Abstract
Congenital anomalies of the kidney and urinary tract (CAKUT) cover a wide range of structural malformations that result from defects in the morphogenesis of the kidney and/or urinary tract. These anomalies account for about 40–50% of children with chronic kidney disease worldwide. Knowledge from genetically modified mouse models suggests that single gene mutations in renal developmental genes may lead to CAKUT in humans. However, until recently only a handful of CAKUT-causing genes were reported, most of them in familial syndromic cases. Recent findings suggest that CAKUT may arise from mutations in a multitude of different single gene causes. We focus here on single gene causes of CAKUT and their developmental origin. Currently more than 20 monogenic CAKUT-causing genes have been identified. High-throughput sequencing techniques make it likely that additional CAKUT-causing genes will be identified in the near future.
Keywords: Congenital Anomalies of the Kidney and Urinary Tract, CAKUT, genetic kidney disease, monogenic disease
Introduction
Congenital anomalies of the kidney and urinary tract (CAKUT) comprise a wide range of structural malformations that result from defects in the morphogenesis of the kidney and/or the urinary tract. These anomalies include among others: renal agenesis, renal hypodysplasia, multicystic dysplastic kidney, hydronephrosis, ureteropelvic junction obstruction, megaureter, ureter duplex, vesicoureteral reflux and posterior urethral valves [1]. CAKUT account for about 40–50% of children with chronic kidney disease [2]. The condition may appear as an isolated feature or as part of a systemic condition that encompasses extra-renal manifestations [3–5]. The notion that CAKUT may be caused by single gene mutations (“monogenic CAKUT”) is suggested by three findings: (1) CAKUT may appear with familial aggregation [6]; (2) monogenic mouse models exhibit CAKUT phenotypes; (3) human multi-organ monogenic syndromes may include CAKUT phenotypes. Recently this notion was corroborated by the discovery of more than 20 single-gene causes for CAKUT in humans [7–11]. Until then only a handful of CAKUT-causing genes had been documented, most of which were identified among familial syndromic cases, including HNF1B (Renal Cysts and Diabetes Syndrome) [4], PAX2 (Renal Coloboma Syndrome) [3], and EYA1 (branchio-oto-renal syndrome) [5]. Recent findings suggest that CAKUT may be caused by a multitude of different disease-causing genes (Table 1), each gene representing a monogenic recessive or dominant cause of CAKUT [3–5, 12–36]. Given this broad genetic locus heterogeneity and the rapidly evolving sequencing technology, it is likely that many novel genes will be identified in the near future.
Table 1.
Single gene causes of human isolated CAKUT and syndromes with a predominant CAKUT phenotype
| Gene Symbol |
Renal Phenotype |
Extra-Renal Phenotype |
Human Disease [OMIM#] | Mouse Model Het / Homd |
Ref | |
|---|---|---|---|---|---|---|
| A. DOMINANT CAKUT | ||||||
| BMP4 | Renal hypodysplasia | Cleft lip, microophthalmia | Microphthalmia, syndromic 6 [*607932] Orofacial cleft 11 [*600625] |
CAKUT | EL | [12] |
| EYA1 | Multicystic dysplastic kidney, renal aplasia | Deafness, ear malformations, branchial cysts | Anterior segment anomalies with or without cataract 113650 Branchiootic syndrome 1 [*602588] Branchiootorenal syndrome 1, with or without cataracts [*113650] Otofaciocervical syndrome [*166780] |
CAKUT | CAKUT | [5] |
| GATA3 | Renal dysplasia | Hypoparathyroidism, heart defects, immune deficiency, deafness | Hypoparathyroidism, sensorineural deafness, and renal dysplasia [*146255] | None | EL | [13, 14] |
| HNF1B | Renal hypodysplasia, single kidney, horseshoe kidney | Diabetes mellitus (MODY5) Hyperuricemia, Hypomagnesaemia, elevated LFT |
Diabetes mellitus, noninsulin-dependent [*125853] Renal cysts and diabetes syndrome [*137920] |
None | EL | [4] |
| KAL1a | Renal agenesis | Micropenis, bilateral cryptorchidism, anosmia | Hypogonadotropic hypogonadism 1 with or without anosmia (Kallmann syndrome 1) [*308700] | N/A | [16] | |
| PAX2 | Vesicoureteral reflux, renal hypoplasia | Optic nerve colobomas, hearing loss | Papillorenal syndrome [*120330] Renal hypoplasia, isolated [*191830] |
CAKUT | CAKUT | [3, 17] |
| RET | Renal agenesis | See OMIM# in the next column | Central hypoventilation syndrome, congenital [*209880] Medullary thyroid carcinoma [*155240] Multiple endocrine neoplasia IIA [*171400] Multiple endocrine neoplasia IIB [*162300] Pheochromocytoma [*171300] Renal agenesis [*191830] |
None | PL, CAKUT | [18, 19] |
| ROBO2 | VUR, ureterovesical junction defects | None | Vesicoureteral reflux 2 [*610878] | None | PL, CAKUT | [20] |
| SALL1 | Renal hypodysplasia, renal agenesis | Limb, ear, anal abnormalities | Townes-Brocks syndrome [*107480] | CAKUT | CAKUT | [21] |
| SIX1 | Renal hypodysplasia, VUR | Deafness, ear defects, branchial cysts | Brachiootic syndrome 3 [*608389] Deafness, autosomal dominant 23 [*605192] |
None | CAKUT | [70] |
| SIX2 | Renal hypodysplasia | None | - | None | PL, CAKUT | [12, 27] |
| SIX5 | Renal hypodysplasia, VUR | Deafness, ear defects, branchial cysts | Branchiootorenal syndrome 2 [*610896] | None | None | [23] |
| SOX17 | VUR, UPJO | None | Vesicoureteral reflux 3 [*613674] | None | EL | [24] |
| TNXB | VUR | Joint hypermobility | Ehlers-Danlos syndrome, autosomal dominant, hypermobility type [*130020] Ehlers-Danlos syndrome, autosomal recessive, due to tenascin × deficiency [*606408] |
None | None | [25] |
| UPK3A | Renal adysplasia | Subtle facial and limb defects | Renal adysplasia [*191830] | None | CAKUT | [26] |
| WNT42 | Renal hypodysplasia | Female-to-male sex reversal, adrenal dysplasia, lung dysplasia (SERKAL) | Mullerian aplasia and hyperandrogenism [*158330] SERKAL syndrome [*611812] |
None | PL, CAKUT | [28–30] |
| CHD1L | Renal hypodysplasia, VUR, UPJO | None | - | N/A | [31] | |
