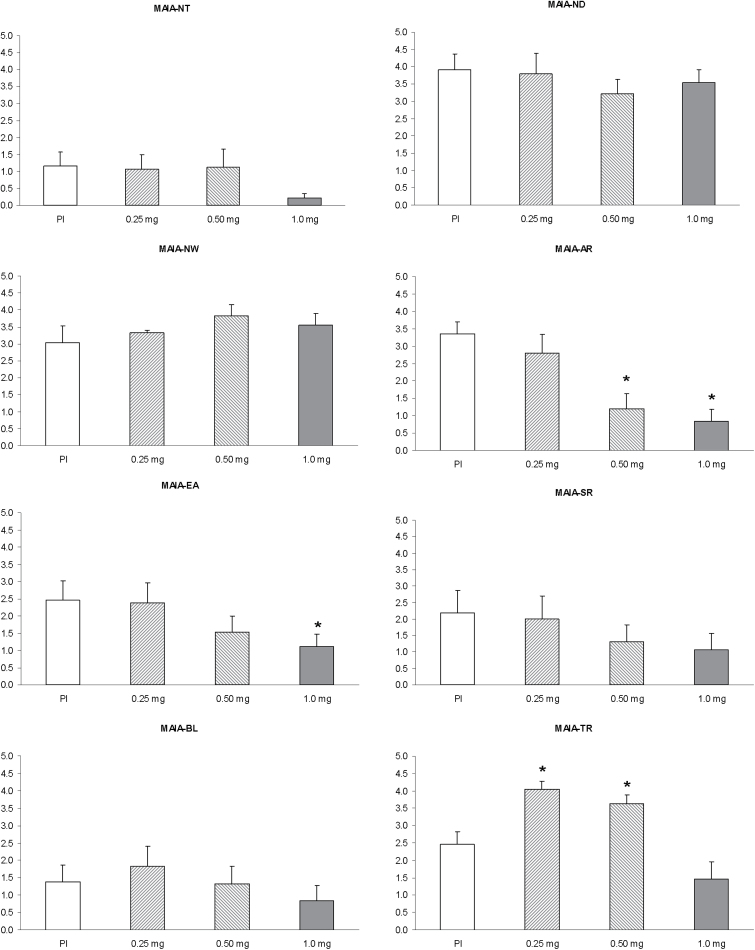
MAIA
Scores on the MAIA subscales are shown in Figure 6 and the results of the statistical analyses in Table 2. A significant effect of dose was observed in the attention regulation (AR, χ2 = 12.58, p = 0.006) and trusting (TR, χ2 = 14.61, p = 0.002) subscales of MAIA (df =3). Post hoc comparisons revealed that AR decreased in a dose-dependent manner, with the medium and high doses showing significantly lower AR than placebo. However, the TR displayed an inverted-U relationship (see Table 2 and Figure 7). The Wilcoxon test showed significant increases at the low and medium doses and a decrease after the high dose. Despite the lack of an overall effect of dose, the post hoc comparisons showed statistically significant decreases in the emotional awareness (EA) subscale at the high dose.
Figure 6.
Mean scores on the eight Multidimensional Assessment for Interoceptive Awareness (MAIA) subscales after administration of placebo, 0.25mg, 0.50mg, and 1mg salvinorin-A. Error bars denote 1 standard error of mean (n=8). AR: Attention regulation; BL: Body listening; EA: Emotional awareness; ND: Not distracting; NT: Noticing; NW: Not worrying; SR: Self-regulation; TR: Trusting. The asterisk indicates significant differences with placebo at p<0.05.
Personal Narratives
General Effects
The most commonly reported themes are shown in Table 3, organized by dose. These included: (a) the sudden onset of effects; (b) changes in bodily sensations; (c) changes in the perception of time, depersonalization, and derealization; (d) modifications of visual and auditory perception; (e) the intensity and brevity of effects; (f) pleasurable effects; and (g) impairment of the capacity to interact with surroundings. The table shows the number of volunteers that described a given effect and includes excerpts from the narratives.
