Abstract
For the non-cardiologist emergency physician and intensivist, performing an accurate estimation of left ventricular ejection fraction (LVEF) is essential for the management of critically ill patients, such as patients presenting with shock, severe respiratory distress or chest pain. Our objective was to develop a semi-quantitative method to improve visual LVEF evaluation. A group of 12 sets of transthoracic echocardiograms with LVEF in the range of 18–64% were interpreted by 17 experienced observers (PRO) and 103 untrained observers or novices (NOV), without previous training in echocardiography. They were asked to assess LVEF by two different methods: i) visual estimation (VIS) by analysing the three classical left ventricle (LV) short-axis views (basal, midventricular and apical short-axis LV section) and ii) semi-quantitative evaluation (base, mid and apex (BMA)) of the same three short-axis views. The results for each of these two methods for both groups (PRO and NOV) were compared with LVEF obtained by radionuclide angiography. The semi-quantitative method (BMA) improved estimation of LVEF by PRO for moderate LV dysfunction (LVEF 30–49%) and normal LVEF. The visual estimate was better for lower LVEF (<30%). In the NOV group, the semi-quantitative method was better than than the visual one in the normal group and in half of the subjects in the moderate LV dysfunction (LVEF 30–49%) group. The visual estimate was better for the lower LVEF (ejection fraction <30%) group. In conclusion, semi-quantitative evaluation of LVEF gives an overall better assessment than VIS for PRO and untrained observers.
Keywords: left ventricular function, echocardiography, left ventricular ejection fraction, wall motion score index
Introduction
Emergency physicians and intensivists are often called on to manage critically ill patients. Bedside echocardiography offers useful information regarding many acute cardiovascular conditions such as tamponade, pulmonary embolism and aortic dissection. However, clinicians encounter coronary heart disease and congestive heart failure far more often, and rapid evaluation of left ventricular ejection fraction (LVEF) is very important in these patients because of its diagnostic and therapeutic implications. In the emergency department (1) or intensive care unit, bedside echocardiography replaces invasive measurements of left ventricular (LV) function. Assessment of LV function is an important skill for physicians of diverse specialties and a key catalyst for the development of hand-held devices (2, 3). In light of this, The American College of Chest Physician has published a statement regarding maintenance of competency in critical care ultrasonography for intensivists (4). As published in the New England Journal of Medicine (5), diagnostic ultrasonography has replaced auscultation as the primary method of evaluating the mechanics of the heart. The broadening use of pocket-size ultrasound machines is changing the world of medical students. Several medical schools have recognised its value and have integrated beside ultrasound in the curriculum of medical students (6). Bedside cardiac ultrasonography is an emerging field, where teaching tools need to be developed and studied. Our article focuses on a new semi-quantitative method to evaluate a crucial cardiac parameter, the LVEF (7, 8, 9, 10). Considering that LVEF is usually assessed by visual estimation (VIS), the aim of our study is to describe a simple semi-quantitative method, base, mid and apex (BMA short axis), to improve VIS of LVEF for non-traditional users. The semi-quantitative technique aims to improve bedside evaluation of LV function by discriminating low, moderately abnormal and normal LVEF in order to limit potential errors and improve quality of care. Accordingly, we evaluated two echocardiographic methods for assessment of LVEF in emergency situations: the first method is simple VIS and the other is a semi-quantitative method (BMA) derived from the wall motion score index (WMSI).
Methods
Setting
The study population consisted of 12 patients from one of our previous studies on the WMSI for LVEF estimation (11). All patients consented to participate in the study as approved by our ethics committee. The 12 patients were selected from a population of 243 patients to represent three different subgroups of LVEF. The first group consisted of four patients with severe LV dysfunction (LVEF ≤30%) with LVEFs of 18, 20, 29 and 30%, respectively, as measured by radionuclide angiography (RNA). The second group consisted of four patients with moderate LV dysfunction (LVEF 31–49%) with LVEFs of 35, 38, 41 and 45%. The third group had normal LV function with LVEFs of 55, 59, 64 and 64%. All patients underwent a standard transthoracic echocardiogram (by experienced echocardiographers) to evaluate WMSI as well as RNA for evaluation of LVEF. The WMSI is a visual semi-quantitative assessment of regional wall motion. The LV is divided into 16 segments. On the basal (mitral) and midventricular (papillary muscle) level, the circumference is divided into six segments and on the apical level into four segments. The score for each segment is graded according to the classical system: normal=1, hypokinaesia=2 and akinaesia=3. The index (WMSI) is calculated by dividing total WMS of the polar map by 16 (the total number of segments). Both studies (Echo and RNA) were interpreted independently in a blinded fashion. Patients with acute myocardial infarction or significant clinical changes in clinical condition between the two tests were excluded. Echocardiographic studies had to display sufficient endocardial definition to allow the evaluation of LV contractility.
