Abstract
Background:
Previous studies have demonstrated that methamphetamine abuse leads to memory deficits and these are associated with relapse. Furthermore, extensive evidence indicates that nicotine prevents and/or improves memory deficits in different models of cognitive dysfunction and these nicotinic effects might be mediated by hippocampal or cortical nicotinic acetylcholine receptors. The present study investigated whether nicotine attenuates methamphetamine-induced novel object recognition deficits in rats and explored potential underlying mechanisms.
Methods:
Adolescent or adult male Sprague-Dawley rats received either nicotine water (10–75 μg/mL) or tap water for several weeks. Methamphetamine (4×7.5mg/kg/injection) or saline was administered either before or after chronic nicotine exposure. Novel object recognition was evaluated 6 days after methamphetamine or saline. Serotonin transporter function and density and α4β2 nicotinic acetylcholine receptor density were assessed on the following day.
Results:
Chronic nicotine intake via drinking water beginning during either adolescence or adulthood attenuated the novel object recognition deficits caused by a high-dose methamphetamine administration. Similarly, nicotine attenuated methamphetamine-induced deficits in novel object recognition when administered after methamphetamine treatment. However, nicotine did not attenuate the serotonergic deficits caused by methamphetamine in adults. Conversely, nicotine attenuated methamphetamine-induced deficits in α4β2 nicotinic acetylcholine receptor density in the hippocampal CA1 region. Furthermore, nicotine increased α4β2 nicotinic acetylcholine receptor density in the hippocampal CA3, dentate gyrus and perirhinal cortex in both saline- and methamphetamine-treated rats.
Conclusions:
Overall, these findings suggest that nicotine-induced increases in α4β2 nicotinic acetylcholine receptors in the hippocampus and perirhinal cortex might be one mechanism by which novel object recognition deficits are attenuated by nicotine in methamphetamine-treated rats.
Keywords: Methamphetamine, nicotine, novel object recognition memory, nicotinic receptors, serotonin transporter
Introduction
Methamphetamine (METH) abuse is a significant public health problem with annual prevalence rate of abuse in 2013 in >1% of adolescents and young adults (Johnston et al., 2014). Extensive clinical evidence indicates that METH abuse causes significant neurocognitive deficits (Kalechstein et al., 2003, 2009; Gonzalez et al., 2004; Hoffman et al., 2006; Cherner et al., 2010; Casaletto et al., 2014). For example, episodic memory is reduced among participants with a history of METH abuse (approximately 11 years), as assessed by performance in learning and recall tests (Casaletto et al., 2014). METH users also present with deficits in learning, motor ability, and working memory tests (Cherner et al., 2010). Neurocognitive deficits occur not only in individuals currently using METH (Simon et al., 2000) but can also persist long after METH is discontinued (4 days to 7 months) (Kalechstein et al., 2003, 2009; Gonzalez et al., 2004; Hoffman et al., 2006; Cherner et al., 2010; Casaletto et al., 2014). Among the different types of neurocognitive deficits caused by METH abuse, METH-associated neurocognitive deficits are greater for episodic memory, executive functions, information processing speed, and motor skills and lesser for attention, working memory, and verbal fluency (Scott et al., 2007). Notably, relapse is associated with episodic memory deficits but not other types of cognitive dysfunction among METH abusers (Simon et al., 2004).
In addition to its impact on cognition, METH abuse causes brain abnormalities in areas important for memory, such as the hippocampus and cortex. For example, Thompson et al. (2004) reported that METH abusers have 7.8% smaller hippocampal volumes than control subjects as assessed by MRI, and these deficits correlated with deficits in deficits on a word recall task. The integrity of hippocampal and cortical neurons can also be assessed by the binding of the serotonin transporter (SERT), a marker highly expressed in these neuronal regions (Lawrence et al., 1993; for review, see Meneses et al., 2011). Loss of presynaptic serotonergic markers such as SERT indicates loss of this population of presynaptic serotonergic terminals. Studies have reported significant loss of serotonergic markers in the hippocampus and cortex of individuals with cognitive dysfunction, such as in METH abuse or Alzheimer’s disease (Chen et al., 1996; Sekine et al., 2006; Ouchi et al., 2009). For example, positron emission tomography scan revealed that SERT densities are reduced in several brain regions of abstinent METH abusers (Sekine et al., 2006).
Novel object recognition (NOR) is an established preclinical model for evaluating recognition memory deficits (for review, see Kinnavane et al., 2014). This test relies on the instinct of rats to preferentially explore novel objects over familiar objects, thus requiring the animals to remember which object is familiar. The perirhinal cortex (PRh) and the hippocampal regions CA1, CA3, and dentate gyrus (DG) are important mediators of NOR (Melichercik et al., 2012; for review, see Kinnavane et al., 2014). Specifically, the PRh-CA1 pathway is important for familiarization of objects, and the PRh-DG-CA3 pathway is important during exploration of novel objects (for review, see Kinnavane et al., 2014). Overall, intact functions of these regions are required for NOR.
In preclinical studies, both contingent and/or noncontingent METH administrations have been shown to impair NOR (McCabe et al., 1987; Belcher et al., 2008; Herring et al., 2008; Tellez et al., 2010; Reichel et al., 2011, 2012). Besides deficits in NOR, these studies in rats revealed significant deficits in SERT density in the hippocampus and PRh (Belcher et al., 2008; Reichel et al., 2012). Both clinical and preclinical evidence suggests that cortical and hippocampal SERT sites are important for learning and memory (Meneses et al., 2011 for review). Furthermore, preclinical evidence suggests a link between SERT sites and NOR. For example, significant NOR deficits occurred in SERT knockout mice (Olivier et al., 2008). Similarly, in METH-treated rats, pretreatment with fluoxetine, a selective SERT inhibitor, attenuated both NOR deficits and hippocampal/cortical SERT density deficits (Tellez et al., 2010). These data suggest that abnormalities in SERT density in the hippocampus and/or PRh might mediate NOR deficits in rats. Overall, METH-induced NOR deficits might be related to abnormalities to the hippocampus and PRh, and these can be assessed via SERT densities.
Extensive evidence from clinical (Jubelt et al., 2008; Newhouse et al., 2012; Sofuoglu et al., 2013; Kalechstein et al., 2014) and preclinical studies (Mizoguchi et al., 2011; Gould et al., 2013) has revealed that nicotinic acetylcholine receptor (nAChR) agonists have cognitive-enhancing properties. This is important because many METH abusers smoke cigarettes (70–80%; Thompson et al., 2004; McCann et al., 2008) and are thus exposed to nicotine (NIC), a nAChR agonist. However, few studies have investigated the impact of NIC exposure on METH-induced memory deficits. Among these, in clinical trials, NIC patch application improved recognition memory or attention in patients with schizophrenia or mild cognitive impairment compared with placebo-treated patients (Jubelt et al., 2008; Newhouse et al., 2012). Activation of α4β2 subtypes of nAChRs also improved working memory in rhesus monkeys that self-administered cocaine (Gould et al., 2013). Further, preclinical studies with systemic or local infusion of NIC indicate the important role of α4β2 subtypes of nAChRs and the hippocampus and PRh in object memory (Melichercik et al., 2012).
The aim of the current studies was 3-fold. First, we investigated whether NIC impacts the NOR deficits caused by METH in rats chronically pre- or posttreated with NIC. Second, we investigated whether any potential cognitive neuroprotection afforded by NIC is mediated by protection of hippocampal and/or PRh serotonergic neurons. Finally, we explored whether α4β2 nAChRs contribute to the impact of NIC on performance in the NOR test. Results revealed that both NIC pre- and posttreatment attenuates METH-induced NOR deficits. This protection was accompanied by an increase in α4β2 nAChR binding but not an attenuation of METH-induced SERT density deficits in the hippocampus and PRh. These findings suggest that NIC-induced increases in α4β2 nAChR binding in the hippocampus and PRh may contribute to the NIC-induced attenuation of NOR deficits caused by METH.
Methods
Animals
Male Sprague-Dawley rats (Charles River Breeding Laboratories, Raleigh, NC), initially weighing 125 to 150g (corresponding to postnatal day [PND] 40), 245 to 270g (corresponding to PND 60; Spear, 2000; Tirelli et al., 2003), or 350 to 415g (corresponding to PND 89) were housed 2 to 3 rats per cage and maintained under a controlled light/dark cycle (14:10 hours) in an ambient environment of 20°C (with the exception of the 6-hour period during which METH or saline vehicle was administered during when the ambient environment was maintained at 24°C). During METH or saline administrations, core body (rectal) temperatures were measured using a digital thermometer (Physitemp Instruments, Clifton, NJ) every 1 hour beginning 30 minutes before the first saline or METH administration and continuing until 30 minutes after the final saline or METH administration. Rats were placed in a cooler environment during METH exposure if their body temperature exceeded 40.5°C and returned to their home cage once their body temperature dropped to 40°C. Food and water were available ad libitum. All experiments were approved by the University of Utah Institutional Animal Care and Use Committee in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals 8 th Edition (Institute of Laboratory Animal Resources, 2011).