Table 3.
Common themes reported by the participants and excerpts from their narratives (n=8)
| 0.25 mg | 0.50 mg | 1 mg | |
|---|---|---|---|
| 1. Sudden onset of effects | 6/8 The effects started after two or three minutes |
7/8 During the first minute, the effects increased very fast |
7/8 The effects started in the first instant after inhalation |
| 2. Changes in bodily sensations | 7/8 Overall, I was feeling a subtle and pleasant tingle in all my body |
7/8 In the first seconds I felt huge effects with an important increase in body temperature, I sweated a lot. I had tingle sensations in all my body |
5/8 It cost me some efforts to bring my mind back to my body: first the eyes, then the mouth, then the halves of the face...it was like a stretched bubble gum |
| 3. Changes in the perception of time, depersonalization, derealization | 2/8 A detachment between myself and the effects on my body happened |
5/8 All of this lasted barely a minute, the temporal axis was creased like an accordion |
8/8 The material world and the sensation of my habitual self faded |
| 4. Modifications of visual and auditory perception | 2/8 I was seeing objects with less definition, like with a plastic texture |
5/8 Inside the bus a group of people were shouting, encouraging my mind to leave my body, and they were feminine voices |
7/8 They knew me and called me by my name, they were telling me positive messages |
| 5. Intensity and brevity of effects | 1/8 The experience has been slight and brief |
4/8 It was very brief but impressive |
8/8 The trip has been very intense |
| 6. Pleasurable effects | 3/8 The state of peace obtained allowed me to think in a very lucid way |
3/8 Good humor is still retained at least until now, an hour and a half later |
6/8 The experience has been positive, nice, and very revealing |
| 7. Impairment of capacity to interact with surroundings | 1/8 I was able to answer the questions without any difficulty |
4/8 It seems that I was answering coherently, but the questions and my answers were nonsense to me |
7/8 When the researcher asked me how intense my experience was, I was unable to answer because at that point I had lost my sense of self |
Other less-frequent themes were also reported. These included the sensation of being in two realities at the same time (n = 5). For example: “I really wanted to be fully in that other reality, it was very familiar, like the reality of my childhood”; laughter (n = 3): “It was very fun to check how absurd the usual world was. I was dying of laughter with the perspective of the existence of other worlds containing life”; becoming an object (n = 3): “I changed from being a square, to a pentagon, to a pyramid”; travel to other dimensions (n = 3): “I have left my body and traveled to another world or dimension”; similarity of the experience with dreams (n = 2): “The experience is like dreaming, and then you have difficulties to remember it”; and changes in dimensionality (n = 2): “Consistent change of dimensionality, I pass from 3D to 2D.” Another recurring theme was perceiving physical beings or presences (n = 4): “There were magical beings in that world”; “I felt the presence of other life forms in that other world.” Sometimes participants perceived that the presences/beings were forces that pulled the body of the participant or talked to them, creating auditory hallucinations.
Detailed Interoceptive Effects
All volunteers experienced profound interoceptive changes and described them extensively in their narratives. Excerpts of these reports are shown in Table 4. Participants experienced being pulled, pressured, or divided; changes in body temperature; tingling; sweating; relaxation; vibrations; loss of contact with the body; and out-of-body experiences. Volunteer reports also referred to the lateralization of effects (n = 2), starting from a specific side of reality: “I had the sensation that the effects of the substance were approaching me, like an air blast, from the left”; “I felt like another reality, just as real or even more authentic, was coming from the right.”
Table 4.
