Summary
We determined brain to liver weight ratio (BLWR) thresholds for fetal growth restriction (FGR) using autopsy information on 395 perinatal deaths comprising stillborn babies who died during labour and neonatal deaths. FGR was defined using two methods: (1) birth weight for gestational age (WGA) less than the 10th percentile; and (2) WGA less than the 10th percentile or discordant birth weight/length. The association between BLWR and FGR was investigated using odds ratios, and classification statistics were calculated for a range of BLWR thresholds. Using WGA, 84 cases (21.3%) were FGR and a further 15 cases (n = 99, 25%) had discordant birth weight/length. The BLWR ranged from 1.02 to 7.30 and was positively associated with FGR. BLWR was not associated with FGR for babies with congenital central nervous system or chromosomal abnormalities. Excluding these, for FGR defined using WGA and discordant birth weight/length, a BLWR threshold of 5.0 was 100% predictive of FGR. A BLWR threshold of 3.0 for babies over 28 weeks gestation and 3.7 for more preterm babies optimised case detection while minimising missed and false positive cases. Additional evidence of FGR should be sought for babies with a BLWR of less than 5.0 to confirm FGR.
Keywords: Autopsy standards, brain to liver weight ratio, fetal growth restriction, perinatal death, small for gestational age
INTRODUCTION
Causes of pathological fetal growth restriction (FGR) are diverse and include many factors such as uteroplacental insufficiency, chronic maternal diseases, congenital and genetic disorders, infections and environmental conditions.1 Correctly identifying FGR in perinatal deaths is important for understanding the clinical pathway that led to the death and the existence of any preventable factors. A diagnosis of FGR in a perinatal death is important information for clinicians and families, and has implications for the management of subsequent pregnancies.
Historically, FGR has been classified as either symmetrical or asymmetrical. In symmetrical FGR, reduced fetal growth is equally reflected across all developing organs and body parts resulting in a small but proportionally shaped infant. In contrast, asymmetrical FGR refers to cases where the rate of growth is unbalanced between different organs and body parts, and where energy is preferentially redirected to vital organs such as the brain and heart at the expense of other organs such as the liver.2,3 Consequently, asymmetrical FGR infants are characterised by disproportionate growth manifested as a low birth weight but with relatively spared length and head circumference.4
More recently, the classification of FGR has been expanded to three main types.5 Type I is symmetrical FGR and is characterised by decreased growth in early pregnancy due to altered cell proliferation of the developing embryo. Type II defines asymmetrical FGR, which is manifested later in pregnancy, generally after 30–32 weeks gestation, and is primarily attributed to reduced cell size. Type III cases of FGR are instances where growth restriction occurs due to a combination of reduced cell proliferation and growth and is a mixture of Type I and Type II FGR. The onset of Type III FGR occurs during the second trimester, the period in which cells are still undergoing proliferation while also starting to increase in size.
FGR represents the failure of babies to reach their genetically determined growth potentials,1 and is associated with an increased risk of mortality and morbidity.1,6,7 Diagnosis of FGR is commonly based on the comparison of estimated fetal weight to population-based standards and is usually defined as a birth weight for gestational age (WGA) below the 10th percentile.8,9 However, difficulties arise in using birth weight percentiles to diagnose FGR in stillborn babies due to the maceration that follows death in utero and the lack of certainty about the gestational age at death.10 Additional information that may be used to assess FGR in and ex utero include fundal height measurements, head circumference to abdominal circumference ratio, femur length to abdominal circumference ratio, amniotic fluid index, maternal arterial umbilical blood flow, placental grading,1,8,9 thigh wasting and brain to liver weight ratio (BLWR) based on information obtained at post-mortem examination.11,12
An increase in BLWR is the result of unbalanced fetal growth in response to stress, where the growth of the brain and other key organs such as the heart are ‘spared’ at the expense of other, less vital organs such as the liver, kidneys, pancreas and skeletal muscle.13,14 Several studies have investigated the relationship between brain to liver weight (or volume) ratios and FGR.11,12,15 Anderson assessed BLWR in fresh stillborn and neonatal deaths using weights obtained from autopsy reports, and found the mean BLWR of infants that displayed normal body weight was 2.8 and that a ratio greater than 4.5 was a strong indicator of dysmature babies with low body weight.11 Mitchell investigated using the BLWR to predict low body weight in stillborn babies, using a birth weight less than the 10th percentile to classify babies as FGR. Mitchell found that the BLWR tended to be elevated in FGR babies; however, the BLWR alone was considered unlikely to be an adequate indicator of FGR due to poor sensitivity and specificity.12 Boito et al. utilised ultrasound technology to estimate brain and liver volumes in a series of pregnancies, and found that developing fetuses that were subsequently classified as FGR at birth displayed a marked elevation in volume ratios at the time of sonographic examination.15
The aim of this study is to determine BLWR thresholds for FGR at autopsy in a normative population of perinatal deaths, comprising stillborn babies who died during labour or in the neonatal period in New South Wales (NSW), Australia.
