Abstract
Background:
Transcranial direct current stimulation over the dorsolateral prefrontal cortex has been shown to be clinically useful in the treatment of drug addiction.
Methods:
We conducted a double-blind randomized clinical trial aiming to assess the effects of bilateral dorsolateral prefrontal cortex transcranial direct current stimulation (left cathodal/right anodal) on crack-cocaine addiction. We defined craving as the primary outcome, and other clinical measurements, including depressive and anxiety symtoms, and quality of life, as secondary outcomes. Seventeen male crack-cocaine users (mean age 30.4±9.8 SD) were randomized to receive 5 sessions of active transcranial direct current stimulation (2 mA, 35cm2, for 20 minutes), every other day, and 19 males (mean age 30.3±8.4 SD) to receive sham-transcranial direct current stimulation (placebo) as control group.
Results:
Craving scores were significantly reduced in the transcranial direct current stimulation group after treatment when compared with sham-transcranial direct current stimulation (P=.028) and baseline values (P=.003), and decreased linearly over 4 weeks (before, during, and after treatment) in the transcranial direct current stimulation group only (P=.047). Changes of anxiety scores towards increase in the sham-transcranial direct current stimulation and decrease in the transcranial direct current stimulation group (P=.03), and of the overall perception of quality of life (P=.031) and of health (P=.048) towards decrease in the sham-transcranial direct current stimulation group and increase in the transcranial direct current stimulation group differed significantly between groups.
Conclusions:
Repetitive bilateral transcranial direct current stimulation over the dorsolateral prefrontal cortex reduced craving for crack-cocaine use, decreased anxiety, and improved quality of life. We hypothesize that transcranial direct current stimulation effects may be associated with increased prefrontal processing and regulation of craving behavior.
Keywords: tDCS, crack-cocaine, dorsolateral prefrontal cortex, craving, quality of life
Introduction
Crack-cocaine is a highly addictive form of cocaine classified as a strong, short-acting stimulant drug (McClelland, 2005). It can be heated and inhaled or smoked (Wallace, 1989), yielding physiological and psychoactive effects that are qualitatively similar to snorting cocaine, but more intense (Hatsukami and Fischman, 1996; McClelland, 2005). It establishes a rapid and more severe dependence and strong withdrawal effects, and prognosis is worse compared with cocaine hydrochloride (Hatsukami and Fischman, 1996; Moura et al., 2014).
The treatment of crack-cocaine dependence imposes a greater challenge compared with snorted cocaine because of the greater addictive profile and more harmful physical and mental consequences. The highly uncontrollable craving to the drug use and, consequently, crack-cocaine binges (Chaves et al., 2011) and the high frequency of relapses are remarkable clinical patterns of this dependence. Pharmacological treatments and bio-psychosocial therapies are of limited efficacy to defeat this drug addiction (McClelland, 2005; De Oliveira et al., 2009; Goldstein and Volkow, 2011; Connolly et al., 2012). Thus, efforts are needed to develop alternative approaches to improve therapeutic success.
The chronic use of crack-cocaine seems to disrupt general cognitive functioning, verbal memory, and attentional resources (De Oliveira et al., 2009). These effects may be reversed after its discontinuation (De Oliveira et al., 2009). Prefrontal cortex (PFC) dysfunction with regard to regulation of limbic reward regions and its involvement in high-order executive function (self-control, salience attribution, and awareness) has been associated with the loss of control over drug use (Goldstein and Volkow, 2011; Connolly et al., 2012), which is an important characteristic of addiction (Garavan et al., 2008). Dorsolateral PFC (dlPFC) activity is highly required when executive control of cognition is required (Kane and Engle, 2002; Enriquez-Geppert et al., 2013). Thus, treatment focusing on dlPFC modulation and possibly improving cognitive control over drug intake may be beneficial in the management of crack-cocaine dependence.
Transcranial direct current stimulation (tDCS) is a noninvasive brain stimulation technique that induces polarity-dependent alterations of cortical excitability (Nitsche and Paulus, 2000; Nitsche et al., 2003, 2007, 2008). Modulation of dlPFC functions with tDCS has been shown to reduce craving for smoking (Fregni et al., 2008a), marijuana (Boggio et al., 2010), and food in healthy subjects (Fregni et al., 2008b). We thus hypothesized that tDCS over the dlPFC might be a promising therapeutic approach to treat drug dependence.
Previous studies from our group showed that anodal tDCS over the left dlPFC reduced craving and depressive symptoms (da Silva et al., 2013) and improved frontal executive functions (Nakamura-Palacios et al., 2012) in severe alcoholic subjects. However, when anodal tDCS was applied repetitively over the left dlPFC, relapses to the use of alcohol were likely increased in alcoholics (da Silva et al., 2013). In addition, Gorini et al. (2014) showed that risk-taking behavior increased after a single session of left dlPFC anodal stimulation, whereas increased safe behavior was observed after right dlPFC anodal stimulation in cocaine users. Therefore, we altered the stimulation protocols in subsequent studies by placing the cathode over the left dlPFC and the anode over the right dlPFC. With this bilateral montage we showed a long-lasting reduction of relapse probability and improvement of perception of quality of life in severe alcoholics after repetitive application (Klauss et al., 2014). Furthermore, we showed that single and repetitive application of tDCS can impact cognitive processing of neutral and especially crack-related visual cues in prefrontal areas (Conti et al., 2014a; Conti and Nakamura-Palacios, 2014b).
Therefore, in this trial we aimed to investigate the clinical effects of repetitive bilateral tDCS (left cathodal/right anodal) of the dlPFC on crack-cocaine addiction measuring craving as the primary outcome. Secondarily, we tested the impact of tDCS on other clinical measurements. We hypothesized that active tDCS would result in a greater reduction of craving compared with sham tDCS.
Methods and Materials
We report this clinical trial according to CONSORT guidelines. This trial was registered under Clinical Trials.gov number NCT02091167.
Trial Design
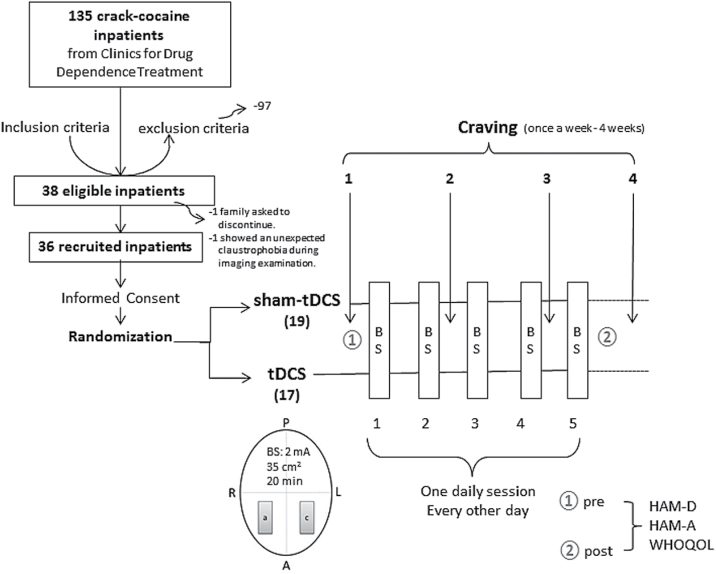
This clinical trial was a parallel randomized sham controlled and single center trial. Subjects were randomly assigned to receive real brain stimulation (tDCS group) or a simulation of this procedure (sham-tDCS group) in a 1:1 ratio (Figure 1) using a computer-generated randomization sequence that was kept with the unblinded study coordinator (not involved in the recruitment) and only revealed to the co-investigator conducting treatments immediately before the first session.
