Abstract
The clinical spectrum of hypertrophic cardiomyopathy (HCM) is complex and includes a variety of phenotypes, which leads to different types of manifestations. Although most of the patients are asymptomatic, a significant proportion of them will develop symptoms or risk of arrhythmias and sudden cardiac death (SCD). Therefore, the objectives of HCM diagnosis and management are to relieve the patients' symptoms (chest pain, heart failure, syncope, palpitations, etc.), prevent disease progression and major cardiovascular complications and SCD. The heterogeneity of HCM patterns, their symptoms and assessment is a challenge for the cardiologist.
Keywords: hypertrophic cardiomyopathy, left ventricular outflow tract obstruction, amyloidosis, cardiac magnetic resonance imaging, Anderson-Fabry's disease, Friedreich's ataxia
Introduction
Hypertrophic cardiomyopathy (HCM) is an inherited heart disease defined by increased left ventricular (LV) wall thickness (≥15 mm in one or more LV myocardial segments), that cannot be explained by abnormal loading conditions (1). It is an autosomal dominant condition, which is present in one in 500 in the general adult population, making it the commonest genetic cardiovascular disease.
The clinical spectrum of HCM is complex and includes a variety of phenotypes, which leads to different types of manifestations. Although most of the patients are asymptomatic, ∼25% will develop symptoms or risk of arrhythmias and sudden cardiac death (SCD) (2).
Therefore, the objectives of HCM diagnosis and management are to relieve the patients' symptoms (chest pain, heart failure, syncope, palpitations, etc.), prevent disease progression and major cardiovascular complications, and SCD (2, 3). The heterogeneity of HCM patterns, their symptoms and assessment are a challenge for the cardiologist.
Diagnosis
The mainstay of the diagnosis is the increased LV wall thickness ≥15 mm (1). The distribution of LV hypertrophy (LVH) is characteristically asymmetric and heterogeneous, but most often involves the interventricular septum more than the posterolateral segments. Symmetric, apical and other atypical distributions are also observed (4, 5). Wall thickness is usually measured in M-mode images. However, M-mode measurements include only basal segments of interventricular septum and posterior wall and an incorrect alignment of the cursor may result in incorrect evaluation. For that reason, measurements of the wall thickness should be performed in all the segments in two-dimensional (2D) images in short-axis views from basal to apex. Asymmetric septal wall hypertrophy is associated with LV outflow tract obstruction (LVOTO) in 20–30% of cases at rest (6). Symmetric hypertrophy appears to be present in ∼4% of HCM cases and should raise the suspicion of other causes of LV thickening (7). The occurrence of apical HCM, however, varies highly in the literature, ranging from 1 to 25% (4, 8). The diagnosis of apical HCM may be missed in standard transthoracic echocardiography and may be revealed with the use of 3D echocardiography, i.v. contrast agents or cardiac magnetic resonance imaging (MRI) (9, 10) (Videos 1 and 2). The apex can form an aneurysm in some of these cases (7) (Videos 3 and 4). Predominant hypertrophy of the middle third of the left ventricle may lead to severe mid-ventricular narrowing and obstruction, which may also be associated with the formation of apical aneurysms, which result from the increased systolic pressures within the cardiac apex from the mid-ventricular obstruction or apical infarction (7, 11).
2D echocardiogram: 4-chamber view showing hypertrophy of the LV apex. Download Video 1 via http://dx.doi.org/10.1530/ERP-15-0007-v1
Download Video 1 (718.9KB, avi)
Apical HCM on parasternal short axis at the level of the apex. There is concentric hypertrophy and collapse of the LV cavity during systole. Download Video 2 via http://dx.doi.org/10.1530/ERP-15-0007-v2
Download Video 2 (438.6KB, avi)
The same patient with HCM scanned without contrast. The use of i.v. contrast offers a better view of the endocardium. Apical aneurysm is clearly visualized on the contrast echocardiogram. Download Video 3 via http://dx.doi.org/10.1530/ERP-15-0007-v3
Download Video 3 (295.5KB, avi)
The same patient with HCM scanned with contrast. The use of i.v. contrast offers a better view of the endocardium. Apical aneurysm is clearly visualized on the contrast echocardiogram. Download Video 4 via http://dx.doi.org/10.1530/ERP-15-0007-v4
Download Video 4 (883.5KB, avi)
Owing to a number of complex mechanisms involving also abnormal dissociation of actin and myosin filaments during the active phase of relaxation in early diastolic filling and LV myocardial properties, HCM is often associated with diastolic dysfunction (12). Decreased tissue Doppler indices-systolic annular velocities and increased E/E′ are typical findings for this (13). In pre-phenotypic gene-positive HCM, the E/E′ ratio has been described as a marker that can distinguish those individuals from controls (14). In later stages, the diastolic dysfunction may result in a restrictive filling pattern with secondary (consecutive) left atrial dilatation, which then may lead to the development of atrial fibrillation (11, 15). Throughout the course of the disease, there may be a ‘burn out’ phase, characterised by LV dilatation and loss of myocardium, which then is replaced by fibrosis, which is thought to be caused by small-vessel ischaemia (7, 16, 17). The absolute LV dilatation may be variably present and does not predict the outcome, but in serial observations increasing dimensions of the LV end-diastolic cavity have been described as well as decreasing septal wall thickness and LV ejection fraction (18).
Features in specific subtypes
The presence of LVH is a finding observed in systemic diseases with cardiac involvement such as Friedreich's ataxia (FRDA) or Noonan's syndrome and metabolic diseases (i.e. Pompe, Fabry, mitochondrial disorders, amyloidosis, etc.) (6).
Anderson–Fabry's disease
Fabry disease is an X-linked lysosomal storage disorder caused by α-galactosidase A deficiency. Cardiac involvement is frequent and there is a strong correlation between age and LVH; all patients above 45 years of age are affected (19). A typical histological feature is the presence of lipid storage in all the cells of the heart, including myocytes, conduction system, valves and endothelium (20).
The common pattern is concentric non-obstructive LVH with end-diastolic wall thickness mildly increased (21), which may develop LV systolic dysfunction as the disease progresses (19, 21, 22, 23, 24).
2D echocardiogram usually shows normal left ventricle systolic function although the diastolic function could be mildly affected. Prominent papillary muscles have been described (25). A ‘Binary sign’, defined as an hyperechogenic interventricular septum border (26), has been described as a characteristic feature of Fabry's disease. Although it could shed light on the diagnosis, this feature has been questioned considering that it is not specific for Fabry's disease and it is highly operator dependent (27, 28). The right ventricle (RV) is commonly affected with hypertrophy but without haemodynamic significance (19, 25). In addition, tissue Doppler imaging (TDI) and strain studies would help to detect early stage of cardiac dysfunction revealing reduced longitudinal and radial LV function. Segmental wall motion abnormality is only present at the advanced stage of the cardiomyopathy and is typically observed in the postero-lateral segments (19, 21, 22, 23, 24). Diastolic dysfunction is usually associated with the presence of LVH although Doppler studies may be able to detect it earlier than the development of LVH (24, 29, 30, 31).
