Abstract
The aims of this study were i) to evaluate mitral and aortic annuli excursion, and aortomitral angle (AMA) during the cardiac cycle in healthy adults using two-dimensional speckle tracking echocardiography, ii) to assess two annuli dynamics and coupling behaviors as an integral, and iii) to detect the relation between two annuli and left ventricular ejection fraction (LVEF). A total of 74 healthy adults underwent transthoracic echocardiography. In the parasternal long-axis view, a number of points were extracted, including right coronary aortic annular, aortomitral fibrous junction, and posterior mitral annular points. The annuli excursion and AMA were measured using a speckle tracking-derived software during the cardiac cycle. During the isovolumic contraction and the isovolumic relaxation phase, annuli excursion and AMA remain stable for a short time. During the systole, annuli excursion increased sharply to the maximum, while AMA narrowed quickly to the minimum value. During the diastole, there are three patterns of decrease in annuli excursion and AMA expansion in different phases. The annuli excursion of three points correlates well with the LVEF (right coronary aortic annulus excursion, r=0.71, P<0.05; non-coronary aortic annulus excursion, r=0.70, P<0.05; posterior mitral annulus excursion, r=0.82, P<0.05). Moreover, there are positive correlations between annuli excursion and the variation of AMA (r=0.60, P<0.05). The annuli excursion and AMA have various regular patterns in healthy adults. The interactions of mitral and aortic annuli correlate with the left ventricular function. Our findings may have relevance to the evaluation of left ventricular function and presurgical planning of patients with valvular diseases.
Keywords: 2D speckle-tracking echocardiography, left ventricular ejection fraction, annulus excursion, aortomitral angle
Introduction
The aortic annulus together with the mitral annulus form the base of left ventricle and they are coupled via aortomitral fibrous tissue. Their physiological function is integral in maintaining normal cardiac performance (1). When the heart muscle contracts, displacement of the two separate valvular units should not be heterogenous (2). The aortomitral angle (AMA) between two annuli planes also changes dynamically during the cardiac cycle (3). However, in previous studies, the aortic and mitral annuli were studied in humans separately as if their functions are independent of each other.
In this study, efforts were made to evaluate the dynamics and interactions of mitral and aortic annuli in humans in a new way. Two-dimensional (2D) speckle tracking echocardiography (STE) can track tissue motion in any direction in a 2D imaging plane. The STE-derived annulus excursion quantification software has been used to assess mitral annulus displacement and its feasibility has been confirmed (4, 5). However, only few studies have examined aortic annulus displacement (6) or AMA using STE. Accordingly, the aims of this study were i) to evaluate mitral and aortic annuli excursion, and AMA during the cardiac cycle using 2D STE technology, ii) to assess two annuli dynamics and coupling behaviors as an integral, and iii) to detect the relation between two annuli and left ventricular ejection fraction (LVEF).
Methods
Study population
A total of 74 people who underwent a health check in our hospital from October 2010 to July 2011 were selected for this study. All of them were referred for the inspection of electrocardiogram, blood pressure, blood glucose, and physical examination to exclude cardiovascular-related diseases. Meanwhile, routine echocardiography was performed to exclude patients who had poor image quality or abnormal cardiac structure or dysfunction (Table 1). Approval to conduct the study was obtained from the institutional review board at the authors' hospital.
Table 1.
Clinical characteristics of study subjects
| Parameter | |
|---|---|
| Age (years) | 37.8±8.5 |
| BSA (m2) | 1.6±0.2 |
| BMI (kg/m2) | 22.7±8.8 |
| Heart rate (beats/min) | 75.4±10.6 |
| SBP (mmHg) | 112±19 |
| DBP (mmHg) | 81±12 |
| LVEDD/BSA (mm/m2) | 27.10±1.99 |
| LAD/BSA (mm/m2) | 17.79±1.30 |
| LVEF (%) | 66.3±6.5 |
BSA, body surface area; SBP, systolic blood pressure; DBP, diastolic blood pressure; LVEDD, left ventricle end-diastolic dimension; LAD, left atrium dimension; LVEF, left ventricular ejection fraction.
