Abstract
Background
Cardiac pump function is often quantified by left ventricular ejection fraction (LVEF) by various imaging modalities. Since the heart is commonly conceptualized as a hydraulic pump, cardiac power describes the hydraulic function of the heart. We aim to describe the prognostic value of resting cardiac power index (CPI) in ambulatory patients with advanced heart failure (HF).
Methods and Results
We calculated CPI in 495 sequential ambulatory patients with advanced HF who underwent invasive hemodynamic assessment with longitudinal follow-up of adverse outcomes (all-cause mortality, cardiac transplantation, or ventricular assist device placement). The median CPI was 0.44 W/m2 [interquartile range 0.37, 0.52]. Over a median of 3.3 years, there were 117 deaths, 104 transplants, and 20 ventricular assist device placements in our cohort. Diminished CPI (<0.44 W/m2) was associated with increased adverse outcomes (Hazard ratio [95% confidence interval] 2.4 [1.8–3.1], p<.0001). The prognostic value of CPI remained significant after adjustment for age, gender, pulmonary capillary wedge pressure, cardiac index, pulmonary vascular resistance, LVEF, and creatinine (HR 1.5 [1.03–2.3], p=0.04). Furthermore, CPI can risk stratify independent of peak oxygen consumption (HR 2.2 [1.4–3.4], p=0.0003).
Conclusion
Resting cardiac power index provides independent and incremental prediction in adverse outcomes beyond traditional hemodynamic and cardio-renal risk factors.
Keywords: Cardiac power index, prognosis, heart failure
INTRODUCTION
The heart is often conceptualized as a muscular hydraulic pump with the ability to generate both flow (“cardiac output”) and pressure. In a purely hemodynamic sense, cardiac output (CO) describes cardiovascular flow through a closed circuit. Cardiac output encompasses not only intrinsic cardiac contractility, but also a complex interplay with vascular compliance and resistance to flow (impedance) in addition to intravascular volume and cardiac filling pressures. The heart and blood vessels are better analogized to a pump creating hydraulic energy and to the pipes that transmit this energy. In the cardiovascular system, during asystole, blood flow eventually slows to a standstill as a result of dissipative effect of turbulence and flow separation. Therefore, the hydraulic energy of the heart can be characterized by cardiac power output (CPO) or cardiac power index (CPI) as the product of flow (CI) and mean arterial pressure. (1) The product of flow output and systemic arterial pressure is the rate of useful work done, or “cardiac power output.”(2)
The heart has a range of power outputs: resting CPO, maximal CPO, and reserve CPO (maximal CPO – resting CPO). As pump dysfunction occurs over time (i.e., incident myocardial infarction, valvular heart disease, myocarditis, etc.), the maximal CPO decreases with corresponding decrements in reserve CPO; and, if severe, is followed by decrements in resting CPO which may lead to severe HF or even cardiogenic shock. (3)
In patients with chronic heart failure (HF), maximal CPO and reserve CPO, measured non-invasively or invasively during cardiopulmonary stress testing (CPX), are strong predictors of mortality.(4–9) When measured in the acute setting, resting CPO can help identify different acute HF syndromes including cardiogenic shock, (3) and is associated with worsening HF and incident mortality. (10, 11) However, there are few data regarding the prognostic impact of resting CPO in chronic HF. (9) Because worsening resting CPO may correlate with HF severity, (3) we hypothesize that invasively measured resting CPO indexed to body surface area (commonly known as CPI), is associated with long-term transplant and ventricular assist device-free survival in an advanced HF cohort.
METHODS
Study Population
This is a retrospective cohort study comprised of ambulatory patients with chronic heart failure seen at the Cleveland Clinic from January 1, 2000, to December 31, 2005. Medical records of all consecutive patients ≥ 18 years old with advanced chronic heart failure (ACHF, >6 months) who had undergone PAC as part of an outpatient assessment. Pulmonary artery catheterization (PAC) was indicated for assessment of disease severity often secondary to progressive signs or symptoms of heart failure. Patients were excluded if they had complex congenital heart disease, were on long-term inotropic drug infusions, or if they were admitted into the hospital directly after PAC for management of decompensated heart failure. The Cleveland Clinic Institutional Review Board approved the study.
Data synthesis and variable definitions
Data abstraction and adjudication has been described previously. (12) If patients had multiple PACs, only data from the first PAC were used. Collected data include demographic characteristics, medical history, drug and device therapy, laboratory values, and underlying heart rhythm. B-type natriuretic peptide (BNP) levels were measured at baseline and at 1- and 6-month follow-up intervals if available. Cardiopulmonary stress test data - including peak-exercise oxygen consumption [Peak VO2] and echocardiographic data were collected if performed within 1 month of the outpatient clinic visit. Cardiopulmonary stress testing was performed according to the recommendations by the American Heart Association. (13) The left ventricular ejection fraction (LVEF) was calculated using the biplane Simpson’s method. Left ventricular end diastolic diameter was measured in the parasternal long axis view. Both tests were read by board-certified cardiologists as part of routine care in accordance with the American Society of Echocardiography guidelines. (14)
Assessment of Hemodynamics
PAC was performed via cannulation of the internal jugular vein under fluoroscopic guidance with the patients in the supine position. Filling pressures including right atrial pressure (RAP), pulmonary arterial pressure, and pulmonary capillary wedge pressure (PCWP) were measured at end-expiration at steady state. Mixed central venous blood gas was collected from the tip of the catheter in the pulmonary artery and cardiac output (CO) was estimated using Fick’s equation and indexed to body surface area (BSA): CO/BSA = cardiac index (CI). Mean arterial pressure was measured non-invasively by an automated blood pressure cuff at the time of PAC. Systemic vascular resistance (SVR) was calculated as the mean arterial pressure – right atrial pressure difference divided by CO. Pulmonary vascular resistance was calculated as: [mean pulmonary arterial pressure (MPAP) – PCWP)]/CO. Cardiac power output (CPO) [W] was calculated by the following equation: CPO = mean arterial pressure [mmHg] × CO [L/min] × K, where K=0.0022 (a conversion factor); and was indexed to body surface area: Cardiac power index (CPI) [W/m2] = CPO [W] / (body surface area [m2]).
