Abstract
Hypertrophic cardiomyopathy (HCM) is a highly heterogeneous disease with varied patterns of hypertrophy. Basal septal hypertrophy and systolic anterior motion (SAM) of the mitral valve (MV) are the key pathophysiological components to left ventricular outflow tract (LVOT) obstruction in HCM. LVOT is associated with higher morbidity and mortality in patients with HCM. Percutaneous septal reduction therapy with alcohol septal ablation (ASA) can lead to a significant improvement in left ventricle haemodynamics, patient symptoms and perhaps prognosis. ASA delivers pure alcohol to an area of myocardium via septal coronary arteries; this creates damage to tissue akin to a myocardial infarction. The basal septal myocardium involved in SAM–septal contact is the target for this iatrogenic infarct. Appropriate patient selection and accurate delivery of alcohol are critical to safe and effective ASA. Securing the correct diagnosis and ensuring suitable cardiac anatomy are essential before considering ASA. Pre-procedural planning and intra-procedural imaging guidance are important to delivering precise damage to the desired area. The procedure is performed worldwide and is generally safe; the need for a pacemaker is the most prominent complication. It is successful in the majority of patients but room for improvement exists. New techniques have been proposed to perform percutaneous septal reduction. We present a review of the relevant pathophysiology, current methods and a summary of available evidence for ASA. We also provide a glimpse into emerging techniques to deliver percutaneous septal reduction therapy.
Keywords: hypertrophic obstructive cardiomyopathy, alcohol septal ablation, non-surgical septal reduction therapy
Background
Hypertrophic cardiomyopathy (HCM) is an inherited disease characterised by otherwise unexplained hypertrophy of the myocardium. It is transmitted in an autosomal dominant pattern with variable penetrance, with an estimated phenotypic prevalence of one in 500 (1). HCM is a highly heterogeneous disease with varied patterns of hypertrophy. The prevalence of left ventricular outflow tract (LVOT) obstruction in HCM is 20–30% at rest (2) and up to 70% with provocation (3). LVOT obstruction is associated with greater levels of dyspnoea, a greater incidence of stroke and higher mortality (2).
LVOT obstruction pathophysiology
Basal septal hypertrophy and systolic anterior motion (SAM) of the mitral valve (MV) are the key components to LVOT obstruction in HCM. Recent magnetic resonance imaging (MRI)-based studies of MV morphology in HCM have highlighted a significantly longer anterior leaflet when compared with controls, this results in coaptation with the posterior leaflet towards its midpoint, leaving a redundant tip (4). This is now suggested to be part of the HCM phenotype.
The asymmetric hypertrophy (ASH) of the basal septum leads to a narrowed left ventricle (LV) outflow tract; this causes rapid acceleration of blood flow apical of the MV. Venturi drag effects through the narrowed LVOT are thought to contribute to pulling the MV apparatus towards the septum. This does not explain SAM of the MV observed at low velocities, hence it is not the sole pathophysiological process. The septal hypertrophy also causes abnormal posteriorly directed flow through the LV (5). This flow circulates around the MV and back towards the LVOT, catching the redundant anterior MV leaflet tip. The MV then moves towards, and in severe cases contacts, the hypertrophied septum. Once mitral–septal contact occurs, the LVOT orifice is narrowed further and greater obstruction to flow develops, resulting in a higher pressure difference. This pressure difference forces the leaflet further against the septum, further narrowing the orifice, exacerbating the haemodynamic abnormalities. This establishes an amplifying feedback loop until systole is complete. The greater the length of time the AMVL is in contact with the septum, the higher the pressure difference (5).
This obstruction has multiple deleterious effects including reduction of forward cardiac output, mitral regurgitation of varying degrees, and load-dependent diastolic dysfunction leading to an increase in LV end-diastolic pressure and subsequent coronary flow abnormalities. These factors contribute to symptoms of dyspnoea, chest pain, pre-syncope and syncope (6, 7, 8).
Alcohol septal ablation
The critical event in this pathophysiological cycle is SAM–septal contact. This therefore becomes the target for percutaneous septal reduction therapy.
Alcohol septal ablation (ASA) damages the basal interventricular septum by inducing a localised infarction with trans-coronary alcohol injection. The LVOT widens, and the basal septum does not move towards the MV in systole (Fig. 1, Videos 1 and 2). This reduces SAM and allows a greater area for blood to escape from the heart (Video 2). There is a reduction in intra-cavity pressure in the LV and hence gradient. The effects can continue to improve for months afterwards. This can partly be explained by the change in diastolic function, the drop in LVOT gradient has been shown to reduce left ventricular hypertrophy distant from the LVOT and therefore cardiac mass (9, 10); this shows that part of the hypertrophy in HOCM is after load dependent and not entirely genetically pre-determined.
Parasternal long-axis view of a 42-year-old male with HOCM, the basal septum at LVOT level measures 27 mm. In systole, the MV apparatus (including the posterior leaflet) moves anteriorly towards the septum. The long anterior leaflet contacts the septum in the LVOT causing severe obstruction to flow out of the LV. Download Video 1 via http://dx.doi.org/10.1530/ERP-14-0058-v1.
Download Video 1 (206.1KB, mov)
An area of the basal septum at the LVOT level has an infarct. The myocardium has shrunk and measures just 13 mm. The LVOT has widened. The SAM of the MV apparatus is now mild with minimal movement of the anterior leaflet towards the LVOT. Download Video 2 via http://dx.doi.org/10.1530/ERP-14-0058-v2.
Download Video 2 (223.1KB, mov)
Parasternal long axis view 6 months post alcohol ablation. The mass of myocardium at the proximal basal septum is reduced. There is less systolic anterior motion of the anterior mitral valve leaflet which no longer contact the septum. Download Video 3 via http://dx.doi.org/10.1530/ERP-14-0058-v3.
Download Video 3 (595KB, avi)
Apical long axis view 6 months post alcohol ablation. Note reduced septal width and SAM. Download Video 4 via http://dx.doi.org/10.1530/ERP-14-0058-v4.
Download Video 4 (609.4KB, avi)
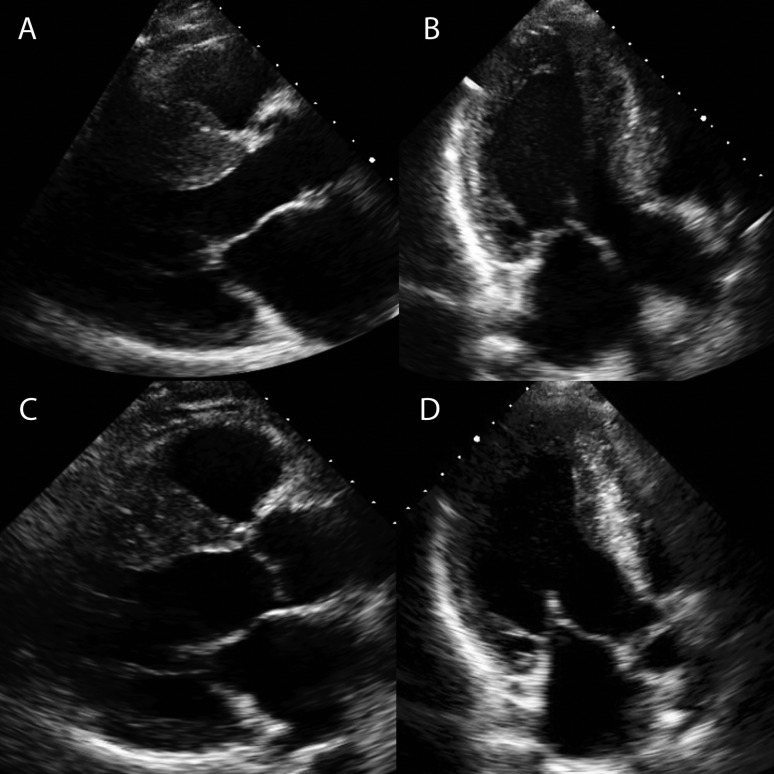
Figure 1.
