Abstract
We evaluated the feasibility and costs of utilising hand-held cardiac ultrasound (HHCU) as part of a community-based pre-participation cardiovascular screening programme. Ninety-seven school children were screened using a personal history, a physical examination, a resting 12-lead electrocardiogram (ECG) and a HHCU. A consultant cardiologist independently reviewed and reported the data. Previously undiagnosed cardiovascular abnormalities were identified in nine participants (9%). An additional three participants (3%) were diagnosed with hypertension. The nine abnormalities were identified at a cost of £460 per finding, with a cost of £43 per participant screened. The marginal cost of adding a HHCU to the personal history, physical examination and ECG was £16 per participant. Pre-participation screening in the community using hand-held echocardiography is practical and inexpensive. The additional sensitivity and specificity provided by the ultrasound may enhance screening programmes, thereby reducing false positives and the need for expensive follow-up testing.
Keywords: screening, echocardiography, athletes, community
Introduction
Despite the known beneficial effects of physical exercise on cardiovascular health, young adults who participate in vigorous exercise are at increased risk of sudden cardiac death (SCD). In addition, the principal causes of SCD usually do not present symptomatically until a fatal episode. Although these events are catastrophic and potentially avoidable, there is still controversy over pre-participation cardiac screening (PPCS) strategies in relation to their efficacy, their cost-effectiveness and the psychosocial effects of false positives. The results of comprehensive research have indicated that PPCS can reduce rates of SCD in young athletes. Despite this, there is no universally adopted screening protocol. The American Heart Association (AHA) currently recommends screening competitive athletes with a targeted personal history, family history and physical examination (1). The European Society of Cardiology (ESC) recommends supplementing this protocol with a 12-lead electrocardiogram (ECG) (2). Although echocardiography can improve the specificity of cardiac screening, the implementation of echocardiography has been limited by cost and accessibility.
The evolution of hand-held cardiac ultrasound (HHCU) devices is increasingly challenging these issues. The American Society of Echocardiography has recognised HHCU as an accurate and reproducible tool for the assessment of cardiac structure and function (3). Moreover, the capability to wirelessly transmit data allows some HHCU devices to be used in both remote areas and in the community. In the present pilot study, we sought to determine the feasibility of using highly portable HHCU devices as part of community cardiac screening in young adults.
Methods
The present prospective pilot study used a cross-sectional design to quantify the relative total cost of HHCU in cardiac screening. Ethical clearance was obtained by the local ethics committee, and written consent was obtained for all participants. Each participant received standardised screening using a focused medical history, a physical examination, a 12-lead ECG and an HHCU device. Clinical practitioners performed the history and physical examination. Cardiac physiologists, who were blinded to the history and physical examination, performed the 12-lead ECG and HHCU scan. The data were reviewed by a blinded consultant cardiologist who determined the requirements for follow-up testing.
Outcome measures
The primary outcome measure was total cost per participant of PPCS screening using a focused medical history, a physical examination, a 12-lead ECG and an HHCU device. The secondary outcome measure was the identification of any cardiac abnormality that required follow-up before medical clearance in sports participation would be granted.
Subjects
One hundred and twenty-three male participants, aged 6–18 years, were enrolled using convenience sampling at a local secondary school. All participants were defined as athletes based on their active involvement in competitive sports at the time of sampling. The sole exclusion criteria was pre-existing cardiovascular disease.
Data collection
Data were collected over 2 consecutive months. Cost figures were calculated using information obtained from the States of Jersey Health and Social Security Department's salary structure data. Primary screening costs included indirect time and travel costs. The cost at follow-up was determined by subsequent secondary care contact and investigations at local rates.
PPCS history
Each participant completed a standardised screening questionnaire as recommended by the AHA consensus panel (1). This questionnaire evaluated both personal and family cardiovascular medical history and was evaluated by a clinical practitioner. If one or more questions were answered ‘yes’, this was considered a positive history screen.
