Abstract
Background:
Preclinical and emerging clinical evidence indicates that varenicline, a nicotinic partial agonist approved for smoking cessation, attenuates alcohol seeking and consumption. Reductions of alcohol craving have been observed under varenicline treatment and suggest effects of the medication on alcohol reward processing, but this hypothesis remains untested.
Methods:
In this double-blind, placebo-controlled randomized experimental medicine study, 29 heavy drinkers underwent a functional magnetic resonance imaging scan after 2 weeks of varenicline (2mg/d) or placebo administration. During functional magnetic resonance imaging, participants performed the Alcohol-Food Incentive Delay task, where they could earn points for snacks or alcohol. At baseline and after 3 weeks of medication, participants underwent intravenous alcohol self-administration sessions in the laboratory.
Results:
During the functional magnetic resonance imaging scan, participants in the varenicline group (N=17) reported lower feelings of happiness and excitement on subjective mood scales when anticipating alcohol reward compared with the placebo group (N=12). Linear mixed effects analysis revealed that anticipation of alcohol reward was associated with significant blood oxygen level dependent activation of the ventral striatum, amygdala, and posterior insula in the placebo group; this activation was attenuated in the varenicline group. The varenicline group showed no difference in intravenous alcohol self-administration relative to the placebo group for either session. Participants with higher insula activation when anticipating alcohol reward showed higher alcohol self-administration behavior across groups.
Conclusions:
Our findings suggest that varenicline decreases blood oxygen level dependent activation in striato-cortico-limbic regions associated with motivation and incentive salience of alcohol in heavy drinkers. This mechanism may underlie the clinical effectiveness of varenicline in reducing alcohol intake and indicates its potential utility as a pharmacotherapy for alcohol use disorders.
Keywords: Alcohol-Food Incentive Delay (AFID) task, intravenous (IV) infusion, heavy drinkers, neuroimaging, ventral striatum
Introduction
Alcohol abuse and dependence contribute to approximately 4% of deaths worldwide and are a major public health burden (Rehm et al., 2009; Rehm et al., 2014). Recent reviews and meta-analyses have shown that pharmacotherapies can reduce drinking among treatment-seeking alcoholics and improve general health (Rosner et al., 2010; Jonas et al., 2014; Pani et al., 2014). Overall effect sizes of existing medications, however, are small, making it important to expand the range of therapeutics and develop personalized treatment approaches (Heilig et al., 2011). One potential medication is the α4β2 nicotinic acetylcholine receptor (nAChR) partial agonist varenicline (Coe et al., 2005), an FDA-approved treatment for smoking cessation (Kuehn, 2006; Tonstad et al., 2006). Recent laboratory studies in humans and animals have shown that varenicline, relative to placebo, reduces alcohol consumption (Steensland et al., 2007; McKee et al., 2009). Further, although not all clinical trials have reported reduced drinking in participants treated with varenicline relative to placebo (Plebani et al., 2013), a review of all trials supported the use of varenicline as a treatment to reduce alcohol consumption (Erwin and Slaton, 2014). Clinical trials have also suggested that varenicline may reduce alcohol craving (Fucito et al., 2011; Litten et al., 2013; Mitchell et al., 2012; Plebani et al., 2013), suggesting that it may reduce motivation for seeking alcohol. This hypothesis presently remains untested.
A proposed mechanism underlying the reinforcement from alcohol consumption is activation of brain reward circuitry, particularly the striatum and midbrain dopamine neurons (Volkow et al., 2002; Koob, 2013). Alcohol potentiates the response of nAChRs to acetylcholine (Cardoso et al., 1999; Zuo et al., 2002), and stimulating nAChRs enhances dopamine release in the striatum, amygdala, and prefrontal cortex (Arqueros et al., 1978; Palotai et al., 2013). Hence, alcohol’s action at nAChRs may play a role in dopamine release in the ventral striatum following alcohol administration (Boileau et al., 2003), which may serve as a target for intervention. For example, direct infusion of varenicline into the nucleus accumbens core in rats led to reduced alcohol intake (Feduccia et al., 2014), suggesting that varenicline’s partial agonist actions at nAChRs may attenuate alcohol’s reinforcing effects in the striatum. A recent study of heavy-drinking smokers showed that varenicline relative to placebo reduced ventral striatal activation in response to smoking cues (Ray et al., 2014), but a study of alcohol-dependent individuals did not find reduced striatal activation in response to alcohol cues (Schacht et al., 2014). Both studies, however, used passive cue-viewing tasks where participants were not required to work for rewards and may therefore not have directly examined neural circuits underlying motivation for alcohol consumption.
The Monetary Incentive Delay (MID) task is widely used to probe the activity of human brain reward circuitry. In this task, participants can earn varying sums of money during a functional MRI (fMRI) scan (Knutson et al., 2001; Bjork et al., 2004). When participants see cues predictive of monetary rewards relative to no reward, they show increased activation in the striatum and amygdala (Hommer et al., 2003). Further, striatal activity scales with the magnitude of the rewards (Knutson et al., 2001). In addition to the striatum and amygdala, the insular cortex has been implicated in craving (Naqvi et al., 2007; Chung and Clark, 2014) and reward anticipation (Craig, 2009). The insula also has a high concentration of nAChRs (Picard et al., 2013), and its activity has been shown to be modulated by varenicline treatment (Sutherland et al., 2013a, 2013b), suggesting that it may be part of the neurocircuitry that subserves alcohol motivation among heavy drinkers. Here, we modified the MID task to examine reward circuitry activity in heavy drinkers while they worked to obtain alcohol or food rewards. The choice of an alcohol reward was to determine how heavy drinkers respond specifically to alcohol rewards rather than rewards in general. The food reward served as a positive control, and it was expected to elicit reward circuitry activation, because food is a primary motivator (along with sex, water, and pain avoidance). Money was not used as a reward, because participants could easily exchange it for alcohol at a later time and thus it may be entangled with alcohol-reward processing. Participants were randomized to receive placebo or varenicline. The modified version, the Alcohol-Food Incentive Delay (AFID) task, was used to examine 3 hypotheses: (1) that the placebo group would show greater activity in the ventral striatum insula and amygdala when anticipating alcohol rewards relative to food rewards or no reward; (2) that varenicline would attenuate activation when anticipating alcohol rewards; and (3) that this reduced activation would correspond to decreased motivation to consume alcohol.