| DSTYK | Renal hypodysplasia, UPJO | Epilepsy in 2 out of 7 affected | - | N/A | [32] | |
| MUC1 | Medullary cystic kidney disease type 1 | - | MCKD1– Medullary cystic kidney disease type1 [#174000] | N/A | [74] | |
| UMOD | Medullary cystic kidney disease type 2 | Hyperuricemia | MCKD2– Medullary cystic kidney disease type2 [#603860] HNFJ2 -Hyperuricemic nephropathy, familial juvenile 2 [#6130925],[#613092] |
none | CAKUTc | [33] |
| B. RECESSIVE CAKUT | ||||||
| ACE | Absence or incomplete differentiation of proximal tubules | Pulmonary hypoplasia (Potter sequence), skull abnormalities | Renal tubular dysgenesis (RTD) [*267430] | None | CAKUT? | [34, 35,71] |
| AGT | Similar to ACE | Similar to ACE | Renal tubular dysgenesis (RTD) [*267430] | None | CAKUT | [34, 35] |
| AGTR1 | Similar to ACE | Similar to ACE | Renal tubular dysgenesis (RTD) [*267430] | None | CAKUT | [34, 35] |
| REN | Similar to ACE | Similar to ACE | Renal tubular dysgenesis [*267430] | None | PL, CAKUT? | [34, 35] |
| FGF20 | Bilateral renal agenesis | None | - | None | CAKUT | [36] |
| TRAP1 | VUR, renal agenesis | VACTERL association | - | N/A | [72] | |
| FRAS1 | Renal agenesis | Cryptophthalmos, nose ear and larynx malformations. Mental retardation and syndactyly manifest occasionally. | Fraser Syndrome [*219000] | None | CAKUT | [73]e |
| FREM2 | Renal agenesis | Cryptophthalmos, nose ear and larynx malformations. Mental retardation and syndactyly manifest occasionally. | Fraser Syndrome [*219000] | None | CAKUT | [74]e |
X-linked recessive
Mode of inheritance can be autosomal dominant or autosomal recessive
Mice lacking uromodulin did not show any clinical or histological feature of MCKD, however transgenic mice that express the C147W mutant uromodulin, corresponding to reported human mutation
C148W, recapitulate most of the uromodulin-associated kidney diseases. ACE angiotensin-converting enzyme; AD autosomal dominant; AR autosomal recessive; EL embryonic lethal; Het Heterozygous; Hom Homozygous; LFT liver function tests; MCDK multicystic dysplastic kidney; MODY 5 maturity onset diabetes of the young type 5; MOI mode of inheritance; N/A not available; OMIM online Mendelian inheritance inman; PL postnatal lethal; Ref Reference; SERKAL sex reversal, kidneys, adrenals, and lungs dysgenesis; UPJO ureteropelvic junction obstruction; VACTERL vertebra, anal, cardiac, trachea-esophageal, renal and limb anomalies; VUR vesicoureteral reflux
The mouse model column in the table refers to the mouse model of each corresponding gene irrespective of whether it has CAKUT phenotype
Hypomorphic recessive mutations have also been shown to cause isolated CAKUT (Kohl, unpublished)
We focus here on single-gene causes of CAKUT and their developmental mechanisms. We mainly discus disease-causing genes related to isolated CAKUT or syndromic forms of CAKUT in which the renal phenotype predominates. Single-gene causes of human congenital urinary bladder diseases are beyond the scope of this review and have been recently reviewed [37].
CAKUT are due to disordered genetic control of kidney development
The pathology of CAKUT is based on the disturbance of normal nephrogenesis, and can be due to genetic abnormalities in renal developmental genes that direct this process [1, 38–41]. In order to understand the genetic basis of human CAKUT it is essential to consider how the normal kidney develops (Figure 1). Kidney development can be divided into the following developmental stages: ureteric bud induction, mesenchymal-to-epithelial transition (MET), renal branching morphogenesis, and nephron patterning and elongation (which include proximal and distal tubule morphogenesis and glomerulogenesis) [1, 38–41]. The underlying molecular control of these developmental stages is governed by a large number of genes and signaling pathways that orchestrate this complex process. Perturbation in each of these steps, as supported by mouse models, can lead to the clinical phenotype of CAKUT. Insights into the related molecular control mechanisms has led to a paradigm shift away from classic anatomic theories to contemporary cell biological and genetic views of the etiology of CAKUT [42].
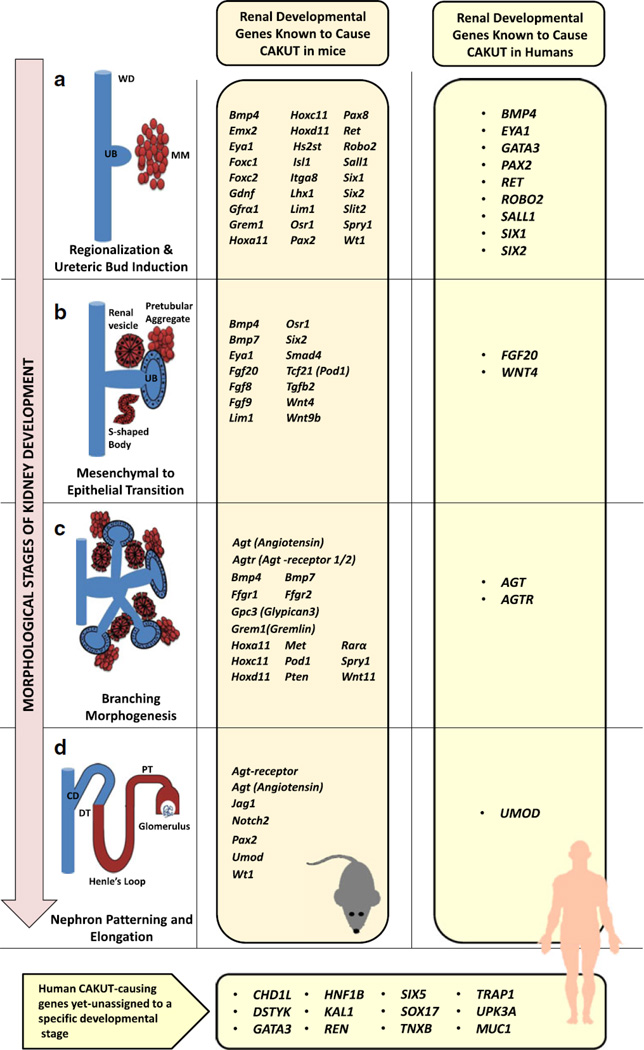
Figure 1. Mechanisms of kidney development and corresponding CAKUT-causing genes in mice and humans.
Steps of nephrogenesis (left column, a to d) and the corresponding CAKUT-causing genes in mice (orange column) and human (right yellow column). The kidney is formed via reciprocal induction between the ureteric bud (UB) and the metanephric mesenchyme (MM) (a). The UB invades the MM cells, which in turn condense around the tip of the branching UB (pre-tubular aggregate). Polarized renal vesicles subsequently develop in mesenchyme-to-epithelial transition (MET) (b). The cells sequentially form comma-shaped and S-shaped bodies and finally give rise to the mature nephron segments (distal and proximal tubule, loop of Henle, and glomerulus) (d). At the same time, the uretric bud branches in a highly reproducible manner and nephrons are induced at each ureteric bud tip (c). These branches eventually form the collecting system, including collecting ducts, renal pelvis, ureter and bladder trigone.
WD Wolffian duct; UB ureteric bud; MM matanephrogenic mesenchyme; CD collecting duct; DT distal tubule; PT proximal tubule.
For many years mouse models have been a key tool in our understanding of the molecular basis of kidney development with numerous mouse models re-capitulating human disease phenotypes. For instance, in mice the following monogenic causes of CAKUT have been described for the following process: (1) Ret and Gdnf for ureteric bud induction [43] (2) Wnt4 for mesenchymal-to-epithelial transition (MET) [44] and (3) Agtr2 (Angiotensin receptor 2) for branching morphogenesis [45]. Figure 1 outlines key steps during normal kidney development and their corresponding CAKUT-causing genes in mice and humans.