MATERIALS AND METHODS
The study population included all singleton stillborn babies that died during labour and neonatal deaths that were reported to the NSW Ministry of Health from 2000 to 2012 and had post-mortem information on brain and liver weights available. Of the 9364 perinatal deaths in 2000 to 2012 that were reported to the NSW Ministry of Health at the time the study commenced, 3007 were singleton neonatal deaths or stillbirths that died during labour. Of these 3007 deaths, a post-mortem had been carried out on 763. The study population comprised 395 deaths for which complete information on birth weight, gestational age and brain and liver weights was available.
Confidential reviews of perinatal deaths are carried out by the NSW Maternal and Perinatal Committee, which is appointed by the Minister for Health to review maternal and perinatal morbidity and mortality in NSW. Obstetric cause-specific death classifications for stillbirths and neonatal deaths are assigned according to the Perinatal Society of Australia and New Zealand Perinatal Death Classification and Neonatal Death Classification.16 Stillbirth is defined as: ‘Death prior to the complete expulsion or extraction from its mother of a product of conception of 20 or more completed weeks of gestation or of 400 g or more birth weight where gestation is not known. The death is indicated by the fact that after such separation the fetus does not breathe or show any other evidence of life, such as beating of the heart, pulsation of the umbilical cord, or definite movement of voluntary muscles.’16 Neonatal death is defined as the death of a live-born baby within 28 days of life.
FGR was defined in two ways:
Birth WGA below the 10th percentile. This was assessed using Australian birth weight percentile standards.17
Birth WGA below the 10th percentile or birth weight and length discordance. As with the first approach, WGA below the 10th percentile was assessed using Australian birth weight percentile standards.17 Birth weight and length discordance was assessed using expected gestational ages based on the baby's birth weight and length, obtained from Australian 50th percentile standards.17,18 Babies were defined as FGR when the expected gestational age for length was at least 4 weeks greater than the expected gestational age for weight, or the birth WGA was below the 10th percentile. The use of a difference of four weeks or more in expected gestational ages to indicate FGR is based on Australian birth weight percentile standards,17 which show that the 10th percentile birth weight for a given gestation corresponds to the 50th percentile birth weight for the gestation 2–4 weeks earlier.
Characteristics of FGR and non-FGR cases for both definitions were compared. Logistic regression was used to calculate the odds ratio (OR) for the association between the BLWR (continuous) and FGR, and to investigate variation by the presence/absence of congenital conditions for both definitions of FGR. To compare the definitions of FGR and investigate the performance of selected BLWR thresholds, 2 by 2 classification tables were constructed by cross-tabulating FGR and a dichotomised BLWR variable where values above the threshold were classified as FGR and those below as non-FGR. BLWR thresholds of interest (2.8, 3.0, 3.5, 4.0, 4.5 and 5.0) were selected a priori to provide a range of values based on those previously reported in the literature and considered clinically relevant. For the selected BLWR thresholds, the following classification statistics and their exact binomial 95% confidence intervals were calculated:
Sensitivity: percentage of true FGR cases classified as FGR.
Specificity: percentage of true non-FGR cases classified as non-FGR.
Positive predictive value (PPV): percentage of cases classified as FGR that are true FGR.
Negative predictive value (NPV): percentage of cases classified as non-FGR that are true non-FGR.
An optimal BLWR threshold was also calculated using Youden's index,19 a measure of overall diagnostic effectiveness that balances the trade-off between sensitivity and specificity. Finally, counts of true positive, true negative, false positive and false negative cases for the study population were obtained by comparing the classification of babies as FGR by selected BLWR thresholds and WGA below the 10th percentile or birth weight/length discordance. Data manipulation and statistical analyses were carried out using SAS Enterprise Guide 5.1 and R 2.15.1.20,21
This study used data collected by the NSW Maternal and Perinatal Committee, which is a quality assurance committee established under the NSW Health Administration Act 1982. As the study conformed to the standards established by the National Health and Medical Research Council for ethical quality review,22 ethics committee approval was not necessary.
RESULTS
The study population of 395 deaths comprised 135 stillbirths and 260 neonatal deaths. Of these 395 babies, 38.5% had a congenital condition, 68.1% were low birth weight (less than 2500 g) and 53.4% were extremely preterm (less than 28 weeks gestation). The BLWR ranged from 1.02 to 7.30 (Table 1).
Table 1.
