Abstract
Clozapine-induced sialorrhea (CIS) affects about one-third of patients treated with clozapine, at times can be stigmatizing, socially embarrassing, disabling, affect quality-of-life, cause poor compliance and can be potentially life-threatening adverse effect. Prompt and effective treatment of CIS may assist treatment tolerability, adherence, and better outcomes in patients with treatment nonresponsive schizophrenia. The beneficial effect of amisulpride augmentation of clozapine therapy for such patients may be enhanced by its anti-salivatory effect on CIS. Current series of five subjects who developed CIS that responded poorly to anticholinergic drugs found drastic improvement in daytime and nocturnal CIS with very low-dose (50-100 mg/day) of amisulpride. Low-dose amisulpride augmentation may also provide strong ameliorating effect on CIS. Nevertheless, a long-term, large-scale study with a broader dose range is warranted to evaluate the stability of this effect across time.
Keywords: adverse effect, amisulpride, clozapine, hypersalivation, sialorrhea
INTRODUCTION
Clozapine is an effective atypical antipsychotic drug, but its use at times may be compromised by troublesome side-effects such as sedation, sialorrhea, weight gain, enuresis, dizziness, besides rare but life-threatening side-effects such as agranulocytosis and myocarditis.[1] Prevalence of clozapine-induced sialorrhea (CIS) ranges between 31% and 72% of patients.[2,3] We report series of five subjects who developed disabling CIS following clozapine therapy that responded poorly to benzhexol, but showed drastic response to very low-dose (50-100 mg/day) of amisulpride. A PubMed search using the keywords: “adverse effect,” “amisulpride,” “clozapine,” “sialorrhea,” “hypersalivation,” to retrieve relevant articles about the concerning physiopathogenesis and treatments suggested for CIS was done supplemented with a manual search of cross references.
CASE REPORT
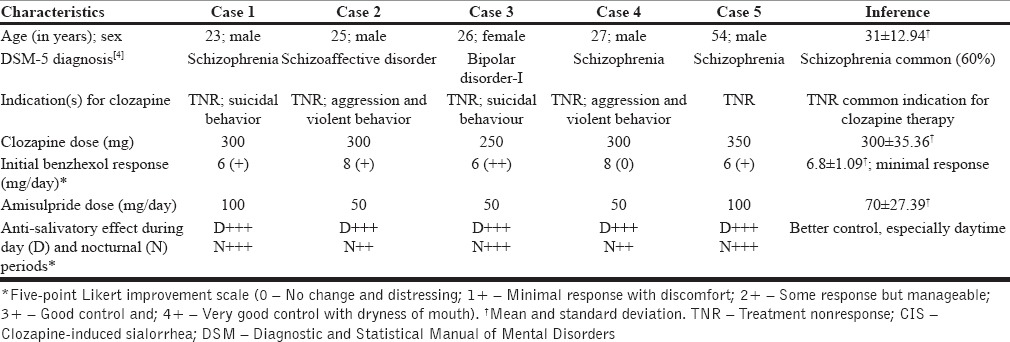
Consecutive subjects (n = 5) with psychiatric illnesses (schizophrenia, schizoaffective disorder, bipolar disorder) diagnosed as per fifth edition of the Diagnostic and Statistical Manual of Mental Disorders diagnostic criteria,[4] who developed debilitating CIS on clozapine therapy (250-350 mg/day) were studied. Indication for clozapine therapy was treatment nonresponsiveness to at least two classes of antipsychotics, including depot typical antipsychotic preparations. Clozapine dose was titrated 12.5-25 mg/day until tolerable or target daily dose of 300 mg, as per prescribing guidelines.[5] Initial benzhexol (6-8 mg/day) for disabling CIS was withdrawn after 1 week due to poor response or intolerable anticholinergic side-effects. Low-dose extended release formulation of amisulpride (50-100 mg/day) was initiated to manage CIS that showed drastic and clinically significant anti-salivatory response during daytime with appreciable effect during nocturnal periods by day-3. Five-point Likert improvement scale (0-no change and distressing; 1+ minimal response with discomfort; 2+ some response but manageable; 3+ good control and; 4+ very good control with dryness of mouth) was utilized to study the anti-salivatory effect of benzhexol and amisulpride for CIS. Table 1 presents the clinical profile of subjects with CIS and its treatment response.
