Abstract
Background:
There has not been conclusive evidence for prevention of brain atrophy by anti-dementia drugs in mild cognitive impairment and Alzheimer’s Disease.
Methods:
Relevant studies were identified through searches of PubMed, databases of the Cochrane Library, and PsycINFO citations up to 16 May, 2015. Only double-blind, randomized, placebo-controlled clinical trials of anti-dementia drugs in patients with mild cognitive impairment or Alzheimer’s Disease were included. Primary outcomes were annualized percent change of total brain volume (%TBV/y), annualized percent change of hippocampal volume (%HV/y), and annualized percent change of ventricular volume (%VV/y) measured by magnetic resonance imaging. Standardized mean difference (SMD) and 95% confidence intervals (CI) were calculated for relevant outcomes.
Results:
Seven randomized, placebo-controlled clinical trials (n=1708) were found to meet the inclusion criteria, including 4 mild cognitive impairment studies (n=1327) and 3 Alzheimer’s Disease studies (n=381) [3 donepezil studies (2 mild cognitive impairment studies and 1 Alzheimer’s Disease study), 1 galantaime study for mild cognitive impairment, 2 mementine studies for Alzheimer’s Disease, and 1 rivastigmine study for mild cognitive impairment]. Pooled anti-dementia drugs showed superior protective outcomes compared with placebo regarding %TBV/y (SMD=-0.21, 95%CI=-0.37 to -0.04, P=.01, N=4, n=624) and %VV/y (SMD=-0.79, 95%CI=-1.40 to -0.19, P=.01, N=3, n=851). However, %HV/y failed to show difference between both groups. Among anti-dementia drugs, donepezil showed significantly greater protective effects than placebo regarding %TBV/y (SMD=-0.43, 95%CI=-0.74 to -0.12, P=.007, N=1, n=164) and %VV/y (SMD=-0.51, 95%CI=-0.73 to -0.29, P<.00001, N=2, n=338). Rivastigmine was also superior to placebo regarding %VV/y (SMD=-1.33, 95%CI=-1.52 to -1.14, P<.00001).
Conclusions:
The results favored the hypothesis that anti-dementia drugs may prevent brain atrophy in patients with mild cognitive impairment and Alzheimer’s Disease.
Keywords: brain atrophy, mild cognitive impairment/Alzheimer’s Disease, magnetic resonance imaging, meta-analysis, anti-dementia drugs
Introduction
Brain atrophy has been shown to be the key pathological change structurally pronounced in Alzheimer’s Disease (AD). Brain volume reduction in patients with AD was significantly associated with dementia severity and cognitive disturbances as well as neuropsychiatric symptoms (Hirono et al., 2000). Brain volume reductions can serve as an anatomical correlate in evaluating the progression of AD and also potentially in evaluating the efficiency of anti-dementia medications.
While anti-dementia medications have been shown to alleviate cognitive decline in AD, they have not been conclusively indicated to have protective effects against brain atrophy in AD. Recent meta-analyses showed that all anti-dementia drugs on the market (cholinesterase inhibitors [donepezil, galantamine, and rivastigmine] and NMDA antagonist [memantine)]) stabilize or slow decline in cognition in AD compared with placebo (Tan et al., 2014). In addition, our recent meta-analysis showed that memantine also had an effect on behavioral and psychological symptoms of dementia compared with placebo (Matsunaga et al., 2014). The current study aimed to provide a more conclusive understanding of the anatomical effect of anti-dementia medications by testing the hypothesis that they are protective against brain atrophy in AD.
While most published studies of anti-dementia medications and brain atrophy report protective effects, there has been no meta-analysis that reported on the protective effect of anti-dementia medications against brain atrophy in patients with mild cognitive impairment (MCI) and/or AD. Therefore, we conducted a meta-analysis of longitudinal, double-blind, randomized, placebo-controlled clinical trials (RCTs) to examine whether anti-dementia drugs had an inhibiting effect on progress brain atrophy in patients with MCI and AD.