Drug Treatments
METH hydrochloride was provided by the National Institute on Drug Abuse (Research Triangle Institute, Research Triangle Park, NC) and administered at 4×7.5mg/kg/injection, s.c., at 2-hour intervals with doses calculated as free-base. (-) NIC (1.010g/mL, Sigma-Aldrich) was administered ad libitum p.o. at concentrations of 10, 20, 50, or 75 µg/mL via the water bottles. Dosing protocols are delineated in the figures. To increase palatability, saccharin (Sweet & Low; Cumberland Packing Corporation, 1%) was added to the animals’ drinking water only in experiments in which the initial NIC concentration was 75 µg/mL or during the highest escalating rate (Figures 1B-C, 2B). In our current studies, NIC water consumption was approximately 34mL/rat/d and tap water consumption was approximately 47mL/rat/d, similarly to previous reports (Bordia et al., 2008). These NIC doses in rats yield plasma concentrations similar to plasma NIC and cotinine concentrations typically found in human smokers (10–50ng/mL for NIC and 300ng/mL for cotinine) (Benowitz, 1994; Matta et al., 2007).
Figure 1.
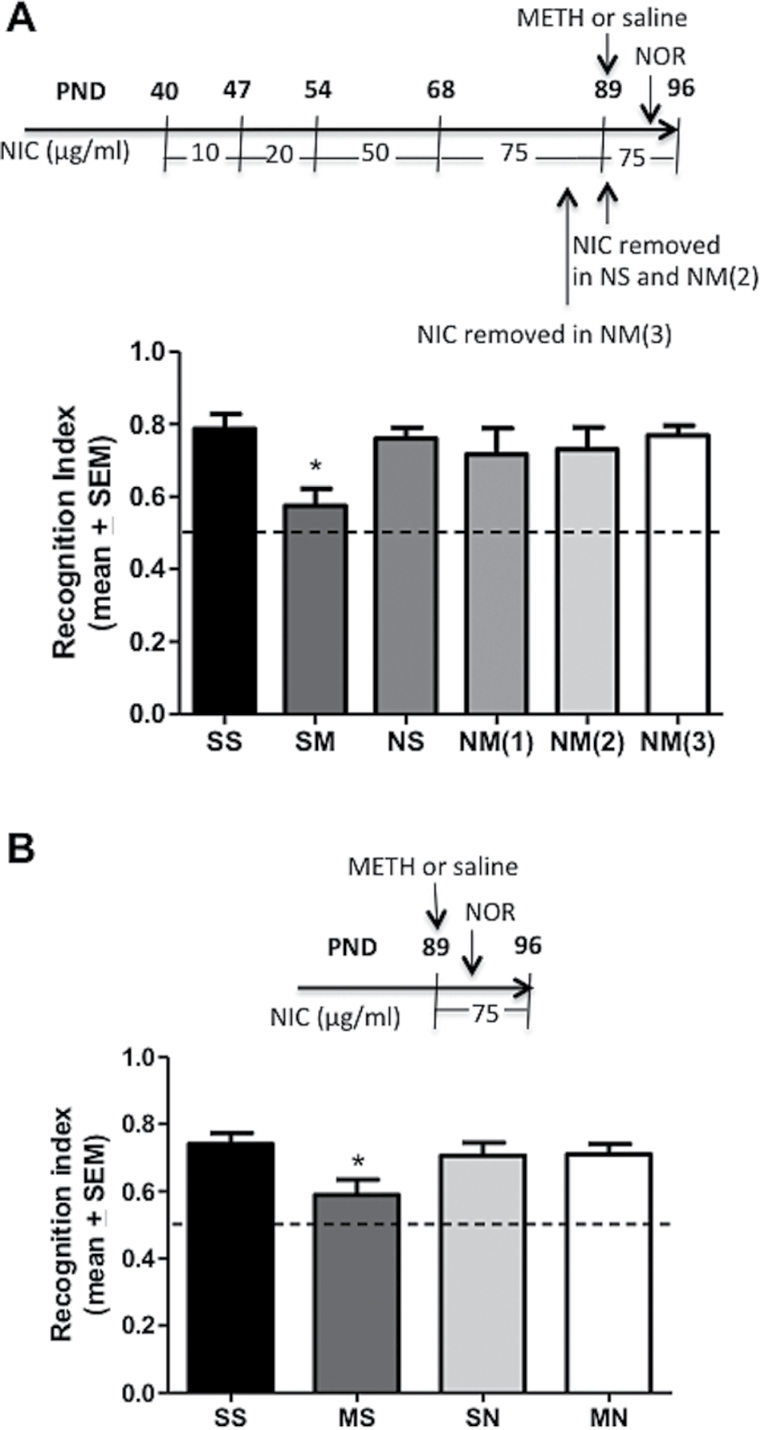
Chronic nicotine (NIC) administration via drinking water initiated during adolescence (A, C) or adulthood (B) attenuates novel object recognition (NOR) deficits caused by methamphetamine (METH). (A) Rats received oral NIC (N) at increasing concentrations via drinking water at doses delineated in figure inset or tap water (S) from postnatal day (PND) 40 to 96 and either saline (S) or METH (M) administrations (4×7.5mg/kg/injection, s.c., 2 hours apart) at PND 89. (B) Rats received oral NIC (N) at increasing concentrations via drinking water at doses delineated in figure inset or tap water (S) from PND 61 to 100 and either saline (S) or METH (M) administrations (4×7.5mg/kg/injection, s.c., 2 hours apart) at PND 93. (C) Rats received oral NIC (N) at increasing concentrations via drinking water at doses delineated in figure inset or tap water (S) from PND 40 to 61 and either saline (S) or METH (M) administrations (4×7.5mg/kg/injection, s.c., 2 hours apart) at PND 54. NOR was assessed 5 days after METH. Data are expressed as mean ±SEM of (A) N=8 to 10, (B) N=8 to 11, or (C) 7 to 10 determinations. *Represent values statistically different from saline controls (P<.05) as well as values not different from the chance exploration of 0.5 illustrated by the dashed line (P<.05). NM,NIC water/METH injections; NS,NIC water/saline injections; SM,tap water/METH injections; SS,tap water/saline injections.
Figure 2.
Chronic nicotine (NIC) administration via drinking water given either as pretreatment (A) or posttreatment (B) attenuates methamphetamine (METH)-induced deficits in novel object recognition (NOR). (A) Postatal day (PND) 40 rats received either tap water (SS and SM groups) or NIC water (10–75 μg/mL) until PND 88 (NM(3) group), PND 89 (NS and NM(2) group), or PND 96 (NM(1) group). METH (4×7.5mg/kg/injection, s.c., 2 hours apart) or saline (1mL/kg/injection) was given at PND 89. In the NM(3) group, NIC water was replaced by tap water 24h prior to the first METH injection. In NS and NM(2) groups NIC water was replaced by tap water 2 hours prior to the first METH or saline injection. (B) PND 89 rats received either METH (4×7.5mg/kg/injection, s.c., 2 hours apart) or saline (1mL/kg/injection) injections, and 2 hours after the last injection they received either tap water (SS and SM groups) or NIC water (75 μg/mL) until PND 96 (NS and NM groups). NOR testing was initiated 3 days after METH or saline injections. Data are expressed as mean±SEM of N=6 to 11 determinations. *Represent values statistically different from saline controls (P<.05) as well as values that are not statistically different from the chance exploration of 0.5 illustrated by the dashed line (P<.05). NM,NIC water/METH injections; NS,NIC water/saline injections; SM,tap water/METH injections; SS,tap water/saline injections.
NOR
After 3 days of recovery from METH or saline administrations, rats underwent a 5-minute habituation session in test apparatus (clear plexiglas open field 45cm wide×26cm high) in which they were allowed to explore the environment without the objects. On the following day (ie, 5 days after METH) and during the familiarization phase, the rats explored 2 identical objects (plastic water bottles 12cm tall) for 3 minutes. The NOR test was conducted 90 minutes later by allowing rats to explore an object from the familiarization phase and a novel object for 3 minutes (polyvinyl chloride pipe, 9cm tall × 5cm wide). The position of the objects was counterbalanced between morning and afternoon sessions, that is, one-half of each group was tested in the morning with the same object placement and the other one-half was tested in the afternoon with a different object placement. The selection of these objects was based upon previously published research (Besheer and Bevins, 2000; Reichel et al., 2012) in which no difference in novel-object preference was observed with this pair of objects. The apparatus and objects were cleaned with CaviCide (Metrex Research) between each rat session. Each session was video recorded for later analysis by experimenters blinded to group treatment. Exploration was defined as sniffing or touching the object with the nose; sitting on or leaning against the object was not counted as exploration.
