Abstract
Topical anesthetics are being widely used in numerous medical and surgical sub-specialties such as anesthesia, ophthalmology, otorhinolaryngology, dentistry, urology, and aesthetic surgery. They cause superficial loss of pain sensation after direct application. Their delivery and effectiveness can be enhanced by using free bases; by increasing the drug concentration, lowering the melting point; by using physical and chemical permeation enhancers and lipid delivery vesicles. Various topical anesthetic agents available for use are eutectic mixture of local anesthetics, ELA-max, lidocaine, epinephrine, tetracaine, bupivanor, 4% tetracaine, benzocaine, proparacaine, Betacaine-LA, topicaine, lidoderm, S-caine patch™ and local anesthetic peel. While using them, careful attention must be paid to their pharmacology, area and duration of application, age and weight of the patients and possible side-effects.
Keywords: Topical anesthesia, Eutectic mixture of local anesthetics, iontophoresis, local anesthetic, skin permeation enhancer, sonophoresis, uses and side-effects of topical anesthetics
Introduction
Injections of local anesthetics are painful. It can worsen needle anxiety, and can cause tissue edema, which distorts the surgical site. Use of topical anesthesia can avoid all these problems and is becoming a routine in clinical practice.
Topical anesthesia is defined as superficial loss of sensation in conjunctiva, mucous membranes, or skin, produced by direct application of local anesthetic solutions, ointments, gels or sprays.
The first local anesthetic (cocaine) was a topical anesthetic and was serendipitously discovered to have anesthetic properties, when Albert Niemann in 1860, like many chemists of that era tested his newly isolated compound and noted that it caused numbing of the tongue.[1] In 1884, Karl Koller, an ophthalmic surgeon, demonstrated that general anesthesia could be avoided for ophthalmic procedures by using cocaine application to the conjunctiva.[2] The discovery of various amide and ester local anesthetics, their topical preparations and delivery systems in due course of time opened the gate of immense possible uses of topical anesthetics.
Mechanism of Action
Topical anesthetics reversibly block nerve conduction near their site of administration by targeting free nerve endings in the dermis or mucosa, thereby producing temporary loss of sensation in a limited area. Nerve impulse conduction is blocked by decreasing nerve cell membrane permeability to sodium ions, possibly by competing with calcium-binding sites that control sodium permeability. This change in permeability decreases depolarization and increases excitability threshold until the ability to generate an action potential is lost.
Pharmacology
Topical anesthetics are weak bases. They are made up of three important components: An aromatic ring, an intermediate length ester or amide linkage and a tertiary amine. The aromatic ring is primarily responsible for the lipid solubility that allows diffusion across the nerve cell membrane, determining the intrinsic property of these agents.[3,4,5] Protein binding of these agents depend on both the aromatic and amine portion.[6]
Onset of action, anesthesia depth, and duration of action are determined by the pKa level, pH level, lipid solubility, protein binding, and vasodilatory effects of the specific local anesthetic. Other factors, which play important roles, are the site of application (faster onset at mucosa and sites with thin stratum corneum), vascularity of tissues in the area applied, the surface area, and duration of application.
Ester-type topical anesthetics are metabolized by plasma cholinesterase and other nonspecific esterases, while amide anesthetics are primarily metabolized in the liver via microsomal enzymes. Ester anesthetics are known to cause allergic manifestations on contact, while it is said to be a rare occurrence with amide anesthetics.[7,8,9] Para-amino benzoic acid (PABA), an ester hydrolysis metabolite is also known to be associated with allergic manifestations.[10]
Skin Penetration Routes
There are three pathways to cross the stratum corneum, which is the main barrier for topical anesthetic agent delivery:[11] Intercellular route (through the intercellular spaces of the cornified keratinocytes), para or transcellular route (through the cornified cells) and transappendageal route or shunt pathway (through the openings of the hair follicles and sweat glands) [Figure 1].
Figure 1.
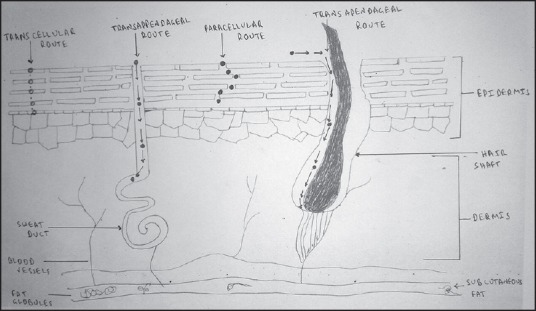
Skin penetration routes
Topical anesthetics are also able to penetrate mucosal surfaces, such as the mouth, genitals, and conjunctiva more easily than through a keratinized surface because of the absence of a stratum corneum.
