Abstract
Objective
To evaluate the safety, tolerability, and comparative pharmacokinetics (PK) of intravenous and oral carbamazepine.
Methods
In this phase 1, open-label study, adult patients with epilepsy on a stable oral carbamazepine dosage (400–2,000 mg/day) were converted to intravenous carbamazepine (administered at 70% of the oral dosage). A 28-day outpatient period preceded an up to 10-day inpatient period and a 30-day follow-up period. Intravenous carbamazepine was administered over 15 or 30 min every 6 h on days 1–7; some patients in the 15-min group were eligible to receive four 2- to 5-min (rapid) infusions on day 8. Patients underwent blood sampling to determine the area under the concentration–time curve (AUC) for carbamazepine and metabolite carbamazepine-10,11-epoxide following oral (day 0) and intravenous carbamazepine administration (days 1, 7, and 8). Bioequivalence was evaluated in patients with normal renal function (creatinine clearance >80 ml/min). Safety assessments were conducted through day 38.
Results
Ninety-eight patients enrolled and 77 completed the PK component. The mean daily oral and intravenous carbamazepine dosage for 64 PK-evaluable patients with normal renal function was 962.5 and 675.1 mg (70% of oral dosage), respectively. Steady-state minimum concentration (Cmin) and overall exposure (AUC0–24) for intravenous carbamazepine infused over 30, 15, or 2–5 min were similar to oral carbamazepine. The 90% confidence intervals (CIs) for the ratios of the adjusted means for AUC0–24, maximum concentration (Cmax), and Cmin were within the 80%–125% bioequivalence range for 30-min intravenous infusions versus oral administration, but exceeded the upper limit for Cmax for the 15-min and rapid infusions. All intravenous carbamazepine infusions were well tolerated.
Significance
Intravenous carbamazepine infusions (70% of oral daily dose) of 30-, 15-, and 2- to 5-min duration, given every 6 h, maintained patients’ plasma carbamazepine concentrations. Intravenous carbamazepine 30-min infusions were bioequivalent to oral carbamazepine in patients with normal renal function; rapid infusions were well-tolerated in this study.
Keywords: Intravenous carbamazepine, Bioequivalence, Cyclodextrin, Epilepsy, Pharmacokinetic
Key Points
Phase I study to evaluate the safety, tolerability, and comparative pharmacokinetics of intravenous and oral carbamazepine.
Patients received intravenous carbamazepine infused at 70% of oral daily dose at 30-, 15-, or 2- to 5-min duration every 6 h for up to 8 days.
Intravenous carbamazepine infusions maintained patient plasma carbamazepine concentrations.
Intravenous carbamazepine 30-min infusions were bioequivalent to oral carbamazepine in patients with normal renal function.
Rapid infusions were well tolerated in this study.
Carbamazepine is an oral antiepileptic drug (AED) that is prescribed worldwide as a first-line treatment for partial seizures and is considered an essential medicine by the World Health Organization.1 Although the mechanism of action of carbamazepine is not known with certainty, the agent is believed to exert its antiepileptic effects through sodium channel modulation.2
The pharmacokinetics (PK) of carbamazepine following oral administration are complex: it is slowly and variably absorbed,3 moderately protein bound (65–85%), and exhibits autoinduction, which causes the initial low clearance rate to double or triple with continued exposure.4–8 Carbamazepine metabolism occurs in the liver, primarily via the cytochrome P450 (CYP) isozyme CYP3A4, to produce the active metabolite carbamazepine-10,11-epoxide.2
Carbamazepine is insoluble in water, and no commercial intravenous replacement formulation is currently available.9 Patients with epilepsy who are unable to take oral carbamazepine because of gastrointestinal illness, dysphagia, injury, surgery, or change in mental status must rely on other AEDs with parenteral formulations to maintain seizure control. However, switching from oral carbamazepine to another AED carries the risk of breakthrough seizures or intolerability due to adverse events (AEs). An intravenous carbamazepine formulation would be clinically useful, as it would allow patients who are temporarily unable to take oral medications to maintain therapeutic concentrations of an effective drug.
Cyclodextrins are large cyclic molecules that improve the aqueous solubility of lipophilic compounds. Specifically, sulfobutylether β-cyclodextrin (SBECD) sodium salt is used in U.S. Food and Drug Administration (FDA)–approved intravenous formulations of voriconazole,10 ziprasidone mesylate,11 aripiprazole,12 and amiodarone HCl.13 Recently, cyclodextrins have been used to improve the aqueous solubility of carbamazepine, thereby permitting the preparation of an intravenous formulation.14
This study assessed the safety, tolerability, steady-state PK, and bioequivalence of intravenous carbamazepine dissolved in SBECD sodium salt given as 30-, 15-, and 2- to 5-min infusions compared to orally administered carbamazepine in adult patients with epilepsy.
Methods
Study design
Trial OV-1015 (NCT01079351) was a phase 1, multicenter, sequential, open-label study designed to assess the safety, tolerability, and PK of intravenous carbamazepine in relation to oral carbamazepine in patients with epilepsy. The study included a 28-day outpatient lead-in period, followed by an up to 10-day/9-night inpatient confinement period, and a 30-day follow-up period (Fig.1). During the lead-in period, patients were required to take their concomitant medications as prescribed and oral carbamazepine every 6, 8, or 12 h, as appropriate. During the confinement period, patients received oral carbamazepine on days −1 and 0. Following their last oral dose on Day 0 (6 or 8 h for patients taking an immediate release [IR] formulation; 12 h for patients receiving an extended release [ER] formulation), patients were administered their first infusion of intravenous carbamazepine dosed at 70% of the their total daily dose (TDD) of oral carbamazepine, delivered as a 30-min infusion. After an independent data-monitoring committee determined that a sufficient number of patients in the 30-min group had tolerated the infusion, patients were then enrolled in the 15-min infusion group. Patients continued to receive intravenous carbamazepine infusions every 6 h (q6 h) through day 7. Some patients who received 15-min infusions had the option of remaining in the confinement period to assess the safety, tolerability, and PK parameters of 2- to 5-min infusions of intravenous carbamazepine on day 8.