Echocardiographic studies
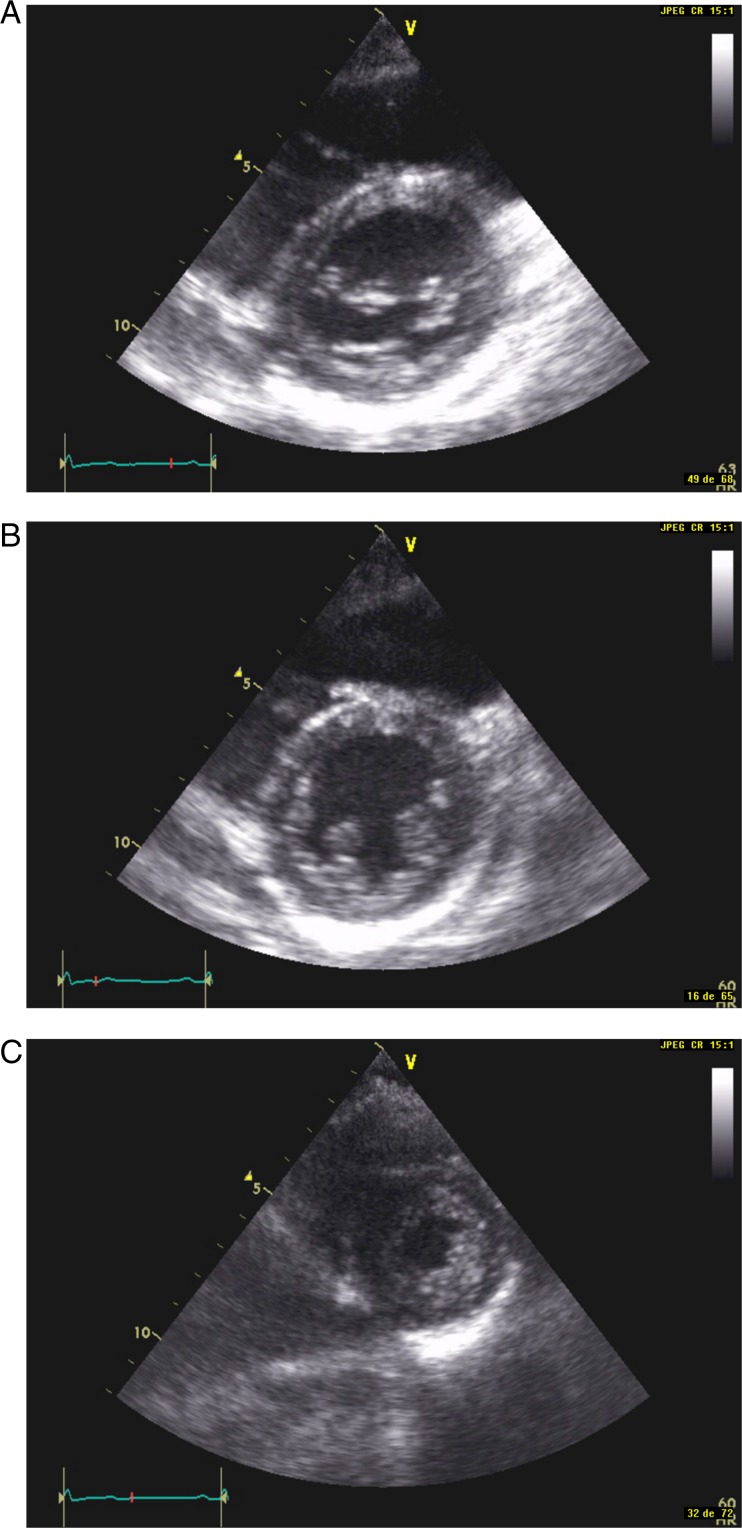
Echocardiographic studies were performed using a commercially available ultrasound system (Hewlett-Packard Sonos 1500 or 5000 (Hewlett Packard, Mississauga, ON, Canada)) with a 2.5 MHz transducer, with all echocardiograms recorded on video home system (VHS) tape. Data collection began in March 2005 and ended in February 2007. Although a complete transthoracic examination was performed in all patients, only the three classical parasternal short-axis views (BMA, see Fig. 1A, B and C respectively) were used (12). These echocardiograms were shown to two different groups of observers, the first one consisting of untrained physicians or medical students (called novices (NOV)) and the second group consisting of individuals experienced in echocardiography (experienced observers (PRO)). The NOV group was composed of 103 individuals without any previous echocardiography training. Their only training consisted of viewing two brief cases: one patient with normal LV function and one patient with LV dysfunction including hypokinaesia (reduced motion and thickening of a LV segment) and akinaesia (absent motion and thickening of a LV segment). They were instructed to assign a LVEF value of 60% if all three short-axis views were normal, a value of 40% in the case of diffuse hypokinaesia, and a value of 20% in the case of diffuse severe hypokinaesia (SH)/akinaesia. In the case of focal hypokinaesia or akinaesia, they were instructed to perform an approximate visual assessment. The PRO group was composed of six cardiologists (5–25 years of experience), 11 cardiology fellows (with a minimum of 3–6 months of recent training) and technicians in echocardiography (5–25 years of experience). Our university teaching hospital laboratory completes a mean of 5000 transthoracic studies/year.