Methods
This systematic review and meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (Moher et al., 2009)
Inclusion Criteria, Search Strategy, Data Extraction, and Outcomes
Inclusion criteria were double-blind RCTs of anti-dementia drugs (donepezil, galantamine, rivastigmine, and memantine) for patients with MCI and AD regarding brain volume measured by magnetic resonance imaging (MRI). Studies were identified through searches of PubMed, databases of the Cochrane Library, and PsycINFO citations up to 16 May, 2015. The following English key words were used: “donepezil” OR “galantamine” OR “memantine” OR “rivastigmine” AND “randomized” OR “random” OR “randomly” AND “imaging” OR “MRI” without language restriction. Cortical Pattern Matching, Diffusion Tensor Imaging, or other techniques that do not report absolute brain volumes were not included. Two authors (T.K. and S.M.) independently extracted, checked, and entered the data into the Review Manager software (Version 5.3 for Windows, Cochrane Collaboration, http://tech.cochrane.org/Revman).
Data Synthesis and Statistical Analysis
Primary outcomes were percent change of total brain volume per year (%TBV/y), percent change of hippocampal volume per year (%HV/y), and percent change of ventricular volume per year (%VV/y) measured by structural MRI. Secondary outcomes were percent change of total brain volume per half year (%TBV/half year) and percent change of hippocampal volume per half year (%HV/half year). We included data from a 26-week RCT (Schmidt et al., 2008) and other data from a 24-week RCT (Krishnan et al., 2003) in %HV/half year. Whereas primarily intention-to-treat (ITT) or modified ITT data (ie, at least 1 dose or at least 1 follow-up assessment) were included, per protocol population studies (Dubois et al., 2015) were also included to increase the sample size as much as possible.
The meta-analyses were performed using Review Manager. In combining the studies, the conservative random effects model by DerSimonian and Laird (DerSimonian and Laird, 1986) was employed, since the underlying effects can differ across studies and populations that are not necessarily homogeneous. Standardized mean difference (SMD) was used whereby the effect sizes (Hedge’s g) were combined with its 95% confidence interval (CI). Cohen’s guideline was adopted such that ESs of 0.2, 0.5, and 0.8 corresponded to small, medium, and large effects, respectively (Cohen, 1988). Since the study of Prins and colleagues (Prins et al., 2014) did not report standard deviation of %TBV/y and %HV/y, we imputed the SD from Schuff et al. (2011) as has been done before (Leucht et al., 2009). The Cochrane risk of bias criteria (Cochrane Collaboration, http://www.cochrane.org/) was used to perform methodological quality control. Significant subgroup difference was tested using the I2 statistic, considering values of ≥50% to reflect a significant subgroup difference. Sensitivity analyses had been planned to determine the reasons for the heterogeneity in cases where I2 values were ≥50% for the primary outcomes. To cope with the potential heterogeneity across studies and populations, several subgroup analyses were conducted. Funnel plots were visually inspected to assess the possibility of publication bias.
Results
Study Characteristics
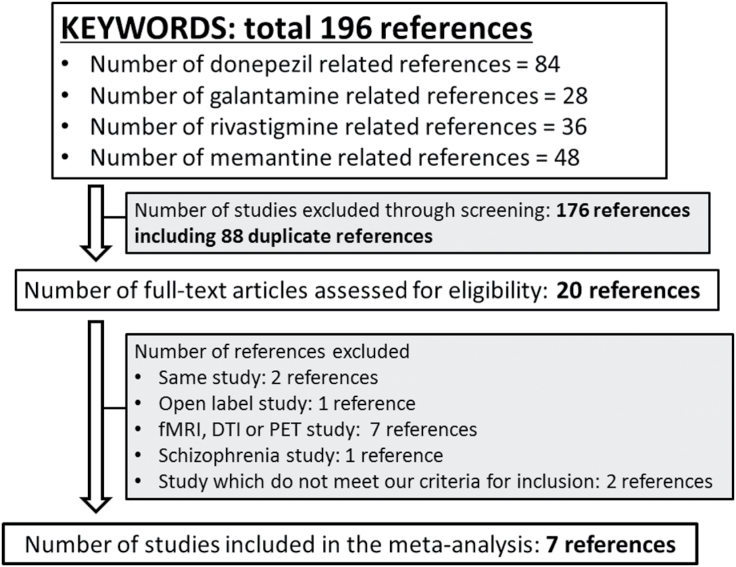
The search using the keywords given above yielded 196 references. Initially, 176 references including 88 duplicate references were excluded from analysis based on title and abstract review. Thirteen references were excluded based on full text examination. Seven RCTs were included in the current meta-analysis (Krishnan et al., 2003; Feldman et al., 2007; Schmidt et al., 2008; Schuff et al., 2011; Wilkinson et al., 2012; Prins et al., 2014; Dubois et al., 2015) (Figure 1). No additional articles were identified by manually searching all articles included in the meta-analysis. These 7 RCTs (n=1708) consisted of 4 MCI studies (n=1327) and 3 AD studies (n=381) (3 donepezil studies [2 MCI studies and 1 AD study], 1 galantaime study for MCI, 2 mementine studies for AD, and 1 rivastigmine study for MCI) (Table 1). All studies were double-blind RCTs that mentioned the required study design details (supplementary Figure S1). Although 6 of 7 RCTs used ITT or modified ITT data, the remaining 1 RCT (Dubois et al., 2015) used per protocol population data. All RCTs were published in English and were industry sponsored. The study duration was 24 weeks in 1 RCT (Krishnan et al., 2003), 48 weeks in 1 RCT (Schuff et al., 2011), 1 year in 3 RCTs, 2 years in 1 RCT (Prins et al., 2014), and 4 years in 1 RCT (Feldman et al., 2007). Other characteristics of the studies are summarized in Table 1.