Effects of ASA on basal septum. (A and B) A 42-year-old male with a septum of 29 mm, severe SAM of the MV and LVOT gradient of 102 mmHg before ASA. See Videos 1 and 2. (C and D) The septum post-ASA; there is a reduction in size of the basal septum. Videos 3 and 4 show reduction in systolic excursion of the septum and SAM. The LVOT gradient post-ASA was 10 mmHg.
Patient selection
Appropriate patient selection is imperative to the performance of effective and safe ASA. The treating team must complete comprehensive clinical assessment. Although no restrictions are placed upon age of patient, it is generally recommended that it is inappropriate to perform ASA in children (11). The long-term risk of ventricular arrhythmia remains unclear, and providing a potentially arrhythmic scar in children is discouraged. If deemed appropriate for ASA, it is then necessary to perform detailed investigation of cardiac anatomy and haemodynamics and explore functional impairment.
Clinical assessment
Assess symptom burden
The primary reason for performing ASA currently is to improve symptomatology. There has been some suggestion that removing LVOT gradients with ASA improves prognosis, but this is not yet secure and should not be the driver to treatment (12, 13). Patients must report NYHA class III dyspnoea (11, 14). Exceptions can be made for patients with class II dyspnoea, disabling non-arrhythmogenic pre-syncope/syncope or chest pain without revascularisation options. Patients must be symptomatic despite optimisation of negatively inotropic medications such as β-blockers, verapamil or disopyramide before consideration of septal reduction therapy.
Rule out phenocopies
The operator must be secure in a diagnosis of genetically determined HCM. It is essential to ensure that the patient does not suffer from any pathology that is amenable to alternative therapies before performing a deliberately destructive procedure.
Trial of right ventricular apical pacing
Some patients with HCM already have a cardiac rhythm management device in situ. In these patients, a trial of dual-chamber right ventricular (RV) apical pacing with a short atrioventricular delay should be performed to assess effects on LVOT gradient and symptoms. Pacing can be effective in a small proportion of patients (15, 16, 17, 18). It is not currently indicated as first-line treatment (11, 14).
Assessment of cardiac anatomy and haemodynamics
Assessment of LVOT gradient
There must be a demonstrable LVOT gradient to correct in order for ASA to be effective. It has been stated in the ACCF/AHA and recent ESC guidelines for the management of HCM that a gradient of ≥50 mmHg is required for a patient to derive benefit from treatment (11, 14).
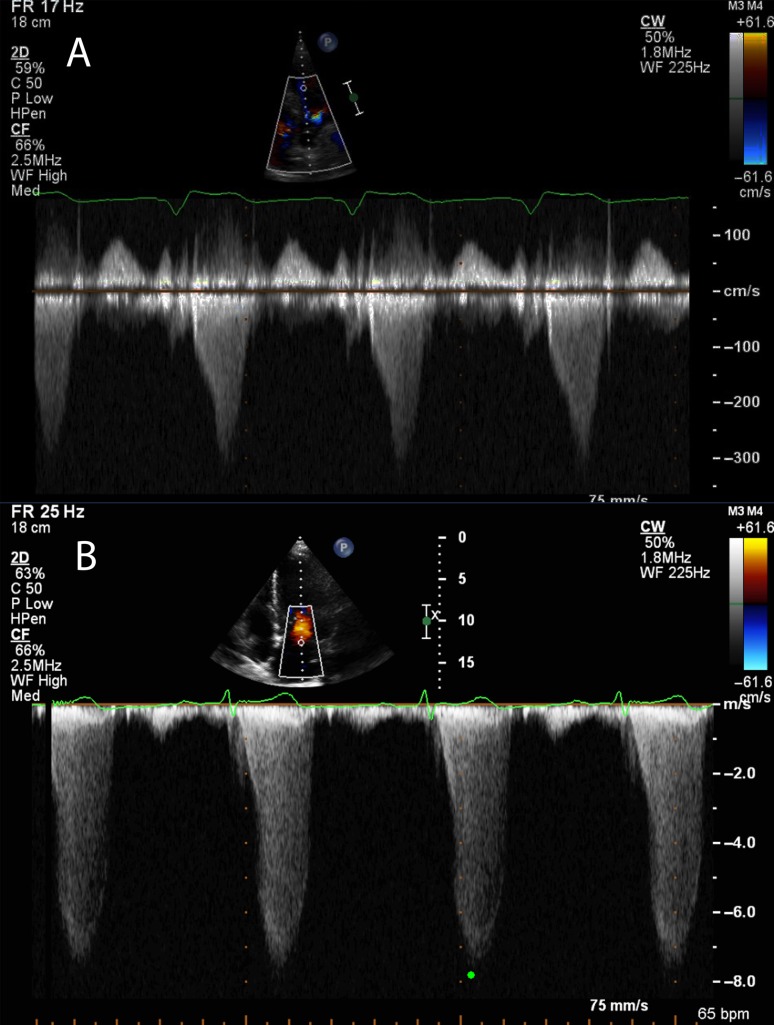
A gradient is calculated using the modified Bernoulli equation based on flow velocities measured with continuous-wave Doppler through the LVOT. The aortic valve and LVOT should be assessed to ensure that there is no stenosis or sub-aortic band responsible for the increased velocities. The envelope shape attributable to LVOT obstruction in SAM-mediated HOCM is typically scimitar shaped with late-flow acceleration. This is related to the pathophysiology described earlier, where the amplifying feedback loop causes accelerated late pressures and hence increased blood velocity (Fig. 1). It is important to ensure that the Doppler signal is not measuring the mitral regurgitation flow that is often associated with SAM; the flow of MR often has high velocities and can lead to erroneously high LVOT gradient calculations (Fig. 2). Pulsed wave Doppler can be used to localise the flow acceleration point, as those with mid-cavity gradients do not derive as much benefit from ASA (19).
Figure 2.
(A) A continuous-wave Doppler trace through the LVOT and aortic valve. Note the late acceleration and the subsequent scimitar-shaped envelope. The peak velocity in this patient was 3.1 m/s. (B) A CW trace through the posteriorly directed MR observed as a result of SAM and failure of coaptation of the MV leaflets. This has a more symmetrical shape and high velocities of 7.9 m/s. If this velocity were used to incorrectly calculate a gradient, the value would be 250 mmHg. Compare this with the correct calculation of just 38 mmHg.
ASA can also be effective for those with a minimal gradient at rest but with a marked increase with Valsalva manoeuvre or exercise (20). Dobutamine causes an apparent LVOT gradient in the normal heart and should not be used in these circumstances (21).
Septal size
The basal septum should be ≥15 mm. There is a risk of ventricular septal defect with iatrogenic infarct in those with a septum <15 mm. Although rare, VSDs have been reported as a complication from ASA (22). The ESC guidelines suggest that a septum of ≥17 mm as a minimum measurement for alcohol ablation to be considered (14). The benefit of ASA in those with a septum >30 mm is not clear. It is generally recommended that myectomy is a more suitable option in these patients (11).
MV and papillary muscle anatomy
MV abnormalities are relatively common in HCM (23). Primary disease of the MV should prompt a surgical referral. Cardiac MRI and echocardiography can be used to ensure that there is no abnormal papillary muscle anatomy such as anterior displacement or direct insertion into valve leaflets that would also require surgical correction (24).
Rule out alternative pathologies requiring surgical intervention
Significant co-morbid cardiac disease requiring surgery such as coronary artery disease, sub-aortic bands or MV abnormalities should prompt a surgical referral. Combined operative therapy incorporating septal myectomy is preferred in these circumstances.
Functional assessment
Pulmonary function tests
Pulmonary disease as the prime driver to dyspnoea must be excluded before progressing to ASA. Removal of the LVOT gradient in these patients is less likely to alleviate symptoms (25). Classification of ≥moderate COPD by global initiative for chronic obstructive lung disease (GOLD) severity scale should prompt optimisation of lung disease management before consideration of ASA (26). A restrictive pattern on PFTs should prompt further pulmonary investigations.
Cardiopulmonary exercise testing
CPEX allows an accurate assessment of the true physical restriction along with providing information on the contribution of pulmonary and cardiac diseases. There must be a baseline abnormal limit to exertion to correct before considering ASA (Table 1).
Table 1.
