Abstract
Intravesical immunotherapy with bacillus Calmette-Guérin (BCG) has been used by urologists worldwide since its approval in 1990 by the US Food and Drug Administration. After 23 yr, it is fair to say that BCG remains the only truly effective intravesical therapy for non–muscle-invasive bladder cancer (NMIBC), with proven effects reducing recurrence and prolonging survival. It would also be fair to say that, as with any longstanding, “old” remedy, many misconceptions and myths have emerged over the years regarding its use, efficacy, and tolerability. These misconceptions regarding BCG often stand in the way of its delivery and optimal care of our patients. In this paper, we aim to dispel some of these myths, following the text of the primary authors' “Critical Discussion Session” at the 2013 annual meeting of the American Urological Association in San Diego, California.
Keywords: Bladder cancer, Urothelial cancer, BCG, Therapy
Myth 1: Bacillus Calmette-Guérin only reduces recurrence rates of non–muscle-invasive bladder cancer
False
BCG is the single most effective intravesical treatment proven to decrease both progression and recurrence based on evidence from high-quality meta-analyses and randomized controlled trials (RCTs) [1–3]. Specifically, using a meta-analysis of 24 RCTs evaluating BCG in >4000 patients with NMIBC, Sylvester et al provided the most convincing evidence that intravesical immunotherapy not only reduces recurrence but also provides an overall 27% risk reduction overall in progression to invasive disease (odds ratio: 0.73; p = 0.001) This benefit was seen in both papillary and carcinoma in situ (CIS) lesions but, importantly, was noted only in trials that utilized maintenance therapy in addition to induction therapy.
With regard to the use of BCG versus mitomycin C (MMC) in preventing progression and recurrence in patients with intermediate- to high-risk disease, Malmstrom et al performed an individual patient data meta-analysis and concluded that “there were no statistically significant differences regarding progression, overall survival, and cancer-specific survival between the two” treatments [4]. What the authors fail to recognize is that there was a clinically relevant difference in outcomes between the groups. Bladder cancer deaths were 5.6% in the BCG group versus 9.3% in the MMC group, and 8.6% of the BCG-treated patients required radical cystectomy compared with 15% of MMC-treated patients. Moreover, this analysis compared maintenance MMC with suboptimal BCG (see Myth 2).
Myth 2: Induction bacillus Calmette-Guérin is sufficient intravesical therapy for non– muscle-invasive bladder cancer
False
Current evidence clearly shows that intravesical BCG should be administered according to the protocol described in SWOG trial 8507 [5]. Induction consists of six weekly intravesical instillations of BCG, followed by maintenance consisting of three weekly treatments at 3 mo and 6 mo and then every 6 mo, for a total of 36 mo. With this regimen, it is possible for approximately 60% of patients who have an initial response to remain tumor free for >5 yr; with maintenance, disease progression can be significantly reduced by 37% in the setting of papillary or CIS lesions [2,5]. The difference in response is significant compared with those who received only induction therapy (approximately only 40% are tumor free at 5 yr), and the “6 + 3” regimen has been validated in other recent RCTs in other countries [6]. Some experts claim that maintenance does not work; they are likely referring to past studies showing differences in patient outcomes with induction BCG alone but compared with suboptimal maintenance schedules (Fig. 1), such as 6-wk induction therapy followed by monthly maintenance [7] or one instillation every 3 mo [8]. The most common “mistake” is simply to follow induction with yet another 6-wk cycle of instillations at 6 mo [7–9]. The risk of recurrence and progression is lifelong in these patients, and induction therapy alone is not enough to produce prolonged immune stimulation necessary for optimal patient outcomes. Furthermore, being an immune-modulatory therapy, the dose and duration of treatment are of paramount importance and likely are related to various factors such as patient age and tumor characteristics. For example, in a recently published study, Oddens et al analyzed full-dose BCG intravesical immunotherapy compared with one-third dose treatments given with a 1- to 3-yr maintenance course (European Organisation for Research and Treatment of Cancer [EORTC] 30962) [10]. They found that full dose for 3 yr was most effective in reducing recurrences of high-risk disease, whereas for intermediate-risk disease, full dose for 1 yr was most effective.
Fig. 1.