Table 1.
Clinical profile of subjects on clozapine-amisulpride combination therapy for CIS (n = 5)
DISCUSSION
Salivation is regulated by both cholinergic and adrenergic tone. Clozapine being a wide-spectrum atypical antipsychotic drug has an affinity for several different receptor types and may, hypothetically, both stimulate and inhibit salivary secretion. CIS is paradoxical, as the drug possesses both potent anticholinergic and adrenolytic activity at the central nervous system. Peripherally, it also acts on muscarinic receptors of acinar cells (especially submandibular glands), independent of central nervous mechanisms, presynaptic intraglandular events, or circulating catecholamines (in rats). The overall actions of clozapine suggest that salivation is increased during sleep and at rest, but is decreased during meals.[6]
However, amelioration of CIS in the present series with the nonanticholinergic and nonadrenolytic agent amisulpride raises the possibility that clozapine activity might be different at the level of the peripheral nervous system. On the other hand, it is possible that peripheral activity of amisulpride may reduce salivary secretion, similar to the peripheral activity of sulpiride that reduces gastric acid secretion by inhibiting gastrin release, and of levosulpride that promotes (gastric) prokinetic activity.[7]
Possible peripheral mechanisms of CIS include M4 agonism, alpha-2 antagonism, and suppression of swallowing reflex.[5] Although, there are various treatment options, none has been demonstrated to be superior.[8] Due to its elusive mechanism, physicians have attempted to treat this side-effect with agents that counteract clozapine's adrenergic and muscarinic properties.[9] Drug treatments used for CIS such as anticholinergic drugs (ipratropium bromide, atropine eye drops, hyoscine, pirenzepine, propantheline, benztropine, benzhexol, glycopyrrolate) and central alpha-2 agonists (clonidine, lofexidine) render added side-effects. Other medications used for CIS include amitriptyline, sulpiride, amisulpride (400 mg/day), quetiapine, and botulinum toxin.[5] Very few reports of CIS responding to low-dose amisulpride (150 mg/day),[1,8] and bupropion,[10] are also quoted.
Although current pharmacotherapy on CIS has focused on anticholinergic agents albeit its capacity to induce cognitive impairment,[11,12] recent studies report concomitant amisulpride augmentation of clozapine therapy (150-400 mg/day) may halve latter dose without recurrence of an acute psychotic symptomatology, in addition to improvement in psychotic symptoms, and disappearance of CIS almost entirely, often leading to good compliance.[1,8,13,14,15] In the current series, an effective anti-salivatory activity for CIS at diurnal periods, even at very low-dose of amisulpride (50-100 mg/day) than quoted in the literature was found for benzhexol nonresponsive and debilitating CIS. This may suggest a potential role for even low-dose amisulpride in the management of CIS. Furthermore, restricting the use of anticholinergic drugs for CIS may lessen the cognitive side-effects of these drugs, thereby improving the overall quality-of-life of those schizophrenia patients with cognitive deficits.[5]
CONCLUSION
