Abstract
Mutations in the presenilin (PSEN) encoding genes (PSEN1 and PSEN2) occur in most early onset familial Alzheimer’s Disease. Despite the identification of the involvement of PSEN in Alzheimer’s Disease (AD) ∼20 years ago, the underlying role of PSEN in AD is not fully understood. To gain insight into the biological function of PSEN, we investigated the role of the PSEN homolog SEL-12 in Caenorhabditis elegans. Using genetic, cell biological, and pharmacological approaches, we demonstrate that mutations in sel-12 result in defects in calcium homeostasis, leading to mitochondrial dysfunction. Moreover, consistent with mammalian PSEN, we provide evidence that SEL-12 has a critical role in mediating endoplasmic reticulum (ER) calcium release. Furthermore, we found that in SEL-12-deficient animals, calcium transfer from the ER to the mitochondria leads to fragmentation of the mitochondria and mitochondrial dysfunction. Additionally, we show that the impact that SEL-12 has on mitochondrial function is independent of its role in Notch signaling, γ-secretase proteolytic activity, and amyloid plaques. Our results reveal a critical role for PSEN in mediating mitochondrial function by regulating calcium transfer from the ER to the mitochondria.
Keywords: Caenorhabditis elegans, calcium, endoplasmic reticulum, mitochondria, presenilin
MITOCHONDRIA are highly dynamic organelles that are responsible for a myriad of functions including ATP production, generation and metabolism of reactive oxygen species (ROS), and apoptosis (Detmer and Chan 2007). Thus cells, in particular muscle cells and neurons due to their high-energy demands, are extremely reliant on mitochondrial function. Mitochondria also play a critical role in calcium homeostasis by acting as a calcium buffer. Calcium, in turn, regulates mitochondrial morphology, activity, and movement. The relevance of mitochondria is highlighted by the fact that many neurodegenerative diseases, such as Parkinson’s and Alzheimer’s Disease (AD) are characterized by abnormal mitochondrial activity and morphology (Cali et al. 2012).
Mutations in the genes encoding presenilins (PSENs), PSEN1 and PSEN2, are the most common cause of early onset familial Alzheimer’s Disease (FAD). PSENs are ∼50-kDa multipass transmembrane proteins that reside predominantly on the endoplasmic reticulum (ER) (Bezprozvanny and Mattson 2008) and have been shown to be enriched in an ER subcompartment that is in contact with mitochondria (Area-Gomez et al. 2009). Although altered PSEN function has been known to have a role in AD for 20 years (Sherrington et al. 1995), the functional consequences of mutations in PSENs are controversial and not understood. The prevailing model for the pathogenesis of AD is the “amyloid hypothesis,” which states that an increase in β amyloid (Aβ) 42 peptide is the major cause of neuronal degradation in AD (Hardy and Selkoe 2002); support for this hypothesis stems from the accumulation of amyloid plaques in the brains of AD patients (Hardy 2006). In addition to the “amyloid hypothesis,” there is a large body of work that suggests that dysregulation of intracellular calcium has a central role in the pathogenesis of AD. Indeed, several laboratories have shown that FAD mutant PSENs affect store-operated calcium influx by increasing the activity or expression of ER calcium channels [e.g., ryanodine receptors (RYR) (Chan et al. 2000), inositol 1,4,5 trisphosphate receptors (IP3R) (Leissring et al. 1999; Stutzmann et al. 2004; Cheung et al. 2008), or sarco-endoplasmic reticulum calcium ATPases (SERCA) (Green et al. 2008a)] and that PSENs themselves have been shown to act as standalone ER calcium leak channels (Tu et al. 2006; Bandara et al. 2013). Moreover, PSENs are a crucial component of the γ-secretase complex, an intramembranous multi-subunit protease. In addition to the cleavage of amyloid precursor protein (APP) into Aβ peptides, γ-secretase has also been implicated in the cleavage of several other transmembrane proteins, including Notch and APP-like proteins (Beel and Sanders 2008).
In this study, we set out to understand the role of PSEN in the regulation of calcium homeostasis and mitochondrial function in the model system Caenorhabditis elegans. The C. elegans PSEN family comprises the three genes hop-1, sel-12, and spe-4 (Levitan and Greenwald 1995; Li and Greenwald 1997; Arduengo et al. 1998). Although hop-1 and sel-12 are widely expressed, including in muscle and neurons (Levitan and Greenwald 1998), the more distantly related spe-4 is exclusively expressed in the male germline (Arduengo et al. 1998). From our studies, we demonstrate that mutations in sel-12 cause increased calcium signaling and mitochondrial fragmentation and dysfunction. Moreover, we find that the mitochondrial defects observed in sel-12 mutants are independent of γ-secretase activity and are caused by ER-mediated calcium transfer to the mitochondria. Furthermore, we find evidence that the structural and functional defects in mitochondria of sel-12 mutants render them susceptible to oxidative stress and reduce their life span.
Materials and Methods
C. elegans strains and genetics
C. elegans were grown on OP50-seeded NGM plates at 20° except where noted. Animals were synchronized for all experiments by bleaching plates containing gravid worms, and the progeny were rocked in M9 buffer for <48 hr. The synchronized L1s were then allowed to grow on NGM plates seeded with OP50 until the stage required for the experiments was reached. For experiments that required sterilization, age-synchronized L4s were moved to 0.5 mg/ml 5-fluorour-aci1-2’-deoxyribose (FUDR) (Sigma) containing NGM plates seeded with OP50. The following strains were used in this study: wild type (N2), sel-12 (ar131, ty11 and ok2078), hop-1 (ar179), ccIs4251, zxIs3, unc-68(r1162), crt-1(jh101), mcu-1(tm6026), egl-19(tak5), egl-19(n2368), akEx885, takIs13, glp-1(e2141), lin-12(ok2215), syIs243, stEx30, and adIs2122.
Levamisole assay
Briefly, 15–20 age-synchronized L4 or young adult worms from each strain were picked onto individual 0.5-mM levamisole-containing NGM plates by someone other than an assay executioner. At each time point, worms were gently prodded on their head with a platinum pick three times, and in the event of a lack of response, they were marked as paralyzed and eliminated from the assay. Levamisole assay was observed for >70 min. Six replicate assays were done.
Channelrhodopsin analysis
We used the C. elegans strain zxIs3 [Punc-47::ChR2(H134R)::YFP + lin-15(+)] that expresses channelrhodopsin (ChR2) in their GABAergic motor neurons (Liewald et al. 2008). This optogenetic study was performed as previously described (Liewald et al. 2008; Fry et al. 2014), and the percentage elongation of the animals was calculated using the equation below.
DNA constructs and transgenesis
The Pmyo-3::R-GECO1 construct was assembled by cloning PCR-amplified R-GECO1 into the vector containing the myo-3 promoter pPD95.86 (a gift from Andrew Fire, Addgene). Using standard procedures, these constructs, along with pJM23 [lin-15(+)], were injected into lin-15(n765ts) animals (Jin et al. 1999). Transgenic progeny were identified by body-wall or pharyngeal muscle fluorescence and lin-15 mutant rescue. Stable integrated lines (takIs13) were generated with γ-irradiation using standard procedures (Jin et al. 1999; Spooner et al. 2012). A full-length sel-12 cDNA that contains a 3′ SL2::TagRFP cassette was generated by Genscript. To make the Pmyo-3::sel-12::SL2::TagRFP construct, this synthesized sel-12 cDNA was placed into a vector containing the myo-3 promoter pPD95.86 (Addgene). To make the Prab-3::sel-12::SL2::TagRFP construct, the myo-3 promoter was replaced with a PCR-amplified rab-3, pan-neuronal promoter (Mahoney et al. 2008). Using standard procedures, these constructs along with the coinjection marker Pttx-3::GFP were injected into N2 animals. Transgenic progeny were identified by appropriate tissue fluorescence.
Calcium imaging
The basal calcium levels in the body-wall muscle of animals were analyzed by expressing R-GECO1 under the myo-3 promoter (Moerman and Fire 1997). Briefly, five age-synchronized sterilized young adults were mounted on a 2.5% agarose in M9 pad and immobilized using Polybead Polystyrene 0.10 μm microspheres (Polysciences). The animals were imaged at 10× magnification using an inverted Zeiss AxioObserver microscope equipped with an Andor Clara CCD camera. For each genotype, we analyzed 50 animals. A Wacom Bamboo tablet and stylus were used to draw freehand selections around each immobilized animal for each image, and their fluorescence intensity was measured using Metamorph software. Relative R-GECO1 fluorescence in each strain compared to wild-type R-GECO1 fluorescence. Protein levels of R-GECO1 in each strain were used to normalize the fluorescence intensity. Protein levels of R-GECO1 were detected using Western blot analysis. Briefly, a large plate of FUDR-sterilized, synchronized day 1 adult worms from each strain were freeze-cracked in RIPA buffer, sonicated, and spun down at 4° to collect supernatant. This supernatant was then used for Western analysis using previously established methods (Kelsh et al. 2014). R-GECO1 levels were probed using a DsRed Polyclonal Antibody (Clontech catalog no. 632496) antibody, and protein loading was verified using an Anti-Actin Antibody, clone C4 antibody (EMD Millipore).
To analyze calcium transients, we utilized the same technique but imaged the worms, one worm/ agar pad/slide, under 63× oil while focusing on a single muscle cell for 50 sec. The fluorescence intensity of the R-GECO1 in this muscle cell was measured over this time period and adjusted for photobleaching. A calcium transient was scored as observed when F/F0 was >5%.