Clinical and diagnostic criteria of patients must fulfil before progression to ASA
| Category | Criteria for progression to ASA |
|---|---|
| Diagnosis | Clinician must be satisfied that the pathology is genetically determined hypertrophic cardiomyopathy in the absence of other causes of LVH |
| Symptom status | NYHA III dyspnoea |
| NYHA II with non-arrhythmic pre-syncope or chest pain | |
| Medications | Symptoms refractory to an adequate trial of negatively inotropic medications |
| LVOT gradient | ≥50 mmHg at rest, Valsalva manoeuvre or with exercise stress |
| LVOT gradient must be due to SAM of the MV. Causes such as sub-aortic band and anomalous papillary muscle architecture must be ruled out | |
| Septal size | ≥15 mm in diastole |
| Alternative indication for surgery | Rule out coronary artery disease, sub-aortic band or MV abnormalities requiring surgical intervention |
| RV apical pacing | Trial of RV pacing if dual-chamber device already in situ |
| Functional testing | >50% predicted FEV1 and FVC on PFTs |
| <90% predicted peak VO2 on CPEX testing | |
| Cardiac restriction on CPEX testing |
Pre-procedural investigation
In order to achieve satisfactory assessment of suitability for ASA, the investigations in Table 2 are completed.
Table 2.
Investigations performed in the workup of patients proposed for ASA
| Category | Test | Rationale |
|---|---|---|
| Haematological | U&Es | Secondary organ dysfunction |
| Often abnormal in phenocopies (HTN, Anderson-Fabry, infiltration) | ||
| CK | Rule out syndromes such as myotonic dystrophy | |
| FBC | Rule out anaemia as a cause of dyspnoea | |
| α-galactosidase | Consider in cases where there is clinical suspicion of Anderson-Fabry disease | |
| ECG | 12-lead ECG | Help distinguish phenocopies: |
| Small QRS – infiltrative myocardial disease | ||
| Excessively large QRS – Pompe's, Danon's syndromes | ||
| 1st degree HB – storage disease | ||
| AV block – infiltration, Anderson-Fabry | ||
| Pre-excitation – Pompe's, Danon's syndromes | ||
| Extreme axis deviation – Noonan's syndromes | ||
| 24-h ECG | Consideration of SCD risk and potential for ICD implantation before ASA | |
| 24-h BP monitor | Consider in cases where a phenocopy of hypertensive heart disease is suspected | |
| Imaging | Echocardiogram | Assess: |
| Septal size | ||
| Extent of SAM | ||
| LVOT gradient extent and localisation, rest and with Valsalva manoeuvre | ||
| Diastolic function | ||
| Cardiac MRI | Assess: | |
| Septal size and distribution of hypertrophy | ||
| Presence of HCM variant such as abnormal direct papillary muscle insertion into MV leaflets, antero-apical displacement of anterior papillary muscle, sub-aortic band | ||
| Assess pre-existing scar; risk of SCD and comparison of iatrogenic scar location post-ASA | ||
| CT coronary angiography | Plan access to septal arteries that supply target myocardium | |
| Rule out significant coronary artery disease that will require CABG | ||
| Functional | PFTs | Rule out significant lung disease as the primary driver to dyspnoea |
| CPEX | Assess: | |
| Functional capacity (baseline to compare with post-ASA) | ||
| Primary cause of dyspnoea | ||
| Prognostic implications |
ASA: the procedure
ASA should be performed by experienced operators; the learning curve in ASA can require the performance of at least 40 procedures (27). This was re-enforced in the ACCF/ACC guidelines, in which it has been stated that an operator must have experience of at least 20 procedures or work in a centre with a cumulative procedural volume of 50 patients (11).
Arterial access is secured and a guidecath manoeuvred to the coronary arteries. Simultaneous monitoring of LV and central aortic pressures can be performed using and end-hole catheter placed at the LV apex and a guidecath in the ascending aorta. This will give continuous LVOT gradient readings throughout the procedure. This usually requires two arterial access points but diagnostic procedures can be carried out with one puncture using a pressure wire sited in the LV apex. LVOT gradient should be measured at rest and following an ectopic beat for the Brockenbrough–Braunwald–Morrow phenomenon. Sometimes a high ectopic burden or runs of ventricular tachycardia from catheter irritation prevent continuous monitoring of the gradient.
A venous sheath should be sited to allow insertion of a temporary pacing wire (TPW) at the RV apex. Septal pacing must be avoided as early activation of the septum can increase LVOT obstruction.
Heparin is used to prevent thromboembolic complication. A guidewire is advanced to the septal artery of choice. This may be a sub-branch of a septal artery as these vessels often supply both the right and left septal myocardia. An over-the-wire balloon is then advanced into the septal artery. Once inflated, this then creates a secure, hollow channel from the operator to the target myocardium.
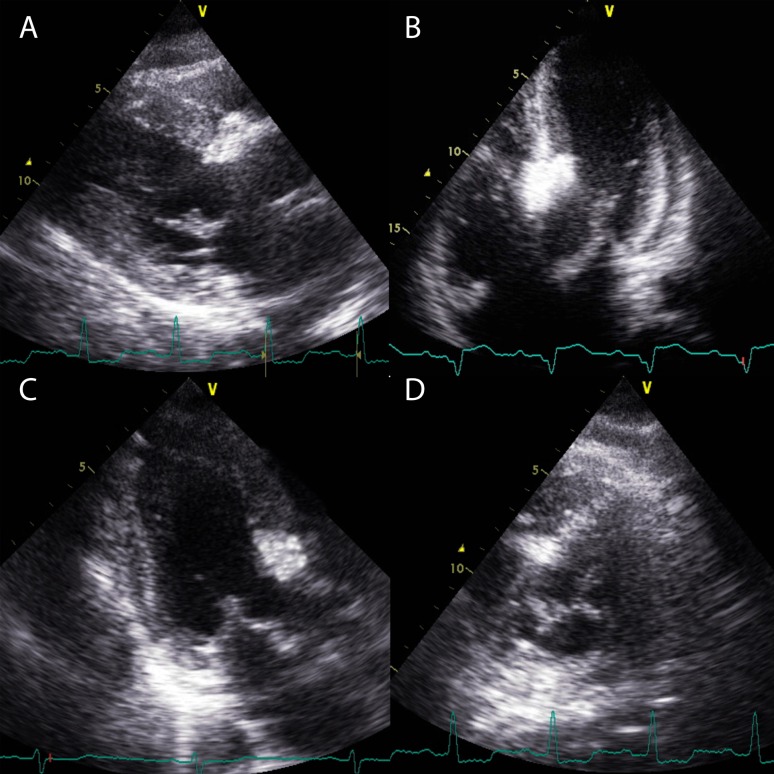
Myocardial contrast echocardiography is then used to demonstrate the area of myocardium supplied by the chosen vessel. Contrast should be injected into the chosen septal artery with continuous echocardiographic screening. An obvious opacification of the area of the septum involved in the contact point for SAM will be observed if the artery is the correct choice (Fig. 3). Multiple projections are required to ensure the correct distribution in three dimensions.
Figure 3.
Myocardial contrast echocardiography. Echocardiographic contrast is injected into a chosen septal artery with continuous echocardiographic screening. Localisation of the contrast can be observed in the basal septum in the parasternal long-axis (A), apical 5-chamber (B) and apical long-axis (C) views. (D) A parasternal short-axis view with contrast visible at 11 O'clock. This is required as an additional view to ensure that the chosen vessel does not supply an area too inferior or anterior in the septum. Contrast should highlight the SAM–septal contact area.