Tissue Preparation
Rats were decapitated 7 days after METH treatment. Brains were hemisected and the right side rapidly removed and frozen in isopentane on dry ice and stored at -80°C. Frozen right hemispheres were sliced at 12 µm thick at the level of the dorsal hippocampus/PRh (3.5mm from bregma, Paxinos and Watson 6th edition) using a cryostat. Eight slices (4/rat) were mounted on each Superfrost Plus glass micro slides (VWR International, Radnor, PA) and stored at -80°C for subsequent use in autoradiography assays. The left hippocampus was dissected on ice, placed in cold sucrose buffer (0.32M sucrose, 3.8mM NaH2PO4, and 12.7mM Na2HPO4) and used for [3H]5-HT uptake as described below. The striatal tissues from these animals were also processed and data published separately.
[125I]RTI-55 Autoradiography
SERT density was assessed via [125I]RTI-55 binding to dorsal hippocampus and PRh slices as previously described (O’Dell et al., 2012). Briefly, slides were thawed on a slide warmer (5–10 minutes) and incubated in sucrose buffer (10mM sodium phosphate, 120mM sodium chloride, 320mM sucrose, pH 7.4) containing 21 pM 125I-RTI-55 (2200 Ci/mmol, PerkinElmer, Waltham, MA). Nonspecific binding was determined by slides incubated in sucrose buffer containing 21 pM [125I]RTI-55 and 100nM fluoxetine. Slides were rinsed twice in ice-cold buffer and distilled water for 2 minutes and air-dried. Sample slides and standard 125I microscale slides (American Radiolabeled Chemicals, St. Louis, MO) were placed on 1 cassette and exposed to same Kodak MR film (Eastman Kodak Co., Rochester, NY) for 24 hours to keep variables constant.
[125I]-Epibatidine Autoradiography
α4β2 nAChR density was assessed via [125I]-epibatidine binding to dorsal hippocampus and PRh slices as previously described (Lai et al., 2005; Huang et al., 2009). Briefly, slides were thawed on a slide warmer (5–10 minutes) and preincubated in binding buffer (50mM Tris, 120mM NaCl, 5mM KCl, 2.5mM CaCl2, 1.0mM MgCl2, pH 7.5) at room temperature for 30 minutes followed by a 40-minute incubation in binding buffer containing 0.015nM [125I]-epibatidine (2200 Ci/mmol, PerkinElmer) in the presence of 100nM αCtxMII. Nonspecific binding was determined by slides incubated in binding buffer containing 0.015nM [125I]-epibatidine plus 0.1mM NIC. Slides were rinsed twice in ice-cold buffer for 5 minutes followed by a 10-second rinse in distilled water. Slides were air-dried. Sample slides and standard 125I microscale slides (American Radiolabeled Chemicals) were placed on 1 cassette and exposed to same Kodak MR film (Eastman Kodak) for 24 hours to keep variables constant.
Synaptosomal [3H]5-HT Uptake
Hippocampal [3H]5-HT uptake was determined using rat hippocampal synaptosomes prepared as previously described (McFadden et al., 2012). Briefly, synaptosomes were prepared by homogenizing freshly dissected hippocampal tissue in ice-cold 0.32M sucrose buffer (pH 7.4) and centrifuged (800 g, 12 minutes, 4°C). The supernatants were centrifuged (22,000 g, 15 minutes, 4°C) and the resulting pellets were resuspended in ice-cold assay buffer (in mM: 126 NaCl, 4.8 KCl, 1.3 CaCl2, 16 sodium phosphate, 1.4 MgSO4, 11 glucose and 1 ascorbic acid; pH 7.4) and 1 μM pargyline. Samples were incubated for 10 minutes at 37°C and the assays initiated by the addition of [3H]5-HT (5nM final concentration). Following incubation for 3 minutes, samples were placed on ice to stop the reaction. Samples were then filtered through GF/B filters (Whatman) soaked previously in 0.05% polyethylenimine. Filters were rapidly washed 3 times with 3mL of ice-cold 0.32M sucrose buffer using a filtering manifold (Brandel). Nonspecific values were determined in the presence of 10 μM fluoxetine. Radioactivity trapped in filters was counted using a liquid scintillation counter. Protein concentrations were determined using the Bio-Rad Protein Assay (Bio-Rad Laboratories).
Statistical Analyses
The recognition index (ie, the ratio of the time rats spend exploring the novel object divided by the time rats spend exploring both objects) was used as the dependent variable for NOR. The exploration index (ie, the ratio of the time rats spent exploring the identical object in familiarization phase located on the position the novel object was 90 minutes later placed in NOR phase divided by the time rats spent exploring both identical objects) was used as the dependent variable for assessment of exploration of the 2 identical objects during familiarization phase. Two independent, blinded raters scored each behavioral test with a reliability correlation of ≥0.94 for the recognition index. For autoradiography, optical densities from 4 replicate slices per rat were quantified using ImageJ software (National Institutes of Health). Specific binding was obtained by subtracting film background from mean density values and converting to fmol/mg using the standard curve generated from 125I standards. The optical densities of the samples were within the linear range of the standards. Statistical analyses were conducted using GraphPad Prism 5.01 software (La Jolla, CA). The exploration and recognition indexes were first compared with the chance exploration value of 0.5 in each group. Values >0.5 indicate higher preference for the novel object. Mean concentrations ± SEM were analyzed using 1-way analysis of variance followed by Tukey HSD posthoc test for determination of significance among groups. Correlation analysis of recognition index and either nAChR or SERT density was achieved by using Pearson correlation coefficient. Differences among groups were considered significant if the probability of error was <5% (P<.05).
Results
NIC administration via drinking water from adolescence to adulthood (ie, PND 40–96) attenuated the NOR deficits caused by repeated high-dose METH injections when administered at PND 89 and with NOR testing performed on PND 94 (Figure 1A) (F3,34=10.01, P<.0001). Similarly, ad libitum exposure to an escalating-dose regimen of NIC attenuated METH-induced deficits in NOR when NIC was administered from PND 61 to 100 and METH at PND 93, with NOR testing on PND 98 (Figure 1B) (F3,30=3.698, P=.0224). METH per se did not induce deficits in NOR when administered at PND 54 (Figure 1C) (F3,31=1.273, P=.3009).
Results presented in Figure 2A demonstrate that NIC pre-treatment via drinking water from adolescence to adulthood (i.e., PND 40 – 89), but discontinued during and after METH, attenuated the NOR deficits caused by METH when METH was administered 2h after NIC removal at PND 89 and NOR testing performed at PND 94 (F5,38=2.342, P<.05). NIC pretreatment from PND 40 to 88, and discontinued during and after METH, also attenuated METH-induced NOR deficits when METH was given 24 hours after NIC removal on PND 89 and NOR testing performed at PND 94 (F5,38=2.342, P<.05). Furthermore, data presented in Figure 2B demonstrate that NIC posttreatment given from PND 89 to 96 initiated 2 hours after last METH injection also attenuated the NOR deficits caused by METH when assessed at PND 94 (F3,35=3.296, P=.0316).
Analysis of object exploration during the familiarization phase shown in Tables 1 and 2 revealed no differences in the animals’ preference for either of the 2 identical objects (PND 40–96, F3,34=0.12; PND 61–100, F3,30=0.09; PND 40-, F3,31=0.45; PND 40–88/89/96, F5,38=0.29; and PND 89–96, F3,35=0.19, ns; Table 1) or in their total exploration of the 2 identical objects (PND 40–96 F3,34=1.07; PND 61–100, F3,30=1.14; PND 40–61, F3,31=0.49; PND 40–88/89/96, F5,38=0.69; and PND 89–96, F3,35=0.56, ns; Table 2). Furthermore, the total exploration time of the novel plus the familiar object during NOR phase was mostly similar among groups (PND 40–96, F3,34=0.11; PND 61–100, F3,30=0.13; PND 40–61, F3,31=2.39; PND 40–88/89/96, F5,38=0.69; and PND 89–96, F3,35=2.93, ns; Table 2).
Table 1.
