Abstract
Right ventricular volumes and ejection fraction are challenging to assess by echocardiography, but are well established as functional and prognostic parameters. Three-dimensional (3D) echocardiography has become widespread and relatively easy to use, making calculation of these parameters feasible in the large majority of patients. We review past attempts to estimate right ventricular volumes, current strengths and weaknesses of 3D echocardiography for this task, and compare with corresponding data from magnetic resonance imaging.
Keywords: right ventricle, volumes, systolic function, echocardiography, 3D echocardiography
Why are right ventricular volumes important?
The right ventricle (RV) far from being ‘forgotten’, as has been suggested, challenged echocardiographic diagnostic efforts from very early on. It is a considerably more complex and more difficult structure to visualize and quantitate than the left ventricle (LV). The main reasons for this are as follows:
RV overall shape, which can be described as a shell or a roughly triangular body covering part of the circumference of the LV, following the shape of the LV, with a crescent-shaped short-axis cross-section. Different from the LV, it has anatomically distinct inflow and outflow tracts, a relatively thin free wall, and is heavily trabecularized.
RV location, which is in the near field of parasternal echocardiographic windows, and in apical views may be obscured by ribs, sternum, or lung, especially if the image is optimized for the LV (Fig. 1).
Figure 1.
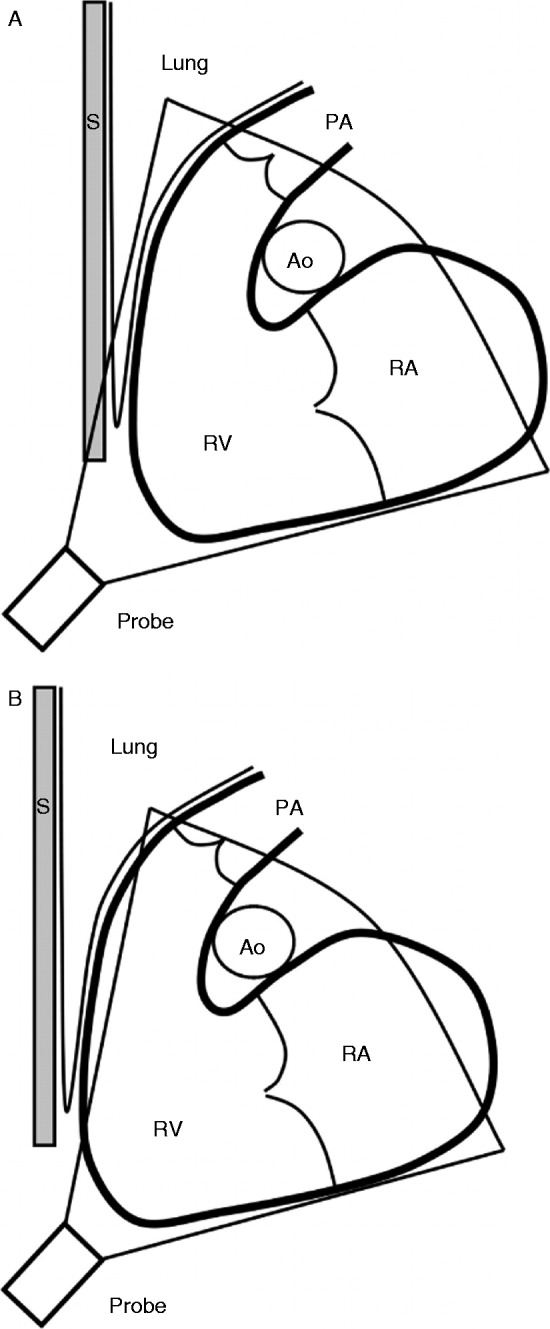
Schematic illustration of the right ventricle and the difficulties to include the whole volume. The inflow and outflow tracts are in the same plane – an oblique sagittal plane – as the apex. The right ventricle (RV) is depicted with the adjacent structures of the right atrium (RA), the pulmonary trunk (PA), the sternum (S), the ascending aorta (Ao), and lung tissue. (A) The sternum, ribs, and lung tissue can shadow the imaging of the RV, in particular the anterior part of the right ventricular outflow tract (RVOT). (B) Either the anterior part of the RV or the apex may not be included in the whole volume when trying to overcome this shadowing, especially if the RV is dilated. Reproduced from Ostenfeld E, Carlsson M, Shahgaldi K, Roijer A & Holm J 2012 Manual correction of semi-automatic three-dimensional echocardiography is needed for right ventricular assessment in adults; validation with cardiac magnetic resonance. Cardiovascular Ultrasound 10 1, published as an open access article by Biomed Central.
Thus, RV ejection fraction, which until recently could only be determined by magnetic resonance imaging (MRI) or radionuclide angiography, contains substantial functional and prognostic information from LV parameters as well as from conventional functional RV parameters independently.
RV ejection fraction is excellent for the assessment of functional consequences of chronic and acute pulmonary hypertension. Kawut et al. (1) found that RV ejection fraction by radionuclide angiography predicted death or lung transplantation in 84 adult patients with pulmonary hypertension, while pulmonary arterial pressures did not. In a study of pulmonary hypertension of different etiologies in children (n=100), absolute RV volumes and RV ejection fraction by MRI were found to predict prognosis more strongly than conventional echocardiographic parameters or pulmonary pressure estimated by echocardiography or measured by right heart catheterization (2). Patients with an RV ejection fraction <44% had a 1-, 2-, and 3-year survival of 87, 78, and 65% respectively, while those with an RV ejection fraction of 44–55% had a survival rate of 97, 97, and 90% for the same intervals and patients with a normal ejection fraction (>55%) had a survival rate of 97% throughout the first 3 years. Recently, a multi-center study in 94 adult patients with pulmonary hypertension of various etiologies demonstrated that MRI-derived RV ejection fraction and its changes over 1 year paralleled changes in functional class (e.g., 6-min walking distance) and survival under contemporary drug therapy with endothelin receptor antagonists and/or phosphodiesterase inhibitors (3). Size and function of the RV are also critical for the management of many forms of congenital heart disease, for example, atrial septal defect or pulmonary regurgitation in operated patients with tetralogy of Fallot (4).
Furthermore, it has increasingly become clear in recent years that, in many ‘left-sided’ clinical scenarios, such as heart failure due to coronary artery disease, the RV co-determines course and prognosis. Larose et al. (5) showed that MRI-determined RV ejection fraction independent of LV ejection fraction and infarct size strongly predicted survival in patients after myocardial infarction (adjusted hazard rate of RV ejection fraction, <40% 3.54 (CI, 1.50–8.36)).
The outcome of heart failure therapy using continuous-flow LV assist devices critically depends on RV function, with RV failure predicting short-term and long-term mortalities (6). It is reasonable to assume that three-dimensional (3D) echocardiographic measurement of volumes and ejection fraction might improve assessment of RV function in this scenario, although such data are lacking.