Echocardiography
All subjects underwent 2D transthoracic echocardiography (iE33 echocardiography system; Philips Medical Systems, Best, The Netherlands) examinations conducted by a single, experienced echocardiographer. To optimize speckle tracking, 2D grayscale harmonic images were obtained at a frame rate of 60 to 80/s. Standard parasternal long-axis and apical chamber views were obtained using a synchronous electrocardiogram in all cases. In each imaging plane, four end-expiratory cardiac cycles were acquired. From the parasternal long-axis view, the end-diastolic dimension of the left ventricle was measured from the left side of the interventricular septum to the left ventricular posterior wall, and the end-systolic dimension of the left atrium was measured from the left atrial anterior wall to the left atrial posterior wall. In apical two- or four-chamber views, the LVEF was assessed according to the modified biplane Simpson's rule (7). Characteristics of subjects and routine echocardiographic data are listed in Table 1.
Annulus excursion and AMA measurement
In the parasternal long-axis view (Fig. 1), aortic and mitral annuli composed the base of the LV, and they were connected to each other via fibrous tissue, which is also called the aortomitral curtain (8). The aortic annulus plane was defined as the plane incorporating both the fibrous junction and the lowest point of the right aortic annulus, and the mitral annulus plane was defined as the plane that included the fibrous junction and the lowest point of the posterior mitral annulus. Annulus excursion was defined as the annular motion toward the LV apex, while AMA referred to the angle between aortic and mitral annuli planes.
Figure 1.
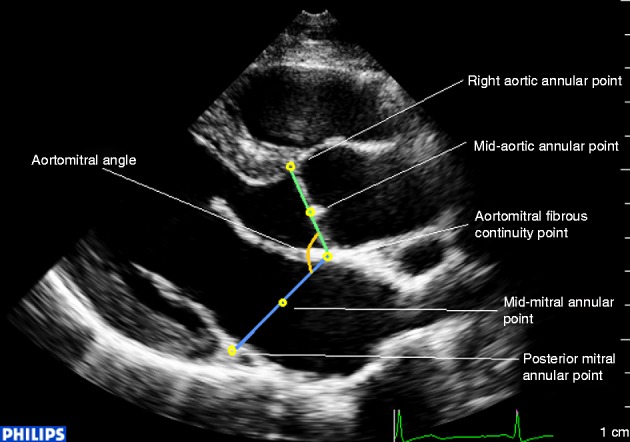
Definition of mitral annulus, aortic annulus, and the angle between them in the parasternal long-axis view.
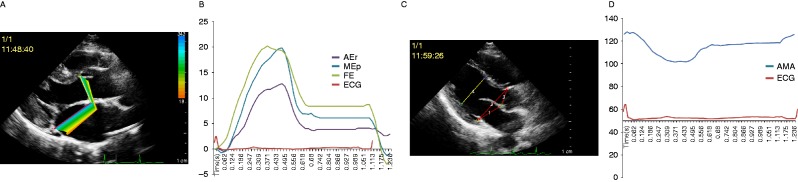
2D echocardiography native data were transferred into QLAB workstation (Philips) for offline analysis. To assess mitral annulus excursion, in the end-diastolic frame, two tracking points were marked manually on the posterior mitral annulus and aortomitral fibrous continuity, and one anchor point was marked outside the imaging plane which was approximately at the same distance from the two tracking points. For the aortic annulus excursion, two points were marked manually on the right coronary aortic annular point and aortomitral fibrous continuity point. The points were tracked frame by frame by synchronous color kinesis imaging using the QLAB software (Fig. 2A). For measuring AMA, three points, including right coronary aortic annular corners, aortomitral curtain, and posterior mitral annular corners, were marked manually, and the software was used to track the distance variation between different points, and the AMA was computed manually (Fig. 2C). The mean value for three heartbeats was calculated. Subsequently, annulus excursion and AMA were plotted as curves varying with time (Fig. 2B and D). Some values were selected for comparison as follows: the maximum right aortic annular point excursion (AEr), the maximum post-mitral annular point (MEp), the maximum fibrous excursion (FE), maximum AMA (AMA(max)), minimum AMA (AMA(min)), and the difference between AMA(max) and AMA(min) (AMA(d)).