Figure 1.
Diagram of the general procedure: eligible crack-cocaine users were recruited from the clinics for treatment of drug dependence, signed the term of consent, and were randomized to receive repetitive bilateral (cathode left/anode right over the dorsolateral prefrontal cortex) transcranial direct current stimulation (tDCS; 2 mA, 35cm2, stimulation for 20min) every other day for a total of 5 sessions. Craving to the use of crack-cocaine was examined once per week for 4 weeks (the week before treatment, during the second and third treatment weeks, and the week after treatment). a, anode; A, anterior; BS, brain stimulation; c, cathode; HAM-D, Hamilton Scale for Depression; HAM-A, Hamilton Scale for Anxiety; L, left; P, posterior; R, right; WHOQOL, Quality of Life.
Participants
A total of 135 crack-cocaine addicted ICD-10 Classification of Mental and Behavioural Disorders and the Diagnostic and Statistical Manual of Mental Disorders, fourth edition subjects, as defined by the DSM-IV, were interviewed for this trial, and 38 subjects meeting our inclusion criteria (see below) agreed to participate in this study. They were recruited from the Clinic for Drug Dependence Treatment from Espírito Santo state, Brazil. One subject declined to participate after the beginning of the study and one subject showed claustrophobia and was excluded. So, 36 subjects successfully completed the study (see diagram flow in the Figure 1).
The inclusion criteria for this study were: (1) male patients over the age of 18 years; (2) met criteria for crack-cocaine dependence according to the ICD-10 Classification of Mental and Behavioural Disorders and the Diagnostic and Statistical Manual of Mental Disorders, fourth edition, as determined by clinical evaluation; (3) in stable clinical condition with no need for inpatient care; (4) able to read, write, and speak Portuguese; and (5) no severe withdrawal signs or symptoms at baseline. Conversely, exclusion criteria included: (1) a condition of intoxication or withdrawal due to a substance other than crack-cocaine, (2) unstable mental or medical disorder or substance abuse or addiction other than crack-cocaine dependence, except nicotine and/or caffeine; (3) a diagnosis of epilepsy, convulsions, or delirium tremens during abstinence from crack-cocaine; (4) a previous history of drug hypersensitivity or adverse reactions to diazepam or other benzodiazepines and haloperidol; (5) any contraindication for electrical brain stimulation procedures such as electronic implants or metal implants.
Ethical approval was provided by the Brazilian Institutional Review Board of the Federal University of Espírito Santo (registration 384.281), Brazil. The study was conducted in strict adherence to the Declaration of Helsinki and is in accordance with the ethical standards of the Committee on Human Experimentation of the Federal University of Espírito Santo, ES, Brazil, where this study was conducted. Subjects were fully informed about the experimental protocol and voluntarily signed an informed consent form before the start of the experiment.
Intervention
The intervention in this clinical trial was noninvasive brain stimulation by transcranial direct current stimulation (tDCS). Direct currents were transferred via a pair of carbonated silicone electrodes (35cm2) with a thick layer of high-conductive EEG gel underneath them according to our previous study (Nakamura-Palacios et al., 2012). The electric current was delivered by an electric stimulator (Striat, Ibramed Indústria Brasileira de Equipamentos Médicos Ltd, São Paulo, Brazil). For tDCS, the cathode was placed over the left dlPFC (F3) while the anode was placed over the right dlPFC (F4) according to the 10–20 international system (Figure 1). In each session, the currents flowed continuously for 20 minutes (2.0 mA).
For sham tDCS, the electrodes were placed at the same positions, but the stimulator was gradually turned off after 20 seconds. In this way, subjects remain blinded to the respective stimulation condition, as the itching sensation typical for tDCS is often only experienced initially during stimulation (Brunoni et al., 2014). A previous study validated the sham procedure of 2 mA tDCS, showing similar blinding efficacy as a placebo pill (Brunoni et al., 2014).
Both groups (sham- and real tDCS) received one session per day, every other day, with a total of 5 sessions (Figure 1). Patients were clinically evaluated (HAM-D, HAM-A, and WHOQOL) before intervention and after completion of the protocol. Furthermore, craving evaluation was performed once per week during the 4 weeks of treatment (Figure 1).
Outcomes
Craving was scored with a brief scale composed of 5 items (1, 2, 4, 5, and 13) from the Obsessive Compulsive Drinking Scale (Anton et al., 1995; Anton et al., 1996; Anton, 2000), which assesses craving in a narrow sense according to De Wildt et al. (2005). These items are identical with those from the Obsessive Compulsive Cocaine Use Scale and Obsessive-Compulsive Cocaine Scale, on which the Obsessive Compulsive Drinking Scale was based, as proposed recently by Hormes et al. (2012) and Vorspan et al. (2012). They allow the quantification of thoughts and feelings (obsessions) and behavioral intention (de Wildt et al., 2005) about cocaine/crack use and are detailed in the appendix (Appendix 1, supplementary Data).
Secondary outcomes consisted of global physical and clinical examination, including the assessment of depressive and anxiety symptoms, and quality of life as listed below and detailed in the appendix (Appendix 1, supplementary Data). For these parameters, the patients were assessed at baseline and after the 5-session treatment period (2 weeks): (1) Hamilton Depression Rating Scale (HAM-D); (2) Hamilton Anxiety Rating Scale (HAM-A); and (3) quality of life of the World Health Organization (WHOQOL-BREF).
All clinical measurements were conducted by one of the experimenters blinded for the brain stimulation procedures; conversely, the experimenter responsible for tDCS application was blinded to all clinical outcomes for the entire period of the study.
Statistical Analyses
We powered the study for a moderate effect size given our hypothesis that tDCS would be associated with a relevant reduction in craving scores. Thus, assuming an effect size of 0.35 considering a repeated-measure ANOVA as principal statistical test with a power of 80% 2-sided probability of a type I error of 5%, a minimum of 32 subjects would be necessary. However, to account for waiving or dropouts expected to be very common in this condition, we increased the estimated sample to 10%, resulting in 36 subjects.
Comparisons of craving, HAM-D, HAM-A, and quality of life scores before and after treatment were analyzed by a repeated-measure ANOVA, including the respective baseline values as moderator variables, the independent group factor (sham-tDCS vs tDCS), and repeated measure factor treatment (before and after tDCS), followed by Bonferroni-corrected posthoc t tests. Regarding the predictability of tDCS intervention on craving scores, a linear regression was employed considering the 4 weeks of treatment.
When the interaction was not statistically significant in the ANOVA, we used exploratory paired t tests to compare results before and after treatment within sham and real tDCS groups. Student t tests for independent measures were used for between-group comparisons considering the extension of changes (final – initial). For Likert-scale data of subjective confidence regarding the treatment they were receiving, the Mann-Whitney test was used for between-group comparison, because these data were not normally distributed (Shapiro-Wilk normality test).
For nonparametric nominal data, chi-square tests were used to compare results between and within sham and real tDCS groups.
