Abstract
Introduction
Prostate biopsies are usually taken from the peripheral rather than anterior region of the prostate. Consequently, tumors originating from the anterior apical region and transition zones may be under-sampled. We examined whether addition of transrectal anterior biopsy (TAB) would improve efficacy of prostate biopsies.
Materials and methods
Simulations of TAB and sextant biopsy (SB) were performed using computer models of 86 autopsy prostates (AP) and 40 radical prostatectomy (RP) specimens. TAB was obtained bilaterally from apex, mid, and base regions by advancing the biopsy needle 5 mm–35 mm beyond the prostatic capsule. A phase I clinical trial with 114 patients was conducted to determine the performance of an extended biopsy protocol consisting of TAB, SB, and laterally-directed biopsy (LDB).
Results
The overall cancer detection rates of SB and TAB were 33% and 55% for AP series (p = 0.00003); 60% and 88% for RP series (p = 0.006). Alternatively, SB + bilateral apical TAB and SB + bilateral mid TAB had cancer detection rates of 45% and 42% for AP series; 80% and 78% for RP series. The extended biopsy protocol detected cancer in 33% (38/114) of patients with 29, 25, and 15 diagnosed by SB, LDB, and bilateral apical TAB, respectively. Patients diagnosed by bilateral apical TAB versus SB (p = 0.01) and LDB (p = 0.02) were statistically significant. Without bilateral apical TAB, the overall cancer detection rate decreased to 30% (34/114).
Conclusions
Inclusion of bilateral TAB from apical region for first time and repeat prostate biopsies may increase diagnosis of prostate cancer. The clinical significance of these findings needs further investigations and clinical follow up.
Keywords: prostate cancer, anterior tumors, transrectal prostate biopsy, anterior apical region, transition zone, computer simulation, clinical validation
Introduction
Prostate cancer accounts for one third of all male malignancies and 10% of cancer deaths in men in the United States.1 In 2013, an estimated 238,590 new cases of prostate cancer will be diagnosed and 29,720 men will die from this disease.1 Aggressive prostate-specific antigen (PSA) based screening and standardization of transrectal ultrasound (TRUS) guided prostate biopsy protocols have resulted in significant stage migration and earlier detection of prostate cancer involving a growing proportion are low-volume posterior peripheral zone tumors.2 Consequently, an increased incidence of predominantly anterior tumors has been observed. These developments have necessitated a reconsideration of prostate biopsy strategies for the diagnosis of anterior prostate cancer and determine clinical significance of these tumors to offer suitable therapeutic options.
Due to the limitations of serum PSA cut off levels to correctly identify prostate cancer patients that require definitive therapy, current prostate biopsy protocols need to increase specificity for clinically threatening cancer without reducing sensitivity.3 Clinically threatening cancers are defined as having volumes ≥ 0.5 cc or a Gleason sum ≥ 7.4 This may be accomplished by modifying the number and locations of biopsy cores taken from the prostate. However, the fact that many men die with and not from prostate cancer has been used to argue against aggressive screening by suggesting it would detect high proportions of latent or clinically non-threatening cancers which could result in unnecessary treatment.5
Previous studies have demonstrated TRUS-guided sextant biopsy (SB) alone may miss as much as 25%–50% of clinically threatening carcinoma while laterally-directed biopsy (LDB) can detect 20% more such tumors than the SB.4,6 Consequently, the current clinical recommendation is an extended-biopsy scheme with at least 8–12 cores that include a combination of SB and LDB without targeting the anterior of the prostate at initial biopsy.7 A common shortcoming of current TRUS-guided biopsy protocols is that they may adequately sample posterior tumors while anterior tumors remain undersampled.
In a series of 281 radical prostatectomy specimens, 38% of prostates contained anterior tumors.8 Computer simulations of reconstructed prostatectomy specimens showed that SB and LDB undersample the anterior transition zone, midline peripheral zone, and inferior portions of the anterior horn in the peripheral zone.9 Our studies found that SB and LDB failed to detect 65% and 80% of anterior tumors, respectively.4,6 An 11-core biopsy scheme combining SB and five alternative area biopsies (including two anterior horn, two transition zone, and one midline) in 362 patients detected 33% more prostate cancer than SB alone (p = 0.001).10 Therefore, anterior tumors may require multiple sets of TRUS-guided biopsies for diagnosis.11
Template guided transperineal mapping biopsies (TMB) adequately sample both posterior and anterior tumors.12,13 One study analyzed 1132 radical prostatectomy specimens where prostate cancer was initially diagnosed in 718 cases by TRUS-guided biopsies and in 414 by TMB.14 This study found that TMB detected proportionally more anterior tumors (16.2% versus 12%, p = 0.046), and identified them at a smaller size (1.4 versus 2.1 cm3, p = 0.03) and stage (extracapsular extension 13% versus 28%, p = 0.03) than TRUS-guided biopsies. However, using the TMB biopsy as a method for initial screening of prostate cancer patients remains controversial due to high costs, access to operative time, and urologist acceptance as well as need for training. Hence, modifications may be necessary for current TRUS-guided biopsy protocols to adequately sample both posterior and anterior tumors.
