Abstract
The humoral response to human immunodeficiency virus (HIV) remains incompletely understood. In this report, we describe biased λ light chain use during the HIV Env glycoprotein (Env) response in HIV infection and vaccination. We examined HIV Env binding (and neutralization) in the context of light chain use in subjects with acute HIV infection, chronic HIV infection, and among HIV vaccinees. In all populations tested, there was a λ chain bias for HIV Env binding antibodies, compared with other HIV antigens (such as p24) or tetanus toxoid. In subjects with chronic HIV infection, a λ bias was noted for neutralization, with λ antibodies accounting for up to 90% of all neutralization activity observed. This is the first report of antibody function in a human infection being tied to light chain use. In HIV infection, antibodies expressing λ light chains tended to have longer CDRL3s, increased light chain contact with HIV Env, and less hypermutation in the heavy chain, compared with antibodies using the κ light chain. These data also support an evolutionary model for the understanding the various κ to λ light chain ratios observed across species and suggest that the λ light chain bias against HIV provides the host an advantage in developing a more efficient humoral response.
Keywords: HIV, humoral immunity, neutralization, antibody, light chain
While there is a good understanding of light chain selection in B-cell development, little is known beyond the phenomenological level about widely variant light chain bias among different species or about its functional significance. For example, the κ to λ light chain (κ/λ) ratio in laboratory mice is 20:1, whereas the ratio is reversed in sheep and 1.9:1 in humans [1]. Moreover, for many years the use of one light chain over another was not thought or known to confer any selective advantage [2–4]. However, reports of light chain bias toward select antigens have emerged over the years, most recently including a λ bias for the V2 epitope of human immunodeficiency virus (HIV) Env, which have led to the hypothesis that antibody function may be related to this bias [5–16]. This idea is still not widely accepted, as different antibody gene families can be selected for against the same antigen [17].
We have been studying the HIV-specific antibody response directly from the plasma of multiple HIV type 1 (HIV)–infected subjects, including elite neutralizers [18–20]. In this study, we investigated the importance of the light chain in the HIV humoral response to Env.
METHODS
Subjects and Samples
Plasma samples were obtained from the following groups: (1) 30 subjects with chronic HIV infection due to clade B (the chronic cohort) [19, 20]; (2) 15 subjects with acute HIV infection due to CRF02_AG and clade G (the acute cohort) [21]; and (3) and 30 subjects from HIV Vaccine Trials Network (HVTN) 205, and 15 subjects from HVTN 094 (the vaccine cohort). All subjects in the vaccine cohort received the GeoVax GOVX-B11, composed of a DNA prime and modified vaccinia Ankara virus boost. The demographic and additional details are found in Table 1 and Supplementary Methods. All studies were approved by institutional review boards, and all individuals provided written informed consent.
Table 1.
Demographic and Clinical Characteristics of the 3 Cohorts
| Characteristic | Chronic (n = 30) | Vaccine (n = 45) | Acute (n = 15) |
|---|---|---|---|
| Location | United States | United States | Nigeria |
| HIV subtype | Clade B | Clade B | Clade G and CRF02_AG |
| Age, y | 43.5 (22–54) | 26 (18–45) | 31 (18–40) |
| Race/ethnicity | |||
| African | 0 | 0 | 100 |
| African American | 80 | 4.4 | 0 |
| Hispanic | 0 | 24.4 | 0 |
| Mixed | 0 | 4.4 | 0 |
| White | 20 | 66.7 | 0 |
| Sex | |||
| Male | 66.7 | 46.7 | 46.7 |
| Female | 33.3 | 53.3 | 53.3 |
| Risk factor for HIV infection | NA | na | |
| Sex | 56.7 | … | … |
| Injection drug use | 43.3 | … | … |
| HIV load, RNA copies/mL | 2233 (<48–6 215 519) | NA | 114 715 (400–513 269) |
| CD4+ T-cell count, cells/µL | 604 (3–1778) | NA | na |
Data are % of subjects or median value (range), unless otherwise indicated. Subjects in the chronic cohort are HIV-infected individuals from Baltimore who are not receiving highly active antiretroviral therapy (n = 30) and include persons with broad HIV neutralization (n = 6), persons with restricted neutralization (n = 18), and persons with no neutralization (n = 6). Subjects in the vaccine cohort are individuals from the HIV Vaccine Trials Network 094 and 205 (n = 45). Subjects in the acute cohort are Nigerian HIV-infected individuals with a diagnosis of acute HIV infection (n = 15), 11 of whom were also followed for at least 1 year after infection.
Abbreviations: HIV, human immunodeficiency virus; NA, not applicable; na, not available.
Proteins and Reagents
Tetanus toxoid antigen was purchased from VWR (Radnor, Pennsylvania), and p24 (3B) was obtained from the National Institutes of Health AIDS Reagents program. Recombinant HIV antigens were expressed by transient transfection of 293T cells, purified by lectin affinity chromatography and dialyzed against PBS, as previously described [22].
Immunoglobulin G (IgG) Purification and Fractionation
Plasma was sequentially purified on a protein A affinity chromatography column (GE Healthcare, Piscataway, New Jersey), an anti-human IgG1 column (Capture Select, Naarden, Netherlands), and an anti-human κ and λ column (Capture Select) to yield purified IgG1 κ and λ fractions. Alternatively, plasma was sequentially purified on a protein A affinity chromatography column and gp120 column to yield anti-gp120 antibodies. Finally, plasma was sequentially purified on a protein A affinity chromatography column, gp120 column, and anti-human κ and λ columns, to yield purified anti-gp120 κ and λ fractions. Details can be found in the Supplementary Methods.
Enzyme-Linked Immunosorbent Assay (ELISA)
Direct and capture ELISAs were modified from those previously described [18] to calculate κ/λ ratios by measuring the κ and λ antibody concentrations in plasma or purified antibody and the binding κ/λ ratio in plasma or purified antibody against tetanus toxoid, p24, and gp120. For direct measurement of κ and λ concentrations, a modified human IgG ELISA was developed by coating half the plates with mouse anti-human κ and half with mouse anti-human λ antibodies at 1 µg/mL (Southern Biotech; Birmingham, Alabama), with human myeloma IgG1 κ and λ of known concentration (Sigma Aldrich, St. Louis, Missouri) as controls. For measurement of binding κ/λ ratios against various antigens, the antigens were either directly coated (tetanus toxoid at 0.5 µg/mL, p24 at 1 µg/mL) or captured (BaL-gp120 at a concentration of 0.15 µg/mL) by antibody D7324 (Cliniqa, San Marcos, California) adsorbed to the solid phase at 2 µg/mL. Details can be found in the Supplementary Methods.