PPCS examination
Each participant completed a standardised screening physical examination as recommended by the AHA consensus panel (1). The examination included: blood pressure (BP) measurement, auscultation of the heart, palpation of the femoral and radial pulses and inspection for characteristic features of Marfan syndrome. BP was considered elevated if it was in excess of the 90th percentile for sex, age and height as defined by the National Heart, Lung, and Blood Institute (4).
12-lead ECG
A cardiac physiologist conducted an ECG at rest on each participant using a GE MAC 5000 HD machine (GE Healthcare, Chalfont Saint Giles, Buckinghamshire, UK) with standard 12-lead placement. Each ECG was analysed immediately by the performing physiologist and later by a blinded cardiology consultant from a remote location. The 2010 European Society of Cardiology Consensus Panel on ECG interpretation in athletes (5) was employed to analyse each ECG recording. An abnormal ECG finding was then considered to be a positive ECG screen.
Hand-held cardiac ultrasound
The final part of the PPCS was HHCU evaluation using a VScan (GE, Horten, Norway) device. Weighing only 390 g and connected to a broad-bandwidth, phased array probe (1.7–3.8 MHz, 120×33×26 mm), the device provides real-time black-and-white 2D and colour Doppler blood flow images. Distance and area measurements were calculated using a touchpad and electronic caliper. Depth and gain were adjusted to optimize image quality. Data were stored on the internal micro-SD card and then transferred to a PC through a USB docking station. Parasternal and apical views were obtained with the patient in a left lateral, recumbent position by qualified cardiac physiologists. The severity of valvular abnormalities (stenotic or regurgitant) were assessed using 2D findings (hyperdynamic left ventricle (LV) or chamber enlargement) and visual, qualitative colour Doppler findings (jet area). The measurements obtained from the image protocol followed British Society of Echocardiography guidelines: inter-ventricular septal thickness during diastole, left ventricular diameter during diastole, left ventricular internal diameter during systole, left ventricular posterior free wall during diastole, left atrial internal diameter and aortic root at the annulus (6). The cardiac physiologist who performed the echocardiogram was asked to report his findings immediately after each scan. Data were then saved and later reviewed by a consultant cardiologist from a remote location. The report of each participant's ultrasound was categorised as: i) normal; ii) having abnormalities consistent with benign physiologic cardiac remodelling common in trained athletes (7); or iii) having diagnostic cardiac abnormalities relevant to increased sports participation risk (including indeterminate cardiac morphology requiring further diagnostic evaluation). All of the participants with abnormalities relevant to increased sports participation risk were referred for further diagnostic testing at follow-up.
Statistical analysis
Data were analysed using Microsoft Excel Software. The estimated total costs of each screening modality were calculated.
Results
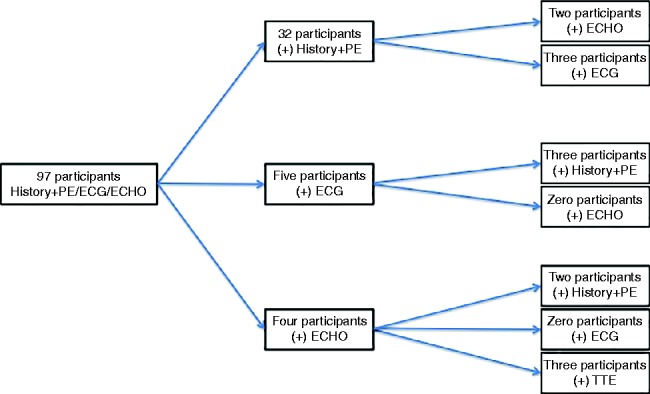
One hundred and twenty-three male participants were enrolled, of which 97 (78%) attended for screening over a 2-month period. Each mode of screening lasted 5±1 min per participant. Review of the data by the consultant cardiologist took 10±2 min per participant. The results are summarized in Table 1. The correlation of the abnormalities to one another is illustrated in Fig. 1.
Table 1.