Characteristics of the study population by FGR status for two definitions of FGR
| Variable/category | FGR (WGA <10th percentile) | FGR (plus discordance)* | Total | ||||
| No | Yes | FGR† | Cases added | FGR† | |||
| n | n | % | n | % | n | % | |
| Congenital condition | |||||||
| No | 197 | 46 | 18.9 | 12 | 23.9 | 243 | 61.5 |
| Yes | |||||||
| All | 114 | 38 | 25.0 | 3 | 27.0 | 152 | 38.5 |
| CNS or CA‡ | 31 | 8 | 20.5 | 1 | 23.1 | 39 | 25.7 |
| Gender | |||||||
| Male | 186 | 48 | 20.5 | 7 | 23.5 | 234 | 59.2 |
| Female | 125 | 36 | 22.4 | 8 | 27.3 | 161 | 40.8 |
| Perinatal death | |||||||
| Stillbirth | 109 | 26 | 19.3 | 5 | 23.0 | 135 | 34.2 |
| Neonatal | 202 | 58 | 22.3 | 10 | 26.2 | 260 | 65.8 |
| Gestational age, weeks | |||||||
| <28 | 179 | 32 | 15.2 | 1 | 15.6 | 211 | 53.4 |
| 28+ | 132 | 52 | 28.3 | 14 | 35.9 | 184 | 46.6 |
| Birth weight, g | |||||||
| <2500 | 207 | 62 | 23.0 | 1 | 23.4 | 269 | 68.1 |
| 2500+ | 104 | 22 | 17.5 | 14 | 28.6 | 126 | 31.9 |
| Brain to liver weight ratio quintiles | |||||||
| 1.02–2.07 | 69 | 10 | 12.7 | 1 | 13.9 | 79 | 20.0 |
| 2.07–2.56 | 70 | 9 | 11.4 | 3 | 15.2 | 79 | 20.0 |
| 2.56–2.96 | 66 | 13 | 16.5 | 4 | 21.5 | 79 | 20.0 |
| 2.96–3.56 | 63 | 16 | 20.3 | 4 | 25.3 | 79 | 20.0 |
| 3.56–7.30 | 43 | 36 | 45.6 | 3 | 49.4 | 79 | 20.0 |
| Birth length percentiles | |||||||
| <3 | 5 | 22 | 81.5 | 0 | 81.5 | 27 | 8.5 |
| 3–10 | 11 | 15 | 57.7 | 0 | 57.7 | 26 | 8.2 |
| 10–50 | 83 | 25 | 23.1 | 0 | 23.1 | 108 | 34.0 |
| 50–90 | 115 | 10 | 8.0 | 10 | 16.0 | 125 | 39.3 |
| 90–97 | 18 | 1 | 5.3 | 3 | 21.1 | 19 | 6.0 |
| >97 | 13 | 0 | 0.0 | 2 | 15.4 | 13 | 4.1 |
| Total | 311 | 84 | 21.3 | 15 | 25.1 | 395 | 100.0 |
FGR, fetal growth restriction; WGA, weight for gestational age.
*FGR defined as WGA <10th percentile or weight/length discordance.
†Denotes percentage of strata group that is FGR.
‡CNS or CA: central nervous system conditions or chromosomal anomalies, which are a subset of all congenital conditions.
Using WGA less than the 10th percentile, 84 (21.3%) babies were defined as FGR, with higher proportions of FGR cases observed in babies with congenital conditions (25.0%), of low birth weight (23.0%), 28 weeks gestation or greater (28.3%), higher BLWR quintiles (3.56–7.30; 45.6%) and birth length less than or equal to the 10th percentile (69.8%). The addition of birth weight/length for gestational age discordance to the WGA definition of FGR identified an extra 15 cases, increasing the total to 99 (25.1%). Comparatively, this led to increases in the proportion of FGR cases observed in babies with a birth weight of 2500 g or more from 17.5% to 28.6%, babies of 28 weeks gestation or greater from 28.3% to 35.9%, and babies with a birth length above the 90th percentile from 3.1% to 18.8% (Table 1).
The study population included 152 babies with congenital conditions of which 25.7% were affected by conditions of the central nervous system (CNS) or had chromosomal anomalies (CA) (Table 1). For the definition of FGR based on WGA only, logistic regression demonstrated that the association between FGR and the BLWR was strongest for babies without congenital conditions (OR 3.3) and without CNS conditions or CA (OR 2.9) (Fig. 1). The association between FGR and the BLWR was weaker when all babies were considered (OR 2.2) and further reduced for babies with congenital conditions (OR 1.5). The association was in the opposite direction for the subgroup of babies with CNS or CA congenital conditions (OR 0.5), although not significantly so. Similar estimates of association were found when using FGR defined with the addition of birth weight/length discordance. CNS conditions and CA therefore were excluded from subsequent analyses.
Fig. 1.
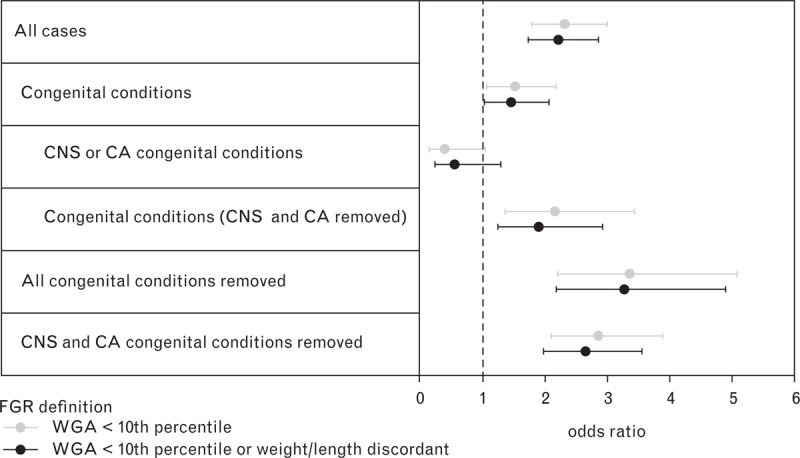
Effect of congenital conditions on the association between the BLWR and FGR by definition of FGR. BLWR, brain to liver weight ratio; CNS or CA, central nervous system conditions or chromosomal anomalies; FGR, fetal growth restriction; WGA, weight for gestational age. Bars represent 95% confidence intervals.
