Abstract
Only limited data are available from which normal ranges of mitral annular (MA) and tricuspid annular (TA) dimensions have been established. Normative data are important to assist the echocardiographer in defining the mechanism of atrioventricular valve regurgitation and to inform surgical planning. This study was conceived to establish normal MA and TA dimensions. Consecutive healthy subjects over the age of 60 were randomly recruited from the community as part of a screening project within South Birmingham. MA and TA dimensions in end-systole and end-diastole were evaluated in the parasternal and apical acoustic windows views using transthoracic echocardiography. Gender (males (M) and females (F))-specific dimensions were then assessed. A total of 554 subjects were screened and 74 with pathology considered to have an effect on annular dimensions were excluded from analysis. The mean age of the remaining 480 subjects was 70±7 years and the majority were female (56%). Dimensions were larger in men than in women and greater at end-diastole than end-systole (both P<0.05). Mean MA diameters at end-systole in the parasternal long axis view (cm) were 3.44 cm (M) and 3.11 cm (F) and at end-diastole 3.15 cm (M) and 2.83 cm (F) respectively. Mean TA diameters (cm) at end-systole in the apical 4 chamber view were 2.84 (M) and 2.80 (F) and at end-diastole 3.15 (M) and 3.01 (F) respectively. The reference ranges derived from this study differ from current published standards and should help to improve distinction of normal from pathological findings, in identifying aetiology and defining the mechanism of regurgitation.
Keywords: transthoracic echocardiography, mitral annulus, mitral regurgitation, tricuspid annulus, tricuspid regurgitation
Introduction
Accurate knowledge of mitral annular (MA) and tricuspid annular (TA) dimensions is important in helping the echocardiographer to define the aetiology and mechanism of atrio-ventricular valve regurgitation. Dilatation of the mitral valve (MV) or tricuspid valve (TV) annuli often contributes to primary (organic) and secondary (functional) regurgitation and knowledge of the extent of dilatation may assist the surgeon in identifying those valves amenable to repair (1). In particular, knowledge of annular dimensions is necessary before surgical planning for annuloplasty (1, 2). Only limited data from small studies are available providing normal ranges of MA and TA dimensions using two-dimensional transthoracic and transoesophageal echocardiography (2DE), yet these continue to be the mainstay of valve assessment despite the growth of 3D echocardiography (3DE) (3, 4). The most widely used dimensions for the MA are obtained from peri-operative studies that identify cut-offs based on outcomes during general anaesthesia but do not comment on normal ranges (4). Direct open measures of annular dimensions are unlikely to be reliable as they represent a non-physiological state.
Tricuspid regurgitation (TR) is a common condition associated with an adverse prognosis, particularly if left untreated following cardiothoracic surgery. A major cause of TR is malcoaptation of the leaflets due to TA dilatation (5). While standard surgical practice has shifted in favour of TV annuloplasty and repair, there are no large-scale data identifying normal ranges for dimensions in systole and diastole on 2DE (5, 6).
In view of the paucity of data from healthy subjects, this study was conceived to establish normal MA and TA dimensions in standard anatomic planes, both in systole and diastole, using transthoracic 2DE.
Methods
A total of 554 consecutive subjects over the age of 60 were randomly recruited from the lists of General Practitioners as part of a screening project within South Birmingham (UK). Assessment of participants was performed by a research physician using a standard protocol including questions on medical history, family history, cardiovascular risk factors, alcohol intake, physical activity and drug history (verified from the computerised records of the practice). The subjects then underwent a physical assessment including measurement of heart rate, and blood pressure and determination of BMI. Participants who were healthy and free of overt cardiovascular disease (including a history of ischaemic heart disease and atrial fibrillation) were invited to undergo echocardiography. Patients aged 60–85 were included in the screening study, the details of which have been published previously (7). Exclusion criteria for this echocardiographic sub-study included mild or more significant valvular heart disease, left ventricular (LV) dysfunction (LV ejection fraction below 50%), and LV dilatation (LV internal dimension in diastole above 5.9 cm (M) and 5.3 cm (F)) and left atrial (LA) dilatation (maximal LA volume above 29 ml). This study was approved by the Local Research Ethics Committee and written informed consent was obtained from all participants.