Exploration Index (Familiarization Phase)
| SEM | ||||||
|---|---|---|---|---|---|---|
| Experiment | Treatment Group | |||||
| SS | SM | NS | NM(1) | NM(2) | NM(3) | |
| PND 40–96 | 0.537 | 0.569 | 0.563 | 0.527 | ||
| (Figure 1A) | (0.095) | (0.061) | (0.033) | (0.031) | ||
| PND 61–100 | 0.547 | 0.518 | 0.524 | 0.513 | ||
| (Figure 1B) | (0.050) | (0.081) | (0.052) | (0.059) | ||
| PND 40–61 | 0.517 | 0.507 | 0.480 | 0.489 | ||
| (Figure 1C) | (0.041) | (0.063) | (0.052) | (0.023) | ||
| PND 40–88/89/96 | 0.540 | 0.512 | 0.522 | 0.474 | 0.532 | 0.563 |
| (Figure 2A) | (0.073) | (0.097) | (0.034) | (0.033) | (0.035) | (0.038) |
| PND 89–96 | 0.569 | 0.570 | 0.552 | 0.514 | ||
| (Figure 2B) | (0.056) | (0.082) | (0.084) | (0.032) | ||
Rats received oral NIC (N) via drinking water or tap water (S) from PND40-96, PND61-100, PND40-61, PND40-88 (NM(3)) 89 (NM(2)), or 96 (NM(1)) or from PND89-96 and either saline (S) or METH (M) injections at doses and ages delineated in Figures 1 and 2 insets and methods. Object familiarization was performed 6 d after saline or METH injections as described in methods. Data are expressed as mean values ± SEM.
Abbreviations: M = methamphetamine; N = nicotine; NM = nicotine/methamphetamine; NS = nicotine/saline; PND = postnatal day; SM = tap water/methamphetamine; SS = tap water/saline.
Table 2.
Total Exploration Time (sec)
| SEM | ||||||
|---|---|---|---|---|---|---|
| Experiment | Familiarization Phase | |||||
| SS | SM | NS | NM(1) | NM(2) | NM(3) | |
| PND 40-96 | 23.7 | 25.1 | 37.6 | 26.5 | ||
| (Figure 1A) | (2.7) | (2.6) | (4.2) | (4.0) | ||
| PND 61-100 | 30.5 | 28.2 | 40.3 | 30.9 | ||
| (Figure 1B) | (8.9) | (4.3) | (3.7) | (3.7) | ||
| PND 40-61 | 37.1 | 32.7 | 38.9 | 39.9 | ||
| (Figure 1C) | (5.7) | (3.8) | (7.6) | (4.2) | ||
| PND 40-88/89/96 | 37.7 | 26.3 | 35.1 | 31.2 | 30.1 | 29.9 |
| (Figure 2A) | (4.1) | (4.5) | (5.8) | (3.9) | (4.8) | (4.1) |
| PND 89-96 | 45.9 | 39.0 | 49.1 | 42.7 | ||
| (Figure 2B) | (5.3) | (4.3) | (8.5) | (4.4) | ||
| Experiment | NOR Phase | |||||
|---|---|---|---|---|---|---|
| SS | SM | NS | NM(1) | NM(2) | NM(3) | |
| PND 40-96 | 29.0 | 26.0 | 35.7 | 23.2 | ||
| (Figure 1A) | (5.7) | (3.1) | (5.8) | (4.1) | ||
| PND 61-100 | 17.8 | 23.5 | 33.0 | 21.3 | ||
| (Figure 1B) | (4.5) | (5.0) | (6.0) | (4.8) | ||
| PND 40-61 | 36.4 | 34.8 | 40.6 | 24.7 | ||
| (Figure 1C) | (4.6) | (5.6) | (5.3) | (2.2) | ||
| PND 40-88/89/96 | 23.5 | 23.6 | 27.0 | 20.5 | 16.9 | 27.6 |
| (Figure 2A) | (3.6) | (4.8) | (4.4) | (7.5) | (2.8) | (6.1) |
| PND 89-96 | 29.2 | 33.5 | 34.6 | 18.3* | ||
| (Figure 2B) | (4.8) | (5.0) | (6.1) | (1.5) | ||
Rats received oral NIC (N) via drinking water or tap water (S) from PND40-96, PND61-100, PND40-61, PND40-88 (NM(3)) 89 (NM(2)), or 96 (NM(1)) or from PND89-96 and either saline (S) or METH (M) injections at doses and ages delineated in Figures 1 and 2 insets and Methods. Object familiarization (familiarization phase) and recognition (NOR phase) were performed 6 d after saline or METH injections as described in methods. Data are expressed as mean values ± SEM.
Abbreviations: M = methamphetamine; N = nicotine; NM = nicotine/methamphetamine; NOR = novel object recognition; NS = nicotine/saline; PND = postnatal day; SM = tap water/methamphetamine; SS = tap water/saline.
Using the same tissues described in Figure 1A, data presented in Figure 3 indicate that NIC administration from PND 40 to 96 does not protect against the persistent (ie, 7 days) METH-induced deficits in SERT density as assessed by [125I]RTI-55 binding to hippocampal CA1 (Figure 3A, F3,34=59.53), CA3 (Figure 3B, F3,34=35.51), DG (Figure 3C, F3,34=61.90), and PRh (Figure 3D, F3,33=56.32) slices. Similarly, using tissues from the same animals described in Figures 1 and 2, data presented in Table 3 indicate that oral NIC administration does not protect against the persistent (ie, 7 days) METH-induced deficits in SERT function as assessed by [3H]5-HT uptake from hippocampal synaptosomes when NIC was given from PND 40 to 96 (F3,32=87.68), PND 61 to 100 (F3,35=15.08), PND 40 to 88/89/96 (F5,42=14.43), or PND 89 to 96 (F3,34=40.04) and METH administered to adult rats (≥PND 89). When METH was administered to young adults at PND 54, NIC treatment from PND 40 to 61 attenuated the persistent (ie, 7 days) METH-induced SERT function deficits (F3,31=20.82) (Table 3).
Figure 3.
Nicotine (NIC) neuroprotection of methamphetamine (METH)-induced memory deficits is not mediated by attenuation of serotonergic deficits. Rats were treated as described in Figure 1. (A) Brains were harvested 7 days after METH or saline injections and serotonin transporter (SERT) binding to hippocampal CA1 region (A), hippocampal CA3 region (B), and hippocampal dentate gyrus (DG) region (C), and (D) perirhinal cortex (PRh) was assessed via [125I]RTI55 autoradiography. Data are expressed as mean values ± SEM of N=8 to 12 determinations. *Represent values that are statistically different from saline controls (P<.05). NM,NIC water/METH injections; NS,NIC water/saline injections; SM,tap water/METH injections; SS,tap water/saline injections.
Table 3.
[3H]5-HT Uptake from Hippocampal Synaptosomes (fmol/mg)
| (SEM) | ||||||
|---|---|---|---|---|---|---|
| Experiment | Treatment Group | |||||
| SS | SM | NS | NM(1) | NM(2) | NM(3) | |
| PND 40-96 | 1.467 | 0.296* | 1.393 | 0.308* | ||
| (Figure 1A) | (0.061) | (0.061) | (0.101) | (0.055) | ||
| PND 61-100 | 0.547 | 0.251* | 0.611 | 0.291* | ||
| (Figure 1B) | (0.045) | (0.048) | (0.050) | (0.042) | ||
| PND 40-61 | 1.048 | 0.286* | 1.293 | 0.611*# | ||
| (Figure 1C) | (0.097) | (0.057) | (0.145) | (0.079) | ||
| PND 40-88/89/96 | 0.611 | 0.160* | 0.599 | 0.286* | 0.313* | 0.336* |
| (Figure 2A) | (0.026) | (0.024) | (0.041) | (0.063) | (0.065) | (0.047) |
| PND 89-96 | 1.455 | 0.542* | 1.528 | 0.653* | ||
| (Figure 2B) | (0.058) | (0.070) | (0.094) | (0.095) | ||
Rats received oral NIC (N) via drinking water or tap water (S) from PND40-96, PND61-100, PND40-61, PND40-88 (NM(3)) 89 (NM(2)), or 96 (NM(1)) or from PND89-96 and either saline (S) or METH (M) injections at doses and ages delineated in Figures 1 and 2 insets and Methods. Hippocampal tissues were harvested 7 d after last METH or saline injection. [3H]5-HT uptake was performed as described in Methods. Data are expressed as mean values ± SEM. *Values significant different from METH-naïve controls (P<.05). #Values significant different from SM.