How can RV volumes be measured by echocardiography?
Two-dimensional echocardiography
While there is no meaningful way to calculate RV volumes from M-mode, extensive research has been carried out to derive RV volumes from two-dimensional (2D) views. In principle, two approaches can be distinguished: area–length and Simpson's rule. Area–length methods calculate volume of a body by a formula of the form: volume=c×A×L, where A is the cross-sectional area in one view, L is the long-axis length in the cross-section, and c is a constant that has to be found empirically. Simpson's rule approaches use two perpendicular planes sharing a long axis to calculate the volume as a stack of elliptical discs. Acquiring such two planes of the RV by 2D echocardiography, however, is very difficult and still neglects the RV outflow tract; therefore, biplane approaches of Simpson's rule were abandoned. Researchers using angiographic right ventriculography had found that RV volumes could be relatively accurately determined from paired X-ray projections in end-systole and end-diastole, as was already a common practice and reasonably validated for the LV (7). Thus, a number of echocardiographic approaches imitating the angiographic methodology were published, with encouraging validation in vitro (8) and in vivo against radionuclide angiography (9). However, these methods were cumbersome, required views that were not well obtainable in many patients, had only a modest accuracy, and were never validated in a substantial number of patients with different diseases. Therefore, RV volume determination by 2D echocardiography remained a research method, and echocardiographically derived RV ejection fraction remained impractical to assess RV function. For routine clinical purposes, the most widely used morphology-based parameters of RV size and function are given as follows (10):
linear parameters such as the antero-posterior diameter in parasternal long- and short-axis views, as well as short-axis diameters in the apical four-chamber view at different levels of the long axis of the RV;
the most popular functional parameter due to its ease of acquisition became the M-mode registration of the cyclic apico-basal motion of the lateral insertion point of the tricuspid valve leaflet (TAPSE). An alternative functional parameter is RV free wall systolic velocity measured by tissue Doppler;
as a surrogate of ejection fraction, RV fractional area change has been used (RV end-diastolic area minus end-systolic area divided by end-diastolic area, with areas measured in the apical four-chamber or ‘RV-optimized’ four-chamber view; Fig. 2). Alternatively, a monoplane Simpson's rule analog of LV ejection fraction is sometimes used, which is derived from the same view. This of course underestimates true RV volumes as the RV outflow tract is not included, but relatively good correlations of RV ejection fraction with an angiographic standard were obtained in a small study in children (11).
Figure 2.
Examples of fractional area change (FAC) (A and C) in a healthy person (FAC=44%) and (B and D) in a patient with pulmonary arterial hypertension (FAC=13%). A and B are at end-diastole. C and D are at end-systole.
Finally, an approach has been used successfully, in which RV 3D data are reconstructed from 2D images that are registered during acquisition in a magnetic field and then mathematically fitted to ‘knowledge-based’ RV shapes (12, 13).
2D parameters have the advantage of relying on routinely acquired standard views. However, as they only consider a section of the RV and imply geometric assumptions, they are fundamentally problematic, and particularly so in pathologically remodeled ventricles. Thus, the limited accuracy and reliability of 2D measures of RV volume has been a major limitation of echocardiographic imaging, in particular with regard to the management of congenital heart disease (e.g., the follow-up of patients with pulmonary regurgitation after surgical correction of tetralogy of Fallot), with MRI now being the recommended modality to assess the RV size and function (4). Another vexing problem in the practical application of echocardiographic RV volume assessment has been the diagnosis of arrhythmogenic RV cardiomyopathy (ARVC), a genetically transmitted disease for which familial screening is recommended. The currently ‘proposed modification’ of the international task force guidelines for the diagnosis of ARVC (14) uses only linear measurements of RV volume by echocardiography, and they only constitute criteria for the diagnosis of ARVC if co-existing with a regional akinesia or dyskinesia/aneurysm of the RV. Nevertheless, only 50% of patients with imaging-positive ARVC by CMR fulfilled echocardiographic ARVC 2010 criteria (15). The overlap between RV dimensions suggestive of ARVC and those of healthy individuals, in particular endurance athletes, is also considerable; Oxborough et al. (16) found that fully 83% of elite endurance runners or cyclists met the RV outflow tract diameter cut-off incorporated in the ‘minor’ ARVC criteria and still 28% met the size requirements for ‘major’ ARVC criteria. In an ironical twist, this has led to the proposal of hypothesis that high levels of exercise may enhance phenotypical penetrance of ARVC genotype carriers that actually lead to a ARVC-like disease (17), or even that there is an ‘exercise-induced ARVC’ (18, 19), without necessitating a desmosomal abnormality. Both 3D echocardiography and MRI seem to offer better discrimination, in particular using RV ejection fraction impairment in addition to absolute volume cut-offs (20, 21).
3D echocardiography
3D echocardiography has over the last 20 years evolved from a challenging experimental technique requiring outstanding image quality, the use of large 3D transducers, and time-consuming post-processing steps, into a modality on the brink of clinical routine usage, with highly improved spatial and temporal resolution as well as a relatively small transducer footprint.
Image acquisition
Current transthoracic 3D transducers, the so-called ‘fully sampled matrix transducers’, while still slightly heavier and larger than 2D transducers, have a similar footprint to standard 2D transducers and are capable of imaging the entire RV with frame rates of 20–30 frames/s.
