Abstract
Petroleum hydrocarbons are the most common environmental pollutants in the world and oil spills pose a great hazard to terrestrial and marine ecosystems. Oil pollution may arise either accidentally or operationally whenever oil is produced, transported, stored and processed or used at sea or on land. Oil spills are a major menace to the environment as they severely damage the surrounding ecosystems. To improve the survival and retention of the bioremediation agents in the contaminated sites, bacterial cells must be immobilized. Immobilized cells are widely tested for a variety of applications. There are many types of support and immobilization techniques that can be selected based on the sort of application. In this review article, we have discussed the potential of immobilized microbial cells to degrade petroleum hydrocarbons. In some studies, enhanced degradation with immobilized cells as compared to free living bacterial cells for the treatment of oil contaminated areas have been shown. It was demonstrated that immobilized cell to be effective and is better, faster, and can be occurred for a longer period
Keywords: Application, bacteria, biodegradation, crude oil, soil.
INTRODUCTION
There is a growing public concern as a wide variety of toxic organic chemicals are being introduced inadvertently or deliberately into the environment. Petroleum hydrocarbons are one of the common examples of these chemicals, which enter the environment frequently and in large volumes through numerous pathways [1].
Oil contamination has become a global problem in industrialized and developing countries. It is one of the most dangerous pollution factors known today. It can cause a threat to the environment. It is very feared by environmen-talists and it's very hard to control if it spills out [2, 3].
There are a lot of methods for treating petroleum conta-minated sites such as mechanical and chemical methods, but these methods generally are expensive and have limited effectiveness. On the other hand, bioremedia-tion is the promising technology for the reduction of these petroleum pollutant areas since it is cost-efficient and will result in to complete mineralization. Bioremediation is a process that degrades environmental pollution by microorga-nisms [4, 5].
In the last few years, the application of biotechnological processes that involvesing microorganisms with the objective of solving environmental pollution problems, is rapidly gro-wing. The researchers have proved that biological methodology is versatile, high stability, broad applications in various areas, economical and efficient for the remediation ofpetroleum [6]. One of the key points'spoints for bioremediation is maintaining high biomass of bacterial populations. To improve the survival and retention of the bioremediation agents in the contaminated sites, bacterial cells must be immobilized. Immobilized cells have been extensively used in the production of useful chemicals, treatment of wastewaters and bioremediation of pollution cause of its longer operating lifetime and enhanced stability and survival of the cells [7, 8].
The use of immobilized cells has been investigated as an alternative technology for environmental applications. These biocatalysts can offer the possibility of a wider and more economical exploitation in industry, waste treatment, medicine, and development of bioprocess and monitoring devices like the biosensor [9].
The many advantages of immobilized cell systems have been reported and a few of the major reasons are listed below [10]:
Providing high biomass.
Providing cell reuse and reducing the costly processes of cell recovery and cell recycle.
Elimination of cell washout problems at high dilution rates.
High flow rates allow high volumetric productivities.
Providing suitable micro environmental conditions.
Improving genetic stability.
Protection against shear damage.
High resistance to toxic chemicals, pH, temperature, solvents and heavy metals.
Decline of maturation time for some products.
Definition of immobilization
An immobilized molecule is one whose movement in space has been restricted either completely or to a small limited region by attachment to a solid structure. In general the term immobilization refers to the act of the limiting movement or making incapable of movement [11]. Immobi-lization can be occurred for enzymes, cellular organelles, animal and plant cells.
Recently the immobilization of whole cells have been developed as biocatalysts in environmental pollutions when are used for multi enzyme systems. They can be classified to three physiological states consist of dead, living and growing states. So we must choose the state more suitable for the application purpose [12]. There are many different the immobilization of whole cell with other biocatalysts such as enzymes. The heat stability and functional stability of immobilized microbial cells have more than enzyme systems. They are not needful tThe processes for the extraction, separation and purification of the enzymes from the cells are not necessary. Not only enzymes due to their protein structure have less stability in extreme conditions, but also in enzymatic systems unwanted reactions can occurbe occurred [13].
The field for immobilization of whole cell, field of their usageis varied from food industry to biomedical sciences. Microorganisms survived on a carrier can be used in continuous and semi-continuous production processes (biosynthesis of vitamins, amino acids, organic acids, production of monoclonal anti-bodies, recovery of heavy metals, whole cell enzymatic reaction and ethanol fermentation), allowing for significant cost decrease due to refillable biocatalyst [14, 15].
History of immobilization
Immobilization is a natural phenomenon existing in the globe. Radwan et al. [16] have provided evidence that the immobilization principle is already found in nature, as microalgal samples collected along the Gulf coast were covered by biofilms of oil utilizing bacteria that help degrade hydrocarbons found in seawater.
Biofilms are surface-attached microbial communities consisting of multiple layers of cells embedded in hydrated matrices [17]. Biofilms spread on surfaces or within natural structuresincluding such as body, a tooth, grains, glass, a water pipe or conduit, etc. [18].This natural phenomenon encouraged humans to utilize it for his their services. In the 1969 for the first time, enzyme immobilization was applied for continuous production of L-amino acids from acyl DL-amino acids. by By immobilizinged aminoacylase enzyme and since the late 1970s, immobilization techniques have been used extensively in many laboratories [19].