Multiplex IgG Assay
The multivariate Luminex assay was developed using a panel of HIV antigens coupled to carboxylated fluorescent beads, as described previously [23]. The panel includes 10 monomeric gp120s, SOSSIP trimer, gp140, gp41, gag p24, RT p51, RT p66, and Vif. Additional details can be found in the Supplementary Methods.
Neutralization Assays
HIV neutralization testing was performed using a luciferase-based assay in TZM.bl cells as previously described [19, 24]. IgG1 κ, IgG1 λ, anti-gp120 κ, and anti-gp120 λ were tested against SF162.LS, 6535.3, RHPA4259.7, REJO4541.67, and MuLV control. Affinity purified samples from subjects with broad HIV neutralization underwent additional testing (equal starting concentrations) against tier 2 clade B pseudoviruses, determined by individual neutralization profiles [19] and sample quantities. Additional details can be found in the Supplementary Methods.
HIV Neutralizing Monoclonal Antibody (mAb) Analysis
The National Center for Biotechnology Information (NCBI) protein database (available at: http://www.ncbi.nlm.nih.gov/protein) was searched for the string “HIV neutralizing antibody” in humans, and those having neutralization in the parent manuscripts were selected. In a separate analysis, 34 HIV Env-reactive mAbs from the HIV GSK PRO HIV-002 study were analyzed [25]. Sequences of heavy and light chains of mAbs were compared to germ line sequences from the IMGT database (available at: http://www.imgt.org) and analyzed for heavy chain CDR3 length, CDRL3 length, and the percentage of amino acid changes in κ versus λ expressing mAbs. Additional details can be found in Supplementary Methods.
Analysis of Antibody-Antigen Contact in κ and λ Anti-HIV Env mAbs
The protein database (available at: http://www.rcsb.org/) was searched for the complexes of anti-HIV Env mAbs with their antigens to calculate the number of heavy and light chain contacts to Env in mAbs. Both the gp120/gp41 protein and peptide antigens were included, and 25 κ and 24 λ mAbs were analyzed. Additional details can be found in the Supplementary Methods.
Statistics
For data with a normal distribution, the Student t test was performed; otherwise, the Mann–Whitney test was used (Mann–Whitney was also used for log-transformed data). All P values were 2-tailed and considered statistically significant if they were <.05. All data were analyzed with GraphPad Prism software (San Diego, California).
RESULTS
Measurement of the κ/λ Ratio on the Basis of κ and λ Antibody Concentrations in Plasma and Purified IgG
We sought to determine the κ/λ ratios in plasma and gp120-specific fractions in the chronic and vaccine cohorts, using an assay that only measures concentration of κ and λ antibodies. In chronic HIV infection, the mean κ/λ ratio in plasma was 1.82 (range, 0.95–3.74), while the mean ratio in affinity-purified anti-gp120 samples was 1.293 (range, 0.09–2.96; P = .002 by the paired t test). Within this cohort, individuals with broad neutralizing antibodies, those with restricted neutralization, and nonneutralizers all exhibited a λ bias in the κ/λ ratio of anti-gp120 samples, and there was no relation between age, race, sex, CD4+ T-cell count, or viral load on the κ/λ ratio (data not shown).
In the vaccine cohort, the mean κ/λ ratio in plasma was 1.83 (range, 0.64–3.68), and the mean ratio in the affinity-purified anti-gp120 fraction was 0.66 (range, 0.12–0.97), which was significant by the paired t test (P = .0001). A summary of this λ bias in the amount of antibodies produced against HIV Env is presented in Figure 1.
Figure 1.
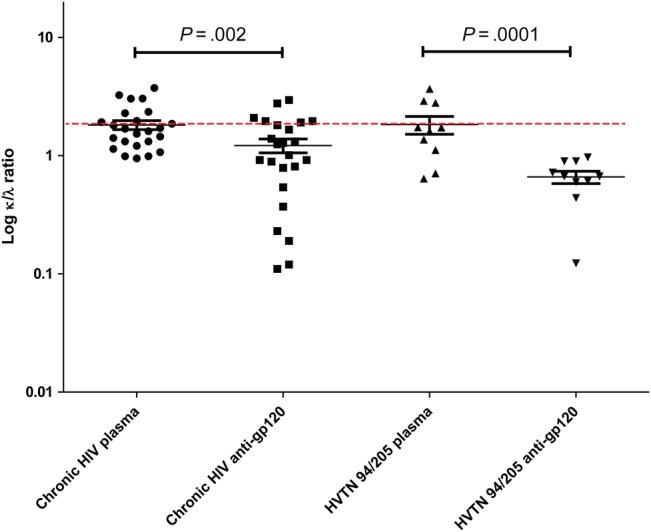
Measurement of the κ to λ light chain (κ/λ) ratio by concentrations from plasma and prepared immunoglobulin (IgG). The concentration of κ and λ antibodies was determined and the κ/λ ratio calculated in plasma and anti-gp120 affinity-purified antibody (see “Materials and Methods” section). In all populations tested, there was a λ bias in the amount of anti-gp120 antibody present, compared with the total antibody response. Data are for 30 patients with chronic human immunodeficiency virus (HIV; 24 had paired samples and were used for analysis) and 19 HIV Vaccine Trials Network (HVTN) vaccinees (10 had paired samples used for analysis). HVTN 94/205 plasma was collected before vaccination (these also serve as HIV-negative controls), while the HVTN 94/205 anti-gp120 were collected after vaccination. The dotted line represents a κ/λ ratio of 1.9 (mean value of plasma κ/λ ratio described in humans). The paired t test was used for analysis.