Figure 1.
Preferred reporting items for systematic reviews and meta-analyses flow diagram.
Table 1.
Study, Patient, and Treatment Characteristics of Included Double-blind, Randomized Controlled Trials
| Study | Total n | Patients (diagnosis) and Inclusion Criteria | Duration | Age (mean ±SD)/ % Male | Race (% White) | MRI (Thickness)/ Segmentation | Outcomes |
|---|---|---|---|---|---|---|---|
| Donepezil | |||||||
| Dubois 2015 (France), Industry, DON=10mg/d (fixed) |
216 (DON=113, PBO=103) | MCI. Age >50 y old, FCSRT total recall <40 or free recall ≤17, CDR=0.5 and preserved cognition and functional performance. | 1 y (about MRI study) | DON=74.13 ±6.40/47.8, PBO=73.67±6.61/47.6 |
NR | 1.5 or 3 Tesla (5mm)/ automated | Percent change of total hippocampal volume/y: DON>PBO, % change of global cerebral volume/y: DON>PBO, % change of ventricular volume/y: DON>PBO |
| Krishnan 2003 (USA), Industry, DON=10mg/d (fixed) | 67 (DON=34, PBO=33) |
Mild to moderate AD (DSM-IV- TR and NINCDS). CDR=1 or 2, MMSE=10~26, Hachinski score≤4 | 24 wk | DON=74.4±7.0/26, PBO=72.4±10.1/30 |
DON=100, PBO=91 | 1.5 Tesla (1.5mm)/ automated | Percent change of total hippocampal volume from baseline to 24 wk: DON>PBO |
| Schuff 2011 (USA), Industry, DON=10mg/d (fixed) | 234 (about MRI study, DON=109, PBO=125) | Amnestic MCI (DSM-, Petersen’s criteria). Age: 45~90 y old, a recent brain scan showing no evidence of focal lesions. CDR=0.5 with a memory score=0.5~1.0, MMSE=24~28, Hachinski score ≤4 | 48 wk | DON=70.6 ±9.8/56.0, PBO=68.4±9.9/56.8 |
DON=95.4, PBO=88.0 | NR (NR)/whole: automated, hippocampus: semiautomated | Percent change of total hippocampal volume/y: DON=PBO, % change of total whole brain atrophy volume/year: DON>PBO, % change of total ventricular volume/year: DON>PBO |
| Galantamine | |||||||
| Prins 2014 (multi- countries), Industry, GAL=16~24mg/d (flexible) | 364 (about MRI study, DON=176, PBO=188) | MCI. Age ≥50 y old, CDR=0.5 with a memory score ≥0.5, and NYU-PRT≤10 | 2 y | GAL=68 ±9.0/57, PBO=69±8.6/46 |
NR | 1.5 Tesla (1.5mm)/ whole: automated, hippocampus: manual | Whole brain atrophy rate/y: GAL>PBO, Hippocampal atrophy rate/y: GAL=PBO |
| Mementine | |||||||
| Schmidt 2008 (Austria), Industry, MEM=20mg/d (fixed) |
36 (MEM= 18, PBO=18) | Probable AD (DSM-IV-TR and NINCDS-ADRDA). Age >50 y old, MMSE=14~22, Hachinski score ≤ 4. | 1 y | MEM=76.5 ±4.81/27.8, PBO=75.8±5.70/44.4 |
NR | 1.5 Tesla (5mm)/ whole: automated, hippocampus: manual | Percent change of brain volume/y: MEM=PBO, % change of hippocampal volume/y: MEM=PBO |
| Wilkinson 2012 (multi- countries), Industry, MEM=20mg/d (fixed) |