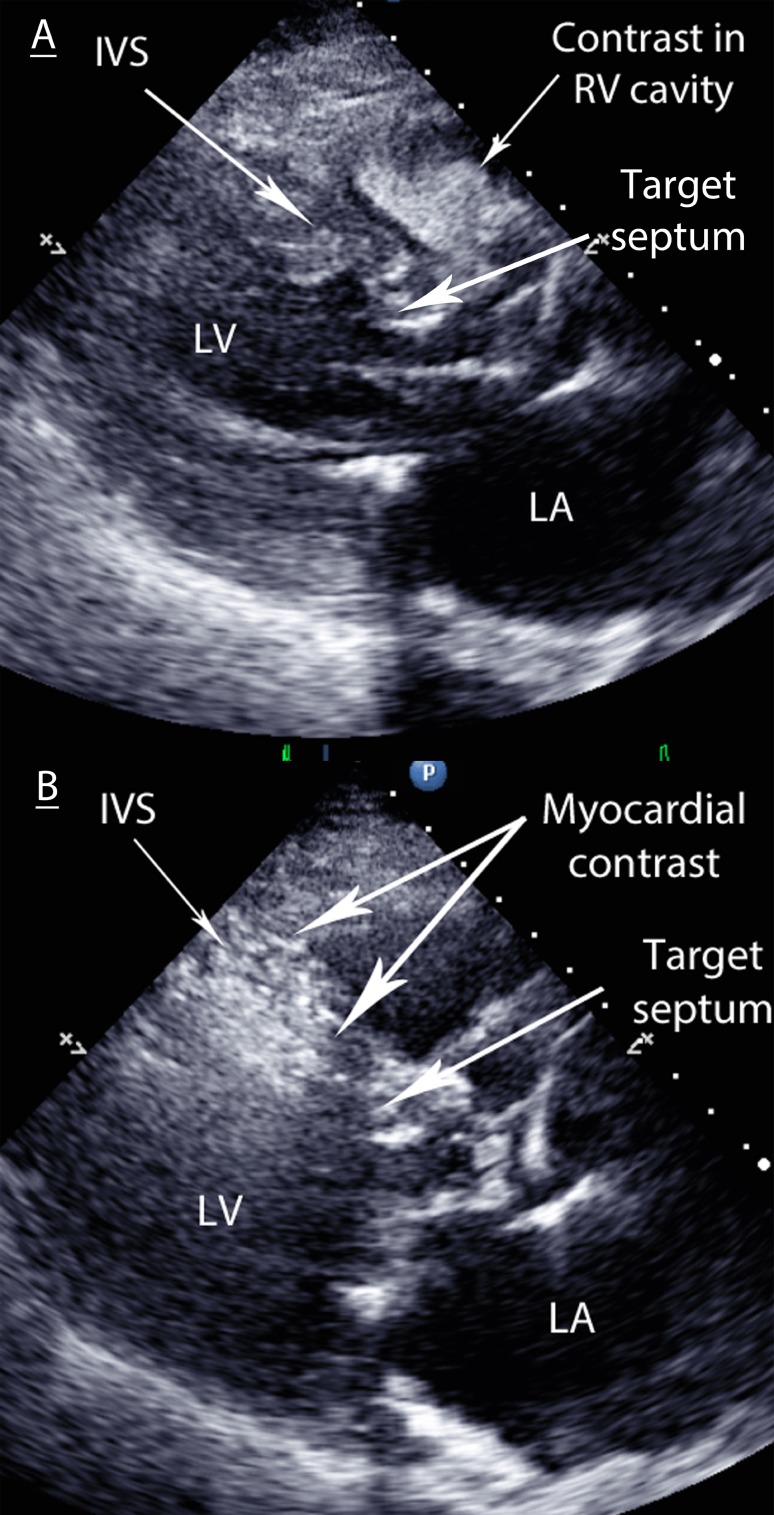
If contrast is observed elsewhere, an alternative artery will need to be explored (Fig. 4).
Figure 4.
(A) Contrast filling the RV cavity. The chosen septal vessel has a connection to the RV cavity via Thebesian veins, therefore the contrast-injected vents directly into the RV. Any alcohol injected herein would drain into systemic circulation and causes minimal myocardial damage. (B) Contrast highlighting the mid-septum. This is too apical and away from the target area at the SAM–septal contact point in the basal septum. Alcohol injection herein would cause an infarct away from the SAM–septal contact area and hence would have no effect on intra-cavity gradients.
The guidewire is then removed and a small amount of angiographic contrast (0.5 ml) injected into the septal artery beyond balloon occlusion to ensure no spill back into the parent artery or collateral connection to other septal or major epicardial vessels.
Once the target artery has been satisfactorily identified, a small volume (1–3 ml) of absolute alcohol is injected slowly in small increments. Analgesia can be given, as injection of alcohol can cause some discomfort. The volume of alcohol injected will depend on the area of myocardium opacified with contrast injection. There is evidence to suggest that small doses (1–2 ml) of alcohol can have equally effective results as large (2–4 ml) doses (28, 29). Balloon occlusion should be maintained for at least 5 min. A final angiographic image is taken to show the septal branch no-reflow and rule out any unwanted coronary artery damage.
The patient is kept under coronary care supervision for 24–48 h with the TPW in situ. If no complete heart block is present at that time, it can be removed. Temporary permanent pacemakers can be used for up to 4 weeks to allow intrinsic conduction to recover. This may reduce the need for permanent system implantation. Hospital stay is 4–5 days if no complication is observed.
Peri-procedural complications of ASA
The most prominent complication associated with ASA is the need for a permanent pacemaker. The risk in large multicentre observations remains around 10% (22, 25, 30, 31, 32). Lower doses of alcohol and increased operator experience appear to reduce this risk. While the ramifications of a pacemaker should not be dismissed, we must bear in mind that RV apical pacing can be an effective treatment to reduce LVOT gradients in some patients. Others require ICD implantation due to risk of SCD. The use of cardiac rhythm management devices in this group may have an independent value and is not necessarily a classic ‘complication’. Patients with long-term pacing seem to have a similar outcome as those without pacing following ASA (31, 33). Other peri-procedural complications have a significantly lower frequency. Peri-procedural ventricular arrhythmia was found in 0.02% and LAD dissection in 0.01% (22). Death is extremely rare, with rates of one in 874 (22), two in 177 (12) (both in cardiogenic shock before ASA), three in 459 (32) and zero in 465 reported (13).
Results
Survival
HCM patients with resting LVOT obstruction have a higher mortality than matched HCM patients without a gradient (2). Recent case series have suggested that removing the gradient may have a beneficial effect on survival (12, 13, 34, 35). These are results from observational studies rather than randomised controlled trials must therefore be taken in context. The observations will, however, go some way to reassuring operators that the iatrogenic scar of ASA does not increase mortality in the medium term.
Risk of ventricular arrhythmia
The fears regarding a pro-arrhythmic risk from the iatrogenic scar formed by ASA do not appear to have been substantiated. There is conflicting evidence but most case series report no increased risk of SCD, ventricular arrhythmias or appropriate ICD therapy (12, 13, 34, 35, 36, 37), while a smaller number claim an increased risk of ventricular arrhythmia (38, 39).
Symptom and gradient resolution
Following the breakthrough studies, multiple early patient series were reported and summarised in a systematic review collated in 2006 (30). In total, 2959 patients were summarised from 42 published studies, although the authors accept that some were duplicated by involvement in more than one report. A baseline assessment revealed a mean age of 53.5 years, an NYHA class of 2.9 despite medical therapy and peak LVOT gradients of 65 mmHg at rest and 125 mmHg on provocation. The mean follow-up period was just over a year. Early mortality (defined as within 30 days) was reported to be 1.5%, with late all-cause mortality at 0.5% (0–9.3%). NYHA status improved significantly to 1.2 (P<0.001). A reduction in angina burden was also observed; the Canadian Cardiovascular Society score reduced from 1.9 to 0.4 (P<0.001). Echocardiographic gradients reduced to 15 mmHg at rest and 31 mmHg on provocation (both P<0.001). Repeat procedures were required in 7% of patients.
A 2011 report from the Multicentre North American registry detailed procedures in 874 patients. All had a resting gradient of ≥30 mmHg or provocable gradient of ≥60 mmHg with advanced symptoms of exertional dyspnoea and/or angina despite medical therapy (40). The mean follow-up duration was 26 months. Seventy-eight per cent of patients suffered NYHA class III or IV dyspnoea before the procedure, with a gradient measuring 70 mmHg at rest and 99 mmHg on provocation. Following ASA, 72.5% of patients were classified as NYHA class I, 23% as class II, 3.9% as class III and 0.65% as class IV. A repeat procedure was required in 12.8% of patients. A mean of peak resting gradients reduced to 35 mmHg post-procedure. The Scandinavian multicentre study included 279 patients with similar results, with NYHA III/IV breathlessness reduced from 94 to 21% and outflow tract gradients falling from 58 to 12 mmHg. Those persisting with NYHA III/IV breathlessness had a high prevalence of co-morbidities including chronic obstructive pulmonary disease and valve disease (25).