Echocardiography
Transthoracic 2DE was performed by an experienced sonographer using a digital commercial harmonic imaging ultrasound system with an S3 3 MHz phased-array transducer (Vivid I, GE Healthcare, Amersham, UK). Resting images of the parasternal long axis (PLAX), parasternal short axis (PSAX) (at mitral leaflet level), apical 4 chamber (A4C) and modified apical 2 chamber (A2C) views were acquired. Image acquisition and measurement of LV, right ventricular (RV), LA and right atrial (RA) internal dimensions and volumes were performed according to the guideline recommendations of the European Society of Echocardiography and the American Society of Echocardiography (ASE) (2, 8). In brief, LV internal dimensions were obtained in the PLAX view in the minor axis at the tips of the mitral leaflets using 2DE. End-diastole was defined as the time in the cardiac cycle when the cardiac dimensions were largest and end-systole as the time in the cardiac cycle when the cardiac dimension was smallest. LV volumes and function were calculated using the biplane modified Simpson's rule in A4C and A2C. When two orthogonal planes could not be identified, a single plane was used. LA volumes were calculated from the biplane area–length model using the maximal planimetered area in the A4C and A2C views. RV internal dimensions were measured in the A4C view optimised to the RV in the mid-cavity and long axis. To estimate inter- and intra-observer variabilities, all variables were measured by the sonographer and research physician (D Jimenez and G Dwivedi) in a subset (n=10) of echocardiograms randomly selected from the total. The inter-and intra-observer variability was calculated as (mean percentage errors) 12 and 9% respectively.
MA measurement
MA dimensions in end-diastole (Fig. 1) and end-systole were evaluated in PLAX (conventional anteroposterior annulus), PSAX (anterolateral commissure–posteromedial commissure) (Fig. 2), A4C (oblique) (Fig. 3) and modified A2C (anatomical commissure–commissure annulus) (Fig. 4) views. The latter image was obtained from the apical view by rotating from the A4C view to obtain an even distribution of P1, A2 and P3 scallops (8). The MV tenting area and tenting distance (Fig. 1) were assessed in the PLAX view. In addition, all the measurements were indexed to body surface area (Mosteller equation). MR was assessed following the European Association of Echocardiography (EAE) guidelines (1).
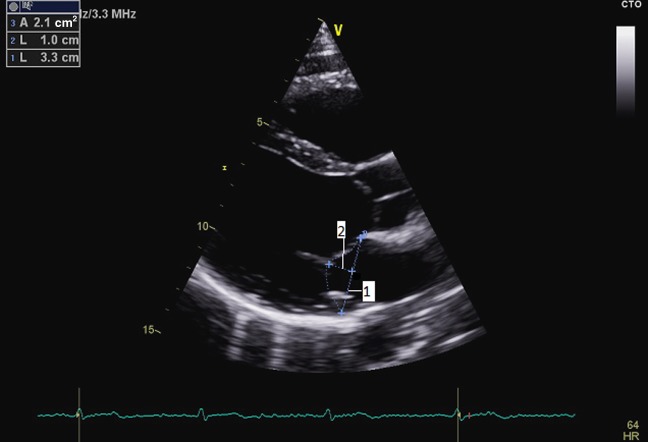
Figure 1.
Parasternal long axis view demonstrating measurements of the mitral annulus in diastole (1), tenting distance (2) and tenting area (dotted line) at end-diastole.
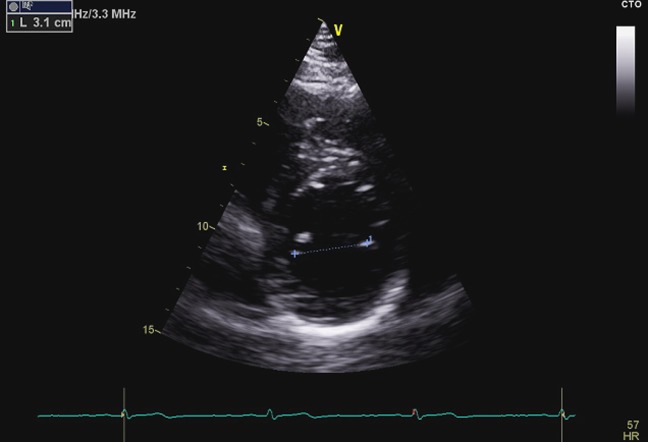
Figure 2.
Parasternal short axis view demonstrating measurement of the anterolateral to posteromedial commissures at end-diastole.
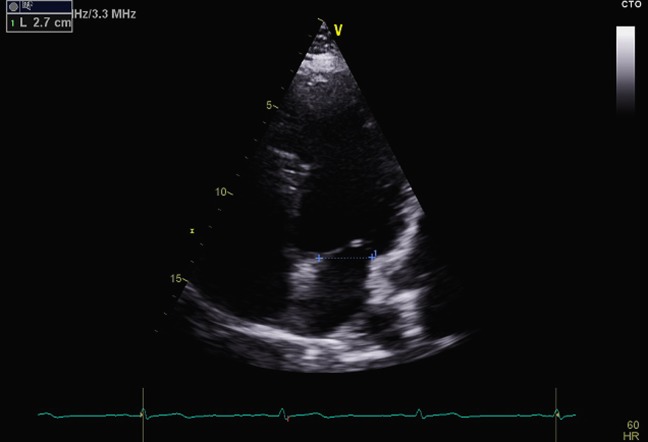
Figure 3.
Apical four-chamber view demonstrating measurement of the mitral annulus at end-diastole.