Figure 1.
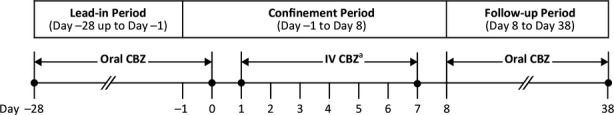
Study design. aPatients received either 30- or 15-min infusions from day 1 to 7, every 6 h. Patients from the 15-min infusion had the option of receiving four 2- to 5-min infusions on day 8.
Patients
Patients who were eligible for participation in this trial had been diagnosed with types of epilepsy for which carbamazepine is indicated (i.e., partial seizures with complex symptomatology [e.g., psychomotor, temporal lobe], generalized tonic–clonic seizures [i.e., grand mal], mixed seizure patterns that include one of the previous seizure types, or other partial or generalized seizures), and were on a stable dosage of oral carbamazepine (IR or ER formulation) for at least 14 days prior to the lead-in period. Doses of all other concomitant medications or interventions that patients were receiving, including AEDs, vagus nerve stimulation, over-the-counter medications, and herbal supplements (provided that they did not interfere with the metabolism of carbamazepine), must have been constant for at least 14 days prior to the lead-in period and remain so throughout the study period. Patients avoided consumption of grapefruit juice during the lead-in and confinement periods.
Patients had to comply with epilepsy medication dosing and maintain an accurate seizure diary. Patients were ineligible to participate if they required daily oral dosages of carbamazepine <400 mg or >2,000 mg; were receiving hormonal birth control; had a QTc interval >450 msec; had an alanine transaminase, aspartate transaminase, or bilirubin concentration ≥3 times the upper limit of normal; had a creatinine clearance <30 ml/min; had a known sensitivity to tricyclic compounds (e.g., amitriptyline, trimipramine, imipramine, or their analogues or metabolites); or had a significant/deteriorating renal disorder.
Trial and consent forms were approved by an institutional review board, and all patients provided written informed consent.
Oral and intravenous carbamazepine
Each patient or the investigative site was responsible for supplying oral carbamazepine. Generic ER and IR formulations of carbamazepine were permitted.
The carbamazepine solution was supplied as 10 mg/ml carbamazepine solubilized with 250 mg/ml SBECD sodium salt (Captisol; Ligand Pharmaceuticals, Inc., La Jolla, CA, U.S.A.) in water, which was sealed in 20 ml single-use vials. The infusion solution was prepared by dilution with 5% dextrose in water. The rationale for infusing at 70% of oral TDD was based on the results from a preliminary PK study (using carbamazepine solubilized in [2-hydroxypropyl]-β-cyclodextrin) that indicated that the absolute bioavailability of oral carbamazepine under steady-state conditions was approximately 70%, with wide intersubject variability (range 40–140%; about two-thirds of patients falling between 50% and 90%).3 By infusing at 70% oral TDD, investigators could reliably ensure a decreased risk of AEs associated with elevated carbamazepine concentrations.
Blood sampling
To assess plasma concentrations and PK parameters of carbamazepine and its metabolite, carbamazepine epoxide, following oral carbamazepine administration, trough blood samples were collected 5 min prior to the patient’s oral dose on days −14, −1, and 0. On day 0, additional samples were drawn at 30- and 60-min following the first oral carbamazepine dose and then at each hour through 12 h. On day 8, a blood sample was collected from patients who received 30- or 15-min intravenous carbamazepine infusions within 5 min of resumption of their oral carbamazepine dose; for patients who were administered 2- to 5-min intravenous carbamazepine infusions, a blood sample was collected within 1 min of resumption of their oral dose of carbamazepine on day 9. On days 8 or 9, samples for all patients were collected at 30 min, and at 1 and 2 h after the first administration of oral carbamazepine, and then again at 3 and 4 h for those patients who received ER oral carbamazepine formulations.
Table S1 describes the blood sampling schedule to evaluate intravenous carbamazepine PK. Blood samples following intravenous carbamazepine administration were obtained in the arm opposite to that used for administration of the intravenous formulation.
Pharmacokinetic assessment
Blood samples (up to 4 ml) were collected at each time point and centrifuged at 1,000 g for 10 min to yield ≥2.5 ml of plasma. The plasma was harvested and stored at −70 ± 10°C until being shipped to a central laboratory (PPD Bioanalytical Labs, Middleton, WI, U.S.A.) for bioanalysis of carbamazepine and carbamazepine epoxide using a validated liquid chromatographic mass spectrometric (LC-MS) method.15 The lower limit of quantitation was 0.05 μg/ml for carbamazepine and carbamazepine epoxide.
Pharmacokinetic parameters
The main PK parameters evaluated for carbamazepine and carbamazepine epoxide included area under the concentration–time curve over 24 h (AUC0–24, calculated for q6 h, q8 h, or q12 h administration by multiplying each AUC value by a factor of 4, 3, or 2, respectively), observed maximum plasma concentration (Cmax) from the first infusion of the day, observed minimum plasma concentration (Cmin), average plasma concentration over the dosing interval (Cav) [Cav = AUC(0–τ)/dosing interval], total steady-state plasma clearance (CL), and time to maximum plasma concentration (Tmax). PK parameters were evaluated using noncompartmental analysis performed on individual data for both oral and intravenous carbamazepine formulations.
