Abstract
Plasmodium falciparum–induced severe malaria remains a continuing problem in areas of endemicity, with elevated morbidity and mortality. Drugs targeting mechanisms involved in severe malaria pathology, including cytoadhesion of infected red blood cells (RBCs) to host receptors and production of proinflammatory cytokines, are still necessary. Human C1-inhibitor (C1INH) is a multifunctional protease inhibitor that regulates coagulation, vascular permeability, and inflammation, with beneficial effects in inflammatory disease models, including septic shock. We found that human C1INH, at therapeutically relevant doses, blocks severe malaria pathogenic processes by 2 distinct mechanisms. First, C1INH bound to glycan moieties within P. falciparum glycosylphosphatidylinositol (PfGPI) molecules on the parasite surface, inhibiting parasite RBC invasion and proinflammatory cytokine production by parasite-stimulated monocytes in vitro and reducing parasitemia in a rodent model of experimental cerebral malaria (ECM) in vivo. Second, C1INH bound to host CD36 and chondroitin sulfate A molecules, interfering with cytoadhesion of infected RBCs by competitive binding to these receptors in vitro and reducing sequestration in specific tissues and protecting against ECM in vivo. This study reveals that C1INH is a potential therapeutic antimalarial molecule able to interfere with severe-disease etiology at multiple levels through specific interactions with both parasite PfGPIs and host cell receptors.
Keywords: malaria, GPI, C1-inhibitor
Human malaria, caused by Plasmodium parasites, remains a devastating disease, resulting in approximately 600 000 deaths each year [1]. Plasmodium falciparum, the deadliest of species able to infect humans, produces mostly clinically mild infections, but a minority of these can progress into a life-threatening severe disease. Syndromes observed during severe malaria can include severe malaria anemia, metabolic acidosis, shock-like syndrome, acute respiratory distress syndrome, placental malaria, and cerebral malaria [2]. While poorly understood, these syndromes are thought to result from the synergistic effects of multiple processes, including accumulation (sequestration) of infected red blood cells in specific organs, local and systemic parasite-induced proinflammatory cytokine and chemokine production, and immune cell activation, migration, and infiltration into inflamed tissues, including brain [3].
Symptoms of malaria parasite infection are a consequence of the blood stage of the parasite life cycle. In the bloodstream, parasites invade and multiply inside RBCs, followed by bursting and reinvasion of new RBCs. RBC invasion is a dynamic and complex process that depends on the initial interaction between parasite ligands at the surface of the merozoite, known as merozoite surface proteins (MSPs) including MSP-1, and host receptors at the surface of the RBC [4]. After successful invasion of a new RBC, the parasite developmental program includes active remodeling of the host cell membrane by expression of parasite proteins, including adhesion molecules, such as P. falciparum erythrocyte membrane protein 1 (PfEMP1), that are responsible for the binding of infected RBCs to the host microvasculature in different organs, resulting in organ-specific parasite sequestration and dysfunction [5, 6]. The targets of these adhesion molecules contributing to the tissue/organ specificity of sequestering infected RBCs are receptors on host cells, including CD36 and intercellular adhesion molecule-1 (ICAM-1) on endothelial cells (ECs) and chondroitin sulfate A (CSA) specifically on placental cells.
Infected RBCs rupture to liberate merozoites and, at the same time, release soluble bioactive toxins, including P. falciparum glycosylphosphatidylinositol (PfGPI). PfGPIs are abundantly produced by developing parasites to coat the merozoite surface and to anchor membrane proteins [7]. PfGPIs that are on merozoites or released into solution upon infected RBC rupture are key mediators of P. falciparum malaria pathogenicity, mainly owing to their proinflammatory effects on host immune cells mediated by their interaction with Toll-like receptors [8]. The toxic effects of PfGPIs are comparable to those induced by gram-negative lipopolysaccharides (LPSs), likely because of their overall structural similarities [9, 10].
Human C1-inhibitor (C1INH), a highly glycosylated protein secreted by the liver into the plasma, is a serine protease inhibitor (serpin) with a broad role in regulation of coagulation, vascular permeability, and inflammation, through inactivation of a variety of proteases, including complement system proteases, contact system (kallikrein-kinin system) proteases, and intrinsic coagulation and fibrinolytic proteases [11]. In addition to these functions, C1INH interacts with a variety of substances independent of its protease inhibitor activity, including extracellular matrix components; the complement component C3; E- and P-selectins on ECs and leukocytes [12, 13]; infectious gram-negative bacteria, such as Escherichia coli, Salmonella typhimurium, Bordetella pertussis, and Serratia marcescens [14, 15]; and gram-negative endotoxin [16, 17].
Plasma-derived human C1INH treatment has a beneficial effect in several inflammatory disease models, including myocardial, hepatic, muscle, brain, and intestinal ischemia and reperfusion injury, thermal injury, sepsis, and septic shock induced by gram-negative endotoxin [15, 18–25]. The effectiveness of human C1INH in endotoxic shock is largely a result of binding to and neutralization of lipid A within the endotoxin LPS [16]. C1INH can also protect against inflammatory insults such as ischemia and reperfusion injury via binding to E- and P-selectins and inhibiting leukocyte-EC adhesion [12, 23].
Here we found that plasma-derived human C1INH therapy can inhibit malaria parasite growth, sequestration, and associated disease pathology through 2 distinct mechanisms involving the novel interaction between C1INH and PfGPIs, as well as competition between infected RBCs and C1INH for host cell receptors involved in cytoadhesion and sequestration.
MATERIALS AND METHODS
Parasites
P. falciparum lines (3D7, FUP, FCR3-CD36, FCR3-CSA, and ItG-ICAM1) were cultured as previously described [50]. Late schizonts were concentrated using gelatin flotation.
Immunofluorescence
Fixed, permeabilized infected RBCs or free, fixed, nonpermeabilized P. falciparum merozoites were incubated with plasma-derived human C1INH (Berinert; CSL Behring) at 100 µg/mL. C1INH was detected using rabbit polyclonal antibody (Dako) at a dilution of 1:1000. P. falciparum MSP-1 was detected using 1:200 dilutions of the monoclonal antibody (mAb) MRA-94 (MR4; ATCC). Specific antibodies were detected with Alexa Fluor 488–labeled goat anti-rabbit immunoglobulin G (IgG) or Alexa Fluor 594–labeled goat anti-mouse (Molecular Probes) at a dilution of 1:1000. The parasite nucleus was stained with DAPI 5 µg/mL (Invitrogen). Slides were analyzed using a fluorescence or confocal microscope.