Primary screening: abnormal findings and final diagnosis.
| Participant | HHE | Medical history and physical examination | ECG | Final diagnosis |
|---|---|---|---|---|
| 1 | N | ↑BP | N | ↑BP |
| 2 | N | ↑BP | N | ↑BP |
| 3 | N | ↑BP | N | ↑BP |
| 4 | N | N | ↑QT | IVR RBBB |
| 5 | N | D CP | ↑QT | N |
| 6 | N | N | TWI V1–V3 | N |
| 7 | N | FH | Partial RBBB | Partial RBBB |
| 8 | N | D Irreg.HB | Bradycardia | Bradycardia |
| 9 | TR and RVD | D | N | TR |
| 10 | AR | N | N | AR |
| 11 | LVD | N | N | N |
| 12 | SD | CP | N | SD |
| 13 | N | D CP | N | N |
| 14 | N | D CP Irreg.HB | N | N |
| 15 | N | D CP | N | N |
| 16 | N | D CP | N | N |
| 17 | N | D CP | N | N |
| 18 | N | D | N | N |
| 19 | N | D | N | N |
| 20 | N | D | N | N |
| 21 | N | D | N | N |
| 22 | N | D CP | N | N |
| 23 | N | CP | N | N |
| 24 | N | D | N | N |
| 25 | N | D | N | N |
| 26 | N | CP Irreg.HB | N | N |
| 27 | N | D Irreg.HB | N | N |
| 28 | N | FH D | N | N |
| 29 | N | CP | N | N |
| 30 | N | CP Irreg.HB | N | N |
| 31 | N | D | N | N |
| 32 | N | CP | N | N |
| 33 | N | CP | N | N |
| 34 | INAD V | D CP | N | N |
| 35 | N | D | N | N |
| 36 | N | D CP Irreg.HB | N | N |
↑BP, blood pressure exceeding the 90th percentile; MR, mitral regurgitation; AR, aortic regurgitation; RV, right ventricle; LV, left ventricle; RBBB, right bundle branch block; HHE, hand-held echocardiogram; RVD, right ventricular dilatation; LVD, left ventricular dilatation; TWI V1–V3, T-wave inversion in leads V1 to V3; FH, family history of cardiac problem at less than 50 years; D, dizziness during or after exercise; Irreg.HB, irregular heartbeat/skipped beats/palpitations; CP, chest pain during exercise; TR, tricuspid regurgitation; N, normal; INAD V, inadequate views; SD, septal dyskinesis; IVD, idioventricular rhythm.
Figure 1.
Correlation of screening findings.
Medical history
A positive medical history was identified in 29 participants (30%) (dizziness, chest pain, and family history). Of these, 3 (10%) had isolated ECG abnormalities (prolonged QT, partial right bundle branch block (RBBB) and sinus bradycardia) and two (7%) had isolated HHCU findings (septal dyskinesis and tricuspid regurgitation with right ventricular dilatation (RVD)). Inadequate HHCU views were obtained in one participant (3%) with a positive medical history. Formal transthoracic echocardiography later ruled out structural abnormalities.
Physical examination
A positive physical examination was identified in nine participants (9%). Three were found to have raised BP that was in excess of the 90th percentile, and six were found to have an irregular heart rate. Only one of the nine positive physical examinations correlated with further screening abnormalities (sinus bradycardia on 12-lead ECG).
Electrocardiogram
A total of five participants (5%) were found to have positive ECG findings (prolonged QT interval (n=2), T-wave inversion, partial RBBB, and sinus bradycardia). Of these, 3 (60%) corresponded to a positive medical history (chest pain, dizziness, and family history). None of the 5 (0%) was found to have abnormalities upon physical examination or HHCU scanning. Further diagnostic testing at cardiology follow-up ruled out cardiovascular abnormalities in two of the five participants (prolonged QT and T-wave inversion V1–V3).
Hand-held cardiac ultrasound
Inadequate views were obtained for one participant (1%) using HHCU. Further diagnostic testing at follow-up revealed no cardiovascular abnormalities in that participant. Overall, four participants (4%) were found to have HHCU abnormalities (tricuspid regurgitation with RVD, aortic regurgitation, left ventricular dilatation (LVD), and septal dyskinesis). Two abnormalities (tricuspid regurgitation with RVD and septal dyskinesis) corresponded to an abnormal medical history (dizziness and chest pain respectively). None of the four participants with ultrasound abnormalities had a positive physical examination or ECG result.