Mitochondrial morphology analysis
Body-wall mitochondrial structure was analyzed using the ccIs4251 strain [Pmyo-3::GFP(NLS)::LacZ (PSAK2) + Pmyo-3::GFP (PSAK4) + dpy-20(+)], which targets GFP to the matrix of the body-wall muscle mitochondria and nucleus and the syIs243 strain [Pmyo-3::TOM20::mRFP + unc-119(+) + pBS SK+], which targets monomeric red fluorescent protein (mRFP) to the outer mitochondrial membrane. These strains were crossed into various genotypes to determine mitochondrial structure. Age-synchronized day 1 adults (nongravid) were immobilized, and their mitochondrial structure was imaged under the 63× oil objective on a Zeiss AxioObserver microscope equipped with a Andor Clara CCD camera. Images were compiled, and the mitochondria were scored as linear, intermediate, or fragmented (Lu et al. 2011) under blinded conditions. Neuronal mitochondria were assessed similarly using akEx885 (Pnmr-1::Su9::GFP; kindly provided by A. V. Maricq).
MitoTracker Red CMXRos (Life Technologies) and tetramethylrhodamine ethyl ester (TMRE) were used to determine the structural and functional integrity of the body-wall muscle mitochondria in various C. elegans strains. Briefly, age-synchronized worms (L4s for Mitotracker and young adults for TMRE) were stained with either 1nμg/ml of Mitotracker or 200 nM of TMRE in M9 with OP50 for 6 hr with shaking at 20°. Worms were then washed four times with M9. Mitotracker and TMRE-stained animals were destained overnight or for 1 hr, respectively. Worms were imaged as day 1 adults as per the protocol described above.
Inhibition of γ-secretase activity
Unseeded NGM plates were coated with 100 μl of 100 μM Compound E (Calbiochem), a γ-secretase inhibitor, and were then seeded with OP50 the next day. Synchronized L1 staged animals were grown on these plates, and day 1 adults were used for analysis (Francis et al. 2002). γ-Secretase inhibition was confirmed by the glp-1-like sterility observed in the hop-1(ar179) mutants that were grown on the Compound E-containing plates.
RNA interference
Age-synchronized L1 animals were allowed to grow to day 1 adults on RNA interference (RNAi) plates seeded with empty vector, itr-1, or drp-1 RNAi-containing bacteria.
Drug treatments
Thapsigargin (Abcam) (Xu et al. 2001), mito-TEMPO (Sigma) (Johnson et al. 2012), and cyclosporine A (Sigma) (Giacomotto et al. 2013) were dissolved in DMSO and added to the NGM media (cooled to 55°) to the appropriate final concentrations, and animals were grown on these plates as previously described.
2′,7′-dichlorodihydrofluorescein diacetate assay
Three replicate 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA) assays (Life Technologies) were used to measure ROS levels in wild type, sel-12(ar131), sel-12(ty11), and sel-12(ok2078) animals. Animals grown on 100-mm NGM plates were washed three times with M9 to remove OP50. Then the animals were washed once in PBS and resuspended in 100 μl of PBS followed by freeze thawing and 10 sec of sonication. After spinning down the sample at 20,000 × g for 10 min at 4°, the protein concentration was determined using a BCA protein assay (Pierce). Twenty-five micrograms of the protein was used for each strain, the samples were incubated with H2DCF-DA at 37° for 4 hr, and a Flex Station 3 Reader (Molecular Devices) was used to measure fluorescence intensity.
Paraquat assay
Sensitivity to paraquat (Aldrich) was analyzed as previously described (Han et al. 2012). Briefly, 50 animals per strain were placed in tubes containing 100 μl of 0, 50, 100, or 150 mM paraquat in M9 buffer and incubated at 20° for 24 hr. Subsequently, the animals were moved to an NGM plate and their survival was observed. Animals were marked as dead if they showed no response to three gentle pats from a platinum pick. These experiments were repeated three times.
Statistical analysis
GraphPad Prism software was used for statistical analysis. Student’s t-test was used for comparing two samples, and one-way ANOVA with Bonferroni posttest was used while making multiple comparisons. The chi-square test was used to compare the mitochondrial structural distribution between strains.
Results
sel-12 mutants are hypersensitive to levamisole
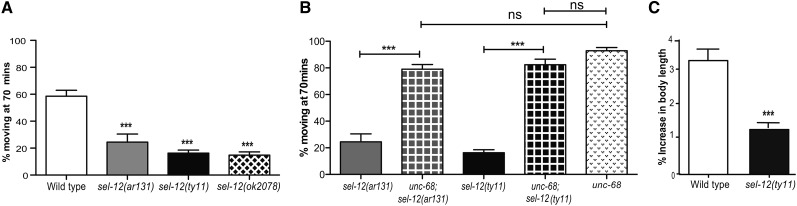
To gain insight into the physiological role that PSEN has in C. elegans, we turned to a simple and widely used pharmacological assay using levamisole, an acetylcholine receptor agonist that promotes progressive paralysis by hypercontraction of the body-wall muscle. We examined whether the null mutations sel-12(ty11) and sel-12(ok2078) or the missense mutation sel-12(ar131), which is a conserved change identified in FAD patients (Tedde et al. 2003), causes a different behavioral response to levamisole compared to wild-type animals. Upon exposure to levamisole for 70 min, only 24.4% of sel-12(ar131), 16.3% of sel-12(ty11), and 14.8% of sel-12(ok2078) animals were moving compared to 58.5% of wild-type animals (Figure 1A). Since previous studies have implicated PSENs in the regulation of calcium (Leissring et al. 1999; Chan et al. 2000; Green et al. 2008b; Brunello et al. 2009; Chakroborty et al. 2009), we next investigated whether reducing calcium signaling in sel-12 mutants could suppress the hypersensitivity of sel-12 mutants to levamisole. Accordingly, we introduced unc-68(r1162), a RYR null mutation, into the sel-12 mutant background and tested levamisole sensitivity. Strikingly, introduction of the unc-68 mutation into the sel-12 mutant background suppressed the elevated sensitivity of sel-12 mutants to levamisole. When exposed to levamisole, 79.1% of unc-68; sel-12(ar131) and 82.4% of unc-68; sel-12(ty11) mutants are motile unlike sel-12(ar131) and sel-12(ty11) mutants, which are 24.4 or 16.3% motile, respectively (Figure 1B). These results taken together suggest that elevated sensitivity of sel-12 mutants to levamisole may result from elevated calcium signaling.
Figure 1.
sel-12 mutants have hyper-excitable muscle. (A) sel-12 mutants are hypersensitive to 0.5 mM of levamisole. (B) Rescue of sel-12 mutant hyper-sensitivity to 0.5 mM of levamisole by unc-68 mutant background. For A and B, data are displayed as mean ± SEM (***P < 0.001 using one-way ANOVA). (C) sel-12 mutants elongate significantly less than wild-type animals upon GABAergic motor neuron activation. n = 30 animals per genotype. Data are displayed as mean ± SEM, and all comparisons have been made to wild type unless otherwise indicated (***P < 0.001 using two-tailed T-tests).
sel-12 mutants are defective in GABAergic inhibitory motor neuron-induced muscle relaxation and have reduced motility
We reasoned that if sel-12 mutants have elevated calcium signaling in the body-wall muscle, their muscle should be less responsive to inhibitory neuronal signaling. To investigate this possibility, we used an optogenetic approach utilizing zxIs3 animals that express ChR2 in their GABAergic inhibitory motor neurons (Liewald et al. 2008). In zxIs3 animals, blue light stimulation of ChR2 will cause the depolarization of GABAergic inhibitory motor neurons, resulting in the relaxation of body-wall muscles and measurable elongation of the animal (Liewald et al. 2008; Fry et al. 2014). If sel-12 animals have elevated cytoplasmic calcium levels, we predict that sel-12 mutants will not elongate to the same extent as wild-type animals. Freely crawling zxIs3 and zxIs3; sel-12(ty11) animals were illuminated with blue light, and their percentage changes in body length were measured. Upon stimulation, wild-type animals extend their body length 3.7% while sel-12 mutants show a significant decrease in their ability to relax and extend 1.5% (Figure 1C). Consistent with this defect in muscle relaxation, sel-12 mutants also show reduced locomotive abilities in both crawling (Supporting Information, Figure S1A) and swimming assays (Figure S1B). These data demonstrate that sel-12 mutants have an inability to elongate normally upon muscle inhibition and have reduced locomotion, which is consistent with sel-12 mutants having elevated calcium signaling.
SEL-12 is required for maintaining cellular calcium homeostasis
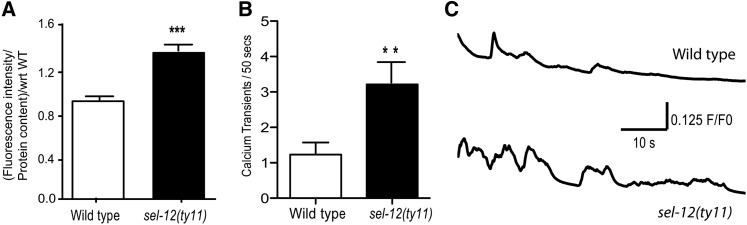
PSEN mutations have been reported to be detrimental to calcium homeostasis in various in vitro and in vivo models (Leissring et al. 1999; Chan et al. 2000; Smith et al. 2005; Green et al. 2008b; Brunello et al. 2009; Chakroborty et al. 2009). Consistent with these observations, our data suggest that there is elevated calcium signaling in the body-wall muscle of sel-12 mutants. To directly measure calcium levels in sel-12 mutants, we generated transgenic animals that express R-GECO1, a red-shifted genetically encoded calcium indicator (GECI) (Zhao et al. 2011), in the cytosol of body-wall muscle. We evaluated calcium levels in the body-wall muscle based on fluorescence intensity of R-GECO1 in sel-12(ty11) mutants. These values were normalized to the R-GECO1 protein content relative to wild-type animals in each strain using Western blot analysis. The relative fluorescence intensity of R-GECO1 with respect to relative protein content in sel-12 mutants is 48.4% higher than in wild-type animals (Figure 2A and Figure S2). Additionally, we examined calcium transients in immobilized intact animals by measuring the fluorescence intensity of R-GECO1 in wild-type and sel-12(ty11) mutant animals. Calcium transients coincide with muscle contraction and are dependent on RYR function (Liu et al. 2011). sel-12 mutants have 3.2 transients/50 sec whereas wild-type animals show only 1.2 transient/50 sec (Figure 2, B and C). The significantly higher fluorescence intensity and increased number of calcium transients indicates that loss of SEL-12 function results in elevated calcium signaling.
