Abstract
Resting echocardiography measurements are poor predictors of exercise capacity and symptoms in patients with heart failure (HF). Stress echocardiography may provide additional information and can be expressed using left ventricular ejection fraction (LVEF), or diastolic parameters (E/E′), but LVEF has some major limitations. Systolic annular velocity (S′) provides a measure of longitudinal systolic function, which is relatively easy to obtain and shows a good relationship with exercise capacity. The objective of this study was to investigate the relationship among S′, E/E′ and LVEF obtained during stress echocardiography and both mortality and hospitalisation. A secondary objective was to compare S′ measured using a simplified two-wall model. A total of 80 patients with stable HF underwent exercise stress echocardiography and simultaneous cardiopulmonary exercise testing. Volumetric and tissue velocity imaging (TVI) measurements were obtained, as was peak oxygen uptake (VO2 peak). Of the total number of patients, 11 died and 22 required cardiac hospitalisation. S′ at peak exertion was a powerful predictor for death and hospitalisation. Cut-off points of 5.3 cm/s for death and 5.7 cm/s for hospitalisation provided optimum sensitivity and specificity. This study suggests that, in patients with systolic HF, S′ at peak exertion calculated from the averaged spectral TVI systolic velocity of six myocardial segments, or using a simplified measure of two myocardial segments, is a powerful predictor of future events and stronger than LVEF, diastolic velocities at rest or exercise and VO2 peak. Results indicate that measuring S′ during exercise echocardiography might play an important role in understanding the likelihood of adverse clinical outcomes in patients with HF.
Keywords: stress echocardiography, tissue Doppler imaging, left ventricular ejection fraction
Introduction
Systolic heart failure (HF) is a major cause of mortality and morbidity, and its prevalence is increasing due to the ageing population (1). It is conventionally defined by demonstrating typical clinical symptoms and signs associated with compatible findings on a resting echocardiogram (2). Patients with systolic dysfunction respond to a range of physical and medical therapies, but nonetheless have a poor prognosis (3). Resting echocardiography and particularly left ventricular ejection fraction (LVEF) has been shown to be a poor predictor of exercise capacity (4, 5). Several studies have suggested the additional value that stress echocardiography has on exercise capacity and symptoms (5, 6, 7) and prognosis (8, 9, 10, 11).
Stress echocardiography adds prognostic value in patients with myocardial ischaemia secondary to coronary artery disease (12, 13). Furthermore, echocardiographic data during exercise provide additional information regarding patients' overall exercise ability (14). Patients with HF demonstrating an increase in LVEF of 5% (in absolute terms) or more during stress echocardiography have a better prognosis (15, 16, 17). However, LVEF can be challenging to obtain reliably and has some major limitations including a lack of reproducibility, dependence on loading conditions and it only describes maximum displacement at the end of systole meaning the longitudinal component of systolic function is not completely described. Systolic annular velocities obtained during stress echocardiography show a strong relationship with exercise tolerance (6, 18) and the change in early diastolic velocity has also proven predictive (19). The relationship between both systolic and diastolic velocities and prognosis, however, remains undetermined. Therefore, the objective of this study was to investigate whether systolic velocity (S′), diastolic reserve (E/E′) and LVEF, all of which describe different aspects of cardiac function, would relate to mortality and cardiac hospitalisation in an established cohort of patients with systolic HF who had undergone stress echocardiography and cardiopulmonary exercise testing as part of a research study.
Methods
This was a retrospective longitudinal study of 80 patients with systolic HF participating in cohort studies undertaken at our institution using the same echocardiography and cardiopulmonary exercise test (CPET) protocols (20) (explained in more detail below). All patients were medically treated for systolic HF and had been stable on medical therapy for at least 1 month before study inclusion. The definition of systolic HF was made by the combination of appropriate symptoms and a depressed LV on echocardiography. Exclusion criteria included unstable angina, symptomatic angina, evidence of reversible ischaemia, PCI (percutaneous coronary intervention) or coronary artery bypass graft (CABG) within the last 6 months, severe lung disease or poor echocardiographic windows. Informed written consent was obtained and all studies were approved by the local ethical committee.
Echocardiography
Echocardiography was performed using a GE Vivid 7 platform (Vingmed-General Electric, Horten, Norway) equipped with a phased-array 3.5 MHz transducer. Two-dimensional, spectral Doppler and tissue velocity imaging (TVI) were obtained at rest and during exercise. LV volumes and LVEF were calculated using Simpson's biplane method in the apical four-chamber and two-chamber views. Transmitral Doppler was obtained by placing a pulsed wave Doppler sample volume at the tips of the mitral leaflets. Pulsed wave Doppler for cardiac output assessment was taken in the five-chamber view 1 cm below the aortic valve. TVI was performed with the sample volume placed at mitral annulus in the three-apical views. Exercise S′ (defined as the highest velocity during systole after the end of isovolumetric contraction) was obtained from six peri-annular sites of the mitral annulus (septal, lateral, inferior, anterior, posterior and anteroseptal). At least three cardiac cycles were obtained and S′ was averaged for each segment and all available S′ were averaged. At instances where a reproducible TVI recording was not achieved, all available recordings were used to calculate the average. Myocardial velocity during early diastole (E′) was measured on the lateral wall because this was laid down in one of the study protocols (because of the inclusion of a proportion of patients who had undergone previous cardiac surgery, in whom septal diastolic velocity might not have been representative). Images were obtained in real time and analysed after each study. Images were stored offline.