Endpoints
The time interval from the outpatient visit to either all-cause mortality, heart transplantation (HT), or ventricular assist device placement was defined as the duration of follow-up. All-cause mortality was assessed by analyzing data from the electronic health record in addition to querying the Social Security Death Index. All endpoints were censored on December 31, 2007.
Statistical Methods
Continuous variables were expressed as either mean ± standard deviation of median (interquartile range [IQR]) where appropriate. The unpaired Student’s t-test or Wilcoxon signed-rank test were used to compare parametric and non-parametric continuous variables, respectively. Categorical variables were expressed as percentage (%) with comparisons via Fisher’s Exact test or the chi-square method. CPI was divided into two partitions stratified by the median in order to make clinical comparisons of prognostic value. Only non-missing data were analyzed. P-values of ≤0.05 were considered significant to reject the null hypothesis that there were no differences in transplant-free survival between subjects stratified by median CPI and, in sub-groups with serial BNP levels or with additional stratification by peak VO2. Independent variables include CPI levels stratified by median CPI and dependent variables include all-cause mortality, cardiac transplantation, ventricular assist device placement and serial BNP. Survival analyses were completed via the Kaplan-Meier method and log-rank analysis to compare transplant-free survival curves of CPI stratified by median CPI for the cohort and a sub-group analysis stratified by median peak VO2. Cox-proportional hazards models were used to compare time-to-event analyses to determine hazard ratios [HR] and 95% confidence intervals [CI] for mortality, cardiac transplantation, and ventricular assist device placement for CPI stratified by median CPI. Multivariable models adjusted for age, gender, PCWP, Fick CI, PVR, creatinine, and LVEF. In a subset, BNP levels were added as an additional covariate. Statistical analyses were performed using JMP Pro version 10 (SAS Institute, Inc., Cary, North Carolina).
RESULTS
Baseline Characteristics
Baseline characteristics of our study cohort are described in Table 1, which are representative of a population with advanced HF. CPI levels were distributed with a right skew (Figure 1). The median CPI was 0.44 [0.37, 0.52] [W/m2]. Lower CPI was associated with features consistent with more advanced disease status, including lower body mass index, higher ICD and mineralocorticoid receptor antagonist use, with lower LVEF and higher LVEDD.
Table 1.
Baseline Characteristics (n=495)
| Variable | Overall | CPI < 0.44 [W/m2] (n=247) | CPI ≥ 0.44 [W/m2] (n=248) | p-value |
|---|---|---|---|---|
| Age [years] | 54 ± 11 | 55 ± 11 | 53 ± 12 | 0.2 |
| Male (%) | 75.8 | 76.1 | 75.4 | 0.9 |
| BMI [kg/m2] | 28 ± 6 | 27 ± 5 | 29 ± 6 | 0.002 |
| ICM (%) | 48.3 | 47 | 49.6 | 0.6 |
| LVEF [%] | 20 [15, 25] | 15 [15, 20] | 20 [15, 30] | <.0001 |
| LVEDD [cm] | 6.6 ± 1.1 | 6.8 ± 1.2 | 6.4 ± 1.0 | <.0001 |
| ICD (%) | 38.8 | 44.1 | 33.5 | 0.02 |
| CRT (%) | 9.5 | 10.9 | 8.1 | 0.3 |
| NYHA Class | ||||
| II (%) | 3.6 | 2.8 | 4.4 | 0.5 |
| III (%) | 90.7 | 91.9 | 89.5 | |
| IV (%) | 5.5 | 5.3 | 5.7 | |
| Medications | ||||
| ACEI/ARB (%) | 88.3 | 87.5 | 87.1 | 1 |
| Beta-blocker (%) | 69.9 | 69.2 | 70.6 | 0.8 |
| MRA (%) | 37.9 | 42.7 | 33.1 | 0.03 |
Abbreviations: CPI: cardiac power index; BMI: body mass index; ICM: ischemic cardiomyopathy; LVEF: left ventricular ejection fraction; LVEDD: left ventricular end diastolic diameter; ICD: implantable cardioverter defibrillator; CRT: cardiac resynchronization therapy; NYHA: New York Heart Association; ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; and MRA: mineralocorticoid receptor antagonist.
Missing values: LVEDD: n=101 and LVEF: n=106
Figure 1. Cardiac Power Index Levels [W/m2] in Study Cohort (n=495).
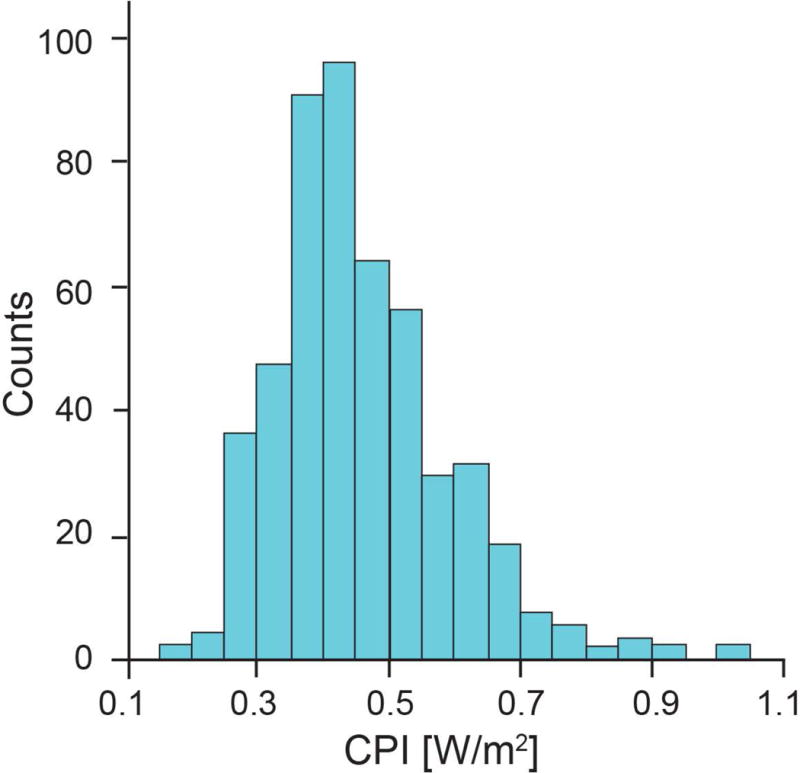
Mean: 0.46 ± 0.13 [W/m2] and Median: 0.44 [0.37, 0.52] [W/m2]. Abbreviation: CPI: cardiac power index
Baseline CPI, Hemodynamics and Lab Values
Baseline hemodynamics are shown stratified by median CPI (Table 2). Lower CPI was associated with lower MAP and CI (p<.0001 for both), but higher RAP, pulmonary arterial pressures, PCWP, SVR, and PVR (p<.001 for all). Lower CPI was associated with higher baseline creatinine (p=0.0008) and BNP (p<.0001). In a subset of patients with available BNP data (n=135) at the time of clinical evaluation, baseline BNP and CPI levels were moderately correlated (Spearman’s rho=−0.43, p<.0001). Lower baseline CPI was associated with higher BNP levels at baseline, and subsequently at 1-month, and 6-month follow-up visits (p<.001 for all) (Figure 2).
