Abstract
The Akt protein kinase, also known as protein kinase B, plays key roles in insulin receptor signalling and regulates cell growth, survival and metabolism. Recently, we described a mechanism to enhance Akt phosphorylation that restricts access of cellular phosphatases to the Akt activation loop (Thr308 in Akt1 or protein kinase B isoform alpha) in an ATP-dependent manner. In the present paper, we describe a distinct mechanism to control Thr308 dephosphorylation and thus Akt deactivation that depends on intramolecular interactions of Akt C-terminal sequences with its kinase domain. Modifications of amino acids surrounding the Akt1 C-terminal mTORC2 (mammalian target of rapamycin complex 2) phosphorylation site (Ser473) increased phosphatase resistance of the phosphorylated activation loop (pThr308) and amplified Akt phosphorylation. Furthermore, the phosphatase-resistant Akt was refractory to ceramide-dependent dephosphorylation and amplified insulin-dependent Thr308 phosphorylation in a regulated fashion. Collectively, these results suggest that the Akt C-terminal hydrophobic groove is a target for the development of agents that enhance Akt phosphorylation by insulin.
Keywords: ceramide, dephosphorylation resistance, insulin sensitivity, protein kinase A, protein kinase B/Akt
INTRODUCTION
The serine/threonine Akt protein kinases (Akt1, Akt2 and Akt3, also known as protein kinase B isoforms α, β and γ) affect multiple cellular functions related to cell growth and survival, differentiation, metabolism and migration [1,2]. Activation of Akt kinases is initiated by docking of the Akt pleckstrin homology (PH) domain to membrane phosphoinositide lipid products PtdIns(3,4,5)P3 or PtdIns(3,4)P2 [1,2]. Akt catalytic activity is further contingent on phosphorylation of two regulatory residues, one at its centrally located activation loop and one at the C-terminal tail. Complex mechanisms regulate the phosphorylation state of these two residues.
Akt activation loop phosphorylation (Thr308 in Akt1) is achieved by the phosphoinositide-dependent kinase-1 (PDK1). The Akt C-terminal tail (Ser473 in Akt1) is phosphorylated by the mTORC2 (mammalian target of rapamycin complex 2) protein kinase, which is composed of the mammalian target of rapamycin catalytic subunit and the RICTOR (Rapamycin-insensitive companion of mTOR) subunit [3,4]. However, knockout of mTORC2 in mice only partially blocks Akt Ser473 phosphorylation, as evidenced in muscle tissues [5,6]. TANK-binding kinase 1 (TBK1) and atypical IκB kinase ε were found to phosphorylate Akt kinases at both the activation loop and the C-terminal tail [7,8]. Similarly, DNA-dependent protein kinase (DNA-PK) can phosphorylate the Akt1 C-terminal tail (Ser473) during the DNA-damage response [9,10]. In addition, mTORC2 was found to phosphorylate additional residues in the extreme Akt1 C-terminus (Ser477 and Thr479) [11]. These sites are also phosphorylated by cyclin-dependent kinase 2 (Cdk2)/cyclin A or by DNA-PK under synchronized cell cycle conditions and DNA-damaging conditions respectively.
Dephosphorylation of Akt kinases is accomplished by at least two phosphatases: the abundantly expressed protein phosphatase 2A (PP2A) [12,13] and PHLPP1/2 (PH domain leucine-rich repeat protein phosphatase). PHLPP1/2 is a member of the PP2C phosphatase family, which selectively dephosphorylates residues located in the C-terminal tails of protein kinase C (PKC) and of Akt kinases [14].
Previously, we showed that Akt dephosphorylation is subject to intrinsic allosteric control via ATP binding to Akt [15]. ATP binding alters the Akt activation loop conformation to enable interaction of the phosphorylated activation loop with other residues located in the kinase domain, including Arg273 (Arg274 in Akt2) resulting in steric hindrance of activation loop dephosphorylation. As a consequence, Akt activation at the plasma membrane is sustained or prolonged. This mechanism is probably responsible for the ‘paradoxical’ phosphorylation of Akt kinases observed during treatment of cells with several Akt-specific ATP competitive inhibitors, including A-443654, GSK690693 and GDC-0068 [16–18]. In the case of Akt2, this mechanism has been shown to be biologically relevant. Specifically, Akt2 plays an important role in glucose metabolism and mitochondrial function [19,20] and the R274H mutation not only compromises the phosphatase-shielding cage but also is associated with severe insulin resistance and diabetes mellitus in humans [21]. Residues analogous to Arg274 also protect PKA (protein kinase A) and PKC kinases from dephosphorylation [22], suggesting that allosteric mechanisms controlling phosphatase access to regulatory residues can increase steady-state phosphorylation of Akt and other members of the protein kinase A, G, and C (AGC) group.
In the present paper, we characterize a second allosteric regulatory mechanism that controls Akt dephosphorylation kinetics and is mediated by intramolecular association of C-terminal sequences of Akt kinases with their kinase domains. We further provide evidence that the strength of interaction of C-terminal sequences with the kinase domain can be exploited to modulate Akt dephosphorylation kinetics. Molecular dials that gate phosphatase access are embedded in different parts of Akt kinase, including the nucleotide-binding pocket, the PH domain and the C-terminal sequences. The intricate interplay of these molecular dials is likely to contribute to insulin and ceramide signalling and they offer novel therapeutic targets to treat diseases ranging from cancer to diabetes.
MATERIALS AND METHODS
Plasmids, peptides and chemicals
Akt1 was fused at the N-terminus with an Src myristoylation signal (Myr, MGSSKSKPKSR) and at the C-terminus with a haemagglutinin (HA) epitope, as described in [15]. To distinguish heterologously expressed Akt from endogenous Akt, a 41-amino-acid large tag (LT), AIDGAGAGALVPRGSKET-AAAKFERQHMDSGAYPYDVPDYA, was fused at Akt C-terminus. The LT tag contained a peptide linker followed by a thrombin cleavage site, S-epitope tag and HA epitope tag. All plasmids were under the control of the cytomegalovirus promoter. Mutant constructs were generated using standard molecular biology strategies and confirmed by sequencing at the core facilities of Kimmel Cancer Center (Philadelphia, PA, U.S.A.). GFP in pFred143 (KH1035) was used in co-transfections to monitor transfection efficiency. H-89 {N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide}, a weak ATP-competitive Akt inhibitor (Km 2.5μM [23]) was purchased from LC Laboratories. The phosphatase inhibitor calyculin A was purchased Cell Signaling Technology. Pervanadate was prepared fresh by mixing equal molar amounts of hydrogen peroxide and NaVO4 [24].
Cell culture and transfection
H9C2 cells derived from rat neonatal hearts (A.T.C.C.) were cultured in M199 medium supplemented with 10% FBS and antibiotics. Human embryonic kidney (HEK) 293 cells were cultured in Dulbecco’s modified Eagle’s essential medium (DMEM) supplemented with 10% FBS and antibiotics. Primary human airway smooth muscle cultures were generated as described previously [25]. Cells were transfected using Fugene-6 HD (Roche) or Lipofectamine 3000 Reagent (Life Technologies) according to the manufacturer’s protocols.
Cell extract Akt dephosphorylation
To maximally phosphorylate Akt1 constructs at the activation loop (Thr308), Akt constructs were co-transfected with PDK1. Transfected cells were stimulated for 15 min with 2 μM insulin, in the presence of the tyrosine phosphatase inhibitor pervanadate (100 μM). To harvest, stimulated cells were washed once with ice-cold PBS before they were placed in a cell culture dish on an ethanol/solid-CO2 bath to flash freeze. Cell-free extracts were prepared by scraping flash-frozen cells into phosphatase assay buffer (50 mM Hepes, pH 7.5, 100 mM NaCl, 1 mM EDTA, 1 mM EGTA, 10 mM NaF, 5 μM H-89 ATP-competitive inhibitor and 0.1% NP40 detergent supplemented with 5 μg/ml leupeptin, 5 μg/ml aprotinin, 10 mM PMSF and 1 mM DTT). H-89 was used in the cell-free system as an ATP-competitive inhibitor against Akt (Km for Akt = 2.5 μM [23]). Total cell-free extracts were incubated at 30°C to initiate dephosphorylation. At the indicated times, a fixed amount of cell extract was removed from incubation and the reaction stopped by adding an equal volume of stop assay buffer (25 mM Tris/HCl, pH 7.6, 137 mM NaCl, 10% glycerol, 1% NP40, 10 mM NaF supplemented with 5 μg/ml leupeptin, 5 μg/ml aprotinin, 10 mM PMSF, 1 mM NaVO4 and 100 nM calyculin, 20 mM β -glycerolphosphate and 1 mM sodium pyrophosphate). After lysis on ice for 10 min, cell lysates were clarified, reduced and denatured by PAGE sample buffer for immunoblotting.
Recombinant Akt and protein kinase A dephosphorylation
Recombinant activated Akt1 (50 ng, Millipore) and recombinant activated PKA catalytic subunit (50 ng, New England Biolabs) was dephosphorylated in 50 μl of optimized phosphatase assay buffer containing non-ionic detergents and supplemented with metal ions (1 mM MnCl2 or 5 mM MgCl2) and phospholipid micelles containing 4 μM PtdIns(3,4,5)P3, 40 μM phosphoserine and 40 μM phosphocholine (Echelon Biosciences or Avanti Polar Lipids). The assays were initiated by adding 60–70 ng of recombinant PP2A catalytic subunit (L309 deletion, Cayman Chemicals) or 40 units of λ-phosphatase (Cell Signaling Technology).
Phosphatase assay with p-nitrophenyl phosphate as substrate
The same conditions used for Akt dephosphorylation were applied to assays using the colorimetric p-nitrophenyl phosphate phosphatase (PNPP) substrate. Assays were set up in untreated 96-well plates (Nunc 80040LE 0910) on ice and warmed at 37°C for 2 min before adding 50 μl of commercially prepared PNPP substrate (Anaspec). Phosphatase activity was measured at 405 nm from 5 to 60 min after incubation at 37°C. Where indicated, 200 ng of PP2A and 40 units of λ-phosphatase were used.
Immunoblotting
Akt phosphorylation (Ser473 and Thr308) was measured as described previously [26]. Briefly, cells were homogenized on ice using a NP40 lysis buffer (25 mM Tris/HCl, pH 7.6, 137 mM NaCl, 10% glycerol, 1% NP40 and 10 mM NaF) freshly supplemented with 1 mM sodium pyrophosphate, 5 μg/ml leupeptin, 5 μg/ml aprotinin, 1 mM EDTA, 10 mM PMSF, 1 mM NaVO4 and 1 mM DTT. Clarified cellular lysates were boiled and separated by SDS/PAGE (4–12% gels) and transferred on to nitrocellulose membranes. For immunoblotting, membranes were blocked for 30 min with Li-Cor blocking buffer and probed with antibodies at 4°C overnight. The blots were subsequently incubated with either IRDye 700 or 800 secondary antibodies conjugated to IR fluorophores for 60 min. Bands were visualized and directly quantified using the Odyssey Infrared Imaging System (Li-Cor). The following antibodies were used at 1:1000 dilution: anti-phospho-proline-rich Akt substrate of 40 kDa (PRAS40) Ser246, anti-phosph-ERK1/2 (extracellular-signal-regulated kinase 1/2), anti-phospho-Akt (Thr308), anti-phospho-Akt (Ser473; from Cell Signaling Technology), anti-total Akt (from BD Biosciences) and anti-GAPDH (glyceraldehyde-3-phosphate dehydrogenase; from Santa Cruz Biotechnology) and anti-HA (from Covance).
