Abstract
Background and Aims:
We proposed a review of present literature and systematic analysis of present literature to summarize the evidence on the use of β-blockers on the outcome of a patient with severe sepsis and septic shock.
Material and Methods:
Medline, EMBASE, Cochrane Library were searched from 1946 to December 2013. The bibliography of all relevant articles was hand searched. Full-text search of the grey literature was done through the medical institution database. The database search identified a total of 1241 possible studies. The citation list was hand searched by both the authors. A total of 9 studies were identified.
Results:
Most studies found a benefit from β-blocker administration in sepsis. This included improved heart rate (HR) control, decreased mortality and improvement in acid-base parameters. Chronic β-blocker usage in sepsis was also associated with improved mortality. The administration of β-blockers during sepsis was associated with better control of HR. The methodological quality of all the included studies, however, was poor.
Conclusion:
There is insufficient evidence to justify the routine use of β-blockers in sepsis. A large adequately powered multi-centered randomized controlled clinical trial is required to address the question on the efficacy of β-blocker usage in sepsis. This trial should also consider a number of important questions including the choice of β-blocker used, optimal dosing, timing of intervention, duration of intervention and discontinuation of the drug. Until such time based on the available evidence, there is no place for the use of β-blockers in sepsis in current clinical practice.
Keywords: β-blockers, cardiac index, heart rate control, mortality, sepsis, systematic review
Introduction
Sepsis is a clinical syndrome that arises from an inflammatory response to infection. The response from the host is associated with immune, hormonal, metabolic, bioenergetics, and autonomic nervous system modification. This is associated with an overall catabolic state, excessive adrenergic stimulation, high catecholamine levels, and myocardial depression. Myocardial injury in sepsis is mediated via excessive catecholaminergic action and cytokine production.[1] β-blockers modulate both these pathways. There are several studies that have shown the benefits of β-blockers in sepsis. Animal studies have shown benefits of β-blockers.[2,3,4] Until date, there is no published systematic review on the effect of β-blockers in sepsis. We sought to summarize the evidence from all human studies on the effect of β-blockers in sepsis.
Material and Methods
Methods of inclusion and analysis were developed in accordance with the Cochrane collaboration guidelines.[5]
Search methods
We identified references for this systematic review using Medline, EMBASE and the Cochrane register of controlled trials with search terms sepsis (MeSH term) OR “β-blockers” (MeSH term) OR “β-adrenergic blocking agent*” (all fields) OR “β-antagonist*” (all fields) OR “β-adrenergic antagonist*” OR “β-adrenoreceptor antagonist*” (all fields) OR “β-adrenergic receptor antagonist* (all fields). Appendix 1 shows the search strategy. No language or publication date restriction was imposed. Medline was searched from 1946 and EMBASE from 1947. Bibliographies of all selected articles were searched. We also searched the grey literature via the medical university database. An additional search was done on the clinical trial database.[6]
Study selection and data extraction
All potential relevant studies were obtained and critically appraised. We used the following inclusion criteria:
Diagnosed sepsis.
On β-blockers or treated with β-blockers during their Intensive Care Unit (ICU) admission.
Adult population (age >18 or older).
Exclusion criteria were:
Pediatric patients.
Animal studies.
Nonseptic patients.
The eligible studies were heterogeneous and, therefore, did not permit statistical pooling.
Data extraction
After independent review of the finalized articles, the following information was extracted. Year of publication, sample size, study population, heart rate (HR) control, mortality rate, adverse incidence and change in metabolic parameters with the administration of β-blockers. The papers were reviewed to confirm the initial diagnosis of sepsis. Individual authors were contacted to clarify overlapping of patients between studies. Authors were also contacted for data on subgroup analysis.
Study selection
Our electronic database search identified 1242 studies for initial abstract review. We identified 31 texts for full-text review. Of these, 19 were excluded because they did not meet the inclusion/exclusion criteria; 10 editorial reviews, 2 non-β-blockers (calcium channel blockers) 5 animal trials and 2 pediatric population [Figure 1]. Three studies which included 2 case series and 1 randomized controlled trial (RCT) were excluded after clarifying that they referred to the same cohort of patients.[7,8,9] This was confirmed from personal correspondence with the author[7] and by identifying identical methodology and patient cohort in 2 studies.[8,9] We requested the author of one study[10] for subgroup analysis, but they did not respond.
Figure 1.