Cardiopulmonary exercise test
A recumbent cycle ergometer (ERG 911 S/L, Schiller, Baar, Switzerland) was used. Peak oxygen uptake (VO2 peak) was used as the main outcome variable as it has been previously shown to be a strong predictor of mortality in patients with systolic HF (21, 22). Patients were asked to continue to take their medication as usual. At the start of the test a 3-min rest period was included followed by a 3 min of warm-up period. Exercise protocols were individually determined based on functional status. Work rate (5, 10, 15 or 20 W) increased every minute until voluntary exhaustion aiming for 6–10 min of exercise. Heart rate, blood pressure and oxygen saturation were monitored throughout. Oxygen uptake, carbon dioxide production and ventilation were continuously measured and derived using a calibrated breath-by-breath analyser (Schiller Powercube AT-104 PC, Ganzhorn, Baar, Switzerland). A respiratory exchange ratio >1 was used to indicate good effort (7). Echocardiography measurements commenced when patients were close to finishing the test when the RER was exceeding 0.95 and if patients were not taking β-blockers in combination with a peak predicted HR >85%. All measurements were taken within 90 s of finishing exercise. Patients were verbally encouraged to exercise until maximal exertion. All tests were performed according to the exercise testing guidelines (23). VO2 peak was expressed as the highest value from an average of 30 s during the final stage of the exercise test.
Follow-up
All-cause mortality and cardiac hospitalisation end points were evaluated by cross-referencing with the hospital information system (which is linked to the UK registry of death), the clinical case notes, contacting the primary care physician and, where necessary, contacting the patient by telephone. No patient was lost to follow-up. Where hospitalisations had occurred, all best endeavours to evaluate the notes were made to ensure that the hospitalisation was HF related. Data on re-admission were based on the primary diagnosis at discharge.
Statistical analyses
All data are expressed as mean±s.d. or percentage for continuous variables and categorical data are expressed as absolute values and percentages. Pearson's correlation coefficients or Spearman's coefficients were used to determine relationship between echocardiographic variables and exercise tolerance. Differences between groups were investigated using the Student's t-test for continuous data and the χ 2 test for categorical data. All variables were assessed for univariate statistical significance using Cox's proportional hazard regression model for mortality and hospitalisation. All significant predictors of outcome were entered into multivariate Cox's proportional model (forward selection) to identify the strongest predictors of mortality or cardiac hospitalisation. Another Cox's regression analysis was performed interchanging the average systolic velocities for the average of the septal and lateral walls. Data from Cox's regression analysis are expressed as hazard ratios (HRs) with 95% CI. Receiver operating characteristics (ROC) curves were used to determine the optimal prognostic threshold value (highest combination of sensitivity/specificity) for mortality and hospitalisation. Interobserver variability was undertaken from 12 randomly selected subjects and intraclass correlations (ICCs) were calculated. All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS version 20.0; SPSS, Inc.). A P value of <0.05 was considered significant.
Results
Baseline characteristics are given in Table 1. A total of 80 patients were included in the study, 11 patients died and 22 required non-elective hospital admissions during a median follow-up of 24 months (range 3–40 months). Of the remaining number of patients, 50 suffered from ischaemic heart disease, and, of them, 16 had a previous CABG and 13 a previous PCI. A proportion of patients (n=23) previously underwent cardiac resynchronisation therapy (CRT) before inclusion in the research study, 30 patients had been clinically approved to receive CRT and the remaining 27 did not qualify for CRT at the time of the study. Although no significant differences were found for the presence of ischaemic heart disease, there was a trend observed for hospitalisation (P=0.25) and survival (P=0.15) (Table 1).
Table 1.
Baseline clinical characteristics. Data are expressed as mean±s.d. or as number (%) of patients
| All patients (n=80) | Survivors (n=69) | Non-survivors (n=11) | No hospitalisation (n=58) | Hospitalisation (n=22) | |
|---|---|---|---|---|---|
| Age (years) | 72±9 | 71±9 | 74±7 | 71±10 | 74±7 |
| Male | 51 (64%) | 44 | 7 | 39 | 12 |
| IHD | 50 (63%) | 41 | 9 | 34 | 16 |
| Hypertension | 23 (29%) | 19 | 4 | 16 | 7 |
| LBBB | 42 (53%) | 38 | 4 | 32 | 10 |
| Diabetes mellitus | 15 (19%) | 14 | 1 | 10 | 5 |
| Valvular heart disease | 9 (11%) | 7 | 2 | 6 | 3 |
| CRT | 23 (29%) | 19 | 4 | 15 | 8 |
| CABG | 16 (20%) | 15 | 1 | 11 | 5 |
| PCI | 13 (16%) | 10 | 3 | 9 | 4 |
| ACE inhibitor | 50 (63) | 42 | 8 | 32 | 18 |
| β-blocker | 57 (71%) | 49 | 8 | 43 | 14 |
| Digoxin | 8 (10%) | 7 | 1 | 6 | 2 |
| Amiodarone | 17 (21%) | 13 | 4 | 11 | 6 |
| ARB | 24 (30%) | 21 | 3 | 21* | 3 |
| Diuretic | 59 (74%) | 49 | 10 | 39* | 20 |
| Statin | 54 (68%) | 49 | 5 | 39 | 15 |
| Serum creatinine (mmol/l) | 105±35.9 | 103±34 | 121±45 | 101±32 | 118±42 |
| Serum sodium (mmol/l) | 139±3.0 | 139±3 | 140±3 | 140±3 | 139±3 |
| Resting heart rate (beats/min) | 68±15 | 67±15 | 71±16 | 68±16 | 67±11 |
| Exercise heart rate (beats/min) | 99±23 | 99±22 | 96±16 | 102±24 | 91±20 |
| Resting cardiac output (l/min) | 3.9±1.4 | 3.9±1.4 | 3.7±1.3 | 3.9±1.4 | 3.9±1.6 |
| Exercise cardiac output (l/min) | 6.7±2.2 | 6.9±2.3 | 5.4±1.6 | 6.9±2.3 | 6.3±2.0 |
*P<0.05; ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; CABG, coronary artery bypass graft; CRT, cardiac resynchronisation therapy; IHD, ischaemic heart disease; LBBB, left bundle branch block; PCI, percutaneous coronary intervention.