Measurement of the Binding κ/λ Ratio Against Tetanus Toxoid, p24, and gp120 Antigens From Plasma
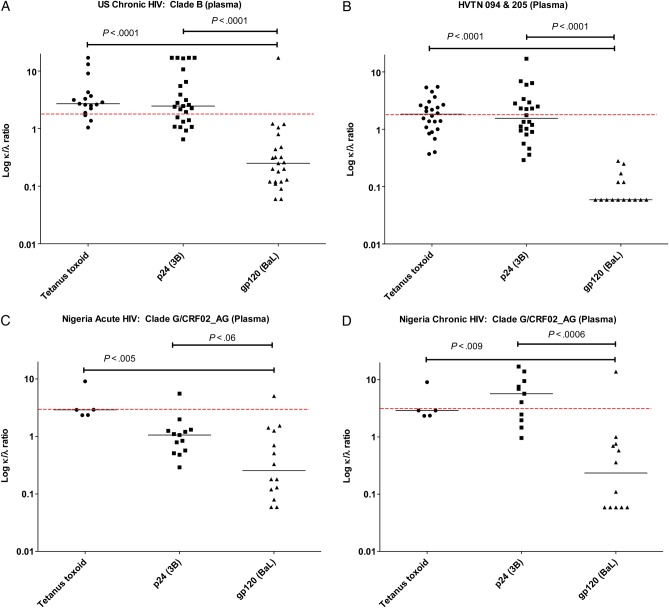
To determine whether the λ bias observed among gp120-specific antibodies was specific to this antigen, we measured κ/λ ratios to gp120, p24, and tetanus toxoid in a binding assay dependent on both the concentration and affinity of antibodies in the chronic, cohort, and acute cohorts. In all groups (including the subgroups of broad neutralizers, restricted neutralizers, and nonneutralizers in the chronic cohort), the binding κ/λ ratio of anti-gp120 antibodies had a λ bias, compared with tetanus toxoid and p24. This difference was significant when comparing responses of gp120 to those of tetanus toxoid and p24 in the chronic cohort (P < .0001 and P < .0001, respectively) and the vaccine cohort (P < .0001 and P < .0001, respectively). This result was also significant between responses to gp120 and those to tetanus toxoid in the acute cohort (P = .005) during acute HIV infection, as well between responses to gp120 and those to both tetanus toxoid and p24 in the acute cohort during the chronic phase of HIV infection (P = .009 and P = .0006, respectively). A summary of these findings is shown in Figure 2.
Figure 2.
Measurement of the κ to λ light chain (κ/λ) ratio against tetanus toxoid, p24, and gp120 antigens from plasma. Plasma from each subject was tested by enzyme-linked immunosorbent assay (ELISA) against the 3 antigens to calculate the κ/λ ratio of reactivity against each antigen for subjects with reactive plasma (see “Materials and Methods” section). In all populations tested, there was a λ bias in the reactivity of plasma to human immunodeficiency virus (HIV) Env, compared with p24 and tetanus toxoid. A, Data are for 27 US subjects with chronic HIV infection. B, Data are for 30 HIV Vaccine Trials Network (HVTN) 094 and 205 vaccinees. C and D, Data are for 14 Nigerian patients during acute HIV infection (C) and chronic HIV infection (D). Plotted data are limited to patients with positive ELISA samples, and Nigerian patients were only tested for tetanus toxoid at 1 time point (although graphed for both the acute and chronic phases of infection). Assays were run in duplicate. The dotted line represents a κ/λ ratio of 1.9 (mean value of plasma κ/λ ratio described in humans). For data with a normal distribution, the Student t test was performed; otherwise, the Mann–Whitney U test was used.
Measurement of the κ/λ Ratio Against HIV Antigens From IgG1 Samples
To determine whether the λ bias was related to a specific HIV Env or a property of HIV Envs in general, we tested samples against a panel of 22 HIV antigens (15 Env related) in a multiplex assay. In the chronic cohort, κ/λ ratios against a panel of HIV antigens were measured by comparing IgG1 κ to IgG1 λ antibodies in the multiplex assay dependent on affinity (fractions adjusted for concentration). Again, a λ bias against all the HIV Envs tested was noted (Figure 3). A similar pattern of λ bias against HIV Env was noted in the multiplex assay when whole plasma or affinity-purified anti-gp120 antibody was tested (data not shown). We tested the statistical significance of the IgG1 K/L ratio from one of these envelopes (BaL-gp120) against the nonenvelope antigens and found the following: p24 3b (P < .0001), RT p51 (P < .0001), RT p66 (P = .0001), Vif (P = .003), Gp41 HXB2 (P = .025), and protease p7 (P = .35).
Figure 3.
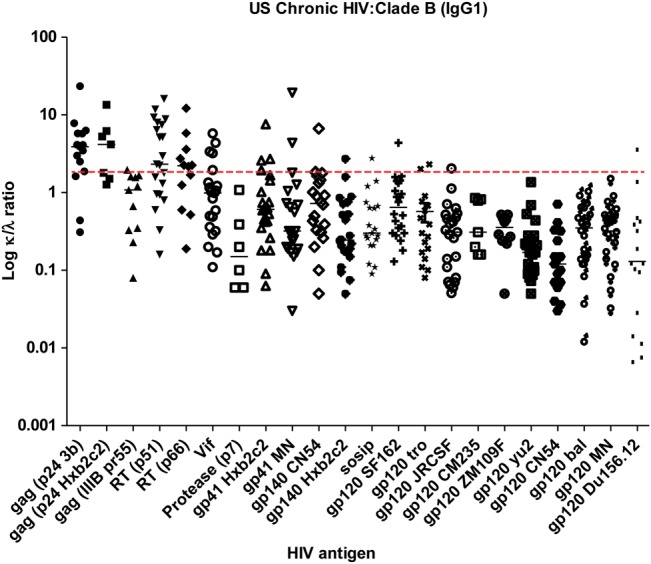
κ to λ light chain (κ/λ) ratio of the immunoglobulin G1 (IgG1) fraction for an expanded set of human immunodeficiency virus (HIV) antigens. Fractionated IgG1 from each subject (n = 25) in the chronic cohort was tested at a known concentration against a multiplexed panel of HIV antigens to calculate the κ/λ ratio for each antigen. The log κ/λ ratio is presented for each antigen tested for samples that were positive. There was a λ bias in the affinity of the IgG1 toward all HIV Envs tested, compared with most other antigens, including gag p24, RT p51, RT p66, and vif. Assays were run in duplicate. The dotted line represents a κ/λ ratio of 1.9, the mean value of the plasma κ/λ ratio described in humans.
