Abstract
Purpose of Review
To review the most recent studies assessing the preparedness of healthcare practitioners to provide anti-HIV pre-exposure prophylaxis (PrEP) and to suggest areas for future implementation research.
Recent Findings
As PrEP is a bio-behavioral intervention, healthcare providers are likely to play a critical role in implementing PrEP in care settings. Studies suggest that many specialized providers are aware of PrEP and support its provision as a public health intervention, though knowledge and acceptance are less among generalists. Therefore, utilization of PrEP by clinicians has been limited to a few early adopters. Concerns about the efficacy and long-term safety of PrEP, and perceived barriers to prescribing PrEP, could limit prescribing behaviors and intentions. Resistance to performing routine HIV risk assessments by clinicians is an additional barrier to implementing PrEP, though innovative tools to help clinicians routinely perform risk assessments are being developed.
Summary
Interventions are needed to engage a broader array of healthcare providers in PrEP provision. Utilizing a framework based on diffusion of innovation theory, this review proposes strategies that can be implemented and evaluated to increase PrEP prescribing by healthcare providers. If resources are invested in training clinicians to provide PrEP, then these stakeholders could enhance the use of PrEP as part of a prevention package by primary providers.
Keywords: HIV, pre-exposure prophylaxis, healthcare providers, risk assessment, implementation
Introduction
Over the past five years, several randomized, controlled studies have demonstrated the efficacy of oral antiretroviral pre-exposure prophylaxis (PrEP) to decrease the transmission of HIV in diverse populations when participants were highly adherent (1–6). Based on the results of these studies, the U.S. Food and Drug Administration approved the use of tenofovir-emtricitabine for use as daily PrEP in 2012 (7), and in 2014, the U.S. Centers for Disease Control and Prevention (CDC) issued comprehensive guidelines recommending that PrEP be included as a prevention option for individuals at high risk for HIV acquisition (8). More recently, demonstration projects in the U.S. (9), Kenya and Uganda (10), and Brazil (11) have shown that many persons at risk for HIV infection are interested in using PrEP, and that many participants are able to take PrEP with high adherence. With encouraging results from these studies and issuance of normative guidelines, for PrEP to have a major public health impact in decreasing HIV incidence, it needs to be provided in primary care settings.
As with any bio-behavioral intervention, healthcare professionals will play a critical role in the successful implementation of PrEP. These “gatekeepers” will need to be informed about the clinical aspects of PrEP management, be willing to prescribe it, trained in its use, and need to be supported with resources to simplify the incorporation of this novel intervention into practice. However, the initial adoption of PrEP by healthcare providers has been slow (12), suggesting a need to understand providers’ attitudes and experiences with PrEP. This review summarizes recent studies assessing providers’ usage and intentions regarding prescribing PrEP, with a focus on 3 interrelated aspects of provider preparedness: 1) awareness of and willingness to prescribe PrEP; 2) challenges with identifying those persons who would benefit from PrEP (i.e., conducting HIV risk assessments) and novel interventions to address and overcome these challenges; and 3) strategies to support the wider appropriate use of PrEP in care settings.
Awareness and Willingness to Prescribe PrEP among U.S. Providers
Many of the recent studies assessing providers’ opinions and PrEP experiences have focused on clinicians in the U.S., which may be an appropriate starting point given the U.S. was the first country in which tenofovir-emtricitabine was approved for clinical use as anti-retroviral PrEP (13). Based on surveys conducted in the last 1–2 years, awareness of PrEP appears to be high among U.S. HIV-care providers, a group of clinicians that might be expected to be among the first to be knowledgeable about PrEP. In a study of 184 providers affiliated with a regional HIV/AIDS educational center in New England, 89% had heard of PrEP (as of 2013), though 25% were not familiar with CDC guidance about PrEP provision that had been issued 2 years before the survey (14). Few respondents (19%) had prescribed PrEP, though half of those who had not prescribed PrEP believed that they would do so in the future. A survey of 515 HIV-care providers and primary care providers (PCPs) in 10 U.S. cities (during 2014–15) found that 97% of HIV-care providers and 76% of PCPs had heard of PrEP, suggesting that awareness was lower among generalist PCPs than HIV specialists (15).
One of the largest studies of provider opinions about PrEP in the U.S. was a national survey of 573 infectious diseases physicians conducted in June to July 2013. Three-fourths of respondents supported the concept that PrEP should be provided to some patients, but 14% were unsure, and 12% did not support PrEP. Only 9% of physicians had prescribed PrEP, 43% had not provided PrEP but would be willing to do so, 34% believed PrEP was not relevant to their practice, and 14% would not provide PrEP in the future. Reasons why respondents would not be willing to provide PrEP included concerns about adherence and selection of resistant viral strains (77%), cost and reimbursement issues (57%), the use of potentially toxic drugs in healthy persons (53%), and a perception that insufficient evidence supporting the efficacy of PrEP existed in real-world settings (16). Overall, the findings of this study highlight that positive attitudes towards PrEP do not necessarily result in actual prescribing experiences or intentions. In addition to theoretical concerns that may cause providers to be cautious about prescribing PrEP, practical barriers could limit prescribing. For example, providers from the New England study cited multiple “real-world” barriers to prescribing PrEP, including a need for more training, few patient requests for PrEP, concerns about insurance coverage for PrEP, and time constraints, among others (14).
