Abstract
Cardiac erosion related to transcatheter atrial septal defect closure devices is of increasing concern. Erosion is reported to have occurred with most of currently available occluder devices. Perhaps due to the very large number of implants worldwide, the Amplatzer (St Jude) occluder is associated with the majority of cardiac erosion events reported in the literature. Best current estimates of the incidence of erosion with the St Jude device are between one and three cases per 1000 implants. Most events occur early after implantation and it is rare, although not unheard of, for events to occur after a year following device insertion. It is important that those involved with closure programmes are vigilant for the problem, because device-related erosion is associated with a significant mortality risk. Despite considerable debate, the risk factors (either patient or device) for erosion remain unclear and require further investigation. Currently available data sets have focussed largely on erosion cohorts and are unable to place these cases in appropriate context with non-erosion closure cases. What is certain is that programmes implanting these devices must take care to implant appropriately sized devices and have in place plans to ensure that patients are both well informed and can access help and advice in the event of developing symptoms.
Keywords: atrium, congenital heart defects, congenital heart disease, device closure
Background
The search for less invasive alternatives to surgery for closing secundum atrial septal defects (ASD) has occupied cardiologists for decades.
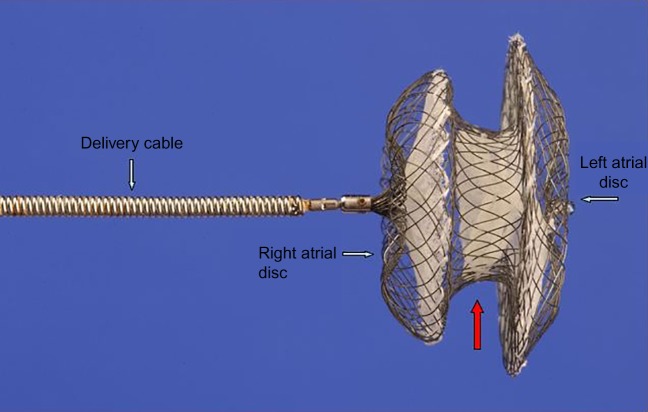
Trans-catheter closure of secundum ASD was first described by King and Mills in 1976 (1). In the mid 1990s, the introduction of the Amplatzer septal occluder (ASO) (http://health.sjm.com/amplatzer-septal-occluder), a nitinol device with a central waist manufactured by the AGA Corporation (Fig. 1) brought this procedure into mainstream cardiological practice. The ASO device was reliable and consistent in its handling characteristics and could easily be recaptured and redeployed without destroying its structure if it was not in an acceptable position initially. Importantly, it was able to deal with the majority of secundum ASDs regardless of their size. It can be said without exaggeration that the introduction of the ASO was a game-changing event for congenital cardiac intervention. More than 200 000 patients worldwide (an estimate based on the number of shipped devices) have subsequently been treated with the device. In addition, partly through experience derived from ASD occlusion, cardiologists developed skills that led to a period of intense development in other aspects of this field.
Figure 1.
Amplatzer septal occluder (ASO): Lateral aspect. Left and right discs (blue arrows), central core (red arrow).
Over time, a number of other devices have become commercially available for ASD closure and deserve consideration with reference to cardiac erosion. These currently include:
Occlutech Figulla occluder (http://www.occlutech.com).
Cera (Lifetech) occluder (http://www.lifetechmed.com).
PFM Nit-Occlud device (http://www.pfmmedical.com/en).
Gore Cardioform septal occluder (formerly Gore Septal Occluder (GSO)) (http://www.goremedical.com/eu/cardioform/).
Cardia Ultrasept (formerly Atriasept 2) (http://www.cardiainc.com).
Cocoon septal occluder (http://sanaremedprod.com/cocoon-septal-occluder/).
Erosion
Cardiac erosion, as a result of mechanical trauma following device implantation, was initially recognised in case reports or small series and in direct communication from physicians to the AGA Corporation (subsequently purchased by St Jude Medical) (2, 3, 4). In most cases, clinical presentation after erosion is with sudden onset of symptoms, e.g., chest pain in association with haemodynamic instability, typically associated with a new pericardial effusion. In some patients, a fistula may be evident on echocardiography (Fig. 2).
Figure 2.