Using cardiac magnetic resonance (CMR) with late gadolinium enhancement technique, replacement fibrosis has been described, especially in the basal inferior and postero-lateral segments (21, 32).
Friedreich's ataxia
FRDA is the commonest hereditary ataxia, autosomal recessively, caused by an inherited expansion of an intronic GAA triplet. Histological features explain that the hypertrophy derives from a striking proliferation of mitochondria within the cardiomyocytes, and a marked loss of contractile fibres (33). The cardiac involvement is high (more than 60% of patient affected) and usually asymptomatic (34). The typical pattern is concentric LVH with an end-diastolic wall thickness of <15 mm and absence of outflow tract obstruction (34, 35) (Video 5).
Parasternal long axis view of patient with FRDA showing concentric LVH without LVOT obstruction. Download Video 5 via http://dx.doi.org/10.1530/ERP-15-0007-v5
Download Video 5 (377.5KB, avi)
In a study of 178 patients, 42% showed concentric remodelling on the echocardiogram, 35% concentric hypertrophy (Video 6), and only 5% eccentric hypertrophy (36). This study also demonstrated a high percentage (>80%) of patients with abnormal diastolic function. Increased relative wall thickness as a sign of concentric remodelling has been discussed in these patients (37, 38).
Patient with FRDA. Parasternal short axis at the mitral valve level, showing concentric LVH involving all segments, more prominent on the anteroseptum. Download Video 6 via http://dx.doi.org/10.1530/ERP-15-0007-v6
Download Video 6 (470.5KB, avi)
There is impaired systolic function but with relatively preserved ejection fraction. Some patients with advanced disease develop a reduced ejection fraction with global hypokinesia and a slightly dilated LV (39). CMR often confirms the LVH and increased LV mass (35). LV mass decreases with longer disease duration (>15 years) suggesting cardiac thinning with prolonged disease (40).
Amyloidosis
Amyloidosis is an infiltrative disease characterised by deposition of amyloid fibrils within the extracellular tissue of one or multiple organs, including the heart. Cardiac amyloidosis pattern is symmetric LV thickening. Longitudinal LV function is severely affected in amyloidosis and this feature can carry diagnostic information (41). Although there are some echocardiographic features commonly associated with amyloidosis, such as ‘sparkling or speckled appearance’ of the LV thickening, there is no objective evidence that they can be used for the diagnosis. RV wall is usually hypertrophied as well. Diastolic function is often impaired with restrictive filling pattern in the advanced stages of the disease (42). Severe atrial dilatation, thickened interatrial septum and pericardial effusion are common findings on cardiac amyloidosis. The severe atrial dilatation is a consequence of the elevated filling pressures; subsequently high E/A and E/e ratios are usually present (42, 43, 44) (Video 7).
Patient with amyloidosis, 4-chamber view showing concentric LVH, more prominent in the septum. There is septal hypokinesia and impairment of the LV systolic function. Loss of longitudinal systolic function. Both atria are dilated. Download Video 7 via http://dx.doi.org/10.1530/ERP-15-0007-v7
Download Video 7 (273.2KB, avi)
CMR shows sub-endocardial or segmental late gadolinium enhancement (LGE) and a highly specific pattern of myocardial and blood-pool gadolinium kinetics caused by similar myocardial and blood T1 signals (45).
There is delayed enhancement in 69% of patients, with the dominant distribution of enhancement being subendocardial, diffuse and not confined to one clear vascular territory (46, 42).
The presence of the above features could raise suspicion of amyloidosis as the aetiology of LVH rather than sarcomeric HCM, especially in the appropriate clinical context (6, 46, 42).
Anatomical abnormalities
There are a number of anatomical abnormalities of the mitral valve and the subvalvular apparatus commonly observed in HCM. Some of them may have their origin in the cardiac morphogenesis and others are more consistent with acquired changes. They play a role in the LVOTO, mid-cavity obstruction and the presence of mitral regurgitation (MR). These abnormalities include papillary muscle abnormalities (hypertrophy, anterior and internal displacement, direct insertion into the anterior mitral valve leaflet) and mitral leaflet abnormalities such as elongation or accessory tissue (1, 47, 48, 49). Systolic anterior motion (SAM) was described as a feature typical for HCM more than 50 years ago. But SAM can also be observed in conditions other than HCM. The LVOTO as a consequence of SAM is a common clinical finding that requires a detailed assessment from anatomical and clinical point of view. The severity of the SAM is related to the duration of leaflet/chordal contact with the septum (severe if >30%) (43). Abnormalities of the mitral valve usually present with excessive leaflet tissue, elongation of the chordal or mitral leaflets, anterior displacement of the mitral apparatus and abnormal insertion of the papillary muscle (directly into the anterior mitral leaflet), prolapse of one or both leaflets (43). The mechanism of the MR is variable. If the MR is solely related to SAM, then it is usually posteriorly directed as a consequence of the mitral leaflet abnormal coaptation (5). Leaflet contact length, posterior leaflet length, ratio of anterior-to-posterior leaflet length and posterior leaflet mobility have been described as significant univariate predictors of MR in relation to mitral SAM (50). Therefore, a meticulous assessment of mitral valve morphology is essential in HCM, particularly in those cases with LVOTO. A correct distinction between functional and primary valve pathology is also crucial for the optimal management of the patient. Transoesophageal echocardiography plays a key role in decision making before any invasive LVOTO therapy and in those cases in which severe MR caused by intrinsic valve abnormalities is suspected (1).
Overlap with other conditions
Differentiation of HCM against hypertensive heart disease, athlete's heart and LV non-compaction (LVNC) can be a challenge. Hypertension alone usually produces concentric remodelling of the left ventricle, isolated increased voltage without repolarisation abnormalities in the 12-lead-ECG and reversibility of hypertrophy after 6–12 months of tight systolic blood pressure control point towards this diagnosis, whereas a family history of HCM, RV hypertrophy, late gadolinium enhancement at the RV insertion points or localised to segments of maximum wall thickness on CMR, a maximum LV wall thickness ≥15 mm in Caucasians and ≥20 mm in Blacks, severe diastolic dysfunction and marked depolarisation abnormalities are more in keeping with HCM (1, 51). In Black people, diagnosis may be assisted by the identification of a non-hypertensive relative or genetic testing (6). Athletes tend to have an enlarged LV cavity, an enlarged left atrium, normal LV filling and relaxation, upright T-waves and a negative family history (52). Female sex, missing response to detraining and systolic anterior movement of the mitral valve are more in favour of the diagnosis of HCM (1). LVNC is characterised by the embryonic pattern of trabeculated myocardium in the left ventricle (53). It is, therefore, defined by prominent LV trabeculae, deep intertrabecular recesses and a thin compact layer (54). Use of contrast echocardiographic agents can be very useful for the delineation of the endocardium and identification of features compatible with LVNC (55).