Final diagnosis following secondary screening
Primary screening identified 12 patients with abnormalities that warranted further diagnostic testing. No additional cardiac abnormalities were identified at follow-up. The participant with left ventricular dysfunction as assessed by HHCU was later found to have normal left ventricular dimensions using formal departmental echocardiography. Further diagnostic testing ruled out significant abnormalities in two participants who were referred with ECG abnormalities (prolonged QT and T-wave inversion V1–V3). This gave a total number of nine true significant abnormalities (hypertension (n=3), bundle branch block pattern (n=2), sinus bradycardia, aortic regurgitation, tricuspid regurgitation, and septal dyskinesis).
Costs
Primary screening took 15±5 min per participant at a cost of £7.50 per 10 min. Cardiologist review of the primary data set took 10±5 min at a cost of £15 per 10 min. The overall cost of the primary screening programme was £4146, or £42.75 per participant. The marginal cost of adding HHCU to routine screening was £1596, or £16.45 per participant. The GE Medical VScan HHCU device and software was purchased by the cardiology department for £1000. The primary screening programme identified 12 abnormalities that required follow-up at a cost of £345 per finding. Cardiology follow-up cost an average £245 for each referred participant. The cost of finding a true abnormality following specialist referral was calculated as £460.
Discussion
We determined that HHCU scanning for community cardiac screening was feasible and cost-effective. The cost of primary screening was calculated at £43 per participant. The marginal cost of incorporating HHCU into the primary screening programme was £16.45 per participant. Primary screening identified abnormalities in 12 participants (12%) who required further diagnostic testing at follow-up before medical clearance for sports participation could be granted. Follow-up ruled out abnormalities in three of the participants, which left 9 (9%) with abnormalities, including valvular heart disease, hypertension, bundle branch block and sinus bradycardia. These abnormalities came at a cost of £460 per finding.
The screening modality with the highest frequency of false positives was ECG. This highlights the poor specificity of ECG in PPCS and supports the findings of previous authors (8, 9, 10). False-positive ECG findings trigger a cascade of further diagnostic investigations that are expensive and can cause emotional distress and sometimes unnecessary disqualification from sports. HHCU screening identified only one false-positive finding (LVD) which required temporary cessation from competitive sports. We were unable to accurately assess the sensitivity and specificity of the HHCU device in the present study, given that only four participants were screened against a ‘gold-standard’ departmental echocardiogram. The ESC currently recognises the 12-lead ECG as a cheap and widely available screening tool for detecting underlying cardiac abnormalities (2). However, the ECG changes that are associated with structural and electrical remodelling through physical conditioning often overlap with those changes identified in hypertrophic cardiomyopathy, which accounts for the leading cause of SCD in the targeted screening population. HHCU can aid clinicians in differentiating between benign physiological and pathological 12-lead ECG changes. The structural cardiovascular abnormalities identified in the present study, which may predispose athletes to later participation risk, would not have been identified if participants had been screened using only the existing protocols of both the AHA and ESC (1, 2).
Previous evaluation of the diagnostic power of HHCU in the hospital setting has been encouraging. HHCU in conjunction with a physical examination has been shown to improve the detection of cardiac abnormalities by as much as 31.5% (11). Particularly relevant to the present study, enhanced sensitivity of HHCU scanning has been previously reported when used by various healthcare professionals, including medical students, cardiac physiologists and cardiologists (12, 13). Despite this, results indicate that diagnostic accuracy is higher when HHCU is carried out by more experienced ultrasonographers (14, 15). Only more recently has the role of HHCU as a screening tool for subclinical cardiovascular abnormalities been evaluated. Singh et al. (16) examined 1023 subjects using HHCU in the largest community-screening study in rural India to date. Abnormalities were categorized as major (n=170) and minor (n=207). The investigators identified 11 subjects with asymmetrical left ventricular hypertrophy. Of these 11, HHCU identified five subjects (45%) with features consistent with LV outflow tract obstruction and systolic anterior motion of mitral valve leaflets (16). With hypertrophic cardiomyopathy being the leading cause of SCD in young athletes, the demonstrated capability of HHCU to correctly identify such structural heart lesions adds weight to its role in PPCS.