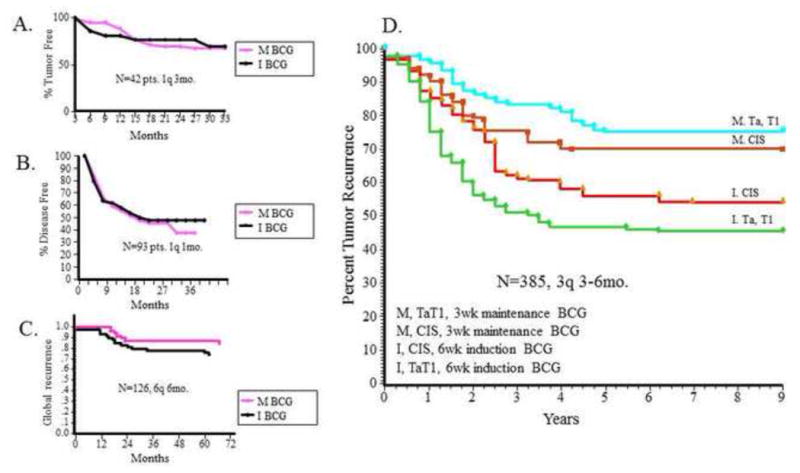
Bacillus Calmette-Guérin (BCG) maintenance is not created equal. (a–c) The left panel depicts Kaplan-Meier curves reflecting recurrence trends in three clinical trials showing no difference between BCG induction alone compared with BCG maintenance (suboptimal schedule) [7–9]. (d) The right panel depicts Kaplan-Meier curves reflecting the importance of true maintenance therapy for tumor recurrence in superficial bladder cancer of all stages [5]. BCG = bacillus Calmette-Guérin; CIS = carcinoma in situ; I = induction; M = maintenance.
Myth 3: Bacillus Calmette-Guérin is indicated only in high-risk disease
False
The use of adjuvant intravesical therapy after transurethral resection of bladder lesions is recommended based on the likelihood of recurrence and progression. The beneficial effect of BCG is greatest in those with both intermediate- and high-risk disease [1], as only those with low-grade and low-volume Ta tumors (low risk) have a <5% probability of progression. High-risk tumors include those with high-grade histology, T1, and CIS lesions. Intermediate-risk tumors include mainly recurrent or high-volume Ta disease. In fact, in a recent well-controlled randomized study by the EORTC 30911, patients with intermediate-risk disease had the most significant decrease in death from bladder cancer (hazard ratio: 0.35 [95% confidence interval, 0.14–0.86]; p = 0.020). This study demonstrated BCG's superiority compared with intravesical epiribucin and provided compelling evidence with long-term end points to recommend the use of BCG for intermediate-risk disease [1].
Myth 4: Most patients are unable to tolerate the “optimal” bacillus Calmette-Guérin regimen
False
This statement is also said as, “My patients can only tolerate induction, so I will avoid maintenance treatment.” Unfortunately, it reflects a lack of diligence on the part of the treating physician. In the aforementioned EORTC 30962 study comparing full-dose and one-third dose intravesical BCG immunotherapy given with a 1-yr or 3-yr maintenance course, there were no significant differences in side effects based on dose or duration of maintenance schedule given [10]. Of the patients included in the study, >90% were able to tolerate intravesical BCG, and only 7.8% had to discontinue treatment because of intolerable toxicity. A recent survey of >100 urologists in both North America and Europe, with data from 971 patients, showed that only 5.2% of subjects needed to stop intravesical BCG therapy because of toxicity [11]. Contrary to previous reports with dismal rates of discontinuation of intravesical treatment ranging from 20% to 80% due to side effects, more contemporary evidence suggests that BCG is, in fact, mostly well tolerated. Patient education, screening and treatment of preexisting bacterial cystitis, delay of intravesical instillation in the face of severe chemical cystitis, and use of a short-course fluoroquinolone antibiotic can help increase tolerability of BCG immunotherapy.
Myth 5: There is no future in bacillus Calmette-Guérin therapy
This statement deserves more than a one-word response. As highlighted by our discussions, BCG therapy administered diligently and with adequate maintenance therapy in patients with intermediate- and high-risk NMIBC positively affects patient outcomes by decreasing recurrence and progression and improving disease-specific survival, as shown by multiple high-quality, well-performed, multi-institutional clinical trials. It is critical, however, to adhere to the principles outlined earlier because mismanaging intravesical therapy can lead to disastrous consequences for our patients. For example, continued BCG in the setting of high-risk recurrence can lead to progression of tumors, including metastatic disease, and can miss the opportunity of cure for the patient. Clearly, BCG therapy is not a substitute for radical cystectomy. However, once the treating community adopts appropriate standards for BCG regimen, we can focus on improvements in patient selection, response assessment, and ways to predict which patients will or will not benefit from intravesical immunotherapy. Furthermore, when this occurs, we can focus our energy toward elucidating the underlying mechanisms of both host and tumor responses to BCG treatment and identify appropriate mechanism-based combination therapies for nonresponders. These are crucial next steps, but they can be adequately studied only when the primary therapy is standardized. Thus, at this time, it remains imperative that we shed our prejudices and misconceptions, dispel the myths surrounding BCG therapy, and provide aggressive, appropriate adjuvant intravesical treatment to improve outcomes in patients with intermediate- and high-risk NMIBC [11].