The amount of crack-rocks consumed per day was missing for 5 patients, and the days of abstinence data were missing for 1 patient. These missing data were imputed by linear regression.
A 2-tailed P ≤ .05 or less was considered to indicate statistical significance. SPSS Statistics Base 17.0 (SPSS Inc) and GraphPad Prism 5.0 (GraphPad Software Inc) were employed for statistical analysis and graphic presentations.
Results
Baseline Data
Baseline socio-demographic characteristics, patterns of drug use, and clinical outcomes are presented in Tables 1 and 2.
Table 1.
Socio-Demographic Characteristics, Patterns of Crack-Cocaine Use, Impression of What Treatment They Were in and Confidence of This Impression, and Adverse Events, for the Total Sample of Drug Users and Subdivided in Users Submitted to Bilateral Repetitive Transcranial Direct Current Stimulation (tDCS: cathode left/anode right dorsolateral Prefrontal Cortex, 2 mA, 35 cm2, 20min, 5 sessions, every other day, n = 17) or placebo (sham-tDCS: n = 19)
|
Crack-Cocaine Users
(n = 36) |
Sham-tDCS
(n = 19) |
tDCS
(n =17) |
P Value | ||||
|---|---|---|---|---|---|---|---|
| Socio-demographic characteristics | |||||||
| Age [mean (SD)] | 30.4 (9.0) | 30.3 (8.4) | 30.4 (9.8) | t(34) = -0.03 | 0.98 | ||
| Gender n (%) | Male | 36 (100%) | 19 (100%) | 17 (100%) | |||
| Years of education [mean (SD)] |
2.4 (0.8) | 2.3 (0.6) | 2.6 (1.0) | t(34) = -1.22 | 0.23 | ||
| Employment situation n (%) |
Formal job | 5 (13.9%) | 3 (15.8%) | 2 (11.8%) | X 2 = 4.91 | 0.30 | |
| Informal job | 2 (5.6%) | 1 (5.3%) | 1 (5.9%) | ||||
| Unemployed | 17 (47.2%) | 6 (31.6%) | 11 (64.7%) | ||||
| Freelance | 6 (16.7%) | 4 (21.1%) | 2 (11.8%) | ||||
| Disease benefit | 6 (16.7%) | 5 (26.3%) | 1 (5.9%) | ||||
| Marital state n (%) |
Single | 23 (63.9%) | 11 (57.9%) | 12 (70.6%) | X 2 = 9.46 | 0.02* | |
| Married | 8 (22.2%) | 7 (36.8%) | 1 (5.9%) | ||||
| Divorced | 4 (11.1%) | 0 (0%) | 4 (23.5%) | ||||
| Common-law marriage | 1 (2.8%) | 0 (0%) | 1 (5.3%) | ||||
| Tobacco use n (%) | Yes | 30 (83.3%) | 14 (73.7%) | 16 (94.1%) | X 2 = 2.70 | 0.10 | |
| No | 6 (16.7%) | 5 (26.3%) | 1 (5.9%) | ||||
| Crack-cocaine use | |||||||
| Age at onset of crack-cocaine use [mean (SD)] | 21.7 (8.0) | 21.0 (7.1) | 22.5 (9.0) | t(34) = -0.56 | 0.58 | ||
| Amount of crack-cocaine used (rocks/d) [mean (SD)] | 13.1 (11.3) | 12.9 (12.0) | 13.4 (11.0) | t(34) = -0.13 | 0.90 | ||
| Days of abstinence before study [mean (SD)] | 34.6 (10.2) | 35.0 (9.9) | 34.2 (10.9) | t(34) = 0.23 | 0.82 | ||
| Impression n (%) | |||||||
| Sham (placebo) treatment tDCS treatment |
3 (8.3%) | 3 (15.8%) | 0 (0%) | X 2 = 2.93 | 0.09 | ||
| 33 (91.7%) | 16 (84.2%) | 17 (100%) | |||||
| Confidence in their impression n (%) |
[mean (SD)] | 4.2 (0.8) | 4.0 (0.9) | 4.4 (0.7) | U = 75.5 | 0.29 | |
| (1) None | 0 (0%) | 0 (0%) | 0 (0%) | ||||
| (2) Little | 1 (3.6%) | 1 (6.7%) | 0 (0%) | ||||
| (3) Medium | 4 (14.3%) | 3 (20.0%) | 1 (7.7%) | ||||
| (4) Very confident | 12 (42.9%) | 6 (40.0%) | 6 (46.2%) | ||||
| (5) Extremely confident | 11 (39.3%) | 5 (33.3%) | 6 (46.2%) | ||||
| Adverse events n (%) | |||||||
| None | 5 (13.9%) | 4 (21.1%) | 1 (5.9%) | X 2 = 6.86 | 0.14 | ||
| Headache | 1 (2.8%) | 1 (5.3%) | 0 (0%) | ||||
| Buzzing | 1 (2.8%) | 0 (0%) | 1 (5.9%) | ||||
| Tingling in the scalp | 26 (72.2%) | 14 (73.7%) | 12 (70.6%) | ||||
| Burning sensation in the scalp | 3 (8.3%) | 0 (0%) | 3 (17.6%) | ||||
Table 2.
Clinical Measurements in Crack-Cocaine Users at the Beginning (Initial) and at the End (Final) of the Treatment with Bilateral Repetitive tDCS or Placebo (sham-tDCS)
| Clinical Measurements | |||||
|---|---|---|---|---|---|
| mean (SD) |
Sham-tDCS
(n = 19) |
tDCS
(n = 17) |
Between-group analysis | ||
| HAM-D | Initial | 4.3 (3.1) | 5.0 (3.0) |
F
interaction
(1,33) = 0.39
p = 0.54 partial η 2 =0.01 |
|
| Final | 3.5 (3.3) | 3.2 (3.2) | |||
| Within-group analysis | t(18) = 1.04, p = 0.31 | t(16) = 2.2, p = 0.04* | |||
| Final-initial | -0.79 (3.31) | -1.65 (3.37) | t(34)=0.77 | p = 0.45 | |
| HAM-A | Initial | 6.0 (4.3) | 7.6 (5.8) |
F
interaction
(1,33) = 3.91
p = 0.056 partial η 2 =0.11 |
|
| Final | 8.7 (6.5) | 6.4 (4.3) | |||
| Within-group analysis | t(18) = -2.07, p = 0.053 | t(16) = 1.07, p = 0.30 | |||
| Final-initial | 2.68 (5.65) | -1.24 (4.75) | t(34) = -2.24 | p = 0.03* | |
HAM-A, Hamilton Anxiety Rating Scale; HAM-D, Hamilton Depression Rating Scale. *P<.05.
Crack-cocaine subjects were young, with an average of 30.4 years old in the total sample, generally low-educated (<3 years of education), mostly unemployed (47.2%), and single (63.9%) (Table 1). In addition, most of them (83.3%) were tobacco smokers (Table 1).
Except for the marital state, which showed differences between groups (P<.02), mostly because of the greater proportion of married subjects in the sham-tDCS group and of divorced subjects in the tDCS groups, no other socio-demographic parameter differed between groups (Table 1).