Carrier selection
The selection of carrier is very important for use in immobilization. Carriers must be have the following criteria [20]:
non toxic, non polluting, non biodegradable.
High cell mass loading capacity.
High mechanical, biological and chemical stability.
Long shelf life.
Adequate function groups.
Low cost price.
Optimum diffusion distance from flowing media to center of carrier.
Easy separation of cells and carrier from media.
Easy to handle and regenerate.
The type of support media used for anoxic biomass immobilization can affect the efficiency of a bioreactor. The number of cells adhering to the support depend on the kind of support.
Immobilization supports are commonly divided into two main groups: organic and inorganic. Organic carriers are such as modified celluloses, dextran, chitosan and agarose, and inorganic carriers are such as zeolite, clay, anthracite, porous glass, activated char-coal, and ceramics [21].
Organic materials are more abundant than inorganic carriers and can be obtained with strictly controlled porosity, but they are usually very sensitive to pressure or pH, and in many cases to both of them. On the other hand, Inorganic supports generally have one major advantage over other materials, namely, their toughness and etc. Most inorganic supports are totally inert, resistance to temperature, pH, chemicals, microbial degradation, and also crushing or abrasion. Given that they do not normally have to be pro-duced by the end-user and may well be naturally occuring occurring (e.g. sand used in methanogenic fluidized beds), the inorganic supports also lendthemselves more conveniently to scale-up [22, 23].
Organic carrier can be divided into natural and synthetic polymers. Some examples of natural carriers that can be used as support include alginate, carrageenan, agar, agarose, chitosan and chitin. A variety of synthetic polymers such as acrylamide, polyurethane, polyvinyl and resins are also used for immobilization [24].
Alginates (polymers made of different proportions and sequences of mannuronic and guluronic acids extracted from brown algae) are the polymers of choice in most systems of immobilization because they are easy to handle, nontoxic to humans, the environment, and the entrapped microorga-nisms, legally safe for human use, available in large quantities and inexpensive. From a physiological perspec-tive, a major advantage of alginate is that immobilized cells do not suffer extreme changes in physicochemical condition during the procedure of immobilization and the gelis trans-parent and permeable [25].
Types of Immobilization
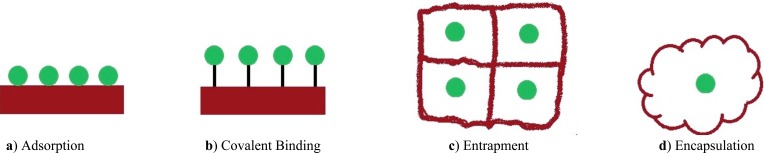
Many different forms of cell immobilization have been used including Adsorption, Covalent Binding, Entrapment and Encapsulation (Fig. 1). Among these methods the Entrapping has been widely investigated [26].
Fig. (1).
Types of Immobilization.
Adsorption
This reversible method for the immobilization of cells is based on the physical interaction between the microorganism and surface of water-insoluble carriers. It is most commonly used for adherence of cell. Immobilization by adsorption is mild, quick, simple, economically advantageous, no need for chemical additives. Moreover it is, easy to perform the process and with thepossibility of reloading of the support. In the interaction between microorganism and the surface of the matrix, weak forces are involved include hydrogen bonds, ionic bonds, hydrophobicbonds and van der Waals forces. It has the disadvantage that the adsorbed enzyme may leak from the carrier during use due to a weak binding force between the enzyme and the carrier. Disadvantages of cells immobilized using the adsorption technique is a the very high rate of leakage from matrix due to weak interactions, unstable interactions, no possibility to control the loading, so the reproducibility is also low [27, 28].
Covalent Binding
The covalent Binding is areversibleeversible immobilization that based on covalent bond formation between activated inorganic carrier and cell in the presence of a binding (cross linking) agent. Covalent method of immobilization is mainly used for enzyme immobilization but it is rarely applied in whole cell immobilization because the toxicity of the coupling agents often RESULTS in loss of cell viability or enzyme activity [29].
Entrapment
Entrapment method is an irreversible immobilization that is based on capturing of particles or cells within a support matrix or inside a hollow fiber. In this type of technique creates a protective barrier is created around the immobilized microbes and prevents the cells leakage from the polymers into surrounding medium while allowing mass transfer of nutrients and metabolites. Entrapment is the mostly applied in cell immobilization. The advantage of entrapment of cell immobilization method is that it is fast, cheap and mild conditions are required for the reaction process. The main disadvantages of this technique are costs of immobilization, injury of support material during usage diffusion limitations, deactivation during immobilization and low loading capacity as biocatalysts [30]. Various types of supports have been used such as agar, chitosan, alginate, celite, carrageenan, cellulose and its derivatives, collagen, gelatin, epoxy resin, photo cross-linkable resins, polyester, polystyrene, polyurethane and acrylic polymers [31]. These matrixes have porous structure, and thus the pollutant and various metabolic products could easily diffuse through into the matrix. Immobilized particle-size to support material pore-size ratio probably is the most important parameter. When the pores are too big the material is leaksing, what which also decreases the loading [32].