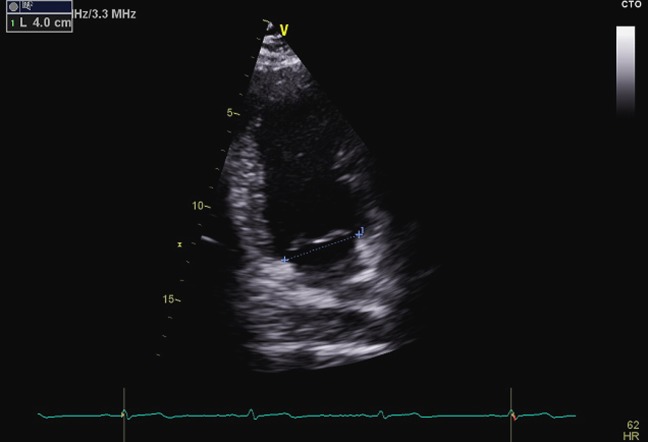
Figure 4.
The modified apical two-chamber view used to measure the anatomical commissure–commissure annulus obtained from the apical view by rotating from the four chamber to obtain an even distribution of P1, A2 and P3 scallops.
TA and right heart measurement
TA dimensions were measured in end-diastole and end-systole in A4C view (Fig. 5). In addition, tricuspid tenting area and distances were measured. RA end-systolic area, RA major and minor dimensions, and RV mid-cavity and longitudinal dimensions were also evaluated as per the recommendations of the ASE (2). Similar to the MV, measurements were also indexed to the body surface area (Mosteller equation). TR was assessed following the EAE guidelines (1).
Figure 5.
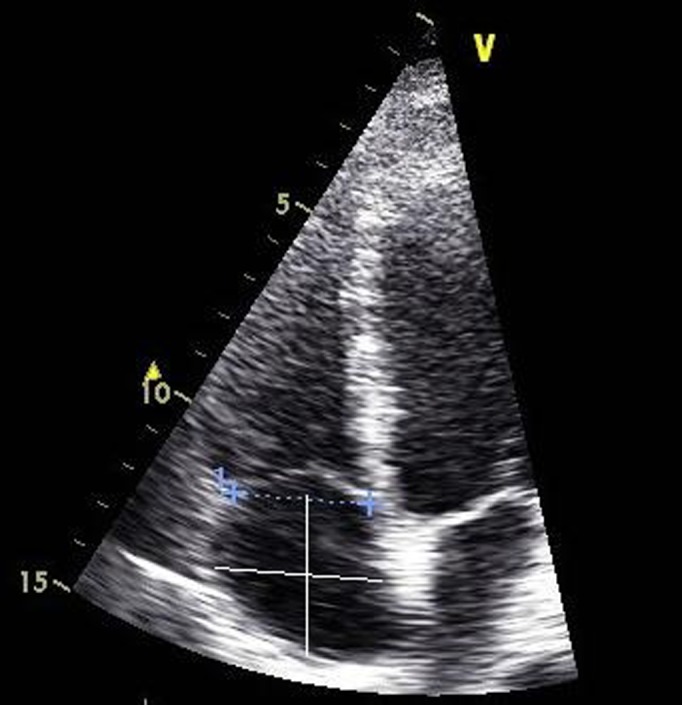
Tricuspid annular dimensions measured at end-diastole in the modified apical four-chamber view to maximise right heart size. Right atrial (RA) major (vertical white line) dimension is from the centre of the tricuspid annulus to the centre of the superior RA wall, parallel to the atrial septum. The RA minor (horizontal white line) dimension is from the mid-level of the RA free wall to the septum, perpendicular to the long axis.
Statistical analysis
Data were expressed as mean±s.d. and also as 5–95% percentile. In addition, minimum and maximum data for each measurement were calculated. Minitab 15 (Minitab Ltd, Coventry, UK) and MedCalc Software (version 12, Mariakerke, Belgium) were used for analysis of the data.
Results
Of the 554 patients screened, 38 with ischaemic heart disease, 21 with atrial fibrillation (previous history or documented at the time of examination) and 15 with moderate or severe valvular heart disease were excluded from the final analyses. In total, 480 subjects were included. The mean age of the subjects was 70 (±7.2) years, the majority were female (56%), with a mean weight of 69 (±12.9) kg and height of 160 (±50) cm. Demographic data are given in Table 1.
Table 1.
Demographic and basic echocardiographic data
| n | 480 |
|---|---|
| Age (years) | 70±7 |
| Male | 211 (44%) |
| Smoker | 144 (30%) |
| Hypertension (≥140/90 mmHg or on treatment) | 105 (22%) |
| Diabetes mellitus | 76 (16%) |
| BMI (kg/m2) | 27±5 |
| BSA (m2) | 1.85 |
| Hypercholesterolaemia (total cholesterol >5.0 mmol/l) | 74 (16%) |
| LA volume (area–length method) (ml) | 17±7 |
| LVIDd (cm) | 4.9±0.6 |
| LVIDs (cm) | 3.2±0.6 |
| LVEDV (ml) | 130±51 |
| LVESV (ml) | 36±29 |
| LVEF (%) | 64±7 |
BSA, body surface area; LA volume, left atrial volume; LVIDd, left ventricular internal dimension in diastole; LVIDs, left ventricular internal dimension in systole; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; LVEF, left ventricular ejection fraction.