They started to use crack-cocaine on average at 21.7 years, consumed on average 13.1 rocks per day, and they were about 34.6 days abstinent before the beginning of the experimental protocol (Table 1). None of these characteristics differed between sham (n=19) and real tDCS groups (n=17) (Table 1).
Patients were kept in a restrictive environment for drug use. Presence of the drug was checked qualitatively in the urine at random intervals during the period of their treatment. All urine samples were found to be negative during the study period.
Subjects were blinded for sham- or real tDCS treatment. When they were asked about their impression of what treatment they had received at the end of the treatment, 33 (91.7%) subjects answered they were exposed to real tDCS (Table 1). That is, only 3 (8.3%) subjects answered they received sham-tDCS. These 3 were from the sham-tDCS group. Interestingly, from the sham-tDCS group, 16 of 19 (84.2%) answered they were receiving real tDCS treatment, whereas all subjects (100%) from the real tDCS group answered positively. In 28 subjects, when they were asked how confident they were with regard to treatment condition, 23 (82.2%) were very to extremely confident in the total sample, 11 (73.3%) from sham-tDCS group, and 12 (92.4%) from tDCS group. There were no statistically significant differences between groups for both the impression and confidence (Table 1). However, it should be noted that this scale was not applied to the first 8 subjects entered in this study due to initial study procedures that prevented application of this scale.
Primary Outcome
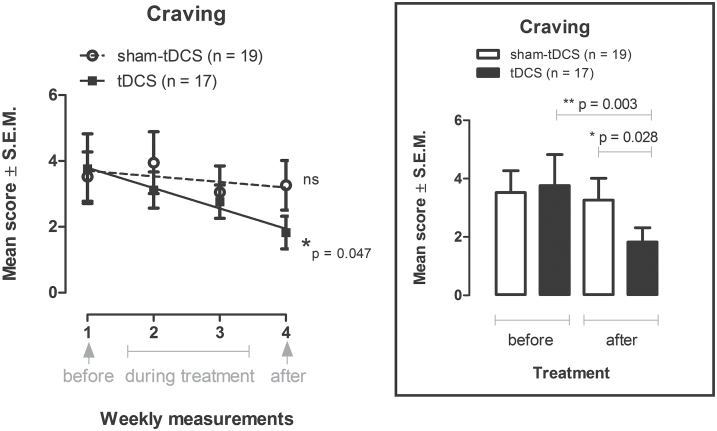
A repeated-measures ANOVA with baseline as moderator variable was conducted to examine the intervention effect on craving (Figure 2, inbox). tDCS and sham-tDCS groups differed on craving over time, and the results of the ANOVA show a significant interaction between groups and treatment [F(1,33)=5.29, P=.028, partial η2=0.14]. Pairwise comparisons by Bonferroni-corrected posthoc tests showed a statistically significant difference between groups after treatment (P=.028) and for craving scores obtained before and after treatment (P=.003) for the real tDCS group only.
Figure 2.
Craving is shown as mean score ± SEMs in the week before treatment, the second and third weeks during the treatment, and the week after treatment with bilateral repetitive transcranial direct current stimulation (tDCS; 2mA, 35cm2: cathode left/anode right over the dorsolateral prefrontal cortex; stimulation for 20min every other day for a total of 5 sessions; n=17) or placebo (sham-tDCS; n=19) in crack-cocaine addicts in the left figure (linear regression: 4.412 - 0.617X, r2=0.058, P=.047). Inbox: mean scores of craving shown the week before and the week after treatment in the real and sham-tDCS groups. *P=.028, **P=.003 (Bonferroni-corrected t tests test following repeated-measures ANOVA adjusted for baseline).
Craving scores decreased linearly from baseline (week before treatment) to the week after treatment only in the tDCS group [linear regression: 4.412 - 0.617X, r2=0.058, F(1,66)=4.089, P=.047] (Figure 2), meaning that the decrease of craving scores could be predicted by active tDCS treatment.
Secondary Outcomes
Depression (indexed by HAM-D) and anxiety (indexed by HAM-A) symptoms were not different between groups, before or after treatment, and no significant interaction was found between groups and treatment factors (Table 2). Scores of HAM-D decreased in both groups after treatment, but this change was statistically significant (P=.04) only for the real tDCS group. For HAM-A, scores increased in the sham-tDCS group almost significantly (P=.053), whereas they decreased in the real tDCS group (Table 2). This opposing change in anxiety scores was statistically different (P=.03) between group analysis.
In general, values of quality of life assessed by WHOQOF-BREF increased after treatment in the real tDCS group but decreased in the sham-tDCS group in Q1 and Q2. There were no significant interactions between groups and treatment factors in the ANOVA (Table 3). However, when considering the extension of changes between final and initial scores, there were significant differences between groups in Q1 (P=.031), which explores the individual’s overall perception of quality of life, and Q2 (P=.048), related to the individual’s overall perception of health (Table 3). Changes in specific domains were not different between groups (Table 3).
Table 3.
Quality of Life (WHOQOF-BREF) in Crack-Cocaine Inpatients at the Beginning (Initial) and at the End (Final) of the Treatment with Bilateral Repetitive tDCS or Placebo (sham-tDCS)
| WHOQOF-BREF | |||||
|---|---|---|---|---|---|
| mean (SD) |
Sham-tDCS
(n = 19) |
tDCS
(n =17) |
Between-group analysis | ||
|
Q1
Individual’s overall perception of quality of life |
Initial | 4.00 (0.82) | 3.65 (1.06) |
F
interaction
(1,33) = 3.85,
p = 0.058 partial η 2 = 0.11 |
|
| Final | 3.95 (0.85) | 4.35 (0.70) | |||
| Within-group analysis | t(18) = 0.27, p = 0.79 | t(16) = -2.51, p = 0.02* | |||
| Final-Initial | -0.526 (0.85) | 0.706 (1.16) | t(34) = -2.26 | p = 0.031* | |
|
Q2
Individual’s overall perception of their health |
Initial | 4.21 (0.79) | 4.06 (0.83) |
F
interaction
(1,33) = 4.04,
p = 0.053 partial η 2 = 0.11 |
|
| Final | 4.00 (0.94) | 4.41 (0.62) | |||
| Within-group analysis | t(18) = 1.17, p = 0.26 | t(16) = -1.69, p = 0.11 | |||
| Final-Initial | -0.211 (0.79) | 0.353 (0.86) | t(34) = -2.05 | p = 0.048* | |
| DOMAINS (transformed scores) | |||||
| Physical health | Initial | 15.85 (2.36) | 15.97 (1.77) |
F
interaction
(1,33) = 0.36,
p = 0.55 partial η 2 =0.01 |
|
| Final | 15.85 (2.26) | 16.27 (1.75) | |||
| Within-group analysis | t(18)= 0.00, p = 1.0 | t(16) = -1.06, p = 0.31 | |||
| Final-initial | -0.0001 (2.77) | 0.3024 (1.18) | t(34) = -0.42 | p = 0.68 | |
| Psychological | Initial | 15.93 (1.89) | 15.37 (2.09) |
F
interaction
(1,33) = 0.82,
p = 0.37 partial η 2 = 0.02 |
|
| Final | 16.17 (1.58) | 16.35 (1.53) | |||
| Within-group analysis | t(18) = -0.65, p = 0.53 | t(16) = -2.25, p = 0.04* | |||
| Final-initial | -0.246 (1.65) | 0.980 (1.80) | t(34) = -1.28 | p = 0.21 | |
| Social relationships | Initial | 14.11 (3.06) | 14.98 (1.80) |
F
interaction
(1,33) = 0.35,
p = 0.56 partial η 2 = 0.01 |
|
| Final | 15.23 (2.93) | 15.14 (2.40) | |||
| Within-group analysis | t(18) = -1.54, p = 0.14 | t(16) = -0.29, p = 0.78 | |||
| Final-initial | 1.122 (3.18) | 0.157 (2.25) | t(34) = 1.04 | p = 0.306 | |
| Environment | Initial | 13.82 (1.67) | 13.26 (1.96) |
F
interaction
(1,33) = 1.33,
p = 0.25 partial η 2 = 0.04 |
|
| Final | 14.63 (2.02) | 14.79 (1.68) | |||
| Within-group analysis | t(18) = -2.19, p = 0.04* | t(16) = -4.83, p = 0.0002*** | |||
| Final-Initial | 0.816 (1.63) | 1.529 (1.30) | t(34) = -1.44 | p = 0.16 | |
WHOQOL-BREF, Abbreviated instrument of quality of life of the World Health Organization (translated to Portuguese). Domains were presented in transformed scores to be comparable with the scores used in the WHOQOL-100. *P<.05, ***P<.001
Both groups showed some changes in the pairwise analyses. When comparing scores after treatment with baseline values (before treatment), a statistically significant change (P=.02) was observed towards a greater mean score after treatment for the first individual question (Q1) in the real tDCS group only (Table 3). From the environmental domain, which showed significant changes for both groups at the global level (P=.04 for sham-tDCS, P=.0002 for the real tDCS group) (Table 3), the “physical environment” item improved for the sham-tDCS group (P=.016), whereas the “physical safety and security” (P=.014), “access to health and social care” (P=.014), and “transport” (P=.027) items improved for the tDCS group. Only the real tDCS group showed a significant increment of the global psychological domain (P=.04) (Table 3), which was mostly due to an increased “self-esteem” item in this group (P=.029).