Sensitivity and PPV for selected BLWR thresholds were very similar for both FGR definitions, with WGA having slightly better sensitivity and the addition of birth weight/length discordance providing a slight increase in PPV (Fig. 2). Higher sensitivity was observed at lower BLWR thresholds, with a value of 64.4% at BLWR of 2.8 using the second definition of FGR which also had the highest PPV of 100% at a threshold of 5.0.
Fig. 2.
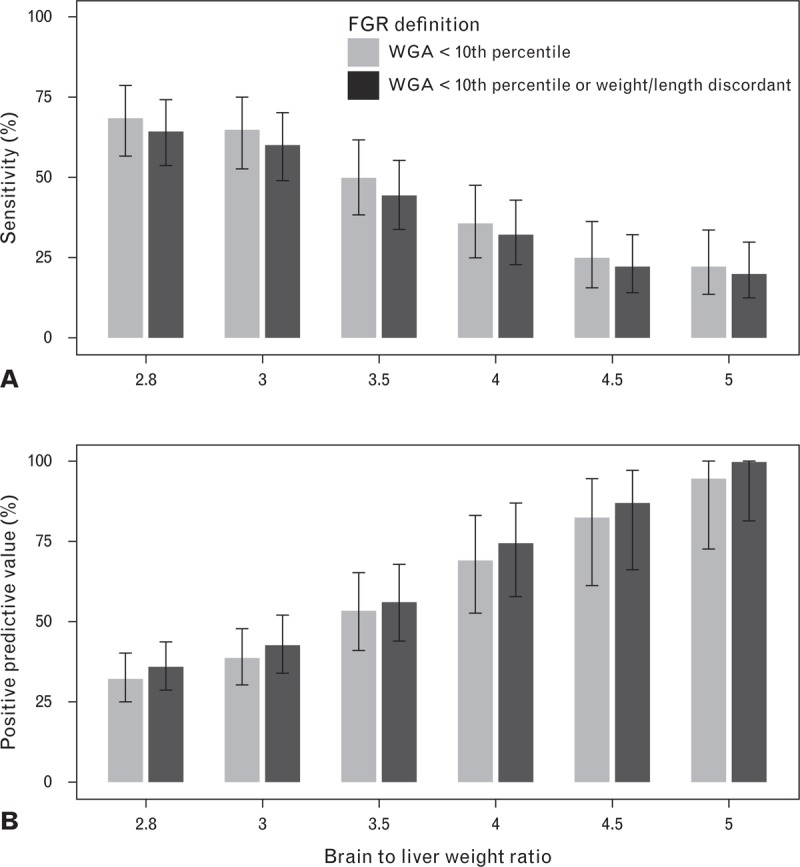
Sensitivity and positive predictive value for selected BLWR thresholds by FGR definition. BLWR, brain to liver weight ratio; FGR, fetal growth restriction; WGA, weight for gestational age. Bars represent 95% confidence intervals.
As the addition of discordant birth weight/length for gestational age to the definition of FGR added only one case of FGR less than 28 weeks gestation compared to 14 cases of 28-plus weeks gestation, subsequent analysis was also stratified by gestational age (Table 2). This revealed a shift from a weaker association between FGR and the BLWR at lower thresholds to a stronger association at higher thresholds for the less the 28 weeks gestational age group compared to babies of 28-plus weeks gestation. The highest sensitivity was found in the older gestational age group (66.1% at a BLWR threshold of 2.8) while in both groups PPV was 100% at a BLWR threshold of 5.0. Sensitivity declined more rapidly with an increasing threshold in the older gestational age group, while the PPV was poorer at lower thresholds in the less than 28 week group. For all thresholds the less than 28 week group showed higher NPV, while the older gestational age group showed higher specificity.
Table 2.