No differences in age, sex, electrolytes, hypertension, left bundle branch block, diabetes, non-significant valvular heart disease, presence of CRT, previous CABG or PCI were observed between those with and without events. A difference was observed for the use of angiotensin II receptor blocker (ARB) and diuretics for patients who have been hospitalised. No other difference in drug history was observed (Table 1).
Of 480 available segments from TVI analysis, 92% were positively identified at rest vs 81% during stress echocardiography. All six myocardial segments were evaluable in 58 patients at rest and 34 patients during exercise. The septal and lateral systolic velocities were identified in all 80 patients at rest and in 78 patients during exercise. The apical long-axis view was most frequently not evaluable, in particular the anteroseptal segment. Nevertheless, in 79 patients at rest and in 72 patients during exercise, at least four segments were accurately identified. The mean TVI value of all six segments correlated well with the mean of the septal and the lateral walls for rest (r=0.93, P<0.001) and exercise (r=0.94, P<0.001).
Maximal exercise tolerance described using VO2 peak was significantly higher in survivors than in non-survivors (1.2±0.4 l/min vs 0.9±0.3 l/min, P=0.04). However, no difference was found between patients who were hospitalised and those who were not (1.2±0.5 l/min vs 1.1±0.3 l/min). A regression analysis showed a weak relationship between VO2 peak and resting and exercise LVEF (r=0.35, P<0.05; r=0.5, P<0.05 respectively), while a moderate correlation was found for exercise S′ (r=0.66, P<0.001).
Echocardiographic, exercise and functional parameters for survivors and non-survivors, as well as those who were and were not hospitalised, are presented in Table 2. There was a significant difference in systolic velocities at rest and exercise, LVEF at rest and exercise, E/E′ at rest and exercise, VO2 peak, and end diastolic dimensions between survivors and non-survivors. Fewer differences were observed between those who were and were not hospitalised, although once again S′, LVEF at rest and end diastolic dimensions remained significant.
Table 2.
Mean echocardiographic, exercise and functional parameters (mean±s.d.)
| Mortality | Survivors | Non-survivors | No hospitalisation | Hospitalisation |
|---|---|---|---|---|
| S′ at rest (cm/s) | 5.4±1.6 | 3.7±1.2 a | 5.3±1.7 | 4.5±1.5 b |
| S′ at exercise (cm/s) | 7.1±2.2 | 4.4±1.3 a | 7.2±2.4 | 5.5±1.6 b |
| LVEF at rest | 33±11 | 24±6 b | 33±10 | 27±11 a |
| LVEF at exercise | 40±14 | 28±8 a | 39±14 | 35±13 |
| E′ at rest (cm/s) | 6.4±2.6 | 5.4±2.1 | 6.4±2.6 | 5.9±2.3 |
| E′ at exercise (cm/s) | 10.1±4.6 | 7.1±2.3 | 10.2±4.7 | 8.5±3.8 |
| E/E′ at rest | 12.6±7.4 | 18.7±9.0 a | 12.6±7.9 | 15.0±7.2 |
| E/E′ at exercise | 12.1±7.2 | 18.2±7.1 a | 12.2±7.7 | 14.8±6.8 |
| VO2 peak (l/min) | 1.2±0.4 | 0.94±0.3 a | 1.2±0.5 | 1.1±0.3 |
| EDV | 158±59 | 180±63 | 161±61 | 162±54 |
| ESV | 108±46 | 139±56 | 109±48 | 121±49 |
| EDD | 5.8±0.8 | 6.5±0.9 b | 5.8±0.8 | 6.3±0.9 b |
| ESD | 4.8±0.9 | 5.6±1.4 | 4.7±1.0 | 5.6±0.9 a |
| LA diameter | 3.9±0.8 | 4.4±0.6 | 3.9±0.8 | 4.2±0.6 |
| NYHA | 2.2±0.8 | 2.5±0.7 | 2.2±0.7 | 2.2±0.8 |
LVEF, left ventricular ejection fraction; S′, systolic velocity; E′, myocardial velocity early diastole; E/E′, transmitral-to-basal early diastolic velocity ratio; VO2 peak, peak oxygen uptake; EDV, end-diastolic volume; ESV, end-systolic volume; EDD, end-diastolic dimensions; ESD, end-systolic dimensions; LA, left atrium; NYHA, New York Heart Association class.
Survivors vs non-survivors or hospitalisation vs no hospitalisation P<0.01.
Survivors vs non-survivors or hospitalisation vs no hospitalisation P<0.05.