Materials and Methods
Participant Characteristics
This was a 3-week, randomized, double-blind, placebo-controlled, experimental study of varenicline in heavy drinkers. Prospective participants underwent a screening visit that consisted of clinical and psychiatric evaluation (Structured Clinical Interview for DSM-IV; First, 2002). Smoking and drinking history were assessed using the Fagerström Test for Nicotine Dependence (Heatherton et al., 1991) and 90-day Timeline Followback (TLFB) (Sobell and Sobell, 1992), respectively. Participants also completed the Alcohol Use Disorders Identification Test (AUDIT; Babor et al., 2001) and the Self-Rating of the Effects of Alcohol (SRE; Schuckit et al., 1997). Participants were included if they consumed an average of >20 drinks/wk for men and 15 drinks/wk for women and were not seeking help for alcohol-related problems.
Participants were excluded if they met any of the following criteria: (1) lifetime history of Axis I mood, anxiety, or substance use disorders (other than alcohol or nicotine use disorders); (2) recent or regular use of illicit or nonprescribed psycho-active substances; (3) history of clinically significant alcohol withdrawal; (4) lifetime history of violence, suicide attempts, or self-injurious behavior; (5) current or chronic medical conditions, including cardiovascular conditions, requiring inpatient treatment or frequent medical visits; (6) use of medications contraindicated with varenicline in the past 90 days or those that may affect the hemodynamic response (eg, antihypertensives) within the past 30 days, or those that may interact with alcohol within 2 weeks prior to the study; (7) metal in body, left-handedness; or claustrophobia (MRI exclusion criteria). The complete list of inclusion/exclusion criteria is available at clinicaltrials.gov. The study protocol was approved by the NIH Combined Neuroscience Institutional Review Board, and participants were enrolled after providing written informed consent.
A total of 49 participants were randomized, but 3 failed to return. Eight participants failed to complete the study because of compliance failure or error (3 varenicline participants, 5 placebo participants). Nine participants were removed from the final sample because of poor imaging data quality (excessive motion artifacts; 4 varenicline participants, 5 placebo participants). A total of 29 participants were included in the final dataset (varenicline: N=17, placebo: N=12). Smokers and nonsmokers were included in the study. Nonsmokers had not smoked in the past year. Smokers smoked daily. Participants were given medication in pill bottles; adherence was monitored by counting the pills remaining.
Study Procedures
Following enrollment, participants underwent 5 study visits 1 week apart. Visit 2 included a baseline intravenous alcohol self-administration (IV-ASA) session. Following this session, participants began medication with varenicline or placebo. The varenicline dose was titrated during the first week (0.5mg/d for the first 3 days, 1mg/d for the next 4 days, 2mg/d for the remaining 14 days of medication). Following 2 weeks of medication, participants completed an fMRI scan. At the end of 3 weeks, participants underwent a second IV-ASA session. Participants were instructed not to drink alcohol in the 48 hours prior to study procedures. Participants were not allowed to smoke once they entered the study facility but could smoke on the study day before arrival to reduce craving or withdrawal effects. Breath carbon monoxide levels were assessed to monitor smoking prior to each study session. Participants were fed lunch approximately 1 hour prior to the IV-ASA sessions and the scan. Participants were paid for their participation in the study.
IV-ASA
Participants arrived around 9:00 am and provided a breathalyzer reading (Drager Safety Inc., Irving, TX) and urine sample to confirm sobriety and absence of illicit substances. After eating a small breakfast, an IV catheter was inserted into a vein in the forearm. IV-ASA used the computer-assisted self-infusion of ethanol system (Zimmermann et al., 2008) and was based on a physiologically based pharmacokinetic model for ethanol (Ramchandani et al., 1999). The session lasted 2.5 hours and consisted of 2 phases: priming and open-bar. During the priming phase, the software prompted participants to push a button 4 times, each resulting in an alcohol infusion that raised breath alcohol concentration (BrAC) by 7.5 mg% in 2.5 minutes. After 10 minutes, participants achieved a peak BrAC of 30 mg%. During the next 15 minutes, the button remained inactive while participants experienced the result of their presses. For the next 2 hours, participants completed the open-bar phase, where they could self-administer alcohol infusions ad libitum. For safety purposes, a BrAC limit was set at 120 mg%. If the participant reached this level, the button became inactive until BrAC fell below the limit. BrAC readings were obtained at approximately 15-minute intervals. After the open-bar phase, the catheter was removed, and the participant was provided a meal and monitored until BrAC fell below 20 mg% when they were released.
fMRI Scanning
The fMRI session was conducted using a General Electric or Siemens Skyra 3T scanner with a 12 or 20 channel head coil, respectively. Structural scans were collected for later coregistration with functional images using the MPRAGE sequence. Two functional scans time-locked to the start of the AFID task were acquired using a T2*-EPIRT sequence (T2*-weighted echoplanar imaging; TR=2000 milliseconds, TE=30 milliseconds, FoV=240mm, 64×64 matrix, 36 axial slices with 0-mm gap, flip angle=90°, total duration: 6 minutes, 20 seconds, 3.75×3.75×3.75mm voxels) that measured changes in blood oxygen level dependent contrast.