Classical studies [46] have also highlighted the importance of the position of the ureteric budding in the development of CAKUT and lead to the “budding hypothesis”. According to this hypothesis the precise position at which the ureteric bud grows out from the mesonephric duct is critical for subsequent normal kidney and urinary tract development. This hypothesis was generated following the anatomical analysis of duplex kidneys which showed that a more severe hypoplasia and dysplasia were closely correlated with mal-displacement of the ureteral orifice. This hypothesis, in part, is supported by the fact that many “early development” genes, involved in the ureteric budding stage, actually lead to CAKUT. In the following sections we will discuss the most important single gene causes of CAKUT in humans in relation to their corresponding function during kidney development.
Human CAKUT-causing genes involved in ureteric bud induction
The products of most genes that if mutated cause CAKUT in humans are involved in the control of the early morphogenesis stages of the kidney i.e. induction of the metanephric mesenchyme by the ureteric bud and mesenchymal-to-epithelial transition. Ureteric budding is promoted by GDNF signaling via its receptor RET. In humans, mutations in RET were initially recognized to cause multiple endocrine neoplasia (MEN) syndrome [47] and Hirschsprung disease [48]. RET mutations were subsequently reported to cause CAKUT in fetuses with bilateral renal hypodysplasia/agenesis [18, 49]. In addition the role of RET as CAKUT causing gene is suggested by the finding that many patients with Hirschprung disease have silent urinary tract defects [50]. Still, data regarding the frequency of RET as a CAKUT-causing gene are conflicting [18, 49]. Mutation analysis of GDNF has been performed in patients with CAKUT. However, no evidence supporting its causative role has been established so far [30, 51]. The GDNF-RET signaling pathway is regulated by multiple circuits. Given the central role of the GDNF-RET signaling pathway in ureteric budding it was likely that mutations in genes that regulate this pathway may result in CAKUT. Indeed, these regulatory mechanisms include transcription factors such as PAX2, EYA1, and SALL1, all of which have been initially recognized to be mutated in small pedigrees with multiple affected individuals with CAKUT and syndrome-specific extra-renal manifestations. Mutations in PAX2 were first identified in patients with Renal Coloboma Syndrome which comprises renal hypodysplasia, optic nerve abnormalities and deafness [3]. To date, more than 55 disease causing mutations of PAX2 have been reported worldwide [17]. Importantly, PAX2 mutations were shown to also lead to isolated CAKUT without optic nerve or hearing abnormalities or with subtle features. In addition, PAX2-mutations were shown to lead to variable renal phenotypes across the spectrum of CAKUT, including renal hypodysplasia, vesicoureteral reflux renal cysts and multcystic dysplastic kidneys as the most common ones [17]. Mutations in EYA1 lead to Branchio-Oto-Renal (BOR) syndrome which is characterized by hearing loss, structural defects of the ear, branchial fistula or cyst and CAKUT, ranging from mild renal hypoplasia to agenesis [5]. Interestingly, mutations in SIX1 and SIX5 have also been identified in patients with EYA1-negative BOR syndrome, and probably represent a more rare underlying etiology [22, 23]. Mutations in SALL1 lead to Townes-Brocks Syndrome (TBS), which is characterized by kidney, anal, ear and thumb abnormalities [21]. An isolated CAKUT phenotype was reported in one patient with a SALL1 mutation [52]. Unpublished data from our lab further support an isolated CAKUT phenotype.
BMP4 is expressed in the mesenchymal cells that surround the Wolffian duct and inhibits GDNF-RET-signaling [38]. Missense mutations in BMP4 were identified in five CAKUT patients [12]. Subsequent functional analysis, using overexpression assays in zebrafish suggested that these mutations affect BMP4 protein function [53].
Human CAKUT-causing genes involved in mesenchymal-to-epithelial transition
Once the ureteric bud invades the metanephrogenic mesenchyme it induces condensation of the metanephrogenic mesenchymal cells around the ureteric bud tips. This step begins the polarization of the mesenchyme to generate the epithelial cells of the nephron in a process named mesenchymal-to-epithelial transition (MET) [38, 39]. WNT proteins, i.e. WNT9b and WNT4 that act within the WNT signaling pathway play a critical role in this process [38, 39]. Furthermore, recent evidence generated from mouse models supports that the WNT-pathway partially is regulated by SIX2 [54]. Interestingly, mutations in WNT4 or SIX2 have been identified in pediatric patients with CAKUT [12, 30]. Other important key players in MET are the fibroblast growth factor (FGF) ligands Fgf8 and Fgf9 (Figure 1) [38, 39]. In addition, in a recent report, on three affected fetuses with bilateral renal agenesis from a consanguineous family, an autosomal recessive loss of function mutation gene was identified in the FGF20 [36].
Human CAKUT-causing genes involved in branching morphogenesis
Renal branching morphogenesis follows the primary ureteric bud outgrowth. The ureteric bud subsequently undergoes serial branching to generate approximately 15 generations of branches. During this time new nephrons are induced at the tip of each branching bud (Figure 1). Several factors have been shown to modulate the UB branching morphogenesis [39]. One of the factors that govern this process is Angiotensin 2. Angiotensin 2 activates both the angiotensin receptor type 1 and 2 on the ureteric bud to stimulate branching. In addition it is required for elongation of the collecting duct [39]. Accordingly, mutations in the genes encoding several components of the renin–angiotensin system: AGT (angiotensinogen), REN (renin), ACE (angiotensin-converting enzyme), and AGTR1 (angiotensin II receptor type 1) have been linked to the distinct severe phenotype of CAKUT in humans of renal tubular dysgenesis [34] (Table 1). This rare fetal autosomal recessive disorder is characterized by early onset of persistent anuria leading to oligohydramnios and the Potter sequence, secondary to the absence or incomplete differentiation of the proximal tubules. Inactivation of different components of the RAS has been performed in mice. While Agtr2 [45] and Agt [55] null mice have CAKUT, this phenotype was not recapitulated in Ace and Ren null mice. These findings illustrate the possible discrepancies between mouse models and human diseases whereas the former exhibit an earlier developmental insult (branching morphogenesis) as compared to the later insult (developmental abnormality of the proximal tubules due to impaired tubular growth and differentiation).
Human CAKUT-causing genes involved in nephron patterning and elongation
Whereas a large amount of research has focused on the initial stages of kidney formation, much less is known about the genetic programs that drive segmentation of the nephron [41]. Indeed, the only possible human CAKUT-causing gene that can be designated under this category is UMOD. The Uromodulin (UMOD) gene encodes the Tamm-Horsfall protein, which is the most abundant urinary protein in humans [56]. UMOD mutations cause a large variety of different kidney syndromes: (1) medullary cystic kidney disease type 2 (MCKD2), (2) familial juvenile hyperuricemic nephropathy (FJHN), and (3) glomerulocystic kidney disease (GCKD) [33]. All of these disorders are inherited in an autosomal dominant mode and may have a CAKUT phenotype. Nevertheless, in a previous study published by our group, no UMOD mutations were identified among 96 patients with isolated CAKUT, implying that it may represent a very rare etiology for this condition [57].