Prompt and effective treatment of CIS may assist with treatment tolerability, adherence, and outcomes in patients with treatment nonresponsive schizophrenia or bipolar disorder. Although small randomized controlled trials of combined use of amisulpride (400 mg/day) and clozapine have shown a beneficial effect on CIS,[14] current series supports that even very low-dose amisulpride (50-100 mg/day) provides strong ameliorating effect on CIS. Nevertheless, a long-term, large-scale study with a broader dose range is warranted to evaluate the stability of this effect across time.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Aggarwal A, Sharma DD. Amisulpride for clozapine-induced sialorrhea. Psychopharmacol Bull. 2009;42:69–71. [PubMed] [Google Scholar]
- 2.Sockalingam S, Shammi C, Remington G. Clozapine-induced hypersalivation: A review of treatment strategies. Can J Psychiatry. 2007;52:377–84. doi: 10.1177/070674370705200607. [DOI] [PubMed] [Google Scholar]
- 3.Soler Roibal MA, Oca Bravo L, Montejo Iglesias LM. The clozapine-induced hypersalivation and its treatment. Actas Esp Psiquiatr. 1999;27:408–11. [PubMed] [Google Scholar]
- 4.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5) 5th ed. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 5.Taylor D, Paton C, Kapur S, editors. Maudsley Prescribing Guidelines. 10th ed. London: Informa Healthcare; 2009. [Google Scholar]
- 6.Ekström J, Godoy T, Riva A. Clozapine: Agonistic and antagonistic salivary secretory actions. J Dent Res. 2010;89:276–80. doi: 10.1177/0022034509356055. [DOI] [PubMed] [Google Scholar]
- 7.Serra J. Levosulpiride in the management of functional dyspepsia and delayed gastric emptying. Gastroenterol Hepatol. 2010;33:586–90. doi: 10.1016/j.gastrohep.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Praharaj SK, Ray P, Gandotra S. Amisulpride improved debilitating clozapine-induced sialorrhea. Am J Ther. 2011;18:e84–5. doi: 10.1097/MJT.0b013e3181c84bbd. [DOI] [PubMed] [Google Scholar]
- 9.Rogers DP, Shramko JK. Therapeutic options in the treatment of clozapine-induced sialorrhea. Pharmacotherapy. 2000;20:1092–5. doi: 10.1592/phco.20.13.1092.35036. [DOI] [PubMed] [Google Scholar]
- 10.Stern RG, Bellucci D, Cursi-Vogel N, Hughley J, Elangovan N. Clozapine-induced sialorrhea alleviated by bupropion - A case report. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1578–80. doi: 10.1016/j.pnpbp.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 11.Liang CS, Ho PS, Shen LJ, Lee WK, Yang FW, Chiang KT. Comparison of the efficacy and impact on cognition of glycopyrrolate and biperiden for clozapine-induced sialorrhea in schizophrenic patients: A randomized, double-blind, crossover study. Schizophr Res. 2010;119:138–44. doi: 10.1016/j.schres.2010.02.1060. [DOI] [PubMed] [Google Scholar]
- 12.Bird AM, Smith TL, Walton AE. Current treatment strategies for clozapine-induced sialorrhea. Ann Pharmacother. 2011;45:667–75. doi: 10.1345/aph.1P761. [DOI] [PubMed] [Google Scholar]
- 13.Kreinin A, Miodownik C, Sokolik S, Shestakova D, Libov I, Bergman J, et al. Amisulpride versus moclobemide in treatment of clozapine-induced hypersalivation. World J Biol Psychiatry. 2011;12:620–6. doi: 10.3109/15622975.2010.527370. [DOI] [PubMed] [Google Scholar]
- 14.Kreinin A, Novitski D, Weizman A. Amisulpride treatment of clozapine-induced hypersalivation in schizophrenia patients: A randomized, double-blind, placebo-controlled cross-over study. Int Clin Psychopharmacol. 2006;21:99–103. doi: 10.1097/01.yic.0000188216.92408.69. [DOI] [PubMed] [Google Scholar]
- 15.Croissant B, Hermann D, Olbrich R. Reduction of side effects by combining clozapine with amisulpride: Case report and short review of clozapine-induced hypersalivation-a case report. Pharmacopsychiatry. 2005;38:38–9. doi: 10.1055/s-2005-837771. [DOI] [PubMed] [Google Scholar]