278 (MEM=134, PBO=144) | Probable AD (NINCDS-ADRDA). Age ≥ 50 y old, MMSE=12~20. Number of patients who also received ChEI: MEM=71.8%, PBO=72.9% | 1 y | MEM=74±9/38, PBO=74±8/48 |
99.6 | 1.5 Tesla (5mm)/ hippocampus: manual, others: semiautomated | Percent change of total brain atrophy rate/y: MEM=PBO, Total brain atrophy rate (mL/y): MEM=PBO, When including data from patients who received only MEM, total brain atrophy rate (mL/y): MEM>PBO |
| Rivastigmine | |||||||
| Feldman 2007 (multi- countries), Industry, RIV=3~12mg/d (flexible) |
513 (about MRI study, RIV=259, PBO=254) |
MCI. Age: 55~85 y old, CDR=0.5 and NYU-PRT <9 | 4 y | RIV=70.3±7.4/46.9, PBO=70.6±7.6/48.6 |
NR | NR (1.5mm)/ hippocampus: semiautomated, ventricle: manual | Mean change in ventricular volume rate from baseline to endpoint (cm3/y): RIV=PBO |
Abbreviations: AD, Alzheimer’s disease; CDR, Clinical Dementia Rating; ChEI, cholinesterase inhibitor; DON, donepezil; DSM-IV-TR, Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision; FCSRT, Free and Cued Selective Reminding Test; GAL, galantamine; MCI, mild cognitive impairment; MEM, mementine; MMSE, Mini-Mental State Examination; MRI, magnetic resonance imaging; NINCDS-ADRDA, National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association; NR; not reported; NYU-PRT, New York University paragraph recall test; PBO, placebo; Petersen’s criteria, Petersen R C, Smith G E, Waring S C. et al Aging, memory, and mild cognitive impairment. Int Psychogeriatr 1997 965–69.69; RIV, rivastigmine.
Medication and Changes in Brain Volumes
Pooled anti-dementia drugs showed a significantly smaller decrease in %TBV/y (SMD=-0.21, 95%CI=-0.37 to -0.04, I2=4%, P=.01, N=4, n=624) and a smaller increase in %VV/y (SMD=-0.79, 95%CI=-1.40 to -0.19, I2=94%, P=.01, N=3, n=851), while %HV/y did not show significant difference. For individual anti-dementia drugs, donepezil showed a lesser decrease in %TBV/y (SMD=-0.43, 95%CI=-0.74 to -0.12, P=.007, N=1, n=164) and a smaller increase in %VV/y (SMD=-0.51, 95%CI=-0.73 to -0.29, I2=0%, P<.00001, N=2, n=338). Rivastigmine also showed a lesser increase in %VV/y (SMD=-1.33, 95%CI=-1.52 to -1.14, P<.00001, N=1, n=513) (Table 2). Visual inspection of the funnel plots for primary outcomes in both treatment groups suggested no publication bias (data not shown). Although there was no significant difference in %TBV/half year and %HV/half year between pooled anti-dementia drugs and placebo groups, donepezil was superior to placebo regarding %HV/half year (SMD=-1.06, 95%CI=-1.59 to -0.53, P<.0001, N=1, n=63) (Table 2).
Table 2.