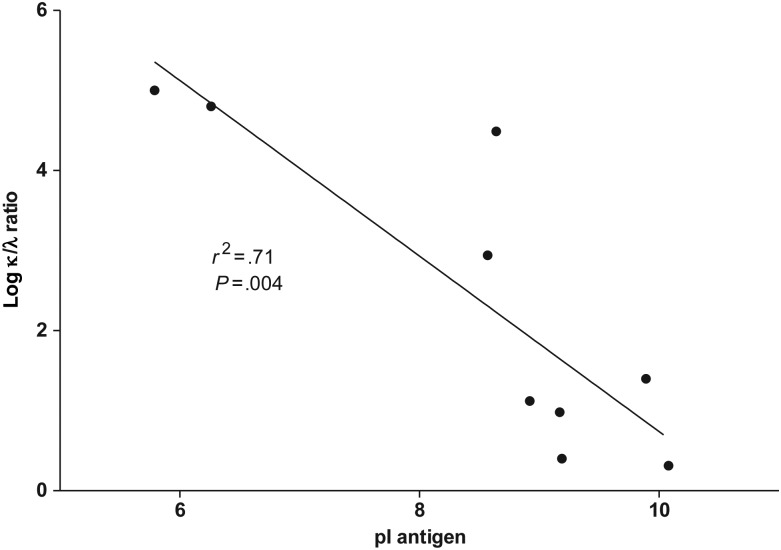
To see whether a correlation existed between the κ/λ ratio and isoelectric point (pI) of each antigen, the mean κ/λ ratios for various antigens were plotted against calculated isoelectric points of each antigen. There was an inverse correlation between antigen pI and κ/λ ratio (P = .004; r2 = 0.71; Figure 4).
Figure 4.
Isoelectric point (pI) of various antigens and κ to λ light chain (κ/λ) ratio. Isoelectric points of various antigens were calculated (available at: http://web.expasy.org/compute_pi/) on the basis of published sequences and compared with mean κ/λ ratios for each antigen. Antigens include tetanus toxoid (pI 5.79) and HIV antigens p24 (pI 6.26), p66 (pI 8.57), p51 (pI 8.64), pr55 (pI 9.17), gp41 (pI 8.92), gp160 (pI 8.96)0, gp120 (pI 9.19), vif (pI 9.89), and p7 (pI 10.08).There was a correlation between the pI of antigen and the κ/λ ratio (a higher pI was associated with a lower κ/λ ratio; P = .004; r2 = .71).
Measurement of Neutralization From κ and λ Fractionated IgG1 and anti-gp120 Antibody Samples
In subjects in the chronic cohort, IgG1 κ and IgG1 λ and anti-gp120 κ and anti-gp120 λ were tested by neutralization against a panel of HIV pseudoviruses. Compared with IgG1 κ antibodies, the IgG1 λ fraction demonstrated significantly enhanced activity, based on the 50% inhibitory concentration (IC50), against the tier 1 virus SF162.LS (P = .007) and the combined tier 2 panel (P = .011; Figure 5A). Among the IgG1 fraction, this difference is consistent with λ antibodies accounting for 85.2% of the tier 1 activity and 90% of the tier 2 activity (see “Materials and Methods” section for the formula). Likewise, among a mixture of all subclasses, anti-gp120 λ demonstrated significantly enhanced activity by IC50 against the tier 1 virus SF162.LS (P = .001) and the combined tier 2 panel (P = .03; Figure 5B). Thus, λ antibodies accounted for 85% of the tier 1 activity and 83.3% of the tier 2 activity in the gp120 fraction. In a separate experiment, the anti-gp120 κ and λ antibodies from 4 subjects with broad HIV neutralization activity were tested against a panel of tier 2 and 3 viruses. In 3 subjects, anti-gp120 λ antibodies had lower IC50 values for the tested viruses (P = .02, P = .001, and P = .0003), whereas the fourth subject had the reverse pattern (P = .0005; this was the only subject among those tested in this study who demonstrated this pattern; Figure 5C).
Figure 5.
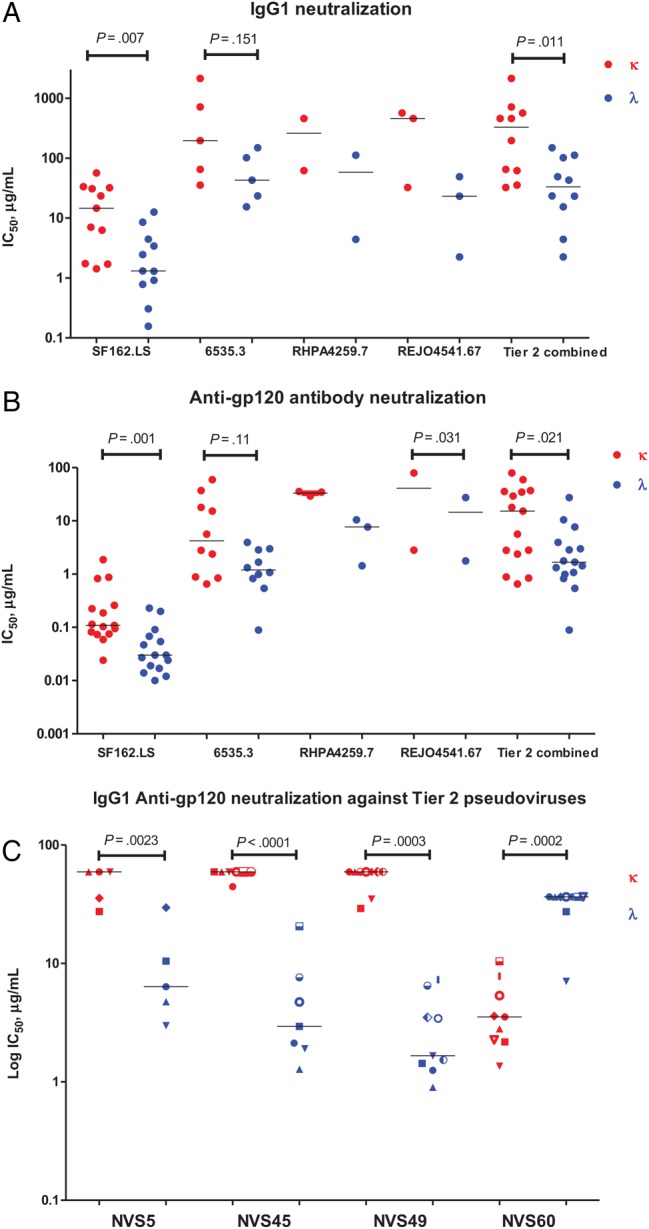