Figure 2.
sel-12 mutants have aberrant calcium homeostasis. (A) Basal cytosolic calcium level is significantly higher in sel-12 mutants compared to wild-type animals. Data are displayed as mean relative intensity of R-GECO1 in wild-type and sel-12 animals normalized to relative R-GECO1 protein concentration ± SEM, and all comparisons have been made to wild type unless otherwise indicated (***P < 0.001 using two-tailed T-tests). (B) Number of calcium transients per 50 sec observed in each strain (n = 9 per strain) (**P < 0.01 using two-tailed t-tests). (C) Representative traces of calcium transients observed in immobilized animals.
Abnormal mitochondrial morphology is observed in sel-12 mutants
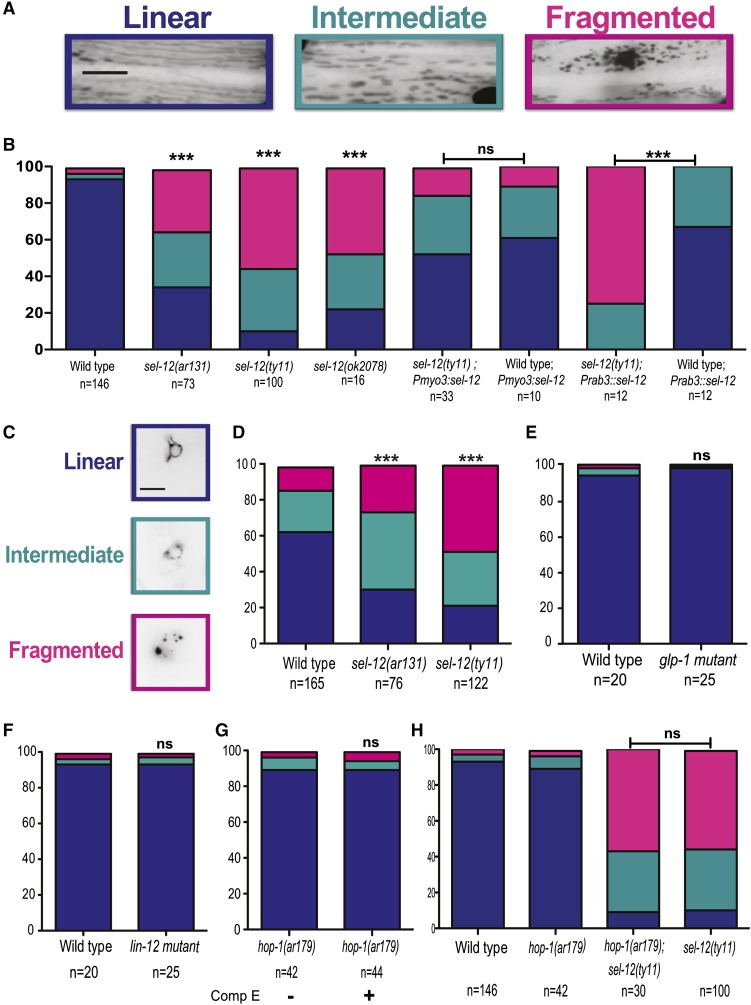
When cytoplasmic calcium levels within cells increase, mitochondria will facilitate the uptake of calcium. To investigate whether the elevated calcium signaling observed in sel-12 mutants leads to an alteration in mitochondrial organization, we examined the morphology of mitochondria in the body-wall muscle of sel-12 mutants. We utilized a transgenic strain, ccIs4251, which expresses green fluorescent protein (GFP) that localizes to the nucleus and the mitochondrial matrix (Fire et al. 1998). Examination of wild-type animals reveals that the majority of mitochondria in the body-wall muscle are organized into a parallel and linear network (Figure 3, A and B). However, the mitochondria in sel-12 mutants are disorganized and display a fragmented appearance (Figure 3, A and B). While we observed only 2.9% fragmentation in the mitochondrial network in wild-type animals, the fragmentation of mitochondria in sel-12(ar131), sel-12(ty11), and sel-12(ok2078) mutants is 34.5, 55.6, and 47.2%, respectively (Figure 3, A and B). We analyzed the organization of body-wall muscle myosin in sel-12 mutants to rule out the possibility of widespread structural damage in the muscle using the strain stEx30, which expresses GFP-labeled body-wall-muscle-specific myosin. Strikingly, the myosin organization in sel-12 mutants is indistinguishable from that of wild-type animals (Figure S3A), indicating that the mitochondrial morphology defects observed in the sel-12 mutants is not the result of comprehensive muscle structural defects. We also examined an additional transgenic strain with mRFP fused to TOMM-20, an outer mitochondrial membrane protein in the body-wall muscle and observed similar mitochondrial fragmentation in sel-12 mutants (Figure S3B). To determine whether SEL-12 has a cell-autonomous role in maintaining mitochondrial morphology, we expressed wild-type sel-12 under a body-wall-muscle-specific promoter (Pmyo-3). Expression of sel-12 in the body-wall muscle is successful in rescuing the mitochondrial fragmentation in sel-12(ty11) animals (Figure 3B). However, the expression of wild-type sel-12 under a pan-neuronal promoter (Prab-3) does not result in rescue of the mitochondrial defects observed in sel-12(ty11) animals (Figure 3B). These data indicate that SEL-12 works via a cell-autonomous mechanism to regulate mitochondrial morphology. Of note, although not as severe as observed in sel-12 mutants, overexpression of sel-12 by the body-wall-muscle-specific or pan-neuronal promoter in wild-type animals leads to an increase in mitochondrial morphology defects (Figure 3B), suggesting that overexpression of sel-12 in the muscle can lead to mitochondrial disorganization and that overexpression of sel-12 in the nervous system can lead to non-cell-autonomous defects. These data indicate that sel-12 needs to be tightly regulated for normal mitochondrial morphology.
Figure 3.
sel-12 mutants have mitochondrial morphology defects. (A) Representative images of the mitochondrial morphologies observed in the body-wall muscle. (B) Quantification of body-wall muscle mitochondrial morphology in wild-type and sel-12 animals. (C) Representative images of the mitochondrial morphologies observed in the PVC neurons. (D) Quantification of PVC neuronal mitochondrial morphology in wild-type and sel-12 animals. (E) Quantification of body-wall muscle mitochondrial morphology in wild-type and glp-1 animals grown at 25°. (F) Quantification of body-wall muscle mitochondrial morphology in wild-type and lin-12 animals. (G) Quantification of body-wall muscle mitochondrial morphology in hop-1 mutants grown on Compound E. (H) Quantification of body-wall muscle mitochondrial morphology in wild-type, hop-1, hop-1;sel-12(ty11), and sel-12(ty11) mutants. For A, B, and E–H, analysis was done using transgenic animals expressing mito::GFP in body-wall muscle cells (ccIs4251). For C and D, analysis was done using transgenic animals expressing mito::GFP in PVC neurons (akEx885). Blue, cyan, and pink represent the percentage of animals displaying linear, intermediate, and fragmented mitochondrial morphology, respectively. n = number of animals imaged per genotype. All comparisons have been made to wild type unless otherwise indicated, and statistical significance was tested using the chi-square test (“ns” indicates P > 0.05, *** P < 0.001). Bar, 10 µm.
Since mutations in human PSENs lead to neuronal dysfunction and loss, we next investigated the impact of sel-12 mutations on neuronal mitochondria. We examined mitochondrial morphology in the PVC interneurons, which are two interneurons that are located in the tail region and are easily and reliably identified. Similar to the mitochondrial morphology observed in the body-wall muscle, we find that 48.4 and 26.3% of PVC neuronal mitochondria in sel-12(ty11) and sel-12(ar131) mutants are fragmented compared to only 12.7% observed in the wild-type animals (Figure 3, C and D). Taken together, these data suggest that sel-12 mutations lead to an alteration of mitochondrial morphology in both the body-wall muscle and neurons.