Figure 2.
(A) Color kinesis-mode display of annular excursion. (B) The dynamic changes in annulus excursion of three points during three cycles. (C) Example of AMA. (D) The dynamic changes in AMA during three cycles.
We also attempted to quantify the above parameters in an apical three-chamber view, however, we had to give up as the image quality in this plane is not so good as that for the parasternal long-axis view.
To evaluate the intra- and inter-observer variability, measurements were repeated in a subset of 20 randomly selected patients, by the same observer together with an additional observer, who were blinded to all prior measurements.
Statistical analyses
Measurements are expressed as mean±s.d. Continuous variables were compared using one-way ANOVA. The relationship among LVEF, annulus excursion, and AMA was studied using correlation analysis (Pearson's correlation). Bland–Altman analysis was performed to determine the bias and limits of agreement between intra- and inter-observer measurements. All statistical analyses were performed using the SPSS software (SPSS, version 16.0, IBM). A value of P<0.05 was considered as statistically significant.
Results
Quantification of annulus excursion and AMA using 2D STE was achieved in all subjects within 20 s. The measurements are listed in Table 2.
Table 2.
Measurements of annuli excursions and aortomitral angles
| Annuli excursion | |
| AEr (mm) | 13.6±2.3 |
| MEp (mm) | 14.7±2.4 |
| FE (mm) | 11.8±1.8 |
| Aortomitral angle | |
| AMA(max) (°) | 131±7 |
| AMA(min) (°) | 112±7 |
| AMA(d) (°) | 19±4 |
AEr, maximum right aortic annular point excursion; MEp, maximum post-mitral annular point; FE, maximum fibrous excursion; AE(mid-A), AE of mid-aortic annular point; AE(mid-M), AE of mid-mitral annular point; AMA(max), maximum AMA; AMA(min), minimum AMA; AMA(d), the difference between AMA(max) and AMA(min).
Dynamic movement of annulus excursion and AMA
The different phases of cardiac cycle were identified by valve movement and a synchronous electrocardiogram. AEr, MEp, FE, and AMA circular variation are illustrated in Fig. 2B and D. During the isovolumic contraction, all annulus excursion parameters and AMA remain stable for a short time. During the systole, annulus excursion increased sharply to the maximum, while AMA narrowed quickly to the minimum. During the isovolumic relaxation, there is another relative stable stage. During the early diastole, annulus excursion decreased and AMA expanded quickly, followed by a slow variation during the mid-diastole, and, at the late diastole, they accelerated suddenly and changed until reaching the initial level.
Comparison of excursion in different annular portions
To evaluate the heterogenous movement of annulus, maximum excursions of three annular points were compared. MEp was bigger than AEr (P=0.003), whereas AEr was bigger than FE (P<0.001).
Correlation analysis
There are good correlations among MEp, AEr, FE, and LVEF (r=0.82, P<0.05, r=0.71, P<0.05, and r=0.70, P<0.05 respectively). The correlation between AMA(d) and AEr is r=0.60, P<0.05. There is no significant correlation between AMA(d) and LVEF.
Intra- and inter-observer variability
The reproducibility tests of annulus excursion and AMA(d) showed no trends and no difference in the variance of intra- and inter-observer variability (Table 3).
Table 3.