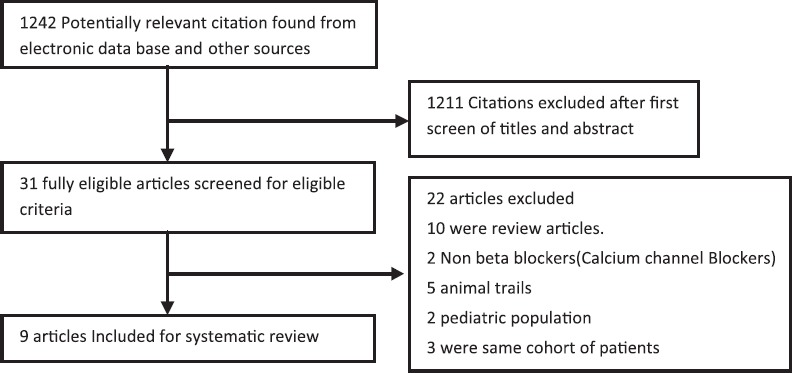
Search flow diagram
Study description
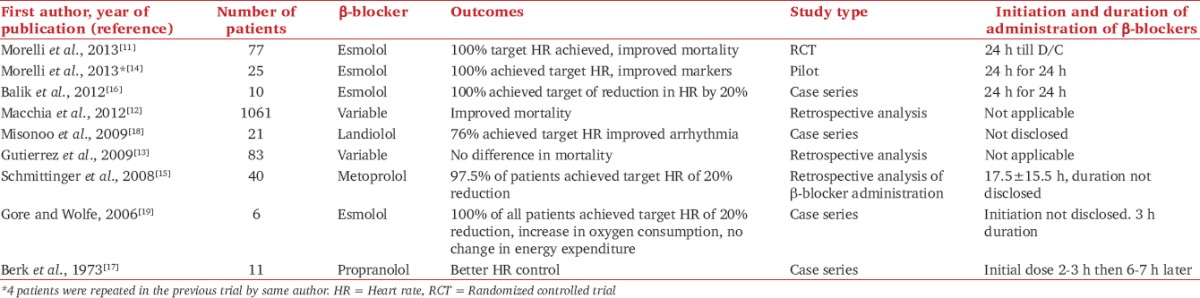
A total of 9 studies were included; of which, 7 were interventional and 2 were observational. These can be further classified as follows: One single center RCT,[11] 2 retrospective observational studies,[12,13] 1 pilot study,[14] 1 retrospective analysis of β-blocker administration,[15] and 4 case series.[16,17,18,19] Table 1 contains analysis of all included studies.
Table 1.
Characteristics of reviewed studies
Results
Hemodynamic parameters
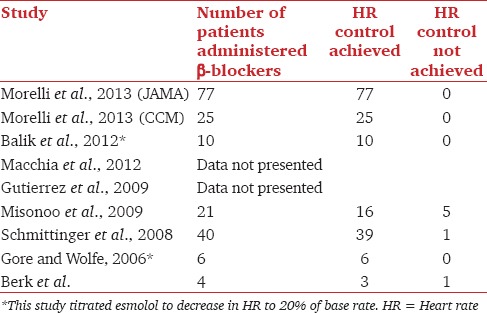
HR reduction was the primary outcome measure in 6 of the 7 interventional studies.[11,14,15,16,18,19] The 2 retrospective studies[12,13] did not provide data on HR control. Table 2 contains data on HR control. In total 179 patients were administered β-blockers and 173 had achieved target HR. In one study,[17] HR data were presented on 4 of the 11 included patients; 3 had better HR control. This is summarized in Table 2.
Table 2.
Heart rate control in reviewed studies
Stroke volume and stroke volume index
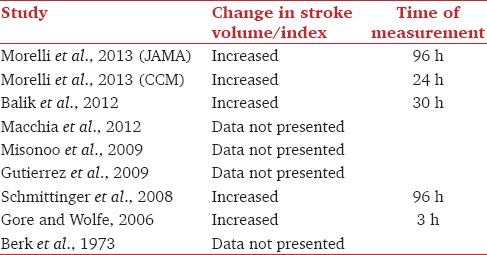
Five studies presented data on stroke volume or stroke volume index.[11,14,15,16,19] These are summarized in Table 3. All values were not statistically significant. Of the interventional studies 2[17,18] had not looked at stroke volume or stroke volume index, 2 retrospective observational studies[12,13] did not provide data on stroke volume or stroke volume index.