The Results of Meta-Analyses
| N | n | I2 | SMD | 95% CI | P | Subgroup Difference | ||
|---|---|---|---|---|---|---|---|---|
| Percent change of total brain volume per year | Donepezil | 1 | 164 | na | -0.43 | -0.74 to -0.12 | 0.007 | I2=25.9%, P=.26 |
| Galantamine | 1 | 242 | na | -0.13 | -0.39 to 0.12 | 0.30 | ||
| Memantine | 2 | 218 | 0% | -0.12 | -0.39 to 0.15 | 0.38 | ||
| All drugs | 4 | 624 | 4% | -0.21 | -0.37 to -0.04 | 0.01 | ||
| Percent change of hippocampal volume per year | Donepezil | 2 | 404 | 77% | -0.29 | -0.71 to 0.13 | 0.17 | I2=0%, P=.40 |
| Galantamine | 1 | 302 | na | 0.03 | -0.19 to 0.26 | 0.77 | ||
| Memantine | 2 | 218 | 9% | -0.01 | -0.33 to 0.32 | 0.97 | ||
| All drugs | 5 | 924 | 62% | -0.14 | -0.36 to 0.09 | 0.25 | ||
| Percent change of ventricular volume per year | Donepezil | 2 | 338 | 0% | -0.51 | -0.73 to -0.29 | <0.00001 | I2=96.8%, P<.00001 |
| Rivastigmine | 1 | 513 | na | -1.33 | -1.52 to -1.14 | <0.00001 | ||
| All drugs | 3 | 851 | 94% | -0.79 | -1.40 to -0.19 | 0.01 | ||
| Percent change of total brain volume per half year | Memantine | 2 | 257 | 0% | -0.02 | -0.27 to 0.22 | 0.86 | na |
| Percent change of hippocampal volume per half year | Donepezil | 1 | 63 | na | -1.06 | -1.59 to -0.53 | <0.0001 | |
| Memantine | 1 | 29 | na | -0.16 | -0.89 to 0.57 | 0.66 | ||
| All drugs | 2 | 92 | 74% | -0.65 | -1.53 to 0.23 | 0.15 | I2=73.8%, P=.05 |
Abbreviations: CI, confidence interval; na, not applicable; SMD, standardized mean difference.
In several subgroup analyses of %TBV/y that included only “total n<100” and “study duration≤1 years,” pooled anti-dementia drugs showed a significantly lesser reduction in %TBV/y compared with placebo (Table 3). Since significant heterogeneities in %HV/y and %VV/y were detected, several sensitivity analyses were performed to identify which confounding factors influence heterogeneity. Only when we performed the sensitivity analysis of %HV/year using data from AD RCTs only did the significant heterogeneity disappear (I2=9) (Table 3). The significant heterogeneities of other sensitivity analyses did not disappear (Table 3). Because 3 subgroups of %VV/y did not differ in their study design (Feldman et al., 2007; Schuff et al., 2011; Dubois et al., 2015) (Table 1), no sensitivity analysis was conducted for %VV/y.
Table 3.
The Results of Subgroup/Sensitivity Analysis
| Subgroup | N | n | I2 | SMD | 95% CI | P | Subgroup Difference | |
|---|---|---|---|---|---|---|---|---|
| Percent change of total brain volume per year | ||||||||
| Diagnosis | MCI | 2 | 406 | 52% | -0.27 | -0.56 to 0.02 | 0.07 | I2=0%, P=.47 |
| AD | 2 | 218 | 0% | -0.12 | -0.39 to 0.15 | 0.38 | ||
| Sample size | Total n≥100 | 3 | 603 | 18% | -0.22 | -0.40 to -0.04 | 0.02 | I2=0%, P=.42 |
| Total n<100 | 1 | 21 | na | 0.14 | -0.73 to 1.01 | 0.75 | ||
| Duration of study | >1 y | 1 | 242 | na | -0.13 | -0.39 to 0.12 | 0.30 | I2=0%, P=.53 |
| ≤1 y | 3 | 382 | 22% | -0.25 | -0.49 to -0.00 | 0.05 | ||
| Percent change of hippocampal volume per year | ||||||||
| Diagnosis | MCI | 3 | 706 | 76% | -0.17 | -0.48 to 0.13 | 0.27 | I2=0%, P=.46 |
| AD | 2 | 218 | 9 | -0.01 | -0.33 to 0.32 | 0.97 | ||
| Sample size | Total n≥100 | 4 | 903 | 69% | -0.12 | -0.35 to 0.12 | 0.34 | I2=0%, P=.49 |
| Total n<100 | 1 | 21 | na | -0.43 | -1.31 to 0.44 | 0.33 | ||
| Duration of study | >1 y | 1 | 302 | na | 0.03 | -0.19 to 0.26 | 0.77 | I2=33.9%, P=.22 |
| ≤1 y | 4 | 622 | 63% | -0.20 | -0.48 to 0.09 | 0.18 | ||
Abbreviations: AD, Alzheimer’s disease; CI, confidence interval; MCI, mild cognitive impairment; na, not applicable; SMD, standardized mean difference.