Reproducibility of annulus excursion and AMA(d) by two-dimensional speckle tracking echocardiography. Values are intraclass correlation coefficients (ICC). ICC was considered as poor when ICC<0.4, fair when ICC=0.40–0.59, good when ICC=0.60–0.74, and excellent when ICC≥0.75. Numbers in parentheses are 95% CIs
| Intra-observer | Inter-observer | |
|---|---|---|
| AEr (mm) | 0.91 (0.84–0.96) | 0.69 (0.23–0.88) |
| MEp (mm) | 0.86 (0.65–0.94) | 0.75 (0.49–0.89) |
| FE (mm) | 0.97 (0.93–0.99) | 0.94 (0.86–0.98) |
| AMA(d) (°) | 0.85 (0.60–0.97) | 0.75 (0.38–0.90) |
AEr, maximum right aortic annular point excursion; MEp, maximum post-mitral annular point; FE, maximum fibrous excursion; AMA(d), difference between maximum and minimum aortomitral angles.
Discussion
The major findings of this study were as follows: i) STE can be used to evaluate annuli movement and AMA. ii) The aortic and mitral annuli vary consistently during the cardiac phases. The variation in AMA reflects the heterogenous movement of mitral and aortic annuli. iii) Annulus excursion is correlated with LVEF and AMA.
Quantification of annulus excursion and AMA using STE
To our knowledge, this is the first study to characterize the left-side annulus dynamics of human heart using STE technology. STE can track tissue motion in echocardiographic imaging loops without any directional limitation, it is a rapid and reproducible method for determining annulus excursion and AMA variation, and it provides a high temporal resolution to ensure the quantification accuracy.
Dynamics of annulus excursion
During the cardiac cycle, the mitral annulus is pulled toward the LV apex in the systole and reversed from the apex in the diastole. This reflects that myocardial contractility is a very efficient mechanism to enhance LV filling and emptying (9, 10). Our result shows that aortic annulus moves toward the LV apex together with mitral annulus and, therefore, we suggest the aortic annular movement should be considered as a part of the LVEF component as well as mitral annulus. It is noteworthy that our results showed the different aortic annulus phases of movement as reported previously in a study by Nakai et al., which illustrated that the aortic annulus moves cranially during the early systole and moves caudally during the remainder of the systole and isovolumic relaxation, and then moves cranially again during the diastole in aortic stenosis patients (6). The discrepancy may be mainly caused by subjects having different disorders.
Furthermore, our data confirmed that the posterior mitral annulus excursion is bigger than that of the anterior aortic annulus, whereas the aortomitral continuity excursion is the smallest. This phenomenon is also corroborated in animal experiments (2). It is possible owing to the component of the aortomitral continuity being the fibrous tissue, the right aortic annulus and the posterior mitral annulus are merged with muscular and fibrous tissues, and the posterior mitral annulus is more free to move with less peripheral restriction (8).
Dynamics of AMA variation
AMA follows a consistent pattern of narrowing and widening with the smallest angle at the end of the systole and the widest angle at the end of diastole (9, 10, 11, 12). The change in the AMA reflects the heterogeneous nature of left cardiac annular movement. Our findings are inconsistent with the data, reported by Sughimoto et al., according to which the AMA changed from 147.1±16.0 to 117.8±8.47 in healthy adults, both being bigger than our measurement. However, the above-mentioned study included only seven subjects and the images were obtained by three-dimensional transesophageal echocardiography with a low frame rate (11). The report by Timek et al. demonstrated that the AMA of sheep changed by 8°±2° during the cardiac cycle, which was far smaller than our result (9). The difference is possibly due to subjects belonging to different species.
Some studies have demonstrated the correlation between AMA and hemodynamics, the narrowing of the AMA brings the left ventricular base closer to the left ventricular outflow tract, narrower AMA can result in systolic anterior motion of the valve leaflets and chordae tendineae (13, 14, 15).
A common shared dynamic physiology of aortic and mitral annuli
Our findings provide evidence that the aortic and mitral valves have synchronous dynamic physiology that is very important for maintaining normal hemodynamics and LV function. It is a well-known fact that the aortic and the mitral annuli belong to the cardiac skeleton that they have a role in the attachment of the myocardial band and for the attachment of the aortic and mitral valves (16, 17). Consequently, if even a small region of myocardium became impaired, the effect will affect annulus excursion and AMA. In agreement with results from previous studies, our results underline the importance of considering the aortic–mitral complex as a single structure (10, 18). Additional knowledge of the left-side valvular structure should contribute toward the development of better approaches for valve apparatus repair or surgery (19, 20, 21).