The principal aim of the present study was not to test the diagnostic accuracy of HHCU but rather to examine the feasibility of incorporating it into existing PPCS protocols in the community. The marginal cost of adding HHCU to the screening programme was £16 (US $27) per participant. Previous research has yielded figures varying from $7 to $400 (17, 18, 19, 20). The variability in the predicted costs reported is clearly affected by the cost of the equipment used, the time spent screening participants and the pricing structure for the staff involved.
We believe that the results of the present study indicate that it is feasible to screen athletes using HHCU as a primary screening tool in the community to enhance the early detection of cardiac abnormalities with potential haemodynamic and arrhythmic complications. Further studies with a greater screening sample are needed to validate the inclusion of HHCU in routine PPCS on a larger scale.
Limitations
One limitation of the present pilot study was the size of the sample screened. Given that only 97 athletes were screened, the heterogeneity of the sample was restricted, and thus we cannot generalise the findings to larger populations. Similarly, only male athletes were screened. Another limitation is the variability in pricing structures for healthcare delivery: the low costs of adding HHCU reflected the low cost of delivering healthcare locally. Despite this limitation, we believe that the pricing structure can be extrapolated to different countries, and respective pricing structures can be developed to evaluate financial feasibility locally. Unlike previous studies (21, 22, 23), we opted not to test the diagnostic performance of the HHCU device against departmental echocardiography. As a result, the misdiagnosis of subtle structural heart abnormalities in particular cannot be excluded. The results of these previous studies have, however, indicated that the HHCU device has a diagnostic power that is at least equivalent to departmental echocardiography when it is used to screen for major structural lesions, including hypertrophic cardiomyopathy (24). Currently, the image resolution of existing HHCU devices is suboptimal for tracking the course of coronary arteries. This is, of course, a significant limitation of the device, given that the second leading cause of SCD in young athletes is anomalous coronary arteries (1). A further limitation of the HHCU device is its failure to calculate outflow tract gradients in the absence of pulsed and continuous wave Doppler. The visual qualitative approach used in the present study to screen for specific HHCU abnormalities adds subjectivity to the interpretation of the gathered data. Despite this, results of previous research have indicated that there is a good correlation between abnormalities identified using the visual qualitative approach and those identified using formal echocardiographic studies (22, 24). Using the same HHCU model, Testuz et al. (24) evaluated the diagnostic accuracy of the visual assessment method by correlating the findings of HHCU and standard echocardiography performed on 104 patients in the acute hospital setting. They concluded that there was moderate to good correlation between the severity of valvular lesions identified by standard echocardiography and that identified by HHCU. Moreover, any differences in the findings (both stenotic and regurgitant) were not thought to be clinically significant.
Conclusion
PPCS using HHCU in the community is practical and inexpensive. The increased portability of HHCU allows cardiac imaging to become a first-line investigation, and it allows PPCS to be moved into the community or school. The additional sensitivity and specificity provided will enhance screening programmes and reduce the need for expensive follow-up testing. The advent of new HHCU devices has opened up a screening opportunity that had been previously closed on the grounds of feasibility. Concerns regarding the cost and availability of routine echocardiography screening are largely based on the assumption that it will be performed by sonographers in specialist centres. The high portability, ease of use, low capital equipment and staffing costs of the HHCU screening programme addresses these concerns. We believe that the addition of HHCU to routine PPCS programmes will reduce false-positive rates and, as a result, the number of unnecessary referrals to specialists. This is desirable for all those involved in the screening process, especially given the psychological and economic effects of false-positive findings.
Declaration of interest
The authors confirm that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
The work was partly funded by a donation from the Jersey Rugby Club.