Adverse Events
We asked subjects about the following adverse effects: headache, neck and scalp pain, tingling, itching, skin redness, burning sensation of the scalp, sleepiness, acute mood changes, trouble concentrating, and others (Brunoni et al., 2011) after treatment. From these potential events, the tingling sensation was reported by 26 subjects (72.2%) in the total sample and equally by the sham- (14 subjects, 73.7%) and real tDCS (12 subjects, 70.6%) groups (Table 1). Three subjects from the tDCS group (17.6%) reported a burning sensation, and one subject (5.9%) from this group reported a tinnitus sensation after treatment. Only one subject from the sham-tDCS group (5.3%) reported headache after treatment. Five subjects (13.9%) from the sham-tDCS group and one from the real tDCS group (5.9%) reported no events at all. No other adverse events were reported by crack-cocaine users from both groups in this study, and no significant difference was found between groups (Table 1).
Discussion
The most important result of this study is that crack-cocaine addicts who received 5 sessions of bilateral dlPFC tDCS (left cathodal/right anodal) every other day showed a significant reduction of craving after treatment when compared with crack-cocaine addicts who received placebo (sham-tDCS) treatment and also to respective baseline values. Secondarily, exploratory analyses show that active and sham tDCS groups also differed regarding changes in anxiety scores, overall perception of quality of life, and health. In all these secondary outcomes, active tDCS resulted in improved outcomes compared with sham tDCS.
The sham- and real tDCS groups were well matched by socio-demographic characteristics such as age, gender, schooling, and occupation and also by characteristics of crack-cocaine use, especially regarding days of abstinence before tDCS intervention. The socio-demographic characteristics of crack-cocaine users and patterns of drug use are similar to those reported in the Brazilian population (Duailibi et al., 2008; Oliveira and Nappo, 2008; Dieckmann et al., 2014; Moura et al., 2014) and also to other countries (Wallace, 1989; Vivancos et al., 2006; Haas et al., 2009; Oliveira et al., 2010).
The main outcome parameter of this study was craving for crack-cocaine. According to Sinha, drug-craving has reemerged as a relevant and important construct in the pathophysiology of addiction with its inclusion in DSM-V as a key clinical symptom of addictive disorders (Sinha, 2013). This is due to recent inputs of neurobiological evidence for craving-related neural activation and clinical evidence supporting its association with drug use, relapse, and recovery processes (Sinha, 2013).
Craving is defined as an uncontrolled urge to consume a drug, a state resulting from the presence of particularly strong obsessions about and irresistible compulsions to use (Robinson and Berridge, 1993; Hormes et al., 2012). It may be considered as a dimensional construct that grows with repetition and increasing levels of drug use (Robinson and Berridge, 1993; Sinha, 2013), even when pleasurable effects are no longer present and after the cessation of withdrawal symptoms (Robinson and Berridge, 1993), and it may also increase in strength over extended periods of abstinence (Robinson and Berridge, 1993; Grimm et al., 2001).
Although definition of craving is still a matter of extensive debate and is beyond the scope of this study, its clinical significance is less controversial (Tiffany and Wray, 2012b). Craving might be the main factor that makes addiction to drugs so difficult to overcome (George and Koob, 2013). For those who are addicted, craving is a very real intrusion in their daily lives, at times dominating their thoughts and generating considerable distress (Tiffany and Wray, 2012b).
In this study, crack-cocaine users treated with real tDCS showed a progressive decrease in craving over the intervention, a pattern not seen in subjects from the sham-tDCS group. By the end of the treatment, the tDCS group showed significantly lower craving scores compared with the sham-tDCS group and also compared with their initial scores (baseline).
There are still no effective therapeutic maneuvers for controlling drug craving in the routine treatment of drug dependence. Recent studies, however, suggest that noninvasive brain stimulation techniques, such as Transcranial Magnetic Stimulation and tDCS, may constitute promising approaches to aid the treatment of substance abuse and drug dependence (Jansen et al., 2013).
Jansen et al (2013) recently conducted a meta-analysis to evaluate the available evidence regarding the effects of noninvasive neurostimulation (Transcranial Magnetic Stimulation and tDCS) of the dlPFC on craving in substance dependence, including highly palatable food. They included 17 eligible studies and found a significant medium effect size favoring active noninvasive neurostimulation over sham stimulation for reduction of craving, with no differences between the techniques, between the various substances of abuse and between substances of abuse and food. They concluded that noninvasive neurostimulation of the dlPFC, irrespectively if magnetic or direct current stimulation, clearly decreases craving.