SEL-12-deficient mitochondrial structural defects are Notch and γ-secretase-independent
PSENs are essential for Notch signaling as it forms part of the γ-secretase complex, a protease that cleaves within the membrane-spanning region of Notch to promote Notch signaling (Struhl and Greenwald 1999). The C. elegans genome encodes two Notch family members, LIN-12 and GLP-1 (Priess 2005; Greenwald 2012). To determine whether the mitochondrial defects observed in sel-12 mutants are caused by the downregulation of Notch signaling, we analyzed mitochondria in glp-1 and lin-12 mutants. Strikingly, the mitochondrial structure in both Notch mutants is similar to those of wild-type animals with glp-1 and lin-12 mutants exhibiting comparable proportions of linear, intermediate, and fragmented mitochondria to wild-type animals (Figure 3, E and F). Since Notch-dependent signaling does not appear to have a role in the SEL-12-deficient mitochondrial phenotype, we next investigated whether γ-secretase activity is required to maintain normal mitochondrial morphology. We used a well-characterized γ-secretase inhibitor called Compound E, which has been previously shown to inhibit all SEL-12-mediated γ-secretase activity but not the γ-secretase activity of HOP-1 (Francis et al. 2002). To completely abolish all γ-secretase activity, we grew hop-1(ar179) null mutants on plates containing Compound E and examined their mitochondria. The mitochondria of γ-secretase-inhibited animals are indistinguishable from wild-type animals (Figure 3G), suggesting that the mitochondrial defects observed in the sel-12 mutants are not mediated by the lack of γ-secretase activity. Furthermore, hop-1(ar179);sel-12(ty11) double-mutant animals show mitochondrial morphology similar to sel-12(ty11) single-mutant animals, suggesting that HOP-1, unlike SEL-12, does not influence mitochondrial structure (Figure 3H).
Elevated cytoplasmic calcium levels alone are not sufficient to cause mitochondrial fragmentation as observed in sel-12 mutants
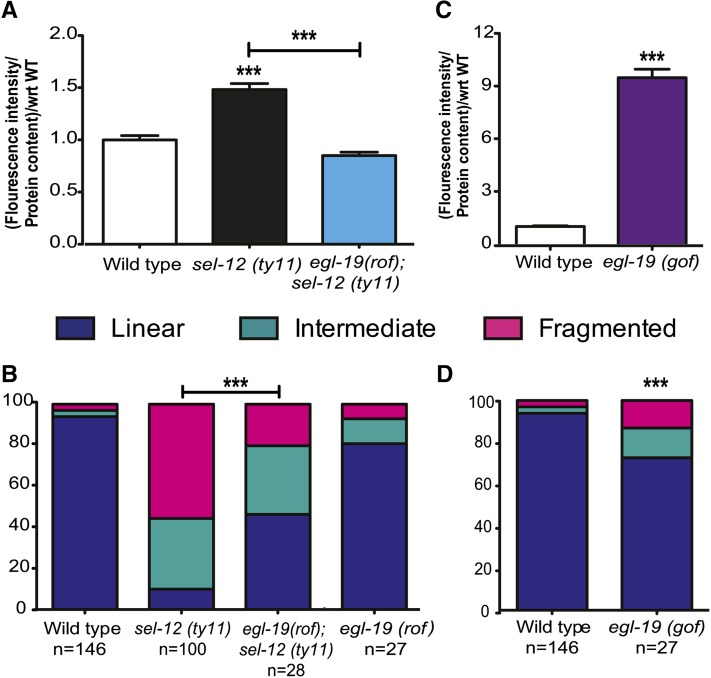
To investigate the cause of mitochondrial fragmentation in sel-12 mutants, we hypothesized that the high cytoplasmic calcium levels and more frequent calcium transients observed in sel-12 mutants are responsible for the mitochondrial morphology disruption. To test this hypothesis, we utilized a reduction of function (rof) mutation in the gene encoding the L-type voltage-gated calcium channel, egl-19, which is critical for muscle contraction (Lee et al. 1997; Spooner et al. 2012). We generated egl-19(rof); sel-12(ty11) double mutants. Using our GECI strain, we found that the egl-19(rof); sel-12 double mutants have cytoplasmic calcium levels that are significantly reduced from sel-12 mutants and similar to those of wild-type animals (Figure 4A and Figure S2). Next, we examined mitochondrial morphology in the double mutants. Consistent with our hypothesis, the mitochondrial morphology in egl-19(rof); sel-12 double mutants is improved compared to sel-12 mutants, with the former having 46.4% of mitochondria in the linear form while sel-12 mutants have only 10.2% in the linear form. Similarly, the introduction of the egl-19(rof) mutation into sel-12 mutants decreases the amount of mitochondrial fragmentation observed in sel-12 mutants from 55.6 to 20.2% (Figure 4B). Despite having cytoplasmic calcium levels similar to wild type, the egl-19(rof); sel-12 double mutants do not have wild-type mitochondria. These data suggest that, while reducing cytoplasmic calcium levels in sel-12 mutants can improve mitochondrial morphology, it does not explain the extent of damage observed in sel-12 mutant animals. We next investigated whether high cytoplasmic calcium levels are sufficient to cause mitochondrial fragmentation by examining the mitochondrial structure in egl-19 gain of function (gof) mutants (Lee et al. 1997). First, using our GECI, we determined that the cytoplasmic calcium levels in egl-19(gof) mutants are ∼10-fold higher than in wild-type animals (Figure 4C and Figure S2). While egl-19(gof) mutants do show significant mitochondrial morphology defects, they did not show mitochondrial defects to the same extent as sel-12 mutants did (Figure 4D), suggesting that increased cytoplasmic calcium alone is not sufficient to explain the mitochondrial phenotype seen in sel-12 mutants.
Figure 4.
Elevated cytoplasmic calcium levels are not sufficient to cause the mitochondrial morphology defects observed in sel-12 mutants. (A) Basal calcium level in wild-type, sel-12, and egl-19(rof);sel-12 animals. (B) Quantification of body-wall muscle mitochondrial morphology in wild-type, sel-12, egl-19(rof);sel-12, and egl-19(rof) animals. (C) Basal calcium level in wild-type and egl-19(gof) animals. (D) Quantification of body-wall muscle mitochondrial morphology in wild-type and egl-19(gof) animals. In A, data are displayed as mean ± SEM (***P < 0.001 using the one-way ANOVA), and all comparisons have been made to wild type unless otherwise indicated. In C, data are displayed as mean ± SEM (***P < 0.001 using two-tailed t-tests). In B and D, analysis was done using transgenic animals expressing mito::GFP in body-wall muscle cells (ccIs4251). n = number of animals imaged per genotype, and statistical significance was tested using the chi-square test (***P < 0.001). Bar, 10 µm.
Reduction of calcium release from the ER can rescue sel-12 mutant mitochondrial defects
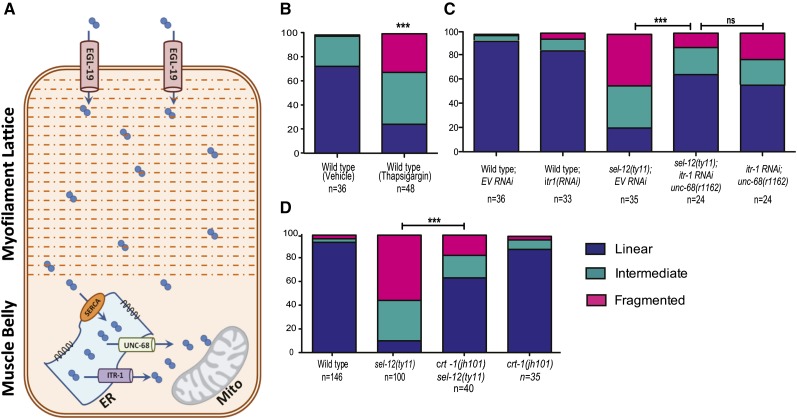
PSENs are found predominantly in the ER membrane and have been implicated in calcium release from the ER and mutant forms or loss of PSEN has been shown to augment ER calcium release (Leissring et al. 1999; Chan et al. 2000; Smith et al. 2005; Chakroborty et al. 2009). EGL-19 voltage-gated calcium channels are located in the plasma membrane and are separated from the mitochondria by the myofilament lattice, which, due to the abundance of calcium-binding proteins, acts as a calcium buffer (Altun 2009; Kim et al. 2009) (Figure 5A). However, the more proximal location of the ER to the mitochondria indicates that ER calcium release may impact mitochondria more than calcium entry from the more distally positioned EGL-19 channels (Figure 5A). To investigate whether exacerbated ER calcium release can result in mitochondrial fragmentation, we grew wild-type animals carrying the mitochondrial GFP marker (ccIs4251) on NGM plates containing 3 μg/ml of thapsigargin. Thapsigargin is a SERCA pump inhibitor that blocks ER calcium uptake and induces calcium release via IP3Rs, thereby increasing the calcium release from the ER and local calcium concentration (Takemura et al. 1989; Xu et al. 2001). Wild-type animals grown on thapsigargin exhibit significant fragmentation compared to vehicle-treated animals, suggesting that excessive ER-calcium release leads to the fragmentation of mitochondria similar to what is observed in sel-12 mutants (Figure 5B).
Figure 5.
Decreasing ER-mediated calcium release improves mitochondrial structure in sel-12 mutant. (A) Cartoon depicting the proximal and distal positioning of ER and EGL-19, respectively, with respect to the mitochondria in the body-wall muscle of C. elegans. (B) Quantification of body-wall muscle mitochondrial morphology in animals grown on NGM plates containing DMSO or 3 μg/ml of thapsigargin. (C) Quantification of body-wall muscle mitochondrial morphology in wild-type, sel-12(ty11),unc-68;sel-12(ty11), and unc-68(r1162) animals grown on RNAi plates seeded with either empty vector (EV) or itr-1 RNAi. (D) Quantification of body-wall muscle mitochondrial morphology in wild-type, sel-12, crt-1;sel-12, and crt-1 animals. In B–D, analysis was done using transgenic animals expressing mito::GFP in body-wall muscle cells (ccIs4251). n = number of animals imaged per genotype. All comparisons have been made to wild type unless otherwise indicated, and statistical significance was tested using the chi-square test (***P < 0.001).