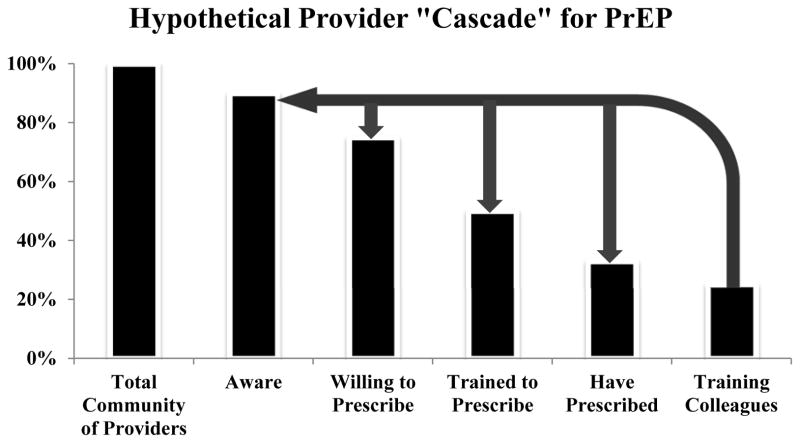
A hypothetical provider “cascade” for PrEP may be a useful construct to conceptualize the various aspects of preparedness (e.g., awareness, willingness, and training) that must be achieved for different types of providers to implement PrEP. (Figure 1)
Figure 1. Hypothetical Provider “Cascade” for PrEP.
Schematic to represent the steps in the continuum of provider knowledge and experience with providing PrEP. This schematic can be applied to the global community of healthcare providers or to specific subgroups of providers with similar geography (e.g., the United States) or professional background (e.g., primary care providers). Heights of bar graphs are hypothetical for the purposes of illustration, given heterogeneity of provider engagement and incomplete data on provider opinions and practices. The arrows represent favorable amplification effects from engaging providers as “trainers” for their colleagues.
Which Providers will Prescribe PrEP in the U.S.?
Qualitative studies with providers conducted in 2012 suggested that a potential barrier to implementing PrEP was a so-called “purview paradox” among HIV-care providers and PCPs. In this study, many HIV-care providers did not perceive themselves to be well-positioned to prescribe PrEP (as some infrequently provided care to HIV-uninfected persons), and PCPs believed that prescribing PrEP would not be feasible in their practices (given limited experience prescribing antiretroviral medications) (17).
However, more recent studies suggest that attitudes and practices with PrEP may be evolving among HIV-care providers and PCPs. A follow-up study of infectious diseases physicians in the U.S. that was conducted in September, 2014, several months after the release of CDC guidelines for PrEP, found that one third of the respondents had prescribed PrEP to sexual partners of their HIV-infected patients. This finding suggested that there may have been an increase in the proportion of physicians who had prescribed PrEP as compared to 1 year prior (18). However, inferring secular trends in prescribing rates from these 2 studies must be done with caution, as these studies did not necessarily enroll the same sample of providers. Another encouraging finding from this study was that a majority of HIV-care providers reported beliefs that counseling their HIV-infected patients about PrEP for partners, offering clinical visits to partners, and prescribing PrEP to partners (when indicated), should be part of their clinical role. The survey of HIV-care providers and PCPs in 10 U.S. cities that was completed in 2015 was also encouraging in that 90% of HIV-care providers and 80% of PCPs indicated they would be willing to prescribe PrEP (15). Therefore, both groups of providers may be increasingly open to prescribing PrEP, with the caveat that clinicians who participate in surveys about HIV prevention may be more willing than others to incorporate HIV prevention strategies, such as PrEP, into their practices. In addition to studies of PCPs and HIV-care providers, surveys to assess attitudes and experiences among practitioners who specialize in the diagnosis and treatment of sexually transmitted infections, as well as practitioners at HIV testing sites, will also be needed. These providers are likely to encounter many persons who may benefit from PrEP, and currently, little is known about their opinions and practices regarding PrEP provision.
Importantly, less than half of the providers in the study of infectious diseases physicians indicated that they felt adequately prepared to prescribe PrEP to persons who inject drugs (PWID) (18). Studies to understand why providers may report different prescribing intentions for patients with specific indications for PrEP, such as PWID, will be important to prevent disparities in access to PrEP. Efforts to ensure equitable prescribing practices may also need to address any implicit behavioral and/or racial biases among clinicians, as a study of medical students detected evidence of implicit biases against racial minorities when making hypothetical prescribing decisions for PrEP (19).