Factors Determining the Dermal Drug Delivery
Drug form
Free bases are lipophilic and can penetrate the stratum corneum on its own whereas the salt forms require special delivery systems to do so.
Melting point and eutectic mixtures
The lower the melting point, the better the penetration is. Eutectic mixtures have a lower melting point, thus better penetration than either component by itself.
Concentration of drug in vehicle
Higher the concentration of drug in the vehicle, higher the rate of penetration.
Skin permeation enhancers
These compounds, promote skin permeability by increasing the permeability of the stratum corneum temporarily and reversibly. They can be:
Solvents, e.g., water, alcohols, glycerol, low molecular weight ethers, sucrose esters,[12] silicone fluids etc.,
Surfactants[13] e.g., ionic, nonionic, bile salts or
Miscellaneous chemicals, e.g., urea, anticholinergic drugs.
Permeation enhancers under trial are eucalyptol, soya bean casein.[14]
Physical means of enhancing permeation
Skin penetration of topically applied anesthetics can be enhanced by following physical measures:
Exfoliation of the skin.
Degreasing by alcohol.
-
By covering the application area with a dressing or patch of nonporous material such as micropore and tegaderm.
Following energy-dependent active measures are being used/tried to enhance drug delivery across the skin.[15]
-
Iontophoresis [Figure 2] (low voltage current to drive charged drugs through skin).[16] The amount of drug delivered via iontophoresis is dependent on the current and the duration of delivery. Lignocaine HCl 10%/adrenaline 0.1% topical iontophoretic patch (LidoSite) is the first Food and Drug Administration (FDA) approved prefilled active anesthetic patch. Disadvantages of ionotrophoresis technique are:
- Can cause skin irritation at higher current densities or upon longer application,[17]
- Prolonged application can also cause electrochemical polarization in the skin, which decreases the magnitude of current flow through the skin,
- The mild electrical sensation can be uncomfortable for some patients.
- The apparatus is expensive and bulky, and
- It cannot be used over large surface areas of the body.
Electroporation (uses short electrical impulses of high voltage to create transient pores in the skin).[18] The electrical pulses are applied only for fraction of a second; the interval between subsequent pulses allows the skin to depolarize.[19] Therefore, polarization of skin does not interfere with the current flow or drug diffusion.
Sonophoresis or phonophoresis [Figure 3] (low frequency, ultrasonic energy to disrupt stratum corneum)[20] — The ultrasound enhances drug delivery by cavitation, micro steaming and heating. The frequencies used can be high in the range of 0.7-16 MHz or low frequency in the range of 20-100 kHz. Low frequency sonophoresis can allow transdermal delivery of both hydrophilic and high molecular mass permeants at therapeutic levels.
Magnetophoresis/magnetokinesis (application of magnetic field to enhance permeation).[21]
Thermal energy (heat à increases skin permeability).
Erbium:YAG laser pretreatment.[22]
Skin pretreatment with a hand-applied, plastic microneedle array.[23]
Figure 2.
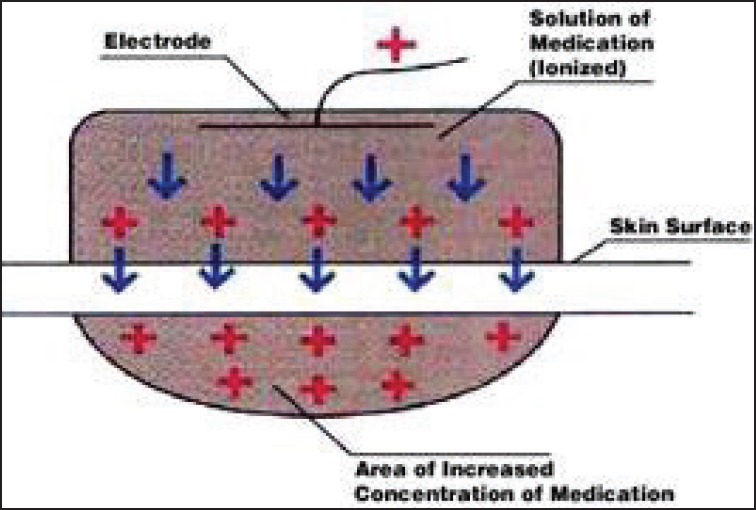
Iontophoresis
Figure 3.
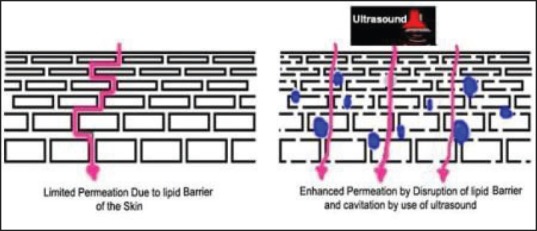
Sonophoresis
Delivery from Lipid Vesicles
Liposomes, niosomes, and transfersomes are examples of lipid vesicles.