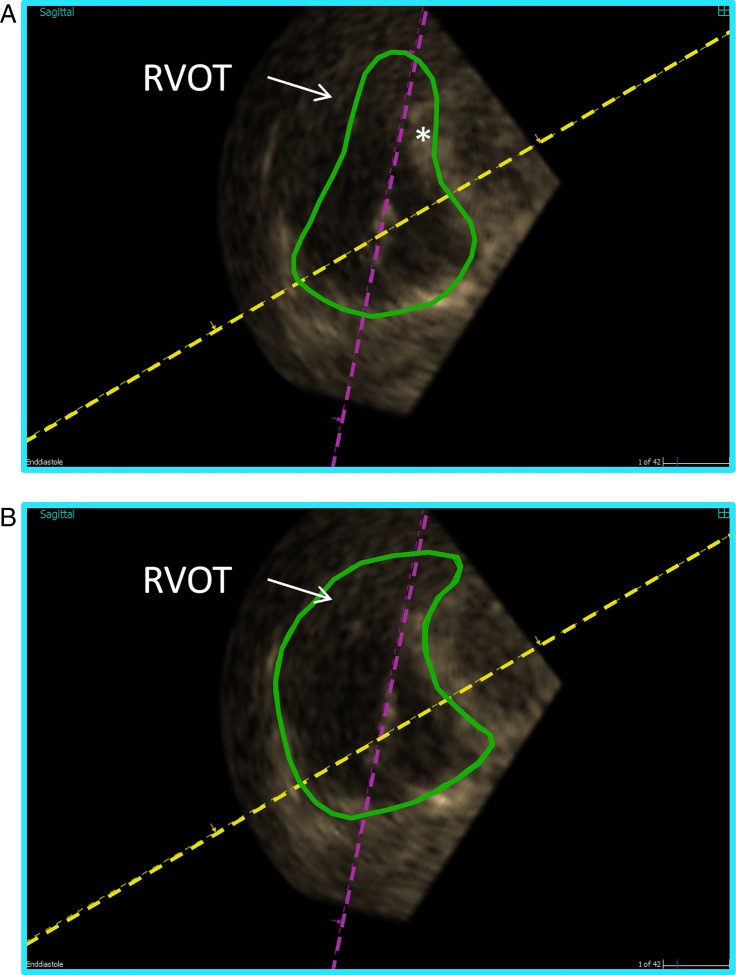
Apical acquisition of volumes is recommended in the adult population. An effort should be made to include the tricuspid valve, the apex, and the outflow tract with the pulmonary valve in the full volume. As it is often difficult to include the whole RV volume in the acquisition sector, a modified apical view can be advantageous. The modified view is off-axis compared with the standard 2D apical four-chamber view. One way (the medial approach) is to move the transducer medially to the RV modified apical four-chamber view and then tilt the transducer cranially and anteriorly – and even rotate – to include the pulmonary valve in the guidance images (Fig. 3). Near-field resolution, ribs, and the sternum are often limiting factors in acquiring adequate images from this view. Another way (the lateral approach) is to displace the transducer laterally with an anterior tilt. To include both the pulmonary and tricuspid valves, it can sometimes be of use to move the transducer further laterally than the RV-focused apical four-chamber view and even to a more cranial intercostal space, as well as rotating the transducer (Fig. 3). The lateral approach is challenged by far-field resolution, ribs, and interpositioned lung tissue (Fig. 1) (22, 23, 24, 25). Foreshortening is not an issue in 3D echocardiography (3DE), as long as the whole volume is included in the dataset, as the axis can be corrected in the post-processing step. In our experience, the feasibility is ∼85% in a non-selected adult population (24, 25). As with 2D echocardiography, focus, depth, and sector should be adjusted to the area of interest to maximize the image quality. Nevertheless, if there is poor 2D image quality, this will also be the case with 3D echocardiography.
Figure 3.
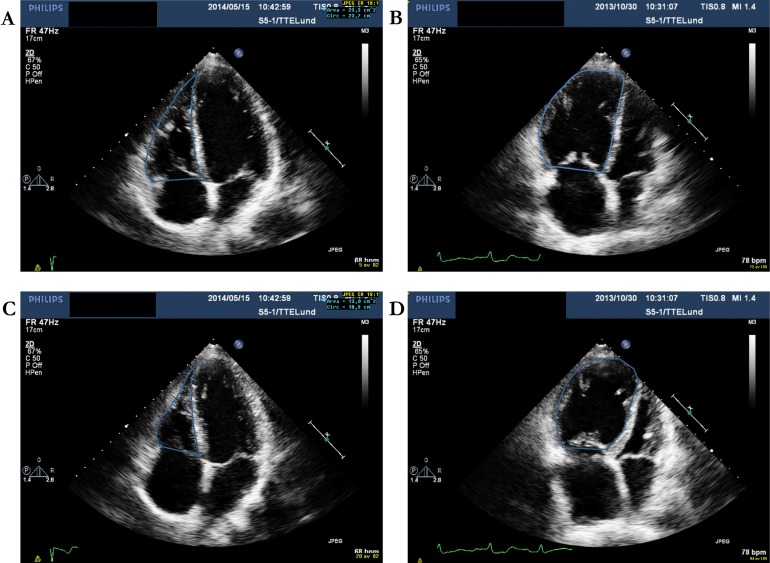
Full-volume three-dimensional datasets cropped to display the cavity of (A) a normal person acquired with the lateral approach and (B) a patient with pulmonary arterial hypertension (PAH) acquired with the medial approach. The right ventricle (RV) and atrium (RA) are enlarged and the septum (*) is bulging into the small left ventricle (LV) in the PAH patient and, at the apex, the right ventricle is larger than the maximum sector angle illustrating the challenge of acquisition of the whole volume.
The 3D dataset for volumetric assessment is acquired by recording the whole heart in automatically created small subvolumes over two to seven heart beats, obtaining one subvolume during each heartbeat. The subvolumes are then electronically merged into one dataset and image. Ideally, this requires equally long heart cycles and no breathing motion during acquisition, otherwise the heart is not in the same position at equivalent time points of each heart beat and ‘stitching artifacts’ can arise. Atrial fibrillation or other irregular rhythms are therefore detrimental. Recently, single-beat acquisition of large pyramidal volumes has become technically feasible, although at the cost of lower spatial and temporal resolution. While, in sinus rhythm, intra- and interpersonal variabilities for measuring LV volumes and EF were the same for single-beat and four-beat acquisition, single-beat acquisition had lower variability than four-beat acquisition in patients with atrial fibrillation (26). The acquisition time for obtaining all four heart chambers, including the RV, by 3D echocardiography is on average <5 min (22, 23).
Image analyses
There are several software packages available for endocardial delineation of cardiac chambers, with some being dedicated to the RV (Figs 4 and 5, Videos 1 and 2). Pronounced trabeculations, prominent moderator band, or anterior papillary muscle are difficult to differentiate from the anterior RV wall using the semi-automatic delineation software, especially with limited image quality (27). Owing to the fact that as much as 25% of the RV volumes may originate from the RV outflow tract (28), semi-automated border tracing can miss out considerable parts of the volume if manual correction is not performed (Fig. 6 and Video 3). The underestimation of volumes in comparison to cardiac MRI decreases when manual correction is performed, yet at the cost of substantially increased time requirements for analysis. Manual correction of semi-automated border delineation can take three to four times longer than the uncorrected delineation, which on average takes <4 min; it is even longer than the time that manual delineation of a transversal stack of magnetic resonance images would take (25).