However, tDCS so far has been shown variable effects on drug craving. Fregni et al. (2008a) showed that in tobacco smokers, stimulation of both left and right dlPFC with anodal tDCS reduced craving after stimulation, but no effects have been found of anodal left or anodal right tDCS over the dlPFC on nicotine craving in more recent studies (Xu et al., 2013; Pripfl and Lamm, 2014). Shahbabaie et al. (2014) found a state-dependent effect of anodal tDCS over the right dlPFC on methamphetamine craving. Anodal tDCS of the left dlPFC slightly improved frontal function and increased the mean amplitude of P3 associated with alcohol-related sounds (Nakamura-Palacios et al., 2012), but when applied repetitively, it tended to increase relapses to alcohol use in alcoholics although it significantly decreased craving to the use of alcohol (da Silva et al., 2013). Bilateral tDCS decreased craving in alcoholics irrespectively of the polarity (anodal left/cathodal right or cathodal left/anodal right) over the dlPFC (Boggio et al., 2008). Similar effects were obtained with repetitive stimulation of a comparable protocol but with the cathode over the left dlPFC and anode over the right dlPFC, reducing relapse probability to the use of alcohol over a 6-month follow-up (Klauss et al., 2014). With cathodal left and anodal right dlPFC stimulation, Fecteau et al. (2014) observed a decrease in the number of cigarettes smoked by tobacco smokers. In this scenario, we found that repetitive bilateral dlPFC tDCS (cathodal left/anodal right) decreased craving for crack-cocaine use.
There are few studies aimed to investigate the effects of tDCS on cocaine/crack-cocaine addiction. Gorini et al. (2014) investigated the effects of modulation of left and right cortical excitability on 2 risk tasks. Cocaine users and healthy controls randomly received left anodal/right cathodal, right anodal/left cathodal, and sham (placebo) stimulation at least 48 hours apart. They observed that cocaine users and control subjects showed increased safe behavior after right dlPFC anodal stimulation, whereas risk-taking behavior increased after left dlPFC anodal stimulation in cocaine users only. They suggest that dependent cocaine users have functional abnormalities in the prefrontal neural networks involved in decision-making and risk-taking behavior. The excessive risk propensity in dependent cocaine users would be due to a hypoactivation of the right dlPFC and a dysbalanced interhemispheric interaction (Gorini et al., 2014).
In the present study, we also used left cathodal/right anodal tDCS over dlPFC but applied it repetitively (5 applications every other day). Our results seem to agree with those reported above, which allow us to suggest that a better craving control induced by brain stimulation could result in less risk-taking behavior, or the other way around, the better control of risk-taking behavior would help to control craving. However, risk-taking and craving may also not be directly related but represent different dimensions converging to a more favorable cognitive control by tDCS-induced modulation of dlPFC function.
A careful assessment of quality of life was made in this study, especially because crack-cocaine addiction has tremendous harmful impacts on the individual’s life, the families, and the community he/she lives in. In this study the individual’s overall perception of quality of life and health improved in crack-cocaine users treated with tDCS after the end of the treatment. By contrast, those who were treated with sham-tDCS showed a change in the opposite direction, that is, towards decrement of quality of life. In addition, the self-esteem of crack-cocaine users from the real tDCS group improved in the psychological domain.
Less craving, less anxiety, and better quality of life are all favorable features to a better outcome of drug dependence treatment. Craving and quality of life have been recommended as a broader array of outcome measures in research on addiction treatment that go beyond the limited measures of drug use (Tiffany et al., 2012a). While craving is a highly salient construct, experienced by the addicted subject as aversive and disruptive to functioning, the addictive process often affects functioning beyond the immediate effects of drug use, including consequences in the domains of health, well-being, psychological functioning, relationships, productivity, and criminality (Tiffany et al., 2012a). Thus, addiction treatments would be more effective if they reduce the impact of negative consequences of drug use. The effects of repetitive bilateral direct current stimulation over the dlPFC seem to nicely fit to these criteria.
There are limitations of this study that need to be taken into account. The sample of crack-cocaine users was relatively small, thus limiting assumptions about the clinical relevance of our findings. Furthermore, patients were about 35 days abstinent of the drug at the beginning of the study, when Obsessive-Compulsive Cocaine Scale scores were possibly already reduced. The reason for this was that during the first month of their drug dependence treatment in the inpatient service, patients usually experience very strong craving and are physically and mentally very unstable. Therefore, they were given some time to stabilize their global status before recruitment to this study. However, this procedure was identical for both randomized groups.
In summary, repetitive bilateral tDCS over the dlPFC (left cathodal and right anodal) reduced craving to crack-cocaine use and anxiety symptoms and improved the overall perception of quality of life and health in crack-cocaine dependents. Further studies should explore further parameters and additional clinical outcomes such as relapses and long-term effects.
Statement of Interest
M.A.N. is member of the advisory board of Neuroelectrics. All other authors (E.K.B., J.K., F.F., and E.M.N.P.) reported no biomedical financial interests or potential conflicts of interest.
Acknowledgments
We want to thank the patients and families who agreed to participate in this study. We thank the Greenhouse Clinic team, especially Dr. Luis Henrique Casagrande, who allowed us to conduct this study in patients from this Clinic for Treatment of Mental Disorders and Drug Dependence, to use its facilities, and made all effort to help us. This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (grant nos. 475232/2013–5, 443824/2014-2, and 466650/2014-0 to E.M.N.P.). E.M.N.P. received a researcher fellowship from Fundação de Amparo à Pesquisa do Espírito Santo. E.K.B. and J.K. were recipients of student fellowships from CNPq and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior.
Appendix 1: Details of Clinical Assessments
Craving (5 items from OCCS)
Craving was scored with a brief scale composed of 5 items (1, 2, 4, 5, and 13) from the Obssessive Compulsive Cocaine Scale (OCCS). The questions of this brief scale are answered on a scale ranging from 0 to 4, resulting in a total score between 0 and 20. The first item (1 of the original scale) asks how much of a person’s time (total per day), when the drug is not used, is occupied by thoughts, ideas, desires, or impulses related to cocaine/crack and its effects, having the possible answers: (0) never; (1) <1h/d; (2) 1–3h/d; (3) 4–8h/d; and (4) >8h/d (or all day long). The second item (2 of the original scale) asks how frequently these thoughts, ideas, desires, or impulses related to cocaine/crack and its effects occur, having as answers: (0) never; (1) no more than 8 times per day; (2) >8 times per day, but most of the day of this person is free of these thoughts; (3) >8 times per day and most of the day the patient is occupied by these thoughts; and (4) these thought are so frequent that he/she cannot count them (almost no hour is free of these thoughts). The third item (4 of the original scale) asks how much distress or disturbance these ideas, thoughts, impulses or desire related to cocaine/crack use cause when the person is under withdrawal, having as answers: (0) no disturbance; (1) slight or infrequent disturbance; (2) moderate or frequent disturbance, but still manageable; (3) severe, very frequent disturbance; (4) extremely disturbing, almost constant or causing disabling distress. The fourth item (5 of the original scale) asks how much effort the person has to make to resist these thoughts, ideas, desires, or impulses, or how much energy he/she has to spend to think of something else when they enter the mind under withdrawal (what matters here is the effort required to resist to the thoughts and not the failure or success to control them), having as answers: (0) intensity is so weak that it requires no effort to resist them; (1) try to resist most of the time; (2) need to make a lot of effort to resist; (3) the effort would be so hard that they give in to these thoughts after a while; (4) They can absolutely not resist and they give in to all these thoughts without trying. Finally, the last item (13 from the original scale) asks about the person’s drive to use cocaine/crack, having as answers: (0) they never feel compelled to use cocaine/crack; (1) some pressure to use cocaine/crack; (2) strong pressure to use cocaine/crack; (3) they feel a very important drive that compels them to use cocaine/crack; (4) they feel a very powerful drive to use cocaine/crack that they cannot resist (Vorspan et al., 2012). This scale was applied in the week before the beginning of the real or sham-tDCS treatment, during the treatment (first and second weeks) and in the week after the end of the treatment.