MA measurements
Tables 2 and 3 list the values of MA in mean (with s.d.), 5–95% percentile and minimum and maximum format for males and females respectively. Tables 4 and 5 list the respective indexed values.
Table 2.
Mitral annulus measurements in men
| Parameter | Mean | s.d. | 5–95 percentile |
|---|---|---|---|
| MA diameter at end-systole PLAX (cm) | 3.15 | 0.49 | 2.5–4.1 |
| MA diameter at end-diastole PLAX (cm) | 3.44 | 0.52 | 2.7–4.5 |
| MA diameter at end-systole PSAX (cm) | 3.10 | 0.41 | 2.4–3.7 |
| MA diameter at end-diastole PSAX (cm) | 3.72 | 0.41 | 3.0–4.3 |
| MA diameter at end-systole A4C (cm) | 3.05 | 0.37 | 2.5–3.8 |
| MA diameter at end-diastole A4C (cm) | 3.32 | 0.40 | 2.7–4.1 |
| MA diameter at end-systole A2C (cm) | 2.94 | 0.31 | 2.5–3.5 |
| MA diameter at end-diastole A2C (cm) | 3.30 | 0.38 | 2.7–4.0 |
| MV tenting distance (cm) | 0.90 | 0.22 | 0.6–1.3 |
| MV tenting area (cm2) | 2.02 | 0.68 | 1.0–3.4 |
A4C, apical 4 chamber; A2C, apical 2 chamber; MV, mitral valve; PLAX, parasternal long axis; PSAX, parasternal short axis.
Table 3.
Mitral annulus measurements in women
| Parameter | Mean | s.d. | 5–95 percentile |
|---|---|---|---|
| MA diameter at end-systole PLAX (cm) | 2.83 | 0.35 | 2.3–3.4 |
| MA diameter at end-diastole PLAX (cm) | 3.11 | 0.40 | 2.5–3.8 |
| MA diameter at end-systole PSAX (cm) | 2.80 | 0.39 | 2.2–3.5 |
| MA diameter at end-diastole PSAX (cm) | 3.34 | 0.43 | 2.8–4.2 |
| MA diameter at end-systole A4C (cm) | 2.77 | 0.31 | 2.3–3.3 |
| MA diameter at end-diastole A4C (cm) | 3.06 | 0.33 | 2.4–3.6 |
| MA diameter at end-systole A2C (cm) | 2.94 | 0.37 | 2.2–3.4 |
| MA diameter at end-diastole A2C (cm) | 3.09 | 0.36 | 2.6–3.8 |
| MV tenting distance (cm) | 0.82 | 0.18 | 0.5–1.1 |
| MV tenting area (cm2) | 1.64 | 0.47 | 1.0–2.4 |
A4C, apical 4 chamber; A2C, apical 2 chamber; MV, mitral valve; PLAX, parasternal long axis; PSAX, parasternal short axis.
Table 4.
Indexed mitral annulus measurements in men
| Parameter (per m2) | Mean index | s.d. | 5–95 percentile index |
|---|---|---|---|
| MA diameter at end-systole PLAX (cm) | 1.59 | 0.25 | 1.3–2.1 |
| MA diameter at end-diastole PLAX (cm) | 1.74 | 0.26 | 1.4–2.3 |
| MA diameter at end-systole PSAX (cm) | 1.57 | 0.21 | 1.2–1.9 |
| MA diameter at end-diastole PSAX (cm) | 1.88 | 0.21 | 1.5–2.2 |
| MA diameter at end-systole A4C (cm) | 1.54 | 0.19 | 1.3–1.9 |
| MA diameter at end-diastole A4C (cm) | 1.68 | 0.20 | 1.4–2.1 |
| MA diameter at end-systole A2C (cm) | 1.48 | 0.16 | 1.3–1.8 |
| MA diameter at end-diastole A2C (cm) | 1.67 | 0.19 | 1.4–2.0 |
| MV tenting distance (cm) | 0.45 | 0.11 | 0.3–0.7 |
| MV tenting area (cm2) | 1.02 | 0.34 | 0.5–1.7 |
A4C, apical 4 chamber; A2C, apical 2 chamber; MV, mitral valve; PLAX, parasternal long axis; PSAX, parasternal short axis.
Table 5.