Modelling structure
Structural figures were prepared using PyMOL (http://www.pymol.org) based on structures of activated Akt2 kinase domain with adenosine 5′-[β, γ-imido]triphosphate (AMP-PNP)/Mn2+ [27,28] and PKA [29].
Calculation of relative free energy contributions
A CHARMM (Chemistry at HARvard Macromolecular Mechanics)-based method [30] was utilized to approximate the free energy contribution of each PDK1-interacting fragment (PIFtide) residue side chain interaction with the kinase domain-binding groove interface. The free energy contributions of the side chains are approximated by first removing peptide backbone atoms, transforming the α-carbon into a methyl group and calculating the linear interaction energy (LIE) difference between the scaled potential energies of the bound and free states [30]. Calculations are performed utilizing the Generalized-Born with Molecular Volume (GBMV) implicit solvent model providing a rigorous treatment of desolvation penalties.
Statistical analysis
A commercial software package was used for statistical analysis (Graph Pad Software). Comparison of means ± S.E.M. was analysed by non-parametric Mann–Whitney test.
RESULTS
Two AGC kinases, Akt and PKA, exhibit differential phosphatase sensitivity
When Akt kinases are ATP-bound, their phosphorylated activation loop (pThr308 in Akt1) interacts with other residues, His194 and Arg273, within the kinase domain and this interaction restricts cellular phosphatases from accessing the phosphorylated site [15]. The amino acid residues required for constructing this ‘phosphatase-shielding cage’ are conserved among AGC kinases, including PKA [22] (Figure 1A). However, unlike Akt kinase, inactive (regulatory subunit-bound) PKA is constitutively phosphorylated at the activation loop via the combined abilities of auto-phosphorylation and phosphatase resistance [31]. PKA activation loop is phosphorylated by PKA itself or by PDK1. In addition, importantly, the fully phosphorylated PKA catalytic subunit becomes highly resistant to phosphatases in cells and in cell-free assays [32–34].
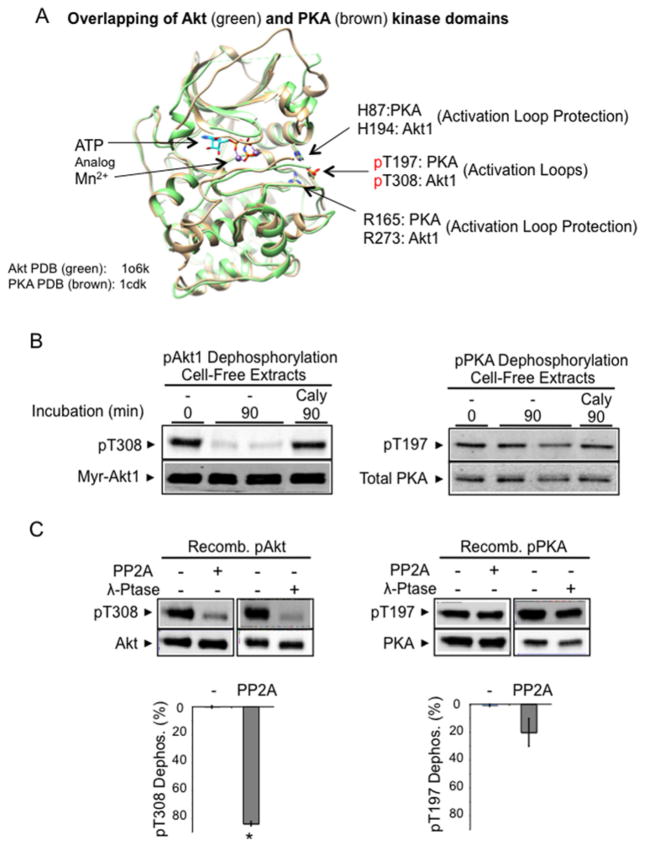
Figure 1. Constitutive dephosphorylation resistance of phospho-PKA, but not phospho-Akt, in a cell-free assay.
(A) Superimposition of Akt kinase domain (Akt2–Mn–AMP-PNP–G–SK3, green, PDB code 1o6k [28]) and PKA kinase domain (PKA–Mn–ATP–PKI, brown, PDB code 1cdk [88]). In the presence of bound ATP analogues, the phosphorylated activation loops (Thr308 in Akt1, Thr197 in PKA) shown are stabilized by invariable histidine (His194 in Akt1, His87 in PKA) and arginine (Arg273 in Akt1, Arg165 in PKA) residues. The structure was modelled on active human Akt2 crystal structures bound to the ATP analogue AMP-PNP and Mn2 + (PDB code 1o6k). Since most of our biochemical studies were performed with Akt1, we indicate the corresponding Akt1 amino acid residues in the structure for clarity. (B) Cell extract dephosphorylation of Akt and PKA. H9C2 cells expressing constitutively phosphorylated Myr-Akt1-HA and phosphorylated endogenous PKA were flash-frozen and extracted on ice. Aliquots of cell extracts without phosphatase inhibitors were incubated at 30°C for 90 min. After incubation, protein extracts were subjected to immunoblot analysis using antibodies detecting phosphorylated Akt (Thr308), HA-tagged Akt, phosphorylated PKA (Thr197) and total PKA. (C) Recombinant phospho-PKA catalytic subunit, but not that of recombinant phospho-Akt, resists dephosphorylation. Recombinant Akt1 and PKA (50 ng) were incubated with PP2A (70 ng of PP2A-C) or pan-substrate bacteriophage λ phosphatase (40 units) for 60 min at 30°C. Images show Akt1 Thr308 phosphorylation, total Akt, PKA Thr197 phosphorylation and total PKA. The histograms show the percentage of pThr308 or pThr197 dephosphorylation. n=4/group. *P < 0.05 compared with PKA dephosphorylation by PP2A.
To directly compare dephosphorylation sensitivity between Akt and PKA using cell-free conditions, H9C2 cell extracts were prepared containing either constitutively phosphorylated Akt1 (Myr-Akt1) or constitutively phosphorylated PKA, using a rapid solid-CO2 freezing/protein extraction method [15]. Incubation of cell extracts at 30°C resulted in 85% of Myr-Akt1 being dephosphorylated by cellular phosphatases within 90 min; inclusion of the phosphatase inhibitor calyculin A inhibited this dephosphorylation (Figure 1B). By contrast and as expected, only 7% of PKA was dephosphorylated following incubation at 30°C for 90 min. It is worth mentioning that transfected Myr-Akt1 expression was much higher than endogenous Akt, but transfection did not affect endogenous Akt response (Supplementary Figure S1).
To rule out the possibility that PKA dephosphorylation resistance was caused either by unknown factors in the cellular milieu or by intramolecular interactions between the PKA catalytic subunit and its regulatory subunit [35], we measured in vitro dephosphorylation using recombinant preparations of Akt and PKA catalytic subunits. When (pre)-phosphorylated recombinant Akt1 or PKA (50 ng each) were incubated with the PP2A catalytic subunit (50 ng), 84% of Akt Thr308 was dephosphorylated within 60 min, yet only 20% of PKA pThr197 was dephosphorylated (Figure 1C). PKA also resisted dephosphorylation more efficiently than did Akt1 when incubated with the pan-substrate bacteriophage λ phosphatase (Figure 1C, second panel). Thus, purified Akt kinase is ‘intrinsically’ sensitive to dephosphorylation, whereas PKA is ‘constitutively’ phosphatase-resistant.
Replacing Akt C-terminus with PIFtide enhances Akt activation loop phosphorylation
As noted earlier, PKA and Akt isoforms are members of AGC kinase family and, as shown for Akt1 and Akt2, their catalytic domains share a high degree of structural and amino acid homology, except at their C-termini (Figure 2A). An active Akt1 structure is not available. We compared the PKA C-terminus to active Akt2 structure with the S474D peptide (PDB code 1o6k). Since most of our biochemical studies were performed with Akt1, we indicated the corresponding Akt1 amino acid residues for clarity.
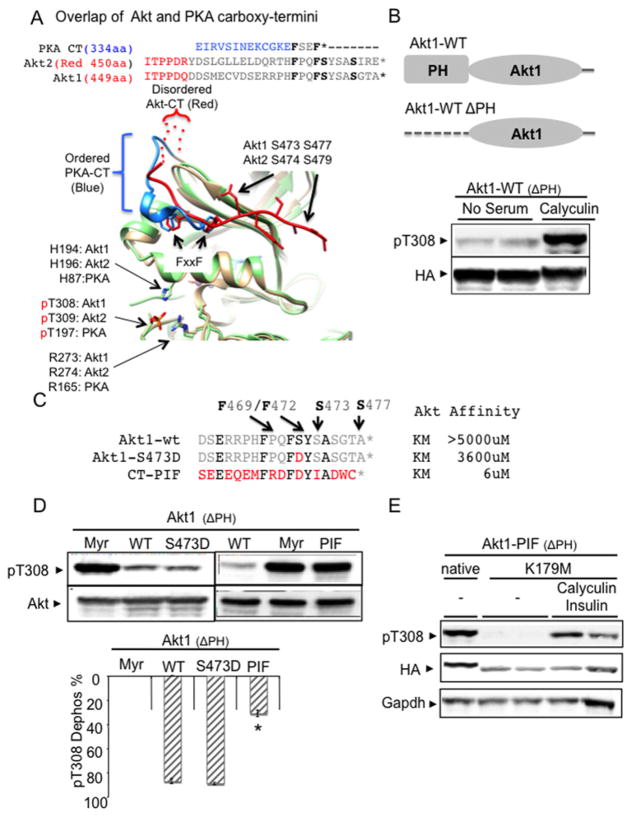
Figure 2. Replacing cytosolic Akt1 C-terminal sequences with PIFtide (SEEEQEMFRDFDYIADWC) and phosphatase inhibitor treatment similarly enhanced Thr308 phosphorylation.