Hamilton Depression Rating Scale (HAM-D)
The severity of depression symptoms was analyzed via a multiple-choice questionnaire. This instrument assesses the severity of depression symptoms such as low mood, insomnia, agitation, anxiety, and weight loss (Hamilton, 1960). The examiner must choose between the possible answers to each question by interviewing the patient and observing the patient’s symptoms. Each question has between 3 and 5 possible answers that increase in severity. In the original scale, the first 17 questions contribute to the total score, while questions 18 to 21 provide additional information about depression (eg, diurnal variation, paranoid symptoms) but are not included in the total score of the scale. This scale was applied at the beginning and at the end of the treatment.
Hamilton Anxiety Rating Scale (HAM-A)
The severity of anxiety symptoms was analyzed via a structured multiple-choice questionnaire (Hamilton, 1959). The scale consists of 14 items, each defined by a series of symptoms, and measures both psychic anxiety (eg, mental agitation and psychological distress) and somatic anxiety (eg, physical complaints related to anxiety). This scale was also applied at the beginning and at the end of the treatment.
Quality of Life
An abbreviated instrument of cross-culturally valid assessment of quality of life of the World Health Organization (WHOQOL-BREF) with 26 questions (Skevington et al., 2004) translated to Portuguese (Fleck et al., 2000) was applied at the beginning and at the end of the treatment. This instrument yields 4 domains (physical health, psychological, social relationships, and environment) and 2 individually scored items regarding overall perception of quality of life (Q1, ie, first question) and health (Q2, ie, second question). The 4 domain scores are scaled in a way that higher scores stand for higher quality of life. These scores were transformed to be comparable with the scores used in the WHOQOL-100 (WHO, 1996).
References
- Anton RF. (2000) Obsessive-compulsive aspects of craving: development of the Obsessive Compulsive Drinking Scale. Addiction 95:S211–217. [DOI] [PubMed] [Google Scholar]
- Anton RF, Moak DH, Latham P. (1995) The Obsessive Compulsive Drinking Scale: a self-rated instrument for the quantification of thoughts about alcohol and drinking behavior. Alcohol Clin Exp Res 19:92–99. [DOI] [PubMed] [Google Scholar]
- Anton RF, Moak DH, Latham PK. (1996) The obsessive compulsive drinking scale: a new method of assessing outcome in alcoholism treatment studies. Arch Gen Psychiatry 53:225–231. [DOI] [PubMed] [Google Scholar]
- Boggio PS, Sultani N, Fecteau S, Merabet L, Mecca T, Pascual-Leone A, Basaglia A, Fregni F. (2008) Prefrontal cortex modulation using transcranial DC stimulation reduces alcohol craving: a double-blind, sham-controlled study. Drug Alcohol Depend 92:55–60. [DOI] [PubMed] [Google Scholar]
- Boggio PS, Zaghi S, Villani AB, Fecteau S, Pascual-Leone A, Fregni F. (2010) Modulation of risk-taking in marijuana users by transcranial direct current stimulation (tDCS) of the dorsolateral prefrontal cortex (DLPFC). Drug Alcohol Depend 112:220–225. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Amadera J, Berbel B, Volz MS, Rizzerio BG, Fregni F. (2011) A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. Int J Neuropsychopharmacol 14:1133–1145. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Schestatsky P, Lotufo PA, Bensenor IM, Fregni F. (2014) Comparison of blinding effectiveness between sham tDCS and placebo sertraline in a 6-week major depression randomized clinical trial. Clin Neurophysiol 125:298–305. [DOI] [PubMed] [Google Scholar]
- Chaves TV, Sanchez ZM, Ribeiro LA, Nappo SA. (2011) Crack cocaine craving: behaviors and coping strategies among current and former users. Rev Saude Publica 45:1168–1175. [DOI] [PubMed] [Google Scholar]
- Connolly CG, Foxe JJ, Nierenberg J, Shpaner M, Garavan H. (2012) The neurobiology of cognitive control in successful cocaine abstinence. Drug Alcohol Depend 121:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti CL, Moscon JA, Fregni F, Nitsche MA, Nakamura-Palacios EM.(2014a. ) Cognitive related electrophysiological changes induced by non-invasive cortical electrical stimulation in crack-cocaine addiction. Int J Neuropsychopharmacol 17:1465–1475. [DOI] [PubMed] [Google Scholar]
- Conti CL, Nakamura-Palacios EM.(2014b) Bilateral transcranial direct current stimulation over dorsolateral prefrontal cortex changes the drug-cued reactivity in the anterior cingulate cortex of crack-cocaine addicts. Brain Stimul 7:130–132. [DOI] [PubMed] [Google Scholar]
- da Silva MC, Conti CL, Klauss J, Alves LG, do Nascimento Cavalcante HM, Fregni F, Nitsche MA, Nakamura-Palacios EM. (2013) Behavioral effects of transcranial direct current stimulation (tDCS) induced dorsolateral prefrontal cortex plasticity in alcohol dependence. J Physiol Paris 107:493–502. [DOI] [PubMed] [Google Scholar]
- De Oliveira LG, Barroso LP, Silveira CM, Sanchez ZV, De Carvalho Ponce J, Vaz LJ, Nappo SA. (2009) Neuropsychological assessment of current and past crack cocaine users. Subst Use Misuse 44:1941–1957. [DOI] [PubMed] [Google Scholar]
- de Wildt WA, Lehert P, Schippers GM, Nakovics H, Mann K, van den Brink W. (2005) Investigating the structure of craving using structural equation modeling in analysis of the obsessive-compulsive drinking scale: a multinational study. Alcohol Clin Exp Res 29:509–516. [DOI] [PubMed] [Google Scholar]
- Dieckmann LH, Ramos AC, Silva EA, Justo LP, Sabioni P, Frade IF, de Souza AL, Galduroz JC. (2014) Effects of biperiden on the treatment of cocaine/crack addiction: a randomised, double-blind, placebo-controlled trial. Eur Neuropsychopharmacol 24:1196–1202. [DOI] [PubMed] [Google Scholar]
- Duailibi LB, Ribeiro M, Laranjeira R. (2008) Profile of cocaine and crack users in Brazil. Cad Saude Publica 24:s545–557. [DOI] [PubMed] [Google Scholar]
- Enriquez-Geppert S, Huster RJ, Herrmann CS. (2013) Boosting brain functions: Improving executive functions with behavioral training, neurostimulation, and neurofeedback. Int J Psychophysiol 88:1–16. [DOI] [PubMed] [Google Scholar]
- Fecteau S, Agosta S, Hone-Blanchet A, Fregni F, Boggio P, Ciraulo D, Pascual-Leone A. (2014) Modulation of smoking and decision-making behaviors with transcranial direct current stimulation in tobacco smokers: a preliminary study. Drug Alcohol Depend 140:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregni F, Liguori P, Fecteau S, Nitsche MA, Pascual-Leone A, Boggio PS.(2008a) Cortical stimulation of the prefrontal cortex with transcranial direct current stimulation reduces cue-provoked smoking craving: a randomized, sham-controlled study. J Clin Psychiatry 69:32–40. [DOI] [PubMed] [Google Scholar]