Providers outside of the USA
As the vast majority of persons at risk for HIV acquisition reside outside the U.S., a major consideration for equitable access to PrEP globally is whether providers in other nations will prescribe PrEP, when PrEP becomes available locally. Currently, few countries have issued normative guidelines for clinicians (13). However, additional nations may develop guidelines in the next few years, given successful PrEP demonstration projects in Brazil (11), Kenya and Uganda (10), and Botswana (20), and various demonstration projects that are being planned (or underway) globally (21). Studies demonstrating the efficacy of “real-world” use of daily PrEP in the UK (the PROUD study) (22) and event-driven PrEP in Canada and Europe (Ipergay) (23) may also prompt guidelines in these locations. As clinicians’ practices may change in response to guidelines, they could potentially be willing to prescribe PrEP medications without formal approval by regulatory bodies. For example, clinicians routinely prescribe antiretroviral medications for post-exposure prophylaxis and prevention of mother- to-child transmission without formal prevention indications. Therefore, studies to assess provider attitudes towards PrEP in multiple geographic regions, particularly in settings where guidelines are anticipated, are needed.
To address this need, several studies have assessed non-American provider opinions about PrEP. In Peru, 58% of 186 providers (mostly attendees of an HIV conference in 2012) were aware of PrEP, with greater awareness among providers caring for large numbers of men who have sex with men (MSM) (24). Forty-five percent of providers reported they would be likely to prescribe oral PrEP at the time of the survey, but 60–70% would be likely to prescribe PrEP if more efficacy data were available or intermittent use were shown to be effective; lack of formal guidelines and concerns about risk compensation were frequently cited concerns. Most (80%) of a heterogeneous sample of 86 Canadian physicians were familiar with PrEP when surveyed in 2012–2013, though less than half were willing to prescribe PrEP (25).
Consistent with these quantitative surveys, a qualitative study of providers from 4 cities in the Americas (San Francisco, New York, Lima, and Rio de Janeiro) completed in 2012 identified a theme that providers were “conflicted” about providing PrEP, as they were eager to enhance HIV prevention for their clients but were also concerned about diverting resources from HIV treatment (26). The need to assess provider opinions about PrEP may be particularly important in areas in which resources for HIV treatment are limited, as providers may perceive ethical dilemmas in terms of resource allocation between PrEP and antiretroviral treatment (27).
In addition to assessments among HIV specialists and PCPs, characterizing attitudes among providers who practice in sexual health clinics will be important. These providers are likely to encounter persons at substantial risk for HIV acquisition, and they might reasonably be expected to be supportive of novel protective interventions for their clients. However, a survey of 328 sexual health practitioners in the UK suggested that these providers shared similar concerns as providers elsewhere, and only half thought PrEP should be available by prescription (28).
Overall, a consistent theme that has emerged from provider assessments in the U.S. and in selected international regions is that many of these stakeholders support PrEP provision as a public health intervention, but residual concerns about negative consequences of PrEP use and practical hurdles may limit prescribing. Moreover, even if providers are informed about PrEP, motivated and trained to prescribe this intervention, and provided with structural supports to overcome practical barriers, an additional consideration is whether frontline clinicians will be able to identify persons who are most likely to benefit from PrEP.
Identifying those persons who would benefit from PrEP
Ideally, providers would have the skills, time and motivation to conduct comprehensive risk assessments as part of routine care for their patients. However, assessments of sexual risk behaviors, sexual orientation, and gender identity, all of which may influence HIV risk, tend to occur infrequently in primary care (29). Multiple factors likely contribute to suboptimal risk assessment practices, including discomfort among providers and patients (30), lack of provider training in communication skills (29), and multiple competing demands on busy clinicians (31).
Healthcare practitioners generally receive minimal training in how to elicit a comprehensive sexual health history, so it is not surprising that practitioners across the continuum of professional development, from medical students to practicing clinicians, express a need for more training in this area (31). Studies suggest that skills-based training could potentially improve providers’ effectiveness at identifying those persons at highest risk for HIV acquisition. A recent study that provided 26 U.S. physicians with sexual history trainings, either as a single 6-hour, in-person training or as 2 1-hour webinars, demonstrated increased frequency of documented sexual history discussions and greater comfort with sexual health discussions (29). The training was based on the theory of planned behavior (i.e., that personal attitudes, social norms, and self-efficacy influence intentions and behaviors) and included information about HIV/STI epidemiology and role-modeling and/or role-playing of effective techniques. This promising pilot study suggests that scale-up of brief, theory-based, interactive trainings for providers could improve risk assessments. In a qualitative study of medical students, participants voiced a need for patient interviews and clinical scenarios focused on sexual health discussions with MSM to improve their comfort with risk assessment for this important population (31). A survey of 1,394 MSM who engaged in online sexual networking and reported interest in using PrEP found that 42% were not comfortable discussing same-sex behaviors with their PCPs, many had never discussed anal sex behaviors or PrEP with PCPs, and three-fourths believed that their PCP would not be willing to prescribe PrEP (32). Therefore, trainings that emphasize culturally-sensitive and nonjudgmental approaches to history-taking could be critical to ensure PrEP uptake in primary care. Medical educators and professional organizations for PCPs should be informed about the results of these studies and encouraged to expand communication curricula about sexual health.