(A) Amino acid composition of Akt1 and Akt2 are highly homologous, especially at the FxxF C-terminal sequences. Only the active Akt2 crystal structure (PDB code 1o6k) is available. Since most of our biochemical studies were performed with Akt1, we indicated both Akt1 and Akt2 amino acid residues in the structure for clarity. Superimposition of active Akt2 crystal structure (PDB code 1o6k) with active PKA crystal structure (PDB code 1cdk). Positioning of the Akt C-terminus (colour red) is distinct from the PKA C-terminus (colour blue). Whereas Akt C-terminal residues (450–466) are disordered, the PKA C-terminus stably interacts with its kinase domain through high-affinity contacts. (B) Phosphatase inhibitor treatment (100 nM calyculin A) increases Thr308 phosphorylation in cytosol-localized Akt (ΔPH-Akt1). PH domain-deleted WT Akt1 (ΔPH-Akt1-WT) was transfected into HEK293 cells and exhibits expression localized to the cytosol. After 48 h, the transfected cells were propagated for an additional 4 h in exogenous growth factor-free medium followed by addition of 100 nM calyculin A for 15 min. Protein extracts were subjected to immunoblot analysis using antibodies to phospho-Akt Thr308 and HA tag. (C) Sequence and known Akt-binding affinity values of Akt1 C-terminal tail (19 amino acids), phosphomimetic aspartic acid replacement of Ser473 (S473D) tail and PIFtide tail from the AGC kinase PRK2 (SEEEQEMFRDFDYIADWC) [37]. The six PIFtide amino acids identical with WT Akt sequences are highlighted. The PIFtide sequences were used to replace the Akt tail in the cytosolic Akt construct (ΔPH-Akt1-PIF). (D) Cytosolic Akt1 containing PIFtide (ΔPH-Akt1-PIF) increases Thr308 phosphorylation. ΔPH-Akt1 with PIFtide replacement (ΔPH-Akt1-PIF) along with membrane-targeting Myr signal (Myr-ΔPH-Akt1) and S473D replacement (ΔPH-Akt1-S473D) constructs were transfected into HEK293 cells. Transfected cells were incubated for 4 h in medium that was free of exogenous growth factors. Protein extracts were subjected to immunoblot analysis using antibodies to phospho-Akt Thr308 and anti-HA. The histogram shows the percentage of reduced pThr308 phosphorylation from maximally phosphorylated Myr-ΔPH-Akt1. ΔPH-Akt1-WT (n=7) compared with ΔPH-Akt1-PIF (n=8), *P < 0.001. See Supplementary Figure S2C for ΔPH-Akt2-S474D replacement data. (E) ATP binding is required for ΔPH-Akt1-PIF to enhance Akt phosphorylation. The ATP-binding lysine residue (Lys179 in full-length human Akt1) was mutated to methionine (K179M) in in ΔPH-Akt1-PIF. HEK293 cells transfected with indicated constructs were incubated for 4 h in medium free of exogenous growth factors, with or without 100 nM calyculin A/2 μM insulin for 30 min.
Structural comparison shows marked differences in intramolecular interactions formed by the respective C-termini. The PKA C-terminus is well-ordered, prior to the FxxF motif (residues 334–344). By comparison, the Akt C-terminus is disordered prior to the FxxF motif (residues 450–455). Using crystal structures of PKA (PDB code 4dg0) and Akt2 (PDB code 1o6k), a CHARMM-based method enabled us to approximate the free energy contribution of each C-terminal residue’s side chain interaction with the kinase domain-binding groove interface (Supplementary Table S1). This analysis reveals that PKA Phe350 of the FxxF motif is involved in several buried electrostatic interactions with residues Gln35, Lys92 and Lys111; consequently, the PKA Phe350 interaction within the kinase domain fold is −0.7 kcal/mol more favourable when compared with the comparable Akt residue (Akt2 Phe473) interactions. These ‘high contact order’ interactions are particularly stabilizing to tertiary structures [36].
Thus structural analysis predicts that PKA C-terminus forms strong intramolecular interactions involving the FxxF motif. By contrast, the Akt2 C-terminus interacts with the kinase domain in a more labile and dynamic fashion. This view is supported by experimental data revealing weak binding of the Akt C-terminus to the kinase domain [37]. The binding affinity of wild-type (WT) Akt C-terminal peptide for the hydrophobic groove on the kinase N-lobe is greater than 5000 μM. Even with phosphomimetic S473D replacement, the C-terminal peptide affinity remains at 3600 μM [37].
We tested phosphatase sensitivity of phospho-Akt in cells by deletion of the PH domain (ΔPH-Akt1), which constitutively localizes Akt in the cytosol. Treating ΔPH-Akt1 expressing cells with a phosphatase inhibitor (calyculin A) restored phosphorylation (Figure 2B), indicating that phosphatase inhibition enhances cytosolic Akt phosphorylation. Transfected ΔPH-Akt1-WT expression was much higher than endogenous Akt (Supplementary Figure S2A).
We next tested the hypothesis that the differences in the strength of the intramolecular interactions of C-terminus FxxF motif may affect the phosphatase protection of activation loop (Akt pThr308). Unlike peptide sequences that correspond to WT Akt C-terminus which has weak affinity (~5000 μM) for binding the Akt kinase domain, a peptide derived from the C-terminus of PKC-related protein kinase 2 (PRK2) (PIFtide) binds Akt at 6 μM [37] (Figure 2C). Thus, we replaced the WT Akt C-terminus with the high-affinity PIF peptide (ΔPH-Akt1-PIF, SEEEQEMFRDFDYIADWC). When expressed in HEK293 cells, the ΔPH-Akt1-PIF construct enhanced Thr308 activation loop phosphorylation to an extent comparable to that observed with membrane-bound Myr-ΔPH-Akt1 (Figure 2D). The expression level of ΔPH-Akt1 mutants was much higher than that of endogenous Akt, but their phosphorylation was not affected by insulin stimulation (Supplementary Figure S2B). Interestingly, phosphomimetic mutations in ΔPH-Akt1 (S473D) or in ΔPH-Akt2 (S474D) failed to enhance Thr308 phosphorylation, probably due to weak ligand affinity even after S473D mutation [37] (Figure 2D; Supplementary Figure S2C).
Previously we demonstrated that ATP-binding site occupancy imparts dephosphorylation resistance to Akt [15]. To test whether the enhanced phosphatase resistance in ΔPH-Akt1-PIF depends on occupation of the ATP-binding pocket, we mutated the ATP-binding lysine residue (Lys179 in the full-length human Akt1) to methionine (K179M) in ΔPH-Akt1-PIF. This construct (K179M-ΔPH-Akt1-PIF) was poorly phosphorylated at Thr308, yet phosphorylation was restored when cells were treated with calyculin A with or without insulin (Figure 2E; Supplementary Figure S2D). Thus, the phosphatase resistance in ΔPH-Akt1-PIF is contingent upon occupation of the ATP-binding pocket previously shown to support Akt dephosphorylation resistance.
Replacing the Akt C-terminus with PIFtide blocks Akt dephosphorylation in cell-free assays
To avoid confounding variables inherent in cell-based studies [38,39], we used cell-free assays to directly test the ability of PIFtide to block phosphatase access to the Akt Thr308 site.
To differentiate transfected full-length Akt from endogenous Akt, we fused full-length Akt1-cDNA with a 41-amino-acid LT. This epitope tag consisted of a flexible linker, a thrombin cleavage site, an S-tag and a HA-tag (Figure 3A). To facilitate Akt dephosphorylation kinetics monitoring in the cell-free assay, we first established conditions that maximally phosphorylated Thr308 in Akt1-LT WT.
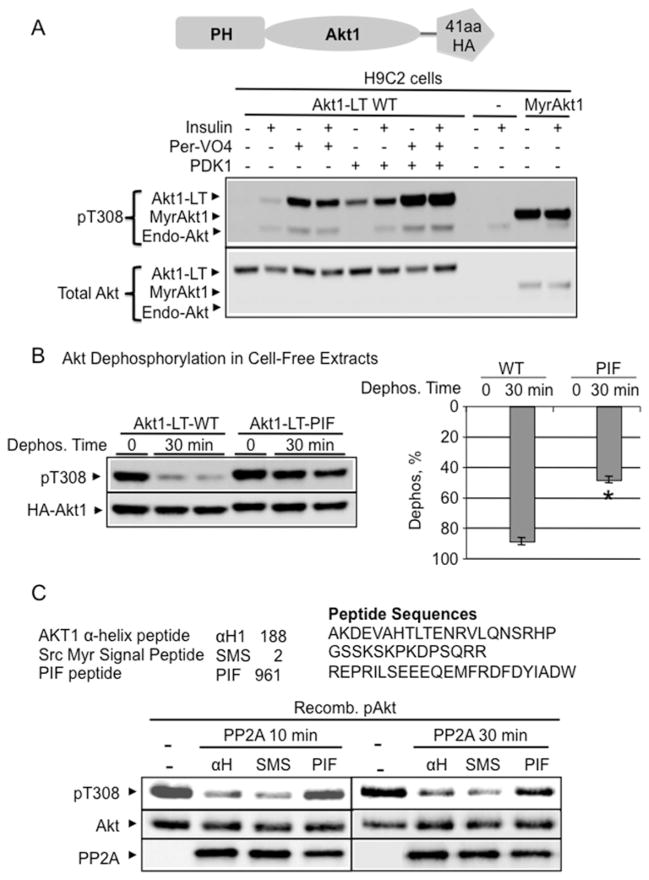
Figure 3. PIFtide enhanced Akt phosphatase resistance in cell-free assays.
(A) To distinguish heterologously expressed Akt from endogenous Akt, a 41-amino-acid LT containing the HA epitope was fused with WT Akt1 (Akt1-LT WT). To define conditions that maximally phosphorylate the Thr308 site, Akt1-LT WT was first co-transfected with the kinase that phosphorylates the Thr308 site (PDK1). Constitutively phosphorylated Myr-Akt1 construct was used as positive control. Then, transfected cells were stimulated for 15 min with 2 μM insulin plus the tyrosine phosphatase inhibitor pervanadate (Per-VO4) (100 μM). Immunoblot analysis using antibodies detecting phosphorylated Akt (Thr308) showed that the combined treatment of insulin/pervanadate/PDK1 maximally phosphorylated Akt1-LT WT. (B) Akt1 with PIFtide replacement resisted dephosphorylation in cell-free extracts. H9C2 cells expressing WT and PIFtide-replaced Akt1-LT constructs were maximally phosphorylated by insulin/pervanadate/PDK1 treatment. Flash-frozen cell extracts were prepared on ice with phosphatase inhibitor-free detergent extraction buffer. The extracts were incubated at 30°C for 30 min. After incubation, protein extracts were subjected to immunoblot analysis using antibodies detecting phosphorylated Akt (Thr308). The histogram shows the percentage of Akt dephosphorylation at 0 min and at 30 min (Dephos. %) (respectively, Akt1-LT WT, n=5, n=10; Akt1-LT-PIF, n=2, n=4). *P < 0.01 compared with Akt1-LT WT at 30 min. (C) Recombinant PIFtide increased phosphatase resistance in recombinant WT-Akt. Amino acid position and composition of synthesized peptides used in the dephosphorylation assay. Pre-phosphorylated recombinant Akt1 (50 ng) was incubated with recombinant PP2A for 10 min or 30 min at 30°C. Assay solution contained 100 μM ATP and 10 μM of indicated peptides. Immunoblot images show Akt1 Thr308 phosphorylation, total Akt1 and PP2A-C protein level. In addition, PIFtide did not affect the catalysis of the universal phosphatase substrate, PNPP (Supplementary Figure S2).
We tested cell treatment conditions in H9C2 cells and showed that combining PDK1 expression with insulin and pervanadate treatment resulted in maximal Thr308 phosphorylation of the Akt1-LT WT construct (Figure 3A). Insulin/pervanadate/PDK1 treatment resulted in Akt1-LT WT phosphorylation at a level comparable to that observed for myristoylated Akt (Figure 3A) and for PIFtide-fused Akt1 (Figure 3B). PDK1/insulin/pervanadate treatment also maximized Akt1-LT WT phosphorylation in HEK293 cells (Supplementary Figure S3A).