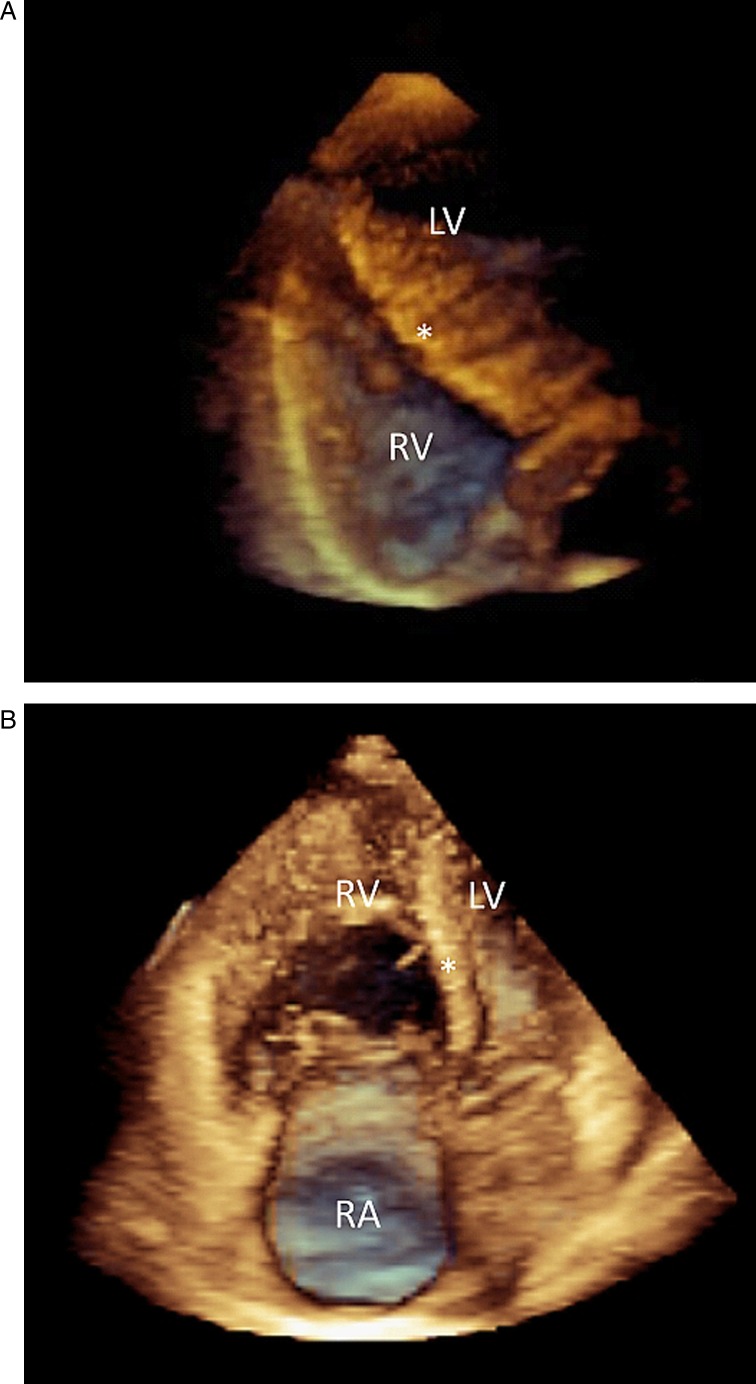
3D echocardiography reconstruction of a right ventricle (green) with normal systolic function (end-diastolic volume 153 ml, end-systolic volume 64 ml, ejection fraction 58 %). The mesh is the right ventricle at end-diastole. The white areas are the pulmonary valve in the upper left side and the tricuspid valve in the upper right side. The right ventricular apex is towards the bottom. Data was processed with dedicated software (4D RV-Function, TomTec Imaging Systems). Download Video 1 via http://dx.doi.org/10.1530/ERP-14-0077-v1
Download Video 1 (1.6MB, avi)
Example of a 3D echocardiographic reconstruction of a patient with pulmonary hypertension (end-diastolic volume 200 ml, end-systolic volume 137 ml, ejection fraction 31 %) showing an enlarged right ventricle (green) with a flattened septum in diastole (mesh) and even more so in systole. Both the longitudinal and lateral function appear altered. Data was processed with dedicated software (4D RV-Function, TomTec Imaging Systems). Download Video 2 via http://dx.doi.org/10.1530/ERP-14-0077-v2
Download Video 2 (1.2MB, avi)
Example of semi-automated delineation of the right ventricle without manual correction. The semi-automated delineation crosses, and hence includes, parts of the septum in the right ventricular volume. On the other hand, the delineation does not follow the anterior part of the right ventricular outflow tract and that part is therefore by default not included in automated volume calculation. Data was processed with dedicated software (4D RV-Function, TomTec Imaging Systems). Download Video 3 via http://dx.doi.org/10.1530/ERP-14-0077-v3
Download Video 3 (1.6MB, avi)
Figure 4.
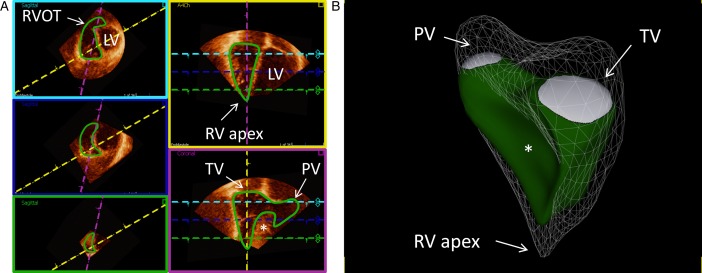
Example of delineation of a normal right ventricle (RV) in end-diastole showing the endocardial contour detection in green. (A) The three left images (magenta, blue, and green boxes) are the short-axis views at different levels with the left ventricle (LV) to the right of the interventricular septum (*). The upper left image (magenta box) is closer to the base and right ventricular outflow tract (RVOT) and the lower image (green box) closer to the apex. The upper right image (yellow box) is a four-chamber view and the lower right image (purple box) is a right ventricular three-chamber view with tricuspid valve (TV), apex, and pulmonary valve (PV) in the same projection. The dashed colored lines represent the plane of the boxes with the corresponding color. (B) Example of a three-dimensional echocardiographic reconstruction of the delineation of the right ventricle seen from the septal side (end-diastolic volume 153 ml, end-systolic volume 64 ml, and ejection fraction 58%). The mesh is the right ventricle at end-diastole, in green at end-systole. Pulmonary valve (PV) is shown in white in the upper left side, tricuspid valve (TV) is shown in the upper right side, and right ventricular (RV) apex toward the bottom. *shows the interventricular septum bulging into RV. Data were processed using a dedicated software (4D RV-Function, TomTec Imaging Systems). See also Video 1.
Figure 5.