Encapsulation
Encapsulation is another irreversible technique similar to entrapment. This method can be achieved by enveloping the biological components within various forms of spherical semi permeable membranes with a selective controlled permeability [33].
The ratio of size of pore of membrane to size of core material is a significant factor in this phenomenon. This limited availability to the microcapsule inside is one of the main advantages of microencapsulation, due to protection of the biocatalyst from the extreme conditions. As most immobilization methods, it prevents biocatalyst leakage, increasing the process efficiency of the processas a result [34].
Application of immobilization techno-logy for use in bioremediation of pollu-tants
The increasing of environmental pollution and the treating contaminated sites are necessary atat present [35]. This review focuses more on the remediation of oil contaminated areas. Bioremediation of crude oil using immobilized cells is rarely studied. All of the methods of immobilization such as Adsorption, Covalent Binding, Entrapment and Encapsulation were tried for bioremediation of crude oil. The high immobilization efficiency of the cells onto the immobilization material and the high affinity between the hydrophobic immobilization material and the substrates caused excellent degradation. Increasing availability of the substrates for the cells and a better interaction between the substrates and the immobilized cells synergistically resulted in developing the degradation rate [36].
Omar and Rehm [37] demonstrated that Candida parapsilosis and Penicillium frequetans when immobilized on granular clay in columns, effectively degraded n-alkanes. They observed that residuals of C12 to C18 alkanes in immobilized bacterial cells system are 13.4 to 32.3% whereas in free bacterial cells system is are 85.9 and 98.9%. Davis and Westlake [38] reported that immobilization of cells onto inert surfaces increased available surface area to facilitate growth of biomass and also enhance degradation rate.
Obuekwe and M. Al-Muttawa [39], an Arthrobacter sp. and a Gram-negative bacillus isolated from Kuwait oil lakes,and then these bacteria incubated with sawdust, Styrofoam or wheat bran, as carriers, under low nutrient conditions, stable exopolysaccharide mediated immobilized cultures were formed. The authors tested the ability to survive and degrade hydrocarbons for 6 weeks at 45 ◦C. Suspensions of free cells degraded less crude oil than freshly immobilized cells.
In other study Quek et al. [40] have reported the immobilization and performance of Rhodococcussp. F92 on polyurethane foam (PUF) in the bioremediation of petroleum hydrocarbons. The immobilized wcells could toere able to degrade a variety of petroleum products such as Arabian light crude, Al-Shaheen crude, diesel and oil slops.
Radwan et al. immobilized oil-utilizing bacteriain biofilms coating macroalgae. This natural immobilization can be protected the bacteria from being washed out and diluted also provided with oxygen, and probably nitrogenous and phosphorus compounds and vitamins for oil-utilizing bacteria [16].
Gentili et al. [41] used chitin and chitosan flakes for immobilization of Rhodococcus corynebacterioides QBTo. This supports are natural, nontoxic, nonpolluting and biodegradable that are obtained from shrimps and crabs. The R. corynebacterioides QBT oimmobilized on chitin and chitosan flakes increased significantly the crude oil biodegradation.
Wiesel et al. [42] observed that a mixed bacterial culture immobilized on granular clay exhibited good growth, and demonstrated equivalent degradation potential of polyaromatic hydrocarbons (PAHs) compared to freely suspended cells in their model soil system.
Diaz et al. [43] used immobilized the bacterial consortium MPD-M on polypropylene fibers for biodegradation of crude oil in water with salinities varying from 0 to 180 g L-1. They observed the immobilized cells significantly increased the biodegradation rate of crude oil compared with free-living cells, the bacterial consortium MPD-M was highly stable in immobilized systems and it was not greatly affected by addition in salinity, also the biodegradation of pristanepristine (PR) and phytane (PH) and of the aromatic fraction was also increased using cells immobilized on polypropylene fibers.
Xu and Lu [44] demonstrated that oil removal in a crude oil-contaminated soil was increased by application of hydrocarbon-degrading bacteria immobilized on peanut hull powder as biocarrier. This biocarrier provides large surface area and strong adsorption capability, in addition improves oxygen diffusion and enhancesed dehydrogenase activity in soil.
Oil-degrading ability of the immobilized bacterial consortium in cocopeat, rice hull powder and sodium alginate capsules was compared by Nunal et al. [45]. They reported that immobilization of the oil-degraders on the surface of cocopeat higher oil reduction, compared to encapsulation in sodium alginate gel. higher oil reduction by the cocopeat-immobilized cells is presumably due to highly sustained microbial population attached to surface of the biocarrier, providing protective niche, the porous nature of cocopeat might allow efficient substrate diffusion, slow release of nutrients, and acceleration of oxygen transfer, thus providing a favorable niche for hydrocarbon utilization. In addition to the encapsulated bacterial cells might not be allowed to replicate inside the alginate matrix and subsequent release into the medium [45].