Study limitations
There are some limitations of our study. First, both mitral valve and aortic valve annuli have complicated three-dimensional shapes, and 2D echocardiography may be limited in its ability to estimate the AMA accurately in three-dimensional space, though it has some advantages of convenience and higher temporal resolution. Secondly, the two annuli are composed of different tissues in different portions and hence we were unable to accurately reflect the complex movement of each part.
Conclusions
2D STE quantification of annulus excursion and AMA is clinically feasible. Both mitral and aortic annulus excursions are correlated with LVEF. Our results underline the importance of considering the aortic–mitral structure as a unique morphological and functional entity, evaluation of which should be taken into consideration during the treatment for left-side heart valvular disease. The methodology described in this article may become an important tool for presurgical planning for patients with valvular disease.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this study.
Funding
This study was supported by the grant from the Science and Technology Pillar Program of Sichuan Province (grant number 2012SZ0028 and 2014SZ0004).
References
- 1. Holen J Nitter-Hauge S Evaluation of obstructive characteristics of mitral disk valve implants with ultrasound Doppler techniques Acta Medica Scandinavica 201 1977. 429–434. 10.1111/j.0954-6820.1977.tb15725.x [DOI] [PubMed] [Google Scholar]
- 2. Goetz WA Lansac E Lim HS Stevens SA Weber PA Duran CMG Kinking of the atrioventricular plane during the cardiac cycle Asian Cardiovascular & Thoracic Annals 14 2006. 394–398. 10.1177/021849230601400509 [DOI] [PubMed] [Google Scholar]
- 3. Flachskampf FA Chandra S Gadipatti A Levine RA Weyman AE Ameling W Hanrath P Thomas JD Analysis of shape and motion of the mitral annulus in subjects with and without cardiomyopathy by echocardiographic 3-dimensional reconstruction Journal of the American Society of Echocardiography 13 2000. 277–287. 10.1067/mje.2000.103878 [DOI] [PubMed] [Google Scholar]
- 4. DeCara JM Toledo E Salgo IS Lammertin G Weinert L Lang RM Evaluation of left ventricular systolic function using automated angle-independent motion tracking of mitral annular displacement Journal of the American Society of Echocardiography 18 2005. 1266–1269. 10.1016/j.echo.2005.07.018 [DOI] [PubMed] [Google Scholar]
- 5. Tsang W Ahmad H Patel AR Sugeng L Salgo IS Weinert L Mor-Avi V Lang RM Rapid estimation of left ventricular function using echocardiographic speckle-tracking of mitral annular displacement Journal of the American Society of Echocardiography 23 2010. 511–515. 10.1016/j.echo.2010.03.003 [DOI] [PubMed] [Google Scholar]
- 6. Nakai H Takeuchi M Yoshitani H Kaku K Haruki N Otsuji Y Pitfalls of anatomical aortic valve area measurements using two-dimensional transesophageal echocardiography and the potential of three-dimensional transesophageal echocardiography European Journal of Echocardiography 111 2010. 369–376. 10.1093/ejechocard/jep220 [DOI] [PubMed] [Google Scholar]
- 7. Lang RM Bierig M Devereux RB Flachskampf FA Foster E Pellikka PA Picard MH Roman MJ Seward J Shanewise JS et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Journal of the American Society of Echocardiography 18 2005. 1440–1463. 10.1016/j.echo.2005.10.005 [DOI] [PubMed] [Google Scholar]
- 8. Silbiger JJ Bazaz R Contemporary insights into the functional anatomy of the mitral valve American Heart Journal 158 2009. 887–895. 10.1016/j.ahj.2009.10.014 [DOI] [PubMed] [Google Scholar]