In addition to skills-based trainings, several novel approaches to help providers identify those persons who would benefit from PrEP are under development. Risk prediction tools for clinical use have been developed for MSM (33) and PWID (34). With these point-of-care tools, responses to several questions about specific patient-reported behaviors are used to generate individualized estimates of HIV risk in real-time. Clinicians can record patients’ responses to questions during in-person interviews, or patients can enter responses directly into the tool before clinic visits (e.g., in waiting rooms). The use of prediction tools may result in more accurate risk assessments than clinician judgment in some cases, which could optimize PrEP prescribing decisions. However, these tools have greater sensitivity than specificity, suggesting that they will be most helpful as screening tools for PrEP. For individuals who have positive screening results, providers would still need to be skilled in detailed risk assessments to make optimal recommendations about PrEP, so these tools are likely to be most helpful when paired with skills-based trainings. A pilot study found that the use of a risk prediction tool for MSM on a computer tablet in a clinic waiting room (“iPad-based risk assessment”) was generally acceptable to patients in 2 U.S. cities, though provider opinions about this approach were not assessed (35). As patients may be more likely to accurately disclose sensitive information during computer-based interviews than during personal interviews with providers (36), and tablet-based behavioral assessments have been successfully utilized with HIV-infected patients and other cohorts (37), additional studies with routine tablet-based assessments in primary care are warranted.
Automated, individualized risk assessments based on the data routinely embedded in patients’ electronic health records (EHRs) offers another promising strategy to identify individuals who may benefit from PrEP. With this approach, the rich array of data available in EHRs is used to determine the EHR profile of patients at high risk of acquiring HIV. Automated algorithms are then developed that can risk-stratify individuals based on their EHR profiles, and these algorithms can be programmed to deliver real-time alerts to clinicians when patients who might benefit from PrEP are identified. Similar algorithms have been successfully developed for the detection and reporting of other STIs in care settings (38). If EHR-based algorithms demonstrate acceptable performance for PrEP, they could provide a potentially scalable, generalizable and automated approach to facilitating risk assessments in primary care settings. As with other risk-prediction tools, these algorithms may not be sufficiently sensitive or specific to replace clinician interviews. If these tools can increase the frequency and accuracy of risk assessment, however, they could complement efforts to train clinicians in sexual history-taking.
Supporting the Diffusion of Innovation for PrEP
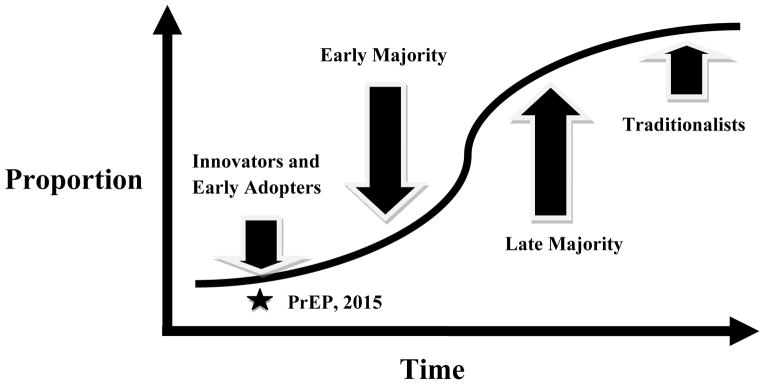
Diffusion of innovation theory describes the factors that influence the adoption of novel technologies by a population of end-users, including medical interventions by clinicians or patients (39). This theory provides a useful framework for charting the trajectory of PrEP uptake in care settings. The temporal uptake of innovations tends to follow an S-shaped curve, with early use limited to a small proportion of the total population (innovators (2.5% of all providers) and early adopters (13.5%), with subsequent expansion to early (34%) and late (34%) majorities, and finally adoption by traditionalists, or “laggards” (16%) (40). (Figure 2) Importantly, widespread adoption of innovations is uncertain during the early stages of diffusion, prior to attainment of an inflection point in adoption. Clinician assessments suggest that PrEP uptake has been limited to innovators and early adopters in the U.S. and only small groups of innovators elsewhere. Therefore, the diffusion of PrEP appears to be at a critical stage, such that engagement of a larger group of early providers will be needed to achieve widespread adoption of PrEP. Of note, the stages of diffusion may not always follow a classic S-shaped curve in healthcare systems where the introduction of new interventions is typically universal, as occurs in some public health care systems.
Figure 2. Temporal Diffusion of PrEP as a Medical Innovation.
The uptake of medical innovations such as PrEP tends to follow an S-shaped curve, with early use limited to a small proportion of the total population (innovators (2.5% of all providers) and early adopters (13.5%), with subsequent expansion to early (34%) and late (34%) majorities, and finally adoption by traditionalists, or “laggards” (16%) (40). For PrEP, clinician assessments suggest that uptake has only occurred among innovators or early adopters, without attainment of an “inflection point,” so widespread adoption remains uncertain. The current state of PrEP adoption by clinicians (i.e., in 2015) is represented by the star. Diffusion of innovation theory suggests several strategies to support adoption of PrEP by larger numbers of clinicians, as described in the text.