A number of series of medium-term results are now available, with time periods ranging from 25 to 141 months (12, 13, 22, 35, 36, 41, 42, 43, 44, 45, 46) (Table 3). Improvement in dyspnoea is observed in most, with a clear trend towards lower LVOT gradients. The procedure does not provide uniform improvement for all, however, with a large recent series displaying a mean post-procedure resting gradient of 35 mmHg (22) (a gradient some would claim justifies repeat treatment) and persistent NYHA class III dyspnoea in 21% (25). There is therefore scope for improvement in treatment and subsequent outcomes for HCM patients with LVOT obstruction refractory to medical therapy.
Table 3.
Long-term results from observational studies post-ASA
| Author | Year | Follow-up (months) | n | Age at ASA (years) | Pre | Post | Complications | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NYHA | Rest grad (mmHg) | NYHA | Rest grad (mmHg) | Death at ASA (%) | Death at follow-up (%) | Death cardiac cause (%) | |||||
| Seggewiss | 2007 | 58 | 100 | 53 | 2.8 | 76 | 1.6 | 0 | 1 | 4 | 2 |
| Kwon | 2008 | 96 | 55 | 63 | 96% ≥3 | 70 | 17% ≥3 | 31 | 24 | 2 | |
| Fernandes | 2008 | 55 | 579 | 54 | 2.8 | 77 | 1.4 | <10 | 9 | 4 | |
| Kuhn | 2008 | 25 | 329 | 58 | 54 | 21 | 1.8 | 8.9 | 3 | ||
| Lyne | 2010 | 141 | 12 | 49 | 2.7 | 70 | 1 | 3 | 0 | 24 | 16 |
| Klopotowski | 2010 | 111 | 61 | 48 | 0 | 5 | 1.6 | ||||
| Jensen | 2011 | 42 | 77 | 61 | 97% ≥3 | 60 | 9% ≥3 | 12 | 0 | 16 | 6.5 |
| Nagueh | 2011 | 26 | 874 | 55 | 78% ≥3 | 70 | 4.5% ≥3 | 35 | 9.3 | 2.8 | |
| Jensen | 2011 | 44 | 279 | 59 | 94% ≥3 | 58 | 21% ≥3 | 12 | 0.3 | 12.2 | |
| Sorajja | 2012 | 68 | 177 | 64 | 100% ≥3 | 73 | 67% =1 | 11 | 1.1 | 14.7 | 2.8 |
| Jensen | 2013 | 100 | 465 | 56 | 86% ≥3 | 60 | 8% ≥3 | 6 | 0 | 9 | 4 |
| Veselka | 2014 | 3 | 436 | 57 | 2.8 | 88 | 1.6 | 21 | 0.2 | 0.7 | |
Comparison to myectomy
According to the 2011 ACC/ACCF guidelines, surgical myectomy remains the treatment of choice for HOCM with symptoms of heart failure. Surgery has a class IIa recommendation, ASA is class IIb (11). This is based on excellent survival, symptom resolution, abolition of gradient and low operative mortality at centres of excellence (47).
Meta-analyses have evaluated non-randomised studies comparing ASA and myectomy at individual institutions (48, 49). The age of the ASA groups tend to be higher than the myectomy group. Baseline NYHA status and echocardiographic characteristics were comparable between groups. Symptom improvement was similar. The only significant differences to come out of detailed statistical analysis were a higher incidence of pacemaker implantation and RBBB in the ASA group, and a small, yet significantly higher, residual LVOT gradient in the ASA group (50).
Owing to limited patient populations, a randomised controlled study to compare mortality benefit between ASA and myectomy will likely never be performed (51).
Alternative methods of non-surgical septal reduction therapy
Alternative methods of inducing damage to the target myocardium that have been explored include injection of glue (52, 53), injection of microspheres (53) and coil embolisation (54, 55). All of these methods damage myocardium by ischaemia. The rich collateralisation of the septum may explain the difficulties in effectively reducing long-term gradients by ischaemia alone. These therapies have not been adopted due to the limited impact on gradients long term.
A significant limitation of ASA is reliance on coronary anatomy to provide access to a target for ablation. Up to 15% of patients have no septal vessel suitable for injection, due to inability to identify or instrument an artery supplying the basal hypertrophy at the SAM–septal contact point (46, 56, 57). Endocardial radiofrequency ablation has been used to deliver damage directly to the endocardial surface of the LV basal septum and bypass this reliance (58, 59, 60).
Lawrenz et al. (59) performed Endocardial Radio frequency Ablation of Septal Hypertrophy (ERASH) in a cohort of 19 adults, eight of whom had ineffective ASA due to inappropriate coronary anatomy. All patients were offered, and declined, surgery. Ablation was performed from the RV septum in ten patients and LV septum in nine patients. Although follow-up imaging showed a minor reduction in the magnitude of septal hypertrophy, 22.6–21.4 mm, deep scars were visible, up to 28 mm, within the septum on cardiac MRI in some. This is in contrast to ASA, where a more marked change in the septal thickness in diastole is observed. Despite a modest effect on septal width, a reduction in resting gradients of 77.7–26.5 mmHg and provoked gradients of 157.5–64.2 mmHg was observed. NYHA status reduced from a mean of 3.0–1.7 (59).
Reduction of septal thickening in systole is proposed to be responsible for the reduced gradient. This akinesia of the basal septum prevents narrowing of the LVOT in systole, reducing SAM and therefore obstruction. The flexibility of ERASH appeals to physicians dealing with HOCM, and early results are comparable to results of early ASA series (61). With a clear learning curve for operators performing ASA (62), this indicates significant promise for ERASH.
Conclusion
ASA provides relief of symptoms attributable to LVOT obstruction in the majority of patients. The success of ASA relies upon appropriate patient selection. While initially viewed as an alternative to myectomy only in patients unfit for surgery, the balance of preference has changed for some operators (63, 64, 65). The improved outcomes of ASA are comparable to myectomy (48, 50), the complication rate has much improved and its availability is much more widespread. Medium-term survival is better than matched HOCM controls and even better than general population-matched controls in some series (12, 35, 66).
While ASA can provide relief to the majority of appropriately selected HOCM patients, up to 25–30% of patients in the largest series are still significantly symptomatic or have persisting LVOT gradients >30 mmHg (22, 25). A further 5–8% of patients selected for ASA cannot undergo the procedure due to limitations of septal arterial supply (46, 56). Few changes have been made in the last decade and developments in the procedure may be required to improve patient outcome.
Newer methods of non-surgical septal reduction therapy (NSRT) therapy may provide greater options for specific subsets of patients within the HOCM cohort (53, 55, 59, 60) and, as these procedures develop, they may well provide a valuable adjunct to the management of HOCM patients.