Table 3.
Stroke volume change in reviewed studies
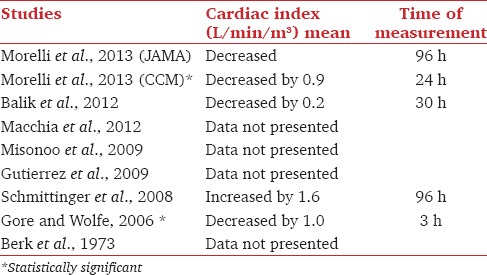
Cardiac index
The data on the cardiac index are summarized in Table 4. Two studies had statistically significant decrease in cardiac output. In one of the case series,[17] cardiac output data were published on 3 of the 11 included patients; of these, cardiac output decreased in 2 patients and in 1 patient cardiac output increased. There were no data on cardiac output or cardiac index presented in 3 studies.[12,13,18]
Table 4.
Cardiac index in reviewed studies
Norepinephrine requirement
There was statistically significant decrease in norepinephrine requirement in one retrospective study[15] and a nonsignificant decrease in three studies.[11,12,16] The other studies have not commented on the norepinephrine requirements.
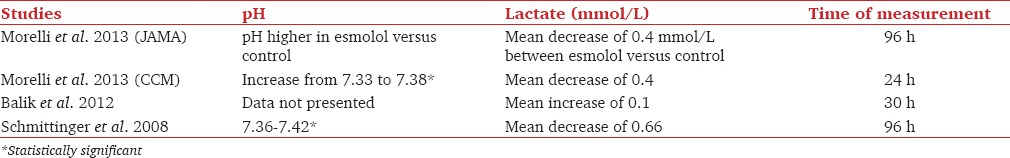
Metabolic variables
The majority of the studies suggest improvement in metabolic variable/s. Data are summarized in Table 5. Gore and Wolfe[19] have reported an increase in adenosine triphosphate/total adenosine phosphate, as well as a statistically significant decrease in glucose, palmitate oxidation, and respiratory quotient. One retrospective analysis[13] reported an initial decrease in serum lactate but a subsequent rise in serum lactate in the β-blocker group as compared to control group. The authors did not comment on the time frame in which the analysis or the intervention compared between the groups. Three studies[12,17,18] did not comment on the metabolic parameters.
Table 5.
Metabolic changes in reviewed studies
Mortality
The RCT[11] showed a statistically significant reduction in mortality in the β-blocker group. One retrospective analysis of patients on β-blockers[12] showed improved survival from 17.8% to 22.1% respectively. This study had not scored the severity of sepsis in both groups. The other retrospective analysis of patients on β-blockers[13] showed no difference in mortality rate with a trend toward the worse outcome, but did not reach statistical significance. One retrospective interventional group[15] and one case series[16] quoted a mortality rate of 33% and 10%, respectively. One case series[17] 7 of the 11 patients had survived. The other studies have not commented on mortality.
Adverse outcome
The retrospective interventional study[15] reported asymptomatic bradycardia in 2 patients, increased norepinephrine requirement in 9 patients and increase in milrinone requirements in 6 patients.
The pilot study[14] administered 500 ml of 6% hydroxyethyl starch in 6 patients. A case series[17] described the use of temporary pacing wire in 1 patient, discontinuing β-blocker because of asymptomatic bradycardia and administration of atropine. This study initiated treatments at HR of 100. The case series[16] reported rebound tachycardia after cessation of esmolol. The rest of the studies have not mentioned significant adverse incidents.
Discussion
This systematic review has revealed some evidence for the benefit of β-blocker in sepsis with limited adverse outcomes. The 9 included studies described 1090 patients on β-blocker prior to ICU admission and 186 patients being administered β-blockers during their ICU stay. β-blocker therapy led to improvement in HR control in 6 studies. There was no significant detrimental effect on MAP. Esmolol was the most commonly used agent in the included studies. Infusions of esmolol had a statistically significant improvement in HR control in 4 studies.[11,14,16,19] Studies that have used esmolol have significantly better HR control than those that used other agents; 100% compared with 89.2%. HR control may be the primary target in future trials as using this target has improved mortality with the limited adverse outcome. However, the target HR value is not yet known; some of the studies targeted a HR of 90 beats per minute, and others targeted a 20% reduction in HR from baseline HR. Increased HR causes shortening of diastolic relaxation time and impairment in diastolic function compromising coronary perfusion thereby leading to cardiac ischemia. Studies have shown that tachycardia is associated with increased incidence of cardiac events in critically ill high-risk patients.[20,21,22] The contribution of HR control alone to the improvement of outcomes in septic patients is currently being addressed by the MODIfY trial (reducing elevated HR in patients with the multiple organ dysfunction syndromes by Ivabradine MODIfY (RCT).