LVOT obstruction
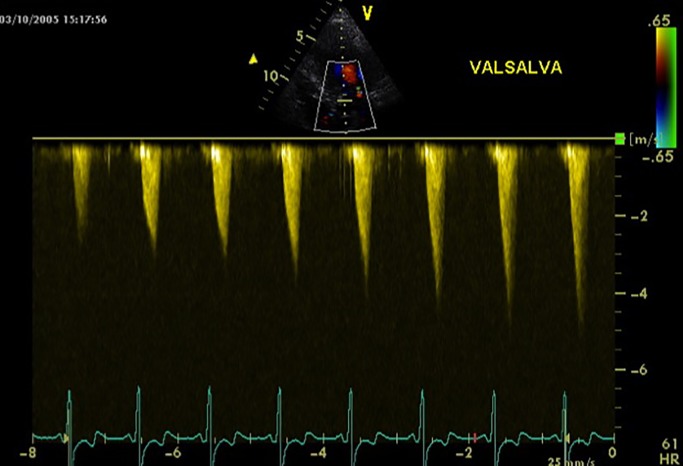
The presence of LVOTO should be suspected in all patients reporting symptoms in their daily activity or during exercise without evidence of resting LVOT pressure gradient. Significant gradient at rest is present in only 25–30% of patients with HCM (56); 75% of HCM patients develop LVOTO at rest or on provocation. The identification of LVOTO has important implications both in the management of symptoms and in the assessment of SCD; therefore, 2D and Doppler echocardiography during Valsalva manoeuvre in the sitting and semi-supine position and standing is recommended in all patients (1) (Fig. 1). Therefore, in addition to standing and Valsalva manoeuvres, some of these patients should be investigated for dynamic LVOTO either by exercise or by the use of glyceryl trinitrate (GTN). Exercise is considered the most physiological and effective modality to precipitate and assess the degree of obstruction. Exercise stress echocardiography is a well-validated safe technique (57, 58, 59) for the assessment of dynamic obstruction in adult asymptomatic and symptomatic HCM population. Several studies demonstrate, by the use of distinct methodologies, that 50–75% of patients with rest peak gradient of ≤30 or 50 mmHg present with ventricular obstruction easily induced by exercise (60, 61, 62). The incidence and severity of exercise-induced LVOT can be increased in post-prandial tests (63). A study has demonstrated that early development of obstruction is associated with higher reduction in the function capacity (64). Another study has shown a progressive increase in the LVOT gradient by a range of physiologic manoeuvres: from standing to Valsalva to exercise (65). The recording of the gradient at the peak of exercise can pose technical difficulties in obtaining the images. Considering the lack of comparative data of stress protocol, according to guidelines, laboratories should develop and validate their own data and ensure that staff are properly trained in the procedure (1). In patients unable to perform an adequate exercise test, sublingual GTN may be used to unmask dynamic obstruction, but it is not physiological and can be poorly tolerated (66). Other pharmacological provocations are not recommended in this clinical setting, being non-physiological and leading to a high rate of false positive: 17–43% of patients can develop LVOT gradient during dobutamine stress echocardiography and this is not predictive of LVOTO in their ‘real life’ (67, 68, 69).
Figure 1.
Continuous Doppler in a HCM patient during Valsalva. The LVOT gradient is progressively increasing during the manoeuvre.
Limitations of echocardiography
Radial contractile function, assessed using the ejection fraction or fractional shortening, is typically normal or supra-normal in patients with HCM both obstructive and non-obstructive. This is also in presence of long-axis function impairment, as demonstrated by the tissue Doppler-derived mitral annular velocities (70). Unfortunately, ejection fraction (EF) is a suboptimal measurement of ventricular function, mainly because of low end-diastolic LV volume. Additionally, the increased wall thickness results in augmented radial wall thickening and overestimation of ventricular systolic function (71, 72). There are suggestions that LV torsion and strain imaging may detect global subtle dysfunction even when traditional measurements are normal (13).
Echocardiography may underestimate the wall thickness when hypertrophy is confined to the anterolateral wall (73), posterior septum or apex (5, 74). In these cases, meticulous imaging and multiple views are mandatory and the use of other imaging modalities (i.e. 3D-echocardiography, contrast echocardiogram, cardiac computed tomography (cardiac CT) and CMR) should be considered (75).
Although the advantage of the use of cardiac MRI in patients with good echocardiography images is limited to the tissue characterisation, this technique can occasionally offer a more precise assessment of the pattern of hypertrophy and submitral valvular apparatus (76), quantification of LV mass (77), detection of myocardial crypts (78), aneurysm and thrombi (79, 80).
Management
Although most patients have a benign course of the disease, HCM can lead to heart failure and sudden death with an annual mortality ranging between 1 and 5% (81). In this context, echocardiography plays a key role in management and risk stratification.
Systolic dysfunction occurs in 10–15% of patients as a result of wall thinning, cavity dilatation and fibrosis, associated with the risk of SCD and overall increased mortality (18). Therefore, baseline and routine assessment of systolic function by means of Biplane Simpson's ejection fraction are important to guide any anti-failure therapy.
In some patients, heart failure is associated with diastolic dysfunction with preserved EF and small LV size. Assessment of diastolic dysfunction by use of Doppler echocardiography, in particular through the use of E/Ea ratio, provides an accurate estimation of filling pressures (82, 83, 84). The E wave/propagation velocity has also been shown to correlate well with invasively measured pressures (83). A mitral annular systolic velocity ≤4 cm/s assessed through TDI has been shown to be an independent predictor of hospitalisation for worsening heart failure (85). Predicting risk is a key component of the management of HCM: wall thickness, LVOTO gradient and left atrium dimension are part of the factors for the risk stratification of HCM patients (86). It is well established that the severity and extent of LVH measured by echocardiography are associated with the risk of SCD: a maximum wall thickness of ≥30 mm has a greater risk of SCD (87, 88, 89). The size of the left atrium is often enlarged and provides important prognostic information (86, 90). The majority of studies have used the anteroposterior LA diameter, but comparable findings are reported using LA volume indexed body also (15, 91, 92).
Despite the significant association between LVTO and SCD (86, 93, 94, 95), the question about the prognostic significance of the provocable LVOTO and the LVOT gradient after treatment remains unresolved. A recent retrospective study has suggested a lower rate of implantable defibrillator insertion, adverse events, medical interventions and septal ablations in patients who show a paradoxical response to exercise defined as a reduction in the LVOT gradient during exercise, therefore reinforcing the importance of echocardiography in risk stratification (96). Echocardiography also plays an important role in guiding interventions: through the use of myocardial contrast echocardiography, the target septal branch is identified during alcohol septal ablation, avoiding alcohol injection into the LV free wall or papillary muscles (97).
Conclusions
Despite some limitations, in the clinical scenario of a patient presenting with LVH, echocardiography plays a central role in the diagnosis of HCM, management of symptoms, risk stratification and decision for treatment. An accurate echocardiographic evaluation is the most helpful imaging test at baseline and follow-up and should be performed by well-trained operators.