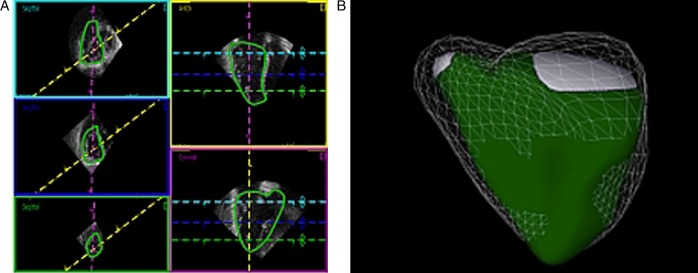
Example of delineation of a patient with pulmonary hypertension in end-diastole. Box, line, and color descriptions are the same as in Fig. 4. (A) The right ventricle is enlarged and the trabeculation is hypertrophied. Trabeculations are included in the volume. The septum is flattened, even in diastole, in the short-axis images. (B) The three-dimensional echocardiographic representation (end-diastolic volume 200 ml, end-systolic volume 137 ml, ejection fraction 31%) shows an enlarged right ventricle with a flattened septum in diastole (mesh) and even more so in systole (green). Both the longitudinal and lateral functions appear to be altered. Data were processed using a dedicated software (4D RV-Function, TomTec Imaging Systems). See also Video 2.
Figure 6.
Example of semi-automated delineation of the right ventricle (A) without manual correction and (B) with manual correction. The endocardial contour detection (green) is enhanced in a basal short-axis view (magenta box, yellow, and purple lines as in Fig. 4A) from a three-dimensional dataset. The semi-automated delineation crosses, and hence includes, parts of the septum (*) in the right ventricular volume. On the other hand, the delineation does not follow the anterior part of the right ventricular outflow tract (RVOT) and that volume is excluded from calculation. See also Video 3.
Importantly, encouraging test–retest reliability for RV 3D echocardiographic volumes has been reported (29, 30), with variabilities of 7% for end-diastolic volumes, 14% for end-systolic volumes, and 8% for ejection fraction when different observers repeated a study on the same subject, using semi-automated border delineating software.
Comparison with MRI
Overall, there is a good to reasonable correlation between RV volumes and ejection fraction measured by MRI and 3D echocardiography (31). Systematic underestimation of all cardiac chamber volumes by 3D echocardiography compared with MRI and also cardiac computed tomography is well established, with less effect on ejection fraction as the absolute volume differences cancel out. Accordingly, an extensive meta-analysis of many small studies directly comparing 3D echocardiography and MRI for RV volumes revealed systematic underestimation of end-systolic RV volume by 5.5 ml, of end-diastolic RV volume by 13.9 ml, and of RV ejection fraction by 0.9% (29, 31).
What is normal?
Several recent publications have sought to establish normal values for RV volumes and ejection fraction (32, 33, 34, 35, 36). While all studies showed dependency of volumes on body surface area and sex, most also showed an age dependency, with a decrease in volumes and a very small increase in ejection fraction with age. This is in accordance with large-scale studies of RV morphology by MRI (37), which furthermore showed an influence of race. Remarkably, normal volumes, whether indexed or not, have been quite different from study to study by 3D echocardiography, at least in part probably due to evolution in transducer technology and software. For example, absolute RV volumes of normals were ∼50% higher in the study by van der Zwaan et al. (34) than in the study by Maffessanti et al. (36). Although the underlying ‘normal’ cohorts were somewhat different in height and body surface area, this seems to be insufficient to explain the discrepancy. Beyond hardware- and software-related factors, differing levels of manual correction of the automated tracking algorithm most probably play a role in this. In our experience, which is supported by observations in the literature (31), manual contour tracking corrections lead to higher calculated volumes by avoiding ‘streamlined’ RV contours. Thus, in the absence of a real standard technique, no definitive normal values can be given at this time (Table 1).
Table 1.
Published RV absolute and indexed volume values by 3D echocardiography from healthy adults
| (33) | (46) | (34) | (35) | |
|---|---|---|---|---|
| RVEDV (ml) | 77±23 | 86±21 | ||
| RVEDVI (ml/m2) | 70±14 | 40±11 | 75±12 | 49±10 |
| RVESV (ml) | 30±12 | 29±11 | ||
| RVESVI (ml/m2) | 33±10 | 16±6 | 33±7 | 16±6 |
| RVEF (%) | 53±10 | 61±10 | 57±4 | 67±8 |
| n | 71 | 166 | 31 | 245 |
| Remarks | RVOT was not included |
RVOT, right ventricular outflow tract; RVEDV, right ventricular end-diastolic volume; RVEDVI, right ventricular end-diastolic volume index; RVESV, right ventricular systolic volume; RVESVI, right ventricular end-systolic index; RVEF, right ventricular ejection fraction.
How do RV volumes and ejection fraction compare with other functional parameters?
As they are difficult to measure, quantitatively estimated RV volumes have played a relatively minor role in clinical practice. This stands in stark contrast to the central importance of LV volumes and ejection fraction in clinical cardiology, from heart failure to valvular heart disease management. Instead, mostly non-volumetric parameters are used for RV function (10), of which all are based on the apical four-chamber view and therefore neglect the RV outflow tract. TAPSE and tissue Doppler measure the longitudinal function of the RV free wall, which is an even more selective way of looking at RV function. Importantly, TAPSE and free wall tissue velocity measure displacement and velocity relative to the stationary transducer, which may produce misleading results. For example, it has been demonstrated that contraction of the LV lateral wall may induce passive motion of the RV free wall because of tethering (tissue continuity) between left and RV free walls (38). Such mechanisms may also be at work to produce the well-known, but poorly explained, postoperative deterioration of TAPSE after heart surgery, which occurs in spite of preserved RV ejection fraction by 3D echocardiography (38, 39). Remarkably, reduction in longitudinal tissue velocity has been noted intraoperatively to coincide with pericardial opening, thereby largely excluding material damage to the myocardium as a cause (40).
Compounding these problems is the variability of the ‘standard’ apical four-chamber view, on which most measurements are based. In the routine examination, the apical four-chamber view is typically fine-tuned to optimize depiction of the LV, avoiding LV foreshortening and excluding the LV outflow tract. There is, however, considerable potential of misrepresenting the RV by a tangential cut, which will result in a falsely too small RV cavity. Therefore, guidelines of the American and European echocardiographic societies (10) have proposed a modification of the standard four-chamber view, the ‘RV-focused view’, which implies rotating the apically positioned transducer to achieve a maximal RV cross-section (especially in the minor axis), while keeping the cardiac apex at the top of the sector and not foreshortening or otherwise distorting the LV.
Speckle tracking-based assessment of global LV function has received much recent attention. It was found more sensitive in detecting early functional impairment than volume-based functional indices such as ejection fraction in several clinical scenarios such as cardiotoxicity of cancer chemotherapy, early cardiomyopathy, and others. Naturally, this paradigm also seems to be attractive for the RV. However, the complexity of RV shape and absence of an established RV segment model, together with the absence of dedicated software, have so far restricted the application of speckle-tracking analysis to the apical four-chamber view, where a portion of the RV free wall (and the septum) can be analyzed (38, 41). Furthermore, real-time 3D strain assessment, allowing for a more comprehensive assessment of the RV, has become technically possible. Recently, in 97 patients with mostly primary pulmonary hypertension, RV ejection fraction correlated highly with 3D RV strain data and both predicted prognosis independently (42), thus confirming earlier data on 3D echocardiography-derived RV ejection fraction and prognosis in patients with pulmonary hypertension (43).