Detailed interoceptive effects extracted from the participants’ narratives. V=volunteer
| Volunteer (n=8) | Dose | ||
|---|---|---|---|
| 0.25 mg | 0.50 mg | 1 mg | |
| V1 | I observed the creases and folds that my body was starting to experience. A force was pressing the right side of my body down and another force was pulling my legs up creating a 90º angle, and my feet were folding parallel to the ceiling, so my sensation was being a square. Visually I wasn’t seeing any image, but that square was conceptually present in my mind | A pressure from my back and another from my leg and my face made me start to fold bidimensionally | While I was still exhaling, the experimental room and the people in the room were the design and the pattern of a dense wool carpet, and then that carpet folded. With my eyes open, I was seeing that huge white fold appear on my foot and slowly approached my head.(...)Somatic sensations, far from being organic, are more close to objects, minerals or plastic materials |
| V2 | After two or three minutes I had the sensation that the effects of the substance were approaching me, like an air blast, from the left. As they were coming they were distorting the objects in the room and also my body and sensations, and I felt this more intensely on my neck. I thought that it would bring me to another world, but while passing through the half of my body the effects disappeared | *No somatic effects reported | We were colorful lines in movement, and the feeling was very pleasant. We were going to fall into the hole of a sink, but I felt that I had to do a lot of strength with my body to get through. There was a force not letting me in, and I had to fight trying to pass. This force changed into a face, into a person of this reality, and I felt its pressure in my arms |
| V3 | I have felt a ‘buzz’, and a relatively intense tachycardia. It has grown, just a little, and have appeared other sensations such as muscular ‘jamming’, or numbness in the hands. I feel a subtle headache, and it is a bit annoying. Otherwise, all good. A little dizzy... but nothing really noticeable | First I felt a ‘buzz’ and then a more intense sensation, it looks ridiculous but I felt I was a mane, and the substance that I had taken was a comb that was combing my body | I felt the sensation as if my face turned feline, and I was vibrating. Then the out of body experience started, and I had the sensation of my mind rising from behind and going to another place, leaving the envelope that was my body. Thus I stopped having muscular sensations, proprioceptive and everything related, both from the face and the body |
| V4 | Right after inhalation I felt something entering my body, and then temperature changes spread throughout my body, starting from my legs and climbing up, and then disappearing | The corporeal sensation was of a force dividing my body in half, and I looked like a comic | I have sweated a lot |
| V5 | *No somatic effects reported | A very potent force split my body in two halves, sending the right side to a faraway world, where I could feel a presence that was trying to take the left side of my body too. The right side was still connected to the rest of the body, so I tried to breathe on those points of connection between the two halves to release the tension and to allow the left part to go to that other world, but it didn’t work | *No somatic effects reported |
| V6 | Warmth. I felt my body heavier, relaxed, wellness in the body, calm, with a subtle tingle on the neck and the head. My mind was also relaxed. I was connected with my body and with the sensations I was feeling | Right after the inhalation I felt a floating sensation, warmth; I need to close my eyes. The intensity of the trip was connected to a force that wouldn’t let me fully trip. That force was doctors and nurses | A lot of corporeal sensations. I have felt a very strong high with a lot of energy to the body. A very potent and tasty feeling. I was enjoying the sensation very much, and I was fully aware of being very high waiting to travel somewhere else |
| V7 | Physically I was feeling a tingle going through my right arm, forehead and head. I have felt a little force that was pressing me against the bed | This time I didn’t have my physical condition as present as the last one, only right after inhalation I felt a subtle tingle. Then everything has been mental and visual, I have stopped paying attention to my body | I have forgotten my body completely, you don’t remember it at any moment |
| V8 | I have felt a warmth in my cheeks, but so subtle that I thought it was only my imagination. So I relaxed thinking that it was the placebo, and then I started to feel a more intense warmth in my thighs. Gradually it increased and appeared in other parts of my body, but it was mild, giving me tranquility, relaxation and peace. Overall, I was feeling a subtle and pleasant tingle in all my body | In the first seconds I felt huge effects with an important increase in body temperature, I sweated a lot. I had tingle sensations in all my body | I wasn’t feeling that in that world I had a physical body. I was an energetic being |
Discussion
The vaporization and inhalation of salvinorin-A proved an effective method of administration, replicating previous research (Johnson et al., 2011; MacLean et al., 2013). At the doses used, salvinorin-A showed a pattern of subjective effects with fast onset and short duration. At all doses, peak effects were reached at 2 minutes after the inhalation (second measurement time point), followed by a progressive decrement, to almost disappearing after 20 minutes. This pattern is analogous to those reported previously (Johnson et al., 2011; MacLean et al., 2013).