Footnotes
(A S Vischer, M C Perez-Tome and S Castelletti contributed equally to this review)
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Funding
A S Vischer was supported by a research grant from the Swiss Heart Rhythm foundation. M C Perez-Tome was supported by a research grant from the Spanish Society of Cardiology. S Castelletti was supported by a research grant from the European Society of Cardiology.
References
- 1. Authors/Task Force members Elliott PM Anastasakis A Borger MA Borggrefe M Cecchi F Charron P Hagege AA Lafont A Limongelli G et al. ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC) European Heart Journal 35 2014. 2733–2779. 10.1093/eurheartj/ehu284 [DOI] [PubMed] [Google Scholar]
- 2. Maron BJ Casey SA Poliac LC Gohman TE Almquist AK Aeppli DM Clinical course of hypertrophic cardiomyopathy in a regional United States cohort Journal of the American Medical Association 281 1999. 650–655. 10.1001/jama.281.7.650 [DOI] [PubMed] [Google Scholar]
- 3. Enriquez AD Goldman ME Management of hypertrophic cardiomyopathy Annals of Global Health 80 2014. 35–45. 10.1016/j.aogh.2013.12.004 [DOI] [PubMed] [Google Scholar]
- 4. Klues HG Schiffers A Maron BJ Phenotypic spectrum and patterns of left ventricular hypertrophy in hypertrophic cardiomyopathy: morphologic observations and significance as assessed by two-dimensional echocardiography in 600 patients Journal of the American College of Cardiology 26 1995. 1699–1708. 10.1016/0735-1097(95)00390-8 [DOI] [PubMed] [Google Scholar]
- 5. Maron MS Maron BJ Harrigan C Buros J Gibson CM Olivotto I Biller L Lesser JR Udelson JE Manning WJ et al. Hypertrophic cardiomyopathy phenotype revisited after 50 years with cardiovascular magnetic resonance Journal of the American College of Cardiology 54 2009. 220–228. 10.1016/j.jacc.2009.05.006 [DOI] [PubMed] [Google Scholar]
- 6. Elliott P McKenna WJ Hypertrophic cardiomyopathy Lancet 363 2004. 1881–1891. 10.1016/S0140-6736(04)16358-7 [DOI] [PubMed] [Google Scholar]
- 7. Hughes SE The pathology of hypertrophic cardiomyopathy Histopathology 44 2004. 412–427. 10.1111/j.1365-2559.2004.01835.x [DOI] [PubMed] [Google Scholar]
- 8. Sperling RT Parker JA Manning WJ Danias PG Apical hypertrophic cardiomyopathy: clinical, electrocardiographic, scintigraphic, echocardiographic, and magnetic resonance imaging findings of a case Journal of Cardiovascular Magnetic Resonance 4 2002. 291–295. 10.1081/JCMR-120003956 [DOI] [PubMed] [Google Scholar]
- 9. Moon JC Fisher NG McKenna WJ Pennell DJ Detection of apical hypertrophic cardiomyopathy by cardiovascular magnetic resonance in patients with non-diagnostic echocardiography Heart 90 2004. 645–649. 10.1136/hrt.2003.014969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Walpot J Pasteuning WH Shivalkar B Apical hypertrophic cardiomyopathy: elegant use of contrast-enhanced echocardiography in the diagnostic work-up Acta Cardiologica 67 2012. 495–497. 10.2143/AC.67.4.2170697 [DOI] [PubMed] [Google Scholar]
- 11. Wigle ED Cardiomyopathy: the diagnosis of hypertrophic cardiomyopathy Heart 86 2001. 709–714. 10.1136/heart.86.6.709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shirani J Pick R Roberts WC Maron BJ Morphology and significance of the left ventricular collagen network in young patients with hypertrophic cardiomyopathy and sudden cardiac death Journal of the American College of Cardiology 35 2000. 36–44. 10.1016/S0735-1097(99)00492-1 [DOI] [PubMed] [Google Scholar]
- 13. Shetty R Samanth J Nayak K Sarang A Thakkar A Evaluation of subtle left ventricular systolic abnormalities in adult patients with hypertrophic cardiomyopathy Journal of Clinical and Diagnostic Research 8 2014. MC05–MC09. 10.7860/JCDR/2014/10185.5287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nagueh SF McFalls J Meyer D Hill R Zoghbi WA Tam JW Quiñones MA Roberts R Marian AJ Tissue Doppler imaging predicts the development of hypertrophic cardiomyopathy in subjects with subclinical disease Circulation 108 2003. 395–398. 10.1161/01.CIR.0000084500.72232.8D [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tani T Tanabe K Ono M Yamaguchi K Okada M Sumida T Konda T Fujii Y Kawai J Yagi T et al. Left atrial volume and the risk of paroxysmal atrial fibrillation in patients with hypertrophic cardiomyopathy Journal of the American Society of Echocardiography 17 2004. 644–648. 10.1016/j.echo.2004.02.010 [DOI] [PubMed] [Google Scholar]
- 16. Kawashima T Yokota Y Yokoyama M Itoh H Pathological analysis of hypertrophic cardiomyopathy simulating dilated cardiomyopathy Acta Pathologica Japonica 43 1993. 304–312. [DOI] [PubMed] [Google Scholar]
- 17. Spirito P Maron BJ Bonow RO Epstein SE Occurrence and significance of progressive left ventricular wall thinning and relative cavity dilatation in hypertrophic cardiomyopathy American Journal of Cardiology 60 1987. 123–129. 10.1016/0002-9149(87)90998-2 [DOI] [PubMed] [Google Scholar]
- 18. Harris KM Spirito P Maron MS Zenovich AG Formisano F Lesser JR Mackey-Bojack S Manning WJ Udelson JE Maron BJ Prevalence, clinical profile, and significance of left ventricular remodeling in the end-stage phase of hypertrophic cardiomyopathy Circulation 114 2006. 216–225. 10.1161/CIRCULATIONAHA.105.583500 [DOI] [PubMed] [Google Scholar]
- 19. Sheppard MN The heart in Fabry's disease Cardiovascular Pathology 20 2011. 8–14. 10.1016/j.carpath.2009.10.003 [DOI] [PubMed] [Google Scholar]
- 20. Desnick RJ Blieden LC Sharp HL Hofschire PJ Moller JH Cardiac valvular anomalies in Fabry disease. Clinical, morphologic, and biochemical studies Circulation 54 1976. 818–825. 10.1161/01.CIR.54.5.818 [DOI] [PubMed] [Google Scholar]
- 21. Weidemann F Niemann M Warnock DG Ertl G Wanner C The Fabry cardiomyopathy: models for the cardiologist Annual Review of Medicine 62 2011. 59–67. 10.1146/annurev-med-090910-085119 [DOI] [PubMed] [Google Scholar]