The diffusion of innovation framework suggests interventions that may facilitate adoption of PrEP. One of the most important strategies is to invest in innovators and early adopters and to promote the visibility of successful early adopter activities (40). In the U.S., a number of clinical sites have reported early successes with PrEP provision, including 3 settings in San Francisco (a private health maintenance organization, an HIV-specific reproductive health program, and a demonstration project at the municipal STI clinic) (41) and a community health center in Boston that specializes in the care of sexual and gender minorities (42). Clinicians at this health center have prescribed PrEP to over 600 patients in total with generally positive experiences, demonstrating that PrEP provision is feasible in primary care (42). Educational programs that summarize these positive experiences for their generalist colleagues could promote uptake by the “early majority” of providers, and are being rolled out in several jurisdictions, most notably New York City and State (43). Peer-to-peer social interactions (sometimes over food, using Grand Rounds or case conference formats) between familiar colleagues are also thought to be critical for widespread adoption. When possible, local champions should also be identified and supported (e.g. with time and funding) so they can “lead by example” (40) and inspire others towards change.
Finally, practitioners must be provided with accurate data on the efficacy and safety data for PrEP, so that frequently-cited concerns about the risk-benefit ratio of PrEP are directly addressed. After the completion of multiple efficacy studies and several demonstration projects, an abundance of data suggests that PrEP is highly protective when taken regularly and is generally safe, even though longer-term safety data are needed. One study directly correlated increased correct knowledge about PrEP with greater likelihood of prescribing it (44). With further education, more providers may come to understand that PrEP screening can offer not only protection from HIV, but it may engage at risk persons who are not otherwise connected to healthcare to access other needed services (45).
Conclusion
Over the past five years, successful PrEP efficacy studies and demonstration projects suggest that PrEP deployment may help decrease HIV incidence globally, particularly when coupled with offering treatment to all people living with HIV (46, 47). However, the increase in the number of PrEP prescribers may continue to be gradual, given a need to enhance numerous aspects of the provider “PrEP cascade” to achieve widespread prescribing. If governments, researchers, and medical educators are willing to commit sufficient resources to train providers, and to develop innovative approaches to HIV risk assessments that can foster the diffusion of PrEP into care settings, then healthcare practitioners could play an important role in promoting wider PrEP use.
Key Points.
Healthcare providers are likely to play a critical role in implementation of PrEP in care settings.
Recent studies suggest that specialist providers are aware of PrEP and support its provision as a public health intervention, but that prescribing has been limited to a minority of “innovators and early adopters.”
Many clinicians are cautious about providing PrEP because of concerns about the efficacy and safety of PrEP; providers also perceive practical barriers to prescribing PrEP in “real-world” settings.
Comprehensive risk assessments will be necessary to identify those persons who would benefit from PrEP, but risk assessment practices are suboptimal among providers due to lack of training, provider and patient discomfort, and time constraints.
Healthcare practitioners could serve as a valuable asset in the roll-out of PrEP if they are provided with training programs about PrEP and sexual history-taking, novel tools to facilitate routine HIV risk assessments, and opportunities to communicate with trusted colleagues about successful experiences with PrEP.
Acknowledgments
Funding
This work was supported by the National Institutes of Mental Health at the National Institutes of Health [K23 MH098795 to D.S.K.] and in part by the Harvard University Center for AIDS Research (CFAR), an NIH funded program [P30 AI060354], which is supported by the following NIH Co-Funding and Participating Institutes and Centers: NIAID, NCI, NICHD, NHLBI, NIDA, NIMH, NIA, NIDDK, NIGMS, FIC, and OAR.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of Interest
K.H.M. has conducted research with unrestricted project support from Gilead Sciences and Merck. D.S. K. has conducted research with unrestricted project support from Gilead Sciences and Bristol Myers Squibb.
References
- 1.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012 Aug 2;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choopanya K, Martin M, Suntharasamai P, Sangkum U, Mock PA, Leethochawalit M, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013 Jun 15;381(9883):2083–90. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 3.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010 Dec 30;363(27):2587–99. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4**.Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015 Feb 5;372(6):509–18. doi: 10.1056/NEJMoa1402269. This study was unable to demonstrate the efficacy of oral and topical pre-exposure prophylaxis with tenofovir-based regimens among young women in sub-Saharan Africa, likely due to low adherence among study participants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012 Aug 2;367(5):423–34. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 6.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012 Aug 2;367(5):411–22. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The New York Times. May 11, 2012. FDA Advisory Committee Supports Approval of Gilead's Truvada® for Reducing the Risk of Acquiring HIV. [Google Scholar]
- 8**.US Public Health Service. [Accessed August 6, 2015];Preexposure Prophylaxis for the Prevention of HIV Infection in the United States - 2014 Clinical Practice Guideline. Available at http://www.cdc.gov/hiv/pdf/PrEPguidelines2014.pdf. This document represents normative guidelines for pre-exposure prophylaxis for clinicians in the U.S.