Neutralization of light chain fractionated samples against clade B tier 1 and tier 2 human immunodeficiency virus (HIV) pseudoviruses. Paired patient immunoglobulin G (IgG) samples were tested for neutralizing activity against the indicated pseudoviruses as described in “Materials and Methods” section. Assays were run in duplicate. The x-axis demonstrates pseudovirus or patient tested. The 50% inhibitory concentration (IC50) titer was calculated as the immunoglobulin concentration that caused a 50% reduction in relative luminescence units (RLU), compared with the virus control wells, after subtraction of cell control RLU (left y-axis). For panels A and B, each circle represents individual patient fractions (red represents κ fractions, and blue represents λ fractions), and for panel C, symbols represent individual pseudoviruses (red represents κ fractions, and blue represents λ fractions). Combined tier 2 refers to IC50 values against 6535.3, RHPA4259.7, and REJO4541. Sixty-seven pseudoviruses were plotted together. A, IgG1 κ and λ fraction testing (n = 15) reveals more potency for neutralization (ie, a lower IC50) in λ fractions. B, Anti-gp120 κ and λ fraction testing (n = 15) reveals more potency for neutralization in λ fractions. C, Anti-gp120 IgG1 κ and λ fractions from 4 patients with known broad neutralizing activity (NVS5, NVS45, NVS49, and NVS60) were tested at equal starting concentrations against an individualized panel of known tier 2 pseudoviruses their plasma has activity against. Three of 4 patients demonstrated more-potent neutralization in the λ fraction.
Differences in κ and λ Anti-HIV Env mAbs
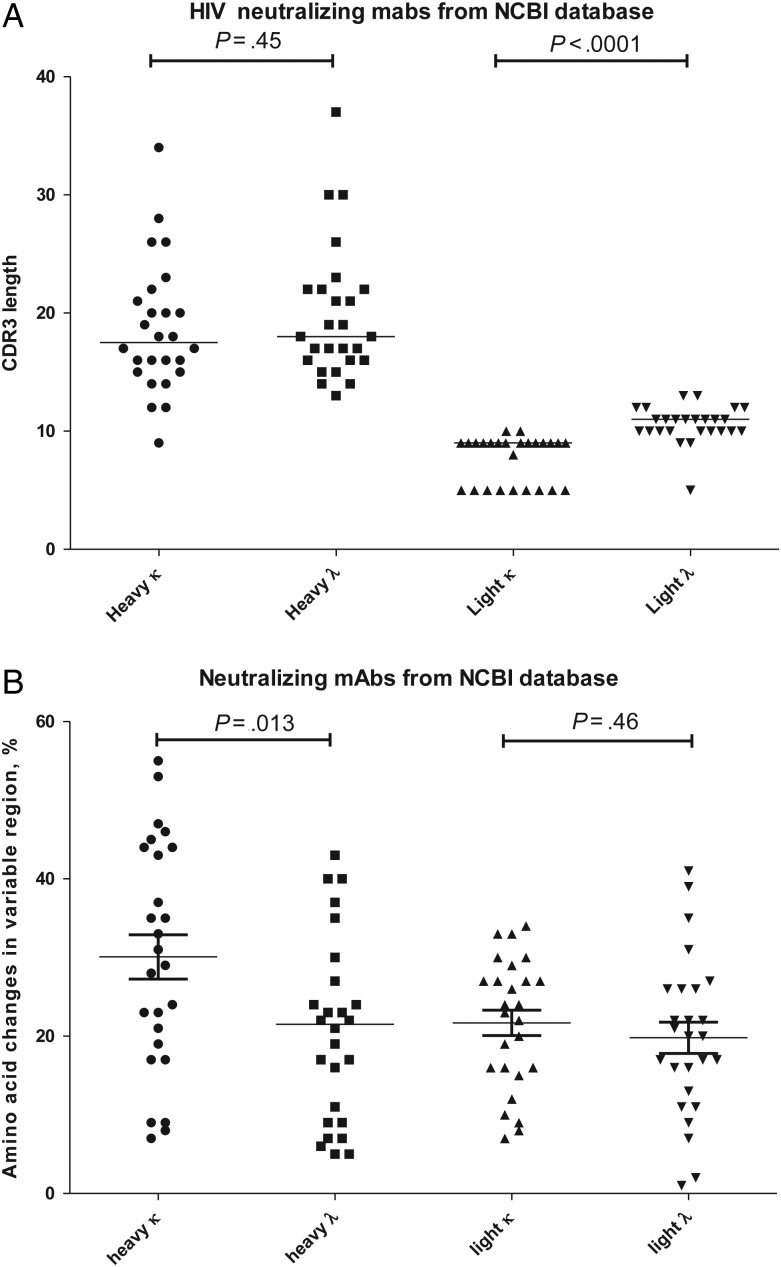
Fifty-two mAbs with HIV neutralizing activity from the NCBI protein database were examined to determine whether there were differences in mAbs that use κ versus λ antibodies. There was no difference observed in the heavy chain CDR3 lengths of mAbs expressing κ versus λ light chain (P = .45); however, the CDL3s of λ light chains were 3 amino acids longer than mAbs expressing κ light chains (10.6 vs 7.7 amino acids; P < .0001; Figure 6A). mAbs expressing the κ light chain had a mean of 21.7% amino acid changes in the light chain variable regions, compared with 19.8 for the λ mAbs (P = .46; Figure 6B). However, there was significantly higher percentage of amino acid changes in the heavy chain variable regions of κ mAbs in comparison to λ mAbs (30 vs 20.7; P = .013; Figure 6B).
Figure 6.