Human CAKUT-causing genes yet-unassigned to a specific developmental stage
The developmental role of some human CAKUT-causing genes is still poorly understood. In this respect we will discuss two important genes, HNF1B and DSTYK. Hepatocyte nuclear factor 1B (HNF1B) is a homeodomain-containing transcription factor. HNF1B is essential factor for embryogenesis of the kidney, pancreas, and liver, and is expressed in the Wolffian duct from a very early developmental stage of the kidney [58]. Mutations in HNF1B have originally been recognized as the cause of the Renal Cysts and Diabetes Syndrome (RCDS) [4]. Subsequently, HNF1B-mutations and deletions were reported among individuals with isolated CAKUT encompassing different renal malformations across its spectrum, such as renal hypodysplasia, multicystic dysplastic kidney, cystic kidney disease, single kidney, and oligomeganephronia [52, 59]. HNF1B mutations have also been recognized to result in genital tract abnormalities, elevated liver function tests, hyperuricemia [60] and hypomagnesaemia [61]. Interestingly, several recent publications showed that contiguous gene deletion in the 17q12 region (which includes the HNF1B transcription factor) has resulted in the clinical combination of autism/schizophrenia and CAKUT [62, 63].
With regard to pathomechanism, a link was identified between HNF1B mutations and autosomal recessive polycystic kidney disease (ARPKD), which is caused by mutations in PKHD1 (polycystic kidney and hepatic disease 1). In mice, it has been shown that Hnf1b binds specifically to the Pkhd1 promoter and stimulates gene transcription. Since Hnf1b directly regulates the transcription of Pkhd1, mutations in HNF1B can inhibit PKHD1 gene expression and therefore may contribute to the formation of renal cysts in humans with RCDS [64].
Currently, HNF1B and PAX2 are considered to be the most frequent CAKUT-causing genes. Still, they are responsible for not more than 5–15% of cases depending on the examined cohort [52, 65, 66].
In a recent study involving 7 affected family members with CAKUT, disease causing mutations were detected in the gene DSTYK [32]. Additional DSTYK mutations were detected in 7 out of 311 (2.3%) unrelated patients with CAKUT. The study demonstrated that DSTYK is a positive regulator of ERK phosphorylation downstream of FGF-receptor activation during kidney development [32].
Finally, another aspect of CAKUT that should be taken into consideration with regard to gene discovery is copy number variations (CNVs). Several lines of evidence support their role in CAKUT [67]. This concept has been highlighted recently in a study involving 522 patients with CAKUT in which 72 distinct known or novel copy-number variations in 87 (16.6%) patients were identified, suggesting that kidney malformations can, in part, result from pathogenic genomic imbalances [68].
Conclusions and future directions
CAKUT are a genetically heterogeneous group of disorders that are caused by mutations in genes involved in the embryogenesis of the kidneys. The malformation phenotypes due to the altered proteins vary from normally appearing kidneys with intact kidney function (i.e., incomplete penetrance) to severe hypodysplasia and end stage kidney disease. In clinical practice the evaluation of patients with CAKUT, in addition to standard care, should include: (1) meticulous evaluation for extra-renal syndrome-specific signs and symptoms (see Table 1); (2) thorough family evaluation for the presence of CAKUT in other family members; (3) referral to genetic counseling. Currently, the most common CAKUT-causing genes are HNF1B and PAX2. Other cases, which often present sporadically, are probably a result of many rare diseases causing genes.
Novel gene discovery for CAKUT is hampered by a high degree of sporadic cases, genetic heterogeneity, lack of genotype-phenotype correlation and phenotypic heterogeneity. For example single gene causes of primary VUR are still elusive despite the fact that multiple disease loci were published [69]. Although primary VUR is one of the most commonly detected CAKUT presentations, its phenotypic case ascertainment is challenging. The fact that improved prenatal ultrasound examinations have resulted in the recognition that often VUR is accompanied by concomitant other congenital kidney and urinary tract abnormalities can provide one explanation for that. However, the advent and progress in sequencing and bioinformatics technologies should ensure that additional CAKUT-causing genes will be described in the near future. This may lead to more relevant etiologic categorization of disease entities than can be provided by ultrasound imaging or histopathology alone. Such assignments may have prognostic implications for patients with CAKUT.
Acknowledgments
F.H. is an Investigator of the Howard Hughes Medical Institute, a Doris Duke Distinguished Clinical Scientist, and the Warren E. Grupe Professor of Pediatrics. This research was supported by grants from the National Institutes of Health (to FH; R01-DK088767) and by the March of Dimes Foundation (6FY11-241). A.V. is a recipient of the Fulbright Post-doctoral Scholar Award for 2013. A.V. is also supported by grants from the Talpiot Medical Leadership Program, Chaim Sheba Medical Center, Tel-Hashomer, Israel and the Manton center Fellowship program, Boston Children’s Hospital, Boston, MA.
References
- 1.Schedl A. Renal abnormalities and their developmental origin. Nature Reviews Genetics. 2007;8:791–802. doi: 10.1038/nrg2205. [DOI] [PubMed] [Google Scholar]
- 2.(2008) North American Pediatric Renal Transplant Cooperative Study (NAPRTCS) 2008 Annual report. Rockville, MD: The EMMES Corporation; 2008. [Google Scholar]
- 3.Sanyanusin P, Schimmenti LA, McNoe LA, Ward TA, Pierpont ME, Sullivan MJ, Dobyns WB, Eccles MR. Mutation of the PAX2 gene in a family with optic nerve colobomas, renal anomalies and vesicoureteral reflux. Nat Genet. 1995;9:358–364. doi: 10.1038/ng0495-358. [DOI] [PubMed] [Google Scholar]