Safety assessments
Safety assessments included clinical laboratory tests (hematology, chemistry, and urinalysis), 3-lead telemetry, 12-lead electrocardiography (ECG) testing, vital sign assessments, physical examinations, seizure monitoring (each patient maintained a daily diary during the outpatient lead-in and confinement periods through day 22 where they recorded all AED doses and any seizure activity; site personnel documented seizure activity during the inpatient confinement period), neurologic examinations, and AE monitoring, including infusion-site reactions. Pooled safety results from this trial and a similarly designed phase III safety study (13181A [NCT01128959]) are described in a separate article.16
Sample size
Sample size estimates were determined using the variability related to oral carbamazepine Cmin, using both individual patient and mean values for bioavailability. Statistical comparisons of mean plasma concentration data before dosing and 6 h after intravenous carbamazepine administration to mean values observed in patients in the preliminary PK study3 were performed. Initially, extensive variability in the bioavailability of oral carbamazepine, along with high interpatient variability in other PK parameters,3 required dosing at least 96 patients to ensure Cmin equivalence to be within the established statistical confidence intervals (CIs) with more than 80% power (α = 0.10). As the study progressed, the observed variability of the intravenous formulation was notably less than that previously reported for the oral formulation. Given this observation, it was determined that data from at least 50 patients would be sufficient to perform bioequivalence testing (described below).
Statistical analyses
It was defined a priori that evaluable PK data from all dosed patients with normal renal function (creatinine clearance [CLCR] >80 ml/min) were to be included in the PK analysis set, with the exception of patients who did not complete the last day of intravenous dosing, had their intravenous dosage adjusted from 70% of their oral carbamazepine TDD, or did not receive equally divided oral doses of carbamazepine (unable to compare PK parameters across a single dosing interval). Per protocol, PK data from patients with CLCR ≤80 ml/min and those patients missing PK data were excluded from the analysis; no imputation method was used. For AUC0–24, Cmax, and Cmin, CIs for the ratios of geometric least squares means were compared by analysis of variance (ANOVA) with treatment as a fixed effect to examine potential bioequivalence (90% CIs between 80% and 125%) between the oral and intravenous regimens.17
The safety set included all patients who received ≥1 dose of intravenous carbamazepine.
Results
Patients
A total of 98 patients were enrolled in the study (30-min infusion group: n = 43; and 15-min infusion group: n = 55) and received ≥1 dose of intravenous carbamazepine; these patients were included in the safety population (Fig. S1). A total of 25 and 73 patients were taking IR and ER carbamazepine, respectively. Of these patients, 95 completed dosing through day 7 (15-min infusion: n = 53; and 30-min infusion: n = 42), and 93 completed through day 38 (15-min infusion: n = 51; and 30-min infusion: n = 42). Twelve patients from the 15-min infusion group received 2- to 5-min infusions on day 8, and all completed dosing and follow-up through day 38. The target duration of 2- to 5-min was achieved in 37 (77.1%) of 48 infusions (range 2–26 min).
Four patients (1 and 3 in the 30- and 15-min infusion groups, respectively) discontinued the study prior to day 22. Reasons for discontinuation were voluntary withdrawal (two patients receiving 15-min infusions), AE (one receiving 30-min infusions), and lost to follow-up (one receiving 15-min infusions).
Twenty-one (21.4%) of 98 patients did not meet the predefined inclusion criteria for the PK analyses: 2 (16.7%) of 12 patients in the 2- to 5-min infusion group; 10 (18.2%) of 55 in the 15-min infusion group; and 11 (25.6%) of 43 in the 30-min infusion group. The reasons these patients did not meet the criteria were early termination (three patients, 3.1%), unevenly split daily dose (9, 9.2%), patient did not take oral dose according to dosing regimen (1, 1.0%), first intravenous dose at day 7 was not between 70 ± 2% of oral dose at day 0 (6, 6.1%), and incomplete PK profile (2, 2.0%).
Of the 98 patients enrolled in the study, 77 had evaluable PK data; a subset of 64 patients who had normal renal function and evaluable PK data for both day 0 (oral) and day 7 (intravenous) were included in the PK population. The demographic data and baseline seizure characteristics for patients in the PK population are presented in Table1. Patient baseline characteristics in the PK population were similar to those in the overall patient population in this trial and the phase III safety study.16
Table 1.
Patient demographics and baseline seizure characteristics (PK population with normal renal function)
| Variable | 30-min infusions | 15-min infusionsa | 2- to 5-min infusionsa | Total |
|---|---|---|---|---|
| n = 23 | n = 41 | n = 10 | n = 64 | |
| Age, years | ||||
| Mean (SD) | 41.0 (11.6) | 43.9 (10.8) | 43.7 (10.0) | 42.8 (11.1) |
| Range | 20–65 | 23–68 | 23–53 | 20–68 |
| Male, n (%) | 12 (52.2) | 19 (46.3) | 4 (40.0) | 31 (48.4) |
| Race, n (%) | ||||
| White | 17 (73.9) | 36 (87.8) | 9 (90.0) | 53 (82.8) |
| Black | 5 (21.7) | 5 (12.2) | 1 (10.0) | 10 (15.6) |
| Asian | 1 (4.3) | 0 | 0 | 1 (1.6) |
| Ethnicity, n (%) | ||||
| Hispanic/Latino | 1 (4.3) | 3 (7.3) | 0 | 4 (6.3) |
| Not Hispanic/Latino | 22 (95.7) | 38 (92.7) | 10 (100.0) | 60 (93.8) |
| Time since last seizure (months) | ||||
| Mean (SD) | 31.0 (45.0) | 33.8 (42.7) | 40.0 (55.2) | 32.7 (43.4) |
| Range | 0.0–203.2 | 0.0–153.3 | 0.1–148.6 | 0.0–203.2 |
| Number of seizures per year | ||||
| Mean (SD) | 86.0 (317.8) | 34.1 (61.8) | 43.8 (63.0) | 54.9 (206.4) |
| Range | 0–1,823 | 0–312 | 0–164 | 0–1,823 |
SD, standard deviation.