AFID Task
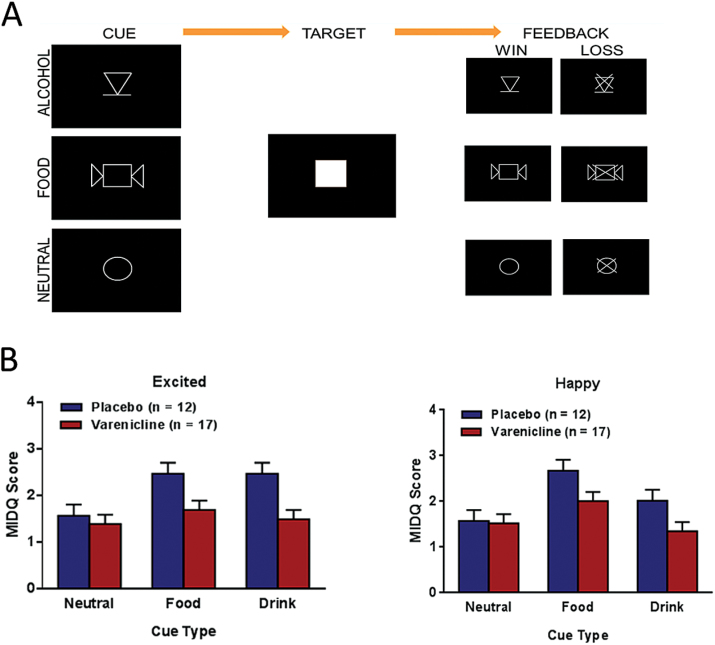
Participants were presented with images (Figure 1A) signaling the chance to win alcohol (intravenous alcohol infusion), food (eg, candy, chips, or granola bars), or no rewards (neutral). Prior to the scan, participants saw the food they could win and were told that they could choose a number of snacks commensurate with the number of food points they earned, and they would redeem them soon after the scan was over, approximately 1 hour after the AFID task. They were told they would receive an alcohol infusion in proportion to the alcohol points they earned while they were still in the scanner, approximately 20 minutes after the end of the AFID task (participants completed another task following alcohol infusion that will be reported in a future manuscript). Participants saw 18 images for each reward type. After a jittered period of time (2000–6000 milliseconds), participants saw a target (ie, a white square) and were instructed to press a button while the target was on the screen to earn a point for that reward type. If the participant was too slow, no points were gained. The target appeared briefly (eg, 200 milliseconds) to make the task difficult. Based on practice runs, the difficulty was adjusted by varying target duration so that each subject would win on approximately two-thirds of trials. Participants were not told of this adjustment. After a jittered period of time (2000–6000 milliseconds), participants saw a picture indicating whether they won or lost.
Figure 1.
Schematic of Alcohol-Food Incentive Delay (AFID) task and subjective results. (A) Visual cues for alcohol (intravenous alcohol infusion), food (highly palatable snacks), or neutral (no rewards) conditions as well as the task sequence. (B) Significant main effects of medication for the items Excited (F1,25=8.16, P =.009) and a trend for a main effect of medication Happy (F1,25 =3.79, P =.063). The varenicline group showed lower scores compared with the placebo group across cue types. Error bars represent SEM.
The MID questionnaire (Bjork et al., 2012) was modified to collect subjective responses immediately following the AFID task while still in the scanner. The AFID questionnaire asked participants to rate their emotional responses when they saw each image (alcohol, food, and neutral) during the task. They were asked to rate their response on a 1–4 Likert scale, where 1 was “slightly or not at all” and 4 was “very” to the following 4 questions: “Were you happy?” “Were you excited?” “Were you unhappy?” “Were you fearful?”
Analysis of IV-ASA Measures
The primary IV-ASA measure was the number of self-administered infusions during each session. BrAC, peak BrAC, and total ethanol administered were also examined. Outcome measures were analyzed using repeated-measures ANCOVA models in SAS PROC MIXED (SAS Institute Inc., Cary, NC) with session (premedication and on-medication) as the within-subjects factor and medication as the between-subjects factor. Smoking status was included as a factor in all models. Age, gender, current diagnosis of an alcohol use disorder, AUDIT score, and number of heavy drinking days from the TLFB were evaluated as potential covariates on a model-by-model basis and were retained in the model if they reached trend level significance (P<.10).
Imaging Processing
Data were preprocessed using Analysis of Functional NeuroImages (AFNI) software (Cox, 1996). Echoplanar images were aligned to anatomical images. Time points with more than 3mm/° motion were censored. Images were spatially smoothed using a 6-mm Gaussian kernel. Voxels were resampled to 3.5mm3. Signal for each voxel was scaled by the mean, so the average intensity was 100; thus, output could be viewed as percent signal change from baseline. A general linear model fit was performed using AFNI’s 3dDeconvolve function, with regressors for alcohol, food, and neutral images for each phase (anticipation, target, hit, miss). Six motion parameters were included in the model as regressors of noninterest.
Brain Imaging Analysis
Brain imaging analysis focused on the anticipatory phase of the task. Linear mixed-effects (LME) analyses were conducted using AFNI’s 3dLME (Chen et al., 2013) for the anticipatory phase of the task. Medication (placebo, varenicline) and image-type (alcohol, food, neutral) were fixed effects in the model and individual participants were treated as random effects. To control for variability from using 2 scanners, a scanner variable was included as a factor (4 varenicline and 3 placebo participants were scanned with the Siemens scanner). LME analysis examined main effect of medication, image-type, and medication-by-image-type interaction. Analyses were performed voxel-wise across the entire brain and then across 3 regions of interest (ROIs): the insula, striatum, and amygdala. These ROIs were chosen because previous studies have shown these regions to activate when processing incentive salience (Knutson et al., 2001; Hommer et al., 2003; Bjork et al., 2004). Volume-threshold adjustment based on Monte Carlo simulations (AFNI’s AlphaSim) was applied to protect family-wise error rate. For a main effect of image-type a threshold an a priori voxel-wise probability of P<.01 in a cluster of 1158 μL (27 voxels) resulted in an a posteriori probability of P<.01. For group-by-condition analyses, an a priori voxel-wise probability of P<.05 in a cluster of 2486 μL (58 voxels) for the entire brain resulted in an a posteriori probability of P<.05. In the ROIs, cluster sizes were considered significant at P<.05 in the following volumes: 771 μL in the insula (18 voxels), 557 μL in the caudate (13 voxels), 515 μL in the putamen (12 voxels), and 300 μL in the amygdala (7 voxels).
Smoking status, scanner, sex, age, number of heavy drinking days from the TLFB, and total SRE score were tested as covariates of significant clusters. We also examined all significant findings among only participants scanned with the General Electric scanner to confirm that effects remained.
Exploratory Analysis of Brain-Behavior Relationships
To explore the behavioral correlates of brain activations identified by LME analysis, linear relationships between brain activation from the identified clusters and alcohol-related measures were examined. These measures included the self-reported scores in response to the alcohol image on the MID questionnaire and the number of infusions self-administered during the second IV-ASA session. Additionally, a voxel-wise exploration of the relationship between brain activation and number of infusions was examined using AFNI’s 3dttest++ program. The same cluster thresholds listed in the LME analysis were applied.
Results
Participant Characteristics
Table 1 shows participant characteristics. Supplemental Figure 1 shows the Consolidated Standards of Reporting Trials flowchart. Randomization groups had comparable Fagerström and AUDIT scores (P>.1) indicating similar smoking and drinking histories. Both groups had mean body mass indices between 25 and 29, indicating participants were overweight. Groups did not differ in their adherence to the medication regimen based counts of remaining pills (P =.23). Based on a 2-sample t test, placebo participants were not significantly older than varenicline participants (P=.07). Since the results of a Shapiro-Wilks test revealed that age was not normally distributed (W=0.85, P<.01), we also performed a nonparametric analysis. A Mann-Whitney U test revealed that the groups did not differ significantly in age (P=.28). Given the trend toward significance in the t test, age was tested as a covariate in subsequent analyses but did not alter any of the results.