There was a trend toward improvement in stroke volume or stroke volume index[11,14,15,19] and decrease in cardiac output.[11,14,15,18,19] Tachycardia worsens myocardial impairment in sepsis, and diastolic filling thereby leading to a decrease in stroke volume.[20] The improvement in stroke volume and stroke volume index in this review could be because the lowering of HR improves diastolic filling and thereby leads to an improvement in stroke volume. HR control also improves myocardial contractility improving stroke volume. Decreases in HR have a comparable decrease in cardiac output possibly because in sepsis cardiac output is predominately dependent on HR.
The β-adrenergic system has a range of effects on cardiac, immune, metabolic and coagulation function. These functions are altered during sepsis.[23] In the pathogenesis of sepsis, there is impairment of oxygen utilization at the mitochondrial level[23,24] and decrease in oxygen delivery to the cellular level. This leads to anaerobic metabolism and hyperlactatemia. In the reviewed studies, there has been an overall trend toward improvement in lactate levels. The mechanism of this could decrease in cellular oxygen expenditure. Improvement in microvascular flow was reported in one study,[14] which could imply an improvement in cellular oxygen delivery.
Catecholamine mediated the hypermetabolic response in septic shock caused increase in resting energy expenditure, extensive protein, and fat catabolism and hyperglycemia. β2-blockers effects include lowering gluconeogenesis, hyperglycemia, proteolysis and resting energy expenditure.[25] β-blockers have been shown to reduce muscle catabolism in burns;[26] it could be postulated that β-blockers have a similar effect in sepsis. However, one study showed that there was no change in muscle catabolism.[19] It is difficult to draw a conclusion on the exact effect of β-blockers in modulating septic metabolic dysfunction.
β adrenergic system modulates the immune system. β2 pathways up regulate the synthesis of anti-inflammatory cytokine and down regulates the synthesis of pro-inflammatory cytokines.[27] The adrenergic system has an effect on monocyte production and immune system apoptosis.[28] Studies have shown a variable effect of β-blockers on the immune system.
Sepsis associated coagulation dysfunction is partly mediated by the adrenergic system. Platelets express adrenoceptors on their surfaces.[29] α2-adrenoceptors enhance platelet aggregation, and β2 receptors reduce platelet aggregation. β-blockers have been demonstrated to decrease platelet aggregation and adhesion.[30] The action of β-blocker is likely to be by multiple mechanisms.
In most studies, β-blockers were started after 24 h of ICU admission based on the theory that the initial compensatory mechanism of sepsis included increase in sympathetic drive to increase HR and systemic vascular resistance and this mechanism would be required during the initial period. However, patients on chronic β-blockers have shown an improvement in mortality.[12] Animal studies showed improvement in survival with early β-blocker therapy[31] prior to the septic insult. This raises the question on the optimal timing of initiation of β-blocker therapy. The duration of infusion of β-blockers has varied from 3 h to the time of discharge in the studies reviewed. This raises the question on what duration should therapy be continued.
The major limitation of the review is that due to the heterogeneous nature of the study it does not permit statistical analysis.
Conclusion
This review of the available evidence suggests that β-blockers may have a role in sepsis. However, at present, we would not recommend the routine use of β-blockers in sepsis until more robust evidence becomes available. This should take the form of a large multi-centered appropriately powered RCT. This trial should also consider the following important aspects in its methodological design:
Choice of drug,
Timing of initiation of therapy,
Duration of therapy, and
Choice of physiological endpoint to target.
This systematic review of the available evidence to date provides the reassurance that such a trial is necessary and feasible.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank Mr. Steve Parton Senior Librarian Keele Medical University.