Figure 1.
(A) Parasternal short-axis view at the mitral valve level. (B) Parasternal short-axis view at the midventricular level. (C) Parasternal short-axis view at the apical level.
Assessment of LV function
LV function was assessed by two different methods: simple VIS and semi-quantitative method (BMA) using segmentation of the LV at three short-axis sections: base (basal level at the mitral level), mid (papillary muscle level) and apical (apical level).
Visual estimation
The two groups of observers (PRO and NOV) analysed the 12 sets of echocardiograms in a random order. Only the three parasternal short-axis views were evaluated: the basal level at the mitral valve (B), the midventricular level at the papillary muscles level (M) and the apical level (A). The observers were asked to quickly analyse the three different levels (a mean of 10–15 beats was shown on tape for each of the three short-axis views) and give a subjective percentage ejection fraction (%EF) that integrated the three levels (e.g. 20 or 45%).
Semi-quantitative method (BMA)
Following visual assessment, 103 unselected novice reviewers received a short introduction to the principle of the simplified WMSI. Considering the linear relation between WMSI and LVEF, we developed a mean approximation for contractility (WMSI) and LVEF to be used in the study. A strong correlation between WMSI and LVEF has been clearly demonstrated in three studies involving a total of 1132 patients using either transthoracic echocardiography or cardiac resonance imaging (13). The WMSI can be translated into LVEF by a mathematical model (regression equation, see Table 1).
Table 1.
Estimation of LVEF according to echo and MRI study
| WMSI | ECHO LVEF (Lebeau 243 patients) | ECHO LVEF (Moller 767 patients) | MRI LVEF (122 patients) |
|---|---|---|---|
| 1.0 | 67 | 64 | 64 |
| 1.1 | 65 | 62 | 62 |
| 1.2 | 62 | 59 | 59 |
| 1.3 | 61 | 56 | 56 |
| 1.4 | 57 | 54 | 54 |
| 1.5 | 54 | 51 | 51 |
| 1.6 | 53 | 48 | 48 |
| 1.7 | 50 | 46 | 46 |
| 1.8 | 47 | 43 | 43 |
| 1.9 | 44 | 41 | 41 |
| 2.0 | 41 | 38 | 38 |
| 2.1 | 39 | 35 | 35 |
| 2.2 | 36 | 33 | 33 |
| 2.3 | 34 | 30 | 30 |
| 2.4 | 31 | 28 | 28 |
| 2.5 | 28 | 25 | 25 |
| 2.6 | 26 | 22 | 22 |
| 2.7 | 24 | 20 | 20 |
| 2.8 | 21 | 17 | 17 |
| 2.9 | 18 | 14 | 14 |
| 3.0 | 15 | 12 | 12 |
Therefore a normal LV function with a score of 1.0 has an echo LVEF of 67% and MRI LVEF of 64% (mean approximation equal or more than 60%). Mild hypokinaesia (MH) with a score of 1.5 has an echo LVEF of 54% and MRI LVEF of 51% (mean approximation=50%). Diffuse hypokinaesia with a score of 2.0 has an echo LVEF of 41% and MRI LVEF of 38% (mean approximation=40%). SH with a score of 2.5 has an echo LVEF of 28% and MRI LVEF of 25% (mean approximation=30%). Diffuse akinaesia has an echo LVEF of 15% and MRI LVEF of 12% (mean approximation=≤20%).
For the purpose of the study, the observers were asked to reevaluate the 12 patients and grade each level (base–midventricular–apex) using a letter: N for normal, H for moderate hypokinaesia and A for akinaesia. Alternatively, if they considered the three scores too rigid they could add two grades: MH and SH. The results were then transformed later by the research team into a global LVEF by averaging the result for each level. For example, in the case of BMA an answer giving mild basal hypokinaesia, moderate midventricular hypokinaesia and apical akinaesia, the calculated LVEF was 37% (e.g. 50%+40%+20%=110/3=37%).