Discussion
To our knowledge, this is the first meta-analysis of anti-dementia drugs longitudinally compared with the placebo for protection from brain atrophy in MCI and AD. Seven studies (n=1708) were identified by this systematic review and included in the meta-analysis. There were 2 main findings in the current study: (1) anti-dementia drugs can prevent decreased total brain volume with small effect size (SMD=-0.21), and (2) anti-dementia drugs can prevent increased ventricular volume in patients with MCI with relatively large effect size (SMD=-0.79). Moreover, although the effect of anti-dementia drugs on prevention of brain atrophy for a half year was unclear, it was demonstrated that anti-dementia drugs had an effect on prevention of brain atrophy for 1 year. Our results suggest that donepezil significantly reduces dilatation in ventricular volume in patients with MCI, which may account for a recent meta-analysis where donepezil was reported to be marginally superior to placebo in Mini–Mental State Examination scores in MCI patients (Tricco et al., 2013).
There were only 3 RCTs included in this meta-analysis that performed an association analysis between baseline-to-endpoint changes in cognitive function tests and baseline-to-endpoint changes in brain volume. MCI studies showed a significant association of %TBV/y to Alzheimer Disease Assessment Scale-cognitive subscale scores per year and of %VV/y, but not %HV/y (Feldman et al., 2007; Schuff et al., 2011). A memantine study also reported that %TBV/y and %HV/y were associated with some cognition tests including ADAS-cog scores (Wilkinson et al., 2012). These associations between brain volume reductions and cognitive declines may suggest that therapeutic effects are anatomically observed. Several studies reported that anti-dementia drugs prevent neuronal apoptosis induced by glutamate and amyloid-β (Rutenberg et al., 1968; Akaike, 2006; Akaike et al., 2010). In addition, anti-dementia drugs are also shown to play a role in maintaining the structure and integrity of amyloid plaques and neurofibrillary tangles (Anand and Singh, 2013; Kumar et al., 2015). Increased neuronal functioning by anti-dementia drugs might mediate the improvement of cognitive function.
On the other hand, the current meta-analysis did not show positive evidence for the protective effect against hippocampal atrophy. Hippocampal volume is considered to be main pathophysiology of MCI and AD (Drago et al., 2011; Fellgiebel and Yakushev, 2011; Jack et al., 2011). Hippocampal atrophy has been reported to be associated with earlier and more severely affected regions in patients with MCI and AD (Drago et al., 2011; Fellgiebel and Yakushev, 2011; Jack et al., 2011; Dubois et al., 2015). Therefore, we expected to see the protective effect also in the hippocampus in addition to the whole brain volume. However, pooled anti-dementia drugs in the current meta-analysis failed to show significantly smaller reduction of %HV/y. It may be due to the nature of the hippocampi that they are a small subpart of the whole brain. Since the unit for a volumetric measurement of the hippocampi and whole brain is identically a “voxel,” hippocampal volume measurements are theoretically less sensitive than whole brain measurement. Therefore, the current results should not be interpreted that hippocampal volume did not show protective effects while whole brain volume did.
The absence of significant protective effect of the hippocampus in the current meta-analysis may be accounted for by the effectiveness difference between mono (cholinesterase inhibitor or memantine) and combination (cholinesterase inhibitor and memantine) therapies, at least to certain extent. One open-label trial showed that memantine was associated with slowing of right hippocampal atrophy and improvement of some cognitive functions in AD patients who received cholinesterase inhibitors (Weiner et al., 2011). However, a posthoc analysis of another memantine study (Wilkinson et al., 2012) showed that combination therapy did not have an inhibiting effect on progressive brain atrophy in patients with AD compared with cholinesterase inhibitors monotherapy. Thus, it remains unclear whether combination therapy has an inhibitory effect on brain atrophy in patients with Alzheimer’s disease. However, our recent meta-analysis (Matsunaga et al., 2015) showed that because combination therapy with cholinesterase inhibitors and memantine was superior to cholinesterase inhibitor monotherapy regarding the scores of cognitive function, behavioral disturbances, activities of daily living, and global assessment scales in moderate to severe AD, combination therapy was exhibited a more beneficial treatment for moderate to severe AD compared with cholinesterase inhibitors monotherapy at the level of clinical practice.