To further investigate the impact of ER calcium release on mitochondrial morphology in sel-12 mutants, we used four different approaches. First, we investigated the effect of RNAi knockdown of the ITR-1/IP3R (to inhibit ER calcium release) in sel-12(ar131), sel-12(ty11), and wild-type animals. Compared to sel-12(ty11) and sel-12(ar131) animals grown on control RNAi, itr-1(RNAi); sel-12(ty11) and itr-1(RNAi); sel-12(ar131) animals show improved mitochondrial structure with significantly increased amounts of linear and decreased amounts of fragmented mitochondria (Figure S4A). Second, we introduced a RYR null mutation unc-68(r1162) into sel-12(ty11) mutants to eliminate the contribution of RYR-mediated calcium release from the ER. Interestingly, the unc-68 single mutants show a higher level of mitochondrial fragmentation than what is observed in wild-type animals (Figure S4A). Nevertheless, from our examination of unc-68; sel-12 double mutants, we find a significant improvement of mitochondrial morphology compared to sel-12(ty11) mutants alone (Figure S4A). Third, since genetic or RNAi ablation of unc-68 or itr-1 does not fully rescue the mitochondrial morphology defects observed in sel-12 mutants (Figure S4A), we used RNAi to knockdown ITR-1/IP3R in the unc-68(r1162); sel-12(ty11) double mutant. Compared to sel-12(ty11) animals grown on control RNAi, itr-1(RNAi); unc-68(r1162); sel-12(ty11) animals show improved mitochondrial structure with linear mitochondria increasing to 65.2 from 20.9% (Figure 5C). Similarly, the amount of fragmented mitochondria observed in sel-12(ty11) is reduced from 43.8 to 12.1% in itr-1(RNAi); unc-68(r1162); sel-12(ty11) animals (Figure 5C). When compared to itr-1 RNAi-treated unc-68(r1162) animals, itr-1(RNAi); unc-68(r1162); sel-12(ty11) animals have similar mitochondrial morphology that is not significantly different, indicating full rescue (Figure 5C). Finally, we sought to reduce ER calcium levels by genetic removal of calreticulin, a critical ER calcium-buffering protein required for normal release of ER calcium (Michalak et al. 1999). We introduced a null calreticulin mutation, crt-1(jh101), into sel-12(ty11) mutants and investigated mitochondrial morphology. Accordingly, reduction of ER calcium results in a robust increase in linear mitochondria; we observed 63.3% linear mitochondria in crt-1; sel-12(ty11) double mutants compared to the 10.2% linear mitochondria in sel-12(ty11) mutants (Figure 5D). Similarly, there is a steep decline in mitochondrial fragmentation from 55.6% in sel-12(ty11) to 17.5% in crt-1;sel-12(ty11) animals (Figure 5D). Together, these data strongly indicate that reduction of calcium release from the ER in sel-12 mutants can restore mitochondrial morphology and indicate that ER calcium release is critical in causing the mitochondrial morphology defects observed in sel-12 mutants.
Decreasing calcium uptake into the mitochondrial matrix reduces sel-12 mutant mitochondrial defects
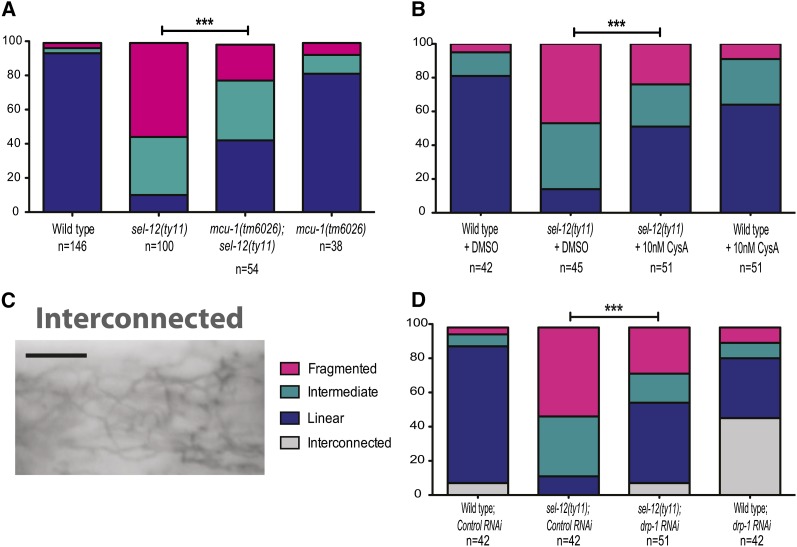
Since mitochondria act as a calcium buffer, this prompted us to investigate whether mitochondrial uptake of calcium in sel-12 mutants mediates the mitochondrial morphology defects observed in sel-12 mutants. Mitochondrial calcium uptake is handled by the conserved mitochondrial calcium uniporter (MCU), a highly selective calcium transporter that is activated when there is a localized increase in calcium concentration (Csordas et al. 2010). To this end, we employed null mcu-1 mutants, which have been previously shown to reduce mitochondrial calcium uptake and buffering (Xu and Chisholm 2014). We generated mcu-1; sel-12(ty11) double mutants and examined their mitochondria. We found that, unlike sel-12 mutants where 10.2% of mitochondria exist in linear structures, the mcu-1; sel-12 double mutants have 42.7% of their mitochondria appear linear (Figure 6A). Furthermore, the amount of mitochondrial fragmentation is reduced from 55.6% in sel-12 mutants to 21.2% in mcu-1; sel-12 double mutants (Figure 6A). Although the rescue of mitochondrial structural defects is highly significant, it is not complete. This is not unexpected since basal levels of mitochondrial calcium are still detected in MCU knockout mice and in mcu-1 null animals despite the impairment of rapid calcium uptake (Pan et al. 2013; Xu and Chisholm 2014). These data indicate that uptake of calcium into the mitochondrial matrix is critical for the mitochondrial morphology defects observed in sel-12 mutants.
Figure 6.
Mitochondrial calcium uptake is responsible for mitochondrial structural disruption in sel-12 mutants. (A) Quantification of body-wall muscle mitochondrial morphology in wild-type, sel-12, mcu-1;sel-12, and mcu-1 animals. (B) Quantification of body-wall muscle mitochondrial morphology in wild-type and sel-12 animals grown on NGM plates containing DMSO or cyclosporine A. (C) Representative image of interconnected mitochondrial phenotype. (D) Quantification of body-wall muscle mitochondrial morphology in wild-type and sel-12 animals grown on RNAi plates seeded with either empty vector (EV) or drp-1 RNAi. Analysis was done using transgenic animals expressing mito::GFP in body-wall muscle cells (ccIs4251). n = number of animals imaged per genotype. Statistical significance was tested using the chi-square test (***P < 0.001). Bar, 10 µm.
Overloading the mitochondria with calcium can trigger the opening of the mitochondrial permeability pore (mPTP), which decreases the membrane potential and causes mitochondrial release of ROS (Brookes et al. 2004). To investigate whether this was the cause of mitochondrial fragmentation in the sel-12 mutants, we treated the animals with an inhibitor of the mPTP, cyclosporine A (CysA) (Broekemeier et al. 1989). Treatment of sel-12 mutants with 10 nM of CysA compared to a control treatment resulted in an increase in linear mitochondria from 13.7 to 51.0% in sel-12 animals and a simultaneous decrease of the mitochondrial fragmentation (Figure 6B). These data indicate that uptake of calcium into the mitochondrial matrix and the opening of the mPTP plays a critical role in the mitochondrial morphology defects observed in sel-12 mutants.
Furthermore, ER-mediated calcium release and subsequent mitochondrial calcium uptake can impact the activity of Drp1, a mitochondrial fission protein (Cribbs and Strack 2007; Cereghetti et al. 2008; Xu et al. 2013). To determine whether DRP-1 is involved in the increased mitochondrial fragmentation observed in sel-12 mutants, we used RNAi to knock down DRP-1 and examined mitochondrial morphology in both wild-type and sel-12(ty11) animals. Wild-type animals grown on drp-1(RNAi) exhibit a high incidence of interconnected and fused mitochondria (Figure 6, C and D), indicating successful suppression of mitochondrial fission. Markedly, sel-12 animals grown on drp-1(RNAi) show reduced fragmentation and increased incidence of linear and interconnected mitochondria (Figure 6D). These data suggest that DRP-1 activity is elevated in sel-12 mutants.
SEL-12 deficiency leads to defects in mitochondrial function
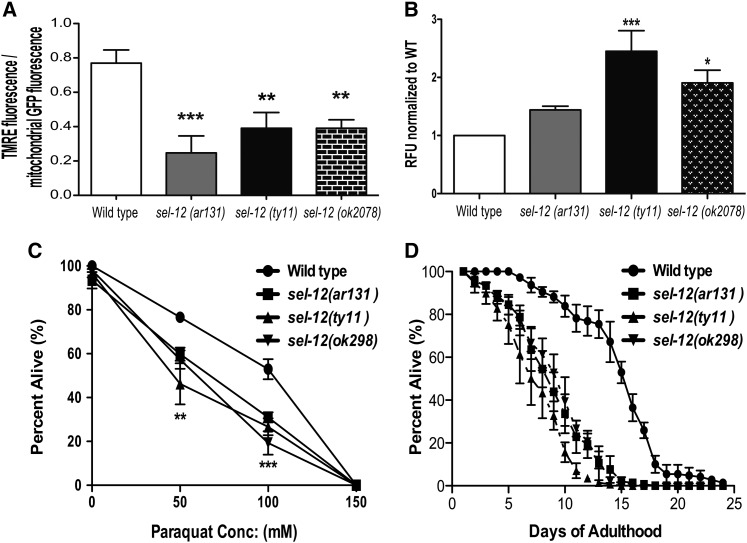
To test whether the mitochondrial fragmentation observed in sel-12 mutants results in defective mitochondrial function, we used TMRE and MitoTracker Red CMXRos to examine the mitochondria in wild-type and sel-12 mutants. TMRE and MitoTracker Red CMXRos are both positively charged, red fluorescent dyes that accumulate in active mitochondria because of their relative negative charge; inactive or damaged mitochondria have decreased membrane potential and will fail to sequester either dye. We found that the ratio of TMRE-labeling intensity to mitochondrial GFP intensity (from ccIs4251) in wild-type animals is significantly higher at 0.77 when compared to 0.25, 0.39, and 0.39 in sel-12(ar131), sel-12(ty11), and sel-12(ok2078) mutants, respectively (Figure 7A). Similarly, MitoTracker CMXRos labeling is greatly reduced in all sel-12 mutants compared to wild-type animals (Figure S5, A and B). These data indicate that the mitochondrial membrane potential in sel-12 mutants is reduced and suggest that mitochondrial function is abrogated.