Indexed mitral annulus measurements in women
| Parameter (per m2) | Mean index | s.d. | 5–95 percentile index |
|---|---|---|---|
| MA diameter at end-systole PLAX (cm) | 1.60 | 0.20 | 1.3–1.9 |
| MA diameter at end-diastole PLAX (cm) | 1.76 | 0.23 | 1.4–2.1 |
| MA diameter at end-systole PSAX (cm) | 1.58 | 0.22 | 1.2–2.0 |
| MA diameter at end-diastole PSAX (cm) | 1.89 | 0.24 | 1.6–2.4 |
| MA diameter at end-systole A4C (cm) | 1.56 | 0.18 | 1.3–1.9 |
| MA diameter at end-diastole A4C (cm) | 1.73 | 0.19 | 1.4–2.0 |
| MA diameter at end-systole A2C (cm) | 1.66 | 0.21 | 1.2–2.0 |
| MA diameter at end-diastole A2C (cm) | 1.75 | 0.20 | 1.5–2.1 |
| MV tenting distance (cm) | 0.46 | 0.10 | 0.3–0.6 |
| MV tenting area (cm2) | 0.93 | 0.27 | 0.6–1.4 |
A4C, apical 4 chamber; A2C, apical 2 chamber; MV, mitral valve; PLAX, parasternal long axis; PSAX, parasternal short axis.
TA and right heart measurements
Tables 6 and 7 list the values relating to the TA in mean (with s.d.), 5–95% percentiles and minimum and maximum format. Tables 8 and 9 list the respective indexed values. There was no association between standard cardiovascular risk factors and MA or TA dimensions.
Table 6.
Tricuspid and right heart measurements in men
| Parameter | Mean | s.d. | 5–95 percentile |
|---|---|---|---|
| TA diameter at end-systole (cm) | 2.84 | 0.39 | 2.3–3.4 |
| TA diameter at end-diastole (cm) | 3.15 | 0.43 | 2.5–3.9 |
| TV-tenting distance (cm) | 0.71 | 0.17 | 0.5–1.1 |
| TV-tenting area (cm2) | 1.20 | 0.36 | 0.8–1.9 |
| RV mid-cavity diameter (cm) | 3.10 | 0.46 | 2.4–3.8 |
| RV longitudinal diameter (cm) | 6.70 | 0.74 | 5.6–8.2 |
| RA end-systolic area (cm2) | 16.0 | 3.50 | 10.8–23.0 |
| RA major dimension (cm) | 5.10 | 0.59 | 4.1–6.2 |
| RA minor dimension (cm) | 3.42 | 0.61 | 2.5–4.4 |
TV, tricuspid valve; RA, right atrium; RV, right ventricle.
Table 7.
Tricuspid and right heart measurements in women
| Parameter | Mean | s.d. | 5–95 percentile |
|---|---|---|---|
| TA diameter at end-systole (cm) | 2.80 | 0.43 | 2.0–3.4 |
| TA diameter at end-diastole (cm) | 3.01 | 0.47 | 2.3–3.9 |
| TV tenting distance (cm) | 0.65 | 0.15 | 0.4–0.9 |
| TV tenting area (cm2) | 1.31 | 0.35 | 0.7–1.8 |
| RV mid-cavity diameter (cm) | 2.90 | 0.43 | 2.2–3.6 |
| RV longitudinal diameter (cm) | 6.15 | 0.77 | 4.8–7.5 |
| RA end-systolic area (cm2) | 13.90 | 3.00 | 8.8–19.8 |
| RA major dimension (cm) | 4.60 | 0.68 | 3.6–5.9 |
| RA minor dimension (cm) | 3.20 | 0.53 | 2.3–4.2 |
TV, tricuspid valve; RA, right atrium; RV, right ventricle.
Table 8.
Indexed tricuspid and right heart measurements in men
| Parameter (per m2) | Mean index | s.d. | 5–95 percentile index |
|---|---|---|---|
| TA diameter at end-systole (cm) | 1.43 | 0.20 | 1.2–1.7 |
| TA diameter at end-diastole (cm) | 1.59 | 0.22 | 1.3–2.0 |
| TV tenting distance (cm) | 0.36 | 0.09 | 0.2–0.6 |
| TV tenting area (cm2) | 0.61 | 0.18 | 0.4–1.0 |
| RV mid-cavity diameter (cm) | 1.57 | 0.23 | 1.2–2.0 |
| RV longitudinal diameter (cm) | 3.38 | 0.37 | 2.8–4.1 |
| RA end-systolic area (cm2) | 8.08 | 1.77 | 5.4–11.6 |
| RA major dimension (cm) | 2.58 | 0.30 | 2.1–3.1 |
| RA minor dimension (cm) | 1.73 | 0.31 | 1.3–2.2 |
TV, tricuspid valve; RA, right atrium; RV, right ventricle.
Table 9.