Appendix 1: Sources searched: Cochrane, EMBASE, Medline

References
- 1.Carlson DL, Willis MS, White DJ, Horton JW, Giroir BP. Tumor necrosis factor-alpha-induced caspase activation mediates endotoxin-related cardiac dysfunction. Crit Care Med. 2005;33:1021–8. doi: 10.1097/01.ccm.0000163398.79679.66. [DOI] [PubMed] [Google Scholar]
- 2.Aboab J, Sebille V, Jourdain M, Mangalaboyi J, Gharbi M, Mansart A, et al. Effects of esmolol on systemic and pulmonary hemodynamics and on oxygenation in pigs with hypodynamic endotoxin shock. Intensive Care Med. 2011;37:1344–51. doi: 10.1007/s00134-011-2236-y. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki T, Morisaki H, Serita R, Yamamoto M, Kotake Y, Ishizaka A, et al. Infusion of the beta-adrenergic blocker esmolol attenuates myocardial dysfunction in septic rats. Crit Care Med. 2005;33:2294–301. doi: 10.1097/01.ccm.0000182796.11329.3b. [DOI] [PubMed] [Google Scholar]
- 4.Hagiwara S, Iwasaka H, Maeda H, Noguchi T. Landiolol, an ultrashort-acting beta1-adrenoceptor antagonist, has protective effects in an LPS-induced systemic inflammation model. Shock. 2009;31:515–20. doi: 10.1097/SHK.0b013e3181863689. [DOI] [PubMed] [Google Scholar]
- 5.Higgins JP, Green S. Cochrane Hand Book for Systematic Review of Interventions Version 5.1.0. The Cochrane Collaboration. 2011. [Last accessed on 2014 Feb 24; Last updated on 2011 Mar]. Available from: http://www.cochrane-handbook.org .
- 6. [Last accessed on 2014 Jan 25]. Available from: http://www.clinicaltrials.gov .
- 7.Morelli A, Ertmer C, Rehberg S, Orecchioni A, Cecchini V, Di Russo A, et al. Heart rate control with esmolol in septic shock: A randomized controlled trial. Intensive Care Med. 2011;37:0342. [Google Scholar]
- 8.Balik M, Rulisek J, Leden P, Zakharchenko M, Otahal M, Bartakova H. Is cardioprotective beta blockade feasible in severe sepsis? A pilot study. Intensive Care Med. 2011;37:0342. [Google Scholar]
- 9.Berk JL, Hagen JF, Dunn JM. The role of beta adrenergic blockade in the treatment of septic shock. Surg Gynecol Obstet. 1970;130:1025–34. [PubMed] [Google Scholar]
- 10.Christensen S, Johansen MB, Tønnesen E, Larsson A, Pedersen L, Lemeshow S, et al. Preadmission beta-blocker use and 30-day mortality among patients in intensive care: A cohort study. Crit Care. 2011;15:R87. doi: 10.1186/cc10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morelli A, Ertmer C, Westphal M, Rehberg S, Kampmeier T, Ligges S, et al. Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: A randomized clinical trial. JAMA. 2013;310:1683–91. doi: 10.1001/jama.2013.278477. [DOI] [PubMed] [Google Scholar]
- 12.Macchia A, Romero M, Comignani PD, Mariani J, D’Ettorre A, Prini N, et al. Previous prescription of ß-blockers is associated with reduced mortality among patients hospitalized in intensive care units for sepsis. Crit Care Med. 2012;40:2768–72. doi: 10.1097/CCM.0b013e31825b9509. [DOI] [PubMed] [Google Scholar]
- 13.Gutierrez J, Hossam A, Lazarezcu R, Kay E, Rundek T. Effect of beta blockers on sepsis outcome. Med Sci Monit. 2009;15:CR499–503. [PubMed] [Google Scholar]
- 14.Morelli A, Donati A, Ertmer C, Rehberg S, Kampmeier T, Orecchioni A, et al. Microvascular effects of heart rate control with esmolol in patients with septic shock: A pilot study. Crit Care Med. 2013;41:2162–8. doi: 10.1097/CCM.0b013e31828a678d. [DOI] [PubMed] [Google Scholar]
- 15.Schmittinger CA, Dünser MW, Haller M, Ulmer H, Luckner G, Torgersen C, et al. Combined milrinone and enteral metoprolol therapy in patients with septic myocardial depression. Crit Care. 2008;12:R99. doi: 10.1186/cc6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balik M, Rulisek J, Leden P, Zakharchenko M, Otahal M, Bartakova H, et al. Concomitant use of beta-1 adrenoreceptor blocker and norepinephrine in patients with septic shock. Wien Klin Wochenschr. 2012;124:552–6. doi: 10.1007/s00508-012-0209-y. [DOI] [PubMed] [Google Scholar]