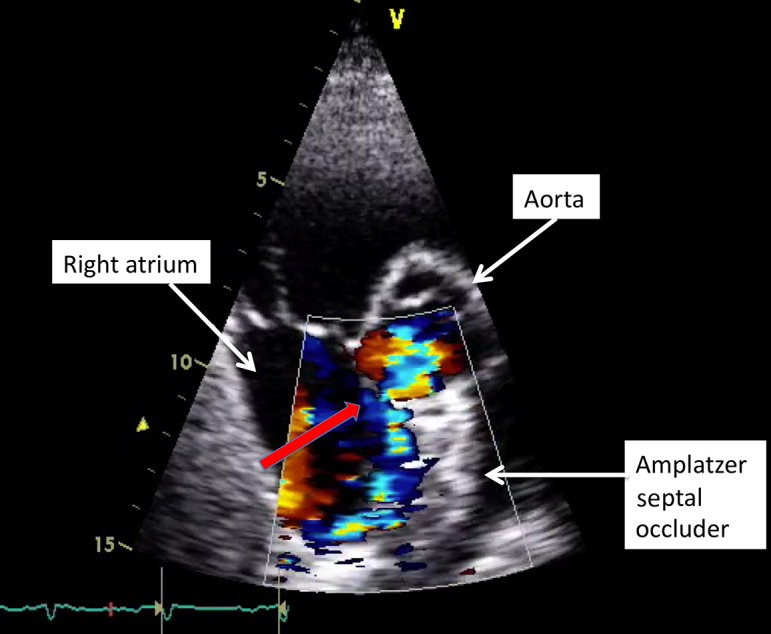
Trans-thoracic echocardiogram (TTE): Short axis view. Amplatzer septal occluder positioned in the atrial septum. Fistula (due to device related erosion) between the aorta and right atrium.
Over a 6-year period (1998–2004), 28 cases of haemodynamic instability, following device insertion, were reported to AGA (5). Data relating to these cases were considered by an AGA-appointed expert panel (5). The majority of patients (19/28, 67%) developed symptoms within 72 h of device implantation, 21/28 (75%) underwent surgery, and the subsequent perforation site was either the left or right atrial roof, the aorta or a combination of those sites. From these data, the AGA Corporation determined the erosion rate at that time in the US to be ∼0.1%. This can at best be considered speculative, given that neither the numerator nor the denominator was accurately known.
Further insights were gained through analysis of the FDA (voluntary) adverse event website, the ‘Manufacturer and User Facility Device Experience’ (MAUDE), and this created additional concern. Cardiac erosion was responsible for a large proportion of events reported to MAUDE (15%). In the 2009 report from DiBardio et al. (6), based on these data, there were ten deaths associated with the 51 cases of cardiac perforation. Although most of the erosions occurred relatively early after device implantation, events were also reported in previously well patients up to 3 years after device closure of the ASD (4). Subsequent case reports have demonstrated that erosions can occur even later after device implantation (7, 8). Analysis of an updated contemporary MAUDE dataset identified over 100 potential erosions leading to a readjustment of the likely incidence of erosion to 1–3 cases per 1000 implants, based on approximate worldwide sales of 250 000 ASO devices (9, 10) As a result, the FDA issued guidelines to both physicians and patients: http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm371145.htm.
In addition to the appropriate advice relating to explicit and thorough counselling of patients prior to implantation, the intervention of the FDA led to a rapid change in the instructions for use (IFU) of the ASO device by St Jude Medical in the US in 2013. Although the altered IFU applied primarily to the US, they were taken seriously in other countries and led to written advice from the company to implanters in other jurisdictions. Despite the lack of definitive evidence relating to the risk factors, the new IFU seemingly removed the option of an interventional approach for many patients and resulted in widespread confusion amongs implanting physicians worldwide, before being rapidly amended.
What are the risk factors for erosion?
A number of potential risk factors have been postulated for the occurrence of ASO-related cardiac erosion. These include deficient aortic and/or superior rim (the rim to the dome of the left atrium), movement of the device within the heart, the insertion of an unnecessarily large or oversized occluder, the insertion of an undersized occluder, patient age and device type.
Deficient rims
A deficient anterior and/or superior rim may bring an edge of a device into contact with either the aorta or the ‘roof’ of the atrium (Fig. 3, Fig. 4) This may then cause tissue trauma and subsequently an erosion. As a result, there is some logic in the argument that a device positioned within a superiorly placed defect may be at a higher risk for eventual erosion. Certainly deficient anterior or superior rims predominate in studies describing the anatomy after cardiac erosion and in AGA's review of erosion cases, deficiency in this area of the heart was present in almost 90% (5, 11). However it is crucially important to remember that deficient rims of this nature are common in ASDs in general, particularly larger ASDs where the prevalence may be as high as 70–80% (11, 12). Data currently in existence (essentially retrospective case series) are insufficient to draw definite conclusions in this regard, and the issue will only be resolved by large, carefully constructed and lengthy longitudinal studies.