Table 1.
Demographic and Alcohol-Drinking History (from 90-Day Timeline Follow-Back Questionnaire) Characterization of the Study Participants
| Placebo | Varenicline | ||||||
|---|---|---|---|---|---|---|---|
| Nonsmoker (n = 6) | Smoker (n = 6) | Total (n = 12) | Nonsmoker (n = 9) | Smoker (n = 8) | Total (n = 17) | Test of Group Differences | |
| N | N | N (%) | N | N | N (%) | Chi-square P-value | |
| Female | 1 | 0 | 1 (8) | 1 | 2 | 3 (18) | 0.62 |
| FHPa | 4 | 2 | 6 (50) | 2 | 2 | 4 (24) | 0.24 |
| Current abuse | 0 | 0 | 0 (0) | 3 | 2 | 5 (29) | 0.06 |
| Current dependence | 1 | 0 | 1 (8) | 2 | 1 | 3 (18) | 0.62 |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | t test P-value | |
| Age (years) | 37.3 (16.6) | 38.5 (11.3) | 37.9 (13.5) | 27.6 (8.1) | 32.3 (10.6) | 29.8 (9.4) | 0.07 |
| Height (cm) | 179.1(11.1) | 175.4 (7.7) | 177.2 (9.3) | 176.5 (3.3) | 174.7 (7.4) | 175.6 (5.5) | 0.95 |
| Weight (kg) | 89.0 (12.2) | 84.2 (11.6) | 86.6 (11.6) | 77.5 (10.2) | 78.0 (10.7) | 77.7 (10.1) | 0.04 |
| Age at first drink | 15.1 (2.8) | 15.4 (6.7) | 15.3 (4.6) | 15.6 (2.7) | 15.9 (1.3) | 15.7 (2.1) | 0.74 |
| Total lifetime drinks | 15,961.8 (13,928) | 53,088.7 (77,424) | 31,431.4 (51,489) | 16,633.2 (16,779) | 24,650.7 (26,777) | 19,934.5 (21,066) | 0.41 |
| AUDITb | 12.5 (4.2) | 14.3 (6.9) | 13.4 (5.5) | 12.2 (3.4) | 16.4 (5.6) | 14.2 (4.9) | 0.93 |
| SRE total scorec | 8.0 (1.1) | 10.7 (1.3) | 9.3 (0.9) | 8.7 (1.4) | 7.5 (1.0) | 8.2 (0.9) | 0.69 |
| Recent drinking history: 90-day timeline followback | |||||||
| Total drinks | 359 (105) | 530 (185) | 444 (169) | 373 (170) | 476 (256) | 418 (211) | 0.97 |
| Drinking days | 66.2 (17.8) | 65.3 (14.7) | 65.8 (15.6) | 59.1 (19.3) | 79.7 (12.1) | 68.1 (19.2) | 0.63 |
| Drinks/ drinking day |
5.7 (2.3) | 8.2 (2.6) | 6.9 (2.7) | 6.7 (3.4) | 5.7 (2.9) | 6.2 (3.1) | 0.84 |
| Heavy drinking days | 34.5 (11.5) | 54.3 (19.7) | 44.4 (18.5) | 34.2 (21.6) | 51.1 (29.7) | 42.2 (26.4) | 0.95 |
| Smoking measures | |||||||
| Cigarettes/ d | 8.2 (4.5) | 7.7 (6.5) | 0.88 | ||||
| FTNDd | 3.5 (2.6) | 3.5 (1.0) | 0.99 | ||||
AUDIT and Fagerström scores are also depicted. Two subjects in the varenicline group and 5 subjects in the placebo group were excluded from analysis due to insufficient data. P-values were calculated based on chi-squared or t tests.
a Family history positive for alcoholism.
b Alcohol Use Disorders Identification Test.
c Self-rating of the effects of alcohol.
b Fagerström test for nicotine dependence.
Self-Reported Mood Scale Measures
There was a main effect of medication (F1, 25=8.16, P =.009) and image-type (F2, 50=6.08, P=.004) in self-reported excitement. Placebo participants reported greater excitement across image-types relative to varenicline participants. Tukey’s posthoc tests revealed that participants were more excited by food (P=.006) and alcohol (P =.02) images relative to neutral images, but alcohol did not differ from food (P>.05) (Figure 1B). There was a main effect of image type on happiness (F2, 50=12.31, P<.001) and a trend for a main effect of medication on happiness (F1, 25=3.6, P=.07). Participants were happier when viewing food images relative to neutral (P<.001) or alcohol (P=.001) images.
IV-ASA Behavior
Both groups self-administered in both sessions at levels that approximated binge consumption, with mean peak BrACs of 83.4 mg% (SEM=7.8) for the baseline session and 85.7 mg% (SEM= 8.6) for the session after 3 weeks of medication. Seventeen of 28 participants (61%) reached the ceiling BrAC of 120mg% during the second session, but rates did not differ by group (varenicline: 62%, placebo: 58%; P=.99). There were no significant effects of medication or a medication-by-session interaction on IV-ASA measures (supplementary Table 2). None of the covariates (smoking, age, gender, current diagnosis of an alcohol use disorder, AUDIT score, and number of heavy drinking days from the TLFB) were significant and were removed from the final model.
Linear Mixed Effects Analysis of fMRI Measures
For a main effect of image type across the entire brain, several clusters survived the threshold, including the hypothesized regions: the striatum and amygdala (supplementary Figure 2). Like in the monetary version of the task (Knutson et al., 2001; Hommer et al., 2003), striatal and amygdalar activation was greater for reward images (alcohol, food) relative to neutral images during the anticipatory phase of the task.