Inhibition of RBC Invasion
Schizonts of P. falciparum FUP or 3D7 strains were cultured with new RBCs in the presence of different concentrations of C1INH or ovalbumin. After 1 and 2 cycles of invasion, the percentage of infected RBCs was established microscopically.
Western Blot
P. falciparum schizonts were suspended in denaturing Laemmli buffer and kept at –80°C until use. Parasite extracts were separated on 4%–20% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and proteins were transferred onto a nitrocellulose membrane (Invitrogen) and blocked overnight. The blot was incubated for 1 hour with C1INH or bovine serum albumin at 200 µg/mL. C1INH was detected with mouse anti-C1INH mAb 288-05 (Abcam) at a dilution of 1:10 000 and goat anti-mouse IgG antibodies conjugated to alkaline phosphatase (Southern Biotech) at a dilution of 1:6000. Bands were visualized with a staining solution (Roche Applied Science).
Binding of C1INH to PfGPI Fragments
A microarray PfGPI chip system with synthetic PfGPI glycan fragments of different lengths has been described [26]. Microarrays were incubated with human C1INH (200 μg/mL) and detected using rabbit polyclonal antibody (Dako) at a dilution of 1:1000. Specific antibodies were detected with Alexa Fluor 488-labeled goat anti-rabbit IgG (Molecular Probes) at a dilution of 1:1000. Slides were read on a GenePix Personal 4100A (Axon Instruments) microarray scanner at 532 nm.
Cytokine Output
Human monocytes in Roswell Park Memorial Institute 1640 medium plus 10% human serum were cocultured with live, P. falciparum schizont or parasites extracts (2 cycles of freezing and thawing) at a cell to parasite ratio of 1:40 in the presence of different concentrations of C1INH. Control monocytes were exposed to equivalent numbers of noninfected RBCs or noninfected RBC extracts. After 18 hours, supernatant levels of interleukin 1β (IL-1β), interleukin 6 (IL-6), and tumor necrosis factor α (TNF-α) were measured by flow cytometry with a cytometric bead array kit (BD Bioscience).
Adhesion Assay
Infected RBCs were added to the C32 human amelanotic melanoma cell line (ATCC CRL-1585), to Chinese hamster ovary (CHO) cells expressing human CD36 (ATCC CRL-2092), or to ICAM-1 (ATCC CRL-2093) cell cultures at the same time or 30 minutes after addition of different concentrations of human C1INH. Bound infected RBCs were fixed, stained, and counted microscopically. The percentage of adherent infected erythrocytes, compared with that among controls without C1INH, was calculated.
C1INH Binding to CD36 and CSA
CHO-CD36 or CHO-CSA cells were incubated with fluorescent-labeled C1INH at 100 µg/mL. After 1 hour at 37°C, cells were washed and fixed, and fluorescence was evaluated. Inhibition of the binding was performed by preincubation of fluorescent-labeled C1INH with soluble recombinant human CD36 (R&D Systems) or soluble CSA (Sigma) at 100 µg/mL for 30 minutes at 37°C before incubation with CHO cells.
In Vivo Parasite Sequestration
Twenty female C57BL6 mice aged 8–10 weeks from Jackson Laboratories were infected with transgenic Plasmodium berghei strain ANKA expressing luciferase/green fluorescent protein under a constitutive promoter [31], and blood samples were collected on day 5 after infection. Late schizonts were concentrated from an overnight culture. Five million schizonts were intravenously injected into each mouse, together with or 30 minutes after intravenous injection of vehicle or 260 µg/mouse of human C1INH. Three hours later, mice were euthanized and perfused with cold saline intracardially. Naive mice were used as controls. The organs were harvested, weighed, and homogenized in an equal volume of luciferase activity assay buffer per milligram of tissue (Invitrogen). Equal volumes were mixed with luciferin substrate and measured in a 96-well luminometer (Biotek Synergy 2).
Experimental Cerebral Malaria
Female C57BL6 mice aged 8–10 weeks were infected with 0.5 million P. berghei strain ANKA–parasitized RBCs/ mouse intravenously. Mice were intravenously injected with vehicle or 260 µg/mouse of C1INH twice daily during days 3–9 after infection. Survival and peripheral parasitemia frequencies were monitored daily. In a separate experiment, infected and vehicle- or C1INH-treated mice were euthanized on day 7 after infection and perfused intracardially with saline, and their brains were harvested, sectioned, and stained with hematoxylin-eosin for histological analysis. Naive mice were used as controls.
Statistical Analysis
Statistical comparison between test and control groups was performed using the 1-way analysis of variance parametric test. A P value of < .05 was considered statistically significant; a P value of < .005 was considered highly statistically significant.
Ethics Statement
This study was performed in strict accordance with the recommendations of the Public Health Services Policy on Humane Care and Use of Laboratory Animals, with all efforts made to minimize animal suffering. The protocols were approved by the Immune Disease Institute Animal Care and Use Committee (IACUC) and the Harvard Medical Area IACUC, both of which are accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
RESULTS
C1INH Binds at the Surface of P. falciparum Merozoites
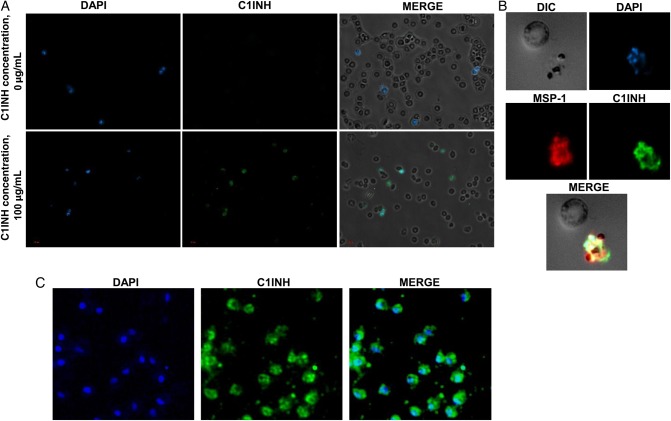
Based on the ability of human C1INH to neutralize LPS [16], together with the structural similarities between LPS and malaria GPIs [10, 9] coating Plasmodium merozoites [7], we hypothesized that C1INH could bind to the surface of this blood-stage invasive form of the parasite. Permeabilized infected and noninfected RBCs were incubated with C1INH, and its binding was detected with specific fluorochrome-conjugated antibodies. Microscopy revealed the specific binding of C1INH to infected cells (Figure 1A). Colocalization of the parasite protein MSP-1, expressed at the merozoite surface, with C1INH in permeabilized infected RBCs (Figure 1B) but not at the membrane of infected or noninfected RBCs (Figure 1A and 1B) further suggested the interaction of C1INH with the parasite membrane. Furthermore, free, fixed, nonpermeabilized P. falciparum merozoites were prepared and exposed to human C1INH. Detection with specific antibodies established that human C1INH binds at the surface of free P. falciparum merozoites (Figure 1C).
