Abstract
Balamuthia mandrillaris , a free-living ameba, causes rare but frequently fatal granulomatous amebic encephalitis (GAE). Few patients have survived after receiving experimental drug combinations, with or without brain lesion excisions. Some GAE survivors have been treated with a multi-drug regimen including miltefosine, an investigational anti-leishmanial agent with in vitro amebacidal activity. Miltefosine dosing for GAE has been based on leishmaniasis dosing because no data exist in humans concerning its pharmacologic distribution in the central nervous system. We describe results of limited cerebrospinal fluid (CSF) and serum drug level testing performed during clinical management of a child with fatal GAE who was treated with a multiple drug regimen including miltefosine. Brain biopsy specimens, CSF, and sera were tested for B. mandrillaris using multiple techniques, including culture, real-time polymerase chain reaction, immunohistochemical techniques, and serology. CSF and serum miltefosine levels were determined using a liquid chromatography method coupled to tandem mass spectrometry. The CSF miltefosine concentration on hospital admission Day 12 was 0.4 μg/mL. The serum miltefosine concentration on Day 37, about 80 hours post-miltefosine treatment, was 15.3 μg/mL. These are the first results confirming some blood-brain barrier penetration by miltefosine in a human, although with low-level CSF accumulation. Further evaluation of brain parenchyma penetration is required to determine optimal miltefosine dosing for Balamuthia GAE, balanced with the drug’s toxicity profile. Additionally, the Balamuthia isolate was evaluated by real-time PCR, demonstrating genetic variability in18S rRNA sequences and possibly signaling the first identification of multiple Balamuthia strains with varying pathogenicities.
Keywords: Balamuthia, granulomatous, encephalitis, miltefosine
Introduction
Balamuthia mandrillaris, a free-living ameba, has been isolated from soil (Schuster et al. 2003; Dunnebacke et al. 2004; Niyyati et al. 2009) and its DNA has been identified in dust by polymerase chain reaction (PCR) (Ahmad et al. 2011). It may also be present in water, having been identified in dogs in contact with stagnant water (Foreman et al. 2004; Finnin et al. 2007). In the United States, the most common disease caused by Balamuthia infections is granulomatous amebic encephalitis (GAE), which in a minority of cases is preceded by or concurrent with the development of Balamuthia-induced skin lesions (CDC unpublished data). It is likely transmitted by inhalation of airborne cysts or by direct contamination through broken skin. Once Balamuthia enters the body, it is believed to spread hematogenously into the central nervous system (CNS) by breaching the blood-brain barrier (Visvesvara et al. 2011; Schuster et al. 2009; Reed et al. 1997). GAE occurs in both immunocompetent and immunocompromised persons of all ages, although Balamuthia GAE does appear to be more common among persons of Hispanic ethnicity (Visvesvara et al. 2011; Schuster et al. 2009, Schuster et al. 2004).
GAE is usually a chronic disease with a prodromal period apparently lasting for several weeks to months. Initial signs and symptoms can include headache, neck stiffness, photophobia, vomiting, personality changes, and tonic-clonic seizures (Visvesvara et al. 2011). More than 200 GAE cases have been reported worldwide (Visvesvara et al. 2011; Schuster et al. 2009). Timely GAE diagnosis is difficult, particularly because of nonspecific symptoms mimicking other types of encephalitis and lack of physician awareness of this disease. Therefore, other cases have likely occurred that were either undiagnosed or misdiagnosed.
Most GAE cases are fatal. Diagnosis is usually made by histochemical methods on biopsied brain tissue obtained late in the clinical course or at autopsy (Visvesvara et al. 2011). Nevertheless, a few patients have survived after biopsies (with or without complete excision of brain lesions) and subsequent treatment with experimental combinations of drugs, including pentamidine isethionate, fluconazole, flucytosine (5-fluorocytosine), sulfadiazine, and a macrolide (azithromycin or clarithromycin) (Deetz et al. 2003; Jung et al. 2004; Bravo et al. 2011; Cary et al. 2010). Miltefosine, an anti-protozoal drug used to treat visceral and cutaneous leishmaniasis, has also shown promise in treating GAE in combination with other anti-amebic drugs (Bravo et al. 2011; Martínez et al. 2010; Centers for Disease Control and Prevention 2010). Miltefosine, a drug approved by the U.S. Food and Drug Administration (FDA) for the treatment of leishmaniasis (Food and Drug Administration 2015), is not sold in the United States but is available from the Centers for Disease Control and Prevention (CDC) for the treatment of free-living amebae infections under an expanded-access investigational new drug protocol in effect with the FDA (Centers for Disease Control and Prevention 2013). However, appropriate miltefosine dosing for GAE treatment has not been established and currently follows dosing regimens recommended for leishmaniasis treatment, which do not account for blood-brain barrier penetration or brain parenchyma accumulation because leishmaniasis is only rarely associated with neurologic pathology (Petersen and Greenlee 2011). Although miltefosine CNS penetration has been demonstrated in rats (Marschner et al. 1992), no data on CNS distribution in humans exists to guide GAE dosing decisions.
We present a case report of a child with fatal Balamuthia GAE and the first data from drug level testing performed in the course of clinical management that demonstrate blood-brain barrier penetration of miltefosine in a human.