- Fregni F, Orsati F, Pedrosa W, Fecteau S, Tome FA, Nitsche MA, Mecca T, Macedo EC, Pascual-Leone A, Boggio PS.(2008b) Transcranial direct current stimulation of the prefrontal cortex modulates the desire for specific foods. Appetite 51:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Kaufman JN, Hester R. (2008) Acute effects of cocaine on the neurobiology of cognitive control. Philos Trans R Soc Lond B Biol Sci 363:3267–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Koob GF. (2013) Control of craving by the prefrontal cortex. Proc Natl Acad Sci U S A 110:4165–4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. (2011) Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci 12:652–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorini A, Lucchiari C, Russell-Edu W, Pravettoni G. (2014) Modulation of risky choices in recently abstinent dependent cocaine users: a transcranial direct-current stimulation study. Front Hum Neurosci 8:661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. (2001) Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature 412:141–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas C, Karila L, Lowenstein W. (2009) [Cocaine and crack addiction: a growing public health problem]. Bull Acad Natl Med 193:947–962; discussion 962–943. [PubMed] [Google Scholar]
- Hatsukami DK, Fischman MW. (1996) Crack cocaine and cocaine hydrochloride. Are the differences myth or reality? JAMA 276:1580–1588. [PubMed] [Google Scholar]
- Hormes JM, Coffey SF, Drobes DJ, Saladin ME. (2012) The Obsessive Compulsive Cocaine Use Scale: development and initial validation of a self-rated instrument for the quantification of thoughts about cocaine use. Drug Alcohol Depend 120:250–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen JM, Daams JG, Koeter MW, Veltman DJ, van den Brink W, Goudriaan AE. (2013) Effects of non-invasive neurostimulation on craving: a meta-analysis. Neurosci Biobehav Rev 37:2472–2480. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. (2002) The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: an individual-differences perspective. Psychon Bull Rev 9:637–671. [DOI] [PubMed] [Google Scholar]
- Klauss J, Penido Pinheiro LC, Silva Merlo BL, de Almeida Correia Santos G, Fregni F, Nitsche MA, Miyuki Nakamura-Palacios E. (2014) A randomized controlled trial of targeted prefrontal cortex modulation with tDCS in patients with alcohol dependence. Int J Neuropsychopharmacol 17:1793–1803. [DOI] [PubMed] [Google Scholar]
- McClelland GT. (2005) The effects and management of crack cocaine dependence. Nurs Times 101:26–27. [PubMed] [Google Scholar]
- Moura HF, Benzano D, Pechansky F, Kessler FH. (2014) Crack/cocaine users show more family problems than other substance users. Clinics (Sao Paulo) 69:497–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura-Palacios EM, de Almeida Benevides MC, da Penha Zago-Gomes M, de Oliveira RW, de Vasconcellos VF, de Castro LN, da Silva MC, Ramos PA, Fregni F. (2012) Auditory event-related potentials (P3) and cognitive changes induced by frontal direct current stimulation in alcoholics according to Lesch alcoholism typology. Int J Neuropsychopharmacol 15:601–616. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. (2000) Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 527 Pt 3:633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, Henning S, Tergau F, Paulus W. (2003) Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol 553:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Doemkes S, Karakose T, Antal A, Liebetanz D, Lang N, Tergau F, Paulus W. (2007) Shaping the effects of transcranial direct current stimulation of the human motor cortex. J Neurophysiol 97:3109–3117. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, Paulus W, Hummel F, Boggio PS, Fregni F, Pascual-Leone A. (2008) Transcranial direct current stimulation: State of the art 2008. Brain Stimul 1:206–223. [DOI] [PubMed] [Google Scholar]
- Oliveira LG, Nappo SA. (2008) [Characterization of the crack cocaine culture in the city of Sao Paulo: a controlled pattern of use]. Rev Saude Publica 42:664–671. [DOI] [PubMed] [Google Scholar]
- Oliveira LG, Ponce Jde C, Nappo SA. (2010) Crack cocaine use in Barcelona: a reason of worry. Subst Use Misuse 45:2291–2300. [DOI] [PubMed] [Google Scholar]
- Pripfl J, Lamm C. (2014) Focused transcranial direct current stimulation (tDCS) over the dorsolateral prefrontal cortex modulates specific domains of self-regulation. Neurosci Res. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. (1993) The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev 18:247–291. [DOI] [PubMed] [Google Scholar]
- Shahbabaie A, Golesorkhi M, Zamanian B, Ebrahimpoor M, Keshvari F, Nejati V, Fregni F, Ekhtiari H. (2014) State dependent effect of transcranial direct current stimulation (tDCS) on methamphetamine craving. Int J Neuropsychopharmacol 17:1591–1598. [DOI] [PubMed] [Google Scholar]
- Sinha R. (2013) The clinical neurobiology of drug craving. Curr Opin Neurobiol 23:649–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany ST, Friedman L, Greenfield SF, Hasin DS, Jackson R.(2012a) Beyond drug use: a systematic consideration of other outcomes in evaluations of treatments for substance use disorders. Addiction 107:709–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany ST, Wray JM.(2012b) The clinical significance of drug craving. Ann N Y Acad Sci 1248:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivancos R, Maskrey V, Rumball D, Harvey I, Holland R. (2006) Crack/cocaine use in a rural county of England. J Public Health (Oxf) 28:96–103. [DOI] [PubMed] [Google Scholar]
- Vorspan F, Bellais L, Romo L, Bloch V, Neira R, Lepine JP. (2012) The Obsessive-Compulsive Cocaine Scale (OCCS): a pilot study of a new questionnaire for assessing cocaine craving. Am J Addict 21:313–319. [DOI] [PubMed] [Google Scholar]
- Wallace BC. (1989) Psychological and environmental determinants of relapse in crack cocaine smokers. J Subst Abuse Treat 6:95–106. [DOI] [PubMed] [Google Scholar]
- Xu J, Fregni F, Brody AL, Rahman AS. (2013) Transcranial direct current stimulation reduces negative affect but not cigarette craving in overnight abstinent smokers. Front Psychiatry 4:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- Fleck MP, Louzada S, Xavier M, Chachamovich E, Vieira G, Santos L, Pinzon V. (2000) [Application of the Portuguese version of the abbreviated instrument of quality life WHOQOL-bref]. Rev Saude Publica 34:178–183. [DOI] [PubMed] [Google Scholar]
- Hamilton M. (1959) The assessment of anxiety states by rating. Br J Med Psychol 32:50–55. [DOI] [PubMed] [Google Scholar]
- Hamilton M. (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry 23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skevington SM, Lotfy M, O’Connell KA. (2004) The World Health Organization’s WHOQOL-BREF quality of life assessment: psychometric properties and results of the international field trial. A report from the WHOQOL group. Qual Life Res 13:299–310. [DOI] [PubMed] [Google Scholar]
- Vorspan F, Bellais L, Romo L, Bloch V, Neira R, Lepine JP. (2012) The Obsessive-Compulsive Cocaine Scale (OCCS): a pilot study of a new questionnaire for assessing cocaine craving. Am J Addict 21:313–319. [DOI] [PubMed] [Google Scholar]
- WHO (1996) Introduction, administration, scoring and generic version of the assessment. Field trial version.