Several limitations to the present analysis have to be noted. First, the number of studies included in this meta-analysis was small. However, we did not include non-RCTs such as case-control studies and prospective cohort studies in the meta-analysis to avoid the increased risk of biases in the meta-analysis (Higgins and Green, 2011). Second, to cope with the study number, studies of MCI and AD were combined. Third, patients with dementia are known to comply poorly with medication regimens (Boada and Arranz, 2013), and therefore the effectiveness of pharmacological interventions for patients with dementia may be limited.
In conclusion, the current results suggested that anti-dementia drugs showed a protective effect against brain atrophy in patients with MCI and AD. However, since number of studies included in the meta-analysis were very small, further study using larger sample will be required to have conclusive interpretations to account for the therapeutic mechanisms between anti-dementia drugs and brain volume.
Statement of Interest
All authors declare they have no direct conflicts of interest relevant to this study. No grants or other funding sources were used for this study. Dr. Kishi has received speaker’s honoraria from Abbott, Astellas, Daiichi Sankyo, Dainippon Sumitomo, Eisai, Eli Lilly, GlaxoSmithKline, Yoshitomi, Otsuka, Meiji, Shionogi, Janssen, Novartis, Tanabe-Mitsubishi, and Pfizer. Dr. Matsunaga has received speaker’s honoraria from Eisai, Janssen, Novartis, Daiichi Sankyo, Ono, Eli Lilly, Takeda, and Otsuka. Dr. Oya has received speaker’s honoraria from Dainippon Sumitomo, Eli Lilly, and Otsuka. Dr. Ikuta has received speaker’s honoraria from Eli Lilly. Dr. Iwata has received speaker’s honoraria from Astellas, Dainippon Sumitomo, Eli Lilly, GlaxoSmithKline, Janssen, Yoshitomi, Otsuka, Meiji, Shionogi, Novartis, and Pfizer and has research grants from GlaxoSmithKline and Otsuka.
Contributions
All authors had full access to all study data and are responsible for the integrity of the data and the accuracy of any data analysis. All authors drafted the final manuscript.
Supplementary Material
Footnotes
This version corrects the title of the article.
References
- Akaike A. (2006) Preclinical evidence of neuroprotection by cholinesterase inhibitors. Alzheimer Dis Assoc Disord 20:S8–11. [DOI] [PubMed] [Google Scholar]
- Akaike A, Takada-Takatori Y, Kume T, Izumi Y. (2010) Mechanisms of neuroprotective effects of nicotine and acetylcholinesterase inhibitors: role of alpha4 and alpha7 receptors in neuroprotection. J Mol Neurosci 40:211–216. [DOI] [PubMed] [Google Scholar]
- Anand P, Singh B. (2013) A review on cholinesterase inhibitors for AD. Arch Pharm Res 36:375–399. [DOI] [PubMed] [Google Scholar]
- Boada M, Arranz FJ. (2013) Transdermal is better than oral: observational research of the satisfaction of caregivers of patients with AD treated with rivastigmine. Dement Geriatr Cogn Disord 35:23–33. [DOI] [PubMed] [Google Scholar]
- Cohen J. (1988) Statistical power analysis in the behavioral sciences. Hillsdale, NJ: Erlbaum. [Google Scholar]
- DerSimonian R, Laird N. (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188. [DOI] [PubMed] [Google Scholar]
- Drago V, et al. (2011) Disease tracking markers for AD at the prodromal (MCI) stage. J Alzheimers Dis 26:159–199. [DOI] [PubMed] [Google Scholar]
- Dubois B, et al. (in press) Donepezil decreases annual rate of hippocampal atrophy in suspected prodromal AD Alzheimers Dement. [DOI] [PubMed]
- Feldman HH, et al. (2007) Effect of rivastigmine on delay to diagnosis of AD from mild cognitive impairment: the InDDEx study. The Lancet Neurology 6:501–512. [DOI] [PubMed] [Google Scholar]
- Fellgiebel A, Yakushev I. (2011) Diffusion tensor imaging of the hippocampus in MCI and early AD. J Alzheimers Dis 26:257–262. [DOI] [PubMed] [Google Scholar]
- Higgins J, Green S. (2011) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 The Cochrane Collaboration www.cochrane-handbook.org.