Patients in the 2- to 5-min infusion group received 15-min infusions on days 1–7, and 2- to 5-min infusions on day 8. Although these patients are counted in the 2- to 5-min and 15-min infusion groups, the total column counts them only once.
Pharmacokinetic analyses of intravenous carbamazepine
Mean steady-state dosage administered and plasma concentrations of carbamazepine
During the study, the mean daily oral and intravenous dosages of carbamazepine for patients with normal renal function were 1,017.4 and 713.0 mg in the 30-min group (n = 23), 931.7 and 653.9 mg in the 15-min group (n = 41), and 960.0 and 673.1 mg in the 2- to 5-min group (n = 10), respectively. The intravenous dosage in each infusion group was ∼70% of the day 0 oral TDD. Figure2 shows the mean plasma concentration–time profiles of carbamazepine after oral administration (day 0) and during the first 6-h intravenous carbamazepine dosing interval (days 1 and 7 for 30- and 15-min durations, days 1 and 8 for the 2- to 5-min duration). The data show that the plasma concentrations associated with intravenous carbamazepine infusion were similar to that of oral carbamazepine on day 0.
Figure 2.
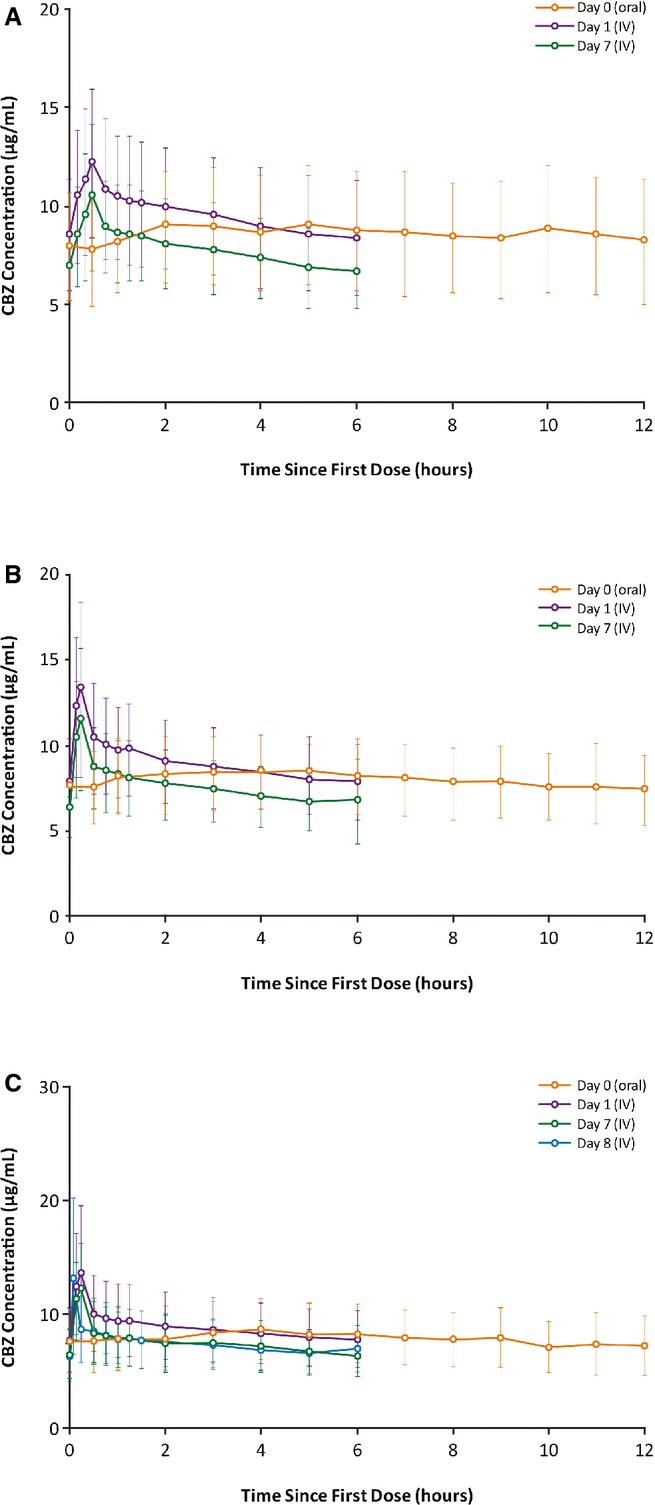
Mean (SD) plasma concentration–time profiles for carbamazepine after oral and intravenous administration over (A) 30 min, (B) 15 min, or (C) 2- to 5-min (patients in the 2- to 5-min infusion group received 15-min infusions on days 1–7, and 2- to 5-min infusions on day 8) in patients with epilepsy with normal renal function. SD, standard deviation.
Steady-state pharmacokinetic parameters and bioequivalence of carbamazepine formulations
Steady-state PK parameters of intravenous and oral carbamazepine for patients with normal renal function are presented in Table2. Mean steady-state values for AUC(0–24) and Cmin for all infusion durations of intravenous carbamazepine and oral carbamazepine were similar; Cmax values following the more rapid infusion rates (15 and 2- to 5-min) were greater than those observed with oral carbamazepine.
Table 2.