Clinical experience with RV volumes and ejection fraction from 3D echocardiography, beyond validation studies with MRI and comparison with other functional echocardiographic parameters, is still limited. In patients with dilated cardiomyopathy, a modest, but independent, correlation of RV ejection fraction with oxygen uptake during a cardiopulmonary exercise test was noted (44). Another study used 3D echocardiography data to divide the RV in patients with secondary pulmonary hypertension and healthy controls into three compartments (inflow and outflow tract, and apex) and calculated the partial ejection fractions as well as the timing of contraction of each of these compartments, finding differences among normals, patients with ischemic heart failure without pulmonary hypertension, and such patients with pulmonary hypertension (45).
Conclusion
Given the increasing use of 3D echocardiography and MRI, it is likely that RV ejection fraction will become a more broadly used functional measure than in the past, where it was impossible to obtain by echocardiography. Potentially useful interventional applications of echocardiographic RV 3D imaging include fusion with fluoroscopic images or electrical activation maps. It is unlikely to replace the quickly and easily obtained parameters: TAPSE, basal RV free wall tissue velocity, and fractional area change. But whenever detailed assessment of the RV is required, in particular in serial examinations of known pathology, 3D echocardiographic determination of RV volumes and ejection fraction will probably become standard. For the assessment of LV systolic function, size (volumes), and ejection fraction are the traditional standard, with longitudinal function assessment by way of tissue Doppler or strain analysis having been added recently. The reverse is true for the RV. Simple measures of longitudinal function such as TAPSE and free wall velocity, as well as fractional area change, as an odd substitute for ejection fraction have been the traditional cornerstone of functional evaluation. For the RV, volumes and ejection fraction are the (echocardiographic) newcomers, which – together with strain measurements – could be the coming standard for RV evaluation by echocardiography.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Funding
This review did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
References
- 1. Kawut SM Horn EM Berekashvili KK Garofano RP Goldsmith RL Widlitz AC Rosenzweig EB Kerstein D Barst RJ New predictors of outcome in idiopathic pulmonary arterial hypertension American Journal of Cardiology 95 2005. 199–203.Erratum in: American Journal of Cardiology 2007 100 562 10.1016/j.amjcard.2004.09.006 [DOI] [PubMed] [Google Scholar]
- 2. Moledina S Pandya B Bartsota M Mortensen KH McMillan M Quyam S Taylor AM Haworth SG Schulze-Neick I Muthurangu V Prognostic significance of cardiac magnetic resonance imaging in children with pulmonary hypertension Circulation. Cardiovascular Imaging 6 2013. 407–414. 10.1161/CIRCIMAGING.112.000082 [DOI] [PubMed] [Google Scholar]
- 3. Peacock AJ Crawley S McLure L Blyth K Vizza CD Poscia R Francone M Iacucci I Olschewski H Kovacs G et al. Changes in right ventricular function measured by cardiac magnetic resonance imaging in patients receiving pulmonary arterial hypertension-targeted therapy: the EURO-MR study Circulation. Cardiovascular Imaging 7 2014. 107–114. 10.1161/CIRCIMAGING.113.000629 [DOI] [PubMed] [Google Scholar]
- 4. Baumgartner H Bonhoeffer P De Groot NM de Haan F Deanfield JE Galie N Gatzoulis MA Gohlke-Baerwolf C Kaemmerer H Kilner P et al. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010) European Heart Journal 31 2010. 2915–2957. 10.1093/eurheartj/ehq249 [DOI] [PubMed] [Google Scholar]
- 5. Larose E Ganz P Reynolds HG Dorbala S Di Carli MF Brown KA Kwong RY Right ventricular dysfunction assessed by cardiovascular magnetic resonance imaging predicts poor prognosis late after myocardial infarction Journal of the American College of Cardiology 49 2007. 855–862. 10.1016/j.jacc.2006.10.056 [DOI] [PubMed] [Google Scholar]
- 6. Hayek S Sims DB Markham DW Butler J Kalogeropoulos AP Assessment of right ventricular function in left ventricular assist device candidates Circulation. Cardiovascular Imaging 7 2014. 379–389. 10.1161/CIRCIMAGING.113.001127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arcilla RA Tsai P Thilenius O Ranniger K Angiographic method for volume estimation of right and left ventricles Chest 60 1971. 446–454. 10.1378/chest.60.5.446 [DOI] [PubMed] [Google Scholar]
- 8. Levine RA Gibson TC Aretz T Gillam LD Guyer DE King ME Weyman AE Echocardiographic measurement of right ventricular volume Circulation 69 1984. 497–505. 10.1161/01.CIR.69.3.497 [DOI] [PubMed] [Google Scholar]
- 9. Starling MR Crawford MH Sorensen SG O'Rourke RA A new two-dimensional echocardiographic technique for evaluating right ventricular size and performance in patients with obstructive lung disease Circulation 66 1982. 612–620. 10.1161/01.CIR.66.3.612 [DOI] [PubMed] [Google Scholar]
- 10. Rudski LG Lai WW Afilalo J Hua L Handschumacher MD Chandrasekaran K Solomon SD Louie EK Schiller NB Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography Journal of the American Society of Echocardiography 23 2010. 685–713. 10.1016/j.echo.2010.05.010 [DOI] [PubMed] [Google Scholar]
- 11. Silverman NH Hudson S Evaluation of right ventricular volume and ejection fraction in children by two-dimensional echocardiography Pediatric Cardiology 4 1983. 197–203. 10.1007/BF02242255 [DOI] [PubMed] [Google Scholar]
- 12. Dragulescu A Grosse-Wortmann L Fackoury C Riffle S Waiss M Jaeggi E Yoo SJ Friedberg MK Mertens L Echocardiographic assessment of right ventricular volumes after surgical repair of tetralogy of Fallot: clinical validation of a new echocardiographic method Journal of the American Society of Echocardiography 24 2011. 1191–1198. 10.1016/j.echo.2011.08.006 [DOI] [PubMed] [Google Scholar]
- 13. Dragulescu A Grosse-Wortmann L Fackoury C Mertens L Echocardiographic assessment of right ventricular volumes: a comparison of different techniques in children after surgical repair of tetralogy of Fallot European Heart Journal Cardiovascular Imaging 13 2012. 596–604. 10.1093/ejechocard/jer278 [DOI] [PubMed] [Google Scholar]