Significant univariate predictors are given in Table 3. None of the clinical characteristics in Table 1 were univariate predictors for death; for hospitalisation, only diuretics were a univariate predictor (Table 3). Aetiology of HF was not a univariate predictor for either mortality or hospitalisation. After all the variables that predicted mortality and hospitalisation (Table 3) on univariate Cox's regression analysis were entered into a forward multivariate Cox's regression model, only exercise S′ emerged as a significant predictor of mortality (HR: 0.36; 95% CI: 0.19–0.67; P=0.001) and hospitalisation (HR: 0.62; 95% CI: 0.45–0.85; P=0.003). This model retained its predictive power when exercise S′ was substituted by the average septum and lateral S′ for mortality (HR: 0.42; 95% CI: 0.24–0.73; P=0.002) and hospitalisation (HR: 0.54; 95% CI: 0.37–0.81; P=0.003).
Table 3.
Univariate predictors of mortality and cardiac admission
| Mortality | HR (95% CI) | P value | Hospitalisation | HR (95% CI) | P value |
|---|---|---|---|---|---|
| S′ at rest (cm/s) | 0.46 (0.29–0.75) | 0.002 | S′ at rest (cm/s) | 0.67 (0.50–0.90) | 0.008 |
| S′ at exercise (cm/s) | 0.47 (0.31–0.71) | <0.0001 | S′ at exercise (cm/s) | 0.65 (0.52–0.83) | <0.0001 |
| LVEF at rest | 0.91 (0.85–0.98) | 0.01 | LVEF at rest | 0.94 (0.90–0.98) | 0.008 |
| LVEF at exercise | 0.93 (0.88–0.98) | 0.01 | E/E′ at exercise | 1.05 (1.00–1.09) | 0.04 |
| E/E′ at rest | 1.07 (1.00–1.14) | 0.03 | EDD | 1.82 (1.03–3.20) | 0.04 |
| E/E′ at exercise | 1.07 (1.01–1.12) | 0.01 | ESD | 2.23 (1.31–3.77) | 0.003 |
| VO2 peak (l/min) | 0.10 (0.01–0.76) | 0.03 | Creatinine | 1.01 (1.00–1.02) | 0.04 |
| ESV | 1.01 (1.00–1.02) | 0.05 | Diuretics | 0.23 (0.05–1.00) | 0.05 |
LVEF, left ventricular ejection fraction; S′, systolic velocity; E′, myocardial velocity early diastole; E/E′, transmitral-to-basal early diastolic velocity ratio; VO2 peak, peak oxygen uptake; ESV, end-systolic volume.
A ROC analysis was performed for mortality and systolic velocity has the largest area under the curve of 0.86 (95% CI: 0.75–0.96). A cut-off of 5.3 cm/s for exercise S′ showed a sensitivity and specificity of 82 and 80%, respectively, for mortality. Similarly, for hospitalisation, a ROC analysis for exercise S′ has an area under the curve of 0.71 (95% CI: 0.58–0.83), which resulted in a cut-off of 5.7 cm/s representing a sensitivity of 59% and a specificity of 74%. Kaplan–Meier curves were generated using these cut-offs (see Fig. 1).
Figure 1.
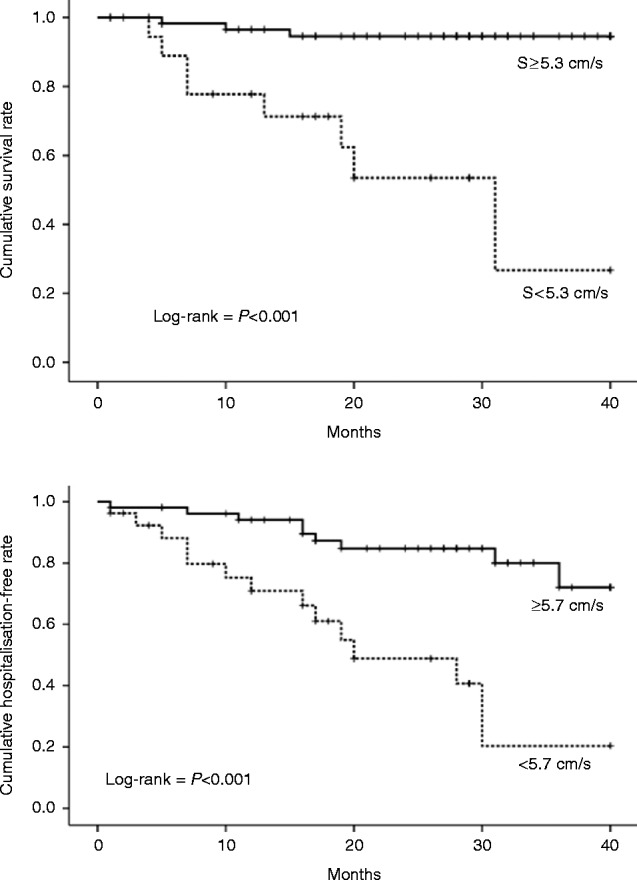
Kaplan–Meier curves for mortality (top) and hospitalisation (bottom).
Interobserver variability measured by ICC was stronger for exercise S′ (ICC: 0.96; 95% CI: 0.88–0.99) compared with exercise LVEF (ICC: 0.57; 95% CI: −0.82 to 0.92), exercise E′ (ICC: 0.83; 95% CI: 0.38–0.95) and exercise E/E′ (ICC: 0.84; 95% CI: 0.40–0.96). Similarly, for the intraobserver variability, the ICC was stronger for TVI-derived parameters, exercise S′ (ICC: 0.97; 95% CI: 0.86–0.99) and exercise E′ (ICC: 0.99; 95% CI: 0.94–0.99) compared with exercise LVEF (ICC: 0.88; 95% CI: 0.50–0.96) and exercise E/E′ (ICC: 0.85; 95% CI: −0.24 to 0.98).