- 9**.Cohen SE, Vittinghoff E, Bacon O, Doblecki-Lewis S, Postle BS, Feaster DJ, et al. High interest in preexposure prophylaxis among men who have sex with men at risk for HIV infection: baseline data from the US PrEP demonstration project. J Acquir Immune Defic Syndr. 2015 Apr 1;68(4):439–48. doi: 10.1097/QAI.0000000000000479. This study demonstrated high interest in pre-exposure prophylaxis among men who have sex with men in 3 U.S. cities as part of a demonstration project. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10**.Baeten J, Heffron R, Kidoguchi L, Mugo N, Katabira E, Bukusi E, et al. Near Elimination of HIV Transmission in a Demonstration Project of PrEP and ART [abstract 24]. Conference on Retroviruses and Opportunistic Infections; February 23–26, 2015; Seattle, Washington. This study demonstrated near-total elimination of HIV transmission within HIV serodiscordant couples with the use of antiretroviral-based prevention strategies. [Google Scholar]
- 11**.Hoagland B, Veloso VG, De Boni RB, Madruga JV, Kallas EG, Fernandes NM, et al. Pre-exposure prophylaxis (PrEP) uptake and associated factors among MSM and TGW in the PrEP Brasil demonstration project [abstract TUACO205LB]. 8th International AIDS Society Conference on HIV Pathogenesis, Treatment, and Prevention; July 19–22, 2015; Vancouver, British Columbia. This study reported high interest in the use of pre-exposure prophylaxis among men and transgender women who have sex with men in a demonstration project conducted in Brazil. [Google Scholar]
- 12.Flash C, Landovitz R, Mera Giler R, Ng L, Magnuson D, Bush Wooley S, et al. Two years of Truvada for pre-exposure prophylaxis utilization in the US. J Int AIDS Soc. 2014;17(4):19730. doi: 10.7448/IAS.17.4.19730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. [Accessed on August 1, 2015];PrEPWatch. Available at http://www.prepwatch.org/prep-access/guidance/
- 14*.Krakower DS, Oldenburg CE, Mitty JA, Wilson IB, Kurth AE, Maloney KM, et al. Knowledge, Beliefs and Practices Regarding Antiretroviral Medications for HIV Prevention: Results from a Survey of Healthcare Providers in New England. PLoS One. 2015;10(7):e0132398. doi: 10.1371/journal.pone.0132398. This study demonstrated that HIV-care providers were generally supportive of pre-exposure prophylaxis but perceived many practical barriers to prescribing it. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15*.Petroll A, Bogart LM, Horvath KJ, Mcauliffe T. PrEP knowledge, attitudes, awareness, and experience among a national sample of US primary care and HIV providers [abstract 41]. 10th International Conference on HIV Treatment and Prevention Adherence; June 28–30, 2015; Miami, Florida. This study demonstrated that generalist primary care providers were less likely to be aware of pre-exposure prophylaxis than HIV specialists, but that both groups would be willing to prescribe it. [Google Scholar]
- 16**.Karris MY, Beekmann SE, Mehta SR, Anderson CM, Polgreen PM. Are we prepped for preexposure prophylaxis (PrEP)? Provider opinions on the real-world use of PrEP in the United States and Canada. Clin Infect Dis. 2014 Mar;58(5):704–12. doi: 10.1093/cid/cit796. This national survey suggested that most infectious diseases physicians in the U.S. supported the provision of pre-exposure prophylaxis, but that few had prescribed this intervention as of 2013, and that many of these physicians reported concerns about pre-exposure prophylaxis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17*.Krakower D, Ware N, Mitty JA, Maloney K, Mayer KH. HIV providers' perceived barriers and facilitators to implementing pre-exposure prophylaxis in care settings: a qualitative study. AIDS Behav. 2014 Sep;18(9):1712–21. doi: 10.1007/s10461-014-0839-3. This study found that HIV specialists and primary care providers perceived other providers to be better positioned to prescribe pre-exposure prophylaxis, which could create a scenario where neither group engages in providing this intervention.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18*.Krakower DS, Beekmann SE, Polgreen PM, Mayer KH. Frontline practices with HIV prevention: a survey of US infectious disease physicians. Conference on Retroviruses and Opportunistic Infections [abstract 1083]; February 23–26, 2015; Seattle, Washington. This national survey suggested that infectious diseases physicians in the U.S. were becoming increasingly open to prescribing pre-exposure prophylaxis as of 2014, after the release of normative guidelines. [Google Scholar]
- 19*.Calabrese SK, Earnshaw VA, Underhill K, Hansen NB, Dovidio JF. The impact of patient race on clinical decisions related to prescribing HIV pre-exposure prophylaxis (PrEP): assumptions about sexual risk compensation and implications for access. AIDS Behav. 2014 Feb;18(2):226–40. doi: 10.1007/s10461-013-0675-x. This study demonstrated implicit racial biases among medical students who were asked to make hypothetical prescribing decisions about pre-exposure prophylaxis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20*.Henderson F, Taylor A, Chirwa L, Williams T, Borkowf C, Kasonde M, et al. Characteristics and oral PrEP adherence in the TDF2 open-label extension in Botswana [abstract TUACO203]. 8th International AIDS Society Conference on HIV Pathogenesis, Treatment, and Prevention; July 19–22, 2015; Vancouver, British Columbia. This study reported high interest in pre-exposure prophylaxis among persons in Botswana who had participated in a prior efficacy study of pre-exposure prophylaxis. [Google Scholar]
- 21. [on August 1, 2015];AIDS Vaccine Advocacy Coalition - Ongoing and Planned PrEP Evaluation Studies. Accessed at http://www.avac.org/resource/ongoing-and-planned-prep-evaluation-studies.