Figure 7.
sel-12 mutants have mitochondrial functional defects. (A) Quantification of body-wall muscle mitochondria staining intensity with TMRE in wild-type and sel-12 animals. n = 30 per strain. (B) H2DCF-DA assay measuring ROS levels relative to wild type. (C) Paraquat sensitivity in wild-type and sel-12 animals after 24 hr of continued exposure. Data are displayed as mean ± SEM, and all comparisons have been made to wild type (*P < 0.05, **P < 0.01, ***P < 0.001 using two-way ANOVA). In B and C, data are from three replicate assays. (D) Life-span assay of FUDR-sterilized wild-type and sel-12 mutant animals. n = 50 per strain. Data are from three replicate assays.
A critical function of the mitochondria is to metabolize ROS generated by mitochondrial oxidative phosphorylation and other cellular sources (Venditti et al. 2013). Since we have found evidence of mitochondrial dysfunction and elevated mPTP opening in sel-12 mutants (Figure 6B), which enhances ROS production (Brookes et al. 2004), we investigated ROS levels in sel-12 mutants. ROS formation was quantified in wild-type and sel-12 mutant animals using the membrane-permeable ROS indicator H2DCF-DA. Consistent with mitochondrial malfunction, we find that sel-12(ar131), sel-12(ty11), and sel-12(ok2078) mutants have 1.5, 2.9, and 2.1 times as much ROS signal as wild-type animals, respectively (Figure 7B). To determine whether elevated ROS levels caused mitochondrial fragmentation in sel-12 mutants, we supplemented sel-12 mutants with mito-TEMPO, a mitochondrial-targeted superoxide-specific antioxidant. Treatment with mito-TEMPO does not result in the rescue of mitochondrial structural defects in the sel-12 mutants (Figure S5C). To explore the consequence of having elevated ROS levels, we exposed sel-12 mutants to paraquat, an ROS generator that has been shown to cause toxicity dependent on endogenous ROS levels (Han et al. 2012). We discovered that all three sel-12 mutants are hypersensitive to paraquat compared to wild-type animals (Figure 7C). These data suggest that mitochondrial function in sel-12 mutants is impaired and that sel-12 mutants are vulnerable to ROS. Furthermore, we found that the life span of sel-12 mutants is significantly shorter compared to that of wild-type animals with all sel-12 mutants dying 10 days prior to wild-type worms (Figure 7D). It should be noted that in a previous study sel-12(ar131) mutants were reported to be hypersensitive to oxidative stress, consistent with our study (Kitagawa et al. 2003). However, these authors also found that a null allele of sel-12 [sel-12(ar171)] was long lived compared to wild-type and sel-12(ar131) mutants. Unlike our current study, in this previous study the sel-12 mutants analyzed also carried an unc-1 mutation, which encodes a stomatin-like protein that has been implicated in gap junction activity, epithelial sodium channel activity, and sensitivity to volatile anesthetics (Rajaraman and Schulder 1999; Sedensky et al. 2001; Chen et al. 2007). The influence of mutations in unc-1 on life span and oxidative stress is unclear. Here, sel-12(ar131), sel-12(ok2078), and sel-12(ty11) all individually show defects in mitochondrial membrane potential (Figure 7A and Figure S5, A and B), elevated ROS (Figure 7B), hypersensitivity to paraquat (Figure 7C), and shortened life spans (Figure 7D). Together, these data indicate that loss of sel-12 function leads to poor mitochondrial function and reduced organismal health.
Discussion
In this study, we provide in vivo evidence that SEL-12, a C. elegans PSEN homolog, is involved in regulating calcium homeostasis and impacts mitochondrial morphology and function. In addition, we have found that the calcium dysregulation observed in SEL-12-deficient animals arises from ER stores and that this defect leads to increased mitochondrial uptake of calcium, which drives the mitochondrial defects observed in sel-12 mutants. Furthermore, we show that the role of SEL-12 in regulating mitochondrial function is independent of its role in γ-secretase function and that the mitochondrial defects observed in sel-12 mutants are caused in part by the opening of the mPTP and elevated DRP-1-dependent mitochondrial fission.
Although the role of PSEN in AD progression remains unclear (Sherrington et al. 1995), similar to our findings, calcium dysregulation has been observed in various models of mutant PSEN function as well as tissue samples from AD patients (Ito et al. 1994; Leissring et al. 1999; Chan et al. 2000; Smith et al. 2005; Tu et al. 2006; Green et al. 2008b). Thus, calcium may have a pivotal role in AD progression. As in other animals, SEL-12 localizes predominantly to the ER (Kovacs et al. 1996; De Strooper et al. 1997; Levitan and Greenwald 1998). Furthermore, recent studies have shown that PSENs are subcellularly localized on the ER to regions where ER and mitochondria contact (Area-Gomez et al. 2009). Moreover, ER–mitochondrial contacts are increased and ER–mitochondrial crosstalk is enhanced in PSEN1 knockout cells, in cells overexpressing the FAD mutant PSEN2, and in fibroblasts from FAD and sporadic AD patients (Zampese et al. 2011; Area-Gomez et al. 2012; Kipanyula et al. 2012). Thus, PSENs are critically positioned where ER and mitochondria communicate and share contents (e.g., calcium, lipids, ATP). Faulty communication between the ER and mitochondria can alter the normal activity of these organelles, leading to cellular dysfunction. This is particularly underlined by our observation of the aggressive mitochondrial fragmentation brought on by thapsigargin treatment compared to the modest structural damage observed as a result of a global increase in cytoplasmic calcium levels in the egl-19(gof) animals. Moreover, our findings also show that reducing ER release and mitochondrial uptake of calcium corrects the mitochondrial defects observed in sel-12 mutants. Taken together, these data indicate that SEL-12 has a critical role in mediating calcium release from the ER and, in the absence of SEL-12 function, excessive ER-mediated release of calcium causes excessive uptake into the mitochondria, resulting in loss of mitochondrial structural organization and function. Thus our current study in C. elegans is in agreement with the exacerbated ER–mitochondrial communication observed in AD patients as well as in PSEN knockout cells (Area-Gomez et al. 2012). Moreover, our data indicate that this enhanced communication between the ER and mitochondria disrupts mitochondrial function, which leads to poor organismal health.
In addition to regulating ER calcium signaling, PSENs are an essential component of the γ-secretase complex. Two well-characterized substrates for γ-secretase are Notch and APP. Inhibition of the γ-secretase blocks Notch-mediated signaling (De Strooper et al. 1999), thus underscoring the importance that PSEN has in promoting Notch function. Furthermore, γ-secretase-mediated cleavage of APP is crucial for generating Aβ peptides, including the toxic Aβ42 (Hardy and Selkoe 2002; Hardy 2006; Bezprozvanny and Mattson 2008). In this study, we found that inhibiting Notch function or γ-secretase activity by genetic and pharmacological approaches does not lead to mitochondrial morphology defects as observed in SEL-12-deficient animals. Thus, our study provides novel evidence that the role of SEL-12 in impacting mitochondrial function is independent of γ-secretase activity. Moreover, the C. elegans homolog of APP, APL-1, is incapable of forming Aβ plaques since it lacks Aβ peptide sequences and β-secretase recognition sites (Daigle and Li 1993; McColl et al. 2012). Furthermore, no Aβ peptides have been detected in C. elegans (McColl et al. 2012). Thus, the mitochondrial defects that we observe in SEL-12-deficient animals are not due to toxic Aβ peptides. It is noteworthy that many mouse models of AD exhibit cognitive decline well before the appearance of amyloid plaques (Lesne et al. 2008). Similarly in humans, plaque load does not correlate with the degree of dementia, and postmortem analyses of some AD patients with severe memory deficits have shown a lack of plaque formation (Terry et al. 1991). Also, neuroimaging techniques have shown extensive plaque formation in people with no cognitive impairment (Nordberg 2008; Villemagne et al. 2008). Furthermore, mitochondria dysfunction is highly reported in AD models and patients and may occur prior to the formation of amyloid plaques (Swerdlow et al. 2010; Cali et al. 2012; Leuner et al. 2012; Schon and Area-Gomez 2013; Sepulveda-Falla et al. 2014). While these previous studies suggest that amyloid plaques might not be required to cause AD pathogenesis but could be a subsequent consequence of cellular (e.g., mitochondria) dysfunction, they are confounded by the presence of amyloid plaques. However, our study of PSEN function in C. elegans, which lack Aβ peptides, provides clear evidence that the mitochondrial morphology and functional defects observed in sel-12 mutants are mediated by ER calcium dysregulation and not by amyloid plaques.