The 0.25mg of salvinorin-A chosen in the present study as the lowest dose was psychoactive (i.e. rated as significantly different from placebo on some measures) in all volunteers. The effects were dose-dependent, with the medium and higher doses leading to more, greater, and longer-lasting perceptual, affective, and somatic effects. The fact that salvinorin-A was psychoactive below doses of one milligram places it in the potency range of LSD. Salvinorin-A induced perceptual and cognitive effects that are typical of 5-HT2A agonists such as LSD and other classical psychedelics, but with intense somatic effects and a strong dissociative component. Some of the somatic modifications showed a biphasic pattern. At low and medium doses, salvinorin-A increased ratings of bodily sensation. However, at the high dose body awareness was strongly decreased, leading to a depersonalization and loss of sense of body ownership.
Assessment scores compare with those obtained by other laboratories. Scores on the HRS subscales confirm the psychedelic profile of salvinorin-A, being in concordance with previous studies both with smoked S. divinorum extracts and pure salvinorin-A. The intensity subscale of HRS shows the high intensity of effects perceived at medium and high doses, consistent with the results of Johnson et al. (2011) and MacLean et al. (2013). Our scores are similar also to studies using serotonergic hallucinogens like ayahuasca (Riba et al., 2001b) or psilocybin (Griffiths et al., 2006).
The narratives of the volunteers included themes that are common to previous studies with salvinorin-A (Siebert, 1994; González et al., 2006; Baggott et al., 2010; Johnson et al., 2011; Addy, 2012; Ranganathan et al., 2012; MacLean et al., 2013; Addy et al., 2015). For instance, the uniqueness of salvia compared to other psychedelics was reported in the present study by two volunteers: “The experience was surprising, how weird it is!,” “This was a completely new experience for me.” This is in concordance with the KOR agonism profile of the substance.
Perceptual Effects
In the present study, significant dose-related effects were found in the instruments measuring alterations in visual and auditory perception. Auditory experiences were quite prominent and included hearing music and being verbally addressed by presences or beings. Visions were also reported frequently in the medium and high doses. These appeared as tunnel or window-like visions, luminous walls and surfaces, metallic objects, geometric patterns, and other worlds of multiple colors: “I was in an immense forest full of fluorescent trees.” As in previous studies (MacLean et al., 2013; Addy et al., 2015), one participant referred to carnival imagery: “There were magical beings in that world, wearing garish dresses, similar to the clothes of a royal court jester.”
Synesthesia
A particular type of visual-proprioceptive synesthesia was experienced that has been previously reported from smoking S. divinorum (Addy et al., 2015). While visual-auditory synesthesia is common with serotonergic and non-serotonergic substances, visual-proprioceptive synesthesia is rarely described (Luke and Terhune, 2013). This was experienced here by two volunteers in three experimental sessions (two in low dose and one in high dose). Objects perceived with eyes open or closed were felt as being associated with the body. For example, seeing external modifications in reality, like a wave, that affects or folds the volunteer’s body: “The effects of the substance were approaching me, like an air blast, from the left. (…) they were distorting the objects in the room and also my body and sensations”. One volunteer described the concept of a square present in the mind that was felt with the body: “A force was pressing the right side of my body (...), so my sensation was being a square. Visually I wasn’t seeing any image, but that square was conceptually present in my mind”.
Effects on Affect
Salvinorin-A led to paradoxical effects on emotion. Volunteers reported improved mood including relaxation and calm, as in previous studies (Hanes, 2001; Bücheler et al., 2005; Baggott et al., 2010; Addy, 2012; MacLean et al., 2013). Participants described positive affect states such as “the experience has been very positive” and “I have enjoyed the experience very much”.