- 4.Lindner TH, Njolstad PR, Horikawa Y, Bostad L, Bell GI, Sovik O. A novel syndrome of diabetes mellitus, renal dysfunction and genital malformation associated with a partial deletion of the pseudo-POU domain of hepatocyte nuclear factor-1beta. Human molecular genetics. 1999;8:2001–2008. doi: 10.1093/hmg/8.11.2001. [DOI] [PubMed] [Google Scholar]
- 5.Abdelhak S, Kalatzis V, Heilig R, Compain S, Samson D, Vincent C, Weil D, Cruaud C, Sahly I, Leibovici M, Bitner-Glindzicz M, Francis M, Lacombe D, Vigneron J, Charachon R, Boven K, Bedbeder P, Van Regemorter N, Weissenbach J, Petit C. A human homologue of the Drosophila eyes absent gene underlies branchio-oto-renal (BOR) syndrome and identifies a novel gene family. Nat Genet. 1997;15:157–164. doi: 10.1038/ng0297-157. [DOI] [PubMed] [Google Scholar]
- 6.Bulum B, Ozcakar ZB, Ustuner E, Dusunceli E, Kavaz A, Duman D, Walz K, Fitoz S, Tekin M, Yalcinkaya F. High frequency of kidney and urinary tract anomalies in asymptomatic first-degree relatives of patients with CAKUT. Pediatr Nephrol. 2013 doi: 10.1007/s00467-013-2530-8. [DOI] [PubMed] [Google Scholar]
- 7.Yosypiv IV. Congenital anomalies of the kidney and urinary tract: a genetic disorder? International Journal of Nephrology. 2012;2012:909083. doi: 10.1155/2012/909083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen F. Genetic and developmental basis for urinary tract obstruction. Pediatr Nephrol. 2009;24:1621–1632. doi: 10.1007/s00467-008-1072-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Renkema KY, Winyard PJ, Skovorodkin IN, Levtchenko E, Hindryckx A, Jeanpierre C, Weber S, Salomon R, Antignac C, Vainio S, Schedl A, Schaefer F, Knoers NV, Bongers EM. Novel perspectives for investigating congenital anomalies of the kidney and urinary tract (CAKUT) Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2011;26:3843–3851. doi: 10.1093/ndt/gfr655. [DOI] [PubMed] [Google Scholar]
- 10.Sanna-Cherchi S, Caridi G, Weng PL, Scolari F, Perfumo F, Gharavi AG, Ghiggeri GM. Genetic approaches to human renal agenesis/hypoplasia and dysplasia. Pediatr Nephrol. 2007;22:1675–1684. doi: 10.1007/s00467-007-0479-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber S. Novel genetic aspects of congenital anomalies of kidney and urinary tract. Current Opinion in Pediatrics. 2012;24:212–218. doi: 10.1097/MOP.0b013e32834fdbd4. [DOI] [PubMed] [Google Scholar]
- 12.Weber S, Taylor JC, Winyard P, Baker KF, Sullivan-Brown J, Schild R, Knuppel T, Zurowska AM, Caldas-Alfonso A, Litwin M, Emre S, Ghiggeri GM, Bakkaloglu A, Mehls O, Antignac C, Network E, Schaefer F, Burdine RD. SIX2 and BMP4 mutations associate with anomalous kidney development. J Am Soc Nephrol. 2008;19:891–903. doi: 10.1681/ASN.2006111282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pandolfi PP, Roth ME, Karis A, Leonard MW, Dzierzak E, Grosveld FG, Engel JD, Lindenbaum MH. Targeted disruption of the GATA3 gene causes severe abnormalities in the nervous system and in fetal liver haematopoiesis. Nat Genet. 1995;11:40–44. doi: 10.1038/ng0995-40. [DOI] [PubMed] [Google Scholar]
- 14.Van Esch H, Groenen P, Nesbit MA, Schuffenhauer S, Lichtner P, Vanderlinden G, Harding B, Beetz R, Bilous RW, Holdaway I, Shaw NJ, Fryns JP, Van de Ven W, Thakker RV, Devriendt K. GATA3 haplo-insufficiency causes human HDR syndrome. Nature. 2000;406:419–422. doi: 10.1038/35019088. [DOI] [PubMed] [Google Scholar]
- 15.Horikawa Y, Iwasaki N, Hara M, Furuta H, Hinokio Y, Cockburn BN, Lindner T, Yamagata K, Ogata M, Tomonaga O, Kuroki H, Kasahara T, Iwamoto Y, Bell GI. Mutation in hepatocyte nuclear factor-1 beta gene (TCF2) associated with MODY. Nat Genet. 1997;17:384–385. doi: 10.1038/ng1297-384. [DOI] [PubMed] [Google Scholar]
- 16.Hardelin JP, Levilliers J, del Castillo I, Cohen-Salmon M, Legouis R, Blanchard S, Compain S, Bouloux P, Kirk J, Moraine C, et al. X chromosome-linked Kallmann syndrome: stop mutations validate the candidate gene. Proc Natl Acad Sci U S A. 1992;89:8190–8194. doi: 10.1073/pnas.89.17.8190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bower M, Salomon R, Allanson J, Antignac C, Benedicenti F, Benetti E, Binenbaum G, Jensen UB, Cochat P, DeCramer S, Dixon J, Drouin R, Falk MJ, Feret H, Gise R, Hunter A, Johnson K, Kumar R, Lavocat MP, Martin L, Moriniere V, Mowat D, Murer L, Nguyen HT, Peretz-Amit G, Pierce E, Place E, Rodig N, Salerno A, Sastry S, Sato T, Sayer JA, Schaafsma GC, Shoemaker L, Stockton DW, Tan WH, Tenconi R, Vanhille P, Vats A, Wang X, Warman B, Weleber RG, White SM, Wilson-Brackett C, Zand DJ, Eccles M, Schimmenti LA, Heidet L. Update of PAX2 mutations in renal coloboma syndrome and establishment of a locus-specific database. Hum Mutat. 2012;33:457–466. doi: 10.1002/humu.22020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skinner MA, Safford SD, Reeves JG, Jackson ME, Freemerman AJ. Renal aplasia in humans is associated with RET mutations. Am J Hum Genet. 2008;82:344–351. doi: 10.1016/j.ajhg.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Y, Houle AM, Letendre J, Richter A. RET Gly691Ser mutation is associated with primary vesicoureteral reflux in the French-Canadian population from Quebec. Hum Mutat. 2008;29:695–702. doi: 10.1002/humu.20705. [DOI] [PubMed] [Google Scholar]
- 20.Lu W, van Eerde AM, Fan X, Quintero-Rivera F, Kulkarni S, Ferguson H, Kim HG, Fan Y, Xi Q, Li QG, Sanlaville D, Andrews W, Sundaresan V, Bi W, Yan J, Giltay JC, Wijmenga C, de Jong TP, Feather SA, Woolf AS, Rao Y, Lupski JR, Eccles MR, Quade BJ, Gusella JF, Morton CC, Maas RL. Disruption of ROBO2 is associated with urinary tract anomalies and confers risk of vesicoureteral reflux. Am J Hum Genet. 2007;80:616–632. doi: 10.1086/512735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohlhase J, Wischermann A, Reichenbach H, Froster U, Engel W. Mutations in the SALL1 putative transcription factor gene cause Townes-Brocks syndrome. Nat Genet. 1998;18:81–83. doi: 10.1038/ng0198-81. [DOI] [PubMed] [Google Scholar]