Neurons are particularly sensitive to mitochondrial function. Indeed, postsynaptic mitochondria clear cytoplasmic calcium by direct uptake of calcium and provide ATP, which is required for calcium extrusion via plasma membrane calcium ATPases or SERCAs. Additionally, mitochondria metabolize ROS species, which can be detrimental to cellular health. In sel-12 mutants, due to irregular calcium homeostasis, we have found evidence of mitochondrial malfunction including loss of mitochondrial membrane potential, elevated ROS, whole-animal susceptibility to ROS, and reduced life span. The generation and accumulation of ROS can lead to neuronal dysfunction and ultimately neurodegeneration. Therefore, understanding the role that PSENs have in mitochondrial function will be crucial to defining its role in AD.
Supplementary Material
Acknowledgments
We thank A. Maricq and A. Fire for reagents and P. McKeown-Longo, Y. Tang, and their lab members for reagents and support. Some nematode strains were provided by the Caenorhabditis Genetics Center, which is funded by National Institutes of Health (NIH) Office of Research Infrastructure Programs (P40 OD010440). The Alzheimer’s Association (NIRG-09-132122) and NIH (GM088213) supported this work.
Footnotes
Communicating editor: M. V. Sundaram
Supporting information is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.115.182808/-/DC1.
Literature Cited
- Altun, Z. F., and D. H. Hall, 2009 Muscle system, introduction. In WormAtlas. Edited for the web by Laura A. Herndon. Last revision: May 2, 2012. DOI: 10.3908/wormatlas.1.6. [Google Scholar]
- Altun, Z. F. and D. H. Hall, 2009 Muscle system, somatic muscle. In WormAtlas. Edited for the web by Laura A. Herndon. Last revision: May 31, 2013. DOI: 10.3908/wormatlas.1.7. [Google Scholar]
- Arduengo P. M., Appleberry O. K., Chuang P., L’Hernault S. W., 1998. The presenilin protein family member SPE-4 localizes to an ER/Golgi derived organelle and is required for proper cytoplasmic partitioning during Caenorhabditis elegans spermatogenesis. J. Cell Sci. 111(Pt 24): 3645–3654. [DOI] [PubMed] [Google Scholar]
- Area-Gomez E., de Groof A. J., Boldogh I., Bird T. D., Gibson G. E., et al. , 2009. Presenilins are enriched in endoplasmic reticulum membranes associated with mitochondria. Am. J. Pathol. 175: 1810–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Area-Gomez E., Del Carmen Lara Castillo M., Tambini M. D., Guardia-Laguarta C., de Groof A. J., et al. , 2012. Upregulated function of mitochondria-associated ER membranes in Alzheimer disease. EMBO J. 31: 4106–4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandara S., Malmersjo S., Meyer T., 2013. Regulators of calcium homeostasis identified by inference of kinetic model parameters from live single cells perturbed by siRNA. Sci. Signal. 6: ra56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beel A. J., Sanders C. R., 2008. Substrate specificity of gamma-secretase and other intramembrane proteases. Cell. Mol. Life Sci. 65: 1311–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I., Mattson M. P., 2008. Neuronal calcium mishandling and the pathogenesis of Alzheimer’s disease. Trends Neurosci. 31: 454–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekemeier K. M., Dempsey M. E., Pfeiffer D. R., 1989. Cyclosporin A is a potent inhibitor of the inner membrane permeability transition in liver mitochondria. J. Biol. Chem. 264: 7826–7830. [PubMed] [Google Scholar]
- Brookes P. S., Yoon Y., Robotham J. L., Anders M. W., Sheu S. S., 2004. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am. J. Physiol. Cell Physiol. 287: C817–C833. [DOI] [PubMed] [Google Scholar]
- Brunello L., Zampese E., Florean C., Pozzan T., Pizzo P., et al. , 2009. Presenilin-2 dampens intracellular Ca2+ stores by increasing Ca2+ leakage and reducing Ca2+ uptake. J. Cell. Mol. Med. 13: 3358–3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cali T., Ottolini D., Brini M., 2012. Mitochondrial Ca(2+) and neurodegeneration. Cell Calcium 52: 73–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereghetti G. M., Stangherlin A., Martins de Brito O., Chang C. R., Blackstone C., et al. , 2008. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc. Natl. Acad. Sci. USA 105: 15803–15808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakroborty S., Goussakov I., Miller M. B., Stutzmann G. E., 2009. Deviant ryanodine receptor-mediated calcium release resets synaptic homeostasis in presymptomatic 3xTg-AD mice. J. Neurosci. 29: 9458–9470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S. L., Mayne M., Holden C. P., Geiger J. D., Mattson M. P., 2000. Presenilin-1 mutations increase levels of ryanodine receptors and calcium release in PC12 cells and cortical neurons. J. Biol. Chem. 275: 18195–18200. [DOI] [PubMed] [Google Scholar]
- Chen B., Liu Q., Ge Q., Xie J., Wang Z. W., 2007. UNC-1 regulates gap junctions important to locomotion in C. elegans. Curr. Biol. 17: 1334–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung K. H., Shineman D., Muller M., Cardenas C., Mei L., et al. , 2008. Mechanism of Ca2+ disruption in Alzheimer’s disease by presenilin regulation of InsP3 receptor channel gating. Neuron 58: 871–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribbs J. T., Strack S., 2007. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep. 8: 939–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordas G., Varnai P., Golenar T., Roy S., Purkins G., et al. , 2010. Imaging interorganelle contacts and local calcium dynamics at the ER-mitochondrial interface. Mol. Cell 39: 121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daigle I., Li C., 1993. apl-1, a Caenorhabditis elegans gene encoding a protein related to the human beta-amyloid protein precursor. Proc. Natl. Acad. Sci. USA 90: 12045–12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B., Beullens M., Contreras B., Levesque L., Craessaerts K., et al. , 1997. Phosphorylation, subcellular localization, and membrane orientation of the Alzheimer’s disease-associated presenilins. J. Biol. Chem. 272: 3590–3598. [DOI] [PubMed] [Google Scholar]
- De Strooper B., Annaert W., Cupers P., Saftig P., Craessaerts K., et al. , 1999. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature 398: 518–522. [DOI] [PubMed] [Google Scholar]
- Detmer S. A., Chan D. C., 2007. Functions and dysfunctions of mitochondrial dynamics. Nat. Rev. Mol. Cell Biol. 8: 870–879. [DOI] [PubMed] [Google Scholar]
- Fire A., Xu S., Montgomery M. K., Kostas S. A., Driver S. E., et al. , 1998. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391: 806–811. [DOI] [PubMed] [Google Scholar]
- Francis R., McGrath G., Zhang J., Ruddy D. A., Sym M., et al. , 2002. aph-1 and pen-2 are required for Notch pathway signaling, gamma-secretase cleavage of betaAPP, and presenilin protein accumulation. Dev. Cell 3: 85–97. [DOI] [PubMed] [Google Scholar]
- Fry A. L., Laboy J. T., Norman K. R., 2014. VAV-1 acts in a single interneuron to inhibit motor circuit activity in Caenorhabditis elegans. Nat. Commun. 5: 5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomotto J., Brouilly N., Walter L., Mariol M. C., Berger J., et al. , 2013. Chemical genetics unveils a key role of mitochondrial dynamics, cytochrome c release and IP3R activity in muscular dystrophy. Hum. Mol. Genet. 22: 4562–4578. [DOI] [PubMed] [Google Scholar]
- Green K. N., Demuro A., Akbari Y., Hitt B. D., Smith I. F., et al. , 2008a SERCA pump activity is physiologically regulated by presenilin and regulates amyloid beta production. J. Gen. Physiol. 132: i1. [DOI] [PubMed] [Google Scholar]
- Green K. N., Demuro A., Akbari Y., Hitt B. D., Smith I. F., et al. , 2008b SERCA pump activity is physiologically regulated by presenilin and regulates amyloid beta production. J. Cell Biol. 181: 1107–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald I., 2012. Notch and the awesome power of genetics. Genetics 191: 655–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S. M., Tsuda H., Yang Y., Vibbert J., Cottee P., et al. , 2012. Secreted VAPB/ALS8 major sperm protein domains modulate mitochondrial localization and morphology via growth cone guidance receptors. Dev. Cell 22: 348–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J., 2006. A hundred years of Alzheimer’s disease research. Neuron 52: 3–13. [DOI] [PubMed] [Google Scholar]
- Hardy J., Selkoe D. J., 2002. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297: 353–356. [DOI] [PubMed] [Google Scholar]
- Ito E., Oka K., Etcheberrigaray R., Nelson T. J., McPhie D. L., et al. , 1994. Internal Ca2+ mobilization is altered in fibroblasts from patients with Alzheimer disease. Proc. Natl. Acad. Sci. USA 91: 534–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Jorgensen E., Hartwieg E., Horvitz H. R., 1999. The Caenorhabditis elegans gene unc-25 encodes glutamic acid decarboxylase and is required for synaptic transmission but not synaptic development. J. Neurosci. 19: 539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D., Allman E., Nehrke K., 2012. Regulation of acid-base transporters by reactive oxygen species following mitochondrial fragmentation. Am. J. Physiol. Cell Physiol. 302: C1045–C1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsh R., You R., Horzempa C., Zheng M., McKeown-Longo P. J., 2014. Regulation of the innate immune response by fibronectin: synergism between the III-1 and EDA domains. PLoS One 9: e102974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Pierce-Shimomura J. T., Oh H. J., Johnson B. E., Goodman M. B., et al. , 2009. The dystrophin complex controls bk channel localization and muscle activity in Caenorhabditis elegans. PLoS Genet. 5: e1000780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipanyula M. J., Contreras L., Zampese E., Lazzari C., Wong A. K., et al. , 2012. Ca2+ dysregulation in neurons from transgenic mice expressing mutant presenilin 2. Aging Cell 11: 885–893. [DOI] [PubMed] [Google Scholar]