However, anxiety scores on the STAI were increased for the low and medium doses, but not for the high dose. This could be due to the ascending order in which active doses were administered, but could also reflect a ceiling effect. There are no direct references in the written reports to moments of anxiety or fear during the experience.
One volunteer reported feelings of being isolated due to not being able to feel the presence of the experimenter in the room, and another volunteer expressed confusion of not being able to understand what was happening. Our results are similar to previous research (Johnson et al., 2011; Ranganathan et al., 2012; MacLean et al., 2013) which reported a lack of negative affect and low anxiety ratings. In contrast, González and coworkers found above average anxiety levels, which could be related to expectancy, previous experience with psychedelics, or the uncontrolled environment of non-laboratory drug use (González et al., 2006).
Interoceptive Effects
Our results suggest biphasic effects of salvinorin-A upon certain aspects of interoception. Results from the MAIA show that salvinorin-A reduced participants’ ability to sustain and control attention to their bodily sensations. Low and medium doses of salvinorin-A acutely led to increases in experiencing one’s body as safe and trustworthy. Increased Trusting scores have been inversely correlated with experiencing chronic low back pain, perceived stress, depression, and trait anxiety; and have been directly correlated with having a mind-body practice and having strong body-listening skills (Mehling et al., 2012, 2013). Thus, in our sample low and medium doses of salvinorin-A produced effects that could be viewed as adaptive, while high doses of salvinorin-A produced maladaptive effects (decreased body awareness).
Dissociative Effects
We observed a strong profile of dissociative effects and disconnection with reality. The body-related dysphoria subscale (LSD) of the ARCI showed lower scores compared to previous studies (González et al., 2006; MacLean et al., 2013) suggesting dissociative effects and a perceived inability to interact with one’s body and surroundings. Further, the high dose of salvinorin-A increased depersonalization and derealization (OSE subscale of APZ) and decreased body and thought control (AIA subscale of APZ).
Paradoxically, the low and medium doses of salvinorin-A increased the MAIA trusting (TR) subscale, indicating an increased experience of one’s body as safe and trustworthy. No other results in our study showed a similar inverted-U-shaped profile. It may be that at lower doses salvinorin-A increases bodily sensations, but that the dissociative effects of the high dose overwhelm the subtle trust-enhancing effects of the low and medium doses.
Neural Substrates of Salvinorin-A Effects
In the encephalon, high levels of KOR are found in the neocortex, the thalamus and the ventral tegmental area (VTA) (Simonin et al., 1995). Despite lower amounts in the hippocampus, KOR activation effectively reduces the activity of excitatory afferents at this level (Chavkin, 2013). It also inhibits dopamine release from VTA afferents to the prefrontal cortex, and the activity of serotonergic and noradrenergic neurotransmission (Schwarzer, 2009). The dynorphin-KOR system has been involved in stress responses, reward and addiction and various psychiatric disorders (Schwarzer, 2009; Tejeda et al., 2012; Chavkin, 2013).
KOR agonism in the temporal and parietal cortices could underlie the visual and auditory modifications (temporal cortex) and the altered experience of the body (parietal cortex). Posterior parietal areas in collaboration with subcortical structures and the premotor cortex play a role in the multi-sensorial experience of the body by codifying it into an axis of reference (e.g. hands, arms, or head). In one study in which the right angular was electrically stimulated, out-of-body experiences were elicited (Blanke et al., 2002). In addition, the medial posterior parietal cortex is a key structure within the default mode network, which has been proposed to be associated with the intimate sense of self (Raichle et al., 2001; Raichle, 2011).