Abbreviations: M = methamphetamine; N = nicotine; NM = nicotine/methamphetamine; NS = nicotine/saline; PND = postnatal day; SM = tap water/methamphetamine; SS = tap water/saline.
Again, using the same tissues described in Figure 1A, data presented in Figure 4 reveal that binge METH administration per se causes long-lasting (ie, 7 days) reduction in α4β2 nAChR density as assessed by [125I]-epibatidine binding to CA1 (Figure 4A) but has no effect on [125I]-epibatidine binding in CA3 (Figure 4B), DG (Figure 4C), or PRh (Figure 4D). NIC increased [125I]-epibatidine binding to CA1 (F3,35=97.71, P<.0001), CA3 (F3,35=80.58, P<.0001), DG (F3,35=18.57, P<.0001), and PRh (F3,34=38.46, P<.0001) in both METH- and saline-treated rats (Figure 4).
Figure 4.
Long-term nicotine (NIC) administration increases α4β2 nicotinic acetylcholine receptor (nAChRs) density in methamphetamine (METH)-treated rats. Rats were treated as described in Figure 1 panel A. Brains were harvested 7 days after METH or saline injections and α4β2 density to hippocampal CA1 region (A), hippocampal CA3 region (B), and hippocampal dentate gyrus (DG) region (C), and (D) perirhinal cortex (PRh) was assessed via [125I]-epibatidine autoradiography. Data are expressed as mean values ± SEM of N=8 to 12 determinations. *Represent values that are statistically different from SS (P<.05). #Represent values that are statistically different from SM (P<.05). NM,NIC water/METH injections; NS,NIC water/saline injections; SM,tap water/METH injections; SS,tap water/saline injections.
Correlation analysis of data presented in Figures 3 and 4 was performed to evaluate possible association of NOR and SERT density (right y-axis) or NOR and α4β2 nAChR density (left y-axis). These data are presented in Figure 5 and demonstrate that NOR does not correlate with SERT density in the CA1 (r(10)=0.04, P=.556, Figure 5A), CA3 (r(10)=0.02, P=.648, Figure 5B), DG (r(10)=0.05, P=.507, Figure 5C), or PRh (r(10)=0.03, P=.590, Figure 5D) in rats treated with oral NIC from PND 40 to 96 and METH administrations at PND 89. Conversely, NOR and α4β2 nAChR density were positively correlated in the CA1 (r(10)=0.66, P=.002), CA3 (r(10)=0.59, P=.006), DG (r(10)=0.63, P=.006), and PRh (r(10)=0.55, P=.009) in these same animals. No correlation between NOR and SERT density or NOR and α4β2 nAChR was found in METH-naïve rats or in NIC-naïve METH-treated rats (data not shown).
Figure 5.
Performance in novel object recognition (NOR) test correlates with α4β2 nicotinic acetylcholine receptor (nAChRs) density, but not with serotonin transporter (SERT) density, in rats treated with nicotine (NIC) and methamphetamine (METH) as described in Figure 1A. Data from NM group presented in Figures 3 and 4 were used for this correlation analysis.
Representative autoradiograms of [125I]RTI-55 and [125I]-epibatidine binding are presented in Figure 6.
Figure 6.
Representative autoradiographs depicting the effects of nicotine (NIC) and methamphetamine (METH) treatments on hippocampus and perirhinal cortex (PRh). (A) Serotonin transporter (SERT) ([125I]RTI55 binding). (B) α4β2 nAChR ([125I]-epibatidine binding). NM,NIC water/METH injections; NS,NIC water/saline injections; SM,tap water/METH injections; SS,tap water/saline injections.
Discussion
To date, extensive literature has demonstrated that NIC administration improves memory function in patients with schizophrenia or mild cognitive impairment (Jubelt et al., 2008; Newhouse et al., 2012), or attenuates memory deficits in laboratory animals induced by sleep deprivation, chronic stress, beta-amyloid infusion, cholinergic lesion, or METH administrations (Yamazaki et al., 2002; Aleisa et al., 2011; Alkadhi, 2011; Mizoguchi et al., 2011; Kruk-Slomka et al., 2014). However, despite evidence indicating that METH abuse is associated with memory impairment (Kalechstein et al., 2003, 2009; Scott et al., 2007; Casaletto et al., 2014), and that many METH addicts are exposed to NIC via cigarette smoking (McCann et al., 2008), few studies have investigated the effects of NIC on the METH-associated memory deficits (Mizoguchi et al., 2011). The present study reveals that long-term oral NIC treatment beginning during either adolescence or adulthood attenuates METH-induced NOR deficits, suggesting that NIC affords cognitive protection. This protective effect of NIC persisted even when NIC was removed 2 or 24 hours prior to METH administrations. Furthermore, oral NIC treatment also attenuated NOR deficits when administered after METH treatment. It is unlikely that the NIC effects on NOR are mediated by serotonergic neurons terminal deficits in the hippocampus and/or PRh, because NIC did not attenuate METH-induced serotonergic deficits. In contrast, NIC increased the density of α4β2 nAChRs in CA1, CA3, DG, and PRh in both saline- and METH-treated rats.
Many studies have demonstrated that NIC prevents memory deficits when administered before a lesion (Yamazaki et al., 2002; Aleisa et al., 2011; Alkadhi, 2011; Srivareerat et al., 2011) or improved memory when administered after a lesion (Jubelt et al., 2008; Mizoguchi et al., 2011; Newhouse et al., 2012). Similarly, the current data indicate that both pre- and/or posttreatment with NIC attenuates METH-induced NOR deficits. These data suggest that the protective effect of NIC achieved via pretreatment might occur by neuroadaptations caused by NIC in a nondamaged system that can ultimately mitigate injury-induced memory loss. This hypothesis is based on findings that chronic NIC administration to healthy individuals (Perry et al., 1999) or non-lesioned rodents (Melichercik et al., 2012; Kruk-Slomka et al., 2014) increases nAChRs binding, leading to increases in long-term potentiation (LTP), a widely accepted process of memory formation (Fujii et al., 1999; Fujii et al., 2000; Welsby et al., 2006, 2009), and improves object recognition memory in normal rats (Melichercik et al., 2012; Kruk-Slomka et al., 2014). Additionally, these positive effects of NIC on memory seem to last several days after NIC removal (Levin and Torry, 1996), perhaps due to long-lasting increases in LTP (Yamazaki et al., 2006; Huang et al., 2008). In previous studies, in vivo NIC pretreatment prevented LTP deficits in area CA1 of the hippocampus in parallel with attenuation of memory deficits induced by cholinergic lesion, chronic stress, or beta-amyloid infusion (Yamazaki et al., 2002; Alkadhi, 2011; Srivareerat et al., 2011).
The protective effect of NIC achieved via posttreatment might occur via effects of NIC on brain derived neurotrophic factor as well as LTP, which ameliorate memory deficits in a damaged system (Yamazaki et al., 2002; Srivareerat et al., 2011). In support of this hypothesis, in laboratory animals, NIC reversed memory deficits induced by cholinergic lesion by augmenting NMDA receptors function and LTP in the CA1 region (Yamazaki et al., 2002). Some clinical studies have shown that NIC treatment improves recognition memory and attention in patients with schizophrenia or mild cognitive impairment, which demonstrates that NIC can ameliorate memory deficits in damaged systems (Levin et al., 1996; Jubelt et al., 2008; Newhouse et al., 2012).
To explore a possible mechanism underlying NIC-induced cognitive neuroprotection, we examined α4β2 nAChRs density in hippocampal and PRh slices. Previous studies have shown that the increases in hippocampal LTP by NIC administration are mediated by α4β2 nAChRs (Fujii et al., 2000; Jia et al., 2010; Nakauchi and Sumikawa, 2012). In fact, patients with dementia display significant reductions in α4β2 nAChRs binding in neocortical and hippocampal regions, and these seem to correlate with progressive cognitive declines (Perry et al., 2000; Colloby et al., 2010). Particularly, in patients with Alzheimer’s disease, there is a loss of α4β2 nAChRs binding, but not of α3 or α7 nAChR subtypes (Perry et al., 2000). The present findings revealed that chronic oral NIC administration abolishes METH-induced deficits in α4β2 nAChRs binding to the CA1 region of the hippocampus and augments α4β2 nAChRs binding to CA3, DG, and PRh. Furthermore, α4β2 nAChR density correlated with NOR performance in animals treated with NIC and METH. In other words, rat performance on the NOR test was directly proportional to α4β2 nAChR density. This specific subtype of nAChR was selected for study, because despite evidence that synaptic plasticity can also be mediated by α7 nAChRs (Halff et al., 2014), studies have demonstrated that α4β2 nAChRs mediate excitatory postsynaptic potentials in the CA1 hippocampus (Bliss and Collingridge, 1993; Bell et al., 2011; Nakauchi and Sumikawa, 2012). Furthermore, in vitro studies by Swant et al. (2010) demonstrated that METH reduces LTP in the CA1 hippocampus, suggesting that, in combination with the present data, METH reduces α4β2 nAChRs, whereby LTP is reduced in the CA1 region. The mechanism by which METH causes deficits in α4β2 nAChRs is unknown, but evidence indicates that METH damages hippocampal and cortical neuron integrity, including serotonergic neurons (McFadden et al., 2012; Reichel et al., 2012), where α4β2 nAChRs are expressed (Seth et al., 2002; Cucchiaro and Commons, 2003), which might lead to reductions in nAChRs density. However, in current studies, METH also reduced SERT binding in the CA3, DG, and PRh without affecting α4β2 binding in these 3 regions. To our knowledge, the differential distribution of α4β2 nAChRs in serotonergic terminals in the CA1, CA3, DG, or PRh has not been demonstrated. Thus, further research is necessary to confirm the speculative mechanism proposed herein. Overall, these data suggest that NIC protection to METH-induced NOR deficits might be mediated by upregulation of α4β2 nAChRs.