Odds ratios and classification statistics for various BLWR thresholds by gestational age∗
| Group (FGR† cases) | BLWR | OR | 95% CI | SN (%) | 95% CI | SP (%) | 95% CI | NPV (%) | 95% CI | PPV (%) | 95% CI |
| All ages (n = 90) | 2.8 | 2.9 | (1.7–4.7) | 64.4 | (53.7–74.3) | 61.3 | (55.1–67.2) | 83.6 | (77.6–88.5) | 36.0 | (28.6–44.0) |
| 3.0 | 4.0 | (2.4–6.7) | 60.0 | (49.1–70.2) | 72.9 | (67.2–78.2) | 84.3 | (79.0–88.8) | 42.9 | (34.1–52.0) | |
| 3.5 | 6.1 | (3.5–10.6) | 44.4 | (34.0–55.3) | 88.3 | (83.9–91.9) | 82.5 | (77.5–86.7) | 56.3 | (44.0–68.1) | |
| 4.0 | 12.2 | (5.6–26.3) | 32.2 | (22.8–42.9) | 96.2 | (93.2–98.2) | 80.8 | (76.0–84.9) | 74.4 | (57.9–87.0) | |
| 4.5 | 25.0 | (7.2–86.7) | 22.2 | (14.1–32.2) | 98.9 | (96.7–99.8) | 79.0 | (74.2–83.2) | 87.0 | (66.4–97.2) | |
| 5.0‡ | – | – | 20.0 | (12.3–29.8) | 100.0 | (98.6–100.0) | 78.7 | (73.9–82.9) | 100.0 | (81.5–100.0) | |
| 3.3§ | 5.4 | (3.2–9.2) | 51.1 | (40.3–61.8) | 83.8 | (78.8–88.0) | 83.5 | (78.5–87.8) | 51.7 | (40.8–62.4) | |
| <28 weeks gestation (n = 28) | 2.8 | 2.1 | (0.9–4.9) | 60.7 | (40.6–78.5) | 58.2 | (49.9–66.1) | 89.0 | (81.2–94.4) | 21.0 | (12.7–31.5) |
| 3.0 | 3.5 | (1.5–8.0) | 60.7 | (40.6–78.5) | 69.3 | (61.3–76.5) | 90.6 | (83.8–95.2) | 26.6 | (16.3–39.1) | |
| 3.5 | 8.9 | (3.7–21.5) | 57.1 | (37.2–75.5) | 86.9 | (80.5–91.8) | 91.7 | (86.0–95.7) | 44.4 | (27.9–61.9) | |
| 4.0 | 18.1 | (6.3–52.3) | 46.4 | (27.5–66.1) | 95.4 | (90.8–98.1) | 90.7 | (85.1–94.7) | 65.0 | (40.8–84.6) | |
| 4.5 | 35.8 | (7.2–178.0) | 32.1 | (15.9–52.4) | 98.7 | (95.4–99.8) | 88.8 | (83.1–93.1) | 81.8 | (48.2–97.7) | |
| 5.0‡ | – | – | 28.6 | (13.2–48.7) | 100.0 | (97.6–100.0) | 88.4 | (82.7–92.8) | 100.0 | (63.1–100.0) | |
| 3.7§ | 13.2 | (5.2–33.5) | 57.1 | (37.2–75.5) | 90.8 | (85.1–94.9) | 92.1 | (86.5–95.8) | 53.3 | (34.3–71.7) | |
| ≥28 weeks gestation (n = 62) | 2.8 | 3.7 | (1.9–7.1) | 66.1 | (53.0–77.7) | 65.5 | (56.0–74.2) | 77.9 | (68.2–85.8) | 51.3 | (39.8–62.6) |
| 3.0 | 5.2 | (2.7–10.2) | 59.7 | (46.4–71.9) | 77.9 | (69.1–85.1) | 77.9 | (69.1–85.1) | 59.7 | (46.4–71.9) | |
| 3.5 | 5.9 | (2.6–13.1) | 38.7 | (26.6–51.9) | 90.3 | (83.2–95.0) | 72.9 | (64.7–80.0) | 68.6 | (50.7–83.1) | |
| 4.0 | 12.8 | (3.5–45.9) | 25.8 | (15.5–38.5) | 97.3 | (92.4–99.4) | 70.5 | (62.7–77.5) | 84.2 | (60.4–96.6) | |
| 4.5 | 24.2 | (3.0–192.2) | 17.7 | (9.2–29.5) | 99.1 | (95.2–100.0) | 68.7 | (61.0–75.7) | 91.7 | (61.5–99.8) | |
| 5.0‡ | – | – | 16.1 | (8.0–27.7) | 100.0 | (96.8–100.0) | 68.5 | (60.8–75.5) | 100.0 | (69.2–100.0) | |
| 3.0§ | 5.2 | (2.7–10.2) | 59.7 | (46.4–71.9) | 77.9 | (69.1–85.1) | 77.9 | (69.1–85.1) | 59.7 | (46.4–71.9) |
BLWR, brain to liver weight ratio; CI, confidence interval; FGR, fetal growth restriction; NPV, negative predictive value; OR, odds ratio; PPV, positive predictive value; SN, sensitivity; SP, specificity.
*Excludes central nervous system conditions or chromosomal anomalies.
†Defined using weight for gestational age <10th percentile or discordant birth weight/length.
‡Odds ratio calculation not possible as positive predictive grouping consists entirely of true positive cases and the denominator for odds calculation is zero.
§Optimal BLWR for classifying FGR based on Youden's index.
Using the second definition of FGR and applying Youden's index, the optimal BLWR threshold for all gestational ages was determined to be 3.3 (Table 2). For babies less than 28 weeks gestation, the optimal threshold was 3.7, while for babies of 28 weeks gestation or greater the optimal threshold was slightly lower at 3.0. Stratifying by gestational age increased the sensitivity and PPV of the optimal BLWR thresholds in both groups compared to all gestational ages, while specificity and NPV improved only for the less than 28 week group.
Using FGR defined as WGA below the 10th percentile or weight/length discordance and excluding CNS conditions or CA, Table 3 shows the study population separated into true positive and negative and false positive and negative groups for selected BLWR thresholds. Overall, a BLWR threshold of 5.0 detected only 18 cases of FGR with no false positive cases, but missed 72 cases of FGR; while a BLWR of 2.8 correctly detected more cases of FGR (n = 58), but incorrectly classified 103 non-FGR cases as FGR. The optimal threshold of 3.3 resulted in 46 FGR cases and 223 non-FGR cases correctly classified, with 43 cases incorrectly classified as FGR (false positive) and 44 cases incorrectly classified as non-FGR (false negative). When optimal thresholds were applied by gestational age, an additional seven cases were correctly classified as FGR and an additional four cases correctly classified as non-FGR, with fewer false positives and false negatives (4 and 7, respectively).