- Kitagawa N., Shimohama S., Oeda T., Uemura K., Kohno R., et al. , 2003. The role of the presenilin-1 homologue gene sel-12 of Caenorhabditis elegans in apoptotic activities. J. Biol. Chem. 278: 12130–12134. [DOI] [PubMed] [Google Scholar]
- Kovacs D. M., Fausett H. J., Page K. J., Kim T. W., Moir R. D., et al. , 1996. Alzheimer-associated presenilins 1 and 2: neuronal expression in brain and localization to intracellular membranes in mammalian cells. Nat. Med. 2: 224–229. [DOI] [PubMed] [Google Scholar]
- Lee R. Y., Lobel L., Hengartner M., Horvitz H. R., Avery L., 1997. Mutations in the alpha1 subunit of an L-type voltage-activated Ca2+ channel cause myotonia in Caenorhabditis elegans. EMBO J. 16: 6066–6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leissring M. A., Paul B. A., Parker I., Cotman C. W., LaFerla F. M., 1999. Alzheimer’s presenilin-1 mutation potentiates inositol 1,4,5-trisphosphate-mediated calcium signaling in Xenopus oocytes. J. Neurochem. 72: 1061–1068. [DOI] [PubMed] [Google Scholar]
- Lesne S., Kotilinek L., Ashe K. H., 2008. Plaque-bearing mice with reduced levels of oligomeric amyloid-beta assemblies have intact memory function. Neuroscience 151: 745–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner K., Muller W. E., Reichert A. S., 2012. From mitochondrial dysfunction to amyloid beta formation: novel insights into the pathogenesis of Alzheimer’s disease. Mol. Neurobiol. 46: 186–193. [DOI] [PubMed] [Google Scholar]
- Levitan D., Greenwald I., 1995. Facilitation of lin-12-mediated signalling by sel-12, a Caenorhabditis elegans S182 Alzheimer’s disease gene. Nature 377: 351–354. [DOI] [PubMed] [Google Scholar]
- Levitan D., Greenwald I., 1998. Effects of SEL-12 presenilin on LIN-12 localization and function in Caenorhabditis elegans. Development 125: 3599–3606. [DOI] [PubMed] [Google Scholar]
- Li X., Greenwald I., 1997. HOP-1, a Caenorhabditis elegans presenilin, appears to be functionally redundant with SEL-12 presenilin and to facilitate LIN-12 and GLP-1 signaling. Proc. Natl. Acad. Sci. USA 94: 12204–12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liewald J. F., Brauner M., Stephens G. J., Bouhours M., Schultheis C., et al. , 2008. Optogenetic analysis of synaptic function. Nat. Methods 5: 895–902. [DOI] [PubMed] [Google Scholar]
- Liu P., Ge Q., Chen B., Salkoff L., Kotlikoff M. I., et al. , 2011. Genetic dissection of ion currents underlying all-or-none action potentials in C. elegans body-wall muscle cells. J. Physiol. 589: 101–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Rolland S. G., Conradt B., 2011. A molecular switch that governs mitochondrial fusion and fission mediated by the BCL2-like protein CED-9 of Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 108: E813–E822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney T. R., Luo S., Round E. K., Brauner M., Gottschalk A., et al. , 2008. Intestinal signaling to GABAergic neurons regulates a rhythmic behavior in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 105: 16350–16355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColl G., Roberts B. R., Pukala T. L., Kenche V. B., Roberts C. M., et al. , 2012. Utility of an improved model of amyloid-beta (Abeta(1)(-)(4)(2)) toxicity in Caenorhabditis elegans for drug screening for Alzheimer’s disease. Mol. Neurodegener. 7: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak M., Corbett E. F., Mesaeli N., Nakamura K., Opas M., 1999. Calreticulin: one protein, one gene, many functions. Biochem. J. 344(Pt 2): 281–292. [PMC free article] [PubMed] [Google Scholar]
- Moerman, D. G., and A. Fire, 1997 Muscle: structure, function, and development, Chapter 16 in C. elegans II, edited by D. L. Riddle, T. Blumenthal, B. J. Meyer, and J. R. Priess. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [PubMed] [Google Scholar]
- Nordberg A., 2008. Amyloid plaque imaging in vivo: current achievement and future prospects. Eur. J. Nucl. Med. Mol. Imaging 35(Suppl 1): S46–S50. [DOI] [PubMed] [Google Scholar]
- Pan X., Liu J., Nguyen T., Liu C., Sun J., et al. , 2013. The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat. Cell Biol. 15: 1464–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priess J. R., 2005. Notch signaling in the C. elegans embryo. (June 25, 2005), WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.4.1, http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaraman V., Schulder M., 1999. Postoperative MRI appearance after transsphenoidal pituitary tumor resection. Surg. Neurol. 52: 592–598, discussion 598–599. [DOI] [PubMed] [Google Scholar]
- Schon E. A., Area-Gomez E., 2013. Mitochondria-associated ER membranes in Alzheimer disease. Mol. Cell. Neurosci. 55: 26–36. [DOI] [PubMed] [Google Scholar]
- Sedensky M. M., Siefker J. M., Morgan P. G., 2001. Model organisms: new insights into ion channel and transporter function. Stomatin homologues interact in Caenorhabditis elegans. Am. J. Physiol. Cell Physiol. 280: C1340–C1348. [DOI] [PubMed] [Google Scholar]
- Sepulveda-Falla D., Barrera-Ocampo A., Hagel C., Korwitz A., Vinueza-Veloz M. F., et al. , 2014. Familial Alzheimer’s disease-associated presenilin-1 alters cerebellar activity and calcium homeostasis. J. Clin. Invest. 124: 1552–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington R., Rogaev E. I., Liang Y., Rogaeva E. A., Levesque G., et al. , 1995. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature 375: 754–760. [DOI] [PubMed] [Google Scholar]
- Smith I. F., Hitt B., Green K. N., Oddo S., LaFerla F. M., 2005. Enhanced caffeine-induced Ca2+ release in the 3xTg-AD mouse model of Alzheimer’s disease. J. Neurochem. 94: 1711–1718. [DOI] [PubMed] [Google Scholar]
- Spooner P. M., Bonner J., Maricq A. V., Benian G. M., Norman K. R., 2012. Large isoforms of UNC-89 (obscurin) are required for muscle cell architecture and optimal calcium release in Caenorhabditis elegans. PLoS One 7: e40182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl G., Greenwald I., 1999. Presenilin is required for activity and nuclear access of Notch in Drosophila. Nature 398: 522–525. [DOI] [PubMed] [Google Scholar]
- Stutzmann G. E., Caccamo A., LaFerla F. M., Parker I., 2004. Dysregulated IP3 signaling in cortical neurons of knock-in mice expressing an Alzheimer’s-linked mutation in presenilin1 results in exaggerated Ca2+ signals and altered membrane excitability. J. Neurosci. 24: 508–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow R. H., Burns J. M., Khan S. M., 2010. The Alzheimer’s disease mitochondrial cascade hypothesis. J. Alzheimers Dis. 20(Suppl 2): S265–S279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura H., Hughes A. R., Thastrup O., Putney J. W., Jr, 1989. Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J. Biol. Chem. 264: 12266–12271. [PubMed] [Google Scholar]
- Tedde A., Nacmias B., Ciantelli M., Forleo P., Cellini E., et al. , 2003. Identification of new presenilin gene mutations in early-onset familial Alzheimer disease. Arch. Neurol. 60: 1541–1544. [DOI] [PubMed] [Google Scholar]
- Terry R. D., Masliah E., Salmon D. P., Butters N., DeTeresa R., et al. , 1991. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 30: 572–580. [DOI] [PubMed] [Google Scholar]
- Tu H., Nelson O., Bezprozvanny A., Wang Z., Lee S. F., et al. , 2006. Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer’s disease-linked mutations. Cell 126: 981–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venditti P., Di Stefano L., Di Meo S., 2013. Mitochondrial metabolism of reactive oxygen species. Mitochondrion 13: 71–82. [DOI] [PubMed] [Google Scholar]
- Villemagne V. L., Fodero-Tavoletti M. T., Pike K. E., Cappai R., Masters C. L., et al. , 2008. The ART of loss: Abeta imaging in the evaluation of Alzheimer’s disease and other dementias. Mol. Neurobiol. 38: 1–15. [DOI] [PubMed] [Google Scholar]
- Xu K., Tavernarakis N., Driscoll M., 2001. Necrotic cell death in C. elegans requires the function of calreticulin and regulators of Ca(2+) release from the endoplasmic reticulum. Neuron 31: 957–971. [DOI] [PubMed] [Google Scholar]
- Xu S., Chisholm A. D., 2014. C. elegans epidermal wounding induces a mitochondrial ROS burst that promotes wound repair. Dev. Cell 31: 48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S., Pi H., Chen Y., Zhang N., Guo P., et al. , 2013. Cadmium induced Drp1-dependent mitochondrial fragmentation by disturbing calcium homeostasis in its hepatotoxicity. Cell Death Dis. 4: e540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampese E., Fasolato C., Kipanyula M. J., Bortolozzi M., Pozzan T., et al. , 2011. Presenilin 2 modulates endoplasmic reticulum (ER)-mitochondria interactions and Ca2+ cross-talk. Proc. Natl. Acad. Sci. USA 108: 2777–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Araki S., Wu J., Teramoto T., Chang Y. F., et al. , 2011. An expanded palette of genetically encoded Ca(2)(+) indicators. Science 333: 1888–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.