Table 2.
Baseline Resting Hemodynamic and Laboratory Values (n=495)
| Variable | Overall | CPI < 0.44 [W/m2] (n=247) | CPI ≥ 0.44 [W/m2] (n=248) | p-value |
|---|---|---|---|---|
| Heart Rate [bpm] | 80 ± 18 | 79 ± 16 | 81 ± 19 | 0.1 |
| MAP [mm Hg] | 86 ± 13 | 81 ± 12 | 91 ± 12 | <.0001 |
| RAP [mm Hg] | 7 [4, 10] | 8 [5, 12] | 6 [3, 9] | <.0001 |
| PASP [mm Hg] | 40 [30, 54] | 45 [32, 57] | 38 [28, 50] | 0.0002 |
| PADP [mm Hg] | 20 [12, 25] | 22 [16, 27] | 18 [12, 22] | <.0001 |
| MPAP [mm Hg] | 27 [19, 35] | 30 [21, 37] | 24 [17, 32] | <.0001 |
| PCWP [mm Hg] | 18 [23, 10] | 20 [13, 25] | 14 [10, 21] | <.0001 |
| Fick CO [L/min] | 4.7 ± 1.3 | 4.1 ± 0.9 | 5.6 ± 1.2 | <.0001 |
| Fick CI [L/min/m2] | 2.4 ± 0.6 | 2.0 ± 0.4 | 2.8 ± 0.5 | <.0001 |
| PVR [WU] | 2.4 ± 1.5 | 2.7 ± 1.7 | 2.0 ± 1.2 | <.0001 |
| SVR [dyn•s/cm5] | 1443 ± 429 | 1530 ± 461 | 1357 ± 376 | <.0001 |
| Hemoglobin [g/dL] | 13.5 ± 1.7 | 13.6 ± 1.6 | 13.4 ± 1.7 | 0.2 |
| Creatinine [mg/dL] | 1.1 [0.9, 1.4] | 1.2 [1.0, 1.5] | 1.0 [0.9, 1.3] | 0.0008 |
| Sodium [mEq/L] | 139 ± 5 | 139 ± 4 | 139 ± 6 | 0.6 |
| BNP [pg/mL] | 348 [137, 782] | 605 [230, 1150] | 228 [81, 484] | <.0001 |
Abbreviations: CPI: cardiac power index; MAP: mean arterial pressure; RAP: right atrial pressure; PASP: pulmonary arterial systolic pressure; PADP: pulmonary arterial diastolic pressure; MPAP: mean pulmonary arterial pressure; PCWP: pulmonary capillary wedge pressure; CO: cardiac output; CI: cardiac index; PVR: pulmonary vascular resistance; SVR: systemic vascular resistance; and BNP: B-type natriuretic peptide.
Missing values: SVR: n=7; PVR: n=8; Hemoglobin: n=47; Creatinine: n=39; Sodium: n=37; and BNP: 360.
Figure 2. Follow-Up B-type Natriuretic Peptide Levels Stratified by Cardiac Power Index Level.