Supplementary data
This is linked to the online version of the paper at http://dx.doi.org/10.1530/ERP-14-0058.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the review.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
References
- 1. Elliott P McKenna WJ Hypertrophic cardiomyopathy Lancet 363 2004. 1881–1891. 10.1016/S0140-6736(04)16358-7 [DOI] [PubMed] [Google Scholar]
- 2. Maron MS Olivotto I Betocchi S Casey SA Lesser JR Losi MA Cecchi F Maron BJ Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy New England Journal of Medicine 348 2003. 295–303. 10.1056/NEJMoa021332 [DOI] [PubMed] [Google Scholar]
- 3. Maron MS Olivotto I Zenovich AG Link MS Pandian NG Kuvin JT Nistri S Cecchi F Udelson JE Maron BJ Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction Circulation 114 2006. 2232–2239. 10.1161/CIRCULATIONAHA.106.644682 [DOI] [PubMed] [Google Scholar]
- 4. Maron MS Olivotto I Harrigan C Appelbaum E Gibson CM Lesser JR Haas TS Udelson JE Manning WJ Maron BJ Mitral valve abnormalities identified by cardiovascular magnetic resonance represent a primary phenotypic expression of hypertrophic cardiomyopathy Circulation 124 2011. 40–47. 10.1161/CIRCULATIONAHA.110.985812 [DOI] [PubMed] [Google Scholar]
- 5. Sherrid MV Chu CK Delia E Mogtader A Dwyer EM Jr An echocardiographic study of the fluid mechanics of obstruction in hypertrophic cardiomyopathy Journal of the American College of Cardiology 22 1993. 816–825. 10.1016/0735-1097(93)90196-8 [DOI] [PubMed] [Google Scholar]
- 6. Maron BJ Maron MS Wigle ED Braunwald E The 50-year history, controversy, and clinical implications of left ventricular outflow tract obstruction in hypertrophic cardiomyopathy from idiopathic hypertrophic subaortic stenosis to hypertrophic cardiomyopathy: from idiopathic hypertrophic subaortic stenosis to hypertrophic cardiomyopathy Journal of the American College of Cardiology 54 2009. 191–200. 10.1016/j.jacc.2008.11.069 [DOI] [PubMed] [Google Scholar]
- 7. Maron BJ Nishimura RA Danielson GK Pitfalls in clinical recognition and a novel operative approach for hypertrophic cardiomyopathy with severe outflow obstruction due to anomalous papillary muscle Circulation 98 1998. 2505–2508. 10.1161/01.CIR.98.23.2505 [DOI] [PubMed] [Google Scholar]
- 8. Ommen SR Nishimura RA What causes outflow tract obstruction in hypertrophic cardiomyopathy? Heart 95 2009. 1725–1726. 10.1136/hrt.2009.174292 [DOI] [PubMed] [Google Scholar]
- 9. Mazur W Nagueh SF Lakkis NM Middleton KJ Killip D Roberts R Spencer WH III Regression of left ventricular hypertrophy after nonsurgical septal reduction therapy for hypertrophic obstructive cardiomyopathy Circulation 103 2001. 1492–1496. 10.1161/01.CIR.103.11.1492 [DOI] [PubMed] [Google Scholar]
- 10. Lakkis N Plana JC Nagueh S Killip D Roberts R Spencer WH III Efficacy of nonsurgical septal reduction therapy in symptomatic patients with obstructive hypertrophic cardiomyopathy and provocable gradients American Journal of Cardiology 88 2001. 583–586. 10.1016/S0002-9149(01)01748-9 [DOI] [PubMed] [Google Scholar]
- 11. Gersh BJ Maron BJ Bonow RO Dearani JA Fifer MA Link MS Naidu SS Nishimura RA Ommen SR Rakowski H et al. ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy: Executive Summary: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Circulation 124 2011. 2761–2796. 10.1161/CIR.0b013e318223e230 [DOI] [PubMed] [Google Scholar]
- 12. Sorajja P Ommen SR Holmes DR Jr Dearani JA Rihal CS Gersh BJ Lennon RJ Nishimura RA Survival after alcohol septal ablation for obstructive hypertrophic cardiomyopathy Circulation 126 2012. 2374–2380. 10.1161/CIRCULATIONAHA.111.076257 [DOI] [PubMed] [Google Scholar]
- 13. Jensen MK Prinz C Horstkotte D van Buuren F Bitter T Faber L Bundgaard H Alcohol septal ablation in patients with hypertrophic obstructive cardiomyopathy: low incidence of sudden cardiac death and reduced risk profile Heart 99 2013. 1012–1017. 10.1136/heartjnl-2012-303339 [DOI] [PubMed] [Google Scholar]
- 14. Elliott PM Anastasakis A Borger MA Borggrefe M Cecchi F Charron P Hagege AA Lafont A Limongelli G et al. ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: The Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC) European Heart Journal 35 2014. 2733–2779. 10.1093/eurheartj/ehu284 [DOI] [PubMed] [Google Scholar]
- 15. Kappenberger L Linde C Daubert C McKenna W Meisel E Sadoul N Chojnowska L Guize L Gras D Jeanrenaud X et al. Pacing in hypertrophic obstructive cardiomyopathy. A randomized crossover study. PIC Study Group European Heart Journal 18 1997. 1249–1256. 10.1093/oxfordjournals.eurheartj.a015435 [DOI] [PubMed] [Google Scholar]
- 16. Gadler F Linde C Juhlin-Dannfelt A Ribeiro A Ryden L Long-term effects of dual chamber pacing in patients with hypertrophic cardiomyopathy without outflow tract obstruction at rest European Heart Journal 18 1997. 636–642. 10.1093/oxfordjournals.eurheartj.a015309 [DOI] [PubMed] [Google Scholar]
- 17. Kappenberger LJ Linde C Jeanrenaud X Daubert C McKenna W Meisel E Sadoul N Chojnowska L Guize L Gras D et al. Clinical progress after randomized on/off pacemaker treatment for hypertrophic obstructive cardiomyopathy. Pacing in Cardiomyopathy (PIC) Study Group Europace 1 1999. 77–84. 10.1053/eupc.1998.0024 [DOI] [PubMed] [Google Scholar]
- 18. Maron BJ Nishimura RA McKenna WJ Rakowski H Josephson ME Kieval RS Assessment of permanent dual-chamber pacing as a treatment for drug-refractory symptomatic patients with obstructive hypertrophic cardiomyopathy. A randomized, double-blind, crossover study (M-PATHY) Circulation 99 1999. 2927–2933. 10.1161/01.CIR.99.22.2927 [DOI] [PubMed] [Google Scholar]
- 19. Cecchi F Olivotto I Nistri S Antoniucci D Yacoub MH Midventricular obstruction and clinical decision-making in obstructive hypertrophic cardiomyopathy Herz 31 2006. 871–876. 10.1007/s00059-006-2928-1 [DOI] [PubMed] [Google Scholar]
- 20. Gietzen FH Leuner CJ Obergassel L Strunk-Mueller C Kuhn H Role of transcoronary ablation of septal hypertrophy in patients with hypertrophic cardiomyopathy, New York Heart Association functional class III or IV, and outflow obstruction only under provocable conditions Circulation 106 2002. 454–459. 10.1161/01.CIR.0000022845.80802.9D [DOI] [PubMed] [Google Scholar]
- 21. Pellikka PA Oh JK Bailey KR Nichols BA Monahan KH Tajik AJ Dynamic intraventricular obstruction during dobutamine stress echocardiography. A new observation Circulation 86 1992. 1429–1432. 10.1161/01.CIR.86.5.1429 [DOI] [PubMed] [Google Scholar]
- 22. Nagueh SF Groves BM Schwartz L Smith KM Wang A Bach RG Nielsen C Leya F Buergler JM Rowe SK et al. Alcohol septal ablation for the treatment of hypertrophic obstructive cardiomyopathy a multicenter North American registry Journal of the American College of Cardiology 58 2011. 2322–2328. 10.1016/j.jacc.2011.06.073 [DOI] [PubMed] [Google Scholar]
- 23. Cavalcante JL Barboza JS Lever HM Diversity of mitral valve abnormalities in obstructive hypertrophic cardiomyopathy Progress in Cardiovascular Diseases 54 2012. 517–522. 10.1016/j.pcad.2012.03.002 [DOI] [PubMed] [Google Scholar]