- 22.Ruf RG, Xu PX, Silvius D, Otto EA, Beekmann F, Muerb UT, Kumar S, Neuhaus TJ, Kemper MJ, Raymond RM, Jr, Brophy PD, Berkman J, Gattas M, Hyland V, Ruf EM, Schwartz C, Chang EH, Smith RJ, Stratakis CA, Weil D, Petit C, Hildebrandt F. SIX1 mutations cause branchio-oto-renal syndrome by disruption of EYA1-SIX1-DNA complexes. Proc Natl Acad Sci U S A. 2004;101:8090–8095. doi: 10.1073/pnas.0308475101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoskins BE, Cramer CH, Silvius D, Zou D, Raymond RM, Orten DJ, Kimberling WJ, Smith RJ, Weil D, Petit C, Otto EA, Xu PX, Hildebrandt F. Transcription factor SIX5 is mutated in patients with branchio-oto-renal syndrome. Am J Hum Genet. 2007;80:800–804. doi: 10.1086/513322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gimelli S, Caridi G, Beri S, McCracken K, Bocciardi R, Zordan P, Dagnino M, Fiorio P, Murer L, Benetti E, Zuffardi O, Giorda R, Wells JM, Gimelli G, Ghiggeri GM. Mutations in SOX17 are associated with congenital anomalies of the kidney and the urinary tract. Hum Mutat. 2010;31:1352–1359. doi: 10.1002/humu.21378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gbadegesin RA, Brophy PD, Adeyemo A, Hall G, Gupta IR, Hains D, Bartkowiak B, Rabinovich CE, Chandrasekharappa S, Homstad A, Westreich K, Wu G, Liu Y, Holanda D, Clarke J, Lavin P, Selim A, Miller S, Wiener JS, Ross SS, Foreman J, Rotimi C, Winn MP. TNXB Mutations Can Cause Vesicoureteral Reflux. J Am Soc Nephrol. 2013 doi: 10.1681/ASN.2012121148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jenkins D, Bitner-Glindzicz M, Malcolm S, Hu CC, Allison J, Winyard PJ, Gullett AM, Thomas DF, Belk RA, Feather SA, Sun TT, Woolf AS. De novo Uroplakin IIIa heterozygous mutations cause human renal adysplasia leading to severe kidney failure. J Am Soc Nephrol. 2005;16:2141–2149. doi: 10.1681/ASN.2004090776. [DOI] [PubMed] [Google Scholar]
- 27.Self M, Lagutin OV, Bowling B, Hendrix J, Cai Y, Dressler GR, Oliver G. Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney. Embo J. 2006;25:5214–5228. doi: 10.1038/sj.emboj.7601381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biason-Lauber A, Konrad D, Navratil F, Schoenle EJ. A WNT4 mutation associated with Mullerian-duct regression and virilization in a 46,XX woman. N Engl J Med. 2004;351:792–798. doi: 10.1056/NEJMoa040533. [DOI] [PubMed] [Google Scholar]
- 29.Mandel H, Shemer R, Borochowitz ZU, Okopnik M, Knopf C, Indelman M, Drugan A, Tiosano D, Gershoni-Baruch R, Choder M, Sprecher E. SERKAL syndrome: an autosomal-recessive disorder caused by a loss-of-function mutation in WNT4. Am J Hum Genet. 2008;82:39–47. doi: 10.1016/j.ajhg.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vivante A, Mark-Danieli M, Davidovits M, Harari-Steinberg O, Omer D, Gnatek Y, Cleper R, Landau D, Kovalski Y, Weissman I, Eisenstein I, Soudack M, Wolf HR, Issler N, Lotan D, Anikster Y, Dekel B. Renal hypodysplasia associates with a WNT4 variant that causes aberrant canonical WNT signaling. J Am Soc Nephrol. 2013;24:550–558. doi: 10.1681/ASN.2012010097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brockschmidt A, Chung B, Weber S, Fischer DC, Kolatsi-Joannou M, Christ L, Heimbach A, Shtiza D, Klaus G, Simonetti GD, Konrad M, Winyard P, Haffner D, Schaefer F, Weber RG. CHD1L: a new candidate gene for congenital anomalies of the kidneys and urinary tract (CAKUT) Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2012;27:2355–2364. doi: 10.1093/ndt/gfr649. [DOI] [PubMed] [Google Scholar]
- 32.Sanna-Cherchi S, Sampogna RV, Papeta N, Burgess KE, Nees SN, Perry BJ, Choi M, Bodria M, Liu Y, Weng PL, Lozanovski VJ, Verbitsky M, Lugani F, Sterken R, Paragas N, Caridi G, Carrea A, Dagnino M, Materna-Kiryluk A, Santamaria G, Murtas C, Ristoska-Bojkovska N, Izzi C, Kacak N, Bianco B, Giberti S, Gigante M, Piaggio G, Gesualdo L, Vukic DK, Vukojevic K, Saraga-Babic M, Saraga M, Gucev Z, Allegri L, Latos-Bielenska A, Casu D, State M, Scolari F, Ravazzolo R, Kiryluk K, Al-Awqati Q, D'Agati VD, Drummond IA, Tasic V, Lifton RP, Ghiggeri GM, Gharavi AG. Mutations in DSTYK and Dominant Urinary Tract Malformations. N Engl J Med. 2013 doi: 10.1056/NEJMoa1214479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hart TC, Gorry MC, Hart PS, Woodard AS, Shihabi Z, Sandhu J, Shirts B, Xu L, Zhu H, Barmada MM, Bleyer AJ. Mutations of the UMOD gene are responsible for medullary cystic kidney disease 2 and familial juvenile hyperuricaemic nephropathy. J Med Genet. 2002;39:882–892. doi: 10.1136/jmg.39.12.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gribouval O, Gonzales M, Neuhaus T, Aziza J, Bieth E, Laurent N, Bouton JM, Feuillet F, Makni S, Ben Amar H, Laube G, Delezoide AL, Bouvier R, Dijoud F, Ollagnon-Roman E, Roume J, Joubert M, Antignac C, Gubler MC. Mutations in genes in the renin-angiotensin system are associated with autosomal recessive renal tubular dysgenesis. Nat Genet. 2005;37:964–968. doi: 10.1038/ng1623. [DOI] [PubMed] [Google Scholar]
- 35.Gribouval O, Moriniere V, Pawtowski A, Arrondel C, Sallinen SL, Saloranta C, Clericuzio C, Viot G, Tantau J, Blesson S, Cloarec S, Machet MC, Chitayat D, Thauvin C, Laurent N, Sampson JR, Bernstein JA, Clemenson A, Prieur F, Daniel L, Levy-Mozziconacci A, Lachlan K, Alessandri JL, Cartault F, Riviere JP, Picard N, Baumann C, Delezoide AL, Belar Ortega M, Chassaing N, Labrune P, Yu S, Firth H, Wellesley D, Bitzan M, Alfares A, Braverman N, Krogh L, Tolmie J, Gaspar H, Doray B, Majore S, Bonneau D, Triau S, Loirat C, David A, Bartholdi D, Peleg A, Brackman D, Stone R, DeBerardinis R, Corvol P, Michaud A, Antignac C, Gubler MC. Spectrum of mutations in the renin-angiotensin system genes in autosomal recessive renal tubular dysgenesis. Hum Mutat. 2012;33:316–326. doi: 10.1002/humu.21661. [DOI] [PubMed] [Google Scholar]
- 36.Barak H, Huh SH, Chen S, Jeanpierre C, Martinovic J, Parisot M, Bole-Feysot C, Nitschke P, Salomon R, Antignac C, Ornitz DM, Kopan R. FGF9 and FGF20 maintain the stemness of nephron progenitors in mice and man. Developmental cell. 2012;22:1191–1207. doi: 10.1016/j.devcel.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woolf AS, Stuart HM, Newman WG. Genetics of human congenital urinary bladder disease. Pediatr Nephrol. 2013 doi: 10.1007/s00467-013-2472-1. [DOI] [PubMed] [Google Scholar]