Table 3.
Number of classified true or false, positive or negative FGR cases for various BLWR thresholds by gestational age∗
| Group | FGR† | FGR based on BLWR | |||||||
| No | Yes | Ratio | No | Yes | FP | FN | TP | TN | |
| All ages | 266 | 90 | 2.8 | 195 | 161 | 103 | 32 | 58 | 163 |
| 3.0 | 230 | 126 | 72 | 36 | 54 | 194 | |||
| 3.5 | 285 | 71 | 31 | 50 | 40 | 235 | |||
| 4.0 | 317 | 39 | 10 | 61 | 29 | 256 | |||
| 4.5 | 333 | 23 | 3 | 70 | 20 | 263 | |||
| 5.0 | 338 | 18 | 0 | 72 | 18 | 266 | |||
| 3.3‡ | 267 | 89 | 43 | 44 | 46 | 223 | |||
| <28 weeks | 153 | 28 | 2.8 | 100 | 81 | 64 | 11 | 17 | 89 |
| 3.0 | 117 | 64 | 47 | 11 | 17 | 106 | |||
| 3.5 | 145 | 36 | 20 | 12 | 16 | 133 | |||
| 4.0 | 161 | 20 | 7 | 15 | 13 | 146 | |||
| 4.5 | 170 | 11 | 2 | 19 | 9 | 151 | |||
| 5.0 | 173 | 8 | 0 | 20 | 8 | 153 | |||
| 3.7‡ | 151 | 30 | 14 | 12 | 16 | 139 | |||
| ≥28 weeks | 113 | 62 | 2.8 | 95 | 80 | 39 | 21 | 41 | 74 |
| 3.0 | 113 | 62 | 25 | 25 | 37 | 88 | |||
| 3.5 | 140 | 35 | 11 | 38 | 24 | 102 | |||
| 4.0 | 156 | 19 | 3 | 46 | 16 | 110 | |||
| 4.5 | 163 | 12 | 1 | 51 | 11 | 112 | |||
| 5.0 | 165 | 10 | 0 | 52 | 10 | 113 | |||
| 3.0‡ | 113 | 62 | 25 | 25 | 37 | 88 | |||
BLWR, brain to liver weight ratio; FGR, fetal growth restriction; FN, false negative; FP, false positive; TN, true negative; TP, true positive.
*Excludes central nervous system conditions or chromosomal anomalies.
†Defined using weight for gestational age <10th percentile or discordant birth weight/length.
‡Optimal BLWR threshold for classifying FGR based on Youden's index.
DISCUSSION
We used information on birth weight and length obtained at post-mortem examination for 395 perinatal deaths in NSW to ascertain a threshold of BLWR that could be used to determine FGR. We assessed the BLWR against two definitions of FGR: first, WGA below the 10th percentile; and second, to improve prediction, WGA below the 10th percentile or birth weight/length discordance. Overall, we found that the BLWR was positively associated with both definitions of FGR. However, in the subset of babies with congenital conditions of the CNS or with CA, BLWR was not associated with FGR, indicating that FGR in these babies does not differentially spare the brain—a finding that is clinically intuitive. Babies with conditions of the CNS or with CA were subsequently excluded from the analysis.
The second definition of FGR that included WGA below the 10th percentile or weight/length is likely to be a better measure of FGR than WGA alone as it takes into account those babies who are of acceptable weight but are disproportionately thin for their length. Compared to WGA alone, this definition gave higher PPVs at all BLWR thresholds. From a clinical perspective, over all gestational ages a BLWR of 5.0 or above correctly predicted the presence of FGR in 100% of cases and a BLWR of less than 5.0 correctly predicted the absence of FGR in 79% of cases. Likewise, a BLWR of 4.0 or above correctly predicted the presence of FGR in 74% of cases, and the absence of FGR in 81% of cases. A BLWR threshold of 3.3 optimised the classification: 52% of cases at or above this threshold were correctly classified as FGR (PPV), while 84% of cases below this threshold were correctly classified as non-FGR (NPV). A higher BLWR was more likely to be predictive of FGR at 28-plus weeks gestation compared to more preterm babies; a BLWR threshold of 3.7 was optimal for babies less than 28 weeks gestation and 3.0 for babies of 28-plus weeks gestation.
This is the largest study published to date investigating the use of the BLWR for determining FGR in a normative population of perinatal deaths. Anderson investigated BLWR for determining FGR using 95 cases in a similar population, while Mitchell used a population of 182 stillbirths.11,12 Stillborn babies who died before the onset of labour were excluded from our study population, addressing any potential confounding associated with maceration following intra-uterine death or uncertain gestational age at death. Further, the exclusion of congenital conditions of the CNS or CA from the study population addresses any substantial confounding of congenital conditions with brain and liver growth. The addition of weight/length discordance to the FGR definition of WGA below the 10th percentile substantially improved the classification of babies greater than 28 weeks gestation. This is consistent with an expected improved ascertainment of Type II and Type III FGR, which both occur later in pregnancy when most fetal growth occurs.