Statistical analysis
All data from each scoring method and from the RNA LVEF were transformed into three ordinal categories: severe (≤30%), moderate (31–49%) and normal (≥50%) LVEF. To assess the global agreement between the two different methods of LVEF scoring and the gold standard RNA, weighted kappa (K w) analysis was done separately for the professional and the novice observers. The analysis was performed with MedCalc version 11.5 (MedCalc, Ostend, Belgium).
To evaluate specific concordance for each patient, an agreement coefficient (between 0 and 100%) was calculated, defined as the total number of agreements (the patient was scored in the same category by RNA and the scorer) on total number of scorings. To evaluate significant difference (number of agreements versus no agreement) between visual scoring and the two semi-quantitative methods, χ 2 statistics were used for novice and the Fisher exact-test with PRO scorers. Statistics of agreements was computed with SPSS version 17.0.
Results
The overall agreement as measured by the K w is higher for PRO than for NOV by a minimum of 0.14–0.22 for all scoring methods (see Table 2). The semi-quantitative methods shows generally higher K w than the visual method for the professionals, but this differences is less clear for the novice scorers.
Table 2.
Results for agreement between RNA and VIS and BMA scoring methods of LVEF evaluation
| Type of observer | VIS | BMA |
|---|---|---|
| PRO | n=19 | n=26 |
| K w | 0.70 (0.63–0.76) | 0.79 (0.74–0.84) |
| NOV | n=107 | n=103 |
| K w | 0.56 (0.53–0.60) | 0.61 (0.57–0.64) |
The percentages of agreements between RNA and LVEF for each estimation method and for each patient are given in Table 3. Differences with P values of less than 0.05 were considered to be statistically significant and are indicated by asterisks. The mean correct classification for the PRO is 73% for the VIS and 81% for the BMA method. The mean correct classification for the NOV is 63% for the VIS and 66% for the BMA method. For the professionals, the difference favouring the semi-quantitative method is for the patients with moderate LV dysfunction (EF between 31 and 49%) and normal EF (≥50%). In novice scorers, the visual method shows better agreement with RNA for the severely ill patients (EF ≤30%), but the semi-quantitative methods seems better for moderate LV dysfunction (in two patients out of four) and for normal subjects.
Table 3.
Percentages of agreement between RNA and left ventricular ejection fraction for each estimation method and for each patient
| Type of observer | Scoring method | |
|---|---|---|
| Patient | VIS | BMA |
| PRO | n=19 | n=26 |
| Patient 1 (EF=18%) | 94.7 | 76.9 |
| Patient 2 (EF=20%) | 100 | 88.5 |
| Patient 3 (EF=29%) | 100 | 88.5 |
| Patient 4 (EF=30%) | 94.7 | 96.2 |
| Patient 5 (EF=35%) | 26.3 | 50.0 |
| Patient 6 (EF=38%) | 42.1 | 73.1* |
| Patient 7 (EF=41%) | 78.9 | 80.8 |
| Patient 8 (EF=43%) | 68.4 | 80.8 |
| Patient 9 (EF=55%) | 63.2 | 92.3* |
| Patient 10 (EF=59%) | 31.6 | 57.7 |
| Patient 11 (EF=64%) | 100 | 96.2 |
| Patient 12 (EF=64%) | 78.9 | 92.3 |
| Mean | 73 | 81 |
| NOV | n=107 | n=103 |
| Patient 1 (EF=18%) | 72.9 | 55.3* |
| Patient 2 (EF=20%) | 87.9 | 73.8* |
| Patient 3 (EF=29%) | 87.9 | 77.7* |
| Patient 4 (EF=30%) | 89.7 | 73.8* |
| Patient 5 (EF=35%) | 34.6 | 63.1* |
| Patient 6 (EF=38%) | 37.4 | 64.1* |
| Patient 7 (EF=41%) | 50.5 | 41.7 |
| Patient 8 (EF=43%) | 55.1 | 51.5 |
| Patient 9 (EF=55%) | 60.7 | 76.7* |
| Patient 10 (EF=59%) | 23.4 | 35.9* |
| Patient 11 (EF=64%) | 86.9 | 92.2 |
| Patient 12 (EF=64%) | 72.9 | 88.3* |
| Mean | 63 | 66 |
*P<0.05.