References
- 1. Maron BJ Thompson PD Ackerman MJ Balady G Berger S Cohen D Dimeff R Douglas PS Glover DW Hutter AM Jr et al. Recommendations and considerations related to preparticipation screening for cardiovascular abnormalities in competitive athletes: 2007 update Circulation 115 2007. 1643–1655. 10.1161/CIRCULATIONAHA.107.181423 [DOI] [PubMed] [Google Scholar]
- 2. Corrado D Pelliccia A Bjørnstad HH Vanhees L Biffi A Borjesson M Panhuyzen-Goedkoop N Deligiannis A Solberg E Dugmore D et al. Cardiovascular pre-participation screening of young competitive athletes for prevention of sudden death: proposal for a common European protocol European Heart Journal 26 2005. 516–524. 10.1093/eurheartj/ehi108 [DOI] [PubMed] [Google Scholar]
- 3. Spencer KT Kimura BJ Korcarz CE Pellikka PA Rahko PS Siegel RJ Focused cardiac ultrasound: recommendations from the American Society of Echocardiography Journal of the American Society of Echocardiography 26 2013. 567–581. 10.1016/j.echo.2013.04.001 [DOI] [PubMed] [Google Scholar]
- 4. Kaltman JR Thompson PD Lantos J Berul CI Botkin J Cohen JT Cook NR Corrado D Drezner J Frick KD et al. Screening for sudden cardiac death in the young. report from the National, Lung, And Blood Institute Working Group Circulation 123 2011. 1911–1918. 10.1161/CIRCULATIONAHA.110.017228 [DOI] [PubMed] [Google Scholar]
- 5. Corrado D Pelliccia A Heidbuchel H Sharma S Link M Basso C Biffi A Buja G Delise P Gussac I et al. Recommendations for interpretation of 12-lead electrocardiogram in the athlete European Heart Journal 31 2010. 243–259. 10.1093/eurheartj/ehp473 [DOI] [PubMed] [Google Scholar]
- 6.Wharton G, Steeds R, Allen J, Brewerton H, Jones R, Kanagala P, Lloyd, G, Masani N, Mathew T, Oxborough D et al 2012 A standard transthoracic echocardiogram. British Society of Echocardiography Education Committee. http://www.bsecho.org/tte-minimum-dataset/ (27 February 2015, date last accessed). [DOI] [PMC free article] [PubMed]
- 7. Baggish AL Hutter AM Jr Wang F Yared K Weiner RB Kupperman E Picard MH Wood MJ et al. Cardiovascular screening in college athletes with and without electrocardiography: a cross-sectional study Annals of Internal Medicine 152 2010. 269–275. 10.7326/0003-4819-152-5-201003020-00004 [DOI] [PubMed] [Google Scholar]
- 8. Serra-Grima R Estorch M Carrio I Subirana M Berna L Prat T Marked ventricular repolarization abnormalities in highly trained athletes' electrocardiograms: clinical and prognostic implications Journal of the American College of Cardiology 36 2000. 1310–1316. 10.1016/S0735-1097(00)00853-6 [DOI] [PubMed] [Google Scholar]
- 9. Maron BJ Bonow RO Seshagiri TN Roberts WC Epstein SE Hypertrophic cardiomyopathy with ventricular septal hypertrophy localized to the apical region of the left ventricle (apical hypertrophic cardiomyopathy) American Journal of Cardiology 49 1982. 1838–1848. 10.1016/0002-9149(82)90200-4 [DOI] [PubMed] [Google Scholar]
- 10. Pelliccia A Maron BJ Culasso F Di Paolo FM Spataro A Biffi A Caselli G Piovano P Clinical significance of abnormal electrocardiographic patterns in trained athletes Circulation 102 2000. 278–284. 10.1161/01.CIR.102.3.278 [DOI] [PubMed] [Google Scholar]
- 11. Galderisi M Santoro A Versiero M Lomoriello VS Esposito R Raia R Farina F Schiattarella PL Bonito M Olibet M et al. Improved cardiovascular diagnostic accuracy by pocket size imaging device in non-cardiologic outpatients: the NaUSiCa (Naples Ultrasound Stethoscope in Cardiology) study. Journal of Cardiovascular Ultrasound. 2010;8:51. doi: 10.1186/1476-7120-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kimura BJ DeMaria AN Technology insight: hand-carried ultrasound cardiac assessment – evolution, not revolution Nature clinical Practice. Cardiovascular Medicine 2 2005. 217–223., quiz 224 10.1038/ncpcardio0154 [DOI] [PubMed] [Google Scholar]