Case report
An 11-year-old Texas boy of Hispanic ethnicity was hospitalized one August with a 3-week history of intermittent nausea, vomiting, progressive lethargy, clumsiness, and right-sided weakness resulting in a fall the day before admission. In retrospect, his parents noted neurologic changes dating back to April when he developed altered penmanship and problems reading and conversing; additionally, his grades slipped during the last 9 weeks of the school year and he experienced intermittent vomiting throughout most of the summer. The patient never complained of headaches or visual changes. His past medical history was otherwise unremarkable. He reportedly liked to dig in soil looking for artifacts, like arrowheads.
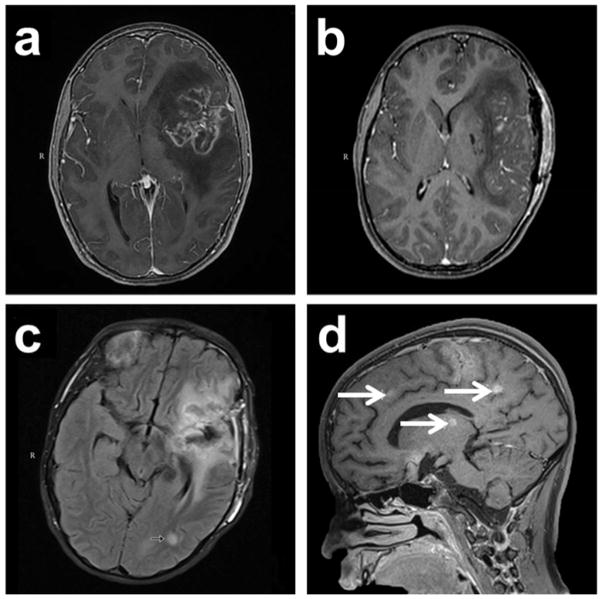
On admission Day 1, magnetic resonance imaging (MRI) of the brain revealed a nonspecific multifocal process predominantly involving the left cerebral hemisphere in the temporal and frontal lobes with severe edema and mass effect resulting in a midline shift (Figure 1). A tumor was suspected so a brain biopsy was performed on Day 2 and an extra-ventricular drain (EVD) was placed to monitor intracranial pressure post-operatively. Hematoxylin and eosin (H&E)-stained tissue sections showed amebic trophozoites and GAE was diagnosed; the patient was given anti-amebic chemotherapy beginning on Day 3. Table 1 summarizes the patient’s multi-drug regimen by hospital day. Indirect immunofluorescence testing (IFA) and real-time PCR performed at CDC later identified the amebae as Balamuthia mandrillaris. The patient also had an elevated anti-Balamuthia serum titer initially 1:64, considered positive for Balamuthia exposure (Schuster et al. 2006b), but rising to 1:256 later in the course of illness.
Fig. 1.
Magnetic resonance imaging (MRI) of the brain during the patient’s clinical course. (a). Initial MRI on admission Day 1 revealed a nonspecific multifocal lesion involving the left frontal temporal lobe with surrounding edema and midline shift. (b). MRI on Day 8 revealed postoperative changes with diminished mass effect and midline shift. (c). MRI on Day 20 (flair axial image) revealed a new lesion in left temporo-occipital white matter (arrow) and further reduction in edema with resolution of midline shift. (d). MRI on Day 26 (T1 sagittal image) revealed numerous new foci throughout the brain (arrows)
Table 1.
Drugs administered to a patient with Balamuthia mandrillaris granulomatous amebic encephalitis, United States, 2010
| Drug | Initiation Date (Hospital Admission Day) | Duration | Dose | Comments |
|---|---|---|---|---|
| Dexamethasone | Day 1 | 25 days | 4mg IV every 6 hours, slowly tapered | Anti-inflammatory drug for cerebral edema |
| Fosphenytioin | Day 1 | 4 days | 100 mg IV every 12 hours | Anticonvulsant drug |
| Liposomal amphotericin B | Day 2 | 3 days | 380 mg IV daily (10 mg/kg/day) | Anti-amebic drug; first administered at initial suspicion of GAE, discontinued in favor of more specific anti-Balamuthia therapy |
| Azithromycin | Day 3 | 58 days | 750 mg IV daily | Anti-amebic drug |
| Fluconazole | Day 3 | 58 days | 400 mg IV daily | Anti-amebic drug adjusted for renal insufficiency |
| 5-fluorocytosine (5FC) | Day 3 | 58 days | 1000 to 1425 mg per nasogastric tube (NG) every 6 hours | Anti-amebic drug adjusted based on levels |
| Pentamidine | Day 3 | 23 days | 160 mg IV daily | Anti-amebic drug discontinued due to pancreatitis and electrolyte problems |
| Sulfadiazine | Day 3 | 58 days | 500 mg PO and NG every 6 hours | Anti-amebic drug |
| Levetiracetam | Day 5 | 12 days | 400mg IV every 12 hours | Anticonvulsant drug |
| Miltefosine | Day 7 | 26.5 days | 50 mg NG twice daily (2.65 mg/kg/day) | Anti-amebic drug discontinued prior to death due to progression of neurologic deterioration, development of multiple organ dysfunction due to presumed drug toxicities, and uncertainty of the contributing role of miltefosine to these toxicities |
| Phenobarbital | Day 14 | 6 days | 55 to 110 mg IV every 12 hours | Anticonvulsant drug |
| Valproate | Day 20 | 4 days | 500 mg IV every 12 hours | Anticonvulsant drug |
| Lorazapam | Day 21 | 23 days | 1.0–2.5 mg IV every 8 hrs, with additional doses as required | Anticonvulsant drug |
| Metronidazole | Day 22 | 39 days | 370 mg IV every 6 hours | Anti-amebic drug for a new brain lesion discovered on MRI on Day 20 |
| Thioridazine | Day 26 | 35 days | 15 mg NG twice daily | Anti-psychotic drug and might also have anti-amebic activity |
| Methylprednisolone | Day 47 | 14 days | 20 mg IV every 6 hours | Anti-inflammatory drug for cerebral edema |