Figure 3.
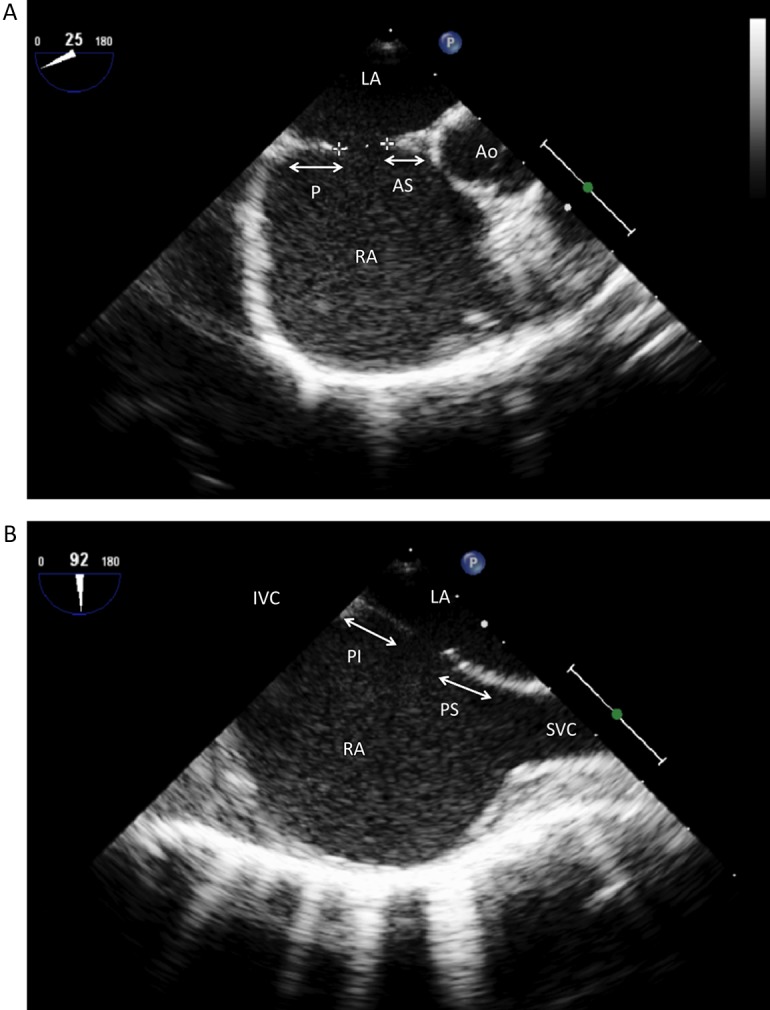
(A) Trans-oesophageal echocardiogram (TOE): Mid-oesophagus, 25° view demonstrating a secundum atrial septal defect (ASD) (crosses). P, posterior margin; AS, antero-superior margin; Ao, Aorta; LA, Left atrium; RA, Right atrium. (B) Trans-oesophageal echocardiogram (TOE): Mid-oesophagus, 90° view demonstrating a secundum ASD. PI, Posterio-inferior margin; PS, Posterio-superior margin; SVC, superior vena cava.
Figure 4.
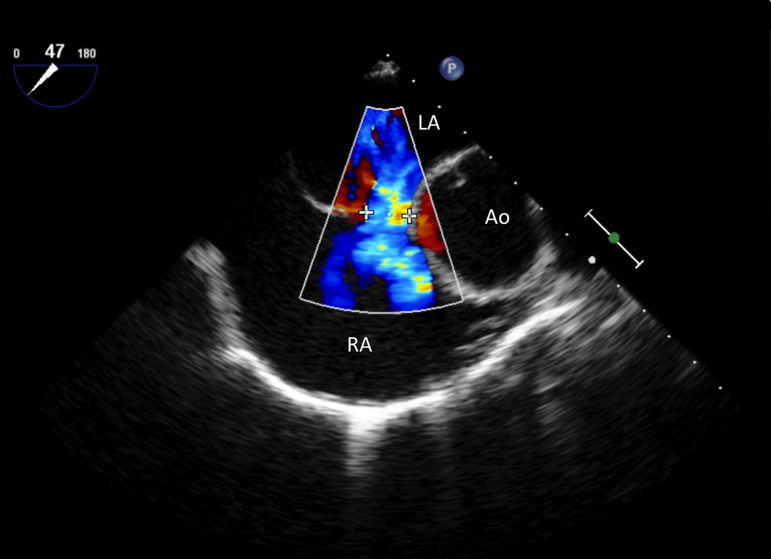
Trans-oesophageal echocardiogram (TOE) with colour Doppler: Mid-oesophagus, 45° view demonstrating an ASD (crosses) with a deficient margin to the aorta. Note the complete lack of tissue to the aorta (Ao), LA, Left atrium; RA, Right atrium.