Mean (%CV) steady-state plasma pharmacokinetic parameters and ratio of geometric least squares means and 90% confidence intervals in patients with normal renal function
| Infusion time/PK parameter | Carbamazepine | CBZ-E | ||||||
|---|---|---|---|---|---|---|---|---|
| IV carbamazepine,a mean (%CV) | Oral carbamazepine,a mean (%CV) | Ratio of geometric LSM | 90% CI ratio | IV carbamazepine,a mean (%CV) | Oral carbamazepine,a mean (%CV) | Ratio of geometric LSM | 90% CI ratio | |
| Day 7: 30-min infusion (n = 23) | ||||||||
| AUC(0–24) (μg h/ml) | 186.4 (28.0) | 204.6 (33.2) | 0.929 | 0.864, 0.998 | 25.2 (37.4) | 35.3 (43.7) | 0.733 | 0.675, 0.795 |
| Cmax (μg/ml) | 10.8 (34.9) | 9.8 (33.7) | 1.099 | 1.010, 1.197 | 1.2 (39.4) | 1.7 (43.8) | 0.731 | 0.661, 0.809 |
| Cmin (μg/ml) | 6.3 (27.2) | 7.21 (34.3) | 0.892 | 0.828, 0.961 | 0.9 (36.2) | 1.2 (44.7) | 0.752 | 0.691, 0.819 |
| Cav (μg/ml) | 7.8 (28.0) | 8.5 (33.2) | — | — | 1.1 (37.4) | 1.5 (43.7) | — | — |
| Day 7: 15-min infusion (n = 41) | ||||||||
| AUC(0–24) (μg h/ml) | 180.1 (25.0) | 191.5 (26.0) | 0.945 | 0.905, 0.986 | 25.6 (67.0) | 31.3 (54.5) | 0.802 | 0.758, 0.848 |
| Cmax (μg/ml) | 11.8 (34.4) | 9.1 (24.9) | 1.273 | 1.174, 1.381 | 1.2 (67.7) | 1.5 (54.2) | 0.787 | 0.740, 0.836 |
| Cmin (μg/ml) | 6.0 (26.7) | 6.7 (28.4) | 0.900 | 0.857, 0.944 | 0.9 (65.6) | 1.1 (57.2) | 0.824 | 0.781, 0.869 |
| Cav (μg/ml) | 7.5 (25.0) | 8.0 (26.0) | — | — | 1.1 (67.0) | 1.3 (54.4) | — | — |
| Day 8: 2- to 5-min infusion (n = 10) | ||||||||
| AUC(0–24) (μg h/ml) | 179.4 (50.9) | 189.9 (32.6) | 0.961 | 0.887, 1.041 | 21.4 (43.4) | 29.7 (44.8) | 0.735 | 0.671, 0.804 |
| Cmax (μg/ml) | 13.4 (51.0) | 9.0 (31.5) | 1.419 | 1.062, 1.896 | 1.0 (43.7) | 1.4 (44.0) | 0.708 | 0.642, 0.780 |
| Cmin (μg/ml) | 6.2 (33.2) | 6.6 (34.6) | 0.947 | 0.865, 1.037 | 0.8 (0.3) | 1.0 (45.4) | 0.769 | 0.684, 0.865 |
| Cav (μg/ml) | 7.5 (28.3) | 7.9 (32.6) | — | — | 0.9 (43.4) | 1.2 (44.8) | — | — |
CBZ-E, carbamazepine-10,11-epoxide; CV, coefficient of variation; IV, intravenous.
IV carbamazepine = day 7 (15- and 30-min infusions) or day 8 (2- to 5-min infusion); oral carbamazepine = day 0.
Compared to oral carbamazepine, bioequivalence criteria were met for AUC(0–24), Cmax, and Cmin for the 30-min intravenous carbamazepine infusion and for AUC(0–24) and Cmin for the 15- and 2- to 5-min infusions, respectively (Table2). For the 15- and 2- to 5-min infusion groups, the Cmax ratio was 1.273 and 1.419, respectively. Hence, 90% CIs of infusion/oral ratios exceeded the upper limit of the accepted 80–125% bioequivalence range.
Plasma concentrations and pharmacokinetic parameters of carbamazepine epoxide
The mean concentration–time profiles of plasma carbamazepine epoxide after oral administration of carbamazepine on day 0, and during the first 6-h intravenous carbamazepine dosing interval on days 1 and 7 are shown in Figure3A (30-min infusion) and Figure3B (15-min infusion); Fig.3C depicts carbamazepine epoxide profiles following intravenous carbamazepine 2- to 5-min infusions on days 1, 7, and 8. Overall metabolite concentrations on days 7 and 8 were lower following intravenous dosing compared to after oral dosing. Across intravenous infusion durations, the AUC(0–24), Cmax, and Cmin values for carbamazepine epoxide were 17.6–29.2% less than those observed with oral carbamazepine (Table2).
Figure 3.