- 22. Linhart A Kampmann C Zamorano JL Sunder-Plassmann G Beck M Mehta A Elliott PM European FOS Investigators Cardiac manifestations of Anderson–Fabry disease: results from the international Fabry outcome survey European Heart Journal 28 2007. 1228–1235. 10.1093/eurheartj/ehm153 [DOI] [PubMed] [Google Scholar]
- 23. Takenaka T Teraguchi H Yoshida A Taguchi S Ninomiya K Umekita Y Yoshidac H Horinouchid M Tabatad K Yonezawa S et al. Terminal stage cardiac findings in patients with cardiac Fabry disease: an electrocardiographic, echocardiographic, and autopsy study Journal of Cardiology 51 2008. 50–59. 10.1016/j.jjcc.2007.12.001 [DOI] [PubMed] [Google Scholar]
- 24. Pieroni M Chimenti C Ricci R Sale P Russo MA Frustaci A Early detection of Fabry cardiomyopathy by tissue Doppler imaging Circulation 107 2003. 1978–1984. 10.1161/01.CIR.0000061952.27445.A0 [DOI] [PubMed] [Google Scholar]
- 25. Weidemann F Strotmann JM Niemann M Herrmann S Wilke M Beer M Voelker W Ertl G Emmert A Wanner C et al. Heart valve involvement in Fabry cardiomyopathy Ultrasound in Medicine & Biology 35 2009. 730–735. 10.1016/j.ultrasmedbio.2008.10.010 [DOI] [PubMed] [Google Scholar]
- 26. Pieroni M Chimenti C De Cobelli F Morgante E Del Maschio A Gaudio C Russo MA Frustaci A Fabry's disease cardiomyopathy Journal of the American College of Cardiology 47 2006. 1663–1671. 10.1016/j.jacc.2005.11.070 [DOI] [PubMed] [Google Scholar]
- 27. Mundigler G Gaggl M Heinze G Graf S Zehetgruber M Lajic N Voigtländer T Mannhalter C Sunder-Plassmann R Paschke E et al. The endocardial binary appearance (‘binary sign’) is an unreliable marker for echocardiographic detection of Fabry disease in patients with left ventricular hypertrophy European Journal of Echocardiography 12 2011. 744–749. 10.1093/ejechocard/jer112 [DOI] [PubMed] [Google Scholar]
- 28. Kounas S Demetrescu C Pantazis AA Keren A Lee PJ Hughes D Mehta A Elliott PM The binary endocardial appearance is a poor discriminator of Anderson–Fabry disease from familial hypertrophic cardiomyopathy Journal of the American College of Cardiology 51 2008. 2058–2061. 10.1016/j.jacc.2008.02.046 [DOI] [PubMed] [Google Scholar]
- 29. Weidemann F Breunig F Beer M Sandstede J Störk S Voelker W Ertl G Knoll A Wanner C Strotmann JM The variation of morphological and functional cardiac manifestation in Fabry disease: potential implications for the time course of the disease European Heart Journal 26 2005. 1221–1227. 10.1093/eurheartj/ehi143 [DOI] [PubMed] [Google Scholar]
- 30. Nishimura RA Appleton CP Redfield MM Ilstrup DM Holmes DR Tajik AJ Noninvasive Doppler echocardiographic evaluation of left ventricular filling pressures in patients with cardiomyopathies: a simultaneous Doppler echocardiographic and cardiac catheterization study Journal of the American College of Cardiology 28 1996. 1226–1233. 10.1016/S0735-1097(96)00315-4 [DOI] [PubMed] [Google Scholar]
- 31. Nagueh SF Bachinski LL Meyer D Hill R Zoghbi WA Tam JW Quiñones MA Roberts R Marian AJ Tissue Doppler imaging consistently detects myocardial abnormalities in patients with hypertrophic cardiomyopathy and provides a novel means for an early diagnosis before and independently of hypertrophy Circulation 104 2001. 128–130. 10.1161/01.CIR.104.2.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Madonna R Cevik C Cocco N Multimodality imaging for pre-clinical assessment of Fabry's cardiomyopathy European Heart Journal Cardiovascular Imaging 15 2014. 1094–1100. 10.1093/ehjci/jeu080 [DOI] [PubMed] [Google Scholar]
- 33. Payne RM Wagner GR Cardiomyopathy in Friedreich ataxia: clinical findings and research Journal of Child Neurology 27 2012. 1179–1186. 10.1177/0883073812448535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Payne RM Peverill RE Cardiomyopathy of Friedreich's ataxia (FRDA) Irish Journal of Medical Science 181 2012. 569–570. 10.1007/s11845-012-0808-7 [DOI] [PubMed] [Google Scholar]
- 35. Parkinson MH Boesch S Nachbauer W Mariotti C Giunti P Clinical features of Friedreich's ataxia: classical and atypical phenotypes Journal of Neurochemistry 126: Suppl 1 2013. 103–117. 10.1111/jnc.12317 [DOI] [PubMed] [Google Scholar]
- 36. Regner SR Lagedrost SJ Plappert T Paulsen EK Friedman LS Snyder ML Perlman SL Mathews KD Wilmot GR Schadt KA et al. Analysis of echocardiograms in a large heterogeneous cohort of patients with Friedreich ataxia American Journal of Cardiology 109 2012. 401–405. 10.1016/j.amjcard.2011.09.025 [DOI] [PubMed] [Google Scholar]
- 37. Weidemann F Rummey C Bijnens B Störk S Jasaityte R Dhooge J Baltabaeva A Sutherland G Schulz JB Meier T et al. The heart in Friedreich ataxia: definition of cardiomyopathy, disease severity, and correlation with neurological symptoms Circulation 125 2012. 1626–1634. 10.1161/CIRCULATIONAHA.111.059477 [DOI] [PubMed] [Google Scholar]
- 38. Dedobbeleer C Rai M Donal E Pandolfo M Unger P Normal left ventricular ejection fraction and mass but subclinical myocardial dysfunction in patients with Friedreich's ataxia European Heart Journal Cardiovascular Imaging 13 2012. 346–352. 10.1093/ejechocard/jer267 [DOI] [PubMed] [Google Scholar]
- 39. Weidemann F Störk S Liu D Hu K Herrmann S Ertl G Niemann M Cardiomyopathy of Friedreich ataxia Journal of Neurochemistry 126: Suppl 1 2013. 88–93. 10.1111/jnc.12217 [DOI] [PubMed] [Google Scholar]
- 40. Rajagopalan B Francis JM Cooke F Korlipara LV Blamire AM Schapira AH Madan J Neubauer S Cooper JM Analysis of the factors influencing the cardiac phenotype in Friedreich's ataxia Movement Disorders 25 2010. 846–852. 10.1002/mds.22864 [DOI] [PubMed] [Google Scholar]
- 41. Zhang L Wang Y Cheng L Wang J Zhou X Liu M Zhang W Zhang M Zhang B Zhi G Value of assessing left ventricular longitudinal systolic peak strain in differential diagnosis of primary cardiac amyloidosis from hypertrophic cardiomyopathy Nan Fang Yi Ke Da Xue Xue Bao 34 2014. 609–616. [PubMed] [Google Scholar]