Device movement in the heart
In theory, a relatively mobile device may exert a ‘cheese wire’ effect within the myocardium. Studies demonstrate that there are different patterns of contact between the device and the myocardium and also that following closure of ASD, the relationship of a device with adjacent tissue changes as the device settles and the heart remodels (13, 14). In addition, particularly ‘dynamic’ ASDs (those that apparently change significantly in size with the cardiac cycle) were a feature of erosion cases in a recently published review (13). There are also anecdotal cases where erosion may have been associated with exaggerated cardiac motion related to exercise (15).
Device size
As with deficient tissue rims, the use of apparently oversized devices within defects predominate within case series of cardiac erosion (5, 11). There are potential issues relating to the retrospective assessment of the appropriateness of device size. However, it is easy to envisage how a device that is significantly too large for a particular defect might protrude into a vulnerable tissue plane, eventually causing damage. To add to the apparent confusion regarding aetiology and risk factors, in direct contrast a published survey of experienced implanters within the CCISC consortium (a US-based quality-assurance data submission group), El-Said and Moore found that in the opinion of over two-thirds of respondents, undersized devices which do not straddle the aorta were at the highest risk of erosion! (16). As a general principle, the use of an adequate- (but no more, and probably no less) sized device should be the aim during implantation; however, sizing ASDs is an imprecise process in which different operators have significantly divergent views. Those closing ASDs including echocardiographers guiding the procedure should be aware of the potential for significant oversizing if balloon interrogation of the ASD is used. As a minimum, if a balloon is deemed necessary, the principles of ‘stop flow,’ i.e., the obliteration of colour flow rather than producing a waist on the balloon due to tissue pressure, should be strictly adhered to.
Patient age
Although erosion is reported in paediatric patients, there appears to be a greater relative risk in adults (5, 10, 11). To our knowledge, there are currently no reports of deaths related to erosion with the ASO in paediatric patients. The reasons for this are not entirely clear, but tissue rigidity would seem to be a potential answer.
Occluder type
Erosions have been reported with the ASO, Occlutech occluder (17), Cardia device (18) as well as with devices that are no longer available on the commercial market (19). Given the relative disparity in the numbers of implants with each device, it is difficult to be absolutely certain that the risk of erosion with ASO is genuinely greater than with other devices. It is important to be aware that as the ASO increases in size, there are changes in wire thickness, leading to ‘jumps’ in stiffness at certain points in the device range, most notably beyond 24 mm. It is noteworthy that the 26 mm ASO device appears to be disproportionally associated with cardiac perforation, adding weight to the argument that device stiffness is an important issue. There have been more than 20 000 implants worldwide with the Gore family of occluders (the HELEX device and Gore Septal Occluder); these devices are relatively soft and compliant and have not been associated with any reported erosions.
Implantation technique
As yet, there is little or no information relating to the conduct of the occlusion procedure and the subsequent risk of early erosion. In all of the previous analyses, no information has been collected about the number of attempts made for implanting a device. It is possible that the risk of erosion may be exacerbated by multiple attempts at deployment of the device prior to the final delivery, leading to local trauma.
What does the echocardiographer need to know?
Experts in echocardiography are an essential part of the team in all areas of ASD closure from pre-procedural assessment, to guiding the procedure, as well as during follow-up after device implantation.
It is clear that there are limited data upon which to delineate risk factors for cardiac erosion following transcatheter ASD occlusion. What information there is available seems to suggest the following:
That the problem is primarily associated with the ASO.
That there may be an association with deficient anterior or superior rims.
That there is additional risk related to increasing device stiffness.