Liposomes are microscopic vesicles, which are composed of one or more lipid bilayers arranged in concentric fashion enclosing an equal number of aqueous compartments capable of entrapping lipid soluble or water-soluble drugs. The lipids used are typically phospholipids such as lecithin. Drug molecules can either be encapsulated in the aqueous space or intercalated into the lipid bilayer depending upon its physicochemical characteristics.[24,25] Studies with radioactive or fluorescence-labeled phospholipids have shown that the liposomes disperse in the upper layers of the stratum corneum, without further penetration into the epidermis, dermis or deeper.[26] Fisher et al. in their study found that 5% liposome-encapsulated tetracaine produced better superficial local anesthesia than 5% eutectic mixture of local anesthetics (EMLA).[27] Disadvantages of liposomes are their instability, and the predisposition of phospholipids to oxidative degradation.
Niosomes (microvesicles) are similar to liposomes, but are prepared from nonionic surfactants. They tend to be smaller in diameter than liposomes, and may have unilamellar (one layer), or multilamellar structures. They are more stable and may provide faster penetration to the stratum corneum than liposomes. They do not go deeper either.
Transfersomes are prepared using bile salt (sodium cholate) molecules. Unlike liposomes, transfersomes appear to be highly deformable and researchers claim that they can transport through pores, which are 5 times smaller than their size.
Various Topical Preparations
Eutectic mixture of local anesthetics
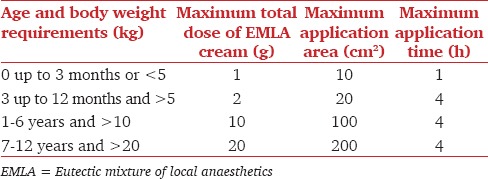
Eutectic mixtures are compounds, which melt at lower temperatures than any of their components, permitting higher concentrations of anesthetics for use. It is 5% oil in water emulsion cream with a melting point of 18°C and consists of 25 mg/mL of lignocaine, 25 mg/mL of prilocaine, a thickener, an emulsifier, and distilled water adjusted to a pH level of 9.4. EMLA is applied in a thick layer (1-2 g/10 cm2, up to a maximal dose of 20 g/200 cm2) to intact skin. Pediatric dosing is shown in Table 1. After application, the area is covered with a patch of tegaderm or clear plastic wrap to facilitate penetration through the stratum corneum. Depth of anesthesia depends on the contact time with EMLA. Anesthetic effect has been shown to reach a maximal depth of 3 mm after a 60-min application, and 5 mm after a 120-min application. Dermal analgesia can be expected to increase for up to 3 h under occlusive dressing and persist for 1-2 h after removal of the cream. EMLA should not be applied to the palms and soles because of variable penetration. EMLA is a pregnancy category B agent, but caution should be exercised when being administered to a nursing mother, because lignocaine is excreted through breast milk (AstraZeneca insert).
Table 1.
Pediatric dosing of EMLA
Tetracaine, adrenaline (epinephrine), and cocaine (TAC)
Consists of 0.5% tetracaine, 0.05% adrenaline, and 11.8% cocaine. It was the first topical anesthetic mixture found to be effective for nonmucosal skin lacerations to the face and scalp. A dose of 1 ml/cm of laceration can be applied using a cotton-tipped applicator with firm pressure that is maintained for 20-40 min. However, it is no longer being used because of general concern about toxicity and expense, and federal regulatory issues involving medications containing cocaine.[28]
Lidocaine, epinephrine, and tetracaine (LET)
Safer and more cost-effective alternative to TAC, contains 4% lignocaine with 0.1% epinephrine and 0.5% tetracaine. LET is used on nonmucosal skin lacerations by placing a few drops directly into the wound. A cotton-tipped applicator with 1-3 mL of the gel or solution is then applied directly to the wound with firm pressure for 15-30 min. It can be safely used in children older than 2 years of age. LET is slightly less effective on extremity lacerations. Because LET contains epinephrine, application to end-arteriolar parts of the body, such as the digits, should be avoided. Caution must also be exercised when contemplating the use of LET in contaminated wounds, complex wounds, or wounds larger than 6 cm. LET and TAC do not work on intact skin.[28]
Bupivanor
It contains 0.48% bupivacaine and 1:26000 norepinephrine. Bupivanor is an effective alternative to TAC and lidocaine infiltration for local anesthesia during laceration repair, especially on the face and scalp.[29]
ELA-max