- 42. Aljaroudi WA Desai MY Tang WH Phelan D Cerqueira MD Jaber WA Role of imaging in the diagnosis and management of patients with cardiac amyloidosis: state of the art review and focus on emerging nuclear techniques Journal of Nuclear Cardiology 21 2014. 271–283. 10.1007/s12350-013-9800-5 [DOI] [PubMed] [Google Scholar]
- 43. Williams LK Frenneaux MP Steeds RP Echocardiography in hypertrophic cardiomyopathy diagnosis, prognosis, and role in management European Journal of Echocardiography 10 2009. iii9–ii14. 10.1093/ejechocard/jep157 [DOI] [PubMed] [Google Scholar]
- 44. Falk RH Quarta CC Dorbala S How to image cardiac amyloidosis Circulation. Cardiovascular Imaging 7 2014. 552–562. 10.1161/CIRCIMAGING.113.001396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Syed IS Glockner JF Feng D Araoz PA Martinez MW Edwards WD Gertz MA Dispenzieri A Oh JK Bellavia D et al. Role of cardiac magnetic resonance imaging in the detection of cardiac amyloidosis JACC. Cardiovascular Imaging 3 2010. 155–164. 10.1016/j.jcmg.2009.09.023 [DOI] [PubMed] [Google Scholar]
- 46. Hansen MW Merchant N MRI of hypertrophic cardiomyopathy: part 2, Differential diagnosis, risk stratification, and posttreatment MRI appearances AJR. American Journal of Roentgenology 189 2007. 1344–1352. 10.2214/AJR.07.2287 [DOI] [PubMed] [Google Scholar]
- 47. Maron MS Olivotto I Harrigan C Appelbaum E Gibson CM Lesser JR Haas TS Udelson JE Manning WJ Maron BJ Mitral valve abnormalities identified by cardiovascular magnetic resonance represent a primary phenotypic expression of hypertrophic cardiomyopathy Circulation 124 2011. 40–47. 10.1161/CIRCULATIONAHA.110.985812 [DOI] [PubMed] [Google Scholar]
- 48. Lee S-P Park K Kim H-K Kim Y-J Sohn D-W Apically displaced papillary muscles mimicking apical hypertrophic cardiomyopathy European Heart Journal Cardiovascular Imaging 14 2013. 128–134. 10.1093/ehjci/jes113 [DOI] [PubMed] [Google Scholar]
- 49. Yang HS Lee KS Chaliki HP Tazelaar HD Lusk JL Chandrasekaran K Tajik AJ Anomalous insertion of the papillary muscle causing left ventricular outflow obstruction: visualization by real-time three-dimensional echocardiography European Journal of Echocardiography 9 2008. 855–860. 10.1093/ejechocard/jen197 [DOI] [PubMed] [Google Scholar]
- 50. Schwammenthal E Nakatani S He S Hopmeyer J Sagie A Weyman AE Lever HM Yoganathan AP Thomas JD Levine RA Mechanism of mitral regurgitation in hypertrophic cardiomyopathy: mismatch of posterior to anterior leaflet length and mobility Circulation 98 1998. 856–865. 10.1161/01.CIR.98.9.856 [DOI] [PubMed] [Google Scholar]
- 51. Shapiro LM Kleinebenne A McKenna WJ The distribution of left ventricular hypertrophy in hypertrophic cardiomyopathy: comparison to athletes and hypertensives European Heart Journal 6 1985. 967–974. [DOI] [PubMed] [Google Scholar]
- 52. Caselli S Maron MS Urbano-Moral JA Pandian NG Maron BJ Pelliccia A Differentiating left ventricular hypertrophy in athletes from that in patients with hypertrophic cardiomyopathy American Journal of Cardiology 114 2014. 1383–1389. 10.1016/j.amjcard.2014.07.070 [DOI] [PubMed] [Google Scholar]
- 53. Sen-Chowdhry S McKenna WJ Left ventricular noncompaction and cardiomyopathy: cause, contributor, or epiphenomenon? Current Opinion in Cardiology 23 2008. 171–175. 10.1097/HCO.0b013e3282fdc939 [DOI] [PubMed] [Google Scholar]
- 54. Arbustini E Weidemann F Hall JL Left ventricular noncompaction: a distinct cardiomyopathy or a trait shared by different cardiac diseases? Journal of the American College of Cardiology 64 2014. 1840–1850. 10.1016/j.jacc.2014.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lampropoulos KM Dounis VG Aggeli C Iliopoulos TA Stefanadis C Contrast echocardiography: contribution to diagnosis of left ventricular non-compaction cardiomyopathy Hellenic Journal of Cardiology 52 2011. 265–272. [PubMed] [Google Scholar]
- 56. Wigle ED Sasson Z Henderson MA Ruddy TD Fulop J Rakowski H Williams WG Hypertrophic cardiomyopathy. The importance of the site and the extent of hypertrophy. A review Progress in Cardiovascular Diseases 28 1985. 1–83. 10.1016/0033-0620(85)90024-6 [DOI] [PubMed] [Google Scholar]
- 57. Morise AP Exercise testing in nonatherosclerotic heart disease: hypertrophic cardiomyopathy, valvular heart disease, and arrhythmias Circulation 123 2011. 216–225. 10.1161/CIRCULATIONAHA.109.914762 [DOI] [PubMed] [Google Scholar]
- 58. Bunch TJ Chandrasekaran K Ehrsam J-E Hammill SC Urban LH Hodge DO Ommen SR Pellikka PA Prognostic significance of exercise induced arrhythmias and echocardiographic variables in hypertrophic cardiomyopathy American Journal of Cardiology 99 2007. 835–838. 10.1016/j.amjcard.2006.10.046 [DOI] [PubMed] [Google Scholar]
- 59. Gimeno JR Tomé-Esteban M Lofiego C Hurtado J Pantazis A Mist B Lambiase P McKenna WJ Elliott PM Exercise-induced ventricular arrhythmias and risk of sudden cardiac death in patients with hypertrophic cardiomyopathy European Heart Journal 30 2009. 2599–2605. 10.1093/eurheartj/ehp327 [DOI] [PubMed] [Google Scholar]
- 60. Cotrim C Loureiro MJ Simões O Miranda R Cordeiro P Ialá M Matias C João I Carrageta M Evaluation of hypertrophic obstructive cardiomyopathy by exercise stress echocardiography. New methodology Revista Portuguesa de Cardiologia 24 2005. 1319–1327. [PubMed] [Google Scholar]
- 61. Maron MS Olivotto I Zenovich AG Link MS Pandian NG Kuvin JT Nistri S Cecchi F Udelson JE Maron BJ Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction Circulation 114 2006. 2232–2239. 10.1161/CIRCULATIONAHA.106.644682 [DOI] [PubMed] [Google Scholar]
- 62. Shah JS Esteban MTT Thaman R Sharma R Mist B Pantazis A Ward D Kohli SK Page SP Demetrescu C et al. Prevalence of exercise-induced left ventricular outflow tract obstruction in symptomatic patients with non-obstructive hypertrophic cardiomyopathy Heart 94 2008. 1288–1294. 10.1136/hrt.2007.126003 [DOI] [PubMed] [Google Scholar]