Discussion
We describe the use of a new simplified semi-quantitative method to estimate LVEF using standard ultrasound imaging views. Using this method, accuracy of LVEF assessment was improved compared with VIS, for both trained and untrained observers.
Non-cardiologists
Previous studies have looked at the effect of minimal training of non-traditional users. Moore and colleagues (14) showed that emergency physicians, with 16 h of didactic teaching and 10 h of hands-on-practice focused on LVEF assessment (low EF ≤30%, mild 30–50% and normal more than 50%) correctly identified the group of EF 69% of the time (percentage of agreement with cardiologists). Another study involved emergency physicians with 3 h of didactic teaching and five supervised examinations specifically oriented on LVEF assessment (low, mild and normal EF). Again, agreement with cardiologist was 66% (15). Melamed and colleagues (9) evaluated the ability of intensivists with limited echocardiographic training to assess LVEF with portable ultrasound machines. Four intensivists received 2 h of didactic teaching and 4 h of hands-on-training in acquisition and interpretation of echocardiographic images. A total of 41 consecutive patients were scanned with a portable machine and intensivists had to classify LVEF in three categories: LVEF >50%, LVEF between 30 and 50% and LVEF <30%. Estimation of LVEF was correct for 72% of the patients. The authors suggested that additional training in image acquisition could improve their performance. Johri and colleagues (16) demonstrated that a simple teaching intervention can successfully diminish interobserver variability in the estimation of LVEF within a group of sonographers and physicians with a spectrum of experience. Cawthorn and colleagues developed and evaluated a novel curriculum for training medical students in the use of focus cardiac ultrasound (FCU). The self-directed module is effective for teaching introductory FCU interpretation skills, while expert-guided training is important for developing scanning technique (6). The results from these studies are in agreement with our findings that VIS of LVEF can have good accuracy even with minimal training. Moreover, our results indicate that the use of semi-quantitative analysis improves the assessment of LVEF by minimally trained physicians.
WMSI
Our method is based on semi-quantitative evaluation of the three LV short-axis levels, using a simple three points (N, H and A) or five points (N, H, A, MH and SH) scale allowing the correlation of LVEF with the WMSI. BMA is derived from the WMS technique, which has been proved to be superior to Simpson biplane LVEF in many studies (17, 18, 19, 20, 21). In our initial study, results of the WMSI were compared with RNA LVEF in 243 patients with abnormal LV contractility. A regression equation was derived to calculate LVEF using the WMSI (RNA LVEF=92.8−25.8×WMSI) and a linear relationship between WMSI and LVEF was demonstrated.
These findings were confirmed by a study by Moller and colleagues (22) from the Mayo Clinic on 767 patients comparing the WMSI with LVEF measured by a semi-quantitative method and VIS by expert echocardiographers. They obtained a regression equation very similar to ours (semi-quantitative LVEF=0.90−0.26×WMSI). Several other groups have validated the use of the WMSI (23, 24, 25, 26). McGowan & Cleland (27) compared VIS of LVEF, Simpson's biplane method and WMSI with the gold standard RNA in a systematic review of 43 studies over a 22-year period (1979–2001) and concluded that all three methods were nearly equivalent.
Semi-quantitative methods (BMA)
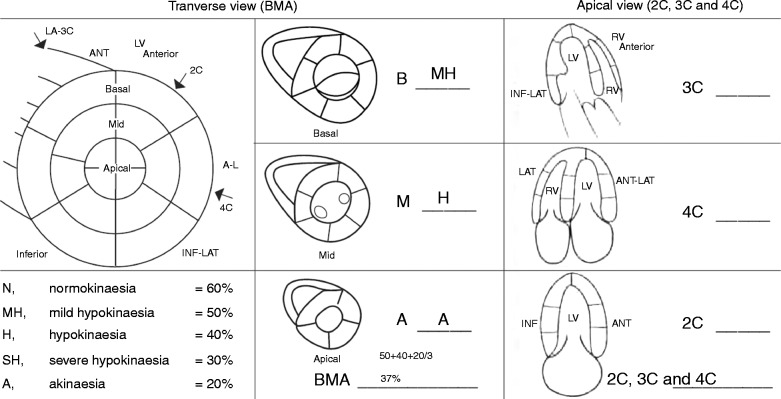
The semi-quantitative method performed differently according to the experience of the operator. Both NOV and experts can easily recognise a very low LVEF. The challenge is when the LVEF is between 30 and 50%; this is where the BMA model can really help both the experts and the NOV. In the PRO group, the BMA method produced better results for the moderate and normal LVEF. The improvement in interpretation of LVEF in the PRO group confirms that the BMA technique is superior to visual assessment. In a previous study (28), we evaluated the visual and semi-quantitative method in a study using BMA and apical views (2 Chamber (2C), 3C and 4C). As in this study, the semi-quantitative method was better than visual assessment for evaluation of moderate and normal LVEF in the novice group; the PRO group fared equally well with either visual or BMA method for all categories. Both methods should be used but the BMA method is better than the apical views method. The advantage of the BMA method is that the three short-axis views are similar to a 3D evaluation of the LV (360° estimation) including all the incidences used in echocardiography for LVEF (long axis, apical (2C, 3C and 4C), sub costal and right parasternal views). The apical views (2C, 3C and 4C) are frequently used for the assessment of LVEF because they are technically easier to do but they are ‘ice pick’ views compared with short-axis views (360° view, see Fig. 2).