To test Akt dephosphorylation kinetics in cell-free extracts, H9C2 cells expressing maximally phosphorylated Akt1-LT WT and Akt1-LT PIF were snap-frozen using an ethanol/solid CO2 bath. Cell extracts were prepared with an EDTA-detergent extraction buffer without phosphatase inhibitors at 4°C. Akt1-LT WT was dephosphorylated by 89 ± 2% within 30 min of incubation at 30°C (Figure 3B). Under the same conditions, Akt with the PIFtide was only dephosphorylated by 48±2% (P < 0.01), suggesting that PIFtide replacement enhanced Akt resistance to phosphatase activity. Unlike transfected constructs, endogenous Akt was similarly dephosphorylated in different cell extract types (Supplementary Figure S3B).
To directly assess whether PIFtide blocks Akt1 dephosphorylation, we incubated the purified PP2A catalytic subunit with recombinant phospho-Akt1 in a solution containing sonicated lipid micelles and PIFtide (Figure 3C). Under these conditions, PIFtide blocked Akt dephosphorylation by PP2A. For a control, we performed dephosphorylation assays in the absence of peptides and also with control peptides (Akt2 S473D C-terminal peptide, α-helical region of Akt peptide, the myristoylated Src peptide and the myristoylated PKA peptide). With the exception of PIFtide, none of the peptides tested affected Akt dephosphorylation significantly (Figure 3C; Supplementary Figure S3C).
To rule out the possibility that PIFtide peptide directly inhibited phosphatases, we determined the effect of PIFtide on dephosphorylation of a universal phosphatase substrate, PNPP. Under conditions identical with those used in the Akt dephosphorylation assay, PIFtide did not affect the ability of PP2A to process PNPP (Figure S3D). Collectively, these data suggest that the Akt C-terminal interaction with PIFtide renders Akt resistant to phosphatase-mediated dephosphorylation.
C-terminal Akt residues can be modified to progressively decrease Akt dephosphorylation
We show the superposition of the C-terminal PIFtide (PDB code 1o6l) and S474D peptide (PDB code 1o6k) in the active Akt2 isoform in Figure 4A. C-terminal sequences surrounding phosphorylation sites of Akt1 (Ser473) and Akt2 (Ser474) are highly homologous (Figure 2A).
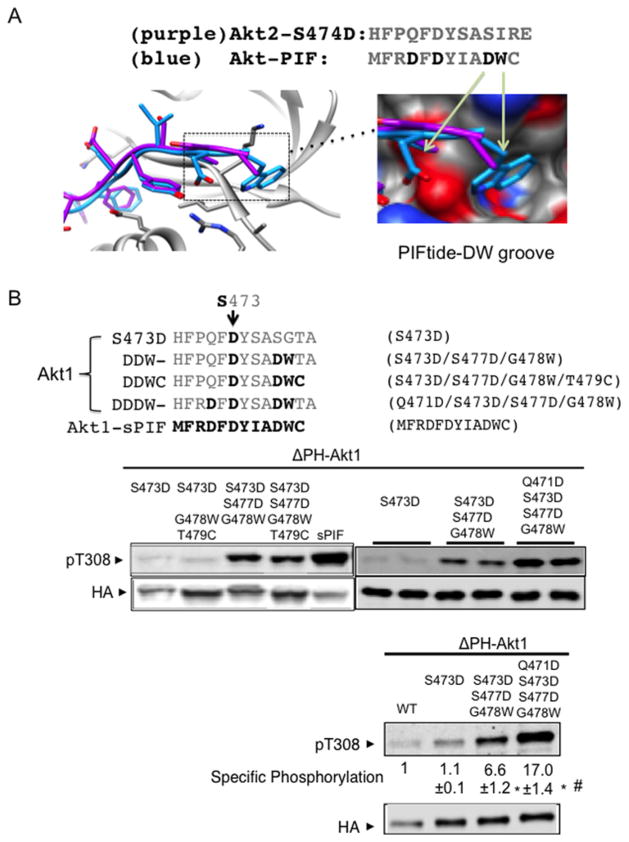
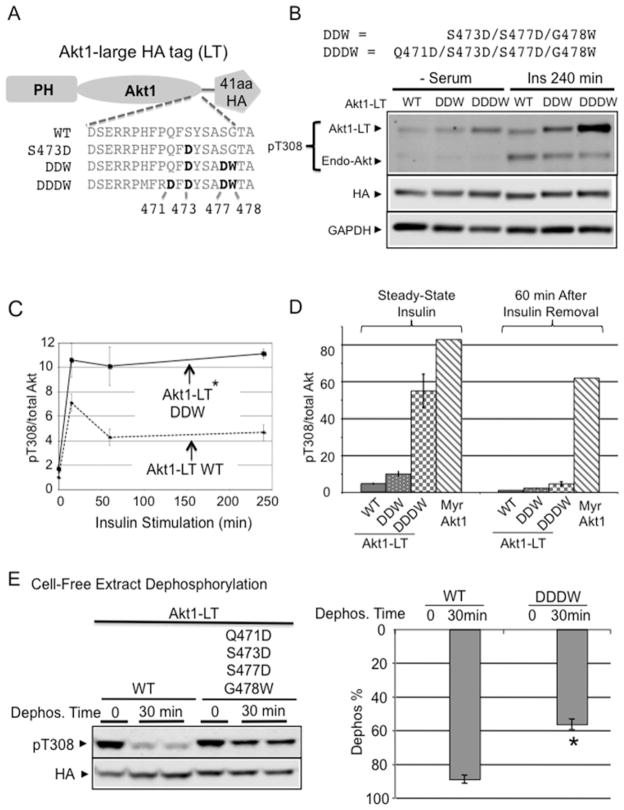
Figure 4. Progressive protection of Akt dephosphorylation by a DDDW targeting groove.
(A) Akt structures were superimposed with Akt C-terminal amino acids (purple) or with PIFtide amino acids (blue). Image depicts the insertion of PIFtide DW residues into an Akt groove. The structure shown is modelled on active human Akt2 crystal structures bound to the ATP analogue AMP-PNP and Mn2 + with S474D mutation (PDB code 1o6k) or with PIFtide fusion (PDB code 1o6l). Most of our biochemical studies were performed with Akt1. When Akt2 structure models were used, we indicate the corresponding Akt1 amino acid residues for clarity. (B) The C-terminally modified cytosolic Akt (ΔPH-Akt1) constructs were transfected into HEK293 cells and incubated for 4 h in medium that was free of exogenous growth factors. Protein extracts were subjected to immunoblot analysis using antibodies to phospho-Akt Thr308 and anti-HA. For quantification, we used a two-colour infrared fluorescence quantitative Western blot system with Li-Cor Odyssey imager to measure band intensity from different blots. In each blot, pThr308 signal and the HA signal in ΔPH-Akt1-WT were normalized to 1. The specific phosphorylation is the ratio of the normalized pThr308 signal to normalized HA signals. *P < 0.001 ΔPH-Akt1-DDW (n=9) or ΔPH-Akt1-DDDW (n=11) compared with ΔPH-Akt1-S473D (n=8). #P < 0.001 ΔPH-Akt1-DDW compared with ΔPH-Akt1-DDDW.
In contrast with the serine/isoleucine motif in the Akt C-terminus, PIFtide contains a charged aspartic acid residue and a bulky tryptophan residue. We hypothesized that these two residues will enhance dephosphorylation protection in Akt kinases. To test this hypothesis, we sequentially replaced Akt1 amino acids in the tail with the corresponding PIFtide amino acids (Figure 4B). Cells expressing these mutants in the ΔPH-Akt1-S473D background revealed that combined S477D/G478W replacement alone enhanced steady-state Thr308 phosphorylation approximately 7-fold (Figure 4B).
We noted that ‘DW’ dipeptide replacement alone was still much less effective than the native PIFtide (Figure 4B), suggesting that additional changes surrounding the FxxF motif (HFPQF as compared to MFRDF) contribute to dephosphorylation protection. Sequential replacement of these residues revealed that adding Q471D to S473D/S477D/G478W (DDDW) further increased phosphorylation from 6.6-fold to more than 17-fold (Figure 4B). Expression level of ΔPH-Akt1 mutants was much higher than that of endogenous Akt, but their phosphorylation were not affected by insulin stimulation (Supplementary Figure S2B). Taken together these results suggest that C-terminal sequences can be progressively modified to restrain phosphorylated Thr308 from cellular phosphatases, thereby increasing activation loop Akt phosphorylation and kinase activity.
A modified Akt C-terminus enhances insulin responsiveness
The preceding C-terminal modifications were tested in an Akt fragment lacking the PH domain, which does not respond to insulin stimulation [1]. To test the effects of C-terminal modification on insulin responses, we introduced the DDW (S473D/S477D/G478W) and DDDW (Q471D/S473D/S477D/G478W) mutations into full-length Akt containing the PH domain (Figure 5A). C-terminally modified Akt1 constructs were fused with a 41-amino-acid LT to differentiate transfected Akt1 mutants from endogenous Akt. After transfecting these constructs into H9C2 cells, both DDW-and DDDW-modified Akt1-LT constructs demonstrated amplified insulin-dependent Thr308 phosphorylation (Figure 5B). Whereas WT Akt1 and the DDW-modified Akt1 responded similarly at acute insulin stimulation, only the DDW-modified Akt1 could sustain insulin-dependent phosphorylation at 4 h after insulin stimulation (Figure 5C).
Figure 5. C-terminal stabilization in full-length Akt enhances insulin responsiveness.
(A) To differentiate from endogenous Akt, a 41-amino-acid LT containing the HA epitope was fused with Akt1 (Akt1-LT). C-terminal mutation constructs in Akt1-LT are shown. (B) Akt1-LT-DDW and Akt1-LT-DDDW progressively improved insulin sensitivity after chronic insulin stimulation (240 min). Indicated Akt1-LT constructs were transfected into H9C2 myoblasts and stimulated with 2 μM insulin for 240 min. Protein extracts were subjected to immunoblot analysis using antibodies to phospho-Akt Thr308 and anti-HA. (C) The graph shows the time course of phospho-Thr308 response after insulin stimulation. Quantification shows the pThr308/total Akt ratio after 4 h of insulin stimulation normalized to Akt1-LT WT. *P < 0.05 Akt1-LT DDW (n=3) compared with Akt1-LT WT (n=4). (D) Unlike phospho-Myr-Akt1, pThr308 in Akt1-LT-DDDW was rapidly dephosphorylated after insulin removal. H9C2 cells were pre-stimulated with insulin for 10 min and then insulin medium was replaced with serum-free medium and incubated for 60 min. Quantification shows pThr308/total Akt ratio after 60 min of insulin removal. (E) DDDW modification provided Thr308 dephosphorylation resistance similar to full-length PIFtide in cell-free extracts. After maximally phosphorylating both AKT1-LT-WT and AKT1-LT-DDDW by insulin/pervanadate/PDK1 treatment, flash-frozen cell extracts were incubated at 30°C for 30 min. After incubation, protein extracts were subjected to immunoblot analysis using antibodies detecting phosphorylated Akt (Thr308) and HA epitope. The histogram shows the percentage of Akt dephosphorylation at 0 min and at 30 min (Dephos. %) (respectively, Akt1-LT WT, n=5, n=10; Akt1-LT-DDDW, n=2, n=5). *P < 0.001 compared with Akt1-LT-WT at 30 min. See also Supplementary Figure S4 for Akt1-LT-PIF compared with Akt1-LT-DDDW assay.