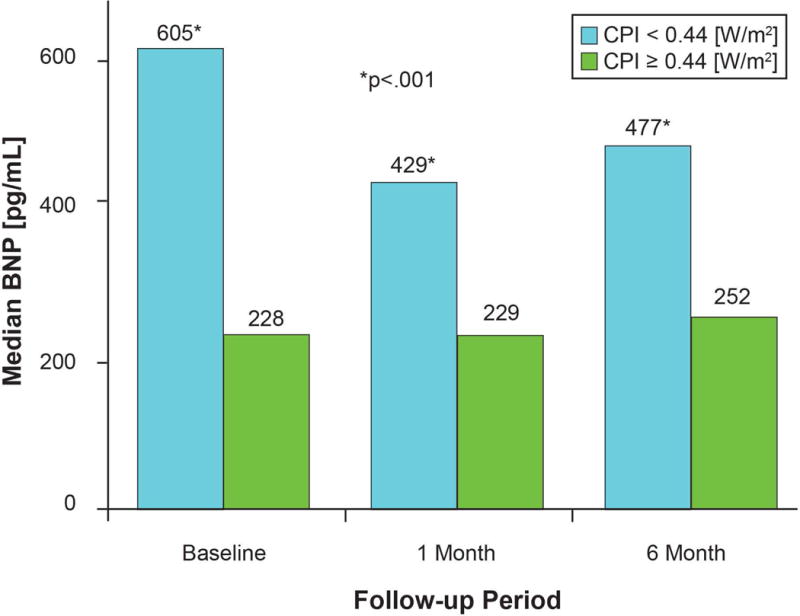
*p<.001. Subset with serial BNP: n=121. Values are expressed as median and p-values calculated via Wilcoxon Signed Rank Test. Abbreviations: BNP: B-type natriuretic peptide and CPI: cardiac power index
CPI and Transplant and Ventricular Assist Device-Free Survival
Of the 495 patients followed for all-cause mortality, cardiac transplantation, or ventricular assist device placement, 234 (47%) patients either died (n=117), underwent cardiac transplantation (n=104), or received a ventricular assist device (n=20) at a median follow-up of 3.3 years after the hemodynamic evaluation. Lower baseline CPI was associated with a significantly lower transplant and ventricular assist device-free survival (Log-rank, chi-square 43.9, p<.0001, Figure 3). CPI below the median predicted a 2.4-fold increase in mortality, cardiac transplantation, or assist device placement (Table 3) when compared to CPI above the median (HR 2.38, [95% CI 1.83–3.11], p<.0001, c-statistic of 0.62 [95% CI 0.59–0.65]). After multivariate adjustment for age, gender, PCWP, Fick CI, creatinine, and LVEF lower CPI still remained independently associated with an increased risk of death, cardiac transplantation, or ventricular assist device placement (HR 1.52 [95% CI 1.03–2.28], p=0.04). Furthermore, lower CPI was still independently associated with an increased risk of death, cardiac transplantation, or ventricular assist device placement when BNP was added to the multivariate adjustment mentioned previously (HR 2.8 [95% CI 1.2–7.3], p=0.02, n=135). Although SVR index (SVRI) is similar to the inverse of CPI, high SVR was associated with a lower hazard ratio was for the composite outcome (HR 1.5, 95% CI 1.1–1.9, p=0.0004). Further, SVRI was modestly correlated to CPI (Spearman rho −0.20, p<.0001) and venous adjusted CPI (MAP-RAP, Spearman rho −0.16, p=0.0003). When analyzed as a continuous predictor, CPI was inversely associated with these adverse outcomes in univariable (per 0.05 unit change, HR 0.80 [95% CI 0.76 – 0.85], p<.0001; c-statistic 0.66 [95% CI 0.62–9.69]) and multivariable analyses (per 0.05 unit change, HR 0.84, [95% CI 0.75 – 0.94], p=0.004).
Figure 3. Kaplan–Meier Estimates of Transplant and Ventricular Assist Device-Free Survival According to Cardiac Power Index Level.
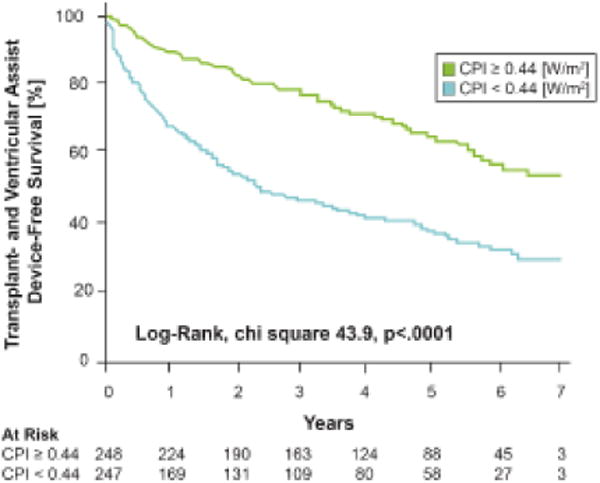
Log-rank, Χ2 43.9, P<0.0001. A total of 117 deaths,104 orthotopic heart transplants, and 20 ventricular assist device placements occurred during a median follow-up of 3.34 years (IQR 1.35, 5.32).
Table 3.
Cox Proportional Hazards Models According to Resting CPI for Death, Mortality, and Ventricular Assist Device Placement
| Variable | Hazard Ratio* | 95% Confidence Interval | P-value |
|---|---|---|---|
| Unadjusted | |||
| Age [years] | 1.02 | 1.004 – 1.03 | 0.006 |
| Male | 1.31 | 0.97 – 1.81 | 0.08 |
| PCWP [mm Hg] | 1.05 | 1.03 – 1.06 | <.0001 |
| Fick CI [L/min/m2] | 0.38 | 0.29 – 0.49 | <.0001 |
| PVR [WU] | 1.22 | 1.14 – 1.29 | <.0001 |
| Creatinine [mg/dL] | 1.64 | 1.32 – 2.01 | <.0001 |
| LVEF [%] | 0.97 | 0.95 – 0.98 | <.0001 |
| CPI† | 2.38 | 1.83 – 3.11 | <.0001 |
| Adjusted | |||
| Age [years] | 1.81 | 0.99 – 1.02 | 0.20 |
| Male | 1.32 | 0.91 – 1.97 | 0.14 |
| PCWP [mm Hg] | 1.01 | 0.99 – 1.03 | 0.16 |
| Fick CI [L/min/m2] | 0.69 | 0.45 – 1.04 | 0.08 |
| PVR [WU] | 1.12 | 1.03 – 1.21 | 0.007 |
| Creatinine [md/dL] | 1.51 | 1.12 – 1.98 | 0.007 |
| LVEF [%] | 0.98 | 0.96 – 1.00 | 0.06 |
| CPI† | 1.52 | 1.03 – 2.28 | 0.04 |
| CPI†‡ | 2.84 | 1.17 – 7.30 | 0.02 |
Per unit change if continuous
CPI ≥ 0.44 vs CPI < 0.44 [W/m2], 241 events
Multivariable adjustment for the previous variables with the addition of BNP in a subset (n=135) with available BNP values.
Abbreviations: CPI: cardiac power index; PCWP: pulmonary capillary wedge pressure; CI: cardiac index; PVR: pulmonary vascular resistance; LVEF: left ventricular ejection fraction; and BNP: B-type natriuretic peptide.