CDR3, CDL3 length, and mutation rate in various anti–human immunodeficiency virus Env monoclonal antibodies (mAbs). A total of 52 neutralizing and broadly neutralizing antibodies in the National Center for Biotechnology Information (NCBI) database were compared for CDR3 length and percentage change in amino acids in the variable regions. A, Correlation between CDR3/CDL3 length and light chain isotype. There was no association between CDR3 length and light chain isotype (P = .45); however, there was a correlation between λ light chain use and CDL3 length (P < .0001). The x-axis groups the mAb variable regions by light chain (heavy or light chain in κ or λ antibodies). The y-axis represents CDR3 or CDL3 amino acid length. B, Correlation between degree of mutation and light chain isotype. There was a correlation between the degree of amino acid mutation in the heavy chain of antibodies expressing κ light chain (there was an increased mutation rate in κ heavy chains; P = .013). The x-axis groups the mAb variable regions by light chain (heavy or light chain in κ or λ antibodies). The y-axis represents the percentage of amino acid changes from the germ line.
To determine whether similar differences in CDRL3 length distributions and somatic hypermutation are found among vaccinees, we analyzed data from the HIV GSK PRO HIV-002 study. In all, 35 HIV-Env reactive mAbs isolated after the first and third vaccinations were analyzed. κ-expressing mAbs had significantly longer heavy chain CDR3s (2 amino acids) and shorter light chain CDRL3s (2 amino acids) than λ expressing mAbs (P = .039 and P < .0001, respectively; Supplementary Figure 1A). The percentage amino acid changes of mAb heavy chains of mAbs were elevated in κ light chains (3.7%), compared with λ light chains (2.4%), but the difference was not significant (P = .15; Supplementary Figure 1B).
Differences in Antibody/Antigen Contacts in κ and λ Anti-HIV Env mAbs
All structures of complexes of anti-HIV Env mAbs with their antigens available in the protein database were analyzed. There were no significant differences in the number of residue/atom contacts between the heavy chain and the Env antigen when antibodies expressing κ (25 mAbs) or λ (24 mAbs) were compared. Antibodies expressing λ chain mediated 27 of 93 of residue/atom contacts in their heavy chains as compared to 26 of 88 of residue/atom contacts mediated by antibodies expressing κ chains (Supplementary Table 1). However, there was a trend toward significantly elevated number of atomic contacts between the λ light chain (14 of 47 of residue/atom contacts; Supplementary Table 1), compared with the κ light chain contacts with their cognate Env epitopes (11 of 34 of residue/atom contacts; Supplementary Table 1) (P = .18 for residue contacts and P = .056 for atom contacts). Structural comparison of representative antibodies expressing the κ or λ light chains are shown in Supplementary Figure 2.
DISCUSSION
In the current study, we found a significant λ bias against HIV Env, compared with other antigens. Using 4 different sample formats (plasma, anti-gp120 antibody, fractionated IgG1, and fractionated anti-gp120 antibody), 4 different assay types (concentration κ/λ ratio, binding κ/λ ratio, multiplex assay, and neutralization assay), and >20 antigens, we found that this bias was observed in individuals with chronic HIV infection (clade B). In the HIV vaccine cohort, there was a pronounced λ bias to HIV Env in the concentration κ/λ ratio and binding κ/λ ratio. Finally, in the acute HIV cohort (clade G and CRF02_AG), there was a λ bias in HIV Env, compared with other antigens, in the binding κ/λ ratio. Limited sample precluded further testing of the vaccine and acute cohorts. However, the results of a λ bias were remarkably consistent across the different subject populations (individuals with acute HIV infection, those with chronic HIV infection, and HIV-negative vaccinees), HIV clades (B, G, and CRF02_AG), and assays tested.
In a previous study, Scheid et al reported a κ preponderance in B cells from elite neutralizers. Interestingly, this bias was absent among the mAbs we analyzed in the NCBI database (after clonally similar antibodies were deleted) and GSK-01 study (κ and λ antibodies were equal). However, our plasma data show a clear bias toward λ antibody production. This discrepancy could be due to the small number of patients [6], the single antigen tested, and possibly selection of B cells in the study by Scheid et al. However, another possibility is the differential regulation of Env-specific memory B cells versus antibody-producing cells.
Our results suggest that, early in HIV infection or after vaccination, there is preferential binding of HIV envelope to B cells that express λ. By the chronic phase of infection, there is not only an increased prevalence of anti-gp120 λ antibodies, but these antibodies exhibit increased gp120 binding and neutralization activity (responsible for up to 90% of plasma neutralization activity).
This study is the first to definitively link light chain use with antibody function in a human infection, in this case HIV neutralization. The light chain is clearly an integral part of the antigen binding site, and biased light chain responses to various antigens have been demonstrated previously [5–16]. Recently, a λ bias has been demonstrated in mAbs targeting the V2 epitope of the antigen used in the RV144 trial [11]. This bias toward antigens has led some to hypothesize a functional advantage. For example, Lucas et al noted oligoclonality in the serum of persons who had received the 23 valent Streptococcus pneumoniae polysaccharide vaccine and hypothesized that this could be tied to antibody functionality [12]. However, a study by the same group challenged the notion of the importance of bias by demonstrating a large repertoire comprising many gene families in the response to S. pneumonia capsular polysaccharide, suggesting that many different paths could achieve the same result [17]. For HIV envelope, it appears that the dominant response occurs through the use of the λ light chain, with likely specific favorable heavy and λ light chain gene family pairings.
Our mAb analysis suggests that the λ light chain provides an increased contribution toward HIV Env binding. Inherently, λ light chains have elongated CDRL3s, compared with κ light chains (7 amino acids in κ light chains vs 8–11 amino acids in λ light chains) [15]. We found that anti-HIV Env mAbs expressing λ had CDRL3 that were found to be 3 amino acids longer (10.6 vs 7.7 amino acids long), suggesting that λ gene families with longer CDRL3s are selected. Furthermore, analysis of complex structures of anti-gp120 antibodies indicates that there may be more λ light chain contacts to Env, compared with κ antibodies. In both panels of mAbs (neutralizing mAbs and mAbs obtained after vaccination), the number of mutations in the light chains was comparable, but fewer heavy chain mutations were present in the λ antibodies. In vaccinees, the heavy chain CDR3s were also shorter in the λ group, suggesting that κ antibodies had to compensate with a higher somatic mutation rate and longer CDR3s in the heavy chain. Indeed, some neutralizing κ mAbs (such as b12 and 8ANC 195) have no light chain contact contributing to antigen binding.