- 9. Timek TA Green GR Tibayan FA Lai DT Rodriguez F Liang D Daughters GT Ingels NB Jr Miller DC Aortomitral annular dynamics Annals of Thoracic Surgery 76 2003. 1944–1950. 10.1016/S0003-4975(03)01078-6 [DOI] [PubMed] [Google Scholar]
- 10. Veronesi F Caiani EG Sugeng L Fusini L Tamborini G Alamanni F Pepi M Lang RM Effect of mitral valve repair on mitral-aortic coupling: a real-time three-dimensional transesophageal echocardiography study Journal of the American Society of Echocardiography 25 2012. 524–531. 10.1016/j.echo.2012.02.002 [DOI] [PubMed] [Google Scholar]
- 11. Sughimoto K Takahara Y Mogi K Liu H Yamazaki K Annular excursion contributes to efficient cardiac output: a three-dimensional echocardiographic approach Journal of Heart Valve Disease 19 2010. 244–248. [PubMed] [Google Scholar]
- 12. Carlsson M Ugander M Mosén H Buhre T Arheden H Atrioventricular plane displacement is the major contributor to left ventrasicular pumping in healthy adults, athletes, and patients with dilated cardiomyopathy American Journal of Physiology. Heart and Circulatory Physiology 292 2007. H1452–H1459. 10.1152/ajpheart.01148.2006 [DOI] [PubMed] [Google Scholar]
- 13. Radermecker M Canivet JL Lancellotti P Limet R The usual causes of left ventricular outflow tract obstruction below the aortic valve in normal ventriculoarterial connection: review of the physiopathology and surgical implications Acta Chirurgica Belgica 105 2005. 475–481. [DOI] [PubMed] [Google Scholar]
- 14. Omoto R Matsumura M Asano H Kyo S Takamoto S Yokote Y Wong M Doppler ultrasound examination of prosthetic function and ventricular blood flow after mitral valve replacement Herz 11 1986. 346–350. [PubMed] [Google Scholar]
- 15. Mihaileanu S Marino JP Chauvaud S Perier P Forman J Vissoat J Julien J Dreyfus G Abastado P Carpentier A Left ventricular outflow obstruction after mitral valve repair (Carpentier's Technique): proposed mechanisms of disease Circulation 78: Suppl I 1988. I78–84. [PubMed] [Google Scholar]
- 16.Gray, Henry 1918. The Heart. Gray's Anatomy 20th edition ch V, 4b, pp 526–539. Ed WH Lewis. London: Longmans. ISBN 613-0-24743-5.
- 17. Torrent-Guasp F Buckberg GD Clemente C Cox JL Coghlan HC Gharib M The structure and function of the helical heart and its buttress wrapping. I. The normal macroscopic structure of the heart Seminars in Thoracic and Cardiovascular Surgery 13 2001. 301–319. [DOI] [PubMed] [Google Scholar]
- 18. Bardia A Subramaniam B Systolic anterior motion: an illustrative case and discussion of management strategy Journal of Perioperative Echocardiography 1 2013. 62–65. 10.5005/jp-journals-10034-1010 [DOI] [Google Scholar]
- 19. Ionasec RI Voigt I Georgescu B Wang Y Houle H Hornegger J Navab N Comaniciu D Personalized modeling and assessment of the aortic–mitral coupling from 4D TEE and CT Medical Image Computing and Computer-Assisted Intervention 12 2009. 767–775. [DOI] [PubMed] [Google Scholar]
- 20. Lansac E Lim KH Shomura Y Goetz WA Lim HS Rice NT Saber H Duran CM Dynamic balance of the aortomitral junction Journal of Thoracic and Cardiovascular Surgery 123 2002. 911–918. 10.1067/mtc.2002.121286 [DOI] [PubMed] [Google Scholar]
- 21. Jensen MO Jensen H Smerup M Levine RA Yoganathan M Nygaard H Hasenkam JM Nielsen SL Saddle-shaped mitral valve annuloplasty rings experience lower forces compared with flat rings Circulation 118: Suppl 14 2008. S250–S255. 10.1161/CIRCULATIONAHA.107.746776 [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a