Overall, the median CPI levels were not different when stratified by age (<50 years (n=140): 0.45 W/m2 [0.38, 0.54]; 50–65 years (n=272): 0.43 [0.37, 0.52] 0.43 W/m2, and >65 years (n=83): 0.42 W/m2 [0.35, 0.50]; p=0.29). Indeed, the point estimates for hazard ratios were different in subgroups by age. In Age <50 years, low CPI was associated with 3.7-fold increased risk of death, cardiac transplantation, or ventricular assist device placement (n=56/140, HR 3.7, 95% CI 2.1–7.1, p<.0001); in age 50–65 years low CPI was associated with a 2.4-fold increased risk of death, cardiac transplantation, or ventricular assist device placement (n=139/272, HR 2.4, 95% CI 1.7–3.5, p<.0001); and in age >65 the were was a non-significant trend towards increased risk of death, cardiac transplantation, or ventricular assist device placement with low CPI (n=46/83, HR 1.6, 0.9–2.8, p=0.14). Patients taking all evidence based therapies (beta-blocker, renin-angiotensin system blocker, and mineralocorticoid antagonist, n=124 (25.1%), below median CPI still remained associated with adverse outcomes (HR 1.99, 95% CI 1.18 – 3.49, p=0.01). In a sensitivity analysis for outcomes censored at 1 year of follow-up (death, n=29; cardiac transplantation, n=65; and ventricular assist device placement, n=10), below median CPI remained associated with an increased risk after multivariable adjustment (HR 2.24, 95% CI 1.12–4.47, p=0.02).
CPI and Transplant and Ventricular Assist Device-Free Survival in a Subset with Cardiopulmonary Stress Testing
174 of the original 495 patients had cardiopulmonary stress testing (Figure 4) and were followed cardiac transplantation, ventricular assist device placement, or all-cause mortality. There was no linear association with baseline CPI and peak VO2 levels (R2=0.001). 85 (49%) patients either died (n=38), underwent cardiac transplantation (n=39), or received a ventricular assist device (n=8) at a median follow-up of 3.1 years after the initial clinical visit. The median peak VO2 was 14.6 [ml/kg/min] and below-median peak VO2 was associated with a trend towards increased mortality (HR 1.47, 95% CI 0.98 – 2.23, p=0.06). Regardless of median stratified peak VO2, lower CPI remained a strong predictor of cardiac transplantation, ventricular assist device placement, or all-cause mortality (Log-Rank, chi-square 21.6, p<0.0001 at 3 years, Figure 5).
Figure 4. Peak VO2 [ml/kg/min] in the Subset with Cardiopulmonary Stress Testing (n=174).
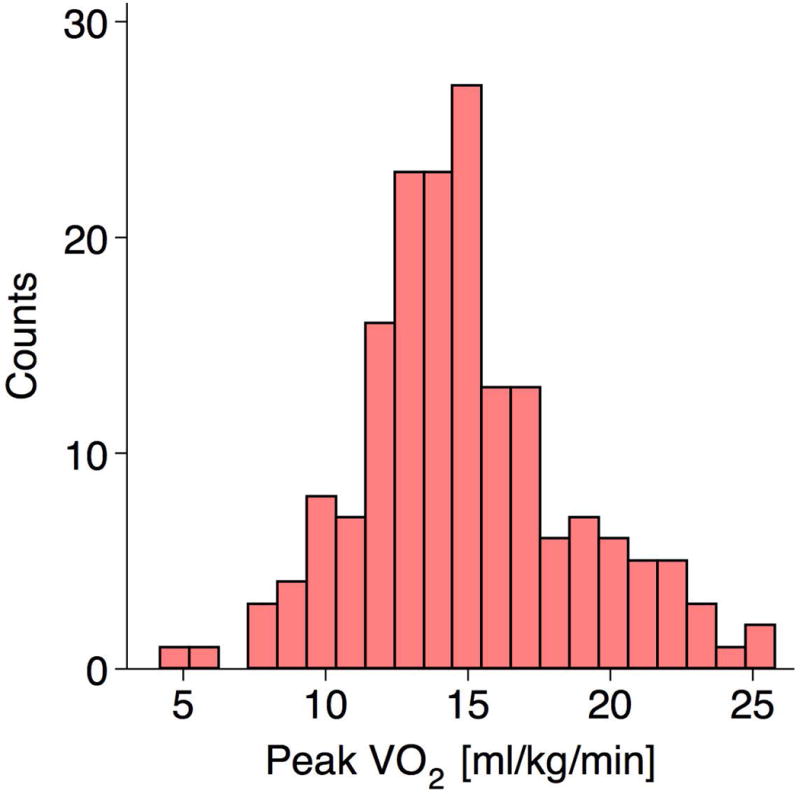
Mean: 14.9 ± 3.8 [ml/kg/min] and Median: 14.6 [12.6, 16.9] [ml/kg/min].
Figure 5. Kaplan-Meier Estimates of Transplant and Ventricular Assist Device-Free Survival According to Cardiac Power Index and Peak VO2.
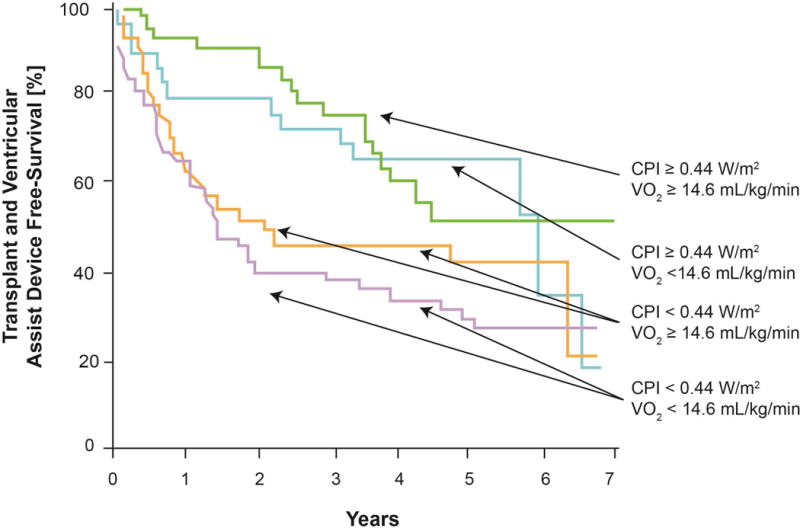
Subset with Peak VO2 measured, n=174. 38 deaths, 39 orthotopic heart transplants, and 8 ventricular assist device placements occurred during a median [interquartile range] follow-up of 3.12 [0.97, 4.83] years. Log-Rank, chi-square 21.6, p<0.0001 for outcomes at 3 years. Units for CPI are W/m2 and Peak VO2 ml/kg/min. Abbreviations: Peak VO2: peak-stress oxygen consumption and CPI: cardiac power index.