- 17.Berk JL, Hagen JF, Maly G, Koo R. The treatment of shock with beta adrenergic blockade. Arch Surg. 1973;104:46–51. doi: 10.1001/archsurg.1972.04180010040011. [DOI] [PubMed] [Google Scholar]
- 18.Misonoo Y, Yajima S, Morisaki H, Suzuki T, Takeda J. Efficacy and safety of beta1 adrenoreceptor blockade by landiolol in sepsis. Crit Care Med. 2009 37/12. [Google Scholar]
- 19.Gore DC, Wolfe RR. Hemodynamic and metabolic effects of selective beta1 adrenergic blockade during sepsis. Surgery. 2006;139:686–94. doi: 10.1016/j.surg.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 20.Sander O, Welters ID, Foëx P, Sear JW. Impact of prolonged elevated heart rate on incidence of major cardiac events in critically ill patients with a high risk of cardiac complications. Crit Care Med. 2005;33:81–8. doi: 10.1097/01.ccm.0000150028.64264.14. [DOI] [PubMed] [Google Scholar]
- 21.Kjekshus JK. Importance of heart rate in determining beta-blocker efficacy in acute and long-term acute myocardial infarction intervention trials. Am J Cardiol. 1986;57:43F–9. doi: 10.1016/0002-9149(86)90888-x. [DOI] [PubMed] [Google Scholar]
- 22.Tuman KJ, McCarthy RJ. Individualizing beta-adrenergic blocker therapy: Patient-specific target-based heart rate control. Anesth Analg. 1999;88:475–6. doi: 10.1097/00000539-199903000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Dünser MW, Hasibeder WR. Sympathetic overstimulation during critical illness: Adverse effects of adrenergic stress. J Intensive Care Med. 2009;24:293–316. doi: 10.1177/0885066609340519. [DOI] [PubMed] [Google Scholar]
- 24.Schmittinger CA, Torgersen C, Luckner G, Schröder DC, Lorenz I, Dünser MW. Adverse cardiac events during catecholamine vasopressor therapy: A prospective observational study. Intensive Care Med. 2012;38:950–8. doi: 10.1007/s00134-012-2531-2. [DOI] [PubMed] [Google Scholar]
- 25.Novotny NM, Lahm T, Markel TA, Crisostomo PR, Wang M, Wang Y, et al. beta-Blockers in sepsis: Reexamining the evidence. Shock. 2009;31:113–9. doi: 10.1097/SHK.0b013e318180ffb6. [DOI] [PubMed] [Google Scholar]
- 26.Herndon DN, Hart DW, Wolf SE, Chinkes DL, Wolfe RR. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345:1223–9. doi: 10.1056/NEJMoa010342. [DOI] [PubMed] [Google Scholar]
- 27.de Montmollin E, Aboab J, Mansart A, Annane D. Bench-to-bedside review: Beta-adrenergic modulation in sepsis. Crit Care. 2009;13:230. doi: 10.1186/cc8026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muthu K, Deng J, Romano F, He LK, Gamelli R, Shankar R, et al. Thermal injury and sepsis modulates beta-adrenergic receptors and cAMP responses in monocyte-committed bone marrow cells. J Neuroimmunol. 2005;165:129–38. doi: 10.1016/j.jneuroim.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 29.Hjemdahl P, Larsson PT, Wallén NH. Effects of stress and beta-blockade on platelet function. Circulation. 1991;84(6 Suppl):VI44–61. [PubMed] [Google Scholar]
- 30.Markel A, Brook JG, Levy Y, Aviram M, Youdim MB. Increased platelet adhesion and aggregation in hypertensive patients: Effect of atenolol. Br J Clin Pharmacol. 1983;16:663–8. doi: 10.1111/j.1365-2125.1983.tb02238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ackland GL, Yao ST, Rudiger A, Dyson A, Stidwill R, Poputnikov D, et al. Cardioprotection, attenuated systemic inflammation, and survival benefit of beta1-adrenoceptor blockade in severe sepsis in rats. Crit Care Med. 2010;38:388–94. doi: 10.1097/CCM.0b013e3181c03dfa. [DOI] [PubMed] [Google Scholar]