Myr of Akt kinase, found in the viral Akt oncogene [40], causes constitutive activation loop phosphorylation that is refractory to serum starvation and phosphoinositide-3-kinase (PI3K) inhibitor treatment [1] (Figure 3A). In the presence of insulin, the specific phosphorylation of the Akt1-LT-DDDW mutant approached that of Myr-Akt1 (Figure 5D). Yet, unlike Myr-Akt1, Akt1-LT-DDDW phosphorylation was reduced 8-fold within 60 min of insulin removal. Thus, C-terminal peptide mimics do not induce constitutive Akt hyperphosphorylation, but rather increase the transient response to insulin.
Next we tested phosphatase resistance of DDDW-modified Akt1-LT in the cell-free dephosphorylation assay. Insulin/pervanadate/PDK1 treatment maximal phosphorylated WT Akt to a level comparable to that of DDDW-modified Akt1-LT. Cell extracts were prepared after rapid freezing. To ensure a lack of protein kinase activity in the extracts, EDTA was included. Upon further incubation of cell extracts at 30°C, 90% of WT Akt1 was dephosphorylated within 30 min. In contrast, only 56% of DDDW-Akt1-LT was dephosphorylated (Figure 5E). Furthermore, DDDW modification provided Thr308 dephosphorylation resistance similar to that caused by full-length PIFtide replacement (Figure 3B; Supplementary Figure S4). Unlike transfected constructs, endogenous Akt were similarly dephosphorylated in different cell extract types (Supplementary Figure S3B). Thus, DDDW modification enables the Akt Thr308 site to resist cellular phosphatases.
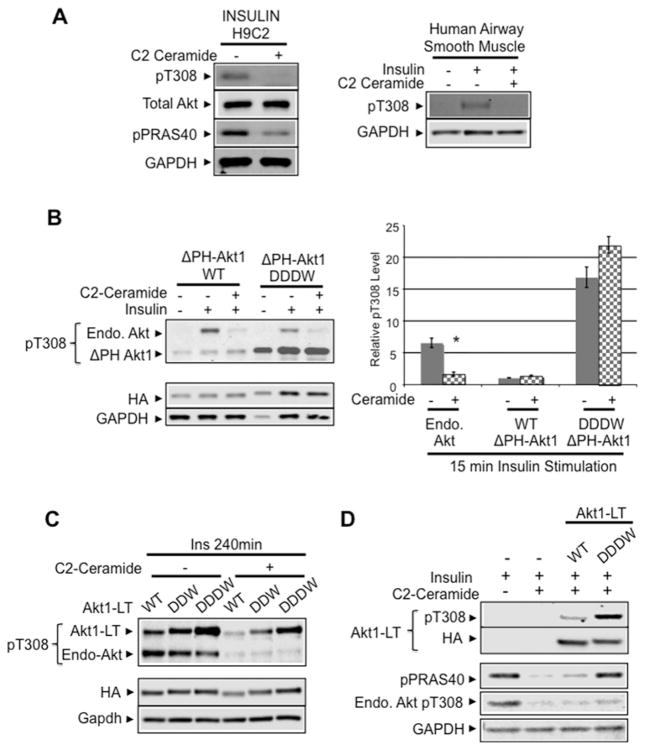
Modified Akt kinase C-terminus renders Akt resistant to C2-ceramide inhibition
Akt kinases link insulin and other growth factors to increased cell growth, metabolism and survival [41–43]. Insulin resistance is associated with induction of the sphingolipid second messenger ceramide and ceramide directly regulates apoptosis susceptibility through inhibition of Akt [44,45]. Indeed, treatment of H9C2 myoblast cells and primary human airway smooth muscle cells for 2 h with 100 μM soluble short-chain ceramide (C2-ceramide) reduced insulin-stimulated endogenous Akt phosphorylation by 90%(Figure 6A).
Figure 6. Modified Akt C-terminus confers resistance to ceramide-mediated inhibition.
(A) C2-ceramide blocks insulin activation of Akt. H9C2 myoblast cells and primary human airway smooth muscle cells were treated for 2 h with 100 μM soluble short-chain ceramide (C2-ceramide). Then, cells were stimulated with 2 μM insulin for 30 min. Protein extracts were subjected to immunoblot analysis using antibodies detecting phosphorylated Akt (Thr308), total Akt and phospho-specific Akt substrate: phospho-PRAS40. GAPDH expression was used as loading control. (B) WT and DDDW-modified ΔPH-Akt1 construct was transfected into H9C2 myoblasts cells and incubated for 2 h with C2-ceramide before insulin stimulation. Immunoblot analysis shows phosphorylated Akt Thr308 levels in both transfected ΔPH-Akt1 constructs (45 kDa) and endogenous Akt (60 kDa). The histogram shows insulin-stimulated Thr308 phosphorylation in the absence or in the presence of C2-ceramide (respectively, endogenous WT Akt, n=11, n=16; ΔPH-Akt1-WT, n=4, n=7; ΔPH-Akt1-DDDW, n=2, n=4). *P < 0.001 compared with endogenous Akt without ceramide. (C) Modified Akt C-terminus resisted ceramide treatment. Akt1-LT WT, Akt1-LT-DDW and Akt1-LT DDDW constructs were transfected into primary human airway smooth muscle cells and were treated for 2 h with 100 μM soluble short-chain ceramide (C2-ceramide). Then, cells were stimulated with 2 μM insulin for 15 min. Immunoblot analysis used antibodies detecting the HA epitope, phosphorylated Akt (Thr308) and GAPDH loading control. (D) DDDW-modified Akt1 enhanced Akt substrate phosphorylation. Akt1-LT WT and Akt1-LT-DDDW constructs were transfected into primary human airway smooth muscle cells. Cells were treated with 100 μM C2-ceramide and stimulated with 2 μM insulin. Immunoblot analysis used antibodies detecting the HA epitope, phosphorylated Akt (Thr308), phospho-specific Akt substrate, phospho-PRAS40 and GAPDH loading control.
To test whether C-terminally modified phosphatase-resistant Akt constructs resist C2-ceramide inhibition, we transfected DDDW-modified constructs into H9C2 cells and treated the cells with C2-ceramide. DDDW-modified ΔPH-Akt1 and full-length Akt1-LT remained highly phosphorylated at Thr308 after C2-ceramide treatment (Figures 6B and 6C). Consistently, Akt1-LT-DDDW also enhanced phosphorylation of the Akt substrate phospho-PRAS40 under C2-ceramide treatment conditions in both primary human airway smooth muscle cells (Figure 6D) and H9C2 cells (Supplementary Figure S5B). Interestingly, in the absence of ceramide, insulin-stimulated pPRAS40 phosphorylation was not further augmented by Akt1-LT-DDDW expression (Supplementary Figures S5A and S5B). These data suggest that targeting Akt dephosphorylation resistance could potentially protect cells from ceramide-mediated insulin resistance.
DISCUSSION
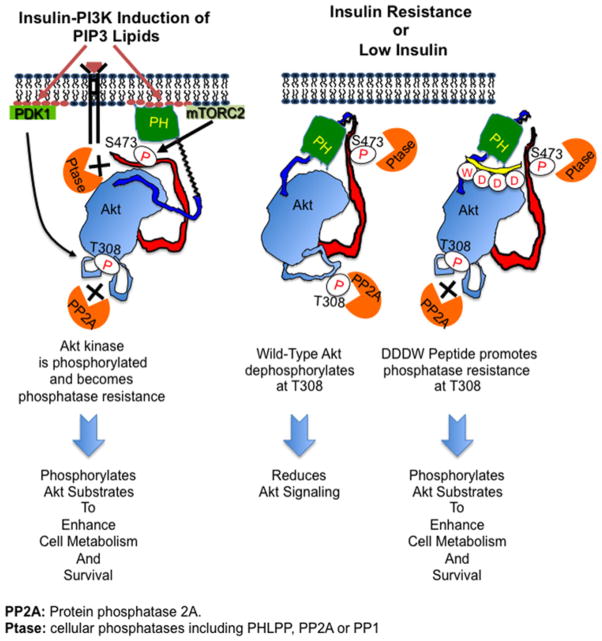
Activation loop phosphorylation is an essential prerequisite for catalytic activity of most AGC kinases [46,47]. In the present study, we demonstrate that the C-terminal signature hydrophobic motifs (FxxF) of the two AGC kinases Akt (protein kinase B) and PKA increase activation loop phosphorylation by restricting access of cellular phosphatases to this site. Whereas C-terminus-dependent dephosphorylation resistance of PKA is constitutive, dephosphorylation resistance of the Akt activation loop is conditional as it depends on membrane-proximal localization and on ATP binding to Akt (Figure 7).
Figure 7. C-terminal stabilization promotes phosphatase-resistance cage formation.
After PtdIns(3,4,5)P3 (PIP3 ) binding to Akt PH domain, membrane-bound Akt is phosphorylated at the C-terminus (Ser473 in Akt1) and at the activation loop (Thr308 in Akt1) by PDK1 and mTORC2 respectively and in a co-ordinated manner. Membrane localization also promotes the formation of a phosphatase-resistant cage to shield both phosphorylated sites from phosphatases. In the absence of phosphatidylinositol (3,4,5)-trisphosphate (PIP3 ), cytosolic phosphorylated Akt is dephosphorylated by phosphatases. DDDW-modified Akt C-terminus promotes phosphatase resistance at the activation loop.
Previous work showed that the Akt C-terminal hydrophobic motif binds Akt with low affinity, even when the regulatory site (Ser473 in Akt1) is phosphorylated [37]. Low-affinity interaction of the Akt C-terminus with the kinase domain is further supported by structural data revealing an inactive ‘open’ conformation with a disordered αC-helix and disordered C-terminal tail [28,37]. By contrast, the C-terminus of PKA (PDB codes 4dg0 and 4dg2) forms high-affinity electrostatic interactions with N-terminal residues (Ser34/Gln35) that should confer significant conformational rigidity on this region; these residues are not conserved in Akt kinases [29]. As shown in the present study, increasing the strength of interaction of C-terminal sequence variants with the kinase domain renders the Akt activation loop resistant to dephosphorylation independent of intracellular localization.
Akt conformational change induced by membrane binding coordinately regulates the phosphorylation of both the activation loop and the C-terminus [1,4]. However, these two sites are phosphorylated by distinct protein kinases (PDK1 and mTORC2). Whereas abolishing activation loop phosphorylation (via PDK1 deletion) does not affect C-terminal phosphorylation in embryonic stem cells [48], abolishing C-terminal phosphorylation blunts activation loop phosphorylation in a cell-type/condition-dependent manner.