Figure 2.
Anatomical correlation between transverse and apical view.
Limitations
While LVEF is a key element in cardiac assessment, it does not always correlate with stroke volume and cardiac output. Other factors, such as diastolic function, valvular heart disease and volume status are outside the scope of our study. Our results indicate that using a few short-axis images of the LV, a reasonable assessment of the LVEF can be achieved. However, we did not assess the ability to acquire images. Adequate image acquisition requires at least 3 months of training (29). Semi-quantitative assessment of the 12 cases was performed after the first reading for VIS of LVEF. The second reading is unlikely to explain the improved results because a different method was used to estimate regional LV function. The analysis was performed rapidly as the observers had only 10–15 images to look at before giving an estimation of LVEF, to recreate the settings in an emergency situation. Finally, this model is intended only for approximate quantification to classify LVEF as severely depressed (≤30%), moderately depressed (31–49%) or normal (≥50%). No hyperkinaesia, dyskinaesia or aneurysms were included in the classification. Only studies with good acquisition and acoustic windows were analysed. The use of contrast may improve endocardial definition and improve the accuracy of currently evaluated methods. This is not meant to replace standard LVEF assessment in the echocardiography laboratory, which has important diagnostic and prognostic implications. Strain and 3D echocardiography are quickly becoming the mainstream. However, these techniques require more expertise than wall motion analysis, and will not be part of cardiac assessment in an emergency setting. For now, LVEF remains essential, and wall motion analysis is a simple and safe semi-quantitative method (30, 31).
Conclusion
VIS is adequate to evaluate LVEF, but our results indicate that semi-quantitative evaluation gives a more accurate assessment by both experienced and non-experienced users. This technique could facilitate training of NOV.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work was supported by the Echocardiography Laboratory of the Hôpital du Sacré-Coeur de Montréal.
References
- 1. Sabia P Abbott RD Afrookteh A Keller MW Touchstone DA Kaul S Importance of two-dimensional echocardiographic assessment of left ventricular systolic function in patients presenting to the emergency room with cardiac-related symptoms Circulation 84 1991. 1615–1624. 10.1161/01.CIR.84.4.1615 [DOI] [PubMed] [Google Scholar]
- 2. Spencer KT Kimura BJ Korcarz CE Pellikka PA Rahko PS Siegel RJ Focused cardiac ultrasound: recommendations from the American Society of Echocardiography Journal of the American Society of Echocardiography 26 2013. 567–581. 10.1016/j.echo.2013.04.001 [DOI] [PubMed] [Google Scholar]
- 3. Choi BG Mukherjee M Dala P Young HA Tracy CM Katz RJ Lewis JF Interpretation of remotely downloaded pocket-size cardiac ultrasound images on a web-enabled smartphone: validation against workstation evaluation Journal of the American Society of Echocardiography 24 2011. 1325–1330. 10.1016/j.echo.2011.08.007 [DOI] [PubMed] [Google Scholar]
- 4. Mayo PH Beaulieu Y Doelken P Feller-Kopman D Harrod C Kaplan A Oropello J Vieillard-Baron A Axler O Lichtenstein D et al. American College of Chest Physicians/La Societe de Reanimation de Langue Francaise: statement on competence in critical care ultrasonography Chest 135 2009. 1050–1060. 10.1378/chest.08-2305 [DOI] [PubMed] [Google Scholar]
- 5. Solomon SD Saldana F Point-of-care ultrasound in medical education – stop listening and look New England Journal of Medicine 370 2014. 1083–1085. 10.1056/NEJMp1311944 [DOI] [PubMed] [Google Scholar]
- 6. Cawthorn TR Nickel C O'Reilly M Kafka H Tam JW Jackson LC Sanfilippo AJ Johri AM Development and evaluation of methodologies for teaching focused cardiac ultrasound skills to medical students Journal of the American Society of Echocardiography 27 2014. 302–309. 10.1016/j.echo.2013.12.006 [DOI] [PubMed] [Google Scholar]