- 24. Rowin EJ Maron BJ Lesser JR Rastegar H Maron MS Papillary muscle insertion directly into the anterior mitral leaflet in hypertrophic cardiomyopathy, its identification and cause of outflow obstruction by cardiac magnetic resonance imaging, and its surgical management American Journal of Cardiology 111 2013. 1677–1679. 10.1016/j.amjcard.2013.01.340 [DOI] [PubMed] [Google Scholar]
- 25. Jensen MK Almaas VM Jacobsson L Hansen PR Havndrup O Aakhus S Svane B Hansen TF Køber L Endresen K et al. Long-term outcome of percutaneous transluminal septal myocardial ablation in hypertrophic obstructive cardiomyopathy: a Scandinavian multicenter study Circulation. Cardiovascular interventions 4 2011. 256–265. 10.1161/CIRCINTERVENTIONS.110.959718 [DOI] [PubMed] [Google Scholar]
- 26. Gomez FP Rodriguez-Roisin R Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines for chronic obstructive pulmonary disease Current Opinion in Pulmonary Medicine 8 2002. 81–86. 10.1097/00063198-200203000-00001 [DOI] [PubMed] [Google Scholar]
- 27. van der Lee C Scholzel B ten Berg JM Geleijnse ML Idzerda HH van Domburg RT Vletter WB Serruys PW ten Cate FJ Usefulness of clinical, echocardiographic, and procedural characteristics to predict outcome after percutaneous transluminal septal myocardial ablation American Journal of Cardiology 101 2008. 1315–1320. 10.1016/j.amjcard.2008.01.003 [DOI] [PubMed] [Google Scholar]
- 28. Veselka J Duchonova R Palenickova J Zemanek D Tiserova M Linhartova K Cervinka P Impact of ethanol dosing on the long-term outcome of alcohol septal ablation for obstructive hypertrophic cardiomyopathy: a single-center prospective, and randomized study Circulation Journal 70 2006. 1550–1552. 10.1253/circj.70.1550 [DOI] [PubMed] [Google Scholar]
- 29. Veselka J Tomasov P Zemanek D Long-term effects of varying alcohol dosing in percutaneous septal ablation for obstructive hypertrophic cardiomyopathy: a randomized study with a follow-up up to 11 years Canadian Journal of Cardiology 27 2011. 763–767. 10.1016/j.cjca.2011.09.001 [DOI] [PubMed] [Google Scholar]
- 30. Alam M Dokainish H Lakkis N Alcohol septal ablation for hypertrophic obstructive cardiomyopathy: a systematic review of published studies Journal of Interventional Cardiology 19 2006. 319–327. 10.1111/j.1540-8183.2006.00153.x [DOI] [PubMed] [Google Scholar]
- 31. Veselka J Krejčí J Tomašov P Durdil V Riedlbauchová L Honěk J Honěk T Zemánek D Outcome of patients after alcohol septal ablation with permanent pacemaker implanted for periprocedural complete heart block International Journal of Cardiology 171 2014. e37–e38. 10.1016/j.ijcard.2013.11.119 [DOI] [PubMed] [Google Scholar]
- 32. Veselka J Lawrenz T Stellbrink C Zemanek D Branny M Januska J Sitar J Dimitrow P Krejci J Dabrowski M et al. Early outcomes of alcohol septal ablation for hypertrophic obstructive cardiomyopathy: a European multicenter and multinational study Catheterization and Cardiovascular Interventions 84 2014. 101–107. 10.1002/ccd.25236 [DOI] [PubMed] [Google Scholar]
- 33. Chang SM Nagueh SF Spencer WH III Lakkis NM Complete heart block: determinants and clinical impact in patients with hypertrophic obstructive cardiomyopathy undergoing nonsurgical septal reduction therapy Journal of the American College of Cardiology 42 2003. 296–300. 10.1016/S0735-1097(03)00623-5 [DOI] [PubMed] [Google Scholar]
- 34. Veselka J Krejčí J Tomašov P Jahnlová D Honěk T Januška J Branny M Zemánek D Survival of patients ≤50 years of age after alcohol septal ablation for hypertrophic obstructive cardiomyopathy Canadian Journal of Cardiology 30 2014. 634–638. 10.1016/j.cjca.2014.03.041 [DOI] [PubMed] [Google Scholar]
- 35. Veselka J Krejci J Tomasov P Zemanek D Long-term survival after alcohol septal ablation for hypertrophic obstructive cardiomyopathy: a comparison with general population European Heart Journal 126 2014. 2374–2380. 10.1093/eurheartj/eht495 [DOI] [PubMed] [Google Scholar]
- 36. Klopotowski M Chojnowska L Malek LA Maczynska R Kukula K Demkow M Witkowski A Dabrowski M Karcz M Baranowski R et al. The risk of non-sustained ventricular tachycardia after percutaneous alcohol septal ablation in patients with hypertrophic obstructive cardiomyopathy Clinical Research in Cardiology 99 2010. 285–292. 10.1007/s00392-010-0116-z [DOI] [PubMed] [Google Scholar]
- 37. Lawrenz T Obergassel L Lieder F Leuner C Strunk-Mueller C Meyer Zu Vilsendorf D Beer G Kuhn H Transcoronary ablation of septal hypertrophy does not alter ICD intervention rates in high risk patients with hypertrophic obstructive cardiomyopathy Pacing and Clinical Electrophysiology 28 2005. 295–300. 10.1111/j.1540-8159.2005.09327.x [DOI] [PubMed] [Google Scholar]
- 38. ten Cate FJ Soliman OI Michels M Theuns DA de Jong PL Geleijnse ML Serruys PW Long-term outcome of alcohol septal ablation in patients with obstructive hypertrophic cardiomyopathy: a word of caution Circulation. Heart Failure 3 2010. 362–369. 10.1161/CIRCHEARTFAILURE.109.862359 [DOI] [PubMed] [Google Scholar]
- 39. Noseworthy PA Rosenberg MA Fifer MA Palacios IF Lowry PA Ruskin JN Sanborn DM Picard MH Vlahakes GJ Mela T et al. Ventricular arrhythmia following alcohol septal ablation for obstructive hypertrophic cardiomyopathy American Journal of Cardiology 104 2009. 128–132. 10.1016/j.amjcard.2009.02.056 [DOI] [PubMed] [Google Scholar]
- 40. Gross CM Schulz-Menger J Kramer J Siegel I Pilz B Waigand J Friedrich MG Uhlich F Dietz R Percutaneous transluminal septal artery ablation using polyvinyl alcohol foam particles for septal hypertrophy in patients with hypertrophic obstructive cardiomyopathy: acute and 3-year outcomes Journal of Endovascular Therapy 11 2004. 705–711. 10.1583/03-1171MR.1 [DOI] [PubMed] [Google Scholar]
- 41. Fernandes VL Nielsen C Nagueh SF Herrin AE Slifka C Franklin J Spencer WH III Follow-up of alcohol septal ablation for symptomatic hypertrophic obstructive cardiomyopathy the Baylor and Medical University of South Carolina experience 1996 to 2007 JACC. Cardiovascular Interventions 1 2008. 561–570. 10.1016/j.jcin.2008.07.005 [DOI] [PubMed] [Google Scholar]
- 42. Kwon DH Kapadia SR Tuzcu EM Halley CM Gorodeski EZ Curtin RJ Thamilarasan M Smedira NG Lytle BW Lever HM et al. Long-term outcomes in high-risk symptomatic patients with hypertrophic cardiomyopathy undergoing alcohol septal ablation JACC. Cardiovascular Interventions 1 2008. 432–438. 10.1016/j.jcin.2008.05.009 [DOI] [PubMed] [Google Scholar]
- 43. Seggewiss H Rigopoulos A Welge D Ziemssen P Faber L Long-term follow-up after percutaneous septal ablation in hypertrophic obstructive cardiomyopathy Clinical Research in Cardiology 96 2007. 856–863. 10.1007/s00392-007-0579-8 [DOI] [PubMed] [Google Scholar]
- 44. Lyne JC Kilpatrick T Duncan A Knight CJ Sigwart U Fox KM Long-term follow-up of the first patients to undergo transcatheter alcohol septal ablation Cardiology 116 2010. 168–173. 10.1159/000318307 [DOI] [PubMed] [Google Scholar]
- 45. Sorajja P Valeti U Nishimura RA Ommen SR Rihal CS Gersh BJ Hodge DO Schaff HV Holmes DR Jr Outcome of alcohol septal ablation for obstructive hypertrophic cardiomyopathy Circulation 118 2008. 131–139. 10.1161/CIRCULATIONAHA.107.738740 [DOI] [PubMed] [Google Scholar]
- 46. Kuhn H Lawrenz T Lieder F Leuner C Strunk-Mueller C Obergassel L Bartelsmeier M Stellbrink C Survival after transcoronary ablation of septal hypertrophy in hypertrophic obstructive cardiomyopathy (TASH): a 10 year experience Clinical Research in Cardiology 97 2008. 234–243. 10.1007/s00392-007-0616-7 [DOI] [PubMed] [Google Scholar]