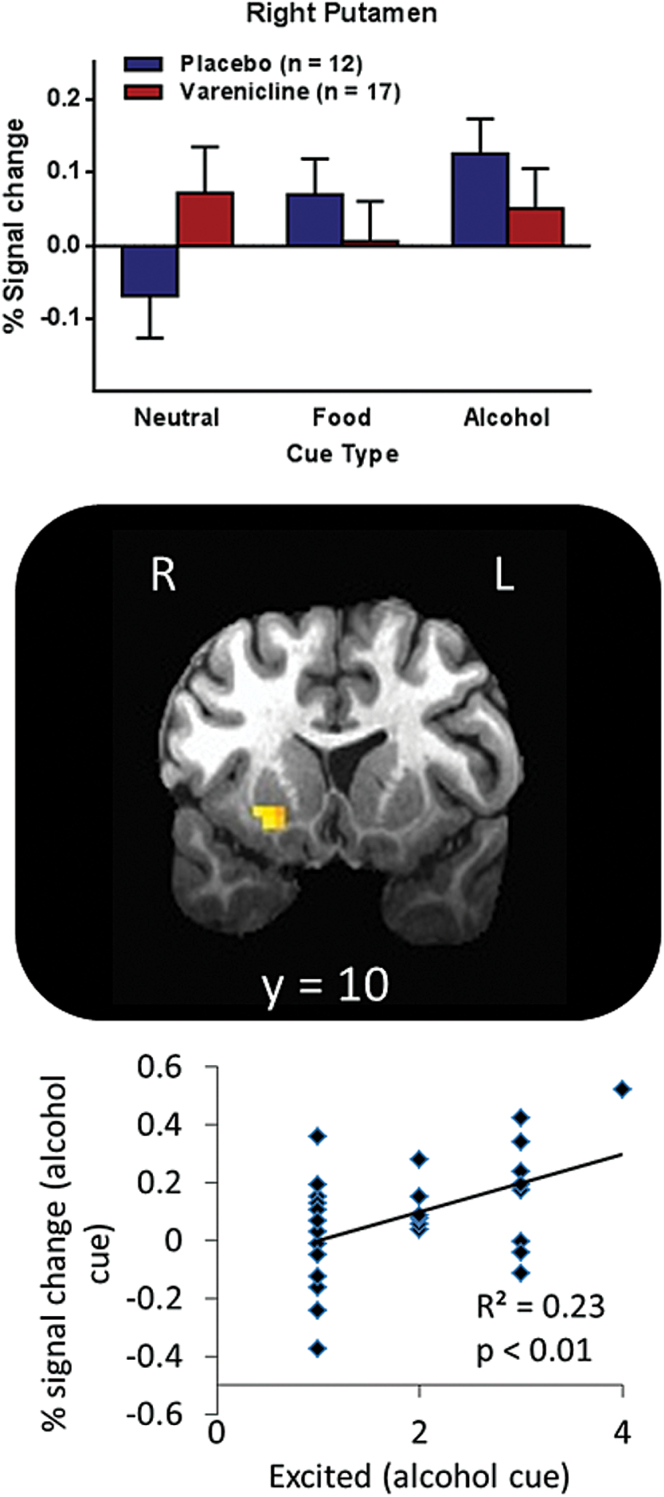
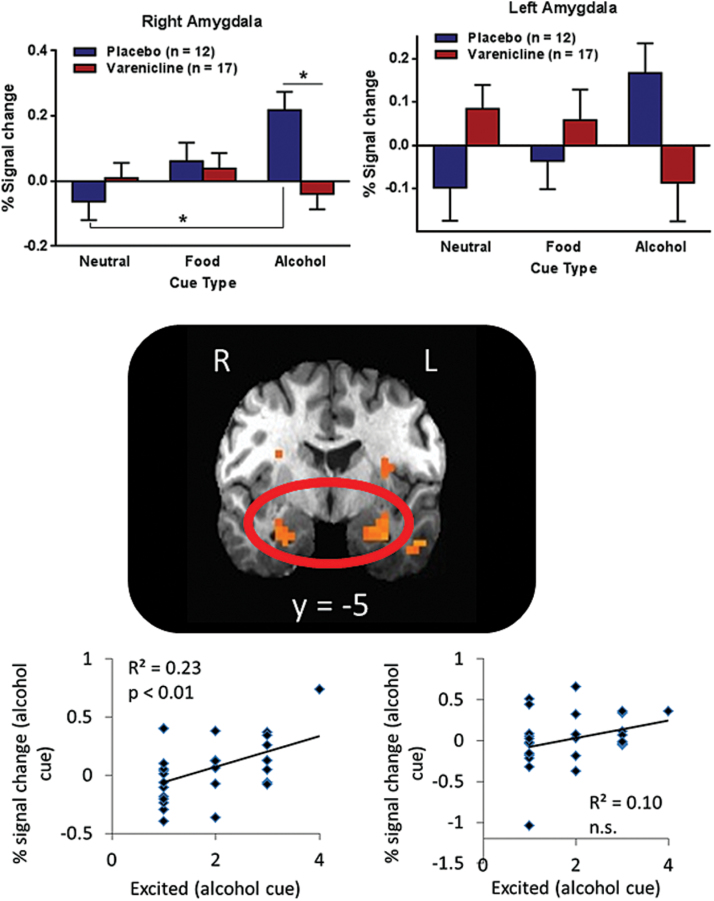
Whole brain voxel-wise analysis revealed several clusters, including the amygdala and insula, with significant medication-by-image-type interactions (supplementary Table 3), where the placebo group showed increased activation when anticipating alcohol relative to neutral rewards, but the varenicline group did not show this increase. When restricted to the ROIs, significant medication-by-image-type interactions appeared in the right posterior insula (Brodmann area 13; x=33, y=-12, z=19, Vol. =1329 µL), right putamen (x=23, y = 10, z=-6, Vol. =686 µL), and bilateral amygdala (left: x=-25, y=-4, z=-17, Vol. =986 µL; right: x=27, y=-5, z=-17, Vol. = 514 µL) (Figure 3). Specifically, for right posterior insula, placebo relative to varenicline participants showed increased response to alcohol images, but varenicline relative to placebo participants showed increased response to food images. For bilateral amygdala (Figure 2) and right putamen (Figure 3), placebo participants showed increased response to alcohol images relative to neutral images, whereas varenicline participants showed roughly equivalent response to all image types. These effects remained significant when covarying for smoking status, scanner, TLFB, SRE, and age; the covariates were not significant. When only examining participants scanned in the General Electric scanner, all effects remained (supplementary Figure 3).
Figure 3.
Varenicline modulates ventral striatal activation. A cluster in the right putamen showed a significant treatment-by-cue-type interaction. The placebo group showed greater activation to alcohol cues relative to neutral cues, whereas the varenicline group showed equivalent activation for both cue types. Individuals with higher putamen activity during the alcohol cue also reported greater excitement when viewing the alcohol cue (R2=0.23, P=.009). Error bars represent SEM.