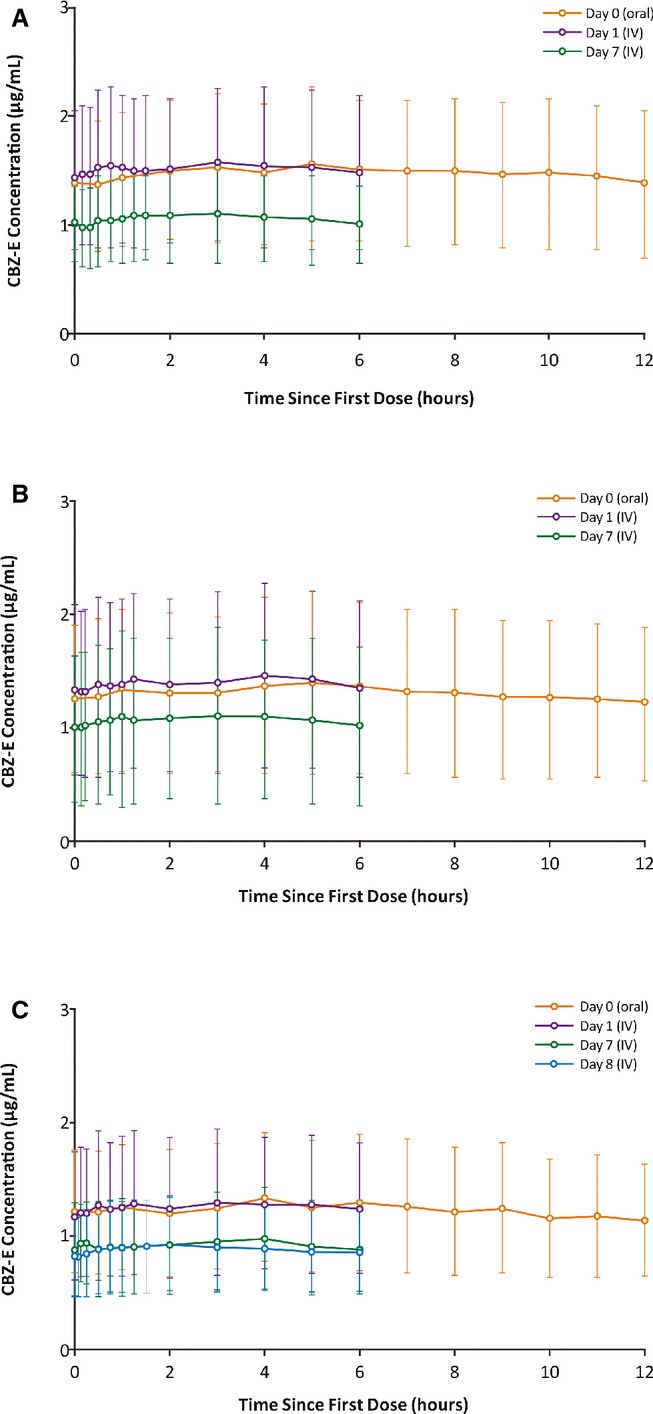
Mean (SD) plasma concentration–time profiles for carbamazepine-10,11-epoxide after oral and intravenous administration over (A) 30 min, (B) 15 min, or (C) 2- to 5-min (patients in the 2- to 5-min infusion group received 15-min infusions on days 1–7, and 2- to 5-min infusions on day 8) in patients with epilepsy with normal renal function. SD, standard deviation.
Safety
During the lead-in period (oral carbamazepine administration), 23 patients (10 and 13 in the 30- and 15-min infusion groups, respectively) experienced 28 AEs (15 and 13, respectively). None of the AEs during the lead-in period occurred at an incidence ≥5%. During the confinement period (intravenous carbamazepine administration), AEs were reported during treatment with intravenous carbamazepine (days 1–7) in 24 (55.8%) of 43 patients in the 30-min infusion group and in 32 (58.2%) of 55 patients in the 15-min infusion group; AEs were reported in 4 (33.3%) of the subset of 12 patients who received 2- to 5-min infusions on day 8. AEs reported at an incidence of ≥5% by infusion group were dizziness (9.3%) in the 30-min group; dizziness (14.5%), cellulitis and rash (7.3% each), ecchymosis, headache, infusion site reaction, and somnolence (5.5% each) in the 15-min group; and dizziness (16.7%) and anemia and muscle spasms (8.3% each) in the 2- to 5-min group (Table3). One patient in the 30-min infusion group experienced a new seizure type (simple partial seizure) on day 7, which resolved without additional treatment. Seizure control was maintained during the switch from oral to intravenous carbamazepine, and back. During the follow-up period (oral carbamazepine), 14 (33.3%) of 42 patients in the 30-min group, 7 (17.9%) of 39 patients in the 15-min group, and 3 (25.0%) of 12 patients in the 2- to 5-min subset group experienced AEs. None of AEs reported during this period in the 30- and 15-min groups occurred at an incidence of ≥5%; in the 2- to 5-min group, folliculitis, pharyngitis, full blood count abnormal, and skin irritations were reported at an incidence of 8.3% each. Carbamazepine Cmax levels following intravenous infusion were not related to the increase in neurologic AEs (Fig. S2).
Table 3.
Adverse events reported in ≥5% of patients in any infusion group (safety population)
| Adverse event, n (%)a | Days 1–7 | Day 8 | |
|---|---|---|---|
| 30-min infusion (n = 43) | 15-min infusion (n = 55) | 2- to 5-min infusion (n = 12)b | |
| Dizziness | 4 (9.3) | 8 (14.5) | 2 (16.7) |
| Cellulitis | 2 (4.7) | 4 (7.3) | 0 |
| Somnolence | 2 (4.7) | 3 (5.5) | 0 |
| Ecchymosis | 1 (2.3) | 3 (5.5) | 0 |
| Headache | 1 (2.3) | 3 (5.5) | 0 |
| Infusion-site reaction | 1 (2.3) | 3 (5.5) | 0 |
| Rash | 0 | 4 (7.3) | 0 |
| Anemia | 1 (2.3) | 0 | 1 (8.3) |
| Muscle spasms | 0 | 0 | 1 (8.3) |
Patients with ≥1 occurrences of the same event were counted only once.
Twelve patients in the 15-min infusion group also received four, 2- to 5-min infusions on day 8. AEs experienced on day 8 were counted separately.