The effects of NIC abstinence on METH-induced NOR deficits were also evaluated in order to investigate how long NIC neuroprotection persists. As demonstrated in the NIC/saline group in Figure 2A, a 5-day abstinence from NIC had no effect on NOR as previously reported (Kenney et al., 2011). Nevertheless, NIC protection against METH-induced NOR deficits remained even 5 to 6 days after the cessation of NIC exposure (eg, NM(2) and NM(3), Figure 2A), indicating that the protective effect of NIC lasts for at least 6 days of NIC abstinence. Others have demonstrated that NIC-induced augmentation in α4β2 nAChRs in the hippocampus remains after 6 days of NIC removal (Gould et al., 2012), suggesting that the potential neuroprotective mechanism of NIC parallels NOR protection. Furthermore, previous studies revealed that chronic NIC pretreatment improved working memory in rats even after 2 weeks of NIC abstinence (Levin and Torry, 1996). In the amygdala, 7 days of oral NIC to mice facilitated LTP induced by high-frequency stimulation, and this facilitation of LTP lasted for at least 72 hours after NIC was stopped (Huang et al., 2008). Similarly, increased NMDA receptors function induced by 10-day NIC in rats lasted for 8 days after NIC removal (Yamazaki et al., 2006). Others have suggested that the longer the NIC exposure, the longer synaptic facilitation lasts (Huang et al., 2008).
Another principal finding of current experiments is that long-term NIC treatment does not attenuate METH-induced serotonergic deficits in the hippocampus and PRh despite affording protection against memory deficits. Previous studies have indicated that METH administrations to rats cause deficits in SERT density in the hippocampus and PRh as well as deficits in the NOR test, suggesting a possible relationship between hippocampal/PRh SERT neurons and NOR (Belcher et al., 2005, 2008; Reichel et al., 2012). However, the present data suggest that such a relationship is unlikely as indicated by data presented in Figure 1C and Table 3 in which METH administration caused significant whole hippocampal SERT deficits, but not NOR deficits, when given to PND 54 rats. Of note, NOR is strongly mediated by PRh functions, and thus it is possible that these animals had no SERT deficits in the PRh, which could explain the lack of NOR deficits. Second, several NIC treatment paradigms attenuated METH-induced NOR deficits independently of attenuation of SERT density deficits. Lastly, our correlation analysis demonstrates that performance in the NOR test is not correlated with SERT density in hippocampal or PRh regions. Thus, current data suggest that NIC neuroprotection to METH-induced memory deficits is not mediated by protection of serotonergic neurons in the hippocampus or PRh cortex.
Notably, the current data also revealed that NIC administration beginning in adolescence attenuates METH-induced hippocampal SERT function deficits when METH was administered to young rats (ie, PND 54), but without a significant effect in older rats (≥PND 89) (Table 3). A possible mechanism by which NIC attenuates METH-induced deficits in SERT function in young, but not older, rats might involve nAChRs. The α4β2 and α7 nAChRs play a role in 5-HT release in the hippocampus, as demonstrated by findings that they are expressed either in the nucleus raphe or serotonergic terminals in the hippocampus (Cucchiaro and Commons, 2003; Aznar et al., 2005). Furthermore, age differences in nAChRs density in the hippocampus and cortex have been reported (Doura et al., 2008). Specifically, these studies found that adolescent rats have a higher density of nAChRs than adult rats. Thus, NIC binding to nAChRs potentially may lead to 5-HT release in the hippocampus/cortex that might differ between adolescents and adults. These age-specific differences in nAChRs may also play a role in NOR, thereby reducing the cognitive deficits caused by METH in adolescent but not adult rodents.
Clinical studies with adolescent METH abusers have demonstrated that executive function is only mildly compromised and verbal memory is not affected, despite several domains of cognitive function being impaired (psychomotor speed, fine motor speed, verbal intelligence, and spatial organization) (King et al., 2010). In line with these findings, previous preclinical studies with adolescent mice showed that 7 or 14 days of noncontingent METH administrations did not impact NOR and synaptic plasticity (North et al., 2012). Of note, METH administrations to PND 51 to 60 do not induce long-term deficits in spatial memory in the Morris water maze (Vorhees et al., 2005). These previous findings are in agreement with current data shown in Figure 1C in which METH administration per se given to PND 54 rats does not impact NOR.
In summary, NIC has cognitive protection and cognitive enhancing properties, and several mechanisms underlying this phenomenon have been suggested, including increases in LTP and brain derived neurotrophic factor levels and reduction in oxidative stress (Soto-Otero et al., 2002; Srivareerat et al., 2011). METH abuse is associated with significant cognitive impairment and, despite the fact that many METH abusers smoke cigarettes and are thus exposed to NIC (McCann et al., 2008), few studies have evaluated the effects of NIC on METH-induced cognitive deficits (Mizoguchi et al., 2011). The findings of the present studies demonstrate that NIC pre-treatment as well as posttreatment attenuate METH-induced NOR deficits in rats. Furthermore, NIC did not attenuate the serotonergic deficits caused by METH but augmented α4β2 nAChRs density in CA1, CA3, and DG hippocampal regions as well as in PRh.
Statement of Interest
METH hydrochloride was provided by the National Institute on Drug Abuse (Research Triangle Institute, Research Triangle Park, NC).
Acknowledgments
The authors thank Dr. Maryka Quik, Dr. Tanuja Bordia, and Dr. Kristen Keefe for their extensive assistance with the autoradiography technique. This work was supported by the National Institutes of Health (grants DA031883, DA11389, DA13367, DA019447, DA036012, GM103801, GM48677); the HHMI Med into Grad Initiative funded by the Howard Hughes Medical Institute (grant 560067777); the American Foundation for Pharmaceutical Education; and the University of Utah Graduate Research Fellowship.