The most likely structure mediating the loss of contact with external reality is the thalamus, which shows high KOR levels in humans (Simonin et al., 1995). Studies in rodents have shown that the centromedian, paraventricular, and centrolateral nuclei of the thalamus are particularly rich in these receptors and cell bodies expressing them (Le Merrer et al., 2009). While the first two nuclei play an important role controlling arousal and the overall level of cortical activity, the paraventricular nucleus is associated with viscero-limbic functions (Van der Werf et al., 2002). Lesions in the centrolateral nucleus lead to widespread reductions in activation of the reticular system, additional thalamic nuclei, and extensive limbic and cortical areas (Raos et al., 1995). A KOR-mediated inhibitory effect of salvinorin-A at this level could block the relay of perceptual and somatic information to the cortex and explain the loss of contact with external reality and with one’s own body as reported by participants.
Alternatively, the claustrum, a sheet of neurons neighboring the insula, also shows high KOR levels. Stiefel and coworkers have proposed that salvinorin-A could disrupt cerebral integration processes taking place at this level and lead to the effects of disconnection from reality induced by the drug (Stiefel et al., 2014). Future studies should use neuroimaging techniques to provide direct evidence of the brain areas targeted by salvinorin-A.
Limitations
The study included a relatively small number of subjects with extensive experience with psychedelics. Thus, our results may not be generalized to less experienced individuals. Additionally, as mentioned in the Methods section, the doses were administered in ascending order. Although the position of the placebo was varied randomly, this design may have influenced the results. In addition, the study had five female and three male participants. Due to the sex differences found in distribution and elimination of salvinorin-A, (Schmidt et al., 2005), the effects may have been more intense in the female than the male participants. However, the small sample size precludes the statistical analysis comparison between the two groups.
Conclusions
The inhalation of vaporized salvinorin-A led to very strong psychotropic effects of rapid onset and short duration. Perceptual modifications included the visual domain, and in contrast with 5HT2A agonists, auditory hallucinations were very common. Also in contrast with the classical serotonergic psychedelics, loss of contact with external reality was prominent with the participants being unreactive to external visual and verbal cues, especially after the medium and high doses. While at the low and medium doses there was an increase in bodily sensations, at 1.0mg there was an almost complete loss of body ownership and an increase in out-of-body experiences. These results suggest that the dynorphins – KOR system may play a previously underestimated role in the regulation of sensory perception, interoception, and the sense of body ownership in humans.
Statement of Interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by grant “PI12/02758” from the “Instituto de Salud Carlos III” of the Spanish Governement, which is co-funded by FEDER. Marta Valle is supported by the “Fondo de Investigación Sanitaria” through grant CP04/00121 from the Spanish Ministry of Health in collaboration with Institut de Recerca de l’Hospital de la Santa Creu i Sant Pau, Barcelona.
The authors would like to thank the study volunteers for their participation.
References
- Addy PH. (2012) Acute and post-acute behavioral and psychological effects of salvinorin A in humans. Psychopharmacology (Berl) 220:195–204. [DOI] [PubMed] [Google Scholar]
- Addy PH, Garcia-Romeu A, Metzger M, Wade J. (2015) The subjective experience of acute, experimentally-induced Salvia divinorum inebriation. J Psychopharmacol 29:426–435. [DOI] [PubMed] [Google Scholar]
- Baggott MJ, Erowid E, Erowid F, Galloway GP, Mendelson J. (2010) Use patterns and self-reported effects of Salvia divinorum: an internet-based survey. Drug Alcohol Depend 111:250–256. [DOI] [PubMed] [Google Scholar]