AEs reported during treatment with intravenous carbamazepine were generally mild (90.6%) or moderate (8.5%). One patient in the 30-min group was reported to have a severe AE of bacteremia that was considered unrelated to intravenous carbamazepine, and the patient was discontinued from the study; no other patient discontinued because of an AE. No severe AEs were reported in patients receiving 2- to 5-min infusions.
During the study, transient elevations in hematology and chemistry values (full blood count abnormal [increased metamyelocytes], blood triglycerides increased, and alanine aminotransferase increased, each AE experienced by one patient, respectively) were considered to be AEs possibly related to intravenous carbamazepine treatment. Four patients experienced AEs of anemia; however, none was considered related to intravenous carbamazepine. Cardiovascular safety results indicated no instances of a QTcF value exceeding 500 msec. Neither oral nor intravenous dosing with carbamazepine appeared to be associated with clinically significant changes in QTc.
Discussion
This trial was conducted to evaluate the steady-state PK, bioequivalence, and safety of intravenous relative to oral carbamazepine. Patients were enrolled into one of two infusion cohorts: 30- and 15-min. Some patients in the 15-min cohort had the option of receiving 2- to 5-min infusions on day 8.
Intravenously administered drugs are essentially 100% bioavailable; therefore, switching to an intravenous formulation from a <100% bioavailable oral formulation, such as carbamazepine, may require dosing adjustments to maintain therapeutic plasma drug concentrations. Based on results from a PK study that evaluated the bioavailability of oral carbamazepine,3 the intravenous carbamazepine dose was reduced to 70% of the oral TDD in this trial. Mean steady-state values for AUC(0–24) and Cmin for all intravenous carbamazepine infusion durations and oral carbamazepine were similar, demonstrating that infusion at 70% of oral TDD divided into four doses given every 6 h was appropriate to maintain a patient’s plasma carbamazepine exposure. The plasma concentration–time profile of carbamazepine epoxide across all intravenous infusion durations were slightly lesser than when following oral dosing. This phenomenon can be explained by decreased first-pass metabolism following intravenous carbamazepine dosing, relative to oral dosing. This difference may be of clinical interest, as elevated carbamazepine epoxide concentrations are associated with a greater incidence of AEs.18
The bioequivalence of intravenous carbamazepine to oral carbamazepine was evaluated for each infusion duration. Intravenous carbamazepine infused over 30 min every 6 h was bioequivalent to oral carbamazepine, as the 90% CIs for AUC(0–24), Cmax, and Cmin ratios were within the accepted bioequivalence range. Although AUC(0–24) and Cmin following 15- and 2- to 5-min infusions met criteria for bioequivalence to oral carbamazepine, it was not surprising that the observed 90% CIs for the Cmax ratio exceeded the upper boundary of the accepted 80–125% range, given the shorter infusion durations. It should be noted that in the European Union, oral carbamazepine is considered a drug with a narrow therapeutic index, and bioequivalence guidelines established by the European Medicines Agency for such drugs use CI limits of 90.00–111.11%.19
Intravenous carbamazepine infusions as rapid as 2- to 5-min, which resulted in greater plasma concentrations, were as well tolerated as oral carbamazepine. Dizziness was the only AE that increased with more rapid intravenous carbamazepine infusions but was not temporally related to transient Cmax levels. Although no other AEs exhibited a similar profile across infusion groups, it is difficult to accurately assess AE trends, as the patients in the 2- to 5-min group were few (n = 12), had been receiving 15-min intravenous carbamazepine infusions immediately prior, and received only four 2- to 5-min infusions over the course of a day. In contrast, patients in the 30- and 15-min infusion groups switched from oral to intravenous carbamazepine and received four infusions/day over 7 days. The incidence of AEs related to infusion were low. One patient experienced a seizure that was considered to be a new type; however, the seizure was brief and resolved within 1 min. Overall, seizure control was maintained during the switch to the intravenous formulation and back to oral carbamazepine. AEs were mild or moderate in intensity, and only one AE of bacteremia was severe and led to discontinuation from the trial. Although elevations in hematology and chemistry values were experienced by three patients following intravenous infusions, all were transient. Treatment with intravenous carbamazepine did not appear to be associated with clinically significant changes in QTc. Further analysis of safety data is reported in an accompanying article describing pooled safety results from this trial and a related phase III safety study.16
In summary, the results from this trial demonstrate that patients with normal renal function who are receiving intravenous carbamazepine administered at 70% of a patient’s oral TDD given every 6 h with infusions of 30-, 15-, and 2- to 5-min, maintained their plasma carbamazepine concentrations. This study also provides evidence that intravenous carbamazepine solubilized in SBECD is well-tolerated and can be used as a bridge therapy for oral carbamazepine formulations in patients with normal renal function with epilepsy who are temporarily unable to take oral medications. The New Drug Application (NDA) for this product is currently under review by the FDA.
Acknowledgments
Manuscript preparation, including editing and formatting of the manuscript, incorporating author comments, preparing tables and figures, and coordinating submission requirements, was provided by Apurva H. Davé, PhD, of Prescott Medical Communications Group (Chicago, IL). This editorial support was funded by Lundbeck LLC.
Biography
Dr. Dwain Tolbert, PhD, FCP, is the executive director of US Clinical Pharmacology and Preclinical Research for Lundbeck LLC. 