- 14. Marcus FI McKenna WJ Sherrill D Basso C Bauce B Bluemke DA Calkins H Corrado D Cox MG Daubert JP et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the Task Force Criteria European Heart Journal 31 2010. 806–814. 10.1093/eurheartj/ehq025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Borgquist R Haugaa KH Gilljam T Bundgaard H Hansen J Eschen O Jensen HK Holst AG Edvardsen T Svendsen JH et al. The diagnostic performance of imaging methods in ARVC using the 2010 Task Force criteria European Heart Journal Cardiovascular Imaging 15 2014. 1219–1225. 10.1093/ehjci/jeu109 [DOI] [PubMed] [Google Scholar]
- 16. Oxborough D Sharma S Shave R Whyte G Birch K Artis N Batterham AM George K The right ventricle of the endurance athlete: the relationship between morphology and deformation Journal of the American Society of Echocardiography 25 2012. 263–271. 10.1016/j.echo.2011.11.017 [DOI] [PubMed] [Google Scholar]
- 17. James CA Bhonsale A Tichnell C Murray B Russell SD Tandri H Tedford RJ Judge DP Calkins H Exercise increases age-related penetrance and arrhythmic risk in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated desmosomal mutation carriers Journal of the American College of Cardiology 62 2013. 1290–1297. 10.1016/j.jacc.2013.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heidbüchel H La Gerche A The right heart in athletes. Evidence for exercise-induced arrhythmogenic right ventricular cardiomyopathy Herzschrittmachertherapie & Elektrophysiologie 23 2012. 82–86. 10.1007/s00399-012-0180-3 [DOI] [PubMed] [Google Scholar]
- 19. La Gerche A Robberecht C Kuiperi C Nuyens D Willems R de Ravel T Matthijs G Heidbüchel H Lower than expected desmosomal gene mutation prevalence in endurance athletes with complex ventricular arrhythmias of right ventricular origin Heart 96 2010. 1268–1274. 10.1136/hrt.2009.189621 [DOI] [PubMed] [Google Scholar]
- 20. Kjaergaard J Hastrup Svendsen J Sogaard P Chen X Bay Nielsen H Køber L Kjaer A Hassager C Advanced quantitative echocardiography in arrhythmogenic right ventricular cardiomyopathy Journal of the American Society of Echocardiography 20 2007. 27–35. 10.1016/j.echo.2006.07.006 [DOI] [PubMed] [Google Scholar]
- 21. Vermes E Strohm O Otmani A Childs H Duff H Friedrich MG Impact of the revision of arrhythmogenic right ventricular cardiomyopathy/dysplasia task force criteria on its prevalence by CMR criteria JACC. Cardiovascular Imaging 4 2011. 282–287. 10.1016/j.jcmg.2011.01.005 [DOI] [PubMed] [Google Scholar]
- 22. Ostenfeld E Shahgaldi K Winter R Willenheimer R Holm J Comparison of different views with three-dimensional echocardiography: apical views offer superior visualization compared with parasternal and subcostal views Clinical Physiology and Functional Imaging 28 2008. 409–416. 10.1111/j.1475-097X.2008.00823.x [DOI] [PubMed] [Google Scholar]
- 23. Lang RM Badano LP Tsang WW Adams DH Agricola E Buck T Faletra FF Franke A Hung J de Isla LP et al. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography European Heart Journal Cardiovascular Imaging 13 2012. 1–46. 10.1093/ehjci/jer316 [DOI] [PubMed] [Google Scholar]
- 24. Nesser HJ Tkalec W Patel AR Masani ND Niel J Markt B Pandian NG Quantitation of right ventricular volumes and ejection fraction by three-dimensional echocardiography in patients: comparison with magnetic resonance imaging and radionuclide ventriculography Echocardiography 23 2006. 666–680. 10.1111/j.1540-8175.2006.00286.x [DOI] [PubMed] [Google Scholar]
- 25. Ostenfeld E Carlsson M Shahgaldi K Roijer A Holm J Manual correction of semi-automatic three-dimensional echocardiography is needed for right ventricular assessment in adults; validation with cardiac magnetic resonance. Cardiovascular Ultrasound. 2012;10:1. doi: 10.1186/1476-7120-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shahgaldi K Manouras A Abrahamsson A Gudmundsson P Brodin LA Winter R Three-dimensional echocardiography using single-heartbeat modality decreases variability in measuring left ventricular volumes and function in comparison to four-beat technique in atrial fibrillation. Cardiovascular Ultrasound. 2010;8:45. doi: 10.1186/1476-7120-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Anwar AM Soliman O van den Bosch AE McGhie JS Geleijnse ML ten Cate FJ Meijboom FJ Assessment of pulmonary valve and right ventricular outflow tract with real-time three-dimensional echocardiography International Journal of Cardiovascular Imaging 23 2007. 167–175. 10.1007/s10554-006-9142-3 [DOI] [PubMed] [Google Scholar]
- 28. Aebischer NM Czegledy F Determination of right ventricular volume by two-dimensional echocardiography Journal of the American Society of Echocardiography 2 1989. 110–118. 10.1016/S0894-7317(89)80073-2 [DOI] [PubMed] [Google Scholar]
- 29. Grapsa J O'Regan DP Pavlopoulos H Durighel G Dawson D Nihoyannopoulos P Right ventricular remodelling in pulmonary arterial hypertension with three-dimensional echocardiography: comparison with cardiac magnetic resonance imaging European Journal of Echocardiography 11 2010. 64–73. 10.1093/ejechocard/jep169 [DOI] [PubMed] [Google Scholar]
- 30. van der Zwaan HB Geleijnse ML Soliman OI McGhie JS Wiegers-Groeneweg EJ Helbing WA Roos-Hesselink JW Meijboom FJ Test-retest variability of volumetric right ventricular measurements using real-time three-dimensional echocardiography Journal of the American Society of Echocardiography 24 2011. 671–679. 10.1016/j.echo.2011.02.007 [DOI] [PubMed] [Google Scholar]
- 31. Shimada YJ Shiota M Siegel RJ Shiota T Accuracy of right ventricular volumes and function determined by three-dimensional echocardiography in comparison with magnetic resonance imaging: a meta-analysis study Journal of the American Society of Echocardiography 23 2010. 943–953. 10.1016/j.echo.2010.06.029 [DOI] [PubMed] [Google Scholar]
- 32. Kjaergaard J Sogaard P Hassager C Quantitative echocardiographic analysis of the right ventricle in healthy individuals Journal of the American Society of Echocardiography 19 2006. 1365–1372. 10.1016/j.echo.2006.05.012 [DOI] [PubMed] [Google Scholar]
- 33. Gopal AS Chukwu EO Iwuchukwu CJ Katz AS Toole RS Schapiro W Reichek N Normal values of right ventricular size and function by real-time 3-dimensional echocardiography: comparison with cardiac magnetic resonance imaging Journal of the American Society of Echocardiography 20 2007. 445–455. 10.1016/j.echo.2006.10.027 [DOI] [PubMed] [Google Scholar]