During the week following hospitalization, the patient became febrile and had increased nausea and vomiting. On Day 5, he had a right-sided focal seizure and received anticonvulsant drugs. On Day 6, he developed twitching in his hands and feet; an electroencephalogram showed no focal seizure activity. He also developed a mild right hemiparesis and was more lethargic but still arousable, alert, and oriented. Miltefosine was started on Day 7. On Day 8, a repeat MRI revealed a diminished mass effect and midline shift with a mild reduction in lesion enhancement but with extensive areas of edema remaining and an acute left putamen lacunar infarct. His clinical condition remained fairly stable for several days and his right-sided weakness and twitching remained unchanged. However, by Day 20, he had newly developed double vision and another MRI that day showed a new lesion in the left temporal occipital white matter, although he had reduced edema and a resolution of the previous midline shift. Approximately 3 weeks post-admission, his mental and neurologic status worsened significantly and he developed pancreatitis, renal impairment, myoglobinurea, and diarrhea. A MRI brain scan on Day 26 showed multiple new foci in the white matter. By Day 32, he had complete right hemiparesis, could say only a few words and follow simple commands, had lost his gag reflex, and had developed aspiration pneumonia. Miltefosine was discontinued on Day 34 (see Table 1). He received a tracheostomy about 5.5 weeks post-admission but never regained full alertness after post-operative sedation was lifted. Follow-up MRIs revealed progressing multifocal lesions in the right temporal/parietal lobe, left parietal lobe/thalamus, posterior fossa, and cerebellum. He was placed in palliative care on Day 49 and by Day 54 showed very little spontaneous respiratory effort. He was pronounced dead 61 days post-admission.
Materials and methods
Several specimens were sent to CDC for further testing, including brain tissue removed at biopsy and autopsy, and cerebrospinal fluid (CSF) and sera obtained during the clinical course. CSF and a portion of fresh brain tissue (macerated) were separately inoculated into human lung fibroblast (HLF) monolayers. The HLF cell cultures were sub-cultured periodically and amebae were harvested and stored at −80°C. The amebae stored at −80°C were thawed and disrupted and the resulting protein antigens were subjected to SDS-PAGE (Kucerova et al. 2011). Separated proteins were silver stained and compared with protein profiles of other isolates (Kucerova et al. 2011). Real-time PCR (Qvarnstrom et al. 2006) was performed on DNA from brain tissue, CSF and cultured amebae, extracted using the DNeasy tissue and blood kit (QIAGEN). Mitochondrial and nuclear small subunit ribosomal genes were amplified and sequenced as previously described (Booton et al. 2003a; Booton et al. 2003b). The mitochondrial gene (16S rRNA) was amplified twice and Sanger sequenced in both directions, resulting in 2- to 6-fold sequencing coverage at all positions. The PCR product from the nuclear gene (18S rRNA) was cloned as described elsewhere since it could not be sequenced directly due to overlapping peaks in the chromatograms (Qvarnstrom 2013). Two clones were sequenced with at least 4-fold coverage at all positions; the two clones differed in just two positions towards the end of the gene. Consensus DNA sequences from the 18S and the 16S rRNA genes were deposited in GenBank with accession numbers JX524850 and JX524851, respectively. Formalin-fixed tissues from brain, optic nerve, lungs, liver, pancreas, and spleen were embedded in paraffin and sectioned. Sections were either stained with H&E or reacted with rabbit anti-Balamuthia antiserum (IFA) as described previously (Visvesvara et al. 1990) and examined using an Olympus BX-60 microscope. Anti-Acanthamoeba serum served as a negative control because anti-Balamuthia antibodies do not cross react with anti-Acanthamoeba antibodies (Kucerova et al. 2011; Huang et al. 1999; Kiderlen et al. 2009). As with brain tissue, CSF was also cultured (Visvesvara et al. 1990; Visvesvara et al. 1993) and tested by real-time PCR. Multiple serum samples were tested for anti-Balamuthia antibodies by IFA (Schuster et al. 2006b). Miltefosine levels in CSF and serum samples were analyzed by a validated liquid chromatography method coupled to tandem mass spectrometry (LC-MS/MS) with slight adaptations (Dorlo et al. 2008a). These samples were diluted (1:20) in blank human sodium EDTA plasma (Bioreclamation Inc., Hicksville, NY, USA) and further processed as previously described (Dorlo et al. 2008a). Samples were quantified based on a calibration line (4–2000 ng/mL) in human plasma. After sample preparation with phenyl-based solid phase extraction (SPE) and chromatographic separation on a Gemini C18 column (150 mm × 4.6 mm, 5 μm) using 10mM NH4OH in methanol: water (95:5, v/v) as a mobile phase, samples were analyzed employing an API365 triple quadrupole mass spectrometer (Sciex, Thornhill, ON, Canada). Accuracy and precision were within ±15% deviation on all concentration levels and the lower limit of quantitation (in plasma) was 4 ng/mL (Dorlo et al. 2008a).