DISCUSSION
Our cohort has several key findings which add to our understanding of the clinical consequences of cardiac hydraulic function in ambulatory patients with advanced HF. First, resting CPI was associated with other common markers of HF disease severity at baseline and over time. Second, in a large cohort of ambulatory patients with advanced HF, we observed a strong association between CPI and transplant and ventricular assist device-free survival. Being the largest series of patients on this topic with long-term follow-up, we had the opportunity to conduct a more rigorous evaluation to understand the determinants of resting CPI. We observed that the prognostic value of CPI remained robust after multivariate adjustment for demographic, laboratory, and adverse hemodynamic risk factors including renal function and BNP. Third, although Peak VO2 is a well known prognostic factor in advanced HF, (15) there was no correlation with CPI and Peak VO2 during CPX. Yet, CPI was independently associated with mortality, incident cardiac transplantation, and incident ventricular assist device placement despite stratification by Peak VO2. These findings highlight the potential for resting CPI to be a useful metric in stratifying risk in patients with advanced HF.
Cardiac output and systemic arterial pressure are both measures of cardiac function, but one does not necessarily predict the other. For example, cardiogenic shock is characterized by both low CO and MAP, whereas distributive shock is characterized by a high CO in the setting of low MAP. CPI, however, is an integration of both measures and is a more accurate representation of cardiac pump efficiency.
In this study, resting CPI was associated with common markers of worsening cardiac dysfunction. Because invasive hemodynamic measurements, including elevated right- and left-sided filling pressures, PVR, and CI, are commonly associated with adverse events including renal dysfunction and death in HF, (16–18) it is not surprising that these factors are associated with and may be secondary to cardiac pump function. Plasma natriuretic peptide levels are markers of myocardial stress and are strongly correlated with adverse outcomes in chronic HF. (19, 20) Renal function, a marker of end organ perfusion, is also strongly associated with both mortality and HF progression in patients with LV dysfunction. (21) Our observation regarding the inverse relationship between baseline resting CPI and baseline BNP or creatinine and higher BNP on follow-up assessment suggested that factors related to cardio-renal disease progression may represent the consequences of cardiac inefficiency and suggest that the incremental prognostic value of CPI is a combined metric of heart failure severity.
The concept of cardiac power has been previously put forward in single-center clinical studies with small sample sizes and event rates. In a series of 50 HF patients with New York Heart Association (NYHA) Class II and III symptoms, (9) patients who had adverse cardiac events (heart failure admission, pulmonary edema, or ventricular arrhythmia) or died during follow-up (21.2 ± 1.2 months, total 19 events) had a non-significant trend towards lower resting CPO. In another series with 219 patients with HF, resting CPO was associated with survival in univariable analyses (total 12 events), but not after multivariable adjustment for other exercise-derived parameters. Our current study provided adequate event rates to demonstrate a robust association with resting cardiac pump function and transplant and ventricular assist device-free survival. This association was independent of hemodynamic and cardiorenal risk factors, and thus supports the hypothesis that resting CPI provides clinically meaningful prognostic information that may be utilized when stratifying ACHF patients for LVAD or heart transplantation.
In patients with HF, the combination of reduced cardiac pumping capacity of the heart in conjunction with dysregulated vascular tone can lead to impaired circulatory delivery of oxygen to the peripheral muscles.(22) It is therefore not surprising that peak VO2 on CPX is associated with peak cardiac pumping performance (23) and as maximal pump function declines mortality risk increases. Indeed, both lower peak exercise CPO and reserve CPO, invasively or non-invasively measured during CPX, are correlated with mortality in patients with varying severities of chronic HF. (4–9) To fully understand how the heart fulfills its performance as a pump, the relationship between the resting pump function (resting CPO [or CPI]) to the peak exercise CPO (and thus reserve CPO) may also clarify the progression of cardiac dysfunction in HF. (1) As cardiac dysfunction progresses, peak exercise and reserve CPO likely lower to a point when cardiac pump function at rest begins to decline (resting CPO). (24)
Results from cardiopulmonary stress testing can be used to risk-stratify patients with advanced HF.(25) Peak exercise VO2 during CPX is a strong prognostic measure with the ability to stratify patients with ACHF during evaluation for HT.(15) Indeed, peak VO2 and peak cardiac pump function are correlated when measured during CPX.(23) However, as our results suggest, resting cardiac pump function may not be related to peak VO2 and was associated with transplant and ventricular assist device-free survival independent of peak VO2. This is congruent with earlier findings that abnormalities in central hemodynamic function are poorly correlated with peak exercise capacity.(26) Furthermore, peak VO2 may be affected by multiple factors including age, gender, and body mass. (27, 28) Whereas, CPI may provide more specific information of cardiac pump function. Therefore, peak cardiac pump function and resting cardiac pump function may provide independent information from each other and these results support the use of CPI when stratifying risk in patients with ACHF. They do not, however, suggest CPX results are any less valuable. In fact, because cardiac pump function can be assessed non-invasively (4, 7, 8) during CPX with good reproducibility, (29) these results support an expanded role for CPX. There are many risk prediction models of patients with heart failure, but few if any have invasive hemodynamic measurements and none incorporate CPI.(30) Based on these results, CPI is a robust prognostic factor in patients with advanced heart failure and provides incremental information to other factors. Its prognostic role needs to be validated in other cohorts.