Cocquempot et al. [46] examined that immobilized cells in PUF better than of immobilized in alginate because of storage stability and microbial activity. The use of polyurethane foams developed due to wide range of porosity, mechanical properties, hydrophobicity and hidrophilicity of polyurethane foams. For example Oh et al. [47] immobilized Yarrowia lipolytica in polyurethane foams for degradation of crude oil.
Liang et al. [48] compared amount of degradation of crude oil in contaminated soil with free-living bacterial cultures and activated carbon biocarrier. RESULTS revealed that immobilization in activated carbon biocarrier increased the biodegradation of crude oil, bacterial population and total microbial activity due to improvement the oxygen, nutrient mass transfer and water holding capacity of the soil.
Immobilized cells are being used in biodegradation of another compounds. Some immobilized cells for use in biodegradation compounds are given in Table 12. Recently, Maliji et al. [49] used natural support such as luffa and sponge for immobilization of Bacillus cereus in diesel Oil degradation. This adsorption system could control pollution and also be easilyy used and with low costs.
Table 1.
Some immobilized cells for use in biodegradation compounds.
| Compounds Degraded | Carriers | Microorganisms | REFERENCES |
|---|---|---|---|
| acrylamide | alginate | Pseudomonas sp. and Xanthomonas maltophilia | [54] |
| Cadmium and Zinc | alginate | Pseudomonas fluorescens G7 | [55] |
| 2-chloroethanol | sand | Pseudomonas putida US2 | [56] |
| cyanuric acid | Granular clay | Pseudomonas sp. NRRL B-12228 | [57] |
| Diesel oil | Polyvinyl alcohol | Hydrocarbon-degrading bacteria | [58] |
| Ethylbenzene | Alginate, agar, polyacrylamide | Pseudomonas fluorescens-CS2 | [59] |
| Mercury | alginate | nitrogen-fixing bacteria (NFB) | [60] |
| naphthalene | alginate, agar and polyacrylamide | Pseudomonas sp. strain NGK 1 | [61] |
| p-Nitrophenol | diatomaceous earth | Pseudomonas sp. | [62] |
| pentachlorophenol | polyurethane | Flavobacterium sp. | [63] |
| Pentachlorophenol | alginate | Phanerochaete chrysosporium | [64] |
| Pentachlorophenol | k-Carrageenan | Pseudomonas sp. UG30 | [65] |
| phenol | Polyvinyl alcohol (PVA) | Acinetobacter sp.strain PD12 | [66] |
| phenol | agar | methanogenic consortium | [67] |
| Phenol,trichloroethane | Chitosan | Pseudomonas putida BCRc14349 | [68] |
| sodium cyanide and acetonitrile | alginate | Pseudomonas putida | [69] |
| Sodium dodecyl sulfare (SDS) | polaycrylamide | Pseudomonas C12B | [70] |
| 2,4,6-trinitrotoluene (TNT) | alginate | Arthrobacter sp. | [71] |
| 2,4,6-Trichlorophenol | k-Carrageenan/gelatin gel | Microbial consortium | [72] |
In some study it wasdemonstrated that the tolerance ability into difficult conditions of immobilized cells was improved due mainly to enhancement modifications of the cell membrance. Ffor example, Kim et al. [50] examined the effect of co-contaminants (phenol) on the biodegradation of pyridine by free and calcium alginate immobilized Pseudomonas putidaMK1 (KCTC 12283). They showed that immobilized cells can effectively increase the tolerance to phenol and RESULTS in increased degradation of pyridine.
In some cases, microbial metabolism of petroleum hydrocarbons may produce toxic metabolites such as naphthenic acids, which can hamper subsequent biodegradation due to their toxicity that represses microbial metabolism [51].
Weir et al. [52] employed Pseudomonas aeruginosa UG14 encapsulatedin alginate, clay and skim milk for degradation of phenanthrene. They observed that survival of encapsulated cells was higher after 30 days whereas free cells endured for 18 days.
O'Reilly et al. [53] investigated the degradation of p-cresol by a Pseudomonas sp. Immobilized in calcium alginate and polyurethane. The RESULTS suggested that polyurethane was a better immobilization matrix than calcium alginate owing to its greater mechanical strength and improved oxygen transfer characteristics.
Some immobilized cells for use in biodegradation compounds
CONCLUSION
Immobilization hydrocarbons degrader's bacteria have high potential to clean up oil contamination and can be facilitating oil biodegradation in polluted environment. In some of studies reported that immobilized cells compared with free living bacteria more effective, have longer shelf life, lower cost price and higher crude oil degrading activity in various areas. In addition immobilized cells increase tolerance ability to unfavorable condition.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors confirm that no conflicts of interest are associated with this article.