Discussion
This study suggests that, in patients with systolic HF, S′ at peak exertion calculated from the averaged spectral TVI systolic velocity of six myocardial segments, or using a simplified measure of two myocardial segments, is a powerful predictor of future events and stronger than LVEF, diastolic velocities at rest or exercise, and VO2 peak. It retains its prognostic value after adjustment for clinical data, peak exercise capacity, functional parameters and other echocardiographic data. Cut-off points of 5.3 cm/s for death and 5.7 cm/s for hospitalisation provided optimum sensitivity and specificity, which were 82 and 80% for mortality and 59 and 74% for hospitalisation. Surprisingly, exercise S′ proved to be a stronger predictor than VO2 peak despite being moderately co-correlated, and VO2 peak having been highly predictive of mortality in other studies (24, 25). This study confirms the limitation of resting echocardiography in predicting either prognosis or exercise capacity in patients with systolic HF (9, 10, 24, 26, 27). Inter- and intraobserver variabilities were excellent for S′ at exercise unlike LVEF at exercise, which was less reliable; this is in agreement with previous studies (28, 29).
The most widely used parameter to characterise HF is resting LVEF, but this measurement can be challenging. The reasons why LVEF relates poorly to both functional capacity (5, 6, 7, 30) and prognosis are complex (8, 9, 10, 11, 24). The reproducibility of measurement remains sub-optimal, due partly to image quality and anatomical factors (31). LVEF is a reflection of whole systolic myocardial displacement and may be more dependent on loading conditions than on other measures such as velocity (6, 31, 32). Furthermore, LVEF poorly describes longitudinal myocardial function, which is often affected early in a variety of heart diseases. Previous studies have documented only a weak relationship between LVEF either at rest or under stress conditions and exercise capacity, which is in agreement with our findings (6). LVEF during dobutamine stress, where loading conditions can be very different, has been shown to predict adverse outcome during echocardiography and nuclear scintigraphy (33, 34, 35). The inability to increase LVEF during stress echocardiography has been shown to be a strong predictor for mortality (15).
Both systolic and diastolic TVI measures, at rest and during exercise, have previously been demonstrated to predict exercise capacity (20, 36, 37). A moderate relationship between VO2 peak and S′ was demonstrated and both resting and stress recorded TVIs were univariate predictors of mortality. The concept of diastolic reserve has gained considerable interest, particularly in patients with HF and preserved ejection fraction, where changes in E and E′ velocities predict exercise performance (37, 38). Resting tissue velocity data, particularly those describing diastole such as E′ and A′, have been shown to have a prognostic value (9, 10, 27). A large study by Grewal et al. (38) found an independent association between left ventricular diastolic dysfunction and exercise capacity. Systolic function was not reported as all patients had a preserved LV. Similar incremental prognostic results have been observed in both systolic HF (9) and HF with preserved LVEF (38, 39) for E′ and E/E′. One complication when analysing all these data is the relatively close relationship between S′ and E′, making it difficult to determine which is the dominant influence. Hence, while the primary end point of these studies may relate to diastolic reserve, there is also a significant positive association with systolic function that may not be fully appreciated. One of the main determinants of early diastolic motion (caused by mitral annular descent) is the release of energy stored during systole, and therefore, S′ and other systolic measures also inevitably describe in part the behaviour of the myocardium during early diastole (40, 41).
Strain imaging is an alternative for describing myocardial deformation (42). An important disadvantage for TVI-derived strain is the low spatial resolution, which results in low reproducibly and, therefore, it is not routinely used in clinical practice (42). Two-dimensional strain speckle tracking has the clear advantage of being angle independent, but requires higher frame rates that may not be sufficient to properly track the increased heart rates at stress. This may result in under sampling, especially during exercise. Recent studies have suggested a potential benefit of speckle track-derived strain and strain rate in detected reversible ischaemia during dobutamine stress echocardiography, but routine use especially during exercise stress echocardiography is limited due to the limited range of heart rates that can be sampled (12, 43).
TVI is available on most echocardiography platforms and peak systolic and diastolic velocities are reproducible and easy to obtain both at rest and on exercise (44). The ability to achieve systolic velocities with a high degree for inter- and intra-reproducibility was confirmed in this study, unlike LVEF. The biggest challenge in achieving averaged TVI measurements is that all walls cannot always be assessed in all patients due to image quality. In this study, more than half of the echocardiographic TVI measures were not satisfactory obtained from all six myocardial walls, and hence the protocols simplified, where only average reading for the septal and lateral walls were tested. This simplified protocol gave results that were not significantly different from the average of all six walls. This may be because, although an underlying segment may be akinetic, unlike deformation imaging, it still shows an apical long-axis velocity because it is tethered to other contracting segments. Thus, the annular velocity at any one point is an aggregate of myocardial contractility in that and adjacent segments.