- 22**.McCormack S, Dunn D. Pragmatic Open-Label Randomised Trial of Preexposure Prophylaxis: The PROUD Study [abstract 22LB]. Conference on Retroviruses and Opportunistic Infections; February 23–26, 2015; Seattle, Washington. This was the first study to demonstrate the efficacy of daily oral pre-exposure prophylaxis in a European population of men who have sex with men. [Google Scholar]
- 23**.Molina JM, Capitant C, Charreau I, Meyer L, Spire B, Pialoux G, et al. On Demand PrEP With Oral TDF-FTC in MSM: Results of the ANRS Ipergay Trial [abstract 23LB]. Conference on Retroviruses and Opportunistic Infections 2015; Seattle, Washington. February 23–26, 2015; This was the first study to demonstrate the efficacy of event-driven oral pre-exposure prophylaxis in a population of men who have sex with men. [Google Scholar]
- 24*.Tang EC, Sobieszczyk ME, Shu E, Gonzales P, Sanchez J, Lama JR. Provider attitudes toward oral preexposure prophylaxis for HIV prevention among high-risk men who have sex with men in Lima, Peru. AIDS Res Hum Retroviruses. 2014 May;30(5):416–24. doi: 10.1089/aid.2013.0212. This study demonstrated that providers in Peru perceived many practical barriers to prescribing pre-exposure prophylaxis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma M, Wilton J, Senn H, Fowler S, Tan DH. Preparing for PrEP: perceptions and readiness of Canadian physicians for the implementation of HIV pre-exposure prophylaxis. PLoS One. 2014;9(8):e105283. doi: 10.1371/journal.pone.0105283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lippman SA, Koester KA, Amico KR, Lama JR, Martinez Fernandes N, Gonzales P, et al. Client and Provider Perspectives on New HIV Prevention Tools for MSM in the Americas. PLoS One. 2015;10(3):e0121044. doi: 10.1371/journal.pone.0121044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27*.Venter F, Allais L, Richter M. Exposure ethics: does HIV pre-exposure prophylaxis raise ethical problems for the health care provider and policy maker? Bioethics. 2014 Jul;28(6):269–74. doi: 10.1111/bioe.12021. This manuscript outlines the ethical implications of pre-exposure prophylaxis for clinicians and policy makers, and argues that pre-exposure prophylaxis should not raise new dilemmas for providers regarding resource allocation. [DOI] [PubMed] [Google Scholar]
- 28*.Desai M, Gafos M, Dolling D, McCormack S, Nardone A. Healthcare providers' knowledge of, attitudes to and practice of pre-exposure prophylaxis for HIV infection. HIV Med. 2015 Jul 14; doi: 10.1111/hiv.12285. This study demonstrated that half of clinicians at genitourinary clinics in the United Kingdom supported the availability of pre-exposure prophylaxis for prescribing. [DOI] [PubMed] [Google Scholar]
- 29*.Lanier Y, Castellanos T, Barrow RY, Jordan WC, Caine V, Sutton MY. Brief sexual histories and routine HIV/STD testing by medical providers. AIDS Patient Care STDS. 2014 Mar;28(3):113–20. doi: 10.1089/apc.2013.0328. The study demonstrated that a brief theory-based training for providers could enhance comfort and practices with STD and HIV risk assessments, which will be critical for identifying persons who may benefit from pre-exposure prophylaxis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sherman MD, Kauth MR, Shipherd JC, Street RL., Jr Communication between VA providers and sexual and gender minority veterans: a pilot study. Psychol Serv. 2014 May;11(2):235–42. doi: 10.1037/a0035840. [DOI] [PubMed] [Google Scholar]
- 31*.Stott DB. The training needs of general practitioners in the exploration of sexual health matters and providing sexual healthcare to lesbian, gay and bisexual patients. Med Teach. 2013 Sep;35(9):752–9. doi: 10.3109/0142159X.2013.801943. This qualitative study found that practitioners expressed a need for more training in sexual health assessments with patients who identify as lesbian, gay or bisexual. [DOI] [PubMed] [Google Scholar]
- 32.Krakower DS, Oldenburg CE, Mimiaga MJ, Novak D, Rosenberger JG, Elsesser S, et al. Patient-provider communication about sexual behaviors and pre-exposure prophylaxis: results from a national online survey of men who have sex with men in the United States [abstract TUPEC506]. 8th International AIDS Society Conference on HIV Pathogenesis, Treatment, and Prevention; July 19–22, 2015; Vancouver, British Columbia. [Google Scholar]
- 33*.Smith DK, Pals SL, Herbst JH, Shinde S, Carey JW. Development of a Clinical Screening Index Predictive of Incident HIV Infection Among Men Who Have Sex With Men in the United States. J Acquir Immune Defic Syndr. 2012 Aug 1;60(4):421–7. doi: 10.1097/QAI.0b013e318256b2f6. This study demonstrates the validity of an HIV risk prediction tool for men who have sex with men, which could help identify those persons who are most likely to benefit from pre-exposure prophylaxis. [DOI] [PubMed] [Google Scholar]