It contains 4 or 5% (ELA-max 5) lignocaine cream in a liposomal matrix and is FDA-approved for the temporary relief of pain resulting from minor cuts and abrasions. ELA-max 5 is marketed for temporary relief of anorectal pain. ELA-max is applied to intact skin for 15-40 min with or without occlusion and provides a longer duration of anesthesia compared to nonliposomal preparations. Maximum area of application is 600 cm2. In children weighing less than 20 kg, a single application of ELA-max cream should not be applied to an area larger than 100 cm2.[30]
Betacaine-LA
It contains lignocaine, prilocaine and phenylephrine. Betacaine-LA is a proprietary anesthetic and exact concentration of its ingredients is a trade secret. The pocket insert of the product reports concentrations of lignocaine and prilocaine to be 4 times that of EMLA and so, it should not be applied to an area larger than 300 cm2 in adults and is not advocated for use in children.[30]
4% tetracaine (amethocaine)
It is a long acting ester anesthetic in lecithin gel base, with a recommended application time of 30-min under occlusive dressing and maximum dose limit of 50 mg.[30]
Topicaine
Topicaine is 4% lignocaine in a gel microemulsion drug delivery system. The recommended application time by the manufacturers is 30-60 min. The maximum area of application is 600 cm2 in adults and 100 cm2 in children.[30]
S-Caine Patch™ and local anesthetic peel
The patch (manufactured by ZARS, Inc., Salt Lake City, UT, US) contains a 1:1 eutectic mixture of 70 mg lignocaine and 70 mg tetracaine base, with a disposable, oxygen activated heating element, which helps in accelerating transcutaneous delivery and analgesic effect of local anesthetics. The heating element generates a controlled level of heating (39°C-41°C) over a period of 2 h.[31,32]
Lidoderm patch
Lidoderm is comprised of an adhesive material containing 5% lignocaine. Each adhesive patch contains 700 mg of lignocaine (50 mg/g adhesive) in an aqueous base. It has been recently approved by the FDA for the treatment of pain caused by postherpetic neuralgia.
Proparacaine or proxymetacaine
About 0.5% solution is suitable for ophthalmic use. With a single drop, the onset of anesthesia usually begins within 30 s, the maximum anesthetic effect is achieved at 5-min and duration of corneal anesthesia is 15-25 min.
Miscellaneous agents with topical anesthetic potential
8-10% capsaicin[33] (act on transient receptor potential vanilloid 1, i.e., the transient receptor potential channel of the vanilloid receptor family subtype 1); tetradotoxin,[34,35] 0.8% nalbuphine,[36] ethyl chloride spray[37] etc.
Clinical applications
For local analgesia on intact skin-EMLA, 4% tetracaine, S-Caine Patch™.
Minimize discomfort prior to injections or before intravenous and arterial line[38] access-EMLA, 4% tetracaine.
For symptomatic relief of chronic pain-heated lidocaine/tetracaine patch has potential utility for managing myofascial trigger points.[39] Successful treatment of trigeminal neuralgia by topical anesthetic oxybuprocaine or proxymetacaine instilled in the eye of the affected side is also reported.[40,41]
To relieve pruritus and pain due to minor burns, skin eruptions (e.g., herpes, sunburn, insect bites), stings, poison ivy, and minor cuts and scratches.-EMLA, lidocaine, epinephrine, and tetracaine (LET), bupivanor, ELA-max.
To assist awake fiberoptic intubation-topical application, “spray as you go” technique, using MADgic device etc., 2% or 4% lignocaine.
-
In ophthalmology and optometry-0.5% proparacaine, 0.4% oxybuprocaine, 2% lignocaine aqueous gel and drops, 0.5% tetracaine.
To numb the outermost layers of the cornea and conjunctiva to:
- Perform a contact/applanation tonometry.
- Perform a Schirmer's test.
- Remove small foreign bodies.
- During procedures as cryotherapy, shave biopsy, curettage of molluscum contagiosa, and laceration repair.
- Cataract phacoemulsification and minor laser surgeries.
- Intravitreal injection.[42]
In dentistry-to numb oral tissues before administering a dental local anesthetic and for symptomatic relief in aphthous stomatitis-2-8% lignocaine, benzocaine 10% and 20%, EMLA.
-
Otorhinolaryngology, for the day care and office based procedures[43] like:
- Topical anesthesia of the tympanic membrane for tympanocentesis; myringotomy; transtympanic injection of gentamicin or steroids; and pressure equalizing tube placement, removal, or manipulation - using topical 80-90% liquefied phenol[44] (provides an effective full-thickness analgesia with an immediate effect), a solution of 8% tetracaine base in 70% isopropyl alcohol[45] or EMLA.[46]
- Indications in nasal cavity include examination using rigid or flexible endoscopes, nasal debridement, control of epistaxis, treatment of nasal fractures,[47] and management of abscesses and hematomas.-Commonly administered in conjunction with a vasoconstricting substance such as 0.05% oxymetazoline for decongestion of mucosal edema.