One modification currently employed by some urologists includes obtaining transrectal anterior biopsy (TAB) bilaterally from the transition zone in addition to the SB and LDB during initial screening. TAB is obtained by advancing the biopsy needle inside the prostate gland through the capsule prior to taking the core. However, specific criteria for TAB such as optimum number of biopsy cores from the anterior region based on prostate gland volume, biopsy needle location and depth of insertion, etc., are not yet established. In this current study, computer simulations were utilized to determine specific criteria for TAB that would improve overall detection rate of clinically threatening prostate cancer. Based on preliminary findings, a non-randomized phase I clinical trial was conducted to validate the diagnostic utility of TAB.
Materials and methods
For computer simulations studies, two consecutive series of prostate specimens were used: 86 autopsy prostates (AP) and 40 radical prostatectomies (RP). Only prostate glands with minimal autolysis, complete transverse sections, and no prior transurethral resection were included in the AP series.4,6 In each specimen, carcinomas were histologically confirmed at autopsy, but none of these had been diagnosed or suspected during life. Patients who had neoadjuvant therapy or were previously incised were excluded from the RP series. Prostate glands were removed en bloc by blunt dissection and fixed in 10% neutral buffered formalin. The entire gland was completely sliced at 4 mm intervals in the transverse plane perpendicular to the prostatic urethra. Each slice was embedded in paraffin and a 5 μm histologic section was cut from the proximal face. The sections were stained with hematoxylin and eosin for routine histological inspection.15 All tumors in each prostate were histologically graded using the Gleason system16 and their locations classified according to McNeal’s zonal anatomy.17 Tumor borders and regions of individual Gleason patterns were dotted on the slide coverslips with a fine point permanent marking pen using a microscope at low magnification. Dotted slides were traced as maps onto acetate sheets. The maps were next transformed into solid 3D computer models using an algorithm with linear interpolation and extrapolation techniques.18 These reconstructed computer models of prostate glands were used in computer simulations to study efficacy of TAB.
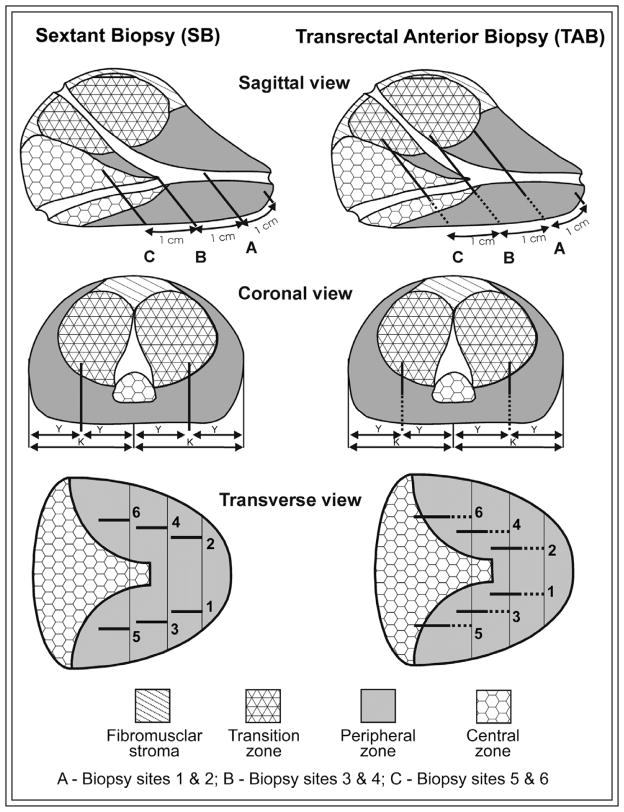
Computer simulations of the SB were then performed, Figure 1.19 Biopsy site 1 is located 1 cm cephalad from the apex of the prostate in the middle of one lobe. Site 2 is located in the contralateral lobe equidistant from the prostate middle groove. Similarly, biopsy sites 3 and 5 are located 2 cm and 3 cm, respectively, cephalad from the apex of the prostate in the middle of the lobe, with sites 4 and 6 being on the contralateral lobe, again equidistant from the prostate middle groove. All simulated needles were 18-gauge with a 15 mm long biopsy core resembling the needle dimensions used in the clinical settings. TAB was simulated using the similar insertion sites of SB described above, Figure 1. A total of six simulated biopsies were taken by inserting the needle through the prostate capsule at 5 mm increments (range 5 mm–35 mm) before taking the biopsy core. For each prostate specimen, the procedure was repeated with TAB until prostate cancer was detected or no further detection was possible.
Figure 1.
Schematic representation of sextant biopsy (on the left) and transrectal anterior biopsy protocols (on the right).