The strong biological evidence underpinning the ‘brain sparing effect’ which leads to elevated BLWR13 suggests that, in the absence of congenital anomalies that alter brain and liver size, most cases of higher than normal BLWR are likely to represent some sort of FGR/malnutrition.23 However, there is currently no recognised single or definite method of ascertaining whether an individual fetus truly has FGR or not. We used WGA and weight/length discordance as the standard for FGR diagnosis. These measures are based on population norms that may fail to capture true FGR cases that meet percentile thresholds while incorrectly flagging constitutionally small but normal infants as FGR. The use of customised growth charts that adjust for maternal characteristics such as height, weight, parity and ethnic group, has been proposed as a way to potentially enhance diagnosis of FGR,24 although the utility and benefits of customised growth chart standards remain uncertain.25 Diagnosis of FGR may also be informed by other clinical information such as serial ultrasound measurements, arterial umbilical blood flow, amniotic fluid index, other body ratios such as head circumference to abdominal circumference and femur length to abdominal circumference, and placental markers of FGR, which were not available for this study. Others combine the above parameters with more clinically and, to some extent, experience based features such as thigh wasting, generalised decrease in subcutaneous body fat and the baby who clinically looks wasted. BLWR failed to show an association with FGR in the subset of infants with congenital conditions of the CNS or CA, and indicates that the BLWR is not useful in the presence of conditions that impact the development of the brain and/or the liver. While BLWR is not a perfect measure of FGR, it would be of diagnostic value where the gestation and/or the date of death are uncertain in the absence of conditions affecting brain and/or liver weights.
Maroun and Graem10 examined the effect of maceration on external measures including body weight and length as well as on internal organs and found no statistically significant difference in body weight between the non-macerated babies and those with various degrees of maceration. It was thought this was possibly due to retention of fluid from organs into body cavities. The finding of effusions is not uncommon in fetuses with varying degrees of maceration. The birth weight is the best indicator of the real body weight as once the fetus has been handled and wrapped, the presence of skin blistering or skin loss may result in significant fluid loss into the wrapping or clothing. The study by Maroun and Graem also analysed organ weights associated with various degrees of maceration. They found that brain weight and, to a slightly lesser extent, heart weight was only slightly affected by varying degrees of maceration, though this is dependent on autopsy technique in removal of brain. The liver, thymus and spleen weights were the most severely affected. The BLWR was on average 2.6 in the non-macerated fetuses, correlating relatively well with our figures, and 4.5 in the severely macerated fetuses comprising those who had been dead in utero for approximately 4 weeks. This is an extraordinarily long period of time compared with usual practice in our population, with antenatal visits usually occurring 4 weeks apart or less in later pregnancy. Furthermore, mothers are often aware of the significance of loss or decrease of fetal movements, tending to report loss or decrease in movements within 24–48 h, particularly in the latter half of the third trimester.
Weight loss of organs such as the liver in maceration will confound the use of the BLWR as a definitive marker of FGR in maceration. However, BLWR could be used judiciously by comparing organ weights with normal values and making an assessment of the degree of maceration and organ weight loss and therefore the significance of the BLWR. Our figure of 5.0 as the BLWR threshold to define all FGR, although missing the detection of some cases, may be a suggestive indicator as to when FGR should be considered in maceration. Diagnosis should not be made without supporting data such as serial ultrasound or flow studies, WGA less than 10th percentile, loss of subcutaneous fat or other suggestive factors including details on perceived degree of maceration and comparison with other organ weights. Discordance between birth weight and length may be used in maceration. Maroun and Graem10 found a small but statistically significant increase in length in the macerated group, which may be a true factor assuming all measurements were done appropriately. We have found that in non-macerated fetuses there is a tendency for measurements to be made without straightening legs appropriately to obtain a true length, possibly partially due to post-mortem rigidity which is not present in the macerated fetus where joints are lax allowing easier measurement of length.
In conclusion, we found that an elevated BLWR is associated with FGR in the absence of congenital CNS conditions or CA, and this association is stronger for babies greater than 28 weeks gestation than more preterm babies. However, the association is not perfect. A BLWR threshold of 5.0 was 100% predictive of FGR, but was associated with a substantial number of missed cases. A BLWR threshold of 3.3 optimised detection of true cases while minimising the number of missed and false positive cases. Optimal thresholds for babies less than and equal to or greater than 28 weeks gestation were 3.7 and 3.0, respectively, and use of these improved the overall performance of BLWR in correctly classifying FGR. The BLWR is a suitable alternative to WGA for diagnosing FGR in circumstances where the weight or gestational age is uncertain and the baby is not affected by CNS conditions or CA. This may include stillborn babies who die before the onset of labour where the liver and brain are not macerated or using multiple factors as discussed above in the hands of an experienced perinatal pathologist. Additional evidence should be sought to make a diagnosis of FGR at a BLWR of less than 5.0 in all cases, as true cases of FGR may be missed below this threshold.
Acknowledgements
Alexandre Stephens was employed by the NSW Ministry of Health on the NSW Biostatistical Officer Training Program at the time this work was conducted. Hospital morbidity and mortality review committees, Perinatal Outcomes Working Party, and the NSW Maternal and Perinatal Committee.
Conflicts of interest and sources of funding: The authors state that there are no conflicts of interest to disclose.