- 63. Nistri S Olivotto I Maron MS Ferrantini C Coppini R Grifoni C Baldini K Sgalambro A Cecchi F Maron BJ β Blockers for prevention of exercise-induced left ventricular outflow tract obstruction in patients with hypertrophic cardiomyopathy American Journal of Cardiology 110 2012. 715–719. 10.1016/j.amjcard.2012.04.051 [DOI] [PubMed] [Google Scholar]
- 64. Nistri S Olivotto I Maron MS Grifoni C Baldini K Baldi M Sgalambro A Cecchi F Maron BJ Timing and significance of exercise-induced left ventricular outflow tract pressure gradients in hypertrophic cardiomyopathy American Journal of Cardiology 106 2010. 1301–1306. 10.1016/j.amjcard.2010.06.057 [DOI] [PubMed] [Google Scholar]
- 65. Joshi S Patel UK Yao S-S Castenada V Isambert A Winson G Chaudhry FA Sherrid MV Standing and exercise Doppler echocardiography in obstructive hypertrophic cardiomyopathy: the range of gradients with upright activity Journal of the American Society of Echocardiography 24 2011. 75–82. 10.1016/j.echo.2010.10.006 [DOI] [PubMed] [Google Scholar]
- 66. Marwick TH Nakatani S Haluska B Thomas JD Lever HM Provocation of latent left ventricular outflow tract gradients with amyl nitrite and exercise in hypertrophic cardiomyopathy American Journal of Cardiology 75 1995. 805–809. 10.1016/S0002-9149(99)80416-0 [DOI] [PubMed] [Google Scholar]
- 67. Pellikka PA Oh JK Bailey KR Nichols BA Monahan KH Tajik AJ Dynamic intraventricular obstruction during dobutamine stress echocardiography. A new observation Circulation 86 1992. 1429–1432. 10.1161/01.CIR.86.5.1429 [DOI] [PubMed] [Google Scholar]
- 68. Roldán FJ Vargas-Barrón J Espinola-Zavaleta N Keirns C Romero-Cárdenas A Severe dynamic obstruction of the left ventricular outflow tract induced by dobutamine Echocardiography 17 2000. 37–40. 10.1111/j.1540-8175.2000.tb00991x [DOI] [PubMed] [Google Scholar]
- 69. Yalçin F Yigit F Erol T Baltali M Korkmaz ME Müderrisoglu H Effect of dobutamine stress on basal septal tissue dynamics in hypertensive patients with basal septal hypertrophy Journal of Human Hypertension 20 2006. 628–630. 10.1038/sj.jhh.1002041 [DOI] [PubMed] [Google Scholar]
- 70. Wigle ED Rakowski H Kimball BP Williams WG Hypertrophic cardiomyopathy. Clinical spectrum and treatment Circulation 92 1995. 1680–1692. 10.1161/01.CIR.92.7.1680 [DOI] [PubMed] [Google Scholar]
- 71. Cramariuc D Gerdts E Davidsen ES Segadal L Matre K Myocardial deformation in aortic valve stenosis: relation to left ventricular geometry Heart 96 2010. 106–112. 10.1136/hrt.2009.172569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Maciver DH A new method for quantification of left ventricular systolic function using a corrected ejection fraction European Journal of Echocardiography 12 2011. 228–234. 10.1093/ejechocard/jeq185 [DOI] [PubMed] [Google Scholar]
- 73. Maron MS Lesser JR Maron BJ Management implications of massive left ventricular hypertrophy in hypertrophic cardiomyopathy significantly underestimated by echocardiography but identified by cardiovascular magnetic resonance American Journal of Cardiology 105 2010. 1842–1843. 10.1016/j.amjcard.2010.01.367 [DOI] [PubMed] [Google Scholar]
- 74. Prasad K Atherton J Smith GC McKenna WJ Frenneaux MP Nihoyannopoulos P Echocardiographic pitfalls in the diagnosis of hypertrophic cardiomyopathy Heart 82: Suppl 3 1999. III8–II15. 10.1136/hrt.82.2008.iii8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Senior R Becher H Monaghan M Agati L Zamorano J Vanoverschelde JL Nihoyannopoulos P Contrast echocardiography: evidence-based recommendations by European Association of Echocardiography European Journal of Echocardiography 10 2009. 194–212. 10.1093/ejechocard/jep005 [DOI] [PubMed] [Google Scholar]
- 76. Rickers C Wilke NM Jerosch-Herold M Casey SA Panse P Panse N Weil J Zenovich AG Maron BJ Utility of cardiac magnetic resonance imaging in the diagnosis of hypertrophic cardiomyopathy Circulation 112 2005. 855–861. 10.1161/CIRCULATIONAHA.104.507723 [DOI] [PubMed] [Google Scholar]
- 77. Olivotto I Maron MS Autore C Lesser JR Rega L Casolo G De Santis M Quarta G Nistri S Cecchi F et al. Assessment and significance of left ventricular mass by cardiovascular magnetic resonance in hypertrophic cardiomyopathy Journal of the American College of Cardiology 52 2008. 559–566. 10.1016/j.jacc.2008.04.047 [DOI] [PubMed] [Google Scholar]
- 78. Brouwer WP Germans T Head MC van der Velden J Heymans MW Christiaans I Houweling AC Wilde AA van Rossum AC Multiple myocardial crypts on modified long-axis view are a specific finding in pre-hypertrophic HCM mutation carriers European Heart Journal Cardiovascular Imaging 13 2012. 292–297. 10.1093/ehjci/jes005 [DOI] [PubMed] [Google Scholar]
- 79. Maron MS Finley JJ Bos JM Hauser TH Manning WJ Haas TS Lesser JR Udelson JE Ackerman MJ Maron BJ Prevalence, clinical significance, and natural history of left ventricular apical aneurysms in hypertrophic cardiomyopathy Circulation 118 2008. 1541–1549. 10.1161/CIRCULATIONAHA.108.781401 [DOI] [PubMed] [Google Scholar]
- 80. Weinsaft JW Kim HW Crowley AL Klem I Shenoy C Van Assche L Brosnan R Shah DJ Velazquez EJ Parker M et al. LV thrombus detection by routine echocardiography: insights into performance characteristics using delayed enhancement CMR JACC. Cardiovascular Imaging 4 2011. 702–712. 10.1016/j.jcmg.2011.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cannan CR Reeder GS Bailey KR Melton LJ Gersh BJ Natural history of hypertrophic cardiomyopathy. A population-based study, 1976 through 1990 Circulation 92 1995. 2488–2495. 10.1161/01.CIR.92.9.2488 [DOI] [PubMed] [Google Scholar]
- 82. Maron BJ Spirito P Green KJ Wesley YE Bonow RO Arce J Noninvasive assessment of left ventricular diastolic function by pulsed Doppler echocardiography in patients with hypertrophic cardiomyopathy Journal of the American College of Cardiology 10 1987. 733–742. 10.1016/S0735-1097(87)80264-4 [DOI] [PubMed] [Google Scholar]
- 83. Nagueh SF Lakkis NM Middleton KJ Spencer WH Zoghbi WA Quiñones MA Doppler estimation of left ventricular filling pressures in patients with hypertrophic cardiomyopathy Circulation 99 1999. 254–261. 10.1161/01.CIR.99.2.254 [DOI] [PubMed] [Google Scholar]