- Blanke O, Ortigue S, Landis T, Seeck M. (2002) Stimulating illusory own-body perceptions. Nature 419:269–270. [DOI] [PubMed] [Google Scholar]
- Bücheler R, Gleiter CH, Schwoerer P, Gaertner I. (2005) Use of nonprohibited hallucinogenic plants: increasing relevance for public health? A case report and literature review on the consumption of Salvia divinorum (Diviner’s Sage). Pharmacopsychiatry 38:1–5. [DOI] [PubMed] [Google Scholar]
- Chavkin C. (2013) Dynorphin--still an extraordinarily potent opioid peptide. Mol Pharmacol 83:729–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig ADB. (2009) How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci 10:59–70. [DOI] [PubMed] [Google Scholar]
- Dittrich A. (1998) The standardized psychometric assessment of altered states of consciousness (ASCs) in humans. Pharmacopsychiatry 31(Supp 2):80–84. [DOI] [PubMed] [Google Scholar]
- González D, Riba J, Bouso JC, Gómez-Jarabo G, Barbanoj MJ. (2006) Pattern of use and subjective effects of Salvia divinorum among recreational users. Drug Alcohol Depend 85:157–162. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Richards WA, McCann U, Jesse R. (2006) Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology (Berl) 187:268–283; disc 284–292. [DOI] [PubMed] [Google Scholar]
- Hanes KR. (2001) Antidepressant effects of the herb Salvia divinorum: a case report. J Clin Psychopharmacol 21:634–635. [DOI] [PubMed] [Google Scholar]
- Johnson MW, MacLean KA, Reissig CJ, Prisinzano TE, Griffiths RR. (2011) Human psychopharmacology and dose-effects of salvinorin A, a kappa opioid agonist hallucinogen present in the plant Salvia divinorum. Drug Alcohol Depend 115:150–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamas X, Farré M, Llorente M, Camí J. (1994) Spanish version of the 49-item short form of the Addiction Research Center Inventory (ARCI). Drug Alcohol Depend 35:203–209. [DOI] [PubMed] [Google Scholar]
- Le Merrer J, Becker JAJ, Befort K, Kieffer BL. (2009) Reward processing by the opioid system in the brain. Physiol Rev 89:1379–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke DP, Terhune DB. (2013) The induction of synaesthesia with chemical agents: a systematic review. Front Psychol 4:753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean KA, Johnson MW, Reissig CJ, Prisinzano TE, Griffiths RR. (2013) Dose-related effects of salvinorin A in humans: dissociative, hallucinogenic, and memory effects. Psychopharmacology (Berl) 226:381–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WR, Sloan JW, Sapira JD, Jasinski DR. (1971) Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther 12:245–258. [DOI] [PubMed] [Google Scholar]
- Mehling WE, Price C, Daubenmier JJ, Acree M, Bartmess E, Stewart A. (2012) The Multidimensional Assessment of Interoceptive Awareness (MAIA). PLOS One 7:e48230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehling WE, Daubenmier J, Price CJ, Acree M, Bartmess E, Stewart AL. (2013) Self-reported interoceptive awareness in primary care patients with past or current low back pain. J Pain Res 6:403–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott J. (1995) Ethnopharmacognosy and human pharmacology of Salvia divinorum and salvinorin A. Curare 18:103–129. [Google Scholar]
- Paulus MP, Stein MB. (2010) Interoception in anxiety and depression. Brain Struct Funct 214:451–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisinzano TE. (2005) Psychopharmacology of the hallucinogenic sage Salvia divinorum. Life Sci 78:527–531. [DOI] [PubMed] [Google Scholar]
- Raichle ME. (2011) The restless brain. Brain Connect 1:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. (2001) A default mode of brain function. Proc Natl Acad Sci USA 98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan M, Schnakenberg A, Skosnik PD, Cohen BM, Pittman B, Sewell RA, D’Souza DC. (2012) Dose-related behavioral, subjective, endocrine, and psychophysiological effects of the κ opioid agonist Salvinorin A in humans. Biol Psychiatry 72:871–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raos VC, Dermon CR, Savaki HE. (1995) Functional anatomy of the thalamic centrolateral nucleus as revealed with the [14C]deoxyglucose method following electrical stimulation and electrolytic lesion. Neuroscience 68:299–313. [DOI] [PubMed] [Google Scholar]