Figure 1.
Human C1-inhibitor (C1INH) binds at the surface of Plasmodium falciparum merozoites. A, Fluorescent microscopy shows specific binding of human C1INH to infected red blood cells (RBCs). Permeabilized infected and uninfected RBCs were incubated with (lower panels) or without (upper panels) a 100-µg/mL solution of human C1INH. Nuclei were stained with DAPI. Binding of human C1INH (green) was detected with specific antibodies. B, Microscopy findings show the binding of human C1INH inside an infected RBC upon permeabilization. No binding was detected on uninfected RBCs or at the membrane of infected cells. The parasite nucleus is stained blue, the parasite merozoite surface protein-1 (MSP-1) is stained red, and human C1INH is stained green. The corresponding merge between the green, blue, and red channels is also shown (C). Fluorescent microscopy findings of free, fixed, nonpermeabilized P. falciparum merozoites demonstrates binding of C1INH to the merozoite surface. The left panel shows nuclear staining with DAPI (blue). C1INH binding was detected and stained green (middle panel). The corresponding merge between the green and the blue channels is shown in the right panel.
C1INH Suppresses Plasmodium Merozoite Invasion
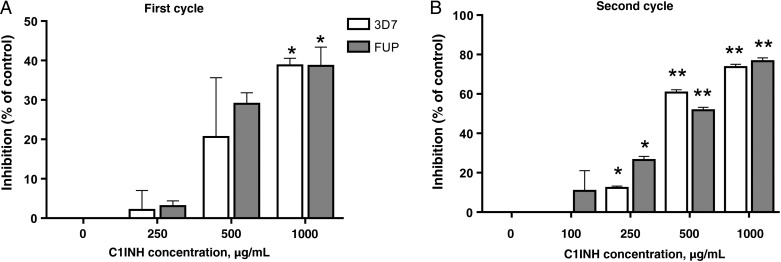
Invasion of RBCs requires an initial weak and reversible contact between the merozoite outer layer and the target cell, possibly involving GPIs and/or GPI-anchored proteins [4]. We investigated whether the binding of human C1INH at the surface of merozoites influenced their ability to infect new target cells. Cultures of RBCs infected with mature P. falciparum stages (ie, schizonts) were exposed to increasing concentrations of C1INH or ovalbumin as a control, and the percentage of invasion inhibition, relative to findings for ovalbumin-treated cultures, was calculated after the first (24 hours later; Figure 2A) and second (72 hours later; Figure 2B) cycles of reinvasion. Parasite invasion was significantly inhibited by C1INH in a dose-dependent manner from the first invasion cycle. A dramatic inhibition of >75%, relative to controls, was achieved after 2 cycles of reinvasion in the presence of 1 mg/mL of C1INH. Similar results were obtained with 2 different P. falciparum strains (3D7 and FUP; Figure 2A and 2B), strongly suggesting that, after rupture of infected RBCs, C1INH is able to bind at the merozoite surface and prevent reinvasion.
Figure 2.
Human C1-inhibitor (C1INH) suppresses Plasmodium falciparum merozoite invasion of red blood cells (RBCs). A and B, RBC invasion inhibition after 1 (A) and 2 (B) cycles of in vitro culture of 2 different P. falciparum strains (FUP and 3D7) in the presence of different concentrations of human C1INH. Results are expressed as the percentage inhibition relative to ovalbumin controls. The figures show 1 representative experiment from 3 that were performed. Error bars show standard errors of the mean. *P < .05 and **P < .0001, versus control.
C1INH Binds Glycan Moieties Within the P. falciparum GPI Molecule
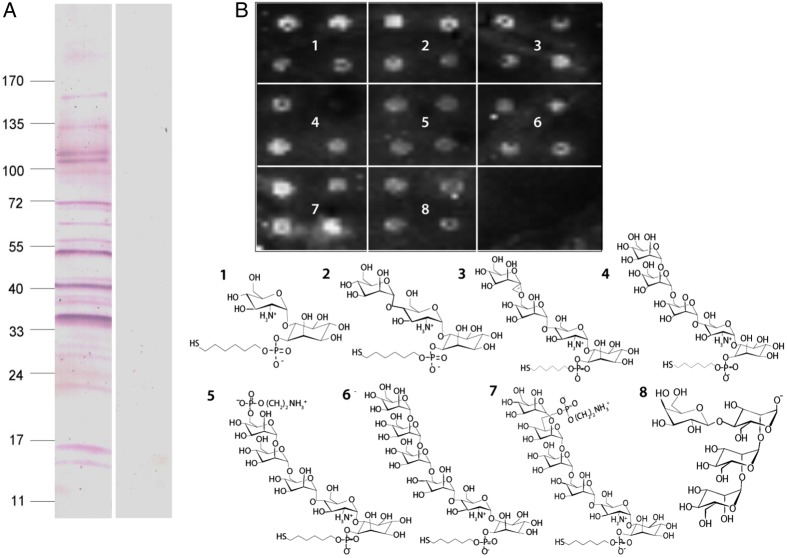
We next asked whether the binding to the merozoite surface is mediated by a direct interaction between C1IHN and PfGPIs or by GPI-anchored proteins at the outer leaflet of the plasma membrane [7]. Western blots of crude P. falciparum schizont extracts revealed binding of C1INH to multiple molecular species (Figure 3A), consistent with either nonspecific binding to multiple different proteins or more-specific interaction with multiple proteins via a shared GPI moiety. To test the latter possibility, we used a microarray PfGPI chip system in which picomolar amounts of synthetic PfGPI glycan fragments of different lengths are attached to the surface of glass slides [26]. C1INH bound all the PfGPI glycan fragments regardless of their length (Figure 3B1–7), as well as a non-PfGPI oligomannan structure (Figure 3B8). This is consistent with C1INH binding to GPI themselves, rather than to surface-bound, GPI-anchored parasite proteins.
Figure 3.