Our research group, as well as other research groups [49], has found that mutating Ser473 to alanine in membrane-bound Akt reduced activation loop phosphorylation by nearly 95% (Supplementary Figure 6B). Similarly, abolishing Ser473 phosphorylation via genetic mTORC2 inhibition (RICTOR subunit deletion or knockdown) blunts activation loop phosphorylation in PTEN (phosphatase and tensin homolog)-deleted mouse prostate glands [50] and in several human cancer cells [3]. These results suggest that lack of Ser473 phosphorylation increases Akt phosphatase sensitivity leading to reduced Thr308 phosphorylation in these cells.
Surprisingly, Ser473 phosphorylation can be uncoupled from Thr308 phosphorylation. Abolishing Akt C-terminal phosphorylation in primary mouse embryonic fibroblast cells fails to reduce Thr308 phosphorylation [5,50–52]. Also, reducing mTORC2 activity blocked both Ser473 and Thr308 phosphorylation in insulin-stimulated 3T3-L1 adipocytes [53], but in brown adipocyte precursor cells, it only blocked Ser473 phosphorylation [54]. It is likely that mouse embryo fibroblasts and brown adipocyte precursor cells possess specific factors that enhance phosphatase resistance even in the absence of Akt C-terminal phosphorylation.
Phosphorylation of the Akt C-terminus regulates catalytic activity [47,55] and substrate selectivity of this kinase [56,57]. As shown in the present study by use of phosphomimetic mutants, phosphorylation of the C-terminus of Akt1 at Ser473 is required but not sufficient for conferring dephosphorylation resistance on the activation loop. The abundantly expressed phosphatase PP2A is the primary phosphatase to dephosphorylate Akt kinases (both the activation loop Thr308 site and the C-terminal Ser473 site) [12,13,58–60]. In addition, the Akt C-terminal site could also be dephosphorylated by a member of the PP2C phosphatase family, PHLPP1/2 [61,62] and by protein phosphatase-1 [63]. Consistently, our earlier study showed that both Thr308 and Ser473 sites can render phosphatase resistant by ATP binding in the presence of recombinant PP2A (Supplementary Figure in [15]). Furthermore, ceramide treatment blocked the phosphorylation at both Thr308 and Ser473 sites (Supplementary Figure S6). Thus, we suggest that one function for Ser473 phosphorylation is to enhance phosphatase resistance at the activation loop. Dephosphorylation kinetics probably affects multiple functions of Akt kinases including insulin-mediated and Akt-dependent modulation of glucose and lipid metabolism [41,43,64]. After insulin stimulation, the Akt2 isoform not only binds phosphoinositide lipids, but is recruited into a GLUT4 (glucose transporter type 4)-containing raft membrane containing potential regulatory proteins such as Cbl (Casitas B-lineage Lymphoma), Crk adapter molecule, flotillin and TC10 (also known as Rho-related GTP-binding protein Q) [65].
Insulin resistance, as observed in diabetes, is associated with reduced Akt activity and up-regulation of the sphingolipid second messenger ceramide [44,45]. Ceramide is known to modestly enhance PP2A activity (~1.5–2-fold) [66], but direct phosphatase activation by ceramide is not the main mechanism for Akt phosphorylation blockade. Instead, ceramide blocks the translocation of the Akt PH domain to the plasma membrane [67,68] via interaction with and/or phosphorylation by PKCζ and membrane rafts [69–72]. Thus, ceramide does not block phosphorylation in ΔPH-Akt constructs.
Along with other research groups, we have shown that the deletion of the Akt PH domain prevents Akt translocation to the plasma membrane [1]. Consequently, expressed ΔPH-Akt1-WT does not respond to growth factor stimulation and is essentially unphosphorylated when expressed in HEK293 cells (Supplementary Figure S2A) and in many other cell types [73–77]. But treating cells with the phosphatase inhibitor calyculin A was sufficient to restore Thr308 phosphorylation in ΔPH-Akt1-WT over 200-fold (Supplementary Figure S2A). Thus, ΔPH-Akt1-WT is already dephosphorylated in the absence of ceramide treatment.
Our earlier studies showed that membrane-localized Akt becomes more phosphatase resistant [15]. Also, ceramide blocked Thr308 phosphorylation in WT Akt in the presence of insulin stimulation (Figure 6C) by preventing the translocation of Akt to the plasma membrane [67,68]. Thus, DDDW-modified Akt1 resisted ceramide treatment by enhancing phosphatase resistance even when not membrane-bound. We show in the present study that a modified Akt C-terminus not only increases steady-state Akt phosphorylation upon insulin exposure, but also imparts resistance to ceramide-dependent Akt dephosphorylation leading to robust Akt1 downstream signalling. These results suggest novel approaches to protect cells against ceramide-associated cytotoxic effects.
Finally, removal of insulin triggers dephosphorylation of C-terminally modified Akt. The insulin-responsive enhancement of Akt phosphorylation is distinct from constitutive phosphorylation as observed in membrane-targeted myristoylated Akt (which confers oncogenic properties) [1,78,79]. Thus, the requirement of physiological insulin to enhance C-terminally modified Akt phosphorylation suggests that Akt agonists based on modified Akt C-termini can be developed that have favourable pharmacological properties and are not encumbered by oncogenic risks.
Akt translocation to the plasma membrane promotes both its phosphorylation and its phosphatase resistance to sustain Akt activity. In addition, other cell membrane compartments, such as mitochondria-associated endoplasmic reticulum and the nucleus, also contain Akt substrates that affect Akt function. For example, Sasaki et al. [80] used subcellular-targeted fluorescent Akt substrate reporter to show that Akt phosphorylation occurs at Golgi and mitochondria subcellular membrane compartments, but not within cytosol. Also, we and others [4] have shown cytosolic Akt is readily dephosphorylated (Figure 2B).
The mechanism for activating Akt in these subcellular compartments is unclear. One possibility is that phosphorylated Akt traverses from the plasma membrane via D3-phosphoinositide-containing membrane vesicles that support Akt phosphorylation and/or phosphatase protection. Alternatively, components of Akt signalling pathway may already reside at these subcellular locations. Specifically, Akt kinase, PI3K, D3-phosphoinositides, PDK1 and mTORC2 have been found in the nucleus [81], at mitochondria-associated endoplasmic reticulum membranes [81–85] and at ClipR-59 lipid/raft associated scaffolding protein in adipocytes [86,87].
In summary, our results demonstrate that differences in dephosphorylation resistance among AGC kinases can be explained in part by differences in the affinity of intramolecular interactions between the C-termini and the kinase domains. Specifically, higher-affinity interactions between these functional domains confer higher degrees of phosphorylation resistance. These findings provide a rational basis for the development of C-terminal peptidomimetics for therapeutic purposes.
Supplementary Material
Acknowledgments
FUNDING
This work was supported by the Prostate Cancer Foundation Ben Franklin-PCF Creativity Award; the Department of Defense [grant numbers W81XWH-12-1-0477 (to U.R.) and HL58506 (to R.B.P.)]; the Diabetes Action Research and Education Foundation Research Grant (to T.O.C.)]; the American Lung Association Biomedical Research Grant (to T.O.C.)]; and the American Heart Association [grant number 14GRNT20480267 (to T.O.C.)].
Abbreviations
- AMP-PNP
adenosine 5′-[β,γ-imido]triphosphate
- DNA-PK
DNA-dependent protein kinase
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HA
haemagglutinin
- HEK
human embryonic kidney
- LT
large tag
- Myr
myristoylation
- PDK1
phosphoinositide-dependent kinase-1
- PH
pleckstrin homology
- PHLPP
PH domain leucine-rich repeat protein phosphatase
- PI3K
phosphoinositide 3-kinase
- PKA/C
protein kinase A/C
- PNPP
p-nitrophenyl phosphate phosphatase
- PP2A
protein phosphatase 2A
- PRK2
PKC-related protein kinase 2
- WT
wild-type
Footnotes
AUTHOR CONTRIBUTION
Tung Chan, Jin Zhang, Roger Armen and Brian Tiegs designed the research. Tung Chan, Jin Zhang, Brian Tiegs, Brian Blumhof, Linda Yan, Nikhil Keny, Morgan Penny and Xue Li performed research. Tung Chan, Jin Zhang, Linda Yan and Morgan Penny contributed new reagents/analytical tools. Tung Chan, Jin Zhang, Ulrich Rodeck, John Pascal, Roger Armen and Raymond Penn analysed data. Tung Chan, Ulrich Rodeck, John Pascal, Roger Armen and Raymond Penn wrote the paper.