Study limitations and areas for further research
A significant weakness of this study is that E′ was measured only in the lateral wall and not averaged over multiple segments and, thus, may have misrepresented patients with regional wall motion abnormalities. Møller et al. (45) reported that E/E′ was a strong independent predictor of death and readmission in a cohort of patients with previous myocardial infarction. Strain analysis either by TVI or by speckle tracking was not used in this study and this might have provided further insights into global and regional deformation. The only CPET variable analysed was VO2 peak. Although VO2 peak correlated with exercise S′, it failed to reach statistical significance as a predictor of hospitalisation. There might be a closer relationship with a submaximal parameter such as oxygen uptake efficiency slope or the ventilatory threshold. All patients were considered if clinically stable from an ischaemic point of view at enrolment, and hence formal wall motion scoring was not undertaken, hence it is possible that this might have produced similar results due to inducible ischaemia. Study population was small and selective as patients were elderly and over half of the patients suffered from stable ischaemic heart disease. Death and hospitalisations were analysed separately as in retrospective analysis, and it can be more challenging to interpret hospitalisation data with absolute certainty and hence a single combined end point was not deemed appropriate.
Conclusion
While contractile reserve is recognised as a predictor of adverse cardiac events, this is the first study to demonstrate that the measurement of peak systolic myocardial velocities derived from TVI during exercise predicts death and hospital admissions to a greater extent than either diastolic reserve or LVEF. A simplified two-wall protocol, which makes evaluation even more straightforward, gave equivalent results. Resting echocardiographic and clinical parameters were less supportive in predicting future events in this study including VO2 peak. While prospective studies should test the hypotheses and particularly the cut-off points identified in this study, the results suggest that measuring S′ during exercise echocardiography might have an important role in understanding the likelihood of adverse clinical outcomes in patients with HF.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
References
- 1.Coronary Heart Disease Statistics 2012 Edition. British Heart Foundation; London: 2012. [Google Scholar]
- 2. McMurray JJ Adamopoulos S Anker SD Auricchio A Böhm M Dickstein K Falk V Filippatos G Fonseca C Gomez-Sanchez MA et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC European Heart Journal 33 2012. 1787–1847. 10.1093/eurheartj/ehs104 [DOI] [PubMed] [Google Scholar]
- 3. Meta-analysis Global Group in Chronic Heart Failure The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis European Heart Journal 33 2012. 1750–1757. 10.1093/eurheartj/ehr254 [DOI] [PubMed] [Google Scholar]
- 4. Benge W Litchfield RL Marcus ML Exercise capacity in patients with severe left ventricular dysfunction Circulation 61 1980. 955–959. 10.1161/01.CIR.61.5.955 [DOI] [PubMed] [Google Scholar]
- 5. Carell ES Murali S Schulman DS Estrada-Quintero T Uretsky BF Maximal exercise tolerance in chronic congestive heart failure: relationship to resting left ventricular function Chest 106 1994. 1746–1752. 10.1378/chest.106.6.1746 [DOI] [PubMed] [Google Scholar]
- 6. Rubis P Podolec P Tomkiewicz-Pajak L Kopec G Olszowska M Tracz W Usefulness of the evaluation of isovolumic and ejection phase myocardial signals during stress echocardiography in predicting exercise capacity in heart failure patients Echocardiography 26 2009. 1050–1059. 10.1111/j.1540-8175.2009.00922.x [DOI] [PubMed] [Google Scholar]
- 7. Witte KK Nikitin NP De Silva R Cleland JG Clark AL Exercise capacity and cardiac function assessed by tissue Doppler imaging in chronic heart failure Heart 90 2004. 1144–1150. 10.1136/hrt.2003.025684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Florea VG Henein MY Anker SD Francis DP Chambers JS Ponikowski P Coats AJ Prognostic value of changes over time in exercise capacity and echocardiographic measurements in patients with chronic heart failure European Heart Journal 21 2000. 146–153. 10.1053/euhj.2000.1737 [DOI] [PubMed] [Google Scholar]
- 9. Wang M Yip G Yu C-M Zhang Q Zhang Y Tse D Kong S-L Sanderson JE Independent and incremental prognostic value of early mitral annulus velocity in patients with impaired left ventricular systolic function Journal of the American College of Cardiology 45 2005. 272–277. 10.1016/j.jacc.2004.09.059 [DOI] [PubMed] [Google Scholar]
- 10. Wang M Yip GW Wang AY Zhang Y Ho PY Tse MK Lam PK Sanderson JE Peak early diastolic mitral annulus velocity by tissue Doppler imaging adds independent and incremental prognostic value Journal of the American College of Cardiology 41 2003. 820–826. 10.1016/S0735-1097(02)02921-2 [DOI] [PubMed] [Google Scholar]
- 11. Grayburn PA Appleton CP DeMaria AN Greenberg B Lowes B Oh J Plehn JF Rahko P St John Sutton M Eichhorn EJ Echocardiographic predictors of morbidity and mortality in patients with advanced heart failure: the Beta-blocker Evaluation of Survival Trial (BEST) Journal of the American College of Cardiology 45 2005. 1064–1071. 10.1016/j.jacc.2004.12.069 [DOI] [PubMed] [Google Scholar]