Human C1-inhibitor (C1INH) binds glycan moieties within the Plasmodium falciparum glycosylphosphatidylinositol (GPI) molecule. A, Extracts of schizont-stage P. falciparum were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto a nitrocellulose membrane. The membrane was incubated with C1INH, and binding was detected using specific anti-C1INH antibodies and antibodies conjugated to alkaline phosphatase. Controls incubated with bovine serum albumin were performed in parallel. The positions of molecular size markers are indicated on the left (values are in kDa). B, Fluorescent readout (upper panel) of C1INH binding to 7 PfGPI glycan fragments of different lengths (panels 1–7) and a non-PfGPI oligomannan structure (panel 8) spotted in quadruplicates, using a synthetic glycan microarray. A scheme of the molecular structure of the fragments used is shown (lower panel).
C1INH Reduces P. falciparum–Induced Proinflammatory Response
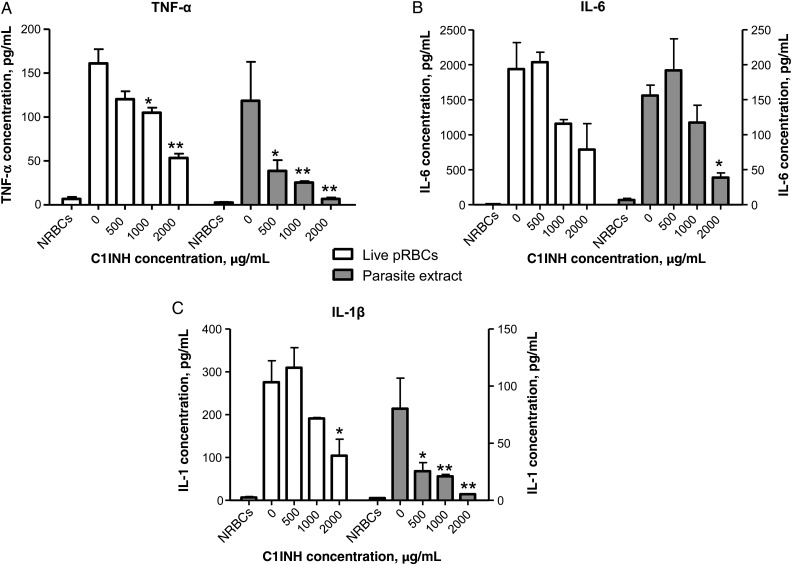
PfGPIs present on merozoites or released in solution upon infected RBC rupture are the major parasite-derived inducers of proinflammatory cytokine production by immune cells [8] associated with the pathology of severe malaria [3, 27]. We next asked whether the interaction between C1INH and PfGPIs affected the proinflammatory activity of blood-stage parasites. To this end, human peripheral blood monocytes were coincubated with RBCs infected with live schizont-stage P. falciparum, or with crude extracts derived from these infected RBCs, for 18 hours in the presence of different concentrations of C1INH, and proinflammatory cytokine levels were measured in the supernatant. Noninfected RBCs or derived extracts were used as negative controls. TNF-α, IL-1β, and IL-6 were all reduced in a dose-dependent manner by C1INH, with an approximately 50%–70% reduction at the highest concentration of 2 mg/mL, using live P. falciparum schizonts, and an approximately 70%–85% reduction, using parasite extracts (Figure 4A–C). These results demonstrate that C1INH decreased the production of parasite-induced pathogenic cytokines by monocytes, presumably via interaction with PfGPIs in the context of either infected RBC extracts or infected RBC bursting and release of soluble parasite material.
Figure 4.
Human C1-inhibitor (C1INH) neutralizes parasite-induced production of proinflammatory cytokines in vitro. A–C, Red blood cells infected with live schizont-stage Plasmodium falciparum (pRBCs; white bars) or schizont-stage parasite extracts (gray bars) were incubated in triplicates with human monocytes for 18 hours at a monocyte to parasite ratio of 1:40. The production of tumor necrosis factor α (TNF-α; A), interleukin 6 (IL-6; B), and interleukin 1β (IL-1β; C) was measured. Noninfected RBCs (NRBCs) were used as negative controls. Cumulative data are means and standard errors of the mean of 3 different experiments. *P < .05 and **P < .005, versus no C1INH.
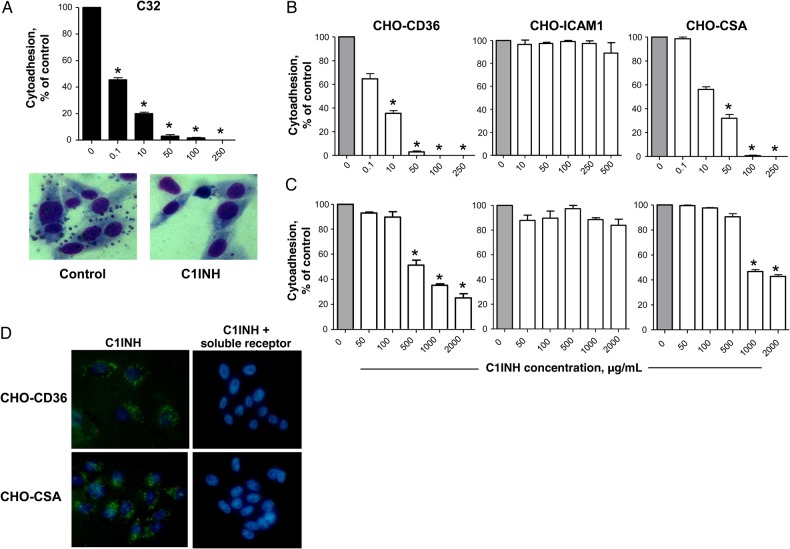
C1INH Blocks P. falciparum–Infected RBC Adhesion to CD36 and CSA
C1INH inhibits leukocyte adhesion to ECs by competitive interaction with E- and P-selectins [12]. P. falciparum–infected RBCs can adhere to host ECs via interaction with cell-surface molecules, including ICAM-1, CD36, and CSA, leading to parasite sequestration [3, 28]. We thus tested the ability of C1INH to interfere with interactions between infected RBCs and host cell receptors in in vitro adhesion assays. We measured adhesion of RBCs infected with the FCR3 P. falciparum strain to C32 human amelanotic melanoma cells expressing multiple host receptors (CD36, ICAM-1, CSA, and thrombospondin) [29, 30]. Adhesion was blocked by C1INH in a dose-dependent manner (Figure 5A). To identify the specificity of host receptor involvement, C1INH-dependent inhibition was tested in adhesion assays with CHO cell lines expressing high levels of human CD36 (CHO-CD36), CSA (CHO-K1), or ICAM-1 (CHO-ICAM-1) and the corresponding P. falciparum strains specific for these cell-surface antigens (FCR3-CD36, FCR3-CSA, and ItG-ICAM1, respectively). Prior addition of C1INH to these CHO cell lines blocked the specific adhesion of infected RBCs to CD36 and CSA in a concentration-dependent manner but did not affect adhesion via ICAM-1 (Figure 5B). Of note, C1INH blocked the cytoadhesion of infected RBCs to the same receptors even when it was added at the same time as parasites, albeit at higher concentrations (Figure 5C). To test the ability of C1INH to competitively inhibit infected RBC adhesion by direct binding to CD36 and CSA host receptors, fluorescence-conjugated C1INH was incubated with CHO cells expressing CD36 or CSA molecules. Fluorochrome-conjugated C1INH bound to CD36- and CSA-expressing CHO cells (Figure 5D, left panels). This binding was abrogated by prior incubation of the fluorescent C1INH with the corresponding soluble receptor (Figure 5D, right panels), demonstrating a direct interaction of C1INH with CD36 and CSA molecules.