Indexed tricuspid and right heart measurements in women
| Parameter (per m2) | Mean index | s.d. | 5–95 percentile index |
|---|---|---|---|
| TA diameter at end-systole (cm) | 1.58 | 0.24 | 1.1–1.9 |
| TA diameter at end-diastole (cm) | 1.70 | 0.27 | 1.3–2.2 |
| TV tenting distance (cm) | 0.37 | 0.08 | 0.2–0.5 |
| TV tenting area (cm2) | 0.74 | 0.20 | 0.4–1.0 |
| RV mid-cavity diameter (cm) | 1.64 | 0.24 | 1.2–2.0 |
| RV longitudinal diameter (cm) | 3.47 | 0.44 | 2.7–4.2 |
| RA end-systolic area (cm2) | 7.85 | 1.69 | 4.9–11.2 |
| RA major dimension (cm) | 2.60 | 0.38 | 2.0–3.3 |
| RA minor dimension (cm) | 1.81 | 0.30 | 1.3–2.4 |
TV, tricuspid valve; RA, right atrium; RV, right ventricle.
Discussion
This study provides the largest sized, population-based dataset available, to our knowledge, to date. Moreover, ranges have been produced that are gender-specific and indexed to body size, variables not accounted for in the previous studies. These values are based on a large and healthy cohort over the age of 60 from the UK, from the first such study, to our knowledge, to recruit over 550 subjects.
Despite the reduced incidence of rheumatic disease in the developed world, the number of MV operations is rising with an increasing average life expectancy leading to a higher prevalence of degenerative MV disease, in conjunction with an advancement in surgical techniques and operator skill in repair (9). A dilated or abnormally shaped MA is a common contributor to both primary (organic) and secondary (functional) MR (1). The measurements provided in this study identify a normal range in both standard echocardiographic planes and in an anatomical axis for the MA, but there are differences when compared with previously published research. The cut-off for annular dilatation used in the current European guidelines for MV assessment is 3.5 cm, based on transoesophageal measurement of the end-diastolic antero-posterior diameter of the MA from a small study of 49 anaesthetised patients scheduled for MV surgery (4). The values obtained in our transthoracic study are similar in the inter-commissural plane but larger in the conventional anterior–posterior plane (2.5–4.1 cm) when compared with results from another small study using 2D transoesophageal echocardiography at ES in 21 normal subjects (2.2–3.6 cm) (10). Although results from a previous, older study had indicated a reasonable correlation between 2D transthoracic measurements and autopsy sizing of the MV annulus (11), results from a more recent 3D study have indicated that a major issue is the wide inter-subject variability of the MV annulus measurements (12). This may contribute to the wider range of values obtained in our study – the mean MA diameters at end-diastole in the PLAX view were 3.44 cm for men and 3.11 cm for women, respectively, but the mean 5–95 percentiles were 2.7–4.5 cm for men and 2.5–3.8 cm for women, and the maximum diameters were 5.6 cm for men and 4.2 cm for women respectively. Two issues should be mentioned that might be relevant. First, while an attempt has been made to account for the normal contraction of the annulus (a decrease in the annular area in systole of approximately 25%) by identifying values for end-systole and end-diastole, a more holistic assessment of the MA is probably required, as the shape of the MV annulus is complicated. This means that no single measure is likely to be sufficient in clinical practice, although this study focused only on normal subjects. Variation in size during the cardiac cycle and limitation of assessment to pre-defined linear dimensions during 2D imaging may contribute to the finding that annular dimensions assessed by 3D may be larger than those defined with 2D echocardiography (13). Secondly, end diastole and end systole were defined in our study with reference to the largest and smallest cardiac chamber dimension identified, as recommended in the guidelines on chamber quantification; hence, there may be further variation between studies that have used the complex of Q, R and S waves or frame after MV closure and frame before MV opening (8). While 3DE may ultimately be preferable for examination of the MV, real-time images are often of lower quality than conventional 2D images, and cyclic variation will result in a greater variability when frame rates are lower; hence, there remains a clear need for the normative data from 2D imaging provided in this study, given the paucity of existing data.
Accurate determination of annular dimensions is of course not only important in identifying the underlying pathophysiological mechanisms but is also vital in predicting the success of a surgical repair. Our data were gathered from patients without MR and therefore do not affect previous evidence that severe annular dilatation (>5 cm in organic MR and =3.7 cm in ischaemic MR) is a predictor of unsuccessful repair (14, 15). This limitation also applies to the measurement of tenting height and tenting volume, which has most frequently been carried out in a surgical series to assess the likelihood of MV repair. Current reference values for 2D imaging are based on the assumption that the MV annulus has a flat, circular geometry, but results from 3D studies have clearly confirmed the saddle shape of the MV apparatus in healthy individuals (16). This non-planar shape means that a tenting distance can be calculated in normal individuals from the annular plane, although the values obtained here are larger than those obtained from previous 3D studies (12). This may reflect the older age of subjects in our study and is likely to partly relate to differences in offline measurement from the annular plane compared with assessment from a single 2D image.