References
- 1.Chan TO, Rittenhouse SE, Tsichlis PN. AKT/PKB and other D3 phosphoinositide-regulated kinases: kinase acvtivation by phosphoinositide-dependent phosphorylation. Annu Rev Biochem. 1999;68:965–1014. doi: 10.1146/annurev.biochem.68.1.965. [DOI] [PubMed] [Google Scholar]
- 2.Fayard E, Xue G, Parcellier A, Bozulic L, Hemmings BA. Protein kinase B (PKB/Akt), a key mediator of the PI3K signaling pathway. Curr Top Microbiol Immunol. 2010;346:31–56. doi: 10.1007/82_2010_58. [DOI] [PubMed] [Google Scholar]
- 3.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 4.Bozulic L, Hemmings BA. PIKKing on PKB: regulation of PKB activity by phosphorylation. Curr Opin Cell Biol. 2009;21:256–261. doi: 10.1016/j.ceb.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Shiota C, Woo JT, Lindner J, Shelton KD, Magnuson MA. Multiallelic disruption of the rictor gene in mice reveals that mTOR complex 2 is essential for fetal growth and viability. Dev Cell. 2006;11:583–589. doi: 10.1016/j.devcel.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Cybulski N, Polak P, Auwerx J, Ruegg MA, Hall MN. mTOR complex 2 in adipose tissue negatively controls whole-body growth. Proc Natl Acad Sci USA. 2009;106:9902–9907. doi: 10.1073/pnas.0811321106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ou YH, Torres M, Ram R, Formstecher E, Roland C, Cheng T, Brekken R, Wurz R, Tasker A, Polverino T, et al. TBK1 directly engages Akt/PKB survival signaling to support oncogenic transformation. Mol Cell. 2011;41:458–470. doi: 10.1016/j.molcel.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie X, Zhang D, Zhao B, Lu MK, You M, Condorelli G, Wang CY, Guan KL. IkappaB kinase epsilon and TANK-binding kinase 1 activate AKT by direct phosphorylation. Proc Natl Acad Sci USA. 2011;108:6474–6479. doi: 10.1073/pnas.1016132108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bozulic L, Surucu B, Hynx D, Hemmings BA. PKBalpha/Akt1 acts downstream of DNA-PK in the DNA double-strand break response and promotes survival. Mol Cell. 2008;30:203–213. doi: 10.1016/j.molcel.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 10.Surucu B, Bozulic L, Hynx D, Parcellier A, Hemmings BA. In vivo analysis of protein kinase B (PKB)/Akt regulation in DNA-PKcs-null mice reveals a role for PKB/Akt in DNA damage response and tumorigenesis. J Biol Chem. 2008;283:30025–30033. doi: 10.1074/jbc.M803053200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu P, Begley M, Michowski W, Inuzuka H, Ginzberg M, Gao D, Tsou P, Gan W, Papa A, Kim BM, et al. Cell-cycle-regulated activation of Akt kinase by phosphorylation at its carboxyl terminus. Nature. 2014;508:541–545. doi: 10.1038/nature13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andjelkovic M, Jakubowicz T, Cron P, Ming XF, Han JW, Hemmings BA. Activation and phosphorylation of a pleckstrin homology domain containing protein kinase (RAC-PK/PKB) promoted by serum and protein phosphatase inhibitors. Proc Natl Acad Sci USA. 1996;93:5699–5704. doi: 10.1073/pnas.93.12.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Resjo S, Goransson O, Harndahl L, Zolnierowicz S, Manganiello V, Degerman E. Protein phosphatase 2A is the main phosphatase involved in the regulation of protein kinase B in rat adipocytes. Cell Signal. 2002;14:231–238. doi: 10.1016/s0898-6568(01)00238-8. [DOI] [PubMed] [Google Scholar]
- 14.Brognard J, Newton AC. PHLiPPing the switch on Akt and protein kinase C signaling. Trends Endocrinol Metab. 2008;19:223–230. doi: 10.1016/j.tem.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan TO, Zhang J, Rodeck U, Pascal JM, Armen RS, Spring M, Dumitru CD, Myers V, Li X, Cheung JY, Feldman AM. Resistance of Akt kinases to dephosphorylation through ATP-dependent conformational plasticity. Proc Natl Acad Sci USA. 2011;108:E1120–E1127. doi: 10.1073/pnas.1109879108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han EK, Leverson JD, McGonigal T, Shah OJ, Woods KW, Hunter T, Giranda VL, Luo Y. Akt inhibitor A-443654 induces rapid Akt Ser-473 phosphorylation independent of mTORC1 inhibition. Oncogene. 2007;26:5655–5661. doi: 10.1038/sj.onc.1210343. [DOI] [PubMed] [Google Scholar]
- 17.Levy DS, Kahana JA, Kumar R. AKT inhibitor, GSK690693, induces growth inhibition and apoptosis in acute lymphoblastic leukemia cell lines. Blood. 2009;113:1723–1729. doi: 10.1182/blood-2008-02-137737. [DOI] [PubMed] [Google Scholar]
- 18.Lin K, Lin J, Wu WI, Ballard J, Lee BB, Gloor SL, Vigers GP, Morales TH, Friedman LS, Skelton N, Brandhuber BJ. An ATP-site on-off switch that restricts phosphatase accessibility of Akt. Sci Signal. 2012;5:ra37. doi: 10.1126/scisignal.2002618. [DOI] [PubMed] [Google Scholar]
- 19.Cheng Z, Tseng Y, White MF. Insulin signaling meets mitochondria in metabolism. Trends Endocrinol Metab. 2010;21:589–598. doi: 10.1016/j.tem.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santi SA, Lee H. Ablation of Akt2 induces autophagy through cell cycle arrest, the downregulation of p70S6K, and the deregulation of mitochondria in MDA-MB231 cells. PLoS One. 2011;6:e14614. doi: 10.1371/journal.pone.0014614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.George S, Rochford JJ, Wolfrum C, Gray SL, Schinner S, Wilson JC, Soos MA, Murgatroyd PR, Williams RM, Acerini CL, et al. A family with severe insulin resistance and diabetes due to a mutation in AKT2. Science. 2004;304:1325–1328. doi: 10.1126/science.1096706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan TO, Pascal JM, Armen RS, Rodeck U. Autoregulation of kinase dephosphorylation by ATP binding in AGC protein kinases. Cell Cycle. 2012;11:475–478. doi: 10.4161/cc.11.3.19059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar CC, Madison V. AKT crystal structure and AKT-specific inhibitors. Oncogene. 2005;24:7493–7501. doi: 10.1038/sj.onc.1209087. [DOI] [PubMed] [Google Scholar]
- 24.Bennett PA, Dixon RJ, Kellie S. The phosphotyrosine phosphatase inhibitor vanadyl hydroperoxide induces morphological alterations, cytoskeletal rearrangements and increased adhesiveness in rat neutrophil leucocytes. J Cell Sci. 1993;106:891–901. doi: 10.1242/jcs.106.3.891. [DOI] [PubMed] [Google Scholar]
- 25.Deshpande DA, Theriot BS, Penn RB, Walker JK. Beta-arrestins specifically constrain beta2-adrenergic receptor signaling and function in airway smooth muscle. FASEB J. 2008;22:2134–2141. doi: 10.1096/fj.07-102459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan TO, Rodeck U, Chan AM, Kimmelman AC, Rittenhouse SE, Panayotou G, Tsichlis PN. Small GTPases and tyrosine kinases coregulate a molecular switch in the phosphoinositide 3-kinase regulatory subunit. Cancer Cell. 2002;1:181–191. doi: 10.1016/s1535-6108(02)00033-8. [DOI] [PubMed] [Google Scholar]
- 27.Davies TG, Verdonk ML, Graham B, Saalau-Bethell S, Hamlett CC, McHardy T, Collins I, Garrett MD, Workman P, Woodhead SJ, et al. A structural comparison of inhibitor binding to PKB, PKA and PKA-PKB chimera. J Mol Biol. 2007;367:882–894. doi: 10.1016/j.jmb.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Yang J, Cron P, Good VM, Thompson V, Hemmings BA, Barford D. Crystal structure of an activated Akt/protein kinase B ternary complex with GSK3-peptide and AMP-PNP. Nat Struct Biol. 2002;9:940–944. doi: 10.1038/nsb870. [DOI] [PubMed] [Google Scholar]
- 29.Bastidas AC, Deal MS, Steichen JM, Keshwani MM, Guo Y, Taylor SS. Role of N-terminal myristylation in the structure and regulation of cAMP-dependent protein kinase. J Mol Biol. 2012;422:215–229. doi: 10.1016/j.jmb.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rahaman O, Estrada TP, Doren DJ, Taufer M, Brooks CL, 3rd, Armen RS. Evaluation of several two-step scoring functions based on linear interaction energy, effective ligand size, and empirical pair potentials for prediction of protein-ligand binding geometry and free energy. J Chem Inf Model. 2011;51:2047–2065. doi: 10.1021/ci1003009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor SS, Ilouz R, Zhang P, Kornev AP. Assembly of allosteric macromolecular switches: lessons from PKA. Nat Rev Mol Cell Biol. 2012;13:646–658. doi: 10.1038/nrm3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bechtel PJ, Beavo JA, Krebs EG. Purification and characterization of catalytic subunit of skeletal muscle adenosine 3′:5′-monophosphate-dependent protein kinase. J Biol Chem. 1977;252:2691–2697. [PubMed] [Google Scholar]
- 33.Shoji S, Titani K, Demaille JG, Fischer EH. Sequence of two phosphorylated sites in the catalytic subunit of bovine cardiac muscle adenosine 3′:5′-monophosphate-dependent protein kinase. J Biol Chem. 1979;254:6211–6214. [PubMed] [Google Scholar]
- 34.Toner-Webb J, van Patten SM, Walsh DA, Taylor SS. Autophosphorylation of the catalytic subunit of cAMP-dependent protein kinase. J Biol Chem. 1992;267:25174–25180. [PubMed] [Google Scholar]
- 35.Endicott JA, Noble ME, Johnson LN. The structural basis for control of eukaryotic protein kinases. Annu Rev Biochem. 2012;81:587–613. doi: 10.1146/annurev-biochem-052410-090317. [DOI] [PubMed] [Google Scholar]
- 36.Fersht AR. Transition-state structure as a unifying basis in protein-folding mechanisms: contact order, chain topology, stability, and the extended nucleus mechanism. Proc Natl Acad Sci USA. 2000;97:1525–1529. doi: 10.1073/pnas.97.4.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang J, Cron P, Thompson V, Good VM, Hess D, Hemmings BA, Barford D. Molecular mechanism for the regulation of protein kinase B/Akt by hydrophobic motif phosphorylation. Mol Cell. 2002;9:1227–1240. doi: 10.1016/s1097-2765(02)00550-6. [DOI] [PubMed] [Google Scholar]
- 38.Alessi DR, Deak M, Casamayor A, Caudwell FB, Morrice N, Norman DG, Gaffney P, Reese CB, MacDougall CN, Harbison D, et al. 3-Phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr Biol. 1997;7:776–789. doi: 10.1016/s0960-9822(06)00336-8. [DOI] [PubMed] [Google Scholar]
- 39.Stokoe D, Stephens LR, Copeland T, Gaffney PR, Reese CB, Painter GF, Holmes AB, McCormick F, Hawkins PT. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science. 1997;277:567–570. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- 40.Bellacosa A, Testa JR, Staal SP, Tsichlis PN. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science. 1991;254:274–277. doi: 10.1126/science.254.5029.274. [DOI] [PubMed] [Google Scholar]
- 41.Gonzalez E, McGraw TE. The Akt kinases: isoform specificity in metabolism and cancer. Cell Cycle. 2009;8:2502–2508. doi: 10.4161/cc.8.16.9335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parker VE, Savage DB, O’Rahilly S, Semple RK. Mechanistic insights into insulin resistance in the genetic era. Diabet Med. 2011;28:1476–1486. doi: 10.1111/j.1464-5491.2011.03463.x. [DOI] [PubMed] [Google Scholar]