- 38.Dressler GR. Advances in early kidney specification, development and patterning. Development. 2009;136:3863–3874. doi: 10.1242/dev.034876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reidy KJ, Rosenblum ND. Cell and molecular biology of kidney development. Seminars in Nephrology. 2009;29:321–337. doi: 10.1016/j.semnephrol.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Faa G, Gerosa C, Fanni D, Monga G, Zaffanello M, Van Eyken P, Fanos V. Morphogenesis and molecular mechanisms involved in human kidney development. Journal of Cellular Physiology. 2012;227:1257–1268. doi: 10.1002/jcp.22985. [DOI] [PubMed] [Google Scholar]
- 41.Vainio S, Lin Y. Coordinating early kidney development: lessons from gene targeting. Nature reviews Genetics. 2002;3:533–543. doi: 10.1038/nrg842. [DOI] [PubMed] [Google Scholar]
- 42.Ichikawa I, Kuwayama F, Pope JCt, Stephens FD, Miyazaki Y. Paradigm shift from classic anatomic theories to contemporary cell biological views of CAKUT. Kidney International. 2002;61:889–898. doi: 10.1046/j.1523-1755.2002.00188.x. [DOI] [PubMed] [Google Scholar]
- 43.Schuchardt A, D'Agati V, Larsson-Blomberg L, Costantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367:380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- 44.Stark K, Vainio S, Vassileva G, McMahon AP. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature. 1994;372:679–683. doi: 10.1038/372679a0. [DOI] [PubMed] [Google Scholar]
- 45.Nishimura H, Yerkes E, Hohenfellner K, Miyazaki Y, Ma J, Hunley TE, Yoshida H, Ichiki T, Threadgill D, Phillips JA, 3rd, Hogan BM, Fogo A, Brock JW, 3rd, Inagami T, Ichikawa I. Role of the angiotensin type 2 receptor gene in congenital anomalies of the kidney and urinary tract, CAKUT, of mice and men. Molecular Cell. 1999;3:1–10. doi: 10.1016/s1097-2765(00)80169-0. [DOI] [PubMed] [Google Scholar]
- 46.Mackie GG, Stephens FD. Duplex kidneys: a correlation of renal dysplasia with position of the ureteral orifice. The Journal of Urology. 1975;114:274–280. doi: 10.1016/s0022-5347(17)67007-1. [DOI] [PubMed] [Google Scholar]
- 47.Santoro M, Carlomagno F, Romano A, Bottaro DP, Dathan NA, Grieco M, Fusco A, Vecchio G, Matoskova B, Kraus MH, et al. Activation of RET as a dominant transforming gene by germline mutations of MEN2A and MEN2B. Science. 1995;267:381–383. doi: 10.1126/science.7824936. [DOI] [PubMed] [Google Scholar]
- 48.Romeo G, Ronchetto P, Luo Y, Barone V, Seri M, Ceccherini I, Pasini B, Bocciardi R, Lerone M, Kaariainen H, et al. Point mutations affecting the tyrosine kinase domain of the RET proto-oncogene in Hirschsprung's disease. Nature. 1994;367:377–378. doi: 10.1038/367377a0. [DOI] [PubMed] [Google Scholar]
- 49.Jeanpierre C, Mace G, Parisot M, Moriniere V, Pawtowsky A, Benabou M, Martinovic J, Amiel J, Attie-Bitach T, Delezoide AL, Loget P, Blanchet P, Gaillard D, Gonzales M, Carpentier W, Nitschke P, Tores F, Heidet L, Antignac C, Salomon R. RET and GDNF mutations are rare in fetuses with renal agenesis or other severe kidney development defects. J Med Genet. 2011;48:497–504. doi: 10.1136/jmg.2010.088526. [DOI] [PubMed] [Google Scholar]
- 50.Pini Prato A, Musso M, Ceccherini I, Mattioli G, Giunta C, Ghiggeri GM, Jasonni V. Hirschsprung disease and congenital anomalies of the kidney and urinary tract (CAKUT): a novel syndromic association. Medicine. 2009;88:83–90. doi: 10.1097/MD.0b013e31819cf5da. [DOI] [PubMed] [Google Scholar]
- 51.Chatterjee R, Ramos E, Hoffman M, VanWinkle J, Martin DR, Davis TK, Hoshi M, Hmiel SP, Beck A, Hruska K, Coplen D, Liapis H, Mitra R, Druley T, Austin P, Jain S. Traditional and targeted exome sequencing reveals common, rare and novel functional deleterious variants in RET-signaling complex in a cohort of living US patients with urinary tract malformations. Human Genetics. 2012;131:1725–1738. doi: 10.1007/s00439-012-1181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weber S, Moriniere V, Knuppel T, Charbit M, Dusek J, Ghiggeri GM, Jankauskiene A, Mir S, Montini G, Peco-Antic A, Wuhl E, Zurowska AM, Mehls O, Antignac C, Schaefer F, Salomon R. Prevalence of mutations in renal developmental genes in children with renal hypodysplasia: results of the ESCAPE study. J Am Soc Nephrol. 2006;17:2864–2870. doi: 10.1681/ASN.2006030277. [DOI] [PubMed] [Google Scholar]
- 53.Tabatabaeifar M, Schlingmann KP, Litwin M, Emre S, Bakkaloglu A, Mehls O, Antignac C, Schaefer F, Weber S. Functional analysis of BMP4 mutations identified in pediatric CAKUT patients. Pediatr Nephrol. 2009;24:2361–2368. doi: 10.1007/s00467-009-1287-6. [DOI] [PubMed] [Google Scholar]
- 54.Park JS, Ma W, O'Brien LL, Chung E, Guo JJ, Cheng JG, Valerius MT, McMahon JA, Wong WH, McMahon AP. Six2 and Wnt regulate self-renewal and commitment of nephron progenitors through shared gene regulatory networks. Developmental cell. 2012;23:637–651. doi: 10.1016/j.devcel.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Okubo S, Niimura F, Matsusaka T, Fogo A, Hogan BL, Ichikawa I. Angiotensinogen gene null-mutant mice lack homeostatic regulation of glomerular filtration and tubular reabsorption. Kidney International. 1998;53:617–625. doi: 10.1046/j.1523-1755.1998.00788.x. [DOI] [PubMed] [Google Scholar]
- 56.Tamm I, Horsfall FL., Jr Characterization and separation of an inhibitor of viral hemagglutination present in urine. Proc Soc Exp Biol Med. 1950;74:106–108. [PubMed] [Google Scholar]
- 57.Wolf MT, Hoskins BE, Beck BB, Hoppe B, Tasic V, Otto EA, Hildebrandt F. Mutation analysis of the Uromodulin gene in 96 individuals with urinary tract anomalies (CAKUT) Pediatr Nephrol. 2009;24:55–60. doi: 10.1007/s00467-008-1016-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Coffinier C, Thepot D, Babinet C, Yaniv M, Barra J. Essential role for the homeoprotein vHNF1/HNF1beta in visceral endoderm differentiation. Development. 1999;126:4785–4794. doi: 10.1242/dev.126.21.4785. [DOI] [PubMed] [Google Scholar]
- 59.Edghill EL, Bingham C, Ellard S, Hattersley AT. Mutations in hepatocyte nuclear factor-1beta and their related phenotypes. J Med Genet. 2006;43:84–90. doi: 10.1136/jmg.2005.032854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bellanne-Chantelot C, Chauveau D, Gautier JF, Dubois-Laforgue D, Clauin S, Beaufils S, Wilhelm JM, Boitard C, Noel LH, Velho G, Timsit J. Clinical spectrum associated with hepatocyte nuclear factor-1beta mutations. Annals of Internal Medicine. 2004;140:510–517. doi: 10.7326/0003-4819-140-7-200404060-00009. [DOI] [PubMed] [Google Scholar]