Results
Balamuthia began to be noticeable within one week in the HLF cell cultures inoculated with CSF and brain tissue. Amebae consumed, multiplied, and destroyed the HLF monolayer initially within two weeks and thereafter within three days on new platings, depending upon the numbers of amebae inoculated. Amebae converted to cysts when all of the monolayer was consumed. Both trophozoites and cysts of the isolate, designated as CDC:V630, were considerably smaller than the index Balamuthia organism originally isolated from a baboon brain (Visvesvara et al. 1990). However, they were morphologically similar to this index organism and other isolates of Balamuthia. Silver-stained protein profiles of cultured amebae revealed a complex pattern and produced major bands ranging from 200 to 10 kDa, but were more or less similar to that of the index isolate (Visvesvara et al. 1990).
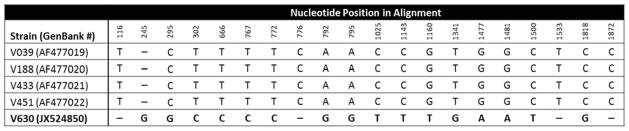
Brain tissue and CSF from the patient, as well as the CDC:V630 culture, tested positive for B. mandrillaris in a real-time PCR assay developed to detect pathogenic free-living amebae in clinical specimens. On further molecular examination of the CDC:V630 culture, the mitochondrial small subunit ribosomal gene (16S rRNA) fragment was 1,075 base pairs in length and exhibited 98.6%–99.5% sequence similarity to corresponding sequences from other isolates of B. mandrillaris, with the highest similarity score to the V451 strain isolated previously (Booton et al. 2003a). This degree of variation is within the range previously found among B. mandrillaris 16S rRNA genes (Booton et al. 2003a). However, the nuclear small subunit ribosomal gene (18S rRNA) showed sequence divergence from existing sequences in the GenBank database (http://www.ncbi.nlm.nih.gov/genbank/index.html); the V630 isolate differed in 20 out of 1,969 nucleotide positions (including 11 transitions, 4 transversions, and 5 indels) from the other B. mandrillaris isolates in GenBank (Figure 2).
Fig. 2.
Variable positions in the 18S rRNA gene sequences from Balamuthia mandrillaris isolates available in GenBank as of 2013. The alignment was made with ClustalW using Geneious v7.1.5. A dash (-) indicate an insertion/deletion. Total length of alignment was 1,973 base pairs. The isolate from the patient described in this report was designated as CDC:V630
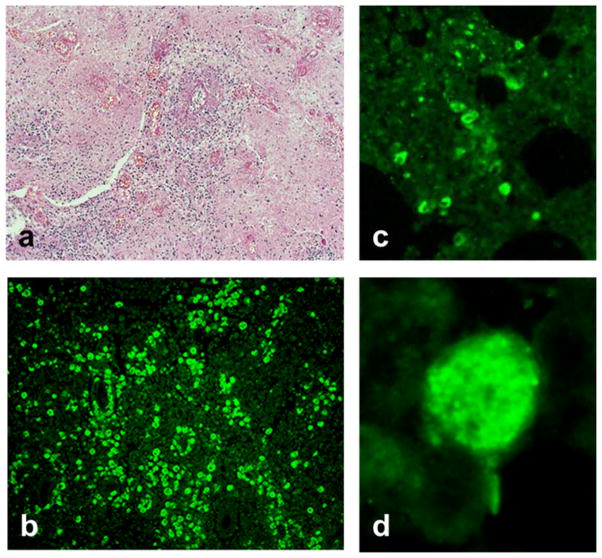
On microscopic examination of H&E-stained sections, the brain and optic nerve appeared necrotic and amebae characterized by single nuclei were seen interspersed with degraded tissue (Figure 3a). Intense necrotic inflammation was evident with large numbers of lymphocytes and polymorphonuclear leukocytes. Abundant amebae were seen in the necrotic optic nerve. Cysts were not evident in the tissue sections. The tissue sections examined by IFA reacted intensely with the rabbit anti-B. mandrillaris serum and produced fluorescence (Figure 3b). Negative reaction was seen with the rabbit anti-Acanthamoeba serum. Sections of the lung stained with H&E showed inflammation but amebae were not readily seen. However, the anti-Balamuthia-treated lung sections revealed a couple of foci of intense fluorescence and closer examination revealed a few glowing amebae (Figure 3c–d). All other tissue sections were negative for amebae.
Fig. 3.
Hematoxylin and eosin (H&E) or indirect immunofluorescence (IFA) staining of tissue sections. (a) A section of brain stained with H&E—Balamuthia amebae are seen interspersed within the tissue at magnification X 100. (b) A similar section of brain reacted with anti-Balamuthia mandrillaris serum in the IFA test at magnification X 100. (c) IFA reactivity of Balamuthia amebae in a section of lung at magnification X 100 indicating dissemination of amebae into the lung. (d) A high power (magnification X 1,000) view of an ameba in the lung by IFA staining
The patient’s serum samples produced high IFA titers for Balamuthia (from 1:64 to 1:256). Serum titers for anti-Balamuthia antibodies range from none to about 1:32 in patients without Balamuthia GAE and a titer of 1:64 or higher is considered a positive immune response (Schuster et al. 2006b).