- 43.Wan M, Birnbaum MJ. Of mice and men: not ExAKTly the same? Cell Metab. 2011;14:722–723. doi: 10.1016/j.cmet.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 44.Bikman BT, Summers SA. Ceramides as modulators of cellular and whole-body metabolism. J Clin Invest. 2011;121:4222–4230. doi: 10.1172/JCI57144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chavez JA, Summers SA. A ceramide-centric view of insulin resistance. Cell Metab. 2012;15:585–594. doi: 10.1016/j.cmet.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 46.Huse M, Kuriyan J. The conformational plasticity of protein kinases. Cell. 2002;109:275–282. doi: 10.1016/s0092-8674(02)00741-9. [DOI] [PubMed] [Google Scholar]
- 47.Pearce LR, Komander D, Alessi DR. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol. 2010;11:9–22. doi: 10.1038/nrm2822. [DOI] [PubMed] [Google Scholar]
- 48.Williams MR, Arthur JS, Balendran A, van der Kaay J, Poli V, Cohen P, Alessi DR. The role of 3-phosphoinositide-dependent protein kinase 1 in activating AGC kinases defined in embryonic stem cells. Curr Biol. 2000;10:439–448. doi: 10.1016/s0960-9822(00)00441-3. [DOI] [PubMed] [Google Scholar]
- 49.Scheid MP, Marignani PA, Woodgett JR. Multiple phosphoinositide 3-kinase-dependent steps in activation of protein kinase B. Mol Cell Biol. 2002;22:6247–6260. doi: 10.1128/MCB.22.17.6247-6260.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guertin DA, Stevens DM, Saitoh M, Kinkel S, Crosby K, Sheen JH, Mullholland DJ, Magnuson MA, Wu H, Sabatini DM. mTOR complex 2 is required for the development of prostate cancer induced by Pten loss in mice. Cancer Cell. 2009;15:148–159. doi: 10.1016/j.ccr.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell. 2006;11:859–871. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 52.Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 53.Hresko RC, Mueckler M. mTOR. RICTOR is the Ser473 kinase for Akt/protein kinase B in 3T3-L1 adipocytes. J Biol Chem. 2005;280:40406–40416. doi: 10.1074/jbc.M508361200. [DOI] [PubMed] [Google Scholar]
- 54.Hung CM, Calejman CM, Sanchez-Gurmaches J, Li H, Clish CB, Hettmer S, Wagers AJ, Guertin DA. Rictor/mTORC2 loss in the Myf5 lineage reprograms brown fat metabolism and protects mice against obesity and metabolic disease. Cell Rep. 2014;8:256–271. doi: 10.1016/j.celrep.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Najafov A, Shpiro N, Alessi DR. Akt is efficiently activated by PIF-pocket-and PtdIns(3,4,5)P3-dependent mechanisms leading to resistance to PDK1 inhibitors. Biochem J. 2012;448:285–295. doi: 10.1042/BJ20121287. [DOI] [PubMed] [Google Scholar]
- 56.Jacinto E, Lorberg A. TOR regulation of AGC kinases in yeast and mammals. Biochem J. 2008;410:19–37. doi: 10.1042/BJ20071518. [DOI] [PubMed] [Google Scholar]
- 57.Cornu M, Albert V, Hall MN. mTOR in aging, metabolism, and cancer. Curr Opin Genet Dev. 2013;23:53–62. doi: 10.1016/j.gde.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 58.Kuo YC, Huang KY, Yang CH, Yang YS, Lee WY, Chiang CW. Regulation of phosphorylation of Thr-308 of Akt, cell proliferation, and survival by the B55alpha regulatory subunit targeting of the protein phosphatase 2A holoenzyme to Akt. J Biol Chem. 2008;283:1882–1892. doi: 10.1074/jbc.M709585200. [DOI] [PubMed] [Google Scholar]
- 59.Padmanabhan S, Mukhopadhyay A, Narasimhan SD, Tesz G, Czech MP, Tissenbaum HA. A PP2A regulatory subunit regulates C. elegans insulin/IGF-1 signaling by modulating AKT-1 phosphorylation. Cell. 2009;136:939–951. doi: 10.1016/j.cell.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hwang JH, Jiang T, Kulkarni S, Faure N, Schaffhausen BS. Protein phosphatase 2A isoforms utilizing Abeta scaffolds regulate differentiation through control of Akt protein. J Biol Chem. 2013;288:32064–32073. doi: 10.1074/jbc.M113.497644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gao T, Furnari F, Newton AC. PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Mol Cell. 2005;18:13–24. doi: 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 62.Brognard J, Sierecki E, Gao T, Newton AC. PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Mol Cell. 2007;25:917–931. doi: 10.1016/j.molcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 63.Thayyullathil F, Chathoth S, Shahin A, Kizhakkayil J, Hago A, Patel M, Galadari S. Protein phosphatase 1-dependent dephosphorylation of Akt is the prime signaling event in sphingosine-induced apoptosis in Jurkat cells. J Cell Biochem. 2011;112:1138–1153. doi: 10.1002/jcb.23033. [DOI] [PubMed] [Google Scholar]
- 64.Bae SS, Cho H, Mu J, Birnbaum MJ. Isoform-specific regulation of insulin-dependent glucose uptake by Akt/protein kinase B. J Biol Chem. 2003;278:49530–49536. doi: 10.1074/jbc.M306782200. [DOI] [PubMed] [Google Scholar]
- 65.Brazil DP, Park J, Hemmings BA. PKB binding proteins. Getting in on the Akt. Cell. 2002;111:293–303. doi: 10.1016/s0092-8674(02)01083-8. [DOI] [PubMed] [Google Scholar]
- 66.Dobrowsky RT, Kamibayashi C, Mumby MC, Hannun YA. Ceramide activates heterotrimeric protein phosphatase 2A. J Biol Chem. 1993;268:15523–15530. [PubMed] [Google Scholar]
- 67.Hajduch E, Balendran A, Batty IH, Litherland GJ, Blair AS, Downes CP, Hundal HS. Ceramide impairs the insulin-dependent membrane recruitment of protein kinase B leading to a loss in downstream signalling in L6 skeletal muscle cells. Diabetologia. 2001;44:173–183. doi: 10.1007/s001250051596. [DOI] [PubMed] [Google Scholar]
- 68.Stratford S, DeWald DB, Summers SA. Ceramide dissociates 3′-phosphoinositide production from pleckstrin homology domain translocation. Biochem J. 2001;354:359–368. doi: 10.1042/0264-6021:3540359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bourbon NA, Sandirasegarane L, Kester M. Ceramide-induced inhibition of Akt is mediated through protein kinase Czeta: implications for growth arrest. J Biol Chem. 2002;277:3286–3292. doi: 10.1074/jbc.M110541200. [DOI] [PubMed] [Google Scholar]
- 70.Powell DJ, Hajduch E, Kular G, Hundal HS. Ceramide disables 3-phosphoinositide binding to the pleckstrin homology domain of protein kinase B (PKB)/Akt by a PKCzeta-dependent mechanism. Mol Cell Biol. 2003;23:7794–7808. doi: 10.1128/MCB.23.21.7794-7808.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hajduch E, Turban S, Le Liepvre X, Le Lay S, Lipina C, Dimopoulos N, Dugail I, Hundal HS. Targeting of PKCzeta and PKB to caveolin-enriched microdomains represents a crucial step underpinning the disruption in PKB-directed signalling by ceramide. Biochem J. 2008;410:369–379. doi: 10.1042/BJ20070936. [DOI] [PubMed] [Google Scholar]
- 72.Blouin CM, Prado C, Takane KK, Lasnier F, Garcia-Ocana A, Ferre P, Dugail I, Hajduch E. Plasma membrane subdomain compartmentalization contributes to distinct mechanisms of ceramide action on insulin signaling. Diabetes. 2010;59:600–610. doi: 10.2337/db09-0897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Franke TF, Yang SI, Chan TO, Datta K, Kazlauskas A, Morrison DK, Kaplan DR, Tsichlis PN. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 74.Datta K, Bellacosa A, Chan TO, Tsichlis PN. Akt is a direct target of the phosphatidylinositol 3-kinase. Activation by growth factors, v-src and v-Ha-ras, in Sf9 and mammalian cells. J Biol Chem. 1996;271:30835–30839. doi: 10.1074/jbc.271.48.30835. [DOI] [PubMed] [Google Scholar]
- 75.Ahmed NN, Grimes HL, Bellacosa A, Chan TO, Tsichlis PN. Transduction of interleukin-2 antiapoptotic and proliferative signals via Akt protein kinase. Proc Natl Acad Sci USA. 1997;94:3627–3632. doi: 10.1073/pnas.94.8.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Andjelkovic M, Alessi DR, Meier R, Fernandez A, Lamb NJ, Frech M, Cron P, Cohen P, Lucocq JM, Hemmings BA. Role of translocation in the activation and function of protein kinase B. J Biol Chem. 1997;272:31515–31524. doi: 10.1074/jbc.272.50.31515. [DOI] [PubMed] [Google Scholar]
- 77.Bellacosa A, Chan TO, Ahmed NN, Datta K, Malstrom S, Stokoe D, McCormick F, Feng J, Tsichlis P. Akt activation by growth factors is a multiple-step process: the role of the PH domain. Oncogene. 1998;17:313–325. doi: 10.1038/sj.onc.1201947. [DOI] [PubMed] [Google Scholar]
- 78.Ahmed NN, Franke TF, Bellacosa A, Datta K, Gonzalez-Portal ME, Taguchi T, Testa JR, Tsichlis PN. The proteins encoded by c-akt and v-akt differ in post-translational modification, subcellular localization and oncogenic potential. Oncogene. 1993;8:1957–1963. [PubMed] [Google Scholar]
- 79.Adam RM, Mukhopadhyay NK, Kim J, Di Vizio D, Cinar B, Boucher K, Solomon KR, Freeman MR. Cholesterol sensitivity of endogenous and myristoylated Akt. Cancer Res. 2007;67:6238–6246. doi: 10.1158/0008-5472.CAN-07-0288. [DOI] [PubMed] [Google Scholar]
- 80.Sasaki K, Sato M, Umezawa Y. Fluorescent indicators for Akt/protein kinase B and dynamics of Akt activity visualized in living cells. J Biol Chem. 2003;278:30945–30951. doi: 10.1074/jbc.M212167200. [DOI] [PubMed] [Google Scholar]
- 81.Martelli AM, Faenza I, Billi AM, Manzoli L, Evangelisti C, Fala F, Cocco L. Intranuclear 3′-phosphoinositide metabolism and Akt signaling: new mechanisms for tumorigenesis and protection against apoptosis? Cell Signal. 2006;18:1101–1107. doi: 10.1016/j.cellsig.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 82.Yang JY, Yeh HY, Lin K, Wang PH. Insulin stimulates Akt translocation to mitochondria: implications on dysregulation of mitochondrial oxidative phosphorylation in diabetic myocardium. J Mol Cell Cardiol. 2009;46:919–926. doi: 10.1016/j.yjmcc.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Giorgi C, Ito K, Lin HK, Santangelo C, Wieckowski MR, Lebiedzinska M, Bononi A, Bonora M, Duszynski J, Bernardi R, et al. PML regulates apoptosis at endoplasmic reticulum by modulating calcium release. Science. 2010;330:1247–1251. doi: 10.1126/science.1189157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Santi SA, Lee H. The Akt isoforms are present at distinct subcellular locations. Am J Physiol Cell Physiol. 2010;298:C580–C591. doi: 10.1152/ajpcell.00375.2009. [DOI] [PubMed] [Google Scholar]
- 85.Betz C, Stracka D, Prescianotto-Baschong C, Frieden M, Demaurex N, Hall MN. Feature Article: mTOR complex 2-Akt signaling at mitochondria-associated endoplasmic reticulum membranes (MAM) regulates mitochondrial physiology. Proc Natl Acad Sci USA. 2013;110:12526–12534. doi: 10.1073/pnas.1302455110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ding J, Du K. ClipR-59 interacts with Akt and regulates Akt cellular compartmentalization. Mol Cell Biol. 2009;29:1459–1471. doi: 10.1128/MCB.00754-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gonzalez E, McGraw TE. Insulin-modulated Akt subcellular localization determines Akt isoform-specific signaling. Proc Natl Acad Sci USA. 2009;106:7004–7009. doi: 10.1073/pnas.0901933106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bossemeyer D, Engh RA, Kinzel V, Ponstingl H, Huber R. Phosphotransferase and substrate binding mechanism of the cAMP-dependent protein kinase catalytic subunit from porcine heart as deduced from the 2.0 A structure of the complex with Mn2 + adenylyl imidodiphosphate and inhibitor peptide PKI(5-24) EMBO J. 1993;12:849–859. doi: 10.1002/j.1460-2075.1993.tb05725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.