Although there is an overall λ light chain bias for the Env response, specific epitopes can have biases, as well. For example, all but 1 CD4 binding site mAb described uses κ. In our data set, although the data present a clear λ light chain bias at the population level, there was a single exception. One individual with a broad HIV neutralization response demonstrated a κ biased neutralization response (even though their plasma binding was λ predominant). From this individual, we have isolated a broadly neutralizing antibody that uses a κ light chain (unpublished data). In light of the population dynamics noted in the current study, one can ask whether targeting antigens (such as the CD4 binding site) with corresponding antibodies that use the κ chain is possible and efficient at the population level, rather than at the individual level.
Since the discovery of the immunoglobulin light chain bias, seminal studies have largely worked out light chain development [26–31]. After successful heavy chain rearrangement, κ rearrangement commences on one chromosome and then the second chromosome if the first rearrangement is nonproductive/autoreactive [30]. In the event when both rearrangements are nonproductive/autoreactive, λ rearrangement commences [30–32]. Finally, naive B cells express IgM with either a κ or λ light chain. The κ/λ ratio in naive B cells is similar to the κ/λ ratio of antibodies found in blood [33] and is thought to be a major determinant affecting the κ/λ ratio in blood.
We found significant interantigen variability in κ/λ ratios, suggesting that κ/λ ratios are not solely dependent on the ratios in naive B cells. In a stochastic model, the responses to all antigens would mirror those of naive B cells. Our studies described above and others have demonstrated light chain bias against various antigens [5, 9, 10], suggesting that the κ/λ ratio for each individual/species is made up of the sum of κ and λ antibodies of individual antigens, each selected nonstochastically. This, of course, is also affected by the available naive B-cell repertoire, which is dependent on the number of functional antibody gene families.
One factor that may be involved in selection of a particular naive B cell (and its corresponding light chain) is the pI of the antigen itself. We did note a correlation between the pI of the antigen and the κ/λ ratio for binding. We are unaware of any inherent differences in these light chains in terms of pI, and we did not note any when testing the pI of the polyclonal κ and λ fractions, as both appear to span the pI range for antibody (data not shown). Thus, this potential factor remains unexplained.
Despite the understanding of the light chain in B-cell development, it is still unknown why different species have widely different κ/λ ratios and whether they have any relation to various infections. During the course of evolution, undoubtedly some biologic threats have left a greater imprint on the host. Lentiviruses may represent one such agent, as they have the capacity to cause a chronic infection that constantly stimulates the immune system, and they are potentially lethal (as shown in simian immunodeficiency virus infection in primates that are not natural hosts). Notably, all known lentiviral hosts (cattle, sheep, horses, cats, and primates) have a general λ bias in their total antibody repertoire [1]. It may be that the inverted κ/λ in these species is related to the ancestral host response against lentiviral envelopes. Selection of gene families that express the λ light chain would affect the naive B-cell population and even skew the responses to antigens that are minimally or not biased in their selection of light chain, simply because of biased availability of the naive B cell.
In conclusion, we found a significant bias toward use of the λ light chain in the anti-HIV Env response in all subject populations (individuals with acute HIV infection, those with chronic HIV infection, and HIV-negative vaccinees) and HIV clades (clade B, clade G, and CRF02_AG) tested. This bias was present for concentration of antibodies produced, affinity toward HIV Env, and neutralization of HIV Env pseudoviruses (up to 90% of neutralization was in the λ antibody fraction). In HIV infection, antibodies expressing λ light chains tended to have longer CDRL3 and less hypermutation in the heavy chain, suggesting increased light chain contacts. The λ light chain bias toward HIV envelope may provide the host an advantage in developing a more efficient humoral response to this lentiviral envelope.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank members of the NVS Study and other research volunteers and staff, as well as the National Institute of Allergy and Infectious Diseases (NIAID) and the NIAID-funded HIV Vaccine Trials Network, for providing vaccine cohort subject specimens from the HVTN 094 and HVTN 205 protocols.
Financial support. This work was supported by the National Institutes of Health (grant 1R01AI110259-01A1 and 1I01BX002358-01A1 to M. M. S. and grant R01AI087181 to G. K. L.), the Bill and Melinda Gates Foundation (grant OPP1033109 to G. K. L., grant OPP1032817 to M. E. A., and grant 38619 to M. S. S.), and the Canadian Institutes of Health Research (postdoctoral fellowship to M. S. P.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Hood L, Gray WR, Sanders BG, Dreyer WJ. Light chain evolution. Cold Spring Harbor Symp Quant Biol 1967; 32:133–46. [Google Scholar]
- 2.Jelonek MT, Chang SJ, Chiu CY, Park MK, Nahm MH, Ward JI. Comparison of naturally acquired and vaccine-induced antibodies to Haemophilus influenzae type b capsular polysaccharide. Infect Immun 1993; 61:5345–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung GH, Kim KH, Daum RS et al. . The V-region repertoire of Haemophilus influenzae type b polysaccharide antibodies induced by immunization of infants. Infect Immun 1995; 63:4219–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung GH, Scott MG, Kim KH et al. . Clonal characterization of the human IgG antibody repertoire to Haemophilus influenzae type b polysaccharide. V. In vivo expression of individual antibody clones is dependent on Ig CH haplotypes and the categories of antigen. J Immunol 1993; 151:4352–61. [PubMed] [Google Scholar]
- 5.Jack RS, Imanishi-Kari T, Rajewsky K. Idiotypic analysis of the response of C57BL/6 mice to the (4-hydroxy-3-nitrophenyl)acetyl group. Eur J Immunol 1977; 7:559–65. [DOI] [PubMed] [Google Scholar]