- 84. Nagueh SF Middleton KJ Kopelen HA Zoghbi WA Quiñones MA Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures Journal of the American College of Cardiology 30 1997. 1527–1533. 10.1016/S0735-1097(97)00344-6 [DOI] [PubMed] [Google Scholar]
- 85. Bayrak F Kahveci G Mutlu B Sonmez K Degertekin M Tissue Doppler imaging to predict clinical course of patients with hypertrophic cardiomyopathy European Journal of Echocardiography 9 2008. 278–283. 10.1093/ejechocard/jen049 [DOI] [PubMed] [Google Scholar]
- 86. OMahony C Jichi F Pavlou M Monserrat L Anastasakis A Rapezzi C Biagini E Gimeno JR Limongelli G McKenna WJ et al. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk–SCD) European Heart Journal 35 2014. 2010–2020. 10.1093/eurheartj/eht439 [DOI] [PubMed] [Google Scholar]
- 87. Monserrat L Elliott PM Gimeno JR Sharma S Penas-Lado M McKenna WJ Non-sustained ventricular tachycardia in hypertrophic cardiomyopathy: an independent marker of sudden death risk in young patients Journal of the American College of Cardiology 42 2003. 873–879. 10.1016/S0735-1097(03)00827-1 [DOI] [PubMed] [Google Scholar]
- 88. Spirito P Bellone P Harris KM Bernabo P Bruzzi P Maron BJ Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy New England Journal of Medicine 342 2000. 1778–1785. 10.1056/NEJM200006153422403 [DOI] [PubMed] [Google Scholar]
- 89. Elliott PM Gimeno Blanes JR Mahon NG Poloniecki JD McKenna WJ Relation between severity of left-ventricular hypertrophy and prognosis in patients with hypertrophic cardiomyopathy Lancet 357 2001. 420–424. 10.1016/S0140-6736(00)04005-8 [DOI] [PubMed] [Google Scholar]
- 90. Guttmann OP Rahman MS O'Mahony C Anastasakis A Elliott PM Atrial fibrillation and thromboembolism in patients with hypertrophic cardiomyopathy: systematic review Heart 100 2014. 465–472. 10.1136/heartjnl-2013-304276 [DOI] [PubMed] [Google Scholar]
- 91. Nistri S Olivotto I Betocchi S Losi MA Valsecchi G Pinamonti B Conte MR Casazza F Galderisi M Maron BJ et al. Prognostic significance of left atrial size in patients with hypertrophic cardiomyopathy (from the Italian Registry for Hypertrophic Cardiomyopathy) American Journal of Cardiology 98 2006. 960–965. 10.1016/j.amjcard.2006.05.013 [DOI] [PubMed] [Google Scholar]
- 92. Tani T Yagi T Kitai T Kim K Nakamura H Konda T Fujii Y Kawai J Kobori A Ehara N et al. Left atrial volume predicts adverse cardiac and cerebrovascular events in patients with hypertrophic cardiomyopathy. Cardiovascular Ultrasound. 2011;9:34. doi: 10.1186/1476-7120-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Elliott P Gimeno J Tomé M McKenna W Left ventricular outflow tract obstruction and sudden death in hypertrophic cardiomyopathy. European Heart Journal. 2006;27:3073. doi: 10.1093/eurheartj/ehl383. (author reply 3073–3074) [DOI] [PubMed] [Google Scholar]
- 94. Olivotto I Maron MS Adabag AS Casey SA Vargiu D Link MS Udelson JE Cecchi F Maron BJ Gender-related differences in the clinical presentation and outcome of hypertrophic cardiomyopathy Journal of the American College of Cardiology 46 2005. 480–487. 10.1016/j.jacc.2005.04.043 [DOI] [PubMed] [Google Scholar]
- 95. Maki S Ikeda H Muro A Yoshida N Shibata A Koga Y Imaizumi T Predictors of sudden cardiac death in hypertrophic cardiomyopathy American Journal of Cardiology 82 1998. 774–778. 10.1016/S0002-9149(98)00455-X [DOI] [PubMed] [Google Scholar]
- 96. Lafitte S Reant P Touche C Pillois X Dijos M Arsac F Peyrou J Montaudon M Ritter P Roudaut R et al. Paradoxical response to exercise in asymptomatic hypertrophic cardiomyopathy: a new description of outflow tract obstruction dynamics Journal of the American College of Cardiology 62 2013. 842–850. 10.1016/j.jacc.2013.06.007 [DOI] [PubMed] [Google Scholar]
- 97. Seggewiss H Gleichmann U Faber L Fassbender D Schmidt HK Strick S Percutaneous transluminal septal myocardial ablation in hypertrophic obstructive cardiomyopathy: acute results and 3-month follow-up in 25 patients Journal of the American College of Cardiology 31 1998. 252–258. 10.1016/S0735-1097(97)00508-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
2D echocardiogram: 4-chamber view showing hypertrophy of the LV apex. Download Video 1 via http://dx.doi.org/10.1530/ERP-15-0007-v1
Download Video 1 (718.9KB, avi)
Apical HCM on parasternal short axis at the level of the apex. There is concentric hypertrophy and collapse of the LV cavity during systole. Download Video 2 via http://dx.doi.org/10.1530/ERP-15-0007-v2
Download Video 2 (438.6KB, avi)
The same patient with HCM scanned without contrast. The use of i.v. contrast offers a better view of the endocardium. Apical aneurysm is clearly visualized on the contrast echocardiogram. Download Video 3 via http://dx.doi.org/10.1530/ERP-15-0007-v3
Download Video 3 (295.5KB, avi)
The same patient with HCM scanned with contrast. The use of i.v. contrast offers a better view of the endocardium. Apical aneurysm is clearly visualized on the contrast echocardiogram. Download Video 4 via http://dx.doi.org/10.1530/ERP-15-0007-v4
Download Video 4 (883.5KB, avi)
Parasternal long axis view of patient with FRDA showing concentric LVH without LVOT obstruction. Download Video 5 via http://dx.doi.org/10.1530/ERP-15-0007-v5
Download Video 5 (377.5KB, avi)
Patient with FRDA. Parasternal short axis at the mitral valve level, showing concentric LVH involving all segments, more prominent on the anteroseptum. Download Video 6 via http://dx.doi.org/10.1530/ERP-15-0007-v6
Download Video 6 (470.5KB, avi)
Patient with amyloidosis, 4-chamber view showing concentric LVH, more prominent in the septum. There is septal hypokinesia and impairment of the LV systolic function. Loss of longitudinal systolic function. Both atria are dilated. Download Video 7 via http://dx.doi.org/10.1530/ERP-15-0007-v7
Download Video 7 (273.2KB, avi)



 This work is licensed under a
This work is licensed under a