That incorrect sizing (either over-sizing or under-sizing) of the device relative to the defect may be an issue.
That the problem is more common and perhaps more serious in adult than in paediatric patients.
These variables can be factored into patient selection for procedures, counselling of patients prior to a procedure and the choice of device used. However, a coherent argument can also be made that at present we do not have enough information to identify the risk factors and therefore material changes to implantation strategy based on uncertain stratification is perhaps an illogical reaction.
For echocardiographers involved with guiding ASD closure procedures, a thorough and accurate delineation of the anatomy is essential. Agreed protocols should be in place with regard to the assessment, ensuring appropriate accurate defect sizing. The echocardiographer should also establish that the device is not unduly affecting the natural anatomy of the heart prior to release, e.g., exerting repetitive compressive force on the aorta. If this occurs, a discussion amongst the implanting team should take place regarding the advisability of device release.
Finally, it is important that protocols are in place with regard to follow-up and surveillance of implanted devices. Vigilance for the presence of pericardial effusion, alterations in device position and any other abnormality, particularly when associated with patient symptoms, are essential. Current recommendations are that patients with devices should remain under indefinite review, even though erosions are by definition unexpected events that cannot necessarily be detected by ‘untargeted’ follow-up. However, continued contact with the implanting team means that patients are more likely to seek appropriate help in the event of worrying symptoms (pain, dizziness, collapse, etc.).
Implications for implantable device technology
Anything other than a cursory analysis of the current available data leads to an uncomfortable conclusion. Despite the fact that there are over 200 000 patients with implants going back almost two decades, previous data collection relating to the implantation and follow-up of ASD devices have been insufficiently robust to identify the risk factors for rare complications of this nature. As a result, discussions about changes to implant practice are based on current ‘received wisdom’ and not hard scientific fact. This is at odds with the issuing of a change in the instructions for use of the ASO device by St Jude Medical in many countries and markets during 2013, which was not based on solid evidence and was both confusing and threatening for physicians.
Clearly, it is essential that future studies be conducted to examine the issue of erosion further. There are also important lessons for the wider industry involved with the implantation of devices (including physicians, the medical device industry and those purchasing these services on behalf of patients) in terms of mandatory data collection. Data collection of this nature will come at a cost but is in the best long-term interests of patients.
Declaration of interest
J D R Thomson BM BS MD FRCP, Consultant to Gore Medical, Proctor of St Jude Medical; S A Qureshi MBChB FRCP, Consultant to Numed Medical, Occlutech and St Jude Medical, Proctor of Venus Medtech and Occlutech.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
References
- 1. King TD Thompson SL Steiner C Mills NL Secundum atrial septal defect. Nonoperative closure during cardiac catheterization Journal of the American Medical Association 235 1976. 2506–2509. 10.1001/jama.1976.03260490024013 [DOI] [PubMed] [Google Scholar]
- 2. Aggoun Y Gallet B Acar P Pulik M Czitrom D Lagier A Laborde F Perforation of the aorta after percutaneous closure of an atrial septal defect with an Amplatz prosthesis, presenting with acute severe hemolysis Archives des Maladies du Coeur et des Vaisseaux 95 2002. 479–482. [PubMed] [Google Scholar]
- 3. Chun DS Turrentine MW Moustapha A Hoyer MH Development of aorta-to-right atrial fistula following closure of secundum atrial septal defect using the Amplatzer septal occluder Catheterization and Cardiovascular Interventions 58 2003. 246–251. 10.1002/ccd.10434 [DOI] [PubMed] [Google Scholar]
- 4. Divekar A Gaamangwe T Shaikh N Raabe M Ducas J Cardiac perforation after device closure of atrial septal defects With the Amplatzer septal occluder Journal of the American College of Cardiology 45 2005. 1213–1218. 10.1016/j.jacc.2004.12.072 [DOI] [PubMed] [Google Scholar]