- 13. Panoulas VF Daigeler AL Malaweera AS Lota AS Baskaran D Rahman S Nihoyannopoulos P et al. Pocket-size hand-held cardiac ultrasound as an adjunct to clinical examination in the hands of medical students and junior doctors European Heart Journal Cardiovascular Imaging 14 2013. 323–330. 10.1093/ehjci/jes140 [DOI] [PubMed] [Google Scholar]
- 14. Choi BG Mukherjee M Dala P Young HA Tracy CM Katz RJ Lewis JF Interpretation of remotely downloaded pocket-size cardiac ultrasound images on a web-enabled smartphone: validation against workstation evaluation Journal of the American Society of Echocardiography 24 2011. 1325–1330. 10.1016/j.echo.2011.08.007 [DOI] [PubMed] [Google Scholar]
- 15. Liebo MJ Israel RL Lillie EO Smith MR Rubenson DS Topol EJ Is pocket mobile echocardiography the next-generation stethoscope? A crosssectional comparison of rapidly acquired images with standard transthoracic echocardiography Annals of Internal Medicine 155 2011. 33–38. 10.7326/0003-4819-155-1-201107050-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Singh S Bansal M Maheshwari P Adams D Sengupta SP Price R Dantin L Smith M Kasliwal RR Pellikka PA et al. American Society of echocardiography: remote echocardiography with web-based assessments for referrals at a distance (ASE-REWARD) study Journal of the American Society of Echocardiography 26 2013. 221–233. 10.1016/j.echo.2012.12.012 [DOI] [PubMed] [Google Scholar]
- 17. Lewis JF Maron BJ Diggs JA Spencer JE Mehrotra PP Curry CL Preparticipation echocardiographic screening for cardiovascular disease in a large, predominantly black population of collegiate athletes American Journal of Cardiology 64 1989. 1029–1033. 10.1016/0002-9149(89)90802-3 [DOI] [PubMed] [Google Scholar]
- 18. Riding NR Sharma S Salah O Khalil N Carré F George KP Hamilton B Chalabi H Whyte GP Wilson MG Systematic echocardiography is not efficacious when screening an ethnically diverse cohort of athletes in West Asia European Journal of Preventive Cardiology 22 2015. 263–270. 10.1177/2047487313506549 [DOI] [PubMed] [Google Scholar]
- 19. Fuller CM Cost effectiveness analysis of screening of high school athletes for risk of sudden cardiac death Medicine and Science in Sports and Exercise 32 2000. 887–890. 10.1097/00005768-200005000-00002 [DOI] [PubMed] [Google Scholar]
- 20. Wyman RA Chiu RY Rahko PS The 5-minute screening echocardiogram for athletes Journal of the American Society of Echocardiography 21 2008. 786–788. 10.1016/j.echo.2007.11.013 [DOI] [PubMed] [Google Scholar]
- 21. Prinz C Voigt JU Diagnostic accuracy of a hand-held ultrasound scanner in routine patients referred for echocardiography Journal of the American Society of Echocardiography 24 2011. 111–116. 10.1016/j.echo.2010.10.017 [DOI] [PubMed] [Google Scholar]
- 22. Andersen GN Haugen BO Graven T Salvesen O Mjolstad OC Dalen H Feasibility and reliability of point-of-care pocket-sized echocardiography European Journal of Echocardiography 12 2011. 665–670. 10.1093/ejechocard/jer108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Biais M Carrie C Delaunay F Morel N Revel P Janvier G Evaluation of a new pocket echoscopic device for focused cardiac ultrasonography in an emergency setting. Critical Care. 2012;16:R82. doi: 10.1186/cc11340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Testuz A Muller H Keller PF Meyer P Stampfli T Sekoranja L Vuille C Burri H Diagnostic accuracy of pocket-size handheld echocardiographs used by cardiologists in the acute care setting European Heart Journal Cardiovascular Imaging 14 2013. 38–42. 10.1093/ehjci/jes085 [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a