Figure 2.
Varenicline modulates amygdala activation. Clusters in the bilateral amygdala showed significant treatment-by-cue-type interactions, where the placebo group showed increased activity in response to an alcohol cue relative to a neutral cue, but the varenicline group did not show increased activity in response to an alcohol cue. In the right amygdala cluster, posthoc analysis showed that the varenicline group had significantly less activation in response to an alcohol cue relative to the placebo group. Activity in the right amygdala cluster during an alcohol cue was positively correlated with self-reported excitement when viewing the alcohol cue (R2=0.23, P=.009). Error bars represent SEM.
Exploratory Analysis of Brain–Behavior Relationships
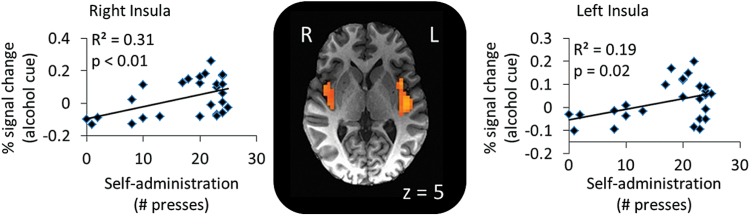
Activation in the right amygdala and right putamen when anticipating an alcohol reward was significantly positively correlated with excitement when viewing the alcohol image (P≤.01) (Figures 2 and 3). Neither left amygdala nor insula activation when anticipating an alcohol reward were correlated with excitement (P>.1). Voxel-wise exploration comparing button presses during the IV-ASA session to brain activation revealed bilateral clusters in the posterior insula (right: Brodmann area 13; x=39, y=-6, z=5, Vol.=1715 µL; left: Brodmann area 13; x=-40, y=-15, z=5, Vol.=5102 µL) that were positively correlated with the number of infusions on the second IV-ASA session (right: R2=0.31, P=.006; left: R2=0.19, P=.02) (Figure 4).
Figure 4.
Insula activation is associated with alcohol consumption. Results of a voxel-wise analysis of correlation between the number of self-infusions during the second visit (button presses). The analysis revealed a positive association between button presses and activity in the bilateral posterior insula in response to the alcohol cue (right: R2=0.31, p=0.006; left: R2=0.19, P=.02).
Discussion
In a sample of heavy drinkers, varenicline reported significantly lower levels of excitement when anticipating alcohol rewards as well as lower neural activity in the amygdala, insula, and ventral striatum when anticipating alcohol rewards. In the placebo group, there was significantly greater activation in the striatum, amygdala, and insula when anticipating alcohol rewards relative to neutral and food rewards; this group also reported greater excitement for alcohol relative to neutral rewards. Insula activation when anticipating an alcohol reward was positively associated with the alcohol self-infusions during the IV-ASA session the following week. These results serve as evidence that task-elicited reward circuitry activation may provide a useful biomarker of incentive salience of alcohol and that blunting this activation may be a mechanism through which varenicline reduces alcohol consumption.
The analysis of the main effect of image-type revealed significant clusters in the striatum and amygdala. Across both groups, participants showed less activation in the ventral striatum and amygdala when anticipating a neutral reward and more activation when anticipating an alcohol reward. As hypothesized, anticipation of a food reward produced more activity than neutral rewards and less activity than alcohol rewards in both regions, suggesting that alcohol was the most valued reward. This interpretation is partially consistent with the self-reported measures of excitement for the 3 reward types, where alcohol generated more excitement than neutral rewards but equivalent excitement relative to food rewards. The original MID also found proportional activity in the amygdala and ventral striatum, where higher rewards elicited greater and smaller rewards elicited lower activity (Knutson et al., 2001; Hommer et al., 2003). Based on the construct validity and the similarity between the AFID and the original MID, we conclude that the AFID task succeeded in measuring neural response to anticipation of alcohol rewards.
In addition to the main effects of the task, there was a medication type by image type interaction in several brain regions. Both the whole brain and small volume analysis found interaction effects in the bilateral amygdala. The small volume analysis also revealed an interaction effect in the ventral striatum. The placebo group showed increased activation when anticipating alcohol relative to neutral rewards, while the varenicline group showed either no increase in activation or decreased activation to alcohol relative to neutral rewards. The effect on activation when anticipating food rewards was less robust, but it appeared that varenicline’s effects (ie, preventing an increase in activation) were stronger for alcohol relative to food rewards, suggesting specificity of action of varenicline to images signaling alcohol reward. These findings are consistent with studies suggesting that the ventral striatum encodes the incentive salience and value of potential rewards (Knutson et al., 2001; Kable and Glimcher, 2009). The results are also consistent with fMRI studies showing blunting of ventral striatum activation to smoking cues in smokers by varenicline (Franklin et al., 2011), although a study examining the effects of varenicline on neural correlates of alcohol cue-reactivity in nontreatment-seeking alcohol dependent individuals did not show significant blunting of striatal responses (Schacht et al., 2014).
The neuroimaging findings were reflected in the self-report measures of excitement for alcohol rewards: the placebo group reported greater excitement when viewing images signaling alcohol relative to neutral rewards, but the varenicline group reported equivalent excitement for all reward types. Self-reported excitement was correlated with ventral striatal and right amygdalar activation. The consistency of the present findings of activation when anticipating alcohol rewards with previous findings using monetary rewards suggests that the task effectively recruited reward circuitry and that varenicline prevented an increase in neural response to anticipating alcohol rewards.
The insula has a high concentration of nAChRs (Picard et al., 2013) and is implicated in craving (Naqvi et al., 2007; Garavan, 2010) and anticipation of both positive and aversive stimuli (Simmons et al., 2011). One prominent theory is that the posterior insula encodes lower-level sensory information and the anterior insula encodes stimulus salience (Craig, 2009). A recent study, however, showed that the body’s glucose levels predicted the posterior insula’s response to food images, suggesting the posterior insula may encode the body’s homeostatic state and motivation for substances to meet the body’s needs (eg, eating food when hungry; Simmons et al., 2013). Possibly consistent with this localization of insula function was a decreased right posterior insula activation in varenicline when anticipating alcohol rewards. Unlike the amygdala and ventral striatum, insula activity was not related to excitement for the alcohol reward. Rather, bilateral posterior insula activation when anticipating alcohol rewards was highest among participants who self-administered more alcohol during the subsequent IV-ASA session. This further suggests that posterior insula activation signals urges to restore homeostatic balance (Paulus, 2007), such as consuming alcohol. Given the reduction in activation when anticipating alcohol rewards among the varenicline group, it is plausible that varenicline reduces posterior insula signaling that drives alcohol seeking.