Mass spectrometry analyses of miltefosine concentrations in the CSF and serum are summarized in Table 2. From Days 7–34, the patient received 50 mg miltefosine per nasogastric tube (NG) twice daily, corresponding to 2.65 mg/kg/day. On Day 12, miltefosine was first detected on analysis of ventricular CSF collected in the external bag of the EVD placed on Day 2 following the brain biopsy. The EVD was removed on admission Day 12 and thereafter no further CSF/ventricular fluid samples were available for testing. The only serum sample was tested for miltefosine on Day 37, about 80 hours after termination of miltefosine treatment when the patient had developed acute renal insufficiency (creatinine 4.2 mg/dL, BUN 87 mg/dL) and pancreatitis, presumed to be due to pentamidine.
Table 2.
Miltefosine levels in the cerebrospinal fluid and serum of a patient with Balamuthia mandrillaris granulomatous amebic encephalitis, United States, 2010
| Specimen Type | Collection Date (Hospital Admission Day) | Days After Initiation of Miltefosine Treatment a | Miltefosine Concentration (μg/mL) b |
|---|---|---|---|
| CSF | Day 6 | −1 | 0 (<LOD) c |
| CSF | Day 6 | −1 | 0 (<LOD) c |
| CSF | Day 12 | 5 | 0.4 |
| Serum | Day 37 | 30 (about 80 hours after last dose) | 15.3 d |
First dose of miltefosine (50 mg twice daily) administered on Day 7 and last dose administered on Day 34.
Analyzed on API365 LC-MS/MS equipment; dilution of CSF and sample pretreatment using phenyl-based solid phase reaction.
LOD = Limit of detection; not determined for miltefosine in CSF but is probably below 20 ng/mL.
At this time, patient had developed acute renal insufficiency (creatinine 4.2 mg/dL, BUN 87 mg/dL) and pancreatitis presumed to be due to pentamidine.
Discussion
B. mandrillaris is the only known species of the genus Balamuthia (Visvesvara et al. 2011). In our patient, the nuclear small subunit ribosomal gene (18S rRNA) of his isolate (CDC:V630) showed quite a few sequence divergences from existing sequences in GenBank. This was unexpected since all previously determined 18S rRNA sequences from B. mandrillaris have been 100% identical, indicating that there is more DNA sequence divergence within the Balamuthia genus than previously thought and that the 16S rRNA gene may not be the best locus to explore that variability. Furthermore, this isolate was considerably smaller in size compared to other B. mandrillaris isolates. These observations might signal the presence of multiple strains of B. mandrillaris with varying pathogenicities, which could help explain the elevated anti-Balamuthia antibody levels observed in some asymptomatic but presumably exposed individuals in Africa and Australia (Huang et al. 1999; Kiderlen et al. 2009).
When Balamuthia exposure results in GAE, it is generally fatal. Many drug combinations have been administered to Balamuthia GAE patients but few have recovered (Deetz et al. 2003; Jung et al. 2004; Bravo et al. 2011; Cary et al. 2010; Martínez et al. 2010; Centers for Disease Control and Prevention 2010) and effective treatment has not yet been established. Our patient was treated with corticosteroids and multiple antimicrobials. One of the antimicrobials given to our patient was miltefosine. Miltefosine is an orally administered alkylphosphocholine compound that was originally developed as an anti-cancer drug (Eibl and Unger 1990). However, for infectious diseases, it has mainly been used as an anti-protozoal drug and the World Health Organization (WHO) lists miltefosine in its essential medicines formulary as an anti-leishmaniasis medicine (World Health Organization, 2015). Miltefosine is currently approved for treatment of leishmaniasis in the United States by FDA (U.S. Food and Drug Administration, 2015). Recently, miltefosine has not only shown good in vitro activity against free-living amebae but has also appeared promising in treating patients with Balamuthia GAE, in combination with other drugs (Bravo et al., 2011; Martínez et al. 2010; Centers for Disease Control and Prevention 2010). Our patient received 53 doses of miltefosine (50 mg BID) over 26.5 days (Days 7–34). After an initial stabilization of symptoms, miltefosine was discontinued 27 days prior to death due to progression of neurologic deterioration, development of multiple organ dysfunction due to presumed drug toxicities, and uncertainty of the contributing role of miltefosine to these toxicities. At the time, no information was available in the literature about CNS penetration of oral miltefosine to guide drug dosing during his clinical course. Drug level testing on available CSF and serum samples was therefore performed.