- 12. Sicari R Nihoyannopoulos P Evangelista A Kasprzak J Lancellotti P Poldermans D Voigt J-U Zamorano JL Stress echocardiography expert consensus statement: European Association of Echocardiography (EAE) (a registered branch of the ESC) European Journal of Echocardiography: the journal of the Working Group on Echocardiography of the European Society of Cardiology 9 2008. 415–437. 10.1093/ejechocard/jen175 [DOI] [PubMed] [Google Scholar]
- 13. Bountioukos M Elhendy A van Domburg RT Schinkel AF Bax JJ Krenning BJ Biagini E Rizzello V Simoons ML Poldermans D Prognostic value of dobutamine stress echocardiography in patients with previous coronary revascularisation Heart 90 2004. 1031–1035. 10.1136/hrt.2003.029025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Agricola E Oppizzi M Pisani M Margonato A Stress echocardiography in heart failure. Cardiovascular Ultrasound. 2004;2:11. doi: 10.1186/1476-7120-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pedone C Bax JJ van Domburg RT Rizzello V Biagini E Schinkel AF Krenning B Vourvouri EC Poldermans D Long-term prognostic value of ejection fraction changes during dobutamine-atropine stress echocardiography Coronary Artery Disease 16 2005. 309–313. 10.1097/00019501-200508000-00008 [DOI] [PubMed] [Google Scholar]
- 16. Bax JJ Poldermans D Elhendy A Cornel JH Boersma E Rambaldi R Roelandt JR Fioretti PM Improvement of left ventricular ejection fraction, heart failure symptoms and prognosis after revascularization in patients with chronic coronary artery disease and viable myocardium detected by dobutamine stress echocardiography Journal of the American College of Cardiology 34 1999. 163–169. 10.1016/S0735-1097(99)00157-6 [DOI] [PubMed] [Google Scholar]
- 17. Werner GS Schaefer C Dirks R Figulla HR Kreuzer H Prognostic value of Doppler echocardiographic assessment of left ventricular filling in idiopathic dilated cardiomyopathy American Journal of Cardiology 73 1994. 792–798. 10.1016/0002-9149(94)90883-4 [DOI] [PubMed] [Google Scholar]
- 18. Ciampi Q Pratali L Porta MD Petruzziello B Manganiello V Villari B Picano E Sicari R Tissue Doppler systolic velocity change during dobutamine stress echocardiography predicts contractile reserve and exercise tolerance in patients with heart failure European Heart Journal Cardiovascular Imaging 14 2013. 102–109. 10.1093/ehjci/jes096 [DOI] [PubMed] [Google Scholar]
- 19. Sekiguchi M Adachi H Oshima S Taniguchi K Hasegawa A Kurabayashi M Effect of changes in left ventricular diastolic function during exercise on exercise tolerance assessed by exercise-stress tissue Doppler echocardiography International Heart Journal 50 2009. 763–771. 10.1536/ihj.50.763 [DOI] [PubMed] [Google Scholar]
- 20. McIntosh RA Silberbauer J Veasey RA Raju P Baumann O Kelly S Beale L Brickley G Sulke N Lloyd GW Tissue Doppler-derived contractile reserve is a simple and strong predictor of cardiopulmonary exercise performance across a range of cardiac diseases Echocardiography 30 2013. 527–533. 10.1111/echo.12084 [DOI] [PubMed] [Google Scholar]
- 21. Arena R Myers J Aslam SS Varughese EB Peberdy MA Peak VO2 and VE/VCO2 slope in patients with heart failure: a prognostic comparison American Heart Journal 147 2004. 354–360. 10.1016/j.ahj.2003.07.014 [DOI] [PubMed] [Google Scholar]
- 22. Poggio R Arazi HC Giorgi M Miriuka SG Prediction of severe cardiovascular events by VE/VCO2 slope versus peak VO2 in systolic heart failure: a meta-analysis of the published literature American Heart Journal 160 2010. 1004–1014. 10.1016/j.ahj.2010.08.037 [DOI] [PubMed] [Google Scholar]
- 23. Gibbons RJ Balady GJ Bricker JT Chaitman BR Fletcher GF Froelicher VF Mark DB McCallister BD Mooss AN O'Reilly MG et al. ACC/AHA 2002 guideline update for exercise testing: summary article. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines) Circulation 106 2002. 1883–1892. 10.1161/01.CIR.0000034670.06526.15 [DOI] [PubMed] [Google Scholar]
- 24. Guazzi M Myers J Peberdy MA Bensimhon D Chase P Pinkstaff S Arena R Echocardiography with tissue Doppler imaging and cardiopulmonary exercise testing in patients with heart failure: a correlative and prognostic analysis International Journal of Cardiology 143 2010. 323–329. 10.1016/j.ijcard.2009.03.053 [DOI] [PubMed] [Google Scholar]
- 25. Francis DP Shamim W Davies LC Piepoli MF Ponikowski P Anker SD Coats AJ Cardiopulmonary exercise testing for prognosis in chronic heart failure: continuous and independent prognostic value from VE/VCO(2) slope and peak VO(2) European Heart Journal 21 2000. 154–161. 10.1053/euhj.1999.1863 [DOI] [PubMed] [Google Scholar]
- 26. Nikitin NP Loh PH de Silva R Ghosh J Khaleva OY Goode K Rigby AS Alamgir F Clark AL Cleland JG Prognostic value of systolic mitral annular velocity measured with Doppler tissue imaging in patients with chronic heart failure caused by left ventricular systolic dysfunction Heart 92 2006. 775–779. 10.1136/hrt.2005.067140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mogelvang R Sogaard P Pedersen SA Olsen NT Marott JL Schnohr P Goetze JP Jensen JS Cardiac dysfunction assessed by echocardiographic tissue Doppler imaging is an independent predictor of mortality in the general population Circulation 119 2009. 2679–2685. 10.1161/CIRCULATIONAHA.108.793471 [DOI] [PubMed] [Google Scholar]