Conflict of Interest
This study was funded by Lundbeck LLC (Deerfield, IL). Drs. Tolbert, Drummond, and Lee are employees of Lundbeck LLC. Drs. Bekersky, Walzer, and Wesche were employees of Lundbeck LLC at the time this study was conducted. Dr. Cloyd has royalty agreements with Lundbeck LLC (intravenous carbamazepine), and the University of Minnesota and Ligand Pharmaceuticals, Inc. (intravenous topiramate), as well as a licensing agreement with the University of Minnesota and Allaysis, LLC (intravenous baclofen). Dr. Cloyd has received grants from Allaysis, LLC, and has consulting agreements with Upsher Smith Laboratories, Inc., and CURx Pharmaceuticals, Inc. Dr. Biton is currently a clinical research investigator for Accera, Inc., Eisai Co., Ltd., Forum Pharmaceuticals, Lundbeck LLC, Marinus Pharmaceuticals, Inc., Pfizer, Inc., SK Life Science Inc., Sunovion Pharmaceuticals Inc., UCB Inc., and Upsher-Smith Laboratories, Inc., and has received consulting/lecture fees from Avigen Inc., Eisai Co., Ltd., GlaxoSmithKline plc, Icagen, Inc., Jazz Pharmaceuticals plc, Lundbeck LLC, Merck & Co., Inc., Ortho-McNeil-Janssen Pharmaceuticals, Inc., Pfizer, Inc., UCB Inc., Upsher-Smith Laboratories, Inc., and Valeant Pharmaceuticals International, Inc. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting Information
Figure S1. Patient flow.
Figure S2. Timing of neurologic AEs relative to PK profile.
Table S1. Blood sampling schedule for 30- and 15-min intravenous carbamazepine infusions.
References
- 2013. WHO Model List of Essential Medicines http://www.who.int/medicines/publications/essentialmedicines/en/index.html. Accessed August 13, 2014.
- Tegretol [package insert] East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2014. [Google Scholar]
- Marino SE, Birnbaum AK, Leppik IE, et al. Steady-state carbamazepine pharmacokinetics following oral and stable-labeled intravenous administration in epilepsy patients: effects of race and sex. Clin Pharmacol Ther. 2012;91:483–488. doi: 10.1038/clpt.2011.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertilsson L, Hojer B, Tybring G, et al. Autoinduction of carbamazepine metabolism in children examined by a stable isotope technique. Clin Pharmacol Ther. 1980;27:83–88. doi: 10.1038/clpt.1980.13. [DOI] [PubMed] [Google Scholar]
- Eichelbaum M, Ekbom K, Bertilsson L, et al. Plasma kinetics of carbamazepine and its epoxide metabolite in man after single and multiple doses. Eur J Clin Pharmacol. 1975;8:337–341. doi: 10.1007/BF00562659. [DOI] [PubMed] [Google Scholar]
- Geradin AP, Abadie FV, Campestrini JA, et al. Pharmacokinetics of carbamazepine in normal humans after single and repeated oral doses. J Pharmacokinet Biopharm. 1976;4:521–535. doi: 10.1007/BF01064556. [DOI] [PubMed] [Google Scholar]
- Levy RH, Pitlick WH, Troupin AS, et al. Pharmacokinetics of carbamazepine in normal man. Clin Pharmacol Ther. 1975;17:657–668. doi: 10.1002/cpt1975176657. [DOI] [PubMed] [Google Scholar]
- Pitlick WH, Levy RH. Time-dependent kinetics I: exponential autoinduction of carbamazepine in monkeys. J Pharm Sci. 1977;66:647–649. doi: 10.1002/jps.2600660511. [DOI] [PubMed] [Google Scholar]
- Serralheiro A, Alves G, Fortuna A, et al. Intranasal administration of carbamazepine to mice: a direct delivery pathway for brain targeting. Eur J Pharm Sci. 2014;60:32–39. doi: 10.1016/j.ejps.2014.04.019. [DOI] [PubMed] [Google Scholar]
- VFEND [package insert] New York, NY: Pfizer; 2014. [Google Scholar]
- GEODON [package insert] New York, NY: Pfizer; 2014. [Google Scholar]
- ABILIFY [package insert] Tokyo, Japan: Otsuka Pharmaceutical Co. Ltd; 2014. [Google Scholar]
- Nexterone [package insert] Deerfield, IL: Baxter Healthcare Corporation; 2011. [Google Scholar]
- Conway JM, White JR, Birnbaum AK, et al. Safety of an IV formulation of carbamazepine. Epilepsy Res. 2009;84:242–244. doi: 10.1016/j.eplepsyres.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hougas WD, Zhang ZP, Nehls CW. 2007. Middleton, WI Madison Bioanalytical Laboratory Quantitation of carbamazepine and carbamazepine 10,11-epoxide in human plasma via PHLC with MS/MS detection. PPD,. Validation Report P626, Project AEEL2.
- Lee D, Kalu U, Halford JJ, et al. Intravenous carbamazepine as short-term replacement therapy for oral carbamazepine in adults with epilepsy: Pooled tolerability results from two open-label trials. Epilepsia. 2015;56:906–914. doi: 10.1111/epi.12991. [DOI] [PubMed] [Google Scholar]
- FDA. Guidance for industry: bioavailability and bioequivalence studies for orally administered drug products – general considerations. Rockville, MD: Center for Drug Evaluation and Research; 2002. . Draft July 2002, [Google Scholar]
- Schoeman JF, Elyas AA, Brett EM, et al. Correlation between plasma carbamazepine-10,11-epoxide concentration and drug side-effects in children with epilepsy. Dev Med Child Neurol. 1984;26:756–764. doi: 10.1111/j.1469-8749.1984.tb08169.x. [DOI] [PubMed] [Google Scholar]
- EMA. 2008. Guideline on the investigation of bioequivalence. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003011.pdf. Accessed 29 January, 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Patient flow.
Figure S2. Timing of neurologic AEs relative to PK profile.
Table S1. Blood sampling schedule for 30- and 15-min intravenous carbamazepine infusions.