Figure 5.
Human C1-inhibitor (C1INH) blocks Plasmodium falciparum–infected red blood cell (RBC) cytoadhesion to host receptors via binding to CD36 and chondroitin sulfate A (CSA). A, Percentage of C32 cells adherent to infected RBCs in the presence of different concentrations of C1INH added at the same time as parasites, compared with controls without C1INH. Data represent mean values and standard errors of the mean of 3 experiments performed. *P < .001, vs control (no C1INH). An image demonstrating infected RBC adherence to C32 cells (left panel) and blocking of adhesion by C1INH (right panel) is also shown. B and C, Percentage of cytoadhesion, relative to that of controls, among RBCs infected with parasites selected to specifically adhere to CD36, ICAM-1, or CSA expressed on Chinese hamster ovary (CHO) cells when human C1INH is added before (B) or concurrently with (C) infected cells. D, Microscopy findings showing the binding of fluorochrome-conjugated human C1INH at the surface of CHO cells (left panels) expressing CD36 (upper panels) or CSA (lower panels). Prior incubation of the fluorescent C1INH with soluble CD36 or CSA abrogates the binding to CHO cells (right panels).
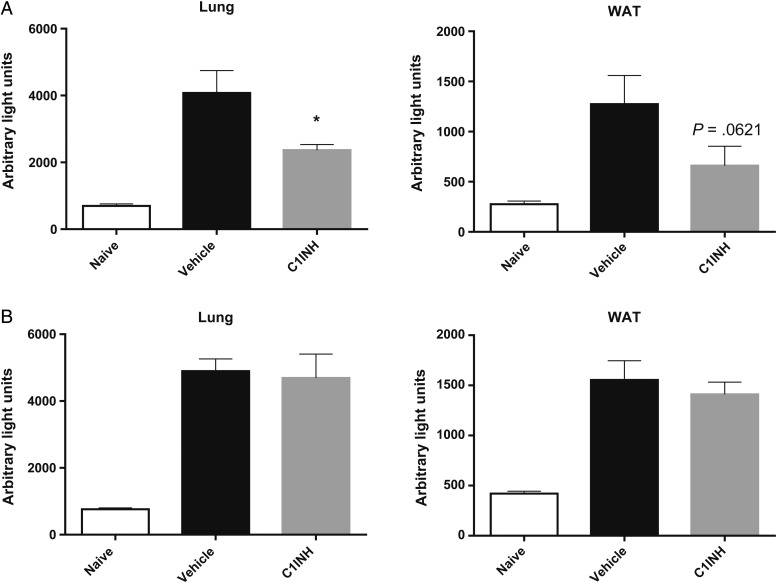
C1INH Reduces CD36-Dependent Parasite Sequestration In Vivo
RBCs infected with P. berghei strain ANKA schizonts are known to adhere to mouse white adipose tissue (WAT) and lung microvasculature in a CD36-dependent manner [31]. We next evaluated the capacity of human C1INH to inhibit CD36-dependent sequestration of infected RBCs in vivo. RBCs harboring mature luciferase-expressing P. berghei strain ANKA were injected intravenously into mice concurrently with or 30 minutes after treatment with vehicle or with 260 µg of human C1INH. Three hours later, mice were intracardially perfused with saline, and WAT and lungs were harvested to measure the ex vivo luciferase activity in organ homogenates as a proxy of the parasite burden in these organs [32]. Intravenous administration of human C1INH before but not concurrently with injection of parasites resulted in reduced luciferase activity in lungs of mice, relative to findings for vehicle-treated controls; a similar trend was observed in WAT (Figure 6A and 6B). These results demonstrate the ability of human C1INH to suppress infected RBC sequestration in vivo, likely via a direct interaction with mouse CD36 at the surface of lung and WAT microvascular ECs.
Figure 6.
Human C1-inhibitor (C1INH) reduces CD36-dependent parasite sequestration in vivo. A, Parasite-derived luciferase signal as a readout of CD36-dependent sequestration of mature luciferase-expressing Plasmodium berghei strain ANKA in lung and white adipose tissue (WAT). Infected cells were injected intravenously in mice previously (A) or concurrently (B) treated with vehicle or 260 µg/mouse of human C1INH. Parasite-derived luciferase signal was measured 3 hours later in harvested organs after perfusion.