Although the right heart plays an important role in the morbidity and mortality of patients with cardiopulmonary diseases, systematic assessment is not uniformly carried out. One of the main reasons for the underassessment of the right heart is the paucity of ultrasound studies providing normal reference values (2). Notably, the most recent ASE guideline was reliant on results from multiple small studies to provide normal right heart values but did not include proposed normal TA dimensions (2). Despite this, TA measurements play an important role in the surgical decision-making process not only for the selection of patients undergoing surgery for TR but also for the selection of the appropriate type of surgical technique (valve plication or ring placement) (17, 18). Current guidelines define the normal TA diameter in adults as 2.8±0.5 cm in the A4C view and significant TA dilation is defined by the diastolic diameter of >2.1 cm/m2 (>3.5 cm) (1). In our study, the mean diastolic TA diameter was 3.15 cm (±0.40 cm) for men (3.01 cm (±0.47 cm) for women) but the 5–95 percentile value was 2.5–3.9 cm for men (2.3–3.9 cm for women) and the maximum diameter was 4.8 cm for men (4.1 cm for women). These values are larger but are more consistent with values from 3D studies (19). Current European guidelines include the suggestion that tricuspid surgery may be indicated (class IIa) when there is mild or moderate regurgitation if the annulus is dilated above 40 mm, which is close to the upper limit of the 95th percentile from our data from normal patients (9). There is a lack of randomised data about the outcomes from TV repair in left-sided disease, and although there is a possibility that a corrective surgery may be helpful at this value, further research is warranted. Moreover, results from 3D studies have consistently demonstrated the TV to be saddle-shaped with a considerable variation between systole and diastole and our study is limited by the fact that the TV annulus was measured only from the apical acoustic window. This means that the assessment was limited only to the more inferior points of the saddle between the septal and lateral aspects of the valve, although the TV annulus can dilate in any plane.
Limitations
First, our study used 2DE. While the advantages of 3DE are increasingly acknowledged in the assessment of the MV and TV, the availability of such technology is not universal and most studies in clinical practice continue to be carried out using 2DE. It is acknowledged that 2DE measurements are known to underestimate the actual MA and TA size compared with 3D measures. However, calculation of TA fractional shortening yields the same result for the two echocardiographic techniques, as the extent of underestimation of TA diameter made by 2DE is similar in diastole and systole (20). Secondly, our recruited subjects were all over the age of 60 and it would have been useful to identify variation in dimensions across other age ranges. It should be noted that 22% of our cohort were on medication for hypertension but that detailed assessment of diastolic function is not available for this cohort, which might affect MA and TA dimensions. Our study did exclude patients with atrial dilatation above current recommended values and this would have meant that few subjects (if any) had chronic, moderate or severe diastolic dysfunction. Moreover, our subjects were all drawn from South Birmingham and it is possible that these results are population-specific; thus, further studies of other diverse populations may be useful.
Conclusion
We have defined reference ranges (gender-specific and indexed) for the TA and MA and other important right heart measurements based on normative percentile values derived from a large healthy population over the age of 60. The reference ranges presented in our study may help in distinguishing normal from pathological findings, in planning for surgical repair of TV and MV and for serial assessment.
Acknowledgements
The authors would like to thank Dr Andrew Blann at the University of Birmingham for providing statistical help.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This study was supported by the British Heart Foundation Project Grant PG/08/076/25549.
References
- 1. Lancellotti P Moura L Pierard LA Agricola E Popescu BA Tribouilloy C Hagendorff A Monin J-L Badano L Zamorano JL et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: mitral and tricuspid regurgitation (native valve disease) European Journal of Echocardiography 11 2010. 307–332. 10.1093/ejechocard/jeq031 [DOI] [PubMed] [Google Scholar]
- 2. Rudski LG Lai WW Afilalo J Hua L Handschumacher MD Chandrasekaran K Solomon SD Louie EK Schiller NB Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography: endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography Journal of the American Society of Echocardiography 23 2010. 685–713. 10.1016/j.echo.2010.05.010 [DOI] [PubMed] [Google Scholar]