- In oral cavity and oropharynx-to assist in awake fiberoptic intubation or laryngoscopy, local examination,[48] closure of a laceration, incision and drainage of a peritonsillar abscess, and treatment of patients who have sustained severe dentoalveolar trauma like maxillomandibular fixation for mandible fractures. The topical anesthetics applied to the mucous membranes in the oral cavity can be useful in alleviating the pain associated with infiltration of local anesthetics, which can be a source of great apprehension for many patients.
- Topical anesthesia of the larynx helps in diagnostic laryngoscopy and bronchoscopy, transnasal esophagogastroduodenoscopy, and placement of an endotracheal tube when awake intubation is indicated either electively or emergently.
For superficial dermatologic, esthetic, and laser procedures like venipuncture,[49] hair and warts removal, split thickness skin graft harvesting,[50] shave or excision biopsy, dermabrasion for tattoo removal, venous leg ulcer debridement, curettage and electrosurgery, treatment of port-wine stains etc.,-EMLA 5%.
For minor penile surgery like circumcision, short frenulum plasty, meatotomy, fulguration of penile and urethral warts. Also for, temporary relief of premature ejaculation when applied to the glans of the penis.[51] -EMLA 5%, benzocaine.
Contraindications
Ester group topical anesthetics are contraindicated in patients with known allergy to PABA, sulfonamides and hair dyes.
Adverse effects
Burning or stinging at the administration site.
Systemic toxicity-due to excess dosage, repeated use, particularly in patients on risk like infants or children or elderly, or patients with liver disease etc. Manifestations can be as follows:
Nonspecific-metallic taste, circumoral numbness, diplopia, tinnitus, dizziness.
Central nervous system (CNS): High plasma concentration can produce CNS excitation (agitation, confusion, muscle twitching, seizure), or CNS depression (drowsiness, obtundation, coma or respiratory arrest). Solutions that contain epinephrine may add to the CNS stimulatory effect.
Cardiovascular: Hypertension, tachycardia, ventricular arrhythmias (ventricular tachycardia, torsades de pointes, ventricular fibrillation, or progressive hypotension, conduction block, bradycardia or asystole. Local anesthetics that contain epinephrine may cause hypertension, tachycardia, and angina.
Treatment as per local anesthetic systemic toxicity treatment guidelines[52] of the American Society of Regional Anesthesia and Pain Medicine (ASRA) and encompasses airway management, cardiovascular support, seizure suppression and use of 20% intralipid.
Allergic reaction to local anesthetics - local anesthetics with a PABA ester-type structure seem to cause most anesthesia-related allergic reactions.
Gag-reflex suppression may occur with oral administration.
Methemoglobinemia (with prilocaine[53] and benzocaine[54]) - signs and symptoms of methemoglobinemia (methemoglobin >1%) include dyspnea, cyanosis, headache, fatigue, exercise intolerance, dizziness and loss of consciousness. Arterial blood with elevated methemoglobin shows a characteristic chocolate-brown color. Severe methemoglobinemia (methemoglobin >50%) manifests as dysrhythmias, seizures, coma and death (>70%). Treatment includes supplemental oxygen, hyperbaric oxygen therapy, exchange transfusion and intravenous administration of the antidote, 1% methylene blue.[55]
Others-skin discoloration, swelling, neuritis, tissue necrosis and sloughing etc.
Because the risk of adverse events with improper application is real, physicians must exercise caution and good judgment while using topical anesthetics.
Conclusion
Topical anesthetics play an important role in decreasing the pain associated with ophthalmological, superficial dermatological, aesthetic and laser procedures, minor surgeries, venipuncture etc. With very wide varieties of agents and delivery devices being improvised upon every day, it seems time is not far off when we can completely abolish use of infiltrative local anesthesia. But, users should be well aware about the pharmacology of the agents being used and possible adverse events.
Footnotes
Source of Support: Nil
Conflicts of Interest: None declared.