Our results must be interpreted in the context of several limitations in our study design. We cannot exclude the presence of selection bias for patients undergoing evaluation and treatment for ACHF at a tertiary care center. However, because of the high severity of illness, this cohort is uniquely powered to compare adverse heart failure outcomes, while still maintaining external validity as a majority of patients were taking evidence based chronic HF therapies during evaluation. Because resting CPI was only analyzed at one time point, it is unknown whether intermediary transient hemodynamic changes correlate with outcomes. However, because BNP levels remain persistently elevated during follow-up, one value of resting CPI is prognostically and phenotypically informative. We cannot exclude that cardiac transplantation and ventricular assist device placement as endpoints may be biased by donor availability and selection criteria for mechanical circulatory support. Moreover, these data were obtained in an era with non-contemporary device therapies. Although, a majority were on renin-angiotensin system blockers, beta-blockers, and a portion on mineralocorticoid antagonists. The QRS duration was not available in all subjects, some were paced so it was not included in the multivariable analysis. The subjects estimated metabolic rate was used in lieu of measured oxygen consumption (at the time of hemodynamic assessment) in order to compute cardiac output by the Fick principle. We also cannot exclude error introduced by calculating the MAP via non-invasive blood pressure measurements, although this bias would be non-differential.
CONCLUSION
Lower resting CPI is associated with higher left and right sided filling pressures and abnormal cardio-renal biomarkers at baseline and follow-up. Resting CPI provides independent and incremental prediction in transplant and ventricular assist device free-survival beyond hemodynamic, demographic and cardio-renal risk factors or cardiopulmonary stress testing. These findings suggest that CPI is a robust phenotypic and prognostic measure and support its use for risk stratification in patients with advanced heart failure.
Acknowledgments
FUNDING
Dr. Tang is supported is supported by National Institutes of Health grants R01HL103931, P20HL113452, and the Cleveland Clinic Clinical Research Unit of the Case Western Reserve University CTSA (UL1TR 000439-06).
Footnotes
CONFLICTS OF INTEREST
There are no relationships to disclose.
References
- 1.Tan LB. Clinical and research implications of new concepts in the assessment of cardiac pumping performance in heart failure. Cardiovasc Res. 1987 Aug;21(8):615–622. doi: 10.1093/cvr/21.8.615. [DOI] [PubMed] [Google Scholar]
- 2.Tan LB. Cardiac pumping capability and prognosis in heart failure. Lancet. 1986 Dec 13;2(8520):1360–1363. doi: 10.1016/s0140-6736(86)92006-4. [DOI] [PubMed] [Google Scholar]
- 3.Cotter G, Moshkovitz Y, Kaluski E, Milo O, Nobikov Y, Schneeweiss A, Krakover R, Vered Z. The role of cardiac power and systemic vascular resistance in the pathophysiology and diagnosis of patients with acute congestive heart failure. Eur J Heart Fail. 2003 Aug;5(4):443–451. doi: 10.1016/s1388-9842(03)00100-4. [DOI] [PubMed] [Google Scholar]
- 4.Lang CC, Karlin P, Haythe J, Lim TK, Mancini DM. Peak cardiac power output, measured noninvasively, is a powerful predictor of outcome in chronic heart failure. Circ Heart Fail. 2009 Jan;2(1):33–38. doi: 10.1161/CIRCHEARTFAILURE.108.798611. [DOI] [PubMed] [Google Scholar]
- 5.Rosenblum H, Helmke S, Williams P, Teruya S, Jones M, Burkhoff D, Mancini D, Maurer MS. Peak cardiac power measured noninvasively with a bioreactance technique is a predictor of adverse outcomes in patients with advanced heart failure. Congest Heart Fail. 2010 Nov-Dec;16(6):254–258. doi: 10.1111/j.1751-7133.2010.00187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scharf C, Merz T, Kiowski W, Oechslin E, Schalcher C, Brunner-La Rocca HP. Noninvasive assessment of cardiac pumping capacity during exercise predicts prognosis in patients with congestive heart failure. Chest. 2002 Oct;122(4):1333–1339. doi: 10.1378/chest.122.4.1333. [DOI] [PubMed] [Google Scholar]
- 7.Williams SG, Cooke GA, Wright DJ, Parsons WJ, Riley RL, Marshall P, Tan LB. Peak exercise cardiac power output; a direct indicator of cardiac function strongly predictive of prognosis in chronic heart failure. Eur Heart J. 2001 Aug;22(16):1496–1503. doi: 10.1053/euhj.2000.2547. [DOI] [PubMed] [Google Scholar]
- 8.Williams SG, Jackson M, Cooke GA, Barker D, Patwala A, Wright DJ, Albuoaini K, Tan LB. How do different indicators of cardiac pump function impact upon the long-term prognosis of patients with chronic heart failure? Am Heart J. 2005 Nov;150(5):983. doi: 10.1016/j.ahj.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 9.Roul G, Moulichon ME, Bareiss P, Gries P, Koegler A, Sacrez J, Germain P, Mossard JM, Sacrez A. Prognostic factors of chronic heart failure in NYHA class II or III: value of invasive exercise haemodynamic data. Eur Heart J. 1995 Oct;16(10):1387–1398. doi: 10.1093/oxfordjournals.eurheartj.a060747. [DOI] [PubMed] [Google Scholar]
- 10.Torre-Amione G, Milo-Cotter O, Kaluski E, Perchenet L, Kobrin I, Frey A, Rund MM, Weatherley BD, Cotter G. Early worsening heart failure in patients admitted for acute heart failure: time course, hemodynamic predictors, and outcome. J Card Fail. 2009 Oct;15(8):639–644. doi: 10.1016/j.cardfail.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Fincke R, Hochman JS, Lowe AM, Menon V, Slater JN, Webb JG, LeJemtel TH, Cotter G. Cardiac power is the strongest hemodynamic correlate of mortality in cardiogenic shock: a report from the SHOCK trial registry. J Am Coll Cardiol. 2004 Jul 21;44(2):340–348. doi: 10.1016/j.jacc.2004.03.060. [DOI] [PubMed] [Google Scholar]