Footnotes
Conflicts of interest: The authors are on the advisory board of Sanofi-Aventis.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sylvester RJ, Brausi MA, Kirkels WJ, et al. Long-term efficacy results of EORTC Genito-Urinary Group randomized phase 3 study 30911 comparing intravesical instillations of epirubicin, bacillus Calmette-Guérin, and bacillus Calmette-Guérin plus isoniazid in patients with intermediate- and high-risk stage Ta T1 urothelial carcinoma of the bladder. Eur Urol. 2010;57:766–73. doi: 10.1016/j.eururo.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sylvester RJ, van der MA, Lamm DL. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urol. 2002;168:1964–70. doi: 10.1016/S0022-5347(05)64273-5. [DOI] [PubMed] [Google Scholar]
- 3.Sylvester RJ, van der Meijden AP, Witjes JA, Kurth K. Bacillus calmette-guerin versus chemotherapy for the intravesical treatment of patients with carcinoma in situ of the bladder: a meta-analysis of the published results of randomized clinical trials. J Urol. 2005;174:86–91. doi: 10.1097/01.ju.0000162059.64886.1c. discussion 91–2. [DOI] [PubMed] [Google Scholar]
- 4.Malmstrom PU, Sylvester RJ, Crawford DE, et al. An individual patient data meta- analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guerin for non-muscle-invasive bladder cancer. Eur Urol. 2009;56:247–56. doi: 10.1016/j.eururo.2009.04.038. [DOI] [PubMed] [Google Scholar]
- 5.Lamm DL, Blumenstein BA, Crissman JD, et al. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol. 2000;163:1124–9. [PubMed] [Google Scholar]
- 6.Hinotsu S, Akaza H, Naito S, et al. Maintenance therapy with bacillus Calmette-Guerin Connaught strain clearly prolongs recurrence-free survival following transurethral resection of bladder tumour for non-muscle-invasive bladder cancer. BJU Int. 2011;108:187–95. doi: 10.1111/j.1464-410X.2010.09891.x. [DOI] [PubMed] [Google Scholar]
- 7.Badalament RA, Herr HW, Wong GY, et al. A prospective randomized trial of maintenance versus nonmaintenance intravesical bacillus Calmette-Guerin therapy of superficial bladder cancer. J Clin Oncol. 1987;5:441–9. doi: 10.1200/JCO.1987.5.3.441. [DOI] [PubMed] [Google Scholar]
- 8.Hudson MA, Ratliff TL, Gillen DP, Haaff EO, Dresner SM, Catalona WJ. Single course versus maintenance bacillus Calmette-Guerin therapy for superficial bladder tumors: a prospective, randomized trial. J Urol. 1987;138:295–8. doi: 10.1016/s0022-5347(17)43125-9. [DOI] [PubMed] [Google Scholar]
- 9.Palou J, Laguna P, Millan-Rodriguez F, Hall RR, Salvador-Bayarri J, Vicente-Rodriguez J. Control group and maintenance treatment with bacillus Calmette-Guerin for carcinoma in situ and/or high grade bladder tumors. J Urol. 2001;165:1488–91. [PubMed] [Google Scholar]
- 10.Oddens J, Brausi M, Sylvester R, et al. Final results of an EORTC-GU Cancers Group randomized study of maintenance bacillus Calmette-Guérin in intermediate- and high-risk Ta, T1 papillary carcinoma of the urinary bladder: one-third dose versus full dose and 1 year versus 3 years of maintenance. Eur Urol. 2013;63:462–72. doi: 10.1016/j.eururo.2012.10.039. [DOI] [PubMed] [Google Scholar]
- 11.Witjes JA, Palou J, Soloway M, et al. Current clinical practice gaps in the treatment of intermediate- and high-risk non-muscle-invasive bladder cancer (NMIBC) with emphasis on the use of bacillus Calmette-Guerin (BCG): results of an international individual patient data survey (IPDS) BJU Int. doi: 10.1111/bju.12012. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]