- 5. Amin Z Hijazi ZM Bass JL Cheatham JP Hellenbrand WE Kleinman CS Erosion of Amplatzer septal occluder device after closure of secundum atrial septal defects: review of registry of complications and recommendations to minimize future risk Catheterization and Cardiovascular Interventions 63 2004. 496–502. 10.1002/ccd.20211 [DOI] [PubMed] [Google Scholar]
- 6. DiBardino DJ McElhinney DB Kaza AK Mayer JE Jr Analysis of the U.S. Food and Drug Administration Manufacturer and User Facility Device Experience database for adverse events involving Amplatzer septal occluder devices and comparison with the Society of Thoracic Surgery congenital cardiac surgery database Journal of Thoracic and Cardiovascular Surgery 137 2009. 1334–1341. 10.1016/j.jtcvs.2009.02.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Diab K Kenny D Hijazi ZM Erosions, erosions and erosions! Device closure of atrial septal defects: how safe is safe? Catheterization and Cardiovascular Interventions 80 2012. 168–174. 10.1002/ccd.24517 [DOI] [PubMed] [Google Scholar]
- 8. Beg K Latson LA Pettersson G Wallace L Qureshi AM Aorta-to-left atrial fistula developing after surgical removal of an atrial septal occlusion device eight years after original implantationN World Journal for Pediatric & Congenital Heart Surgery 6 2015. 320–323. 10.1177/2150135114561688 [DOI] [PubMed] [Google Scholar]
- 9.U.S. FDA. FDA Executive Summary Memorandum – May 24, 2012: Circulatory System Advisory Panel Meeting – Transcatheter ASD Occluders: Clinical Update and Review of Events [pdf]. May 24, 2012. (available at: http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/CirculatorySystemDevicesPanel/ucm300073.htm)
- 10. Moore J Hegde S El-Said H Beekman R III Benson L Bergersen L Holzer R Jenkins K Ringel R Rome J Transcatheter device closure of atrial septal defects. A safety review JACC. Cardiovascular Interventions 5 2013. 433–442. 10.1016/j.jcin.2013.02.005 [DOI] [PubMed] [Google Scholar]
- 11. Amin Z Echocardiographic predictors of cardiac erosion after Amplatzer septal occluder placement Catheterization and Cardiovascular Interventions 83 2014. 84–92. 10.1002/ccd.25175 [DOI] [PubMed] [Google Scholar]
- 12. Podnar T Martanovic P Gavora P Masura J Morphological variations of secundum-type atrial septal defects: feasibility for percutaneous closure using Amplatzer septal occluders Catheterization and Cardiovascular Interventions 53 2001. 386–391. 10.1002/ccd.1187 [DOI] [PubMed] [Google Scholar]
- 13. Kitano M Yazaki S Sugiyama H Yamada O The influence of morphological changes in Amplatzer device on the atrial and aortic walls following transcatheter closure of atrial septal defects Journal of Interventional Cardiology 22 2009. 83–91. 10.1111/j.1540-8183.2008.00421.x [DOI] [PubMed] [Google Scholar]
- 14. Bhaya M Mutluer F Mahan E Mahan L Hsiung MC Yin WH Wei J Tsai SK Zhao GY Yin WH Live/Real time three-dimensional transesophageal echocardiography in percutaneous closure of atrial septal defects Echocardiography 30 2013. 345–353. 10.1111/echo.12106 [DOI] [PubMed] [Google Scholar]
- 15. Santini F Morjan M Onorati F Morando G Faggian G Mazzucco A Life-threatening isometric-exertion related cardiac perforation 5 years after Amplatzer septal defect closure: should isometric activity be limited in septal occluder holders? Annals of Thoracic Surgery. 2012;93:671. doi: 10.1016/j.athoracsur.2011.07.068. [DOI] [PubMed] [Google Scholar]
- 16. El-Said HG Moore JW Erosion by the Amplatzer septal occluder: experienced operator opinions at odds with manufacturer recommendations? Catheterization and Cardiovascular Interventions 73 2009. 925–930. 10.1002/ccd.21931 [DOI] [PubMed] [Google Scholar]
- 17. Wagdi P Closure of intra-atrial septal communications: Adverse events and lessons learned Cardiology Research 2 2011. 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hernández Pérez FJ Fernández Díaz JA García Montero C García Touchard A Oteo Domínguez JF Domínguez Puente JR Goicolea Ruigómez J Late aortic perforation with a fractured ATRIASEPT II device resulting in life-threatening tamponade. EuroIntervention. 2013;9:532. doi: 10.4244/EIJV9I4A86. [DOI] [PubMed] [Google Scholar]
- 19. Happel CM Laser KT Sigler M Kececioglu D Sandica E Haas NA Single center experience: implantation failures, early, and late complications after implantation of a partially biodegradable ASD/PFO-device (BioStar®) Catheterization and Cardiovascular Interventions 85 2015. 990–997. 10.1002/ccd.25783 [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a