REFERENCES
- 1.Hassanshahian M., Emtiazi G., Caruso G., Cappello S. Bioremediation (bioaugmentation/biostimulation) trials of oil polluted seawater: a mesocosm simulation study. Mar. Environ. Res. 2014;95:28–38. doi: 10.1016/j.marenvres.2013.12.010. a. [DOI] [PubMed] [Google Scholar]
- 2.Tebyanian H., Hassanshahian M., Kariminik A. Hexadecane-degradation by Teskumurella and Stenotrophomonas strains isolated from hydrocarbon contaminated soils. Jundishapur J. Microbiol. 2013;26(7):82–91. [Google Scholar]
- 3.Hassanshahian M., Ahmadinejad M., Tebyanian H., Kariminik A. Isolation and characterization of alkane degrading bacteria from petroleum reservoir waste water in Iran (Kerman and Tehran provenances). Mar. Pollut. Bull. 2013;73(1):300–305. doi: 10.1016/j.marpolbul.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Hassanshahian M., Zeynalipour M.S., Musa F.H. Isolation and characterization of crude oil degrading bacteria from the Persian Gulf (Khorramshahr provenance). Mar. Pollut. Bull. 2014;82(1-2):39–44. doi: 10.1016/j.marpolbul.2014.03.027. b. [DOI] [PubMed] [Google Scholar]
- 5.Hasanshahian M., Emtiazi G. Investigation of alkane biodegradation usingthe microtiter plate method and correlation between biofilm formation,biosurfactant production and crude oil biodegradation. Int. Biodeterior. Biodegradation. 2008;62:170–178. doi: 10.1016/j.ibiod.2008.01.004. [DOI] [Google Scholar]
- 6.Ghanavati H., Emtiazi G., Hassanshahian M. Synergism effects of phenol-degrading yeast and ammonia-oxidizing bacteria for nitrification in coke wastewater of Esfahan Steel Company. Waste Manag. Res. 2008;26(2):203–208. doi: 10.1177/0734242X07079874. [DOI] [PubMed] [Google Scholar]
- 7.Cassidy M.B., Lee H., Trevors J.T. Immobilized microbial cells: a review. J. Ind. Microbiol. Biotechnol. 1996;16:79–101. [Google Scholar]
- 8.Scott C.H. Immobilized cells: a review of recent literature. Enzyme Microb. Technol. 1987;9:66–79. doi: 10.1016/0141-0229(87)90145-1. [DOI] [Google Scholar]
- 9.Margaritis A., Merchant F.J. Advances in ethanol production using immobilized cell systems. Crit. Rev. Biotechnol. 1984;2:339–393. [Google Scholar]
- 10.Martin A.M., editor. Bioconversion of waste materials to industrial products. New York: Springer; 1998. [DOI] [Google Scholar]
- 11.Zhang Y.Q., Tao M.L., Shen W.D., Zhou Y.Z., Ding Y., Ma Y., Zhou W.L. Immobilization of L-asparaginase on the microparticles of the natural silk sericin protein and its characters. Biomaterials. 2004;25(17):3751–3759. doi: 10.1016/j.biomaterials.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 12.Rahman R.N., Ghaza F.M., Salleh A.B., Basri M. Biodegradation of hydrocarbon contamination by immobilized bacterial cells. J. Microbiol. 2006;44(3):354–359. [PubMed] [Google Scholar]
- 13.Stolarzewicz I., Bialecka-Florjanczyk E., Majewska E., et al. Immobilization of yeast on polymeric supports. Chem Biochem Eng. 2011;25:135–144. [Google Scholar]
- 14.Ohta T., Ogbonna J.C., Tanaka H., et al. Development of a fermentation method using immobilized cells under unsterile conditions. 2. Ethanol and L-lactic acid production without heat and filter sterilization. Appl. Microbiol. Biotechnol. 1994;42:246–260. doi: 10.1007/BF00902724. [DOI] [Google Scholar]
- 15.Mrudula S., Shyam N. Immobilization of Bacillus megaterium MTCC 2444 by Ca-alginate entrapment method for enhanced alkaline protease production. Braz. Arch. Biol. Technol. 2012;55:135–144. doi: 10.1590/S1516-89132012000100017. [DOI] [Google Scholar]
- 16.Radwan S.S., Al-Hasan R.H., Salamah S., et al. Bioremediation of oily sea water by bacteria immobilized in biofilms coating microalgae. Int Biodet Biodeg. 2002;50:55–59. doi: 10.1016/S0964-8305(02)00067-7. [DOI] [Google Scholar]
- 17.Kierek-Pearson K., Karatan E. Biofilm development in bacteria. Adv. Appl. Microbiol. 2005;57:79–111. doi: 10.1016/S0065-2164(05)57003-5. [DOI] [PubMed] [Google Scholar]
- 18.Carpentier B., Cerf O. Biofilms and their consequences, with particular reference to hygiene in the food industry. J. Appl. Bacteriol. 1993;75(6):499–511. doi: 10.1111/j.1365-2672.1993.tb01587.x. [DOI] [PubMed] [Google Scholar]