- Hirono N, Kitagaki H, Kazui H, Hashimoto M, Mori E. (2000) Impact of white matter changes on clinical manifestation of AD: a quantitative study. Stroke 31:2182–2188. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr., Vemuri P, Wiste HJ, Weigand SD, Aisen PS, Trojanowski JQ, Shaw LM, Bernstein MA, Petersen RC, Weiner MW, Knopman DS, AD Neuroimaging I. (2011) Evidence for ordering of Alzheimer disease biomarkers. Arch Neurol 68:1526–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan KR, Charles HC, Doraiswamy PM, Mintzer J, Weisler R, Yu X, Perdomo C, Ieni JR, Rogers S. (2003) Randomized, placebo-controlled trial of the effects of donepezil on neuronal markers and hippocampal volumes in AD. Am J Psychiatry 160:2003–2011. [DOI] [PubMed] [Google Scholar]
- Kumar A, Singh A, Ekavali (2015) A review on AD pathophysiology and its management: an update. Pharmacol Rep 67:195–203. [DOI] [PubMed] [Google Scholar]
- Leucht S, Komossa K, Rummel-Kluge C, Corves C, Hunger H, Schmid F, Asenjo Lobos C, Schwarz S, Davis JM. (2009) A meta-analysis of head-to-head comparisons of second-generation antipsychotics in the treatment of schizophrenia. Am J Psychiatry 166:152–163. [DOI] [PubMed] [Google Scholar]
- Matsunaga S, Kishi T, Iwata N.(2014) Combination therapy with cholinesterase inhibitors and memantine for AD: a systematic review and meta-analysis Int J Neuropsychopharmacol 28;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga S, Kishi T, Iwata N.(2015) Memantine monotherapy for AD: a systematic review and meta-analysis. PLoS One 10:e0123289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins ND, van der Flier WA, Knol DL, Fox NC, Brashear HR, Nye JS, Barkhof F, Scheltens P. (2014) The effect of galantamine on brain atrophy rate in subjects with mild cognitive impairment is modified by apolipoprotein E genotype: post-hoc analysis of data from a randomized controlled trial. Alzheimers Res Ther 6:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutenberg HL, Schwartz H, Soloff LA. (1968) Norepinephrine- and heparin-induced changes in plasma free fatty acids: a comparison between patients with ischemic heart disease and normal young adults. Am Heart J 76:183–192. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Ropele S, Pendl B, Ofner P, Enzinger C, Schmidt H, Berghold A, Windisch M, Kolassa H, Fazekas F. (2008) Longitudinal multimodal imaging in mild to moderate Alzheimer disease: a pilot study with memantine. J Neurol Neurosurg Psychiatry 79:1312–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuff N, Suhy J, Goldman R, Xu Y, Sun Y, Truran-Sacrey D, Murthy A. (2011) An MRI substudy of a donepezil clinical trial in mild cognitive impairment. Neurobiol Aging 32:2318 e2331–2341. [DOI] [PubMed] [Google Scholar]
- Tan CC, Yu JT, Wang HF, Tan MS, Meng XF, Wang C, Jiang T, Zhu XC, Tan L. (2014) Efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of AD: a systematic review and meta-analysis. J Alzheimers Dis 41:615–631. [DOI] [PubMed] [Google Scholar]
- Tricco AC, Soobiah C, Berliner S, Ho JM, Ng CH, Ashoor HM, Chen MH, Hemmelgarn B, Straus SE. (2013) Efficacy and safety of cognitive enhancers for patients with mild cognitive impairment: a systematic review and meta-analysis. CMAJ 185:1393–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner MW, Sadowsky C, Saxton J, Hofbauer RK, Graham SM, Yu SY, Li S, Hsu HA, Suhy J, Fridman M, Perhach JL. (2011) Magnetic resonance imaging and neuropsychological results from a trial of memantine in AD. Alzheimers Dement 7:425–435. [DOI] [PubMed] [Google Scholar]
- Wilkinson D, Fox NC, Barkhof F, Phul R, Lemming O, Scheltens P. (2012) Memantine and brain atrophy in AD: a 1-year randomized controlled trial. J Alzheimers Dis 29:459–469. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.