- 34. van der Zwaan HB Geleijnse ML McGhie JS Boersma E Helbing WA Meijboom FJ Roos-Hesselink JW Right ventricular quantification in clinical practice: two-dimensional vs three-dimensional echocardiography compared with cardiac magnetic resonance imaging European Journal of Echocardiography 12 2011. 656–664. 10.1093/ejechocard/jer107 [DOI] [PubMed] [Google Scholar]
- 35. Tamborini G Marsan NA Gripari P Maffessanti F Brusoni D Muratori M Caiani EG Fiorentini C Pepi M Reference values for right ventricular volumes and ejection fraction with real-time three-dimensional echocardiography: evaluation in a large series of normal subjects Journal of the American Society of Echocardiography 23 2010. 109–115. 10.1016/j.echo.2009.11.026 [DOI] [PubMed] [Google Scholar]
- 36. Maffessanti F Muraru D Esposito R Gripari P Ermacora D Santoro C Tamborini G Galderisi M Pepi M Badano LP Age-, body size-, and sex-specific reference values for right ventricular volumes and ejection fraction by three-dimensional echocardiography: a multicenter echocardiographic study in 507 healthy volunteers Circulation. Cardiovascular Imaging 6 2013. 700–710. 10.1161/CIRCIMAGING.113.000706 [DOI] [PubMed] [Google Scholar]
- 37. Kawut SM Lima JA Barr RG Chahal H Jain A Tandri H Praestgaard A Bagiella E Kizer JR Johnson WC et al. Sex and race differences in right ventricular structure and function: the multi-ethnic study of atherosclerosis-right ventricle study Circulation 123 2011. 2542–2551. 10.1161/CIRCULATIONAHA.110.985515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Giusca S Dambrauskaite V Scheurwegs C D'hooge J Claus P Herbots L Magro M Rademakers F Meyns B Delcroix M et al. Deformation imaging describes right ventricular function better than longitudinal displacement of the tricuspid ring Heart 96 2010. 281–288. 10.1136/hrt.2009.171728 [DOI] [PubMed] [Google Scholar]
- 39. Tamborini G Muratori M Brusoni D Celeste F Maffessanti F Caiani EG Alamanni F Pepi M Is right ventricular systolic function reduced after cardiac surgery? A two- and three-dimensional echocardiographic study. European Journal of Echocardiography 10 2009. 630–634. 10.1093/ejechocard/jep015 [DOI] [PubMed] [Google Scholar]
- 40. Unsworth B Casula RP Kyriacou AA Yadav H Chukwuemeka A Cherian A Stanbridge Rde L Athanasiou T Mayet J Francis DP The right ventricular annular velocity reduction caused by coronary artery bypass graft surgery occurs at the moment of pericardial incision American Heart Journal 159 2010. 314–322. 10.1016/j.ahj.2009.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Levy PT Sanchez Mejia AA Machefsky A Fowler S Holland MR Singh GK Normal ranges of right ventricular systolic and diastolic strain measures in children: a systematic review and meta-analysis Journal of the American Society of Echocardiography 27 2014. 549–560. 10.1016/j.echo.2014.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Smith BC Dobson G Dawson D Charalampopoulos A Grapsa J Nihoyannopoulos P Three-dimensional speckle tracking of the right ventricle: toward optimal quantification of right ventricular dysfunction in pulmonary hypertension Journal of the American College of Cardiology 64 2014. 41–51. 10.1016/j.jacc.2014.01.084 [DOI] [PubMed] [Google Scholar]
- 43. Grapsa J Gibbs JS Cabrita IZ Watson GF Pavlopoulos H Dawson D Gin-Sing W Howard LS Nihoyannopoulos P The association of clinical outcome with right atrial and ventricular remodelling in patients with pulmonary arterial hypertension: study with real-time three-dimensional echocardiography European Heart Journal Cardiovascular Imaging 13 2012. 666–672. 10.1093/ehjci/jes003 [DOI] [PubMed] [Google Scholar]
- 44. D'Andrea A Gravino R Riegler L Salerno G Scarafile R Romano M Cuomo S Del Viscovo L Ferrara I De Rimini ML et al. Right ventricular ejection fraction and left ventricular dyssynchrony by 3D echo correlate with functional impairment in patients with dilated cardiomyopathy Journal of Cardiac Failure 17 2011. 309–317. 10.1016/j.cardfail.2010.11.005 [DOI] [PubMed] [Google Scholar]
- 45. Calcutteea A Chung R Lindqvist P Hodson M Henein MY Differential right ventricular regional function and the effect of pulmonary hypertension: three-dimensional echostudy Heart 97 2011. 1004–1011. 10.1136/hrt.2010.208900 [DOI] [PubMed] [Google Scholar]
- 46. Aune E Baekkevar M Rodevand O Otterstad JE The limited usefulness of real-time 3-dimensional echocardiography in obtaining normal reference ranges for right ventricular volumes. Cardiovascular Ultrasound. 2009;7:35. doi: 10.1186/1476-7120-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
3D echocardiography reconstruction of a right ventricle (green) with normal systolic function (end-diastolic volume 153 ml, end-systolic volume 64 ml, ejection fraction 58 %). The mesh is the right ventricle at end-diastole. The white areas are the pulmonary valve in the upper left side and the tricuspid valve in the upper right side. The right ventricular apex is towards the bottom. Data was processed with dedicated software (4D RV-Function, TomTec Imaging Systems). Download Video 1 via http://dx.doi.org/10.1530/ERP-14-0077-v1
Download Video 1 (1.6MB, avi)
Example of a 3D echocardiographic reconstruction of a patient with pulmonary hypertension (end-diastolic volume 200 ml, end-systolic volume 137 ml, ejection fraction 31 %) showing an enlarged right ventricle (green) with a flattened septum in diastole (mesh) and even more so in systole. Both the longitudinal and lateral function appear altered. Data was processed with dedicated software (4D RV-Function, TomTec Imaging Systems). Download Video 2 via http://dx.doi.org/10.1530/ERP-14-0077-v2
Download Video 2 (1.2MB, avi)
Example of semi-automated delineation of the right ventricle without manual correction. The semi-automated delineation crosses, and hence includes, parts of the septum in the right ventricular volume. On the other hand, the delineation does not follow the anterior part of the right ventricular outflow tract and that part is therefore by default not included in automated volume calculation. Data was processed with dedicated software (4D RV-Function, TomTec Imaging Systems). Download Video 3 via http://dx.doi.org/10.1530/ERP-14-0077-v3
Download Video 3 (1.6MB, avi)



 This work is licensed under a
This work is licensed under a