References
- Aleisa AM, Helal G, Alhaider IA, Alzoubi KH, Srivareerat M, Tran TT, Al-Rejaie SS, Alkadhi KA. (2011) Acute nicotine treatment prevents REM sleep deprivation-induced learning and memory impairment in rat. Hippocampus 21:899–909. [DOI] [PubMed] [Google Scholar]
- Alkadhi KA. (2011) Chronic stress and Alzheimer’s disease-like pathogenesis in a rat model: prevention by nicotine. Curr Neuropharmacol 9:587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aznar S, Kostova V, Christiansen SH, Knudsen GM. (2005) Alpha 7 nicotinic receptor subunit is present on serotonin neurons projecting to hippocampus and septum. Synapse 55:196–200. [DOI] [PubMed] [Google Scholar]
- Belcher AM, O’Dell SJ, Marshall JF. (2005) Impaired object recognition memory following methamphetamine, but not p-chloroamphetamine- or d-amphetamine-induced neurotoxicity. Neuropsychopharmacology 30:2026–2034. [DOI] [PubMed] [Google Scholar]
- Belcher AM, Feinstein EM, O’Dell SJ, Marshall JF. (2008) Methamphetamine influences on recognition memory: comparison of escalating and single-day dosing regimens. Neuropsychopharmacology 33:1453–1463. [DOI] [PubMed] [Google Scholar]
- Bell KA, Shim H, Chen CK, McQuiston AR. (2011) Nicotinic excitatory postsynaptic potentials in hippocampal CA1 interneurons are predominantly mediated by nicotinic receptors that contain alpha4 and beta2 subunits. Neuropharmacology 61:1379–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL. (1994) Biomarkers of cigarette smoking. In: Smoking and tobacco control monograph 7: The FTC cigarette test method for determining tar, nicotine and carbon monoxide yields of US cigarettes pp 93–111. Bethesda, MD: National Institutes of Health. [Google Scholar]
- Besheer J, Bevins RA. (2000) The role of environmental familiarization in novel-object preference. Behav Processes 50:19–29. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361:31–39. [DOI] [PubMed] [Google Scholar]
- Bordia T, Campos C, Huang L, Quik M. (2008) Continuous and intermittent nicotine treatment reduces L-3,4-dihydroxyphenylalanine (L-DOPA)-induced dyskinesias in a rat model of Parkinson’s disease. J Pharmacol Exp Ther 327:239–247. [DOI] [PubMed] [Google Scholar]
- Casaletto KB, Obermeit L, Morgan EE, Weber E, Franklin DR, Grant I, Woods SP. (2014) Depression and executive dysfunction contribute to a metamemory deficit among individuals with methamphetamine use disorders. Addict Behav 40c:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CP, Alder JT, Bowen DM, Esiri MM, McDonald B, Hope T, Jobst KA, Francis PT. (1996) Presynaptic serotonergic markers in community-acquired cases of Alzheimer’s disease: correlations with depression and neuroleptic medication. J Neurochem 66:1592–1598. [DOI] [PubMed] [Google Scholar]
- Cherner M, Suarez P, Casey C, Deiss R, Letendre S, Marcotte T, Vaida F, Atkinson JH, Grant I, Heaton RK. (2010) Methamphetamine use parameters do not predict neuropsychological impairment in currently abstinent dependent adults. Drug Alcohol Depend 106:154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colloby SJ, Perry EK, Pakrasi S, Pimlott SL, Wyper DJ, McKeith IG, Williams ED, O’Brien JT. (2010) Nicotinic 123I-5IA-85380 single photon emission computed tomography as a predictor of cognitive progression in Alzheimer’s disease and dementia with Lewy bodies. Am J Geriatr Psychiatry 18:86–90. [DOI] [PubMed] [Google Scholar]
- Cucchiaro G, Commons KG. (2003) Alpha 4 nicotinic acetylcholine receptor subunit links cholinergic to brainstem monoaminergic neurotransmission. Synapse 49:195–205. [DOI] [PubMed] [Google Scholar]
- Doura MB, Gold AB, Keller AB, Perry DC. (2008) Adult and periadolescent rats differ in expression of nicotinic cholinergic receptor subtypes and in the response of these subtypes to chronic nicotine exposure. Brain Res 1215:40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S, Ji Z, Morita N, Sumikawa K. (1999) Acute and chronic nicotine exposure differentially facilitate the induction of LTP. Brain Res 846:137–143. [DOI] [PubMed] [Google Scholar]
- Fujii S, Ji Z, Sumikawa K. (2000) Inactivation of alpha7 ACh receptors and activation of non-alpha7 ACh receptors both contribute to long term potentiation induction in the hippocampal CA1 region. Neurosci Lett 286:134–138. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Rippeth JD, Carey CL, Heaton RK, Moore DJ, Schweinsburg BC, Cherner M, Grant I. (2004) Neurocognitive performance of methamphetamine users discordant for history of marijuana exposure. Drug Alcohol Depend 76:181–190. [DOI] [PubMed] [Google Scholar]
- Gould RW, Garg PK, Garg S, Nader MA. (2013) Effects of nicotinic acetylcholine receptor agonists on cognition in rhesus monkeys with a chronic cocaine self-administration history. Neuropharmacology 64:479–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ, Portugal GS, Andre JM, Tadman MP, Marks MJ, Kenney JW, Yildirim E, Adoff M. (2012) The duration of nicotine withdrawal-associated deficits in contextual fear conditioning parallels changes in hippocampal high affinity nicotinic acetylcholine receptor upregulation. Neuropharmacology 62:2118–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halff AW, Gomez-Varela D, John D, Berg DK. (2014) A novel mechanism for nicotinic potentiation of glutamatergic synapses. J Neurosci 34:2051–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring NR, Schaefer TL, Gudelsky GA, Vorhees CV, Williams MT. (2008) Effect of +-methamphetamine on path integration learning, novel object recognition, and neurotoxicity in rats. Psychopharmacology (Berl) 199:637–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman WF, Moore M, Templin R, McFarland B, Hitzemann RJ, Mitchell SH. (2006) Neuropsychological function and delay discounting in methamphetamine-dependent individuals. Psychopharmacology (Berl) 188:162–170. [DOI] [PubMed] [Google Scholar]
- Huang LZ, Parameswaran N, Bordia T, Michael McIntosh J, Quik M. (2009) Nicotine is neuroprotective when administered before but not after nigrostriatal damage in rats and monkeys. J Neurochem 109:826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Kandel ER, Levine A. (2008) Chronic nicotine exposure induces a long-lasting and pathway-specific facilitation of LTP in the amygdala. Learn Mem 15:603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Yamazaki Y, Nakauchi S, Ito K, Sumikawa K. (2010) Nicotine facilitates long-term potentiation induction in oriens-lacunosum moleculare cells via Ca2+ entry through non-alpha7 nicotinic acetylcholine receptors. Eur J Neurosci 31:463–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE. (2014) Monitoring the future results on adolescent drug use: overview of key findings, 2013. Ann Arbor: Institute for Social Research, The University of Michigan. [Google Scholar]
- Jubelt LE, Barr RS, Goff DC, Logvinenko T, Weiss AP, Evins AE. (2008) Effects of transdermal nicotine on episodic memory in non-smokers with and without schizophrenia. Psychopharmacology (Berl) 199:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalechstein AD, Newton TF, Green M. (2003) Methamphetamine dependence is associated with neurocognitive impairment in the initial phases of abstinence. J Neuropsychiatry Clin Neurosci 15:215–220. [DOI] [PubMed] [Google Scholar]
- Kalechstein AD, De la Garza R, 2nd, Newton TF, Green MF, Cook IA, Leuchter AF. (2009) Quantitative EEG abnormalities are associated with memory impairment in recently abstinent methamphetamine-dependent individuals. J Neuropsychiatry Clin Neurosci 21:254–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalechstein AD, Mahoney JJ, 3rd, Verrico CD, De La Garza R., 2nd (2014) Short-term, low-dose varenicline administration enhances information processing speed in methamphetamine-dependent users. Neuropharmacology 85:493–498. [DOI] [PubMed] [Google Scholar]
- Kenney JW, Adoff MD, Wilkinson DS, Gould TJ. (2011) The effects of acute, chronic, and withdrawal from chronic nicotine on novel and spatial object recognition in male C57BL/6J mice. Psychopharmacology (Berl) 217:353–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G, Alicata D, Cloak C, Chang L. (2010) Neuropsychological deficits in adolescent methamphetamine abusers. Psychopharmacology (Berl) 212:243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnavane L, Albasser MM, Aggleton JP. (2014) Advances in the behavioural testing and network imaging of rodent recognition memory. Behav Brain Res. 10.1016/j.bbr.2014.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruk-Slomka M, Michalak A, Budzynska B, Biala G. (2014) A comparison of mecamylamine and bupropion effects on memory-related responses induced by nicotine and scopolamine in the novel object recognition test in mice. Pharmacol Rep 66:638–646. [DOI] [PubMed] [Google Scholar]