- 19.Chibata I., Tosa T., Sato T., et al. Preparation and industrial application of immobilized aminoacylase. Proc Int Ferment Tec Symp 4th. 1972:383–9. [Google Scholar]
- 20.Zacheus O.M., Iivanainen E.K., Nissinen T.K., et al. Bacterial biofilm formation on polyvinyl chloride, polyethylene and stainless steel exposed to ozonated water. Water Res. 2000;34:63–70. doi: 10.1016/S0043-1354(99)00113-X. [DOI] [Google Scholar]
- 21.Lu J., Toy P.H. Organic polymer supports for synthesis and for reagent and catalyst immobilization. Chem. Rev. 2009;109(2):815–838. doi: 10.1021/cr8004444. [DOI] [PubMed] [Google Scholar]
- 22.Magner E. Immobilisation of enzymes on mesoporous silicate materials. Chem. Soc. Rev. 2013;42(15):6213–6222. doi: 10.1039/c2cs35450k. [DOI] [PubMed] [Google Scholar]
- 23.Ispas C., Sokolov I., Andreescu S. Enzyme-functionalized mesoporous silica for bioanalytical applications. Anal. Bioanal. Chem. 2009;393(2):543–554. doi: 10.1007/s00216-008-2250-2. [DOI] [PubMed] [Google Scholar]
- 24.Hartman M. Ordered mesoporous materials for bioadsorption and biocatalysis. Chem. Mater. 2005;17:4577–4593. doi: 10.1021/cm0485658. [DOI] [Google Scholar]
- 25.Buque E.M., Chin-Joe I., Straathof A.J., et al. Immobilization affects the rate and enantioselectivity of 3-oxo-ester reduction by baker's yeast. Enzyme Microb. Technol. 2002;31:656–664. doi: 10.1016/S0141-0229(02)00161-8. [DOI] [Google Scholar]
- 26.Jack T.R., Zajic J.E. The immobilization of whole cells. Adv. Biochem. Eng. Biotechnol. 1977;5:125–145. [Google Scholar]
- 27.Leon R., Fernandes P., Pinheiro H.M., et al. Whole-cell biocatalysis in organic media. Enzyme Microb. Technol. 1998;23:483–500. doi: 10.1016/S0141-0229(98)00078-7. [DOI] [Google Scholar]
- 28.Argin-Soysal S., et al. Effect of surface characteristics and xanthan polymers on the immobilization of Xanthomonas campestris to fibrous matrices. J. Food Sci. 2004;69:441–448. [Google Scholar]
- 29.Groboillot A., Boadi D.K., Poncelet D., Neufeld R.J. Immobilization of cells for application in the food industry. Crit. Rev. Biotechnol. 1994;14(2):75–107. doi: 10.3109/07388559409086963. [DOI] [PubMed] [Google Scholar]
- 30.Trelles J.A., Rivero C.W. Whole cell entrapment techniques. Methods Mol. Biol. 2013;1051:365–374. doi: 10.1007/978-1-62703-550-7_24. [DOI] [PubMed] [Google Scholar]
- 31.Lopez A., Lazaro N., Marques A.M. The interphase technique: a simple method of cell immobilization in gel-beads. J. Microbiol. Methods. 1997;30:231–234. doi: 10.1016/S0167-7012(97)00071-7. [DOI] [Google Scholar]
- 32.Verma M., Brar S.K., Blais J.F., et al. Aerobic biofiltration processes-advances in wastewater treatment. Pract. Period. Hazard. Toxic Radioact. Waste Manage. 2006;10:264–276. doi: 10.1061/(ASCE)1090-025X(2006)10:4(264). [DOI] [Google Scholar]
- 33.Bickerstaff G.F., Jr . Immobilization of enzymes and cells. Methods in Biotechnology. New Jersey: Humana Press; 1997. [Google Scholar]
- 34.Park J.K., Chang H.N. Microencapsulation of microbial cells. Biotechnol. Adv. 2000;18(4):303–319. doi: 10.1016/S0734-9750(00)00040-9. [DOI] [PubMed] [Google Scholar]
- 35.Hassanshahian M., Emtiazi G., Cappello S. Isolation and characterization of crude-oil-degrading bacteria from the Persian Gulf and the Caspian Sea. Mar. Pollut. Bull. 2012;64(1):7–12. doi: 10.1016/j.marpolbul.2011.11.006. b. [DOI] [PubMed] [Google Scholar]
- 36.Wilson N.G., Bradley G. Enhanced degradation of petroleum (slovene diesel) in an aqueous system by immobilized pseudomonas fluorescens. J. Appl. Microbiol. 1996;80:99–104. [Google Scholar]
- 37.Omar S.H., Rehm H.J. Degradation of n-alkanes by Candida parapsilosis and Penicillium frequentans immobilized on granular clay and aquifer sand. Appl. Microbiol. Biotechnol. 1988;28(1):103–108. doi: 10.1007/BF00250507. [DOI] [Google Scholar]
- 38.Davies J.S., Westlake D.W. Crude oil utilization by fungi. Can. J. Microbiol. 1979;25(2):146–156. doi: 10.1139/m79-023. [DOI] [PubMed] [Google Scholar]
- 39.Obuekwe C.O., Al-Muttawa M. Self-immobilized bacterial cultures with potential for application as ready-to-use seeds for petroleum bioremediation. Biotechnol. Lett. 2001;23:1025–1032. doi: 10.1023/A:1010544320118. [DOI] [Google Scholar]