The presence of miltefosine in our patient’s CSF 5 days after initiation of miltefosine treatment indicated penetration of miltefosine through the blood-brain barrier, although disruption of the barrier due to Balamuthia infection could not be excluded. Moderate brain tissue penetration of miltefosine was previously demonstrated in rats (Marschner et al. 1992) but has never been evaluated in humans and was not evaluated directly in our patient. However, some conclusions can still be suggested with our limited CSF and serum samples. Our patient’s serum level approximately 80 hours after miltefosine discontinuation was 15.3 μg/mL. Taking into account the known miltefosine initial elimination half-life of approximately 7 days (Dorlo et al. 2008b; Dorlo et al. 2012b), the extrapolated steady-state serum concentration at the time of miltefosine discontinuation would have approximated 21.3 μg/mL. The in vitro minimum amebacidal concentration (MAC) of miltefosine for some Balamuthia isolates has been shown to be 16.3 μg/mL (40 μM), with variation in sensitivity between strains (Schuster et al. 2006a). Although this value might not be comparable between labs due to the use of different assay methodologies and is likely difficult to extrapolate to the in vivo situation, in particular because of the use of protein-rich culture media (Schuster and Visvesvara 1996), the approximated serum miltefosine level at the time of discontinuation exceeded this MAC. However, this MAC value must be interpreted with caution. Miltefosine is 95% protein-bound (Dorlo et al. 2008b, Dorlo et al. 2012a). Consequently, only 5% of the total serum concentration represents free drug. If the pharmacodynamics of anti-amebic agents is similar to antibacterial agents, then only the free drug fraction would be expected to be pharmacologically active. The original in vitro MAC experiments used protein-rich culture media (Schuster and Visvesvara 1996) but did not account for the effects of potential protein binding in the MAC calculations. Therefore, the MAC represents the total miltefosine concentration, not the free miltefosine concentration, and cannot be used as a basis of comparison for the latter. Penetration of miltefosine from serum into CSF and brain parenchymal tissue is of additional and particular importance. The miltefosine CSF level in our patient on Day 12 after 9 doses (50 mg per dose) of the drug over 5 days was 0.4 μg/mL, a level much less than that expected to be efficacious in the treatment of Balamuthia infection. Assuming that the serum concentration was between 10–20 μg/mL on Day 12, given the 7-day half-life and the fact that steady state of the drug had not yet been reached, this suggests only 2–4% passage across the blood-brain-barrier. Further, depending upon the concentration of protein in CSF, the free drug level was likely substantially less than the total drug level achieved in CSF. While these results may suggest that miltefosine would not be effective in the treatment of meningitis (measured CSF levels), miltefosine might still be effective in treating encephalitis (unmeasured brain parenchymal levels) since the primary infection site for Balamuthia is brain parenchyma, not the meninges. There is precedence for other agents that pass poorly into CSF, such as macrolides, penetrating well into brain parenchyma (Nau et al. 2010; Jaruratanasirikul et al. 1996). Despite our patient’s measured drug levels, miltefosine has shown promise in treating other patients with Balamuthia GAE, in combination with other drugs (Bravo et al., 2011; Martínez et al. 2010; Centers for Disease Control and Prevention 2010). It is possible that the observed success of miltefosine in reported GAE cases may be due to superior penetration into brain parenchyma when compared to CSF. Moreover, in addition to its direct amebacidal effects, previously demonstrated miltefosine immunomodulatory effects may also play a role (Wadhone et al. 2009).
Conclusion
Balamuthia GAE, while rare, continues to have devastating outcomes and high mortality. Even with rapid diagnosis and interventional therapy, our case was fatal. The ameba isolated and cultured from this patient differed in size and ribosomal DNA sequences from previously identified B. mandrillaris isolates, suggesting there may be multiple strains of B. mandrillaris that may affect pathogenicity and drug resistance. Further investigation of Balamuthia through strain typing and genome sequencing should be considered.
CSF drug level testing performed during clinical management of a child with fatal Balamuthia GAE demonstrated for the first time that miltefosine, an anti-leishmanial agent with in vitro amebacidal activity, penetrates the blood-brain barrier to a limited extent in a human. Although miltefosine might hold promise as an adjunct to the current experimental drug cocktail now used to treat Balamuthia GAE, more research is needed to find the most effective doses and optimal drug combination. Given our patient’s death after receiving 2.65 mg/kg/day of miltefosine and considering that pediatric patients need a higher mg/kg dosage compared to adults to achieve a similar level of miltefosine exposure (Dorlo et al. 2012b), a higher daily miltefosine dosage might be considered in future Balamuthia GAE cases if the apparent moderate gastrointestinal and moderate liver toxicity caused by this drug can be controlled.
Footnotes