- Lai A, Parameswaran N, Khwaja M, Whiteaker P, Lindstrom JM, Fan H, McIntosh JM, Grady SR, Quik M. (2005) Long-term nicotine treatment decreases striatal alpha 6* nicotinic acetylcholine receptor sites and function in mice. Mol Pharmacol 67:1639–1647. [DOI] [PubMed] [Google Scholar]
- Lawrence JA, Olverman HJ, Shirakawa K, Kelly JS, Butcher SP. (1993) Binding of 5-HT1A receptor and 5-HT transporter ligands in rat cortex and hippocampus following cholinergic and serotonergic lesions. Brain Res 612:326–329. [DOI] [PubMed] [Google Scholar]
- Levin ED, Torry D. (1996) Acute and chronic nicotine effects on working memory in aged rats. Psychopharmacology (Berl) 123:88–97. [DOI] [PubMed] [Google Scholar]
- Levin ED, Wilson W, Rose JE, McEvoy J. (1996) Nicotine-haloperidol interactions and cognitive performance in schizophrenics. Neuropsychopharmacology 15:429–436. [DOI] [PubMed] [Google Scholar]
- Matta SG, et al. (2007) Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl) 190:269–319. [DOI] [PubMed] [Google Scholar]
- McCabe RT, Gibb JW, Wamsley JK, Hanson GR. (1987) Autoradiographic analysis of muscarinic cholinergic and serotonergic receptor alterations following methamphetamine treatment. Brain Res Bull 19:551–557. [DOI] [PubMed] [Google Scholar]
- McCann UD, Kuwabara H, Kumar A, Palermo M, Abbey R, Brasic J, Ye W, Alexander M, Dannals RF, Wong DF, Ricaurte GA. (2008) Persistent cognitive and dopamine transporter deficits in abstinent methamphetamine users. Synapse 62:91–100. [DOI] [PubMed] [Google Scholar]
- McFadden LM, Hunt MM, Vieira-Brock PL, Muehle J, Nielsen SM, Allen SC, Hanson GR, Fleckenstein AE. (2012) Prior methamphetamine self-administration attenuates serotonergic deficits induced by subsequent high-dose methamphetamine administrations. Drug Alcohol Depend. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melichercik AM, Elliott KS, Bianchi C, Ernst SM, Winters BD. (2012) Nicotinic receptor activation in perirhinal cortex and hippocampus enhances object memory in rats. Neuropharmacology 62:2096–2105. [DOI] [PubMed] [Google Scholar]
- Meneses A, Perez-Garcia G, Ponce-Lopez T, Tellez R, Castillo C. (2011) Serotonin transporter and memory. Neuropharmacology 61:355–363. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H, Ibi D, Takase F, Nagai T, Kamei H, Toth E, Sato J, Takuma K, Yamada K. (2011) Nicotine ameliorates impairment of working memory in methamphetamine-treated rats. Behav Brain Res 220:159–163. [DOI] [PubMed] [Google Scholar]
- Nakauchi S, Sumikawa K. (2012) Endogenously released ACh and exogenous nicotine differentially facilitate long-term potentiation induction in the hippocampal CA1 region of mice. Eur J Neurosci 35:1381–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhouse P, Kellar K, Aisen P, White H, Wesnes K, Coderre E, Pfaff A, Wilkins H, Howard D, Levin ED. (2012) Nicotine treatment of mild cognitive impairment: a 6-month double-blind pilot clinical trial. Neurology 78:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North A, Swant J, Salvatore MF, Gamble-George J, Prins P, Butler B, Mittal MK, Heltsley R, Clark JT, Khoshbouei H. (2012) Chronic methamphetamine exposure produces a delayed, long-lasting memory deficit. Synapse. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell SJ, Galvez BA, Ball AJ, Marshall JF. (2012) Running wheel exercise ameliorates methamphetamine-induced damage to dopamine and serotonin terminals. Synapse 66:71–80. [DOI] [PubMed] [Google Scholar]
- Olivier JD, Jans LA, Korte-Bouws GA, Korte SM, Deen PM, Cools AR, Ellenbroek BA, Blokland A. (2008) Acute tryptophan depletion dose dependently impairs object memory in serotonin transporter knockout rats. Psychopharmacology (Berl) 200:243–254. [DOI] [PubMed] [Google Scholar]
- Ouchi Y, Yoshikawa E, Futatsubashi M, Yagi S, Ueki T, Nakamura K. (2009) Altered brain serotonin transporter and associated glucose metabolism in Alzheimer disease. J Nucl Med 50:1260–1266. [DOI] [PubMed] [Google Scholar]
- Perry DC, Davila-Garcia MI, Stockmeier CA, Kellar KJ. (1999) Increased nicotinic receptors in brains from smokers: membrane binding and autoradiography studies. J Pharmacol Exp Ther 289:1545–1552. [PubMed] [Google Scholar]
- Perry E, Martin-Ruiz C, Lee M, Griffiths M, Johnson M, Piggott M, Haroutunian V, Buxbaum JD, Nasland J, Davis K, Gotti C, Clementi F, Tzartos S, Cohen O, Soreq H, Jaros E, Perry R, Ballard C, McKeith I, Court J. (2000) Nicotinic receptor subtypes in human brain ageing, Alzheimer and Lewy body diseases. Eur J Pharmacol 393:215–222. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Schwendt M, McGinty JF, Olive MF, See RE. (2011) Loss of object recognition memory produced by extended access to methamphetamine self-administration is reversed by positive allosteric modulation of metabotropic glutamate receptor 5. Neuropsychopharmacology 36:782–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Ramsey LA, Schwendt M, McGinty JF, See RE. (2012) Methamphetamine-induced changes in the object recognition memory circuit. Neuropharmacology 62:1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I. (2007) Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev 17:275–297. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Ouchi Y, Takei N, Yoshikawa E, Nakamura K, Futatsubashi M, Okada H, Minabe Y, Suzuki K, Iwata Y, Tsuchiya KJ, Tsukada H, Iyo M, Mori N. (2006) Brain serotonin transporter density and aggression in abstinent methamphetamine abusers. Arch Gen Psychiatry 63:90–100. [DOI] [PubMed] [Google Scholar]
- Seth P, Cheeta S, Tucci S, File SE. (2002) Nicotinic--serotonergic interactions in brain and behaviour. Pharmacol Biochem Behav 71:795–805. [DOI] [PubMed] [Google Scholar]
- Simon SL, Domier C, Carnell J, Brethen P, Rawson R, Ling W. (2000) Cognitive impairment in individuals currently using methamphetamine. Am J Addict 9:222–231. [DOI] [PubMed] [Google Scholar]
- Simon SL, Dacey J, Glynn S, Rawson R, Ling W. (2004) The effect of relapse on cognition in abstinent methamphetamine abusers. J Subst Abuse Treat 27:59–66. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, DeVito EE, Waters AJ, Carroll KM. (2013) Cognitive enhancement as a treatment for drug addictions. Neuropharmacology 64:452–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Otero R, Mendez-Alvarez E, Hermida-Ameijeiras A, Lopez-Real AM, Labandeira-Garcia JL. (2002) Effects of (-)-nicotine and (-)-cotinine on 6-hydroxydopamine-induced oxidative stress and neurotoxicity: relevance for Parkinson’s disease. Biochem Pharmacol 64:125–135. [DOI] [PubMed] [Google Scholar]
- Spear LP. (2000) The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 24:417–463. [DOI] [PubMed] [Google Scholar]
- Srivareerat M, Tran TT, Salim S, Aleisa AM, Alkadhi KA. (2011) Chronic nicotine restores normal Abeta levels and prevents short-term memory and E-LTP impairment in Abeta rat model of Alzheimer’s disease. Neurobiol Aging 32:834–844. [DOI] [PubMed] [Google Scholar]
- Swant J, Chirwa S, Stanwood G, Khoshbouei H. (2010) Methamphetamine reduces LTP and increases baseline synaptic transmission in the CA1 region of mouse hippocampus. PLoS One 5:e11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellez R, Rocha L, Castillo C, Meneses A. (2010) Autoradiographic study of serotonin transporter during memory formation. Behav Brain Res 212:12–26. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W, London ED. (2004) Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci 24:6028–6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirelli E, Laviola G, Adriani W. (2003) Ontogenesis of behavioral sensitization and conditioned place preference induced by psychostimulants in laboratory rodents. Neurosci Biobehav Rev 27:163–178. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Reed TM, Morford LL, Fukumura M, Wood SL, Brown CA, Skelton MR, McCrea AE, Rock SL, Williams MT. (2005) Periadolescent rats (P41-50) exhibit increased susceptibility to D-methamphetamine-induced long-term spatial and sequential learning deficits compared to juvenile (P21-30 or P31-40) or adult rats (P51-60). Neurotoxicol Teratol 27:117–134. [DOI] [PubMed] [Google Scholar]
- Welsby PJ, Rowan MJ, Anwyl R. (2006) Nicotinic receptor-mediated enhancement of long-term potentiation involves activation of metabotropic glutamate receptors and ryanodine-sensitive calcium stores in the dentate gyrus. Eur J Neurosci 24:3109–3118. [DOI] [PubMed] [Google Scholar]
- Welsby PJ, Rowan MJ, Anwyl R. (2009) Intracellular mechanisms underlying the nicotinic enhancement of LTP in the rat dentate gyrus. Eur J Neurosci 29:65–75. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Hamaue N, Sumikawa K. (2002) Nicotine compensates for the loss of cholinergic function to enhance long-term potentiation induction. Brain Res 946:148–152. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Jia Y, Niu R, Sumikawa K. (2006) Nicotine exposure in vivo induces long-lasting enhancement of NMDA receptor-mediated currents in the hippocampus. Eur J Neurosci 23:1819–1828. [DOI] [PubMed] [Google Scholar]