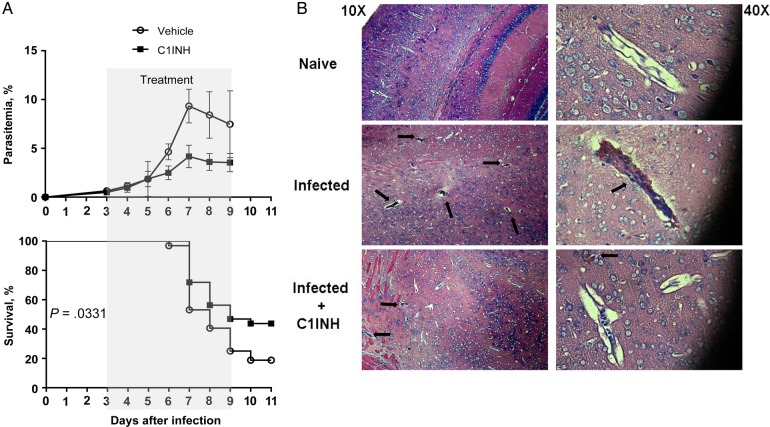
C1INH Protects Against Experimental Cerebral Malaria
Infection of susceptible C57BL/6 mice with P. berghei strain ANKA results in neurovascular dysfunction and death due in part to accumulation of infected RBCs and leukocytes in brain microvasculature, combined with an exaggerated proinflammatory response [33]. This model of experimental cerebral malaria (ECM) mimics multiple aspects of human cerebral malaria [34]. Based on its ability to suppress parasite-induced proinflammatory cytokines in vitro (Figure 4) and parasite cytoadhesion and sequestration in vitro and in vivo, respectively (Figures 5 and 6), we next evaluated the effect of C1INH treatment on ECM outcome. Mice infected with P. berghei strain ANKA were treated intravenously with vehicle or 260 µg of human C1INH twice daily during days 3–9 after infection. The frequency of peripheral parasitemia was reduced by the third day of treatment, and overall survival significantly improved in mice treated with C1INH, compared with control mice that received vehicle (Figure 7A). Histological analysis of brain sections after perfusion revealed that C1INH treatment induced a drastic reduction in the number of microvessels obstructed with parasitized RBCs and leukocytes by day 7 after infection. Brains from C1INH-treated animals were similar to those from naive, uninfected mice, explaining in part the survival advantage of C1INH-treated mice (Figure 7B). Together, these data suggest that protection from ECM upon treatment may result from the activity of C1INH against multiple pathogenic mechanisms leading to severe malaria.
Figure 7.
Human C1-inhibitor (C1INH) protects against experimental cerebral malaria. A, Peripheral parasitemia and survival of mice injected intravenously with Plasmodium berghei strain ANKA on day 0 and treated with vehicle or 260 µg/mouse of human C1INH twice daily during days 3–9 after infection. Cumulative data are means and standard errors of the mean of 3 different experiments. Sample size: 32 mice per group. B, Representative images of hematoxylin-eosin–stained section of brain tissue from naive and infected mice treated with vehicle or human C1INH as described in panel A and harvested on day 7 after infection, after perfusion. Black arrows show brain microvessels obstructed with leukocytes and infected and uninfected red blood cells. Original magnifications ×10 (left) and ×40 (right) are shown.
DISCUSSION
We are the first to demonstrate that human C1INH interacts directly with PfGPIs. Functionally, this binding at the surface of the merozoite significantly decreased invasion of RBCs by merozoites and blunted the proinflammatory response of host immune cells to intact infected RBCs or infected RBC extracts. We also found that C1INH binds specific host cell surface proteins and competitively inhibits infected RBC adhesion via these same molecules. These 2 mechanisms of C1INH action on parasite GPIs and host cell receptor molecules likely combined to prevent severe malaria symptoms in an ECM model in vivo.
During merozoite invasion of RBCs, an initial weak and reversible contact between the coat surrounding the merozoite surface and the RBC is established. This process requires recognition of RBCs by parasite ligands, including GPI-anchored parasite proteins [4]. The binding of C1INH to GPIs at the merozoite surface may be sufficient to perturb such interactions or to otherwise prevent the molecular processes associated with RBC invasion.
C1INH was found to bind directly to glycan moieties within PfGPIs, which results in neutralization of the parasite toxin proinflammatory activity. However, this binding was not specific to Plasmodium GPIs, since a control oligomannan structure found on Leishmania also produced a signal, pointing to a lectin-like activity of C1INH as responsible for the interaction observed. The importance of neutralizing malarial toxins has been highlighted in a recent study showing that neutralizing antibodies directed against parasite GPIs prevented pathology and mortality in an ECM model, promoting the idea of an antitoxin-based malaria treatment [35]. Importantly, GPI molecules appear to be essential for parasite survival [36, 37], and their structure is conserved among different geographic isolates of P. falciparum [38]. Further studies will be necessary to fully characterize the interaction with and subsequent neutralization of PfGPIs by C1INH.
Human C1INH can also interfere with leukocyte adhesion and rolling by competitive binding to selectins at the surface of vascular ECs [12, 18]. Here we found that C1INH could also block the adhesion of infected RBCs to host cells via specific cell surface receptors in vitro, including CD36 and CSA but not ICAM-1. In vivo, this action reduced CD36-dependent accumulation of infected RBCs in highly sequestering tissues. This mechanism of C1INH action likely contributed to the observed reduction in peripheral parasitemia and the protection from ECM-associated neuropathology.
While our data indicate that binding of C1INH to host and parasite molecules occurs independently, neuroprotection in the ECM model in vivo was likely due to the combined effects of these molecular interactions. For example, binding of C1INH to GPIs inhibited reinvasion of new RBCs, reducing parasite expansion, but it also reduced cytokine expression by host immune cells, protecting against pathological local and systemic inflammation. By preventing CD36-dependent cytoadhesion of infected RBCs, C1INH also likely prevented sequestration-dependent local inflammation and promoted clearance of infected RBCs in spleen, further reducing overall parasite burden.
Endogenous C1INH is present in human plasma at a mean concentration of about 250 µg/mL, and the concentration can increase up to 2.5-fold during an acute inflammatory response [21]. Why is this level unable to control P. falciparum malaria symptoms in humans? Activation of the complement and coagulation cascades resulting in an increased C1INH antigen/activity ratio occur during severe malaria [39–42]. This could lead to a functional decrease in C1INH levels in plasma below those required for its potential antimalarial activities. Consistent with this possibility, low plasma levels of active C1INH are observed in patients with severe malaria and may contribute to the severity of the disease [43, 44]. Also possible is the existence of a mechanism of C1INH neutralization by malaria parasites, such as the C1INH-inactivating elastase reported in Pseudomonas aeruginosa [45].
The expanding resistance of malaria parasites to antimalarial drugs emphasizes the urgent need for therapeutic approaches directed against >1 pathogenic mechanism, as demonstrated by C1INH. Induction of resistance by therapeutic interventions such as C1INH is unlikely because the potential targets are highly conserved parasite molecules or of host origin. Of note, the high C1INH concentrations used in our experiments have been safely reached in multiple experimental models of acute inflammation [24, 46], as well as in patients [47–49]. Further work is necessary to establish its beneficial effect in people infected with P. falciparum.
In conclusion, this study reveals 2 distinct molecular mechanisms by which a novel antimalarial compound, C1INH, can prevent pathology associated with severe malaria and may pave the way for developing affordable derivatives with improved efficacy.