Despite the robust neuroimaging findings, there were no significant differences between groups on alcohol self-administration measures following 3 weeks of medication. The 120 mg% safety limit on peak alcohol levels during the IV-ASA session resulted in a ceiling effect and may have limited the ability to measure potential differences in self-administration during the session. The study sample consisted of heavy drinkers who regularly consume alcohol quantities at or above the safety limit; a number of participants reached this ceiling during the session. Future studies using a higher ceiling or progressive ratio paradigms that self-limit alcohol levels while assessing motivation for alcohol consumption may allow greater variance and therefore facilitate examination of medication effects on IV-ASA.
There are several limitations to the present study. It was conducted in nontreatment-seeking heavy drinkers, most of whom were not dependent, and there was no assessment of long-term outcomes of varenicline administration. Thus, it is unclear whether the observed differences in neural response to anticipation of alcohol rewards would persist and result in long-term benefits, or whether the results would extend to a dependent population. Nonetheless, clinical trials have shown that varenicline can reduce drinking relative to placebo in dependent and nondependent drinkers (Mitchell et al., 2012; Litten et al., 2013; Erwin and Slaton, 2014), and the present study suggests a possible mechanism that could explain those findings. Medication adherence was verified by counting the remaining pills for each participant, but measurement of varenicline levels in biological samples would provide more accurate measures of compliance. Scanning was conducted only at a single time-point, after 2 weeks of medication, so it is unclear if groups’ neural responses during the AFID task differed at baseline. Nonetheless, since groups were randomized, we expect baseline differences would account for only a small portion of the observed differences. Future longitudinal studies will help determine how neural processing of alcohol rewards changes across the course of treatment. The use of multiple scanners introduced additional variance into our neuroimaging measures. Our results, however, remained significant when controlling for an effect of scanner. Further, groups were balanced on scanner type, so any hardware effects should be equally represented in both groups. Thus, we conclude that our results are best explained as the result of medication regimen, not an effect of hardware.
The present findings indicate that short-term administration of varenicline in heavy drinkers reduces the incentive salience of alcohol rewards in brain regions that are key to the motivating and rewarding effects of alcohol. This mechanism may underlie the clinical effectiveness of varenicline in reducing alcohol intake and indicates its potential utility as a pharmacotherapy for alcohol use disorders, particularly in individuals that are reward-drinkers. Additionally, since varenicline is not eliminated via hepatic metabolism, it may be particularly useful for patients with liver impairment. The results also suggest that the AFID task may be used to obtain biomarkers of incentive salience for alcohol seeking, which could help evaluate medications as well as identify patients most likely to respond to pharmacotherapy, thus facilitating the development of personalized treatment for alcohol use disorders.
Statement of Interest
The authors declare no conflicts of interest.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the NIH Clinical Center Alcohol Clinic (Tom Lionetti and Dave Spero in particular), 5SWS Day Hospital staff for clinical support, and NMR Center staff for technological support. The authors also acknowledge David Herion, MD, (NIH Clinical Center) for critical input during the design and development of the study protocol; Ashley Smith, PhD, and Jodi Gilman, PhD (NIAAA) for fMRI task programming support; David T. George, MD (NIAAA), for medical support; laboratory research staff for data collection support; and the study participants for their participation in the study.
This study was supported by the NIAAA Division of Intramural Clinical and Biological Research (Z1A AA000466). Medication supply was provided by Pfizer Inc. under agreement with Dr. Bartlett. Development of the CAIS software used for the IV-ASA session was supported by Sean O’Connor, MD, at the Indiana Alcohol Research Center (NIH P60 AA007611).
This work was presented in part at the 52nd Annual Meeting of American College of Neuropsychopharmacology (December 11, 2013, Hollywood, FL) and the Research Society on Alcoholism Meeting (June 21, 2014, Bellevue, WA).
References
- Arqueros L, Naquira D, Zunino E. (1978) Nicotine-induced release of catecholamines from rat hippocampus and striatum. Biochem Pharmacol 27:2667–2674. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M (1993) Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption – II. Addiction 88:791–804. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. (2004) Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J Neurosci 24:1793–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Chen G, Hommer DW. (2012) Mesolimbic recruitment by nondrug rewards in detoxified alcoholics: effort anticipation, reward anticipation, and reward delivery. Human Brain Mapping 33:2174–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau I, Assaad J-M, Pihl RO, Benkelfat C, Leyton M, Diksic M, Tremblay RE, Dagher A. (2003) Alcohol promotes dopamine release in the human nucleus accumbens. Synapse 49:226–231. [DOI] [PubMed] [Google Scholar]
- Cardoso RA, Brozowski SJ, Chavez-Noriega LE, Harpold M, Valenzuela CF, Harris RA. (1999) Effects of ethanol on recombinant human neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther 289:774–780. [PubMed] [Google Scholar]
- Chen G, Saad ZS, Britton JC, Pine DS, Cox RW. (2013) Linear mixed-effects modeling approach to FMRI group analysis. Neuroimage 73:176–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung T, Clark DB. (2014) Insula white matter volume linked to binge drinking frequency through enhancement motives in treated adolescents. Alcohol Clin Exp Res 38:1932–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe JW, et al. (2005) Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem 48:3474–3477. [DOI] [PubMed] [Google Scholar]
- Cox RW. (1996) AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29:162–173. [DOI] [PubMed] [Google Scholar]
- Craig AD. (2009) How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci 10:59–70. [DOI] [PubMed] [Google Scholar]