- 7. Jones AE Tayal VS Sullivan DM Kline JA Randomized, controlled trial of immediate versus delayed goal-directed ultrasound to identify the cause of nontraumatic hypotension in emergency department patients Critical Care Medicine 32 2004. 1703–1708. 10.1097/01.CCM.0000133017.34137.82 [DOI] [PubMed] [Google Scholar]
- 8. Labovitz AJ Noble VE Bierig M Goldstein SA Jones R Kort S Porter TR Spencer KT Tayal VS Wei K Focused cardiac ultrasound in the emergent setting: a consensus statement of the American Society of Echocardiography and American College of Emergency Physicians Journal of the American Society of Echocardiography 23 2010. 1225–1230. 10.1016/j.echo.2010.10.005 [DOI] [PubMed] [Google Scholar]
- 9. Melamed R Sprenkle MD Ulstad VK Herzog CA Leatherman JW Assessment of left ventricular function by intensivists using hand-held echocardiography Chest 135 2009. 1416–1420. 10.1378/chest.08-2440 [DOI] [PubMed] [Google Scholar]
- 10. Reant P Dijos M Arsac F Mignot A Cadenaule F Aumiaux A Jimenez C Dufau M Prevost A Pillois X et al. Validation of a new bedside echoscopic heart examination resulting in an improvement in echo-lab workflow Archives of Cardiovascular Diseases 104 2011. 171–177. 10.1016/j.acvd.2011.01.003 [DOI] [PubMed] [Google Scholar]
- 11. Lebeau R Di Lorenzo M Amyot R Veilleux M Lemieux R Sauve C A new tool for estimating left ventricular ejection fraction derived from wall motion score index Canadian Journal of Cardiology 19 2003. 397–404. [PubMed] [Google Scholar]
- 12. Lang RM Bierig M Devereux RB Flachskampf FA Foster E Pellikka PA Picard MH Roman MJ Seward J Shanewise JS et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology Journal of the American Society of Echocardiography 18 2005. 1440–1463. 10.1016/j.echo.2005.10.005 [DOI] [PubMed] [Google Scholar]
- 13. Lebeau R Serri K Morice MC Hovasse T Unterseeh T Piechaud JF Garot J Assessment of left ventricular ejection fraction using the wall motion score index in cardiac magnetic resonance imaging Archives of Cardiovascular Diseases 105 2012. 91–98. 10.1016/j.acvd.2012.01.002 [DOI] [PubMed] [Google Scholar]
- 14. Moore CL Rose GA Tayal VS Sullivan DM Arrowood JA Kline JA Determination of left ventricular function by emergency physician echocardiography of hypotensive patients Academic Emergency Medicine 9 2002. 186–193. 10.1111/j.1553-2712.2002.tb00242.x [DOI] [PubMed] [Google Scholar]
- 15. Randazzo MR Snoey ER Levitt MA Binder K Accuracy of emergency physician assessment of left ventricular ejection fraction and central venous pressure using echocardiography Academic Emergency Medicine 10 2003. 973–977. 10.1111/j.1553-2712.2003.tb00654.x [DOI] [PubMed] [Google Scholar]
- 16. Johri AM Picard MH Newell J Marshall JE King ME Hung J Can a teaching intervention reduce interobserver variability in LVEF assessment: a quality control exercise in the echocardiography lab JACC. Cardiovascular Imaging 4 2011. 821–829. 10.1016/j.jcmg.2011.06.004 [DOI] [PubMed] [Google Scholar]
- 17. Dahlslett T Karlsen S Grenne B Eek C Sjoli B Skulstad H Smiseth OA Edvardsen T Brunvand H Early assessment of strain echocardiography can accurately exclude significant coronary artery stenosis in suspected non-ST-segment elevation acute coronary syndrome Journal of the American Society of Echocardiography 27 2014. 512–519. 10.1016/j.echo.2014.01.019 [DOI] [PubMed] [Google Scholar]
- 18. Dall'Armellina E Morgan TM Mandapaka S Ntim W Carr JJ Hamilton CA Hoyle J Clark H Clark P Link KM et al. Prediction of cardiac events in patients with reduced left ventricular ejection fraction with dobutamine cardiovascular magnetic resonance assessment of wall motion score index Journal of the American College of Cardiology 52 2008. 279–286. 10.1016/j.jacc.2008.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Duncan RF Dundon BK Nelson AJ Pemberton J Williams K Worthley MI Zaman A Thomas H Worthley SG A study of the 16-segment regional wall motion scoring index and biplane Simpson's rule for the calculation of left ventricular ejection fraction: a comparison with cardiac magnetic resonance imaging Echocardiography 28 2011. 597–604. 10.1111/j.1540-8175.2011.01394.x [DOI] [PubMed] [Google Scholar]