- 28. Nikitin NP Witte KK Thackray SD de Silva R Clark AL Cleland JG Longitudinal ventricular function: normal values of atrioventricular annular and myocardial velocities measured with quantitative two-dimensional color Doppler tissue imaging Journal of the American Society of Echocardiography 16 2003. 906–921. 10.1016/S0894-7317(03)00279-7 [DOI] [PubMed] [Google Scholar]
- 29. McGowan JH Cleland JG Reliability of reporting left ventricular systolic function by echocardiography: a systematic review of 3 methods American Heart Journal 146 2003. 388–397. 10.1016/S0002-8703(03)00248-5 [DOI] [PubMed] [Google Scholar]
- 30. Clark AL Swan JW Laney R Connelly M Somerville J Coats AJ The role of right and left ventricular function in the ventilatory response to exercise in chronic heart failure Circulation 89 1994. 2062–2069. 10.1161/01.CIR.89.5.2062 [DOI] [PubMed] [Google Scholar]
- 31. Donal E Coquerel N Bodi S Kervio G Schnell F Daubert J-C Carré F Importance of ventricular longitudinal function in chronic heart failure European Journal of Echocardiography 12 2011. 619–627. 10.1093/ejechocard/jer089 [DOI] [PubMed] [Google Scholar]
- 32. Marwick TH Stress echocardiography Heart 89 2003. 113–118. 10.1136/heart.89.1.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Otasevic P Popovic ZB Vasiljevic JD Pratali L Vlahovic-Stipac A Boskovic SD Tasic N Neskovic AN Head-to-head comparison of indices of left ventricular contractile reserve assessed by high-dose dobutamine stress echocardiography in idiopathic dilated cardiomyopathy: five-year follow up Heart 92 2006. 1253–1258. 10.1136/hrt.2005.073999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Paraskevaidis IA Adamopoulos S Kremastinos DT Dobutamine echocardiographic study in patients with nonischemic dilated cardiomyopathy and prognostically borderline values of peak exercise oxygen consumption: 18-month follow-up study Journal of the American College of Cardiology 37 2001. 1685–1691. 10.1016/S0735-1097(01)01194-9 [DOI] [PubMed] [Google Scholar]
- 35. Ramahi TM Longo MD Cadariu AR Rohlfs K Slade M Carolan S Vallejo E Wackers FJ Dobutamine-induced augmentation of left ventricular ejection fraction predicts survival of heart failure patients with severe non-ischaemic cardiomyopathy European Heart Journal 22 2001. 849–856. 10.1053/euhj.2001.2654 [DOI] [PubMed] [Google Scholar]
- 36. Rubis P Podolec P Kopec G Olszowska M Tracz W The dynamic assessment of right-ventricular function and its relation to exercise capacity in heart failure European Journal of Heart Failure 12 2010. 260–267. 10.1093/eurjhf/hfp200 [DOI] [PubMed] [Google Scholar]
- 37. Podolec P Rubís P Tomkiewicz-Pajak L Kopeć G Tracz W Usefulness of the evaluation of left ventricular diastolic function changes during stress echocardiography in predicting exercise capacity in patients with ischemic heart failure Journal of the American Society of Echocardiography 21 2008. 834–840. 10.1016/j.echo.2007.12.008 [DOI] [PubMed] [Google Scholar]
- 38. Grewal J McCully RB Kane GC Lam C Pellikka PA Left ventricular function and exercise capacity Journal of the American Medical Association 301 2009. 286–294. 10.1001/jama.2008.1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Little WC Oh JK Echocardiographic evaluation of diastolic function can be used to guide clinical care Circulation 120 2009. 802–809. 10.1161/CIRCULATIONAHA.109.869602 [DOI] [PubMed] [Google Scholar]
- 40. Pacileo G Calabrò P Limongelli G Russo MG Pisacane C Sarubbi B Calabrò R Left ventricular remodeling, mechanics, and tissue characterization in congenital aortic stenosis Journal of the American Society of Echocardiography 16 2003. 214–220. 10.1067/mje.2003.10 [DOI] [PubMed] [Google Scholar]
- 41. Notomi Y Martin-Miklovic MG Oryszak SJ Shiota T Deserranno D Popovic ZB Garcia MJ Greenberg NL Thomas JD Enhanced ventricular untwisting during exercise a mechanistic manifestation of elastic recoil described by Doppler tissue imaging Circulation 113 2006. 2524–2533. 10.1161/CIRCULATIONAHA.105.596502 [DOI] [PubMed] [Google Scholar]
- 42. Dandel M Lehmkuhl H Knosalla C Suramelashvili N Hetzer R Strain and strain rate imaging by echocardiography – basic concepts and clinical applicability Current Cardiology Reviews 5 2009. 133–148. 10.2174/157340309788166642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Moonen M Lancellotti P Zacharakis D Pierard L The value of 2D strain imaging during stress testing Echocardiography 26 2009. 307–314. 10.1111/j.1540-8175.2008.00864.x [DOI] [PubMed] [Google Scholar]
- 44. Nikitin NP Witte KK Application of tissue Doppler imaging in cardiology Cardiology 101 2004. 170–184. 10.1159/000076694 [DOI] [PubMed] [Google Scholar]
- 45. Møller JE Søndergaard E Poulsen SH Seward JB Appleton CP Egstrup K Color M-mode and pulsed wave tissue Doppler echocardiography: powerful predictors of cardiac events after first myocardial infarction Journal of the American Society of Echocardiography 14 2001. 757–763. 10.1067/mje.2001.113367 [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a