Notes
Acknowledgments. We thank Dr J. L. Perignon (Institut Pasteur, Paris, France), for helpful discussions and suggestions associated with this work; Dr J. Smith (SBRI, Seattle, WA), for providing ItG-ICAM1 and FCR3-CSA parasites; Dr A. Moreno (Pitie-Salpetriere Hospital, Paris, France), for P. berghei strain ANKA parasites; and the Malaria Research and Reference Reagent Resource, American Type Culture Collection, for providing 3D7A parasites.
Financial support. This work was supported by the National Institutes of Health (grants AI057366 and HD22082), the Harvard T. H. Chand School of Public Health (Yerby postdoctoral fellowship to P. M.), the Max-Planck Society (funding to P. H. S. and F. K.), the Swiss National Science Foundation (grant 205320-120260 to P. H. S.), and the Infectious Disease Program of the Singapore-MIT Alliance for Research and Technology (support to M. D.-S.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization (WHO). WHO malaria report 2014. Geneva: WHO, 2014. [Google Scholar]
- 2.Mackintosh CL, Beeson JG, Marsh K. Clinical features and pathogenesis of severe malaria. Trends Parasitol 2004; 20:597–603. [DOI] [PubMed] [Google Scholar]
- 3.Schofield L, Grau GE. Immunological processes in malaria pathogenesis. Nat Rev Immunol 2005; 5:722–35. [DOI] [PubMed] [Google Scholar]
- 4.Cowman AF, Crabb BS. Invasion of red blood cells by malaria parasites. Cell 2006; 124:755–66. [DOI] [PubMed] [Google Scholar]
- 5.Miller LH, Baruch DI, Marsh K, Doumbo OK. The pathogenic basis of malaria. Nature 2002; 415:673–9. [DOI] [PubMed] [Google Scholar]
- 6.Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science 1996; 272:1502–4. [DOI] [PubMed] [Google Scholar]
- 7.Gilson PR, Nebl T, Vukcevic D et al. Identification and stoichiometry of glycosylphosphatidylinositol-anchored membrane proteins of the human malaria parasite Plasmodium falciparum. Mol Cell Proteomics 2006; 5:1286–99. [DOI] [PubMed] [Google Scholar]
- 8.Gowda DC. TLR-mediated cell signaling by malaria GPIs. Trends Parasitol 2007; 23:596–604. [DOI] [PubMed] [Google Scholar]
- 9.Caro HN, Sheikh NA, Taverne J, Playfair JH, Rademacher TW. Structural similarities among malaria toxins insulin second messengers, and bacterial endotoxin. Infect Immun 1996; 64:3438–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jakobsen PH, Baek L, Jepsen S. Demonstration of soluble Plasmodium falciparum antigens reactive with Limulus amoebocyte lysate and polymyxin B. Parasite Immunol 1988; 10:593–606. [DOI] [PubMed] [Google Scholar]
- 11.Davis AE III, Cai S, Liu D. The biological role of the C1 inhibitor in regulation of vascular permeability and modulation of inflammation. Adv Immunol 2004; 82:331–63. [DOI] [PubMed] [Google Scholar]
- 12.Cai S, Davis AE III. Complement regulatory protein C1 inhibitor binds to selectins and interferes with endothelial-leukocyte adhesion. J Immunol 2003; 171:4786–91. [DOI] [PubMed] [Google Scholar]
- 13.Chang NS, Boackle RJ, Leu RW. Characterization of C1 inhibitor binding to neutrophils. Immunology 1991; 73:95–101. [PMC free article] [PubMed] [Google Scholar]
- 14.Mejia P, Davis AE. C1 inhibitor suppresses the endotoxic activity of a wide range of lipopolysaccharides and interacts with live gram-negative bacteria. Shock 2012; 38:220–5. [DOI] [PubMed] [Google Scholar]
- 15.Davis AE, Lu F, Mejia P. C1 inhibitor, a multi-functional serine protease inhibitor. Thromb Haemost 2010; 104:886–93. [DOI] [PubMed] [Google Scholar]
- 16.Liu D, Cai S, Gu X, Scafidi J, Wu X, Davis AE III. C1 inhibitor prevents endotoxin shock via a direct interaction with lipopolysaccharide. J Immunol 2003; 171:2594–601. [DOI] [PubMed] [Google Scholar]
- 17.Mejia P, Davis AE III. C1 inhibitor suppresses the endotoxic activity of a wide range of lipopolysaccharides and interacts with live gram-negative bacteria. Shock 2012; 38:220–5. [DOI] [PubMed] [Google Scholar]
- 18.Bergamaschini L, Gobbo G, Gatti S et al. Endothelial targeting with C1-inhibitor reduces complement activation in vitro and during ex vivo reperfusion of pig liver. Clin Exp Immunol 2001; 126:412–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Storini C, Rossi E, Marrella V et al. C1-inhibitor protects against brain ischemia-reperfusion injury via inhibition of cell recruitment and inflammation. Neurobiol Dis 2005; 19:10–7. [DOI] [PubMed] [Google Scholar]
- 20.Caliezi C, Wuillemin WA, Zeerleder S, Redondo M, Eisele B, Hack CE. C1-Esterase inhibitor: an anti-inflammatory agent and its potential use in the treatment of diseases other than hereditary angioedema. Pharmacol Rev 2000; 52:91–112. [PubMed] [Google Scholar]
- 21.Davis AE, Mejia P, Lu F. Biological activities of C1 inhibitor. Mol Immunol 2008; 45:4057–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buerke M, Murohara T, Lefer AM. Cardioprotective effects of a C1 esterase inhibitor in myocardial ischemia and reperfusion. Circulation 1995; 91:393–402. [DOI] [PubMed] [Google Scholar]
- 23.Lu F, Chauhan AK, Fernandes SM, Walsh MT, Wagner DD, Davis AE III. The effect of C1 inhibitor on intestinal ischemia and reperfusion injury. Am J Physiol Gastrointest Liver Physiol 2008; 295:G1042–9. [DOI] [PubMed] [Google Scholar]
- 24.Jansen PM, Eisele B, de Jong IW et al. Effect of C1 inhibitor on inflammatory and physiologic response patterns in primates suffering from lethal septic shock. J Immunol 1998; 160:475–84. [PubMed] [Google Scholar]
- 25.Liu D, Lu F, Qin G, Fernandes SM, Li J, Davis AE III. C1 inhibitor-mediated protection from sepsis. J Immunol 2007; 179:3966–72. [DOI] [PubMed] [Google Scholar]