- 61.Adalat S, Woolf AS, Johnstone KA, Wirsing A, Harries LW, Long DA, Hennekam RC, Ledermann SE, Rees L, van't Hoff W, Marks SD, Trompeter RS, Tullus K, Winyard PJ, Cansick J, Mushtaq I, Dhillon HK, Bingham C, Edghill EL, Shroff R, Stanescu H, Ryffel GU, Ellard S, Bockenhauer D. HNF1B mutations associate with hypomagnesemia and renal magnesium wasting. J Am Soc Nephrol. 2009;20:1123–1131. doi: 10.1681/ASN.2008060633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moreno-De-Luca D, Mulle JG, Kaminsky EB, Sanders SJ, Myers SM, Adam MP, Pakula AT, Eisenhauer NJ, Uhas K, Weik L, Guy L, Care ME, Morel CF, Boni C, Salbert BA, Chandrareddy A, Demmer LA, Chow EW, Surti U, Aradhya S, Pickering DL, Golden DM, Sanger WG, Aston E, Brothman AR, Gliem TJ, Thorland EC, Ackley T, Iyer R, Huang S, Barber JC, Crolla JA, Warren ST, Martin CL, Ledbetter DH. Deletion 17q12 is a recurrent copy number variant that confers high risk of autism and schizophrenia. Am J Hum Genet. 2010;87:618–630. doi: 10.1016/j.ajhg.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Loirat C, Bellanne-Chantelot C, Husson I, Deschenes G, Guigonis V, Chabane N. Autism in three patients with cystic or hyperechogenic kidneys and chromosome 17q12 deletion. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2010;25:3430–3433. doi: 10.1093/ndt/gfq380. [DOI] [PubMed] [Google Scholar]
- 64.Hiesberger T, Bai Y, Shao X, McNally BT, Sinclair AM, Tian X, Somlo S, Igarashi P. Mutation of hepatocyte nuclear factor-1beta inhibits Pkhd1 gene expression and produces renal cysts in mice. J Clin Invest. 2004;113:814–825. doi: 10.1172/JCI20083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thomas R, Sanna-Cherchi S, Warady BA, Furth SL, Kaskel FJ, Gharavi AG. HNF1B and PAX2 mutations are a common cause of renal hypodysplasia in the CKiD cohort. Pediatr Nephrol. 2011;26:897–903. doi: 10.1007/s00467-011-1826-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Madariaga L, Moriniere V, Jeanpierre C, Bouvier R, Loget P, Martinovic J, Dechelotte P, Leporrier N, Thauvin-Robinet C, Jensen UB, Gaillard D, Mathieu M, Turlin B, Attie-Bitach T, Salomon R, Gubler MC, Antignac C, Heidet L. Severe Prenatal Renal Anomalies Associated with Mutations in HNF1B or PAX2 Genes. Clinical journal of the American Society of Nephrology : CJASN. 2013;8:1179–1187. doi: 10.2215/CJN.10221012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weber S, Landwehr C, Renkert M, Hoischen A, Wuhl E, Denecke J, Radlwimmer B, Haffner D, Schaefer F, Weber RG. Mapping candidate regions and genes for congenital anomalies of the kidneys and urinary tract (CAKUT) by array-based comparative genomic hybridization. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2011;26:136–143. doi: 10.1093/ndt/gfq400. [DOI] [PubMed] [Google Scholar]
- 68.Sanna-Cherchi S, Kiryluk K, Burgess KE, Bodria M, Sampson MG, Hadley D, Nees SN, Verbitsky M, Perry BJ, Sterken R, Lozanovski VJ, Materna-Kiryluk A, Barlassina C, Kini A, Corbani V, Carrea A, Somenzi D, Murtas C, Ristoska-Bojkovska N, Izzi C, Bianco B, Zaniew M, Flogelova H, Weng PL, Kacak N, Giberti S, Gigante M, Arapovic A, Drnasin K, Caridi G, Curioni S, Allegri F, Ammenti A, Ferretti S, Goj V, Bernardo L, Jobanputra V, Chung WK, Lifton RP, Sanders S, State M, Clark LN, Saraga M, Padmanabhan S, Dominiczak AF, Foroud T, Gesualdo L, Gucev Z, Allegri L, Latos-Bielenska A, Cusi D, Scolari F, Tasic V, Hakonarson H, Ghiggeri GM, Gharavi AG. Copy-number disorders are a common cause of congenital kidney malformations. Am J Hum Genet. 2012;91:987–997. doi: 10.1016/j.ajhg.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Puri P, Gosemann JH, Darlow J, Barton DE. Genetics of vesicoureteral reflux. Nature reviews Urology. 2011;8:539–552. doi: 10.1038/nrurol.2011.113. [DOI] [PubMed] [Google Scholar]
- 70.Ruf RG, Berkman J, Wolf MT, Nurnberg P, Gattas M, Ruf EM, Hyland V, Kromberg J, Glass I, Macmillan J, Otto E, Nurnberg G, Lucke B, Hennies HC, Hildebrandt F. A gene locus for branchio-otic syndrome maps to chromosome 14q21.3-q24.3. J Med Genet. 2003;40:515–519. doi: 10.1136/jmg.40.7.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Esther CR, Marino EM, Howard TE, Machaud A, Corvol P, Capecchi MR, Bernstein KE. The critical role of tissue angiotensin-converting enzyme as revealed by gene targeting in mice. J Clin Invest. 1997;99:2375–2385. doi: 10.1172/JCI119419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McGregor L, Makela V, Darling SM, Vrontou S, Chalepakis G, Roberts C, Smart N, Rutland P, Prescott N, Hopkins J, Bentley E, Shaw A, Roberts E, Mueller R, Jadeja S, Philip N, Nelson J, Francannet C, Perez-Aytes A, Megarbane A, Kerr B, Wainwright B, Woolf AS, Winter RM, Scambler PJ. Fraser syndrome and mouse blebbed phenotype caused by mutations in FRAS1/Fras1 encoding a putative extracellular matrix protein. Nat Genet. 2003;34:203–208. doi: 10.1038/ng1142. [DOI] [PubMed] [Google Scholar]
- 73.Jadeja S, Smyth I, Pitera JE, Taylor MS, van Haelst M, Bentley E, McGregor L, Hopkins J, Chalepakis G, Philip N, Perez Aytes A, Watt FM, Darling SM, Jackson I, Woolf AS, Scambler PJ. Identification of a new gene mutated in Fraser syndrome and mouse myelencephalic blebs. Nat Genet. 2005;37:520–525. doi: 10.1038/ng1549. [DOI] [PubMed] [Google Scholar]
- 74.Kirby A, Gnirke A, Jaffe DB, Barešová V, Pochet N, Blumenstiel B, Ye C, Aird D, Stevens C, Robinson JT, Cabili MN, Gat-Viks I, Kelliher E, Daza R, DeFelice M, Hůlková H, Sovová J, Vylet'al P, Antignac C, Guttman M, Handsaker RE, Perrin D, Steelman S, Sigurdsson S, Scheinman SJ, Sougnez C, Cibulskis K, Parkin M, Green T, Rossin E, Zody MC, Xavier RJ, Pollak MR, Alper SL, Lindblad-Toh K, Gabriel S, Hart PS, Regev A, Nusbaum C, Kmoch S, Bleyer AJ, Lander ES, Daly MJ. Mutations causing medullary cystic kidney disease type 1 lie in a large VNTR in MUC1 missed by massively parallel sequencing. Nat Genet. 2013;45(3):299–303. doi: 10.1038/ng.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]