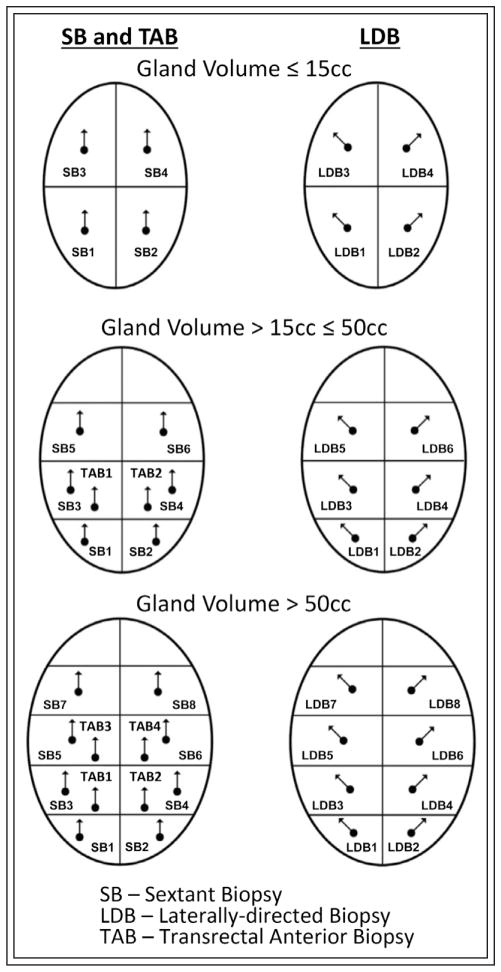
A non - randomized clinical trial was conducted at the University of Colorado Hospital and Denver Health & Hospital Administration between November 2002 and March 2008 to study the efficacy of TAB.20 To satisfy standard of care requirements, an extended biopsy protocol was developed for this clinical trial consisting of SB, LDB, and TAB, Figure 2. A total of 114 patients were selected and agreed to participate in the study. Patients qualified for the study by having either an elevated PSA for their age, increasing PSA velocity, or abnormal digital rectal examination (DRE). For this extended-biopsy scheme, 8, 14, or 20 biopsy cores were taken depending on the size of the prostate gland, Figure 2. Prostates that were less than or equal to 15 cc had eight biopsies. The eight biopsies were taken from the prostate apex and base bilaterally, and then two more LDB were taken at the apex and base, bilaterally. In these are smaller glands, the anterior prostate was not targeted by TAB. Prostates that were greater than 15 cc but less than or equal to 50 cc received 14 biopsies. The 14 biopsies were taken from the following locations: the apex, mid, and base, bilaterally as in SB; three more LDB bilaterally at the apex, mid and base; and bilateral TAB from apical region. Prostates that were greater than 50 cc received 20 biopsies. The 20 biopsies were taken from the apex, distal mid gland, proximal mid gland, and base, bilaterally; four more LDB at the apex, distal mid gland, proximal mid gland, and base, bilaterally; and bilateral TAB from apical and mid regions.
Figure 2.
Schematic representation of extended biopsy protocol utilized in the clinical trial based on prostate volume combining sextant biopsy, laterally-directed biopsy, and transrectal anterior biopsy.
Clinically threatening carcinoma is defined as a lesion(s) with volume ≥ 0.5 cc or Gleason sum ≥ 7 without extracapsular extension.4,21 Computer simulations were used to determine overall cancer detection rates, detection rates for clinically threatening and non-threatening cancer of SB and TAB. Sensitivity, specificity, positive predictive values (PPV), and negative predictive values (NPV) were determined for two biopsy schemes. Clinical trial data was used to determine overall cancer detection rates of different biopsy schemes and their statistical significance. Statistical analyses were performed using t-tests, Fisher’s exact test, non-parametric 2-tailed Wilcoxon Rank Sum, McNemar’s test, and logistics regression (LR). All analyses were performed using STATVIEW (Abacus Concepts, CA, USA) and SAS (SAS Institute, Cory, NC, USA). Differences between groups were considered to be statistically significant when p ≤ 0.05.
Results
Table 1 summarizes baseline patient information for the AP and RP series utilized in computer simulation studies. The patients in the AP series were older and had smaller prostate glands than patients in the RP series. The older age is not unexpected given that these are autopsy specimens. The smaller prostate size is the result of sampling bias. The larger autopsy prostates often did not fix properly in formalin and had to be discarded due to autolysis. The AP series had smaller tumor volumes and a lower proportion of clinically threatening carcinoma than RP series (37% versus 80%). The AP series also had a lower proportion of capsular perforation (17% versus 48%). Only 44/84 patients in AP series had pre-mortem serum available to determine PSA. According to our definition, 32/86 (37%) patients in AP series and 32/40 (80%) patients in RP series had clinically threatening disease, Table 1.
TABLE 1.
Baseline patient information
| Baseline information | AP series (n = 86) | RP series (n = 40) |
|---|---|---|
| Age mean ± S.D. (range) years | 67.4 ± 9.7 (36–87) | 58.6 ± 7.0 (45–70)* |
| PSA mean ± S.D. (range) ng/mL | 7.6 ± 7.9 (0.1–42.2)$ | 6.23 ± 3.23 (0.4–16.8) |
| Median PSA ng/mL | 3.5 | 4.9 |
| Prostate volume mean ± S.D. (range) cm3 | 25.8 ± 10.6 (9.1–53.4) | 37.2 ± 11.4 (19.8–67.0) |
| Median prostate volume cm3 | 23.3 | 