- 26.Kamena F, Tamborrini M, Liu X et al. Synthetic GPI array to study antitoxic malaria response. Nat Chem Biol 2008; 4:238–40. [DOI] [PubMed] [Google Scholar]
- 27.de Souza JB, Riley EM. Cerebral malaria: the contribution of studies in animal models to our understanding of immunopathogenesis. Microbes Infect 2002; 4:291–300. [DOI] [PubMed] [Google Scholar]
- 28.Baruch DI, Ma XC, Singh HB, Bi X, Pasloske BL, Howard RJ. Identification of a region of PfEMP1 that mediates adherence of Plasmodium falciparum infected erythrocytes to CD36: conserved function with variant sequence. Blood 1997; 90:3766–75. [PubMed] [Google Scholar]
- 29.Pouvelle B, Buffet PA, Lépolard C, Scherf A, Gysin J. Cytoadhesion of Plasmodium falciparum ring-stage-infected erythrocytes. Nat Med 2000; 6:1264–8. [DOI] [PubMed] [Google Scholar]
- 30.Eda S, Eda K, Prudhomme JG, Sherman IW. Inhibitory activity of human lactoferrin and its peptide on chondroitin sulfate A-, CD36-, and thrombospondin-mediated cytoadherence of Plasmodium falciparum-infected erythrocytes. Blood 1999; 94:326–32. [PubMed] [Google Scholar]
- 31.Franke-Fayard B, Janse CJ, Cunha-Rodrigues M et al. Murine malaria parasite sequestration: CD36 is the major receptor, but cerebral pathology is unlinked to sequestration. Proc Natl Acad Sci U S A 2005; 102:11468–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mejia P, Treviño-Villarreal JH, Hine C et al. Dietary restriction protects against experimental cerebral malaria via leptin modulation and T cell mTORC1 suppression. Nat Comm 2015; 6:6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amante FH, Haque A, Stanley AC et al. Immune-mediated mechanisms of parasite tissue sequestration during experimental cerebral malaria. J Immunol 2010; 185:3632–42. [DOI] [PubMed] [Google Scholar]
- 34.de Souza JB, Hafalla JC, Riley EM, Couper KN. Cerebral malaria: why experimental murine models are required to understand the pathogenesis of disease. Parasitology 2010; 137:755–72. [DOI] [PubMed] [Google Scholar]
- 35.Schofield L, Hewitt MC, Evans K, Siomos MA, Seeberger PH. Synthetic GPI as a candidate anti-toxic vaccine in a model of malaria. Nature 2002; 418:785–9. [DOI] [PubMed] [Google Scholar]
- 36.Naik RS, Davidson EA, Gowda DC. Developmental stage-specific biosynthesis of glycosylphosphatidylinositol anchors in intraerythrocytic Plasmodium falciparum and its inhibition in a novel manner by mannosamine. J Biol Chem 2000; 275:24506–11. [DOI] [PubMed] [Google Scholar]
- 37.Sanders PR, Kats LM, Drew DR et al. A set of glycosylphosphatidyl inositol-anchored membrane proteins of Plasmodium falciparum is refractory to genetic deletion. Infect Immun 2006; 74:4330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berhe S, Schofield L, Schwarz RT, Gerold P. Conservation of structure among glycosylphosphatidylinositol toxins from different geographic isolates of Plasmodium falciparum. Mol Biochem Parasitol 1999; 103:273–8. [DOI] [PubMed] [Google Scholar]
- 39.Roestenberg M, McCall M, Mollnes TE et al. Complement activation in experimental human malaria infection. Trans R Soc Trop Med Hyg 2007; 101:643–9. [DOI] [PubMed] [Google Scholar]
- 40.Nyakoe NK, Taylor RP, Makumi JN, Waitumbi JN. Complement consumption in children with Plasmodium falciparum malaria. Malar J 2009; 8:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wenisch C, Spitzauer S, Florris-Linau K et al. Complement activation in severe Plasmodium falciparum malaria. Clin Immunol Immunopathol 1997; 85:166–71. [DOI] [PubMed] [Google Scholar]
- 42.Helegbe GK, Goka BQ, Kurtzhals JA et al. Complement activation in Ghanaian children with severe Plasmodium falciparum malaria. Malar J 2007; 6:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clemens R, Pramoolsinsap C, Lorenz R, Pukrittayakamee S, Bock HL, White NJ. Activation of the coagulation cascade in severe falciparum malaria through the intrinsic pathway. Br J Haematol 1994; 87:100–5. [DOI] [PubMed] [Google Scholar]
- 44.Arinola G, Ezeh C. C1 inhibitor, C3 activator, IgG, IgA, and IgM titers in Nigerian sickle cell disease patients with Plasmodium falciparum. Iran J Immunol 2007; 4:44–9. [PubMed] [Google Scholar]
- 45.Catanese J, Kress LF. Enzymatic inactivation of human plasma C1-inhibitor and alpha 1-antichymotrypsin by Pseudomonas aeruginosa proteinase and elastase. Biochim Biophys Acta 1984; 789:37–43. [DOI] [PubMed] [Google Scholar]
- 46.Birnbaum J, Klotz E, Spies CD et al. The combinations C1 esterase inhibitor with coagulation factor XIII and N-acetylcysteine with tirilazad mesylate reduce the leukocyte adherence in an experimental endotoxemia in rats. Clin Hemorheol Microcirc 2008; 40:167–76. [PubMed] [Google Scholar]
- 47.Igonin AA, Protsenko DN, Galstyan GM et al. C1-esterase inhibitor infusion increases survival rates for patients with sepsis. Crit Care Med 2012; 40:770–7. [DOI] [PubMed] [Google Scholar]
- 48.Dorresteijn MJ, Visser T, Cox LA et al. C1-esterase inhibitor attenuates the inflammatory response during human endotoxemia. Crit Care Med 2010; 38:2139–45. [DOI] [PubMed] [Google Scholar]
- 49.de Zwaan C, Kleine AH, Diris JH et al. Continuous 48-h C1-inhibitor treatment, following reperfusion therapy, in patients with acute myocardial infarction. Eur Heart J 2002; 23:1670–7. [DOI] [PubMed] [Google Scholar]
- 50.Trager W, Jensen JB. Human malaria parasites in continuous culture. Science 1976; 193:673–5. [DOI] [PubMed] [Google Scholar]