References
- 1.Biscoping J, Bachmann-Mennenga MB. Local anesthetics from ester to isomer. Anasthesiol Intensivmed Notfallmed Schmerzther. 2000;35:285–92. doi: 10.1055/s-2000-324. [DOI] [PubMed] [Google Scholar]
- 2.Jackson T, McLure HA. Pharmacology of local anesthetics. Ophthalmol Clin North Am. 2006;19:155–61. doi: 10.1016/j.ohc.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Covino BG. Local anesthesia 1. N Engl J Med. 1972;286:975–83. doi: 10.1056/NEJM197205042861805. [DOI] [PubMed] [Google Scholar]
- 4.Covino BG. Local anesthesia 2. N Engl J Med. 1972;286:1035–42. doi: 10.1056/NEJM197205112861906. [DOI] [PubMed] [Google Scholar]
- 5.Covino BG. Local anesthetic agents for peripheral nerve blocks. Anaesthesist. 1980;29:33–7. [PubMed] [Google Scholar]
- 6.Covino BG. Physiology and pharmacology of local anesthetic agents. Anesth Prog. 1981;28:98–104. [PMC free article] [PubMed] [Google Scholar]
- 7.Suhonen R, Kanerva L. Contact allergy and cross-reactions caused by prilocaine. Am J Contact Dermat. 1997;8:231–5. [PubMed] [Google Scholar]
- 8.Fuzier R, Lapeyre-Mestre M, Mertes PM, Nicolas JF, Benoit Y, Didier A, et al. Immediate- and delayed-type allergic reactions to amide local anesthetics: Clinical features and skin testing. Pharmacoepidemiol Drug Saf. 2009;18:595–601. doi: 10.1002/pds.1758. [DOI] [PubMed] [Google Scholar]
- 9.Kluger N, Raison-Peyron N, Michot C, Guillot B, Bessis D. Acute bullous irritant contact dermatitis caused by EMLA ® cream. Contact Dermatitis. 2011;65:181–3. doi: 10.1111/j.1600-0536.2011.01944.x. [DOI] [PubMed] [Google Scholar]
- 10.Mackie BS, Mackie LE. The PABA story. Australas J Dermatol. 1999;40:51–3. doi: 10.1046/j.1440-0960.1999.00319.x. [DOI] [PubMed] [Google Scholar]
- 11.Adriani J, Dalili H. Penetration of local anesthetics through epithelial barriers. Anesth Analg. 1971;50:834–41. doi: 10.1213/00000539-197150050-00027. [DOI] [PubMed] [Google Scholar]
- 12.Cázares-Delgadillo J, Naik A, Kalia YN, Quintanar-Guerrero D, Ganem-Quintanar A. Skin permeation enhancement by sucrose esters: A pH-dependent phenomenon. Int J Pharm. 2005;297:204–12. doi: 10.1016/j.ijpharm.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 13.Shokri J, Nokhodchi A, Dashbolaghi A, Hassan-Zadeh D, Ghafourian T, Barzegar Jalali M. The effect of surfactants on the skin penetration of diazepam. Int J Pharm. 2001;228:99–107. doi: 10.1016/s0378-5173(01)00805-5. [DOI] [PubMed] [Google Scholar]
- 14.Tian W, Hu Q, Xu Y, Xu Y. Effect of soybean-lecithin as an enhancer of buccal mucosa absorption of insulin. Biomed Mater Eng. 2012;22:171–8. doi: 10.3233/BME-2012-0704. [DOI] [PubMed] [Google Scholar]
- 15.Shipton EA. New delivery systems for local anaesthetics-part 2. Anesthesiol Res Pract 2012. 2012 doi: 10.1155/2012/289373. 289373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenbaum SS. Iontophoresis as a tool for anesthesia in dermatologic surgery: An overview. Dermatol Surg. 2001;27:1027–30. doi: 10.1046/j.1524-4725.2001.01856.x. [DOI] [PubMed] [Google Scholar]
- 17.Kanebako M, Inagi T, Takayama K. Transdermal delivery of indomethacin by iontophoresis. Biol Pharm Bull. 2002;25:779–82. doi: 10.1248/bpb.25.779. [DOI] [PubMed] [Google Scholar]
- 18.Sammeta SM, Vaka SR, Murthy SN. Transdermal drug delivery enhanced by low voltage electropulsation (LVE) Pharm Dev Technol. 2009;14:159–64. doi: 10.1080/10837450802471180. [DOI] [PubMed] [Google Scholar]
- 19.Wallace MS, Ridgeway B, Jun E, Schulteis G, Rabussay D, Zhang L. Topical delivery of lidocaine in healthy volunteers by electroporation, electroincorporation, or iontophoresis: An evaluation of skin anesthesia. Reg Anesth Pain Med. 2001;26:229–38. doi: 10.1053/rapm.2001.22633. [DOI] [PubMed] [Google Scholar]
- 20.Zempsky WT, Robbins B, McKay K. Reduction of topical anesthetic onset time using ultrasound: A randomized controlled trial prior to venipuncture in young children. Pain Med. 2008;9:795–802. doi: 10.1111/j.1526-4637.2008.00421.x. [DOI] [PubMed] [Google Scholar]