- 12.Mullens W, Abrahams Z, Skouri HN, Taylor DO, Starling RC, Francis GS, Young JB, Tang WH. Prognostic evaluation of ambulatory patients with advanced heart failure. Am J Cardiol. 2008 May 1;101(9):1297–1302. doi: 10.1016/j.amjcard.2007.12.031. [DOI] [PubMed] [Google Scholar]
- 13.Guazzi M, Adams V, Conraads V, Halle M, Mezzani A, Vanhees L, Arena R, Fletcher GF, Forman DE, Kitzman DW, Lavie CJ, Myers J. EACPR/AHA Scientific Statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation. 2012 Oct 30;126(18):2261–2274. doi: 10.1161/CIR.0b013e31826fb946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hahn RT, Abraham T, Adams MS, Bruce CJ, Glas KE, Lang RM, Reeves ST, Shanewise JS, Siu SC, Stewart W, Picard MH. Guidelines for performing a comprehensive transesophageal echocardiographic examination: recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr. 2013 Sep;26(9):921–964. doi: 10.1016/j.echo.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 15.Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH, Jr, Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991 Mar;83(3):778–786. doi: 10.1161/01.cir.83.3.778. [DOI] [PubMed] [Google Scholar]
- 16.Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, Young JB, Tang WH. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009 Feb 17;53(7):589–596. doi: 10.1016/j.jacc.2008.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Unverferth DV, Magorien RD, Moeschberger ML, Baker PB, Fetters JK, Leier CV. Factors influencing the one-year mortality of dilated cardiomyopathy. Am J Cardiol. 1984 Jul 1;54(1):147–152. doi: 10.1016/0002-9149(84)90320-5. [DOI] [PubMed] [Google Scholar]
- 18.Morley D, Brozena SC. Assessing risk by hemodynamic profile in patients awaiting cardiac transplantation. Am J Cardiol. 1994 Feb 15;73(5):379–383. doi: 10.1016/0002-9149(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 19.Anand IS, Fisher LD, Chiang YT, Latini R, Masson S, Maggioni AP, Glazer RD, Tognoni G, Cohn JN. Changes in brain natriuretic peptide and norepinephrine over time and mortality and morbidity in the Valsartan Heart Failure Trial (Val-HeFT) Circulation. 2003 Mar 11;107(9):1278–1283. doi: 10.1161/01.cir.0000054164.99881.00. [DOI] [PubMed] [Google Scholar]
- 20.James SK, Lindahl B, Siegbahn A, Stridsberg M, Venge P, Armstrong P, Barnathan ES, Califf R, Topol EJ, Simoons ML, Wallentin L. N-terminal pro-brain natriuretic peptide and other risk markers for the separate prediction of mortality and subsequent myocardial infarction in patients with unstable coronary artery disease: a Global Utilization of Strategies To Open occluded arteries (GUSTO)-IV substudy. Circulation. 2003 Jul 22;108(3):275–281. doi: 10.1161/01.CIR.0000079170.10579.DC. [DOI] [PubMed] [Google Scholar]
- 21.Dries DL, Exner DV, Domanski MJ, Greenberg B, Stevenson LW. The prognostic implications of renal insufficiency in asymptomatic and symptomatic patients with left ventricular systolic dysfunction. J Am Coll Cardiol. 2000 Mar 1;35(3):681–689. doi: 10.1016/s0735-1097(99)00608-7. [DOI] [PubMed] [Google Scholar]
- 22.Astrand PO. Quantification of exercise capability and evaluation of physical capacity in man. Prog Cardiovasc Dis. 1976 Jul-Aug;19(1):51–67. doi: 10.1016/0033-0620(76)90008-6. [DOI] [PubMed] [Google Scholar]
- 23.Franciosa JA, Leddy CL, Wilen M, Schwartz DE. Relation between hemodynamic and ventilatory responses in determining exercise capacity in severe congestive heart failure. Am J Cardiol. 1984 Jan 1;53(1):127–134. doi: 10.1016/0002-9149(84)90696-9. [DOI] [PubMed] [Google Scholar]
- 24.Cotter G, Williams SG, Vered Z, Tan LB. Role of cardiac power in heart failure. Curr Opin Cardiol. 2003 May;18(3):215–222. doi: 10.1097/00001573-200305000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013 Oct 15;128(16):e240–327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 26.Sullivan MJ, Higginbotham MB, Cobb FR. Increased exercise ventilation in patients with chronic heart failure: intact ventilatory control despite hemodynamic and pulmonary abnormalities. Circulation. 1988 Mar;77(3):552–559. doi: 10.1161/01.cir.77.3.552. [DOI] [PubMed] [Google Scholar]
- 27.Elmariah S, Goldberg LR, Allen MT, Kao A. Effects of gender on peak oxygen consumption and the timing of cardiac transplantation. J Am Coll Cardiol. 2006 Jun 6;47(11):2237–2242. doi: 10.1016/j.jacc.2005.11.089. [DOI] [PubMed] [Google Scholar]
- 28.Cicoira M, Davos CH, Francis DP, Doehner W, Zanolla L, Franceschini L, Piepoli MF, Coats AJ, Zardini P, Poole-Wilson PA, Anker SD. Prediction of mortality in chronic heart failure from peak oxygen consumption adjusted for either body weight or lean tissue. J Card Fail. 2004 Oct;10(5):421–426. doi: 10.1016/j.cardfail.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 29.Jakovljevic DG, Seferovic PM, Nunan D, Donovan G, Trenell MI, Grocott-Mason R, Brodie DA. Reproducibility of cardiac power output and other cardiopulmonary exercise indices in patients with chronic heart failure. Clin Sci (Lond) 2012 Feb;122(4):175–181. doi: 10.1042/CS20110355. [DOI] [PubMed] [Google Scholar]
- 30.Rahimi K, Bennett D, Conrad N, Williams TM, Basu J, Dwight J, Woodward M, Patel A, McMurray J, MacMahon S. Risk prediction in patients with heart failure: a systematic review and analysis. JACC Heart failure. 2014 Oct;2(5):440–446. doi: 10.1016/j.jchf.2014.04.008. [DOI] [PubMed] [Google Scholar]