- Feduccia AA, Simms JA, Mill D, Yi HY, Bartlett SE. (2014) Varenicline decreases ethanol intake and increases dopamine release via neuronal nicotinic acetylcholine receptors in the nucleus accumbens. Br J Pharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer R. L., Gibbon M., Williams J. B.W. (2002) Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P). New York: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Franklin T, Wang Z, Suh JJ, Hazan R, Cruz J, Li Y, Goldman M, Detre JA, O’Brien CP, Childress AR. (2011) Effects of varenicline on smoking cue-triggered neural and craving responses. Arch Gen Psychiatry 68:516–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H. (2010) Insula and drug cravings. Brain Struct Funct 214:593–601. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. (1991) The Fagerstrom test for nicotine dependence - a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict 86:1119–1127. [DOI] [PubMed] [Google Scholar]
- Heilig M, Goldman D, Berrettini W, O’Brien CP. (2011) Pharmacogenetic approaches to the treatment of alcohol addiction. Nat Rev Neurosci 12:670–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommer DW, Knutson B, Fong GW, Bennett S, Adams CM, Varnera JL. (2003) Amygdalar recruitment during anticipation of monetary rewards: an event-related fMRI study. Ann N Y Acad Sci 985:476–478. [DOI] [PubMed] [Google Scholar]
- Jonas DE, Amick HR, Feltner C, Bobashev G, Thomas K, Wines R, Kim MM, Shanahan E, Gass CE, Rowe CJ, Garbutt JC. (2014) Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA 311:1889–1900. [DOI] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. (2009) The neurobiology of decision: consensus and controversy. Neuron 63:733–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. (2001) Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci 21:RC159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. (2013) Theoretical frameworks and mechanistic aspects of alcohol addiction: alcohol addiction as a reward deficit disorder. Curr Top Behav Neurosci 13:3–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn BM. (2006) FDA speeds smoking cessation drug review. JAMA 295:614. [DOI] [PubMed] [Google Scholar]
- Litten RZ, Ryan ML, Fertig JB, Falk DE, Johnson B, Dunn KE, Green AI, Pettinati HM, Ciraulo DA, Sarid-Segal O, Kampman K, Brunette MF, Strain EC, Tiouririne NA, Ransom J, Scott C, Stout R. (2013) A double-blind, placebo-controlled trial assessing the efficacy of varenicline tartrate for alcohol dependence. J Addict Med 7:277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Harrison EL, O’Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, Picciotto MR, Petrakis IL, Estevez N, Balchunas E. (2009) Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol Psychiatry 66:185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. (2007) Damage to the insula disrupts addiction to cigarette smoking. Science 315:531–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palotai M, Bagosi Z, Jaszberenyi M, Csabafi K, Dochnal R, Manczinger M, Telegdy G, Szabo G. (2013) Ghrelin and nicotine stimulate equally the dopamine release in the rat amygdala. Neurochem Res 38:1989–1995. [DOI] [PubMed] [Google Scholar]
- Pani PP, Trogu E, Pacini M, Maremmani I. (2014) Anticonvulsants for alcohol dependence. Cochrane Database Syst Rev 2:CD008544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP. (2007) Decision-making dysfunctions in psychiatry--altered homeostatic processing? Science 318:602–606. [DOI] [PubMed] [Google Scholar]
- Picard F, Sadaghiani S, Leroy C, Courvoisier DS, Maroy R, Bottlaender M. (2013) High density of nicotinic receptors in the cingulo-insular network. Neuroimage 79:42–51. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, Bolane J, Li TK, O’Connor S. (1999) A physiologically-based pharmacokinetic (PBPK) model for alcohol facilitates rapid BrAC clamping. Alcohol ClinExpRes 23:617–623. [PubMed] [Google Scholar]
- Ray LA, Courtney KE, Ghahremani DG, Miotto K, Brody A, London ED. (2015) Varenicline, naltrexone, and their combination for heavy-drinking smokers: preliminary neuroimaging findings. Am J Drug Alcohol Abuse 41:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. (2009) Alcohol and global health: global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 373:2223–2233. [DOI] [PubMed] [Google Scholar]
- Rehm J, Dawson D, Frick U, Gmel G, Roerecke M, Shield KD, Grant B. (2014) Burden of disease associated with alcohol use disorders in the United States. Alcohol Clin Exp Res 38:1068–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner S, Hackl-Herrwerth A, Leucht S, Vecchi S, Srisurapanont M, Soyka M. (2010) Opioid antagonists for alcohol dependence. Cochrane Database Syst Rev:CD001867. [DOI] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Randall PK, Li X, Henderson S, Myrick H. (2014) Varenicline effects on drinking, craving and neural reward processing among non-treatment-seeking alcohol-dependent individuals. Psychopharmacology (Berl) 231:3799–3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Tipp JE. (1997) The self-rating of the effects of alcohol (SRE) form as a retrospective measure of the risk for alcoholism. Addiction 92:979–988. [PubMed] [Google Scholar]
- Simmons AN, Stein MB, Strigo IA, Arce E, Hitchcock C, Paulus MP. (2011) Anxiety positive subjects show altered processing in the anterior insula during anticipation of negative stimuli. Hum Brain Mapp 32:1836–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons WK, Rapuano KM, Kallman SJ, Ingeholm JE, Miller B, Gotts SJ, Avery JA, Hall KD, Martin A. (2013) Category-specific integration of homeostatic signals in caudal but not rostral human insula. Nat Neurosci 16:1551–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. (1992) Timeline follow-back. In: Measuring alcohol consumption, pp41–72. New York: Springer. [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. (2007) Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc Natl Acad Sci U S A 104:12518–12523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, Carroll AJ, Salmeron BJ, Ross TJ, Stein EA. (2013a) Insula’s functional connectivity with ventromedial prefrontal cortex mediates the impact of trait alexithymia on state tobacco craving. Psychopharmacology (Berl) 228:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, Carroll AJ, Salmeron BJ, Ross TJ, Hong LE, Stein EA. (2013b) Down-regulation of amygdala and insula functional circuits by varenicline and nicotine in abstinent cigarette smokers. Biol Psychiatry 74:538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonstad S, Tonnesen P, Hajek P, Williams KE, Billing CB, Reeves KR. (2006) Effect of maintenance therapy with varenicline on smoking cessation: a randomized controlled trial. JAMA 296:64–71. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. (2002) Role of dopamine in drug reinforcement and addiction in humans: results from imaging studies. Behav Pharmacol 13:355–366. [DOI] [PubMed] [Google Scholar]
- Zimmermann US, Mick I, Vitvitskyi V, Plawecki MH, Mann KF, O’Connor S. (2008) Development and pilot validation of computer-assisted self-infusion of ethanol (CASE): a new method to study alcohol self-administration in humans. Alcohol Clin Exp Res 32:1321–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y, Kuryatov A, Lindstrom JM, Yeh JZ, Narahashi T. (2002) Alcohol modulation of neuronal nicotinic acetylcholine receptors is alpha subunit dependent. Alcohol Clin Exp Res 26:779–784. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.