- 21.Sammeta SM, Repka MA, Narasimha Murthy S. Magnetophoresis in combination with chemical enhancers for transdermal drug delivery. Drug Dev Ind Pharm. 2011;37:1076–82. doi: 10.3109/03639045.2011.559659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baron ED, Harris L, Redpath WS, Shapiro H, Hetzel F, Morley G, et al. Laser-assisted penetration of topical anesthetic in adults. Arch Dermatol. 2003;139:1288–90. doi: 10.1001/archderm.139.10.1288. [DOI] [PubMed] [Google Scholar]
- 23.Duan D, Moeckly C, Gysbers J, Novak C, Prochnow G, Siebenaler K, et al. Enhanced delivery of topically-applied formulations following skin pre-treatment with a hand-applied, plastic microneedle array. Curr Drug Deliv. 2011;8:557–65. doi: 10.2174/156720111796642318. [DOI] [PubMed] [Google Scholar]
- 24.Gregoriadis G. The carrier potential of liposomes in biology and medicine (second of two parts) N Engl J Med. 1976;295:765–70. doi: 10.1056/NEJM197609302951406. [DOI] [PubMed] [Google Scholar]
- 25.Fendler JH, Romero A. Liposomes as drug carriers. Life Sci. 1977;20:1109–20. doi: 10.1016/0024-3205(77)90481-7. [DOI] [PubMed] [Google Scholar]
- 26.Lieb LM, Ramachandran C, Egbaria K, Weiner N. Topical delivery enhancement with multilamellar liposomes into pilosebaceous units: I. In vitro evaluation using fluorescent techniques with the hamster ear model. J Invest Dermatol. 1992;99:108–13. doi: 10.1111/1523-1747.ep12611886. [DOI] [PubMed] [Google Scholar]
- 27.Fisher R, Hung O, Mezei M, Stewart R. Topical anaesthesia of intact skin: Liposome-encapsulated tetracaine vs EMLA. Br J Anaesth. 1998;81:972–3. doi: 10.1093/bja/81.6.972. [DOI] [PubMed] [Google Scholar]
- 28.Kundu S, Achar S. Principles of office anesthesia: Part II. Topical anesthesia. Am Fam Physician. 2002;66:99–102. [PubMed] [Google Scholar]
- 29.Smith GA, Strausbaugh SD, Harbeck-Weber C, Shields BJ, Powers JD, Hackenberg D. Comparison of topical anesthetics without cocaine to tetracaine-adrenaline-cocaine and lidocaine infiltration during repair of lacerations: Bupivacaine-norepinephrine is an effective new topical anesthetic agent. Pediatrics. 1996;97:301–7. [PubMed] [Google Scholar]
- 30.Friedman PM, Mafong EA, Friedman ES, Geronemus RG. Topical anesthetics update: EMLA and beyond. Dermatol Surg. 2001;27:1019–26. doi: 10.1046/j.1524-4725.2001.01855.x. [DOI] [PubMed] [Google Scholar]
- 31.Sethna NF, Verghese ST, Hannallah RS, Solodiuk JC, Zurakowski D, Berde CB. A randomized controlled trial to evaluate S-Caine patch for reducing pain associated with vascular access in children. Anesthesiology. 2005;102:403–8. doi: 10.1097/00000542-200502000-00025. [DOI] [PubMed] [Google Scholar]
- 32.Sawyer J, Febbraro S, Masud S, Ashburn MA, Campbell JC. Heated lidocaine/tetracaine patch (Synera, Rapydan) compared with lidocaine/prilocaine cream (EMLA) for topical anaesthesia before vascular access. Br J Anaesth. 2009;102:210–5. doi: 10.1093/bja/aen364. [DOI] [PubMed] [Google Scholar]
- 33.Haanpää M, Treede RD. Capsaicin for neuropathic pain: Linking traditional medicine and molecular biology. Eur Neurol. 2012;68:264–75. doi: 10.1159/000339944. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz DM, Fields HL, Duncan KG, Duncan JL, Jones MR. Experimental study of tetrodotoxin, a long-acting topical anesthetic. Am J Ophthalmol. 1998;125:481–7. doi: 10.1016/s0002-9394(99)80188-3. [DOI] [PubMed] [Google Scholar]
- 35.Wang L, Shankarappa SA, Tong R, Ciolino JB, Tsui JH, Chiang HH, et al. Topical drug formulations for prolonged corneal anesthesia. Cornea. 2013;32:1040–5. doi: 10.1097/ICO.0b013e31828cbfe6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee CH, Lin SL, Chi TT, Chang SH, Wang HC. Effect of topical administration of 0.8% nalbuphine on the cornea in dogs after phacoemulsification. J Vet Med Sci. 2013;75:1041–7. doi: 10.1292/jvms.12-0125. [DOI] [PubMed] [Google Scholar]
- 37.Soueid A, Richard B. Ethyl chloride as a cryoanalgesic in pediatrics for venipuncture. Pediatr Emerg Care. 2007;23:380–3. doi: 10.1097/01.pec.0000278396.25129.3f. [DOI] [PubMed] [Google Scholar]