References
- 1.Bamfo JE, Odibo AO. Diagnosis and management of fetal growth restriction. J Pregnancy 2011; 2011:640715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peleg D, Kennedy CM, Hunter SK. Intrauterine growth restriction: identification and management. Am Fam Physician 1998; 58:453–460.466-457. [PubMed] [Google Scholar]
- 3.Vandenbosche RC, Kirchner JT. Intrauterine growth retardation. Am Fam Physician 1998; 58:1384–1390.1393-4. [PubMed] [Google Scholar]
- 4.Rosenberg A. The IUGR newborn. Semin Perinatol 2008; 32:219–224. [DOI] [PubMed] [Google Scholar]
- 5.Nardozza LM, Araujo Junior E, Barbosa MM, et al. Fetal growth restriction: current knowledge to the general Obs/Gyn. Arch Gynecol Obstet 2012; 286:1–13. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein IM, Horbar JD, Badger GJ, et al. Morbidity and mortality among very-low-birth-weight neonates with intrauterine growth restriction. The Vermont Oxford Network. Am J Obstet Gynecol 2000; 182:198–206. [DOI] [PubMed] [Google Scholar]
- 7.Zeitlin J, Ancel PY, Saurel-Cubizolles MJ, et al. The relationship between intrauterine growth restriction and preterm delivery: an empirical approach using data from a European case-control study. BJOG 2000; 107:750–758. [DOI] [PubMed] [Google Scholar]
- 8.Bamberg C, Kalache KD. Prenatal diagnosis of fetal growth restriction. Semin Fetal Neonatal Med 2004; 9:387–394. [DOI] [PubMed] [Google Scholar]
- 9.Doubilet PM, Benson CB. Sonographic evaluation of intrauterine growth retardation. Am J Roentgenol 1995; 164:709–717. [DOI] [PubMed] [Google Scholar]
- 10.Maroun LL, Graem N. Autopsy standards of body parameters and fresh organ weights in nonmacerated and macerated human fetuses. Pediatr Dev Pathol 2005; 8:204–217. [DOI] [PubMed] [Google Scholar]
- 11.Anderson JM. Increased brain weight-liver weight ratio as a necropsy sign of intrauterine undernutrition. J Clin Pathol 1972; 25:867–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell ML. Fetal brain to liver weight ratio as a measure of intrauterine growth retardation: analysis of 182 stillborn autopsies. Mod Pathol 2001; 14:14–19. [DOI] [PubMed] [Google Scholar]
- 13.Giussani DA. The vulnerable developing brain. Proc Natl Acad Sci USA 2011; 108:2641–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peebles DM. Fetal consequences of chronic substrate deprivation. Semin Fetal Neonatal Med 2004; 9:379–386. [DOI] [PubMed] [Google Scholar]
- 15.Boito S, Struijk PC, Ursem NT, et al. Fetal brain/liver volume ratio and umbilical volume flow parameters relative to normal and abnormal human development. Ultrasound Obstet Gynecol 2003; 21:256–261. [DOI] [PubMed] [Google Scholar]
- 16.Perinatal Society of Australia and New Zealand (PSANZ) Perinatal Mortality Group. PSANZ Clinical Practice Guideline for Perinatal Mortality. 2nd ed, 2009. April http://www.psanz.com.au/special-interest/perinatal-mortality-group/psanzcpg. [Google Scholar]
- 17.Dobbins TA, Sullivan EA, Roberts CL, et al. Australian national birthweight percentiles by sex and gestational age. Med J Aust 2012; 197:291–294. [DOI] [PubMed] [Google Scholar]
- 18.Beeby PJ, Bhutap T, Taylor LK. New South Wales population-based birthweight percentile charts. J Paediatr Child Health 1996; 32:512–518. [DOI] [PubMed] [Google Scholar]
- 19.Lopez-Raton M, Rodriguez-Alvarez M. OptimalCutpoints: An R package for selecting optimal cutpoints in diagnostic tests. J Stat Software 2014; 61: 1–36. http://CRAN.R-project.org/package=OptimalCutpoints. [Google Scholar]
- 20.SAS Institute Inc. SAS Enterprise Guide 5.1. Cary, NC: SAS/STAT, 2012. [Google Scholar]
- 21.R Core-Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing, 2012. [Google Scholar]
- 22.The National Health and Medical Research Council, the Australian Research Council and the Australian Vice-Chancellors’ Committee. National Statement on Ethical Conduct in Human Research 2007 (Updated May 2013). Canberra: NHMRC, 2013. [Google Scholar]
- 23.Marton T, Hargitai B, Bowen C, et al. Elevated brain weight/liver weight ratio in normal body weight centile term perinatal deaths: an indicator of terminal intrauterine malnourishment. Pediatr Dev Pathol 2013; 16:267–271. [DOI] [PubMed] [Google Scholar]
- 24.Gardosi J. Customised assessment of fetal growth potential: implications for perinatal care. Arch Dis Child Fetal Neonatal Ed 2012; 97:F314–F317. [DOI] [PubMed] [Google Scholar]
- 25.Hutcheon JA, Walker M, Platt RW. Assessing the value of customized birth weight. Am J Epidemiol 2011; 173:459–467. [DOI] [PubMed] [Google Scholar]