- 47. Dearani JA Ommen SR Gersh BJ Schaff HV Danielson GK Surgery insight: septal myectomy for obstructive hypertrophic cardiomyopathy – the Mayo Clinic experience Nature Clinical Practice. Cardiovascular Medicine 4 2007. 503–512. 10.1038/ncpcardio0965 [DOI] [PubMed] [Google Scholar]
- 48. Alam M Dokainish H Lakkis NM Hypertrophic obstructive cardiomyopathy-alcohol septal ablation vs. myectomy: a meta-analysis European Heart Journal 30 2009. 1080–1087. 10.1093/eurheartj/ehp016 [DOI] [PubMed] [Google Scholar]
- 49. Leonardi RA Kransdorf EP Simel DL Wang A Meta-analyses of septal reduction therapies for obstructive hypertrophic cardiomyopathy: comparative rates of overall mortality and sudden cardiac death after treatment Circulation. Cardiovascular interventions 3 2010. 97–104. 10.1161/CIRCINTERVENTIONS.109.916676 [DOI] [PubMed] [Google Scholar]
- 50. Agarwal S Tuzcu EM Desai MY Smedira N Lever HM Lytle BW Kapadia SR Updated meta-analysis of septal alcohol ablation versus myectomy for hypertrophic cardiomyopathy Journal of the American College of Cardiology 55 2010. 823–834. 10.1016/j.jacc.2009.09.047 [DOI] [PubMed] [Google Scholar]
- 51. Olivotto I Ommen SR Maron MS Cecchi F Maron BJ Surgical myectomy versus alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Will there ever be a randomized trial? Journal of the American College of Cardiology 50 2007. 831–834. 10.1016/j.jacc.2007.05.018 [DOI] [PubMed] [Google Scholar]
- 52. Matos GF Hammadeh R Francois C McCarthy R Leya F Controlled myocardial infarction induced by intracoronary injection of n-butyl cyanoacrylatein dogs: a feasibility study Catheterization and Cardiovascular Interventions 66 2005. 244–253. 10.1002/ccd.20486 [DOI] [PubMed] [Google Scholar]
- 53. Oto A Aytemir K Okutucu S Kaya EB Deniz A Cil B Peynircioglu B Kabakci G Cyanoacrylate for septal ablation in hypertrophic cardiomyopathy Journal of Interventional Cardiology 24 2011. 77–84. 10.1111/j.1540-8183.2010.00605.x [DOI] [PubMed] [Google Scholar]
- 54. Lanzino G Kanaan Y Perrini P Dayoub H Fraser K Emerging concepts in the treatment of intracranial aneurysms: stents, coated coils, and liquid embolic agents Neurosurgery 57 2005. 449–459. 10.1227/01.NEU.0000170538.74899.7F [DOI] [PubMed] [Google Scholar]
- 55. Durand E Mousseaux E Coste P Pilliere R Dubourg O Trinquart L Chatellier G Hagège A Desnos M Lafont A Non-surgical septal myocardial reduction by coil embolization for hypertrophic obstructive cardiomyopathy: early and 6 months follow-up European Heart Journal 29 2008. 348–355. 10.1093/eurheartj/ehm632 [DOI] [PubMed] [Google Scholar]
- 56. Faber L Welge D Fassbender D Schmidt HK Horstkotte D Seggewiss H One-year follow-up of percutaneous septal ablation for symptomatic hypertrophic obstructive cardiomyopathy in 312 patients: predictors of hemodynamic and clinical response Clinical Research in Cardiology 96 2007. 864–873. 10.1007/s00392-007-0578-9 [DOI] [PubMed] [Google Scholar]
- 57. Chan W Williams L Kotowycz MA Woo A Rakowski H Schwartz L Overgaard CB Angiographic and echocardiographic correlates of suitable septal perforators for alcohol septal ablation in hypertrophic obstructive cardiomyopathy Canadian Journal of Cardiology 30 2014. 912–919. 10.1016/j.cjca.2014.04.008 [DOI] [PubMed] [Google Scholar]
- 58. Riedlbauchova L Janousek J Veselka J Ablation of hypertrophic septum using radiofrequency energy: an alternative for gradient reduction in patient with hypertrophic obstructive cardiomyopathy? Journal of Invasive Cardiology 25 2013. E128–E132. [PubMed] [Google Scholar]
- 59. Lawrenz T Borchert B Leuner C Bartelsmeier M Reinhardt J Strunk-Mueller C Meyer Zu Vilsendorf D Schloesser M Beer G Lieder F et al. Endocardial radiofrequency ablation for hypertrophic obstructive cardiomyopathy: acute results and 6 months' follow-up in 19 patients Journal of the American College of Cardiology 57 2011. 572–576. 10.1016/j.jacc.2010.07.055 [DOI] [PubMed] [Google Scholar]
- 60. Sreeram N Emmel M de Giovanni JV Percutaneous radiofrequency septal reduction for hypertrophic obstructive cardiomyopathy in children Journal of the American College of Cardiology 58 2011. 2501–2510. 10.1016/j.jacc.2011.09.020 [DOI] [PubMed] [Google Scholar]
- 61. Gietzen FH Leuner CJ Raute-Kreinsen U Dellmann A Hegselmann J Strunk-Mueller C Kuhn HJ Acute and long-term results after transcoronary ablation of septal hypertrophy (TASH). Catheter interventional treatment for hypertrophic obstructive cardiomyopathy European Heart Journal 20 1999. 1342–1354. 10.1053/euhj.1999.1520 [DOI] [PubMed] [Google Scholar]
- 62. Kimmelstiel CD Maron BJ Role of percutaneous septal ablation in hypertrophic obstructive cardiomyopathy Circulation 109 2004. 452–456. 10.1161/01.CIR.0000114144.40315.C0 [DOI] [PubMed] [Google Scholar]
- 63. Yacoub MH Surgical versus alcohol septal ablation for hypertrophic obstructive cardiomyopathy: the pendulum swings Circulation 112 2005. 450–452. 10.1161/CIRCULATIONAHA.105.553313 [DOI] [PubMed] [Google Scholar]
- 64. Hess OM Sigwart U New treatment strategies for hypertrophic obstructive cardiomyopathy: alcohol ablation of the septum: the new gold standard? Journal of the American College of Cardiology 44 2004. 2054–2055. 10.1016/j.jacc.2004.08.047 [DOI] [PubMed] [Google Scholar]
- 65. Parakh N Bhargava B Golden jubilee of hypertrophic cardiomyopathy: is alcohol septal ablation the gold standard? Cardiovascular Revascularization Medicine 10 2009. 172–178. 10.1016/j.carrev.2009.02.006 [DOI] [PubMed] [Google Scholar]
- 66. Jensen MK Prinz C Horstkotte D van Buuren F Bitter T Faber L Bundgaard H Alcohol septal ablation in patients with hypertrophic obstructive cardiomyopathy: low incidence of sudden cardiac death and reduced risk profile Heart 99 2012. 1012–1017. 10.1136/heartjnl-2012-303339 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Parasternal long-axis view of a 42-year-old male with HOCM, the basal septum at LVOT level measures 27 mm. In systole, the MV apparatus (including the posterior leaflet) moves anteriorly towards the septum. The long anterior leaflet contacts the septum in the LVOT causing severe obstruction to flow out of the LV. Download Video 1 via http://dx.doi.org/10.1530/ERP-14-0058-v1.
Download Video 1 (206.1KB, mov)
An area of the basal septum at the LVOT level has an infarct. The myocardium has shrunk and measures just 13 mm. The LVOT has widened. The SAM of the MV apparatus is now mild with minimal movement of the anterior leaflet towards the LVOT. Download Video 2 via http://dx.doi.org/10.1530/ERP-14-0058-v2.
Download Video 2 (223.1KB, mov)
Parasternal long axis view 6 months post alcohol ablation. The mass of myocardium at the proximal basal septum is reduced. There is less systolic anterior motion of the anterior mitral valve leaflet which no longer contact the septum. Download Video 3 via http://dx.doi.org/10.1530/ERP-14-0058-v3.
Download Video 3 (595KB, avi)
Apical long axis view 6 months post alcohol ablation. Note reduced septal width and SAM. Download Video 4 via http://dx.doi.org/10.1530/ERP-14-0058-v4.
Download Video 4 (609.4KB, avi)



 This work is licensed under a
This work is licensed under a