- 6.Smith FI, Cumano A, Licht A, Pecht I, Rajewsky K. Low affinity of kappa chain bearing (4-hydroxy-3-nitrophenyl)acetyl (NP)-specific antibodies in the primary antibody repertoire of C57BL/6 mice may explain lambda chain dominance in primary anti-NP responses. Mol Immunol 1985; 22:1209–16. [DOI] [PubMed] [Google Scholar]
- 7.Sarnesto A, Ranta S, Vaananen P, Makela O. Proportions of Ig classes and subclasses in rubella antibodies. Scand J Immunol 1985; 21:275–82. [DOI] [PubMed] [Google Scholar]
- 8.Zhou J, Lottenbach KR, Barenkamp SJ, Lucas AH, Reason DC. Recurrent variable region gene usage and somatic mutation in the human antibody response to the capsular polysaccharide of Streptococcus pneumoniae type 23F. Infect Immun 2002; 70:4083–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Briault S, Courtois-Capella M, Duarte F, Aucouturier P, Preud'Homme JL. Isotypy of serum monoclonal immunoglobulins in human immunodeficiency virus-infected adults. Clin Exp Immunol 1988; 74:182–4. [PMC free article] [PubMed] [Google Scholar]
- 10.Insel RA, Adderson EE, Carroll WL. The repertoire of human antibody to the Haemophilus influenzae type b capsular polysaccharide. Int Rev Immunol 1992; 9:25–43. [DOI] [PubMed] [Google Scholar]
- 11.Wiehe K, Easterhoff D, Luo K et al. . Antibody light-chain-restricted recognition of the site of immune pressure in the RV144 HIV-1 vaccine trial is phylogenetically conserved. Immunity 2014; 41:909–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lucas AH, Granoff DM, Mandrell RE, Connolly CC, Shan AS, Powers DC. Oligoclonality of serum immunoglobulin G antibody responses to Streptococcus pneumoniae capsular polysaccharide serotypes 6B, 14, and 23F. Infect Immun 1997; 65:5103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baxendale HE, Goldblatt D. Correlation of molecular characteristics, isotype, and in vitro functional activity of human antipneumococcal monoclonal antibodies. Infect Immun 2006; 74:1025–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muller S, Wang H, Silverman GJ, Bramlet G, Haigwood N, Kohler H. B-cell abnormalities in AIDS: stable and clonally-restricted antibody response in HIV-1 infection. Scand J Immunol 1993; 38:327–34. [DOI] [PubMed] [Google Scholar]
- 15.Bridges SL., Jr Frequent N addition and clonal relatedness among immunoglobulin lambda light chains expressed in rheumatoid arthritis synovia and PBL, and the influence of V lambda gene segment utilization on CDR3 length. Mol Med 1998; 4:525–53. [PMC free article] [PubMed] [Google Scholar]
- 16.David D, Zouali M. Variable region light chain genes encoding human antibodies to HIV-1. Mol Immunol 1995; 32:77–88. [DOI] [PubMed] [Google Scholar]
- 17.Zhou J, Lottenbach KR, Barenkamp SJ, Reason DC. Somatic hypermutation and diverse immunoglobulin gene usage in the human antibody response to the capsular polysaccharide of Streptococcus pneumoniae Type 6B. Infect Immun 2004; 72:3505–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guan Y, Sajadi MM, Kamin-Lewis R et al. . Discordant memory B cell and circulating anti-Env antibody responses in HIV-1 infection. Proc Natl Acad Sci U S A 2009; 106:3952–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sajadi MM, Guan Y, DeVico AL et al. . Correlation between circulating HIV-1 RNA and broad HIV-1 neutralizing antibody activity. J Acquir Immune Defic Syndr 2011; 57:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sajadi MM, Lewis GK, Seaman MS, Guan Y, Redfield RR, DeVico AL. Signature biochemical properties of broadly cross-reactive HIV-1 neutralizing antibodies in human plasma. J Virol 2012; 86:5014–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charurat M, Nasidi A, Delaney K et al. . Characterization of acute HIV-1 infection in high-risk Nigerian populations. J Infect Dis 2012; 205:1239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fouts TR, Godfrey K, Reitz M, Hone D, Lewis GK, DeVico AL. Expression and characterization of a single-chain polypeptide analogue of the human immunodeficiency virus type 1 gp120-CD4 receptor complex. J Virol 2000; 74:111427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown EP, Licht AF, Dugast AS et al. . High-throughput, multiplexed IgG subclassing of antigen-specific antibodies from clinical samples. J Immunol Methods 2012; 386:117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li M, Gao F, Mascola JR et al. . Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol 2005; 79:10108–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moody MA, Yates NL, Amos JD et al. . HIV-1 gp120 vaccine induces affinity maturation in both new and persistent antibody clonal lineages. J Virol 2012; 86:7496–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radic MZ, Erikson J, Litwin S, Weigert M. B lymphocytes may escape tolerance by revising their antigen receptors. J Exp Med 1993; 177:1165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gay D, Saunders T, Camper S, Weigert M. Receptor editing: an approach by autoreactive B cells to escape tolerance. J Exp Med 1993; 177:999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tiegs SL, Russell DM, Nemazee D. Receptor editing in self-reactive bone marrow B cells. J Exp Med 1993; 177:1009–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halverson R, Torres RM, Pelanda R. Receptor editing is the main mechanism of B cell tolerance toward membrane antigens. Nat Immunol 2004; 5:645–50. [DOI] [PubMed] [Google Scholar]
- 30.Korsmeyer SJ, Hieter PA, Ravetch JV, Poplack DG, Waldmann TA, Leder P. Developmental hierarchy of immunoglobulin gene rearrangements in human leukemic pre-B-cells. Proc Natl Acad Sci U S A 1981; 78:7096–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hieter PA, Korsmeyer SJ, Waldmann TA, Leder P. Human immunoglobulin kappa light-chain genes are deleted or rearranged in lambda-producing B cells. Nature 1981; 290:368–72. [DOI] [PubMed] [Google Scholar]
- 32.van der Burg M, Tumkaya T, Boerma M, de Bruin-Versteeg S, Langerak AW, van Dongen JJ. Ordered recombination of immunoglobulin light chain genes occurs at the IGK locus but seems less strict at the IGL locus. Blood 2001; 97:1001–8. [DOI] [PubMed] [Google Scholar]
- 33.Rolink A, Streb M, Melchers F. The kappa/lambda ratio in surface immunoglobulin molecules on B lymphocytes differentiating from DHJH-rearranged murine pre-B cell clones in vitro. Eur J Immunol 1991; 21:2895–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.