- 20. Klein P Holman ER Versteegh MI Boersma E Verwey HF Bax JJ Dion RA Klautz RJ Wall motion score index predicts mortality and functional result after surgical ventricular restoration for advanced ischemic heart failure European Journal of Cardio-Thoracic Surgery 35 2009. 847–852.(discussion 852–853) 10.1016/j.ejcts.2008.12.046 [DOI] [PubMed] [Google Scholar]
- 21. Yao SS Qureshi E Sherrid MV Chaudhry FA Practical applications in stress echocardiography: risk stratification and prognosis in patients with known or suspected ischemic heart disease Journal of the American College of Cardiology 42 2003. 1084–1090. 10.1016/S0735-1097(03)00923-9 [DOI] [PubMed] [Google Scholar]
- 22. Moller JE Hillis GS Oh JK Reeder GS Gersh BJ Pellikka PA Wall motion score index and ejection fraction for risk stratification after acute myocardial infarction American Heart Journal 151 2006. 419–425. 10.1016/j.ahj.2005.03.042 [DOI] [PubMed] [Google Scholar]
- 23. Berning J Rokkedal Nielsen J Launbjerg J Fogh J Mickley H Andersen PE Rapid estimation of left ventricular ejection fraction in acute myocardial infarction by echocardiographic wall motion analysis Cardiology 80 1992. 257–266. 10.1159/000175011 [DOI] [PubMed] [Google Scholar]
- 24. Galasko GI Basu S Lahiri A Senior R A prospective comparison of echocardiographic wall motion score index and radionuclide ejection fraction in predicting outcome following acute myocardial infarction Heart 86 2001. 271–276. 10.1136/heart.86.3.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rifkin RD Koito H Comparison with radionuclide angiography of two new geometric and four nongeometric models for echocardiographic estimation of left ventricular ejection fraction using segmental wall motion scoring American Journal of Cardiology 65 1990. 1485–1490. 10.1016/0002-9149(90)91360-I [DOI] [PubMed] [Google Scholar]
- 26. Thune JJ Kober L Pfeffer MA Skali H Anavekar NS Bourgoun M Ghali JK Arnold JM Velazquez EJ Solomon SD Comparison of regional versus global assessment of left ventricular function in patients with left ventricular dysfunction, heart failure, or both after myocardial infarction: the valsartan in acute myocardial infarction echocardiographic study Journal of the American Society of Echocardiography 19 2006. 1462–1465. 10.1016/j.echo.2006.05.028 [DOI] [PubMed] [Google Scholar]
- 27. McGowan JH Cleland JG Reliability of reporting left ventricular systolic function by echocardiography: a systematic review of 3 methods American Heart Journal 146 2003. 388–397. 10.1016/S0002-8703(03)00248-5 [DOI] [PubMed] [Google Scholar]
- 28. Lebeau R Potter BJ Sas G Moustafa S Di Lorenzo M Soulieres V Beaulieu Y Sauvé C Amyot R Serri K Performance of a simplified wall motion score index method for noncardiologists to assess left ventricular ejection fraction ISRN Emergency Medicine 2012 2012. 1–5. 10.5402/2012/309470 [DOI] [Google Scholar]
- 29. Beller GA Bonow RO Fuster V F. American College of Cardiology A. American Heart C. American College of Physicians Task Force on Clinical, and Training ACCF Recommendations for Training in Adult Cardiovascular Medicine Core Cardiology Training (COCATS 3) (revision of the 2002 COCATS Training Statement) Journal of the American College of Cardiology 51 2008. 335–338. 10.1016/j.jacc.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 30. Castel AL Szymanski C Delelis F Levy F Menet A Mailliet A Marotte N Graux P Tribouilloy C Marechaux S Prospective comparison of speckle tracking longitudinal bidimensional strain between two vendors Archives of Cardiovascular Diseases 107 2014. 96–104. 10.1016/j.acvd.2014.01.007 [DOI] [PubMed] [Google Scholar]
- 31. Costa SP Beaver TA Rollor JL Vanichakarn P Magnus PC Palac RT Quantification of the variability associated with repeat measurements of left ventricular two-dimensional global longitudinal strain in a real-world setting Journal of the American Society of Echocardiography 27 2014. 50–54. 10.1016/j.echo.2013.08.021 [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a