- 40.Quek E., Ting Y.P., Tan H.M. Rhodococcus sp. F92 immobilized on polyurethane foam shows ability to degrade various petroleum products. Bioresour. Technol. 2006;97(1):32–38. doi: 10.1016/j.biortech.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 41.Gentili A.R., Cubitto M.A., Ferrero M., et al. Bioremediation of crude oil polluted seawater by a hydrocarbondegrading bacterial strain immobilized on chitin and chitosan flakes. Int. Biodeterior. Biodegradation. 2006;57:222–228. doi: 10.1016/j.ibiod.2006.02.009. [DOI] [Google Scholar]
- 42.Wiesel I., Wubker S.M., Rehm H.J. Degradation of polycyclic aromatic hydrocarbons by an immobilized mixed bacterial culture. Appl. Microbiol. Biotechnol. 1993;39:110–116. doi: 10.1007/BF00166858. [DOI] [Google Scholar]
- 43.Díaz M.P., Boyd K.G., Grigson S.J., Burgess J.G. Biodegradation of crude oil across a wide range of salinities by an extremely halotolerant bacterial consortium MPD-M, immobilized onto polypropylene fibers. Biotechnol. Bioeng. 2002;79(2):145–153. doi: 10.1002/bit.10318. [DOI] [PubMed] [Google Scholar]
- 44.Xu Y., Lu M. Bioremediation of crude oil-contaminated soil: comparison of different biostimulation and bioaugmentation treatments. J. Hazard. Mater. 2010;183(1-3):395–401. doi: 10.1016/j.jhazmat.2010.07.038. [DOI] [PubMed] [Google Scholar]
- 45.Nunal S.N., Santander D.E., Leon S.M., et al. Bioremediation of oil-contaminated seawater and sediment by an oil-degrading bacterial Consortium. Biocontrol Sci. 2014;19(1):11–22. doi: 10.4265/bio.19.11. [DOI] [PubMed] [Google Scholar]
- 46.Cocquempot M.F., Thomasset B., Barbotin J.N. Comparative stabilization of biological photosystems by several immobilization procedures. Eur J Appl Microbiol Biotechnol. 1981;11:193–198. doi: 10.1007/BF00505866. [DOI] [Google Scholar]
- 47.Oh Y.S., Maeng J., Kim S.J. Use of microorganism-immobilized polyurethane foams to absorb and degrade oil on water surface. Appl. Microbiol. Biotechnol. 2000;54(3):418–423. doi: 10.1007/s002530000384. [DOI] [PubMed] [Google Scholar]
- 48.Liang Y., Zhang X., Dai D., et al. Porous biocarrier enhanced biodegradation of crude oil contaminated soil. Int. Biodeterior. Biodegradation. 2009;63:80–87. doi: 10.1016/j.ibiod.2008.07.005. [DOI] [Google Scholar]
- 49.Maliji D., Olama Z., Holail H. Environmental studies on the microbial degradation of oil hydrocarbons and its application in Lebanese oil polluted coastal and marine ecosystem. Int J Curr Microbiol App Sci. 2013;2(6):1–18. [Google Scholar]
- 50.Kim M.K., Singleton I., Yin C.R., Quan Z.X., Lee M., Lee S.T. Influence of phenol on the biodegradation of pyridine by freely suspended and immobilized Pseudomonas putida MK1. Lett. Appl. Microbiol. 2006;42(5):495–500. doi: 10.1111/j.1472-765X.2006.01910.x. [DOI] [PubMed] [Google Scholar]
- 51.Lu M., Zhang Z., Qiao W., Wei X., Guan Y., Ma Q., Guan Y. Remediation of petroleum-contaminated soil after composting by sequential treatment with Fenton-like oxidation and biodegradation. Bioresour. Technol. 2010;101(7):2106–2113. doi: 10.1016/j.biortech.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 52.Weir S.C., Dupuis S.P., Providenti M.A., Lee H., Trevors J.T. Nutrient-enhanced survival of and phenanthrene mineralization by alginate-encapsulated and free Pseudomonas sp. UG14Lr cells in creosote-contaminated soil slurries. Appl. Microbiol. Biotechnol. 1995;43(5):946–951. doi: 10.1007/BF02431932. b. [DOI] [PubMed] [Google Scholar]
- 53.O’Reilly K.T., Crawford R.L. Kinetics of p-cresol degradation by an immobilized Pseudomonas sp. Appl. Environ. Microbiol. 1989;55(4):866–870. doi: 10.1128/aem.55.4.866-870.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nawaz M.S., Franklin W., Cerniglia C.E. Degradation of acrylamide by immobilized cells of a Pseudomonas sp. and Xanthomonas maltophilia. Can. J. Microbiol. 1993;39(2):207–212. doi: 10.1139/m93-029. [DOI] [PubMed] [Google Scholar]
- 55.Sarin Ch., Sarin S. Removal of Cadmium and Zinc from Soil using immobilized cell of biosurfactant producing bacteria. Environ-mentasia. 2010;3(2):49–53. [Google Scholar]