- 38.Ruetzler K, Sima B, Mayer L, Golescu A, Dunkler D, Jaeger W, et al. Lidocaine/tetracaine patch (Rapydan) for topical anaesthesia before arterial access: A double-blind, randomized trial. Br J Anaesth. 2012;109:790–6. doi: 10.1093/bja/aes254. [DOI] [PubMed] [Google Scholar]
- 39.Rauck R, Busch M, Marriott T. Effectiveness of a heated lidocaine/tetracaine topical patch for pain associated with myofascial trigger points: Results of an open-label pilot study. Pain Pract. 2013;13:533–8. doi: 10.1111/papr.12017. [DOI] [PubMed] [Google Scholar]
- 40.Vassilouthis J. Relief of trigeminal neuralgia by proparacaine. J Neurol Neurosurg Psychiatry. 1994;57:121. doi: 10.1136/jnnp.57.1.121-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spaziante R, Cappabianca P, Saini M, de Divitiis E. Topical ophthalmic treatment for trigeminal neuralgia. J Neurosurg. 1995;82:699–700. doi: 10.3171/jns.1995.82.4.0699. [DOI] [PubMed] [Google Scholar]
- 42.Gambrell J, Schaal S. Topical anesthesia for intravitreal injection. Expert Opin Drug Deliv. 2012;9:731–3. doi: 10.1517/17425247.2012.685156. [DOI] [PubMed] [Google Scholar]
- 43.Jourdy DN, Kacker A. Regional anesthesia for office-based procedures in otorhinolaryngology. Anesthesiol Clin. 2010;28:457–68. doi: 10.1016/j.anclin.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 44.Weisskopf A. Phenol anesthesia for myringotomy. Laryngoscope. 1983;93:114. doi: 10.1288/00005537-198301000-00022. [DOI] [PubMed] [Google Scholar]
- 45.Hoffman RA, Li CL. Tetracaine topical anesthesia for myringotomy. Laryngoscope. 2001;111:1636–8. doi: 10.1097/00005537-200109000-00027. [DOI] [PubMed] [Google Scholar]
- 46.Luotonen J, Laitakari K, Karjalainen H, Jokinen K. EMLA in local anaesthesia of the tympanic membrane. Acta Otolaryngol Suppl. 1992;492:63–7. doi: 10.3109/00016489209136812. [DOI] [PubMed] [Google Scholar]
- 47.Atighechi S, Baradaranfar MH, Akbari SA. Reduction of nasal bone fractures: A comparative study of general, local, and topical anesthesia techniques. J Craniofac Surg. 2009;20:382–4. doi: 10.1097/SCS.0b013e31819b945f. [DOI] [PubMed] [Google Scholar]
- 48.Resch K, Schilling C, Borchert BD, Klatzko M, Uden D. Topical anesthesia for pediatric lacerations: A randomized trial of lidocaine-epinephrine-tetracaine solution versus gel. Ann Emerg Med. 1998;32:693–7. doi: 10.1016/s0196-0644(98)70069-1. [DOI] [PubMed] [Google Scholar]
- 49.Hopkins CS, Buckley CJ, Bush GH. Pain-free injection in infants. Use of a lignocaine-prilocaine cream to prevent pain at intravenous induction of general anaesthesia in 1-5-year-old children. Anaesthesia. 1988;43:198–201. [PubMed] [Google Scholar]
- 50.Goodacre TE, Sanders R, Watts DA, Stoker M. Split skin grafting using topical local anaesthesia (EMLA): A comparison with infiltrated anaesthesia. Br J Plast Surg. 1988;41:533–8. doi: 10.1016/0007-1226(88)90013-6. [DOI] [PubMed] [Google Scholar]
- 51.Gyftopoulos KI. The efficacy and safety of topical EMLA cream application for minor surgery of the adult penis. Urol Ann. 2012;4:145–9. doi: 10.4103/0974-7796.102658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neal JM, Bernards CM, Butterworth JF, 4th, Di Gregorio G, Drasner K, Hejtmanek MR, et al. ASRA practice advisory on local anesthetic systemic toxicity. Reg Anesth Pain Med. 2010;35:152–61. doi: 10.1097/AAP.0b013e3181d22fcd. [DOI] [PubMed] [Google Scholar]
- 53.Adams V, Marley J, McCarroll C. Prilocaine induced methaemoglobinaemia in a medically compromised patient. Was this an inevitable consequence of the dose administered? Br Dent J. 2007;203:585–7. doi: 10.1038/bdj.2007.1045. [DOI] [PubMed] [Google Scholar]
- 54.Vallurupalli S, Manchanda S. Risk of acquired methemoglobinemia with different topical anesthetics during endoscopic procedures. Local Reg Anesth. 2011;4:25–8. doi: 10.2147/LRA.S22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cortazzo JA, Lichtman AD. Methemoglobinemia: A Review and Recommendations for Management. J Cardiothorac Vasc Anesth. 2014;28:1055–9. doi: 10.1053/j.jvca.2013.02.005. [DOI] [PubMed] [Google Scholar]