- Riba J, Rodríguez-Fornells A, Barbanoj MJ. (2002) Effects of ayahuasca on sensory and sensorimotor gating in humans as measured by P50 suppression and prepulse inhibition of the startle reflex, respectively. Psychopharmacology (Berl) 165:18–28. [DOI] [PubMed] [Google Scholar]
- Riba J, Rodríguez-Fornells A, Strassman RJ, Barbanoj MJ. (2001a) Psychometric assessment of the Hallucinogen Rating Scale. Drug Alcohol Depend 62:215–223. [DOI] [PubMed] [Google Scholar]
- Riba J, Rodríguez-Fornells A, Urbano G, Morte A, Antonijoan R, Montero M, Callaway JC, Barbanoj MJ. (2001b) Subjective effects and tolerability of the South American psychoactive beverage Ayahuasca in healthy volunteers. Psychopharmacology (Berl) 154:85–95. [DOI] [PubMed] [Google Scholar]
- Roth BL, Baner K, Westkaemper R, Siebert D, Rice KC, Steinberg S, Ernsberger P, Rothman RB. (2002) Salvinorin A: a potent naturally occurring nonnitrogenous kappa opioid selective agonist. Proc Natl Acad Sci USA 99:11934–11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MD, Schmidt MS, Butelman ER, Harding WW, Tidgewell K, Murry DJ, Kreek MJ, Prisinzano TE. (2005) Pharmacokinetics of the plant-derived kappa-opioid hallucinogen salvinorin A in nonhuman primates. Synap N Y N 58:208–210. [DOI] [PubMed] [Google Scholar]
- Schwarzer C. (2009) 30 years of dynorphins--new insights on their functions in neuropsychiatric diseases. Pharmacol Ther 123:353–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seisdedos N. (2002) STAI Cuestionario de ansiedad estado-rasgo. Adaptación española del cuestionario y redacción del manual. Madrid, Spain: TEA Ediciones, SA. [Google Scholar]
- Siebert DJ. (1994) Salvia divinorum and salvinorin A: new pharmacologic findings. J Ethnopharmacol 43:53–56. [DOI] [PubMed] [Google Scholar]
- Simonin F, Gaveriaux-Ruff C, Befort K, Matthes H, Lannes B, Micheletti G, Mattei MG, Charron G, Bloch B, Kieffer B. (1995) kappa-Opioid receptor in humans: cDNA and genomic cloning, chromosomal assignment, functional expression, pharmacology, and expression pattern in the central nervous system. Proc Natl Acad Sci USA 92:7006–7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. (1970) Manual for the state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologists. [Google Scholar]
- Stiefel KM, Merrifield A, Holcombe AO. (2014) The claustrum’s proposed role in consciousness is supported by the effect and target localization of Salvia divinorum. Front Integr Neurosci 8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassman RJ, Qualls CR, Uhlenhuth EH, Kellner R. (1994) Dose-response study of N,N-dimethyltryptamine in humans. II. Subjective effects and preliminary results of a new rating scale. Arch Gen Psychiatry 51:98–108. [DOI] [PubMed] [Google Scholar]
- Tejeda HA, Shippenberg TS, Henriksson R. (2012) The dynorphin/κ-opioid receptor system and its role in psychiatric disorders. Cell Mol Life Sci 69:857–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdés LJ, Díaz JL, Paul AG. (1983) Ethnopharmacology of ska María Pastora (Salvia divinorum, Epling and Játiva-M.). J Ethnopharmacol 7:287–312. [DOI] [PubMed] [Google Scholar]
- Van der Werf YD, Witter MP, Groenewegen HJ. (2002) The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Brain Res Rev 39:107–140. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Clark L, Dunn BD. (2012) The role of interoception in addiction: a critical review. Neurosci Biobehav Rev 36:1857–1869. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Strain EC, Abreu ME, Bigelow GE. (2001) Enadoline, a selective kappa opioid agonist: comparison with butorphanol and hydromorphone in humans. Psychopharmacology (Berl) 157:151–162. [DOI] [PubMed] [Google Scholar]