- 56.Overmeyer C., Rehm H.J. Biodegradation of 2-chloroethanol by freely suspended and adsorbed immobilized Pseudomonas putida US2 in soil. Appl. Microbiol. Biotechnol. 1995;43(1):143–149. doi: 10.1007/BF00170636. [DOI] [PubMed] [Google Scholar]
- 57.Ernst C., Rehm H.J. Development of a continuous system for the degradation of a cyanuric acid by absorbed Pseudomonas sp. NRRL B-12228. Appl. Microbiol. Biotechnol. 1995;43(1):150–155. doi: 10.1007/BF00170637. [DOI] [PubMed] [Google Scholar]
- 58.Cunningham C.J., Ivshina V.I., Kuyukina M.S., et al. Bioremediation of diesel-contaminated soil by microorganisms immobilized in polyvinylalcohol. Int. Biodeterior. Biodegradation. 2004;54:167–174. doi: 10.1016/j.ibiod.2004.03.005. [DOI] [Google Scholar]
- 59.Parameswarappa S., Karigar C., Nagenahalli M. Degradation of ethylbenzene by free and immobilized Pseudomonas fluorescens-CS2. Biodegradation. 2008;19(1):137–144. doi: 10.1007/s10532-007-9121-y. [DOI] [PubMed] [Google Scholar]
- 60.Tariq A., Latif Z. Bioremediation of mercury compounds by using immobilized nitrogen fixing bacteria. Int. J. Agric. Biol. 2014;16:1129–1134. [Google Scholar]
- 61.Manohar S., Karegoudar T.B. Degradation of naphthalene by cells of Pseudomonas sp. strain NGK 1 immobilized in alginate, agar and polyacrylamide. Appl. Microbiol. Biotechnol. 1998;49(6):785–792. doi: 10.1007/s002530051247. [DOI] [Google Scholar]
- 62.Heitkamp M., Camel L.V., Reuter T., et al. Biodegradation of p-Nitrophenol in an aqueous waste stream by immobilized bacteria. Appl Environ Microb; 1990. pp. 2967–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O’Reilly K.T., Crawford R.L. Degradation of pentachlorophenol by polyurethane-immobilized Flavobacterium cells. Appl. Environ. Microbiol. 1989;55(9):2113–2118. doi: 10.1128/aem.55.9.2113-2118.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin J.E., Wang H.Y., Hickey R.F., JE L Use of coimmobilized biological systems to degrade toxic organic compounds. Biotechnol. Bioeng. 1991;38(3):273–279. doi: 10.1002/bit.260380309. [DOI] [PubMed] [Google Scholar]
- 65.Cassidy M.B., Mullineers H., Lee H., et al. Mineralization of pentachlorophenol in a contaminated soil by Pseudomonas sp. UG30 cells encapsulatedin k-carrageenan. J. Ind. Microbiol. Biotechnol. 1997;19:43–48. doi: 10.1038/sj.jim.2900415. [DOI] [Google Scholar]
- 66.Wang Y., Tian Y., Han B., Zhao H.B., Bi J.N., Cai B.L. Biodegradation of phenol by free and immobilized Acinetobacter sp. strain PD12. J. Environ. Sci. (China) 2007;19(2):222–225. doi: 10.1016/S1001-0742(07)60036-9. [DOI] [PubMed] [Google Scholar]
- 67.Dwyer D.F., Krumme M.L., Boyd S.A., Tiedje J.M. Kinetics of phenol biodegradation by an immobilized methanogenic consortium. Appl. Environ. Microbiol. 1986;52(2):345–351. doi: 10.1128/aem.52.2.345-351.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen Y.M., Lin T.F., Huang C., Lin J.C., Hsieh F.M. Degradation of phenol and TCE using suspended and chitosan-bead immobilized Pseudomonas putida. J. Hazard. Mater. 2007;148(3):660–670. doi: 10.1016/j.jhazmat.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 69.Chapatwala K., Babu G.R., Armstead E.R., et al. A kinetic study on the bioremediation of sodium cyanide and acetonitrile by free and immobilized cells of Pseudomonas putida. Appl. Biochem. Biotechnol. 1995;51-52:717–726. doi: 10.1007/BF02933472. [DOI] [Google Scholar]
- 70.White G.F., Thomas O.R. Immobilization of the surfactant degrading bacterium Pseudomonas C12B in polaycrylamide gel. II.Optimizing SDS-degrading activity and stability. Enzyme Microb. Technol. 1990;12:969–975. doi: 10.1016/0141-0229(90)90119-B. [DOI] [PubMed] [Google Scholar]
- 71.Tope A.M., Jamil K., Baggi T.R. Transformation of 2,4,6-trinitrotoluene (TNT) by immobilized and resting cells of Arthrobacter sp. J Hazard Subst Res. 1999;2(3):1–8. [Google Scholar]
- 72.Gardin H., Pauss A. Kappa-carrageenan/gelatin gel beads for the co-immobilization of aerobic and anaerobic microbial communities degrading 2,4,6-trichlorophenol under air-limited conditions. Appl. Microbiol. Biotechnol. 2001;56(3-4):517–523. doi: 10.1007/s002530000581. [DOI] [PubMed] [Google Scholar]