- 34*.Smith DK, Pan Y, Rose CE, Pals SL, Mehta SH, Kirk GD, et al. A Brief Screening Tool to Assess the Risk of Contracting HIV Infection Among Active Injection Drug Users. J Addict Med. 2015 May-Jun;9(3):226–32. doi: 10.1097/ADM.0000000000000123. This study demonstrates the validity of an HIV risk prediction tool for persons who inject drugs, which could help identify those persons who may benefit from pre-exposure prophylaxis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35*.Jones J, Stephenson R, Smith DK, Toledo L, La Pointe A, Taussig J, et al. Acceptability and willingness among men who have sex with men (MSM) to use a tablet-based HIV risk assessment in a clinical setting. Springerplus. 2014;3:708. doi: 10.1186/2193-1801-3-708. This study found that patients were willing to report sexual behaviors into computerized tablets as part of routine care, which could facilitate HIV risk assessments. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Langhaug LF, Cheung YB, Pascoe SJ, Chirawu P, Woelk G, Hayes RJ, et al. How you ask really matters: randomised comparison of four sexual behaviour questionnaire delivery modes in Zimbabwean youth. Sex Transm Infect. 2011 Mar;87(2):165–73. doi: 10.1136/sti.2009.037374. [DOI] [PubMed] [Google Scholar]
- 37.Jensen RE, Rothrock NE, DeWitt EM, Spiegel B, Tucker CA, Crane HM, et al. The role of technical advances in the adoption and integration of patient-reported outcomes in clinical care. Med Care. 2015 Feb;53(2):153–9. doi: 10.1097/MLR.0000000000000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vogel J, Brown JS, Land T, Platt R, Klompas M. MDPHnet: secure, distributed sharing of electronic health record data for public health surveillance, evaluation, and planning. Am J Public Health. 2014 Dec;104(12):2265–70. doi: 10.2105/AJPH.2014.302103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rogers EM. Diffusion of innovations. 5. New York: Free Press; 2003. [Google Scholar]
- 40.Berwick DM. Disseminating innovations in health care. JAMA. 2003 Apr 16;289(15):1969–75. doi: 10.1001/jama.289.15.1969. [DOI] [PubMed] [Google Scholar]
- 41**.Liu A, Cohen S, Follansbee S, Cohan D, Weber S, Sachdev D, et al. Early experiences implementing pre-exposure prophylaxis (PrEP) for HIV prevention in San Francisco. PLoS Med. 2014 Mar;11(3):e1001613. doi: 10.1371/journal.pmed.1001613. This study reported successful early experiences with provision of pre-exposure prophylaxis in 3 clinics in San Francisco. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mayer K, Krakower D, Levine K, Grasso C, Gelmam M. Significant increases in HIV pre-exposure prophylaxis (PrEP) uptake in Boston, a Boston Community Health Center in 2014: who are the recent users? [abstract TUPEC508]. 8th International AIDS Society Conference on HIV Pathogenesis, Treatment, and Prevention; July 19–22, 2015; Vancouver, British Columbia. [Google Scholar]
- 43.New York State Department of Health. [Accessed on August 6, 2015];Pre Exposure Prophylaxis (PrEP) and Non-Occupational Post-Exposure Prophylaxis (PEP) Available at https://www.health.ny.gov/diseases/aids/general/prep/index.htm.
- 44*.Blumenthal J, Jain S, Krakower D, Sun X, Young J, Mayer K, et al. Knowledge is Power! Increased Provider Knowledge Scores Regarding Pre-exposure Prophylaxis (PrEP) are Associated with Higher Rates of PrEP Prescription and Future Intent to Prescribe PrEP. AIDS Behav. 2015 May;19(5):802–10. doi: 10.1007/s10461-015-0996-z. This survey found that increased knowledge was associated with prescribing experiences and intentions regarding pre-exposure prophylaxis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45*.Golub SA. PrEP Can “Do More”: Synergistic effects on primary care, insurance and mental health [abstract 174]. 10th International Conference on HIV Treatment and Prevention Adherence; June 28–30, 2015; Miami, Florida. This study demonstrated that participants in a study of pre-exposure prophylaxis derived comprehensive benefits from their participation in the study, including increased engagement in primary care and improved psychological well-being. [Google Scholar]
- 46.Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. 2015 Jul 20; doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.A Trial of Early Antiretrovirals and Isoniazid Preventive Therapy in Africa. N Engl J Med. 2015 Jul 20; doi: 10.1056/NEJMoa1507198. [DOI] [PubMed] [Google Scholar]