Conflict of Interest
The authors declare that they have no conflicts of interest.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- Ahmad AF, Andrews PW, Kilvington S. Development of a nested PCR for environmental detection of the pathogenic free-living amoeba Balamuthia mandrillaris. J Eukaryot Microbiol. 2011;58:269–271. doi: 10.1111/j.1550-7408.2011.00541.x. [DOI] [PubMed] [Google Scholar]
- Booton GC, Carmichael JR, Visvesvara GS, Byers TJ, Fuerst P. Genotyping of Balamuthia mandrillaris based on nuclear 18S and mitochondrial 16S rRNA genes. Am J Trop Med Hyg. 2003a;68:65–69. [PubMed] [Google Scholar]
- Booton GC, Carmichael JR, Visvesvara GS, Byers TJ, Fuerst PA. Identification of Balamuthia mandrillaris by PCR assay using the mitochondrial 16S rRNA gene as a target. J Clin Microbiol. 2003b;41:453–455. doi: 10.1128/JCM.41.1.453-455.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo FG, Alvarez PJ, Gotuzzo E. Balamuthia mandrillaris infection of the skin and central nervous system: an emerging disease of concern to many specialties in medicine. Curr Opin Infect Dis. 2011;24:112–117. doi: 10.1097/QCO.0b013e3283428d1e. [DOI] [PubMed] [Google Scholar]
- Cary LC, Maul E, Potter C, Wong P, Nelson PT, Given C, 2nd, Robertson W., Jr Balamuthia mandrillaris meningoencephalitis: survival of a pediatric patient. Pediatrics. 2010;125:e699–703. doi: 10.1542/peds.2009-1797. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Balamuthia mandrillaris transmitted through organ transplantation—Mississippi, 2009. MMWR Morb Mortal Wkly Rep. 2010;59:1165–1170. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Investigational drug available directly from CDC for the treatment of infections with free-living amebae. MMWR Morb Mortal Wkly Rep. 2013;62:666. [PMC free article] [PubMed] [Google Scholar]
- Deetz TR, Sawyer MH, Billman G, Schuster FL, Visvesvara GS. Successful treatment of Balamuthia amoebic encephalitis: presentation of two cases. Clin Infect Dis. 2003;37:1304–1312. doi: 10.1086/379020. [DOI] [PubMed] [Google Scholar]
- Dorlo TP, Balasegaram M, Beijnen JH, de Vries PJ. Miltefosine: a review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. J Antimicrob Chemother. 2012a;67:2576–2597. doi: 10.1093/jac/dks275. [DOI] [PubMed] [Google Scholar]
- Dorlo TPC, Hillebrand MJX, Rosing H, Eggelte TA, de Vries PJ, Beijnen JH. Development and validation of a quantitative assay for the measurement of miltefosine in human plasma by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2008a;865:55–62. doi: 10.1016/j.jchromb.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Dorlo TP, Huitema AD, Beijnen JH, de Vries PJ. Optimal dosing of miltefosine in children and adults with visceral leishmaniasis. Antimicrob Agents Chemother. 2012b;56:3864–3872. doi: 10.1128/AAC.00292-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorlo TP, van Thiel PP, Huitema AD, Keizer RJ, de Vries HJ, Beijnen JH, de Vries PJ. Pharmacokinetics of miltefosine in Old World cutaneous leishmaniasis patients. Antimicrob Agents Chemother. 2008b;52:2855–2860. doi: 10.1128/AAC.00014-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnebacke TH, Schuster FL, Yagi S, Booton GC. Balamuthia mandrillaris from soil samples. Microbiology. 2004;150:2837–2842. doi: 10.1099/mic.0.27218-0. [DOI] [PubMed] [Google Scholar]
- Eibl H, Unger C. Hexadecylphosphocholine: a new and selective antitumor drug. Cancer Treat Rev. 1990;17:233–242. doi: 10.1016/0305-7372(90)90053-i. [DOI] [PubMed] [Google Scholar]
- Finnin PJ, Visvesvara GS, Campbell BE, Fry DR, Gasser RB. Multifocal Balamuthia mandrillaris infection in a dog in Australia. Parasitol Res. 2007;100:423–426. doi: 10.1007/s00436-006-0302-0. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration. Impavido/Miltefosine—Label and Approval History. [Accessed 01 July 2015];Drugs@FDA. 2015 http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Label_ApprovalHistory#apphist.
- Foreman O, Sykes J, Ball L, Yang N, De Cock H. Disseminated infection with Balamuthia mandrillaris in a dog. Vet Pathol. 2004;41:506–510. doi: 10.1354/vp.41-5-506. [DOI] [PubMed] [Google Scholar]
- Huang ZH, Ferrante A, Carter RF. Serum antibodies to Balamuthia mandrillaris, a free-living amoeba recently demonstrated to cause granulomatous amoebic encephalitis. J Infect Dis. 1999;179:1305–1208. doi: 10.1086/314731. [DOI] [PubMed] [Google Scholar]
- Jaruratanasirikul S, Hortiwakul R, Tantisarasart T, Phuenpathom N, Tussanasunthornwong S. Distribution of azithromycin into brain tissue, cerebrospinal fluid, and aqueous humor of the eye. Antimicrob Agents Chemother. 1996;40:825–826. doi: 10.1128/aac.40.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Schelper RL, Visvesvara GS, Chang HT. Balamuthia mandrillaris meningoencephalitis in an immunocompetent patient: an unusual clinical course and a favorable outcome. Arch Pathol Lab Med. 2004;128:466–468. doi: 10.5858/2004-128-466-BMMIAI. [DOI] [PubMed] [Google Scholar]
- Kiderlen AF, Radam E, Tata PS. Assessment of Balamuthia mandrillaris-specific serum antibody by flow cytometry. Parasitol Res. 2009;104:663–670. doi: 10.1007/s00436-008-1243-6. [DOI] [PubMed] [Google Scholar]