- 3. Triulzi M Gillam LD Gentile F Newell JB Weyman AE Normal adult cross-sectional echocardiographic values: linear dimensions and chamber areas Echocardiography 1 1984. 403–426. 10.1111/j.1540-8175.1984.tb00173.x [DOI] [Google Scholar]
- 4. Caldarera I Van Herwerden LA Taams MA Bos E Roelandt JR Multiplane transoesophageal echocardiography and morphology of regurgitant mitral valves in surgical repair European Heart Journal 16 1995. 999–1006. [DOI] [PubMed] [Google Scholar]
- 5. Foster GP Dunn AK Abraham S Ahmadi N Sarraf G Accurate measurement of mitral annular dimensions by echocardiography: importance of correctly aligned imaging planes and anatomic landmarks Journal of the American Society of Echocardiography 22 2009. 458–463. 10.1016/j.echo.2009.02.008 [DOI] [PubMed] [Google Scholar]
- 6. Badano LP Agricola E Perez de Isla L Gianfagna P Zamorano JL Evaluation of the tricuspid valve morphology and function by transthoracic real-time three-dimensional echocardiography European Journal of Echocardiography 10 2009. 477–484. 10.1093/ejechocard/jep044 [DOI] [PubMed] [Google Scholar]
- 7. Mahadevan G Dwivedi G Williams L Steeds RP Frenneaux M Epidemiology and diagnosis of heart failure with preserved left ventricular ejection fraction: rationale and design of the study European Journal of Heart Failure 14 2012. 106–112. 10.1093/eurjhf/hfr153 [DOI] [PubMed] [Google Scholar]
- 8. Lang RM Bierig M Devereux RB Flachskampf FA Foster E Pellikka PA Picard MH Roman MJ Seward J Shanewise JS et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology Journal of the American Society of Echocardiography 18 2005. 1440–1463. 10.1016/j.echo.2005.10.005 [DOI] [PubMed] [Google Scholar]
- 9. Vahanian A Alfieri O Andreotti F Antunes MJ Baron-Esquivias G Baumgartner H Borger MA Carrel TP De Bonis M Evangelista A et al. Guidelines on the management of valvular heart disease (version 2012) European Heart Journal 33 2012. 2451–2496. 10.1093/eurheartj/ehs109 [DOI] [PubMed] [Google Scholar]
- 10. Fyrenius A Engvall J Janerot-Sjoberg B Major and minor axes of the normal mitral annulus Journal of Heart Valve Disease 10 2001. 146–152. [PubMed] [Google Scholar]
- 11. Gutgesell HP Bricker JT Colvin EV Latson LA Hawkins EP Atrioventricular valve anular diameter: two-dimensional echocardiographic–autopsy correlation American Journal of Cardiology 53 1984. 1652–1655. 10.1016/0002-9149(84)90596-4 [DOI] [PubMed] [Google Scholar]
- 12. Sonne C Sugeng L Watanabe N Weinert L Saito K Tsukiji M Yoshida K Takeuchi M Mor-Avi V Lang RM Age and body surface area dependency of mitral valve and papillary apparatus parameters: assessment by real-time three-dimensional echocardiography European Journal of Echocardiography 10 2009. 287–294. 10.1093/ejechocard/jen237 [DOI] [PubMed] [Google Scholar]
- 13. Anwar AM Soliman OI ten Cate FJ Nemes A McGhie JS Krenning BJ van Geuns RJ Galema TW Geleijnse ML True mitral annulus diameter is underestimated by two-dimensional echocardiography as evidenced by real-time three-dimensional echocardiography and magnetic resonance imaging International Journal of Cardiovascular Imaging 23 2007. 541–547. 10.1007/s10554-006-9181-9 [DOI] [PubMed] [Google Scholar]
- 14. Omran AS Woo A David TE Feindel CM Rakowski H Siu SC Intraoperative transesophageal echocardiography accurately predicts mitral valve anatomy and suitability for repair Journal of the American Society of Echocardiography 15 2002. 950–957. 10.1067/mje.2002.121534 [DOI] [PubMed] [Google Scholar]
- 15. Lancellotti P Marwick T Pierard LA How to manage ischaemic mitral regurgitation Heart 94 2008. 1497–1502. 10.1136/hrt.2007.134833 [DOI] [PubMed] [Google Scholar]
- 16. Watanabe N Ogasawara Y Yamaura Y Kawamoto T Toyota E Akasaka T Yoshida K Quantitation of mitral valve tenting in ischemic mitral regurgitation by transthoracic real-time three-dimensional echocardiography Journal of the American College of Cardiology 45 2005. 763–769. 10.1016/j.jacc.2004.11.048 [DOI] [PubMed] [Google Scholar]
- 17. De Simone R Lange R Tanzeem A Gams E Hagl S Adjustable tricuspid valve annuloplasty assisted by intraoperative transesophageal color Doppler echocardiography American Journal of Cardiology 71 1993. 926–931. 10.1016/0002-9149(93)90908-U [DOI] [PubMed] [Google Scholar]
- 18. Dreyfus GD Corbi PJ Chan KM Bahrami T Secondary tricuspid regurgitation or dilatation: which should be the criteria for surgical repair? Annals of Thoracic Surgery 79 2005. 127–132. 10.1016/j.athoracsur.2004.06.057 [DOI] [PubMed] [Google Scholar]
- 19. Ton-Nu T-T Levine RA Handschumacher MD Dorer DJ Yosefy C Fan D Hua L Jiang L Hung J Geometric determinants of functional tricuspid regurgitation Circulation 114 2006. 143–149. 10.1161/CIRCULATIONAHA.106.611889 [DOI] [PubMed] [Google Scholar]
- 20. Kwan J Kim GC Jeon MJ Kim DH Shiota T Thomas JD Park KS Lee WH 3D geometry of a normal tricuspid annulus during systole: a comparison study with the mitral annulus using real-time 3D echocardiography European Journal of Echocardiography 8 2007. 375–383. 10.1016/j.euje.2006.07.010 [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a