- Kucerova Z, Sriram R, Wilkins PP, Visvesvara GS. Identification of antigenic targets for immunodetection of Balamuthia mandrillaris. Clin Vaccine Immunol. 2011;18:1297–1301. doi: 10.1128/CVI.05082-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner M, Kötting J, Eibl H, Unger C. Distribution of hexadecylphosphocholine and octadecyl-methyl-glycero-3-phosphocholine in rat tissues during steady-state treatment. Cancer Chemother Pharmacol. 1992;31:18–22. doi: 10.1007/BF00695989. [DOI] [PubMed] [Google Scholar]
- Martínez DY, Seas C, Bravo C, Legua P, Ramos C, Cabello AM, Gotuzzo E. Successful treatment of Balamuthia mandrillaris amoebic infection with extensive neurological and cutaneous involvement. Clin Infect Dis. 2010;51:e7–11. doi: 10.1086/653609. [DOI] [PubMed] [Google Scholar]
- Nau R, Sörgel F, Eiffert H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin Microbiol Rev. 2010;23:858–883. doi: 10.1128/CMR.00007-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyyati M, Lorenzo-Morales J, Rezaeian M, Martin-Navarro CM, Haghi AM, Maciver SK, Valladares B. Isolation of Balamuthia mandrillaris from urban dust, free of known infectious involvement. Parasitol Res. 2009;106:279–281. doi: 10.1007/s00436-009-1592-9. [DOI] [PubMed] [Google Scholar]
- Petersen CA, Greenlee MHW. Neurologic manifestations of Leishmania spp. infection. J Neuroparasitology. 2011;2 doi: 10.4303/jnp/N110401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qvarnstrom Y, Nerad TA, Visvesvara GS. Characterization of a new pathogenic Acanthamoeba species, A. byersi n. sp., isolated from a human with fatal amoebic encephalitis. J Eukaryot Microbiol. 2013;60:626–633. doi: 10.1111/jeu.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qvarnstrom Y, Visvesvara GS, Sriram R, da Silva AJ. Multiplex real-time PCR assay for simultaneous detection of Acanthamoeba spp., Balamuthia mandrillaris, and Naegleria fowleri. J Clin Microbiol. 2006;44:3589–3595. doi: 10.1128/JCM.00875-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed RP, Cooke-Yarborough CM, Jaquiery AL, Grimwood K, Kemp AS, Su JC, Forsyth JRL. Fatal granulomatous amoebic encephalitis caused by Balamuthia mandrillaris. Med J Aust. 1997;167:82–84. doi: 10.5694/j.1326-5377.1997.tb138785.x. [DOI] [PubMed] [Google Scholar]
- Schuster FL, Dunnebacke TH, Booton GC, Yagi S, Kohlmeier CK, Glaser C, Vugia D, Bakardjiev A, Azimi P, Maddux-Gonzalez M, Visvesvara GS. Environmental isolation of Balamuthia mandrillaris associated with a case of amebic encephalitis. J Clin Microbiol. 2003;41:3175–3180. doi: 10.1128/JCM.41.7.3175-3180.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster FL, Glaser C, Honarmand S, Maguire JH, Visvesvara GS. Balamuthia amebic encephalitis risk, Hispanic Americans. Emerg Infect Dis. 2004;10:1510–1512. doi: 10.3201/eid1008.040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster FL, Guglielmo BJ, Visvesvara GS. In-vitro activity of miltefosine and voriconazole on clinical isolates of free-living amoebas: Balamuthia mandrillaris, Acanthamoeba spp., and Naegleria fowleri. J Eukaryot Microbiol. 2006a;53:121–126. doi: 10.1111/j.1550-7408.2005.00082.x. [DOI] [PubMed] [Google Scholar]
- Schuster FL, Honarmand S, Visvesvara GS, Glaser CA. Detection of antibodies against free-living amoebae Balamuthia mandrillaris and Acanthamoeba species in a population of patients with encephalitis. Clin Infect Dis. 2006b;42:1260–1265. doi: 10.1086/503037. [DOI] [PubMed] [Google Scholar]
- Schuster FL, Visvesvara GS. Axenic growth and drug sensitivity studies of Balamuthia mandrillaris, an agent of amebic meningoencephalitis in humans and other animals. J Clin Microbiol. 1996;34:385–388. doi: 10.1128/jcm.34.2.385-388.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster FL, Yagi S, Gavali S, Michelson D, Raghavan R, Blomquist I, Glastonbury C, Boll en AW, Scharnhorst D, Reed SL, Kuriyama S, Visvesvara GS, Glaser CA. Under the radar: Balamuthia amebic encephalitis. Clin Infect Dis. 2009;7:879–887. doi: 10.1086/597260. [DOI] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration. Impavido (Miltefosine) [Accessed 27 March 2015];Drugs@FDA—FDA Approved Drug Products. 2014 http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.DrugDetails.
- Visvesvara GS, Martinez AJ, Schuster FL, Leitch GJ, Wallace SV, Sawyer TK, Anderson M. Leptomyxid ameba, a new agent of amebic meningoencephalitis in humans and animals. J Clin Microbiol. 1990;28:2750–2756. doi: 10.1128/jcm.28.12.2750-2756.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvesvara GS, Roy S, Maguire JH. Pathogenic and opportunistic free-living amebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia pedata. In: Guerrant RL, Walker DH, Weller PF, editors. Tropical infectious diseases—principles, pathogens, & practice. 3. Elsevier; Churchill Livingstone, Philadelphia: 2011. pp. 707–713.pp. 707–713. [Google Scholar]
- Visvesvara GS, Schuster FL, Martinez AJ. Balamuthia mandrillaris, N. G., N. Sp., agent of amebic meningoencephalitis in humans and animals. J Eukaryot Microbiol. 1993;40:504–514. doi: 10.1111/j.1550-7408.1993.tb04943.x. [DOI] [PubMed] [Google Scholar]
- Wadhone P, Maiti M, Agarwal R, Kamat V, Martin S, Saha B. Miltefosine promotes IFN-gamma-dominated anti-leishmanial immune response. J Immunol. 2009;182:7146–7154. doi: 10.4049/jimmunol.0803859. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Miltefosine (Inclusion)—Adults and Children. 18th Expert Committee on the Selection and Use of Essential Medicines; World Health Organization; 2011. [Accessed 27 March 2015]. http://www.who.int/selection_medicines/committees/expert/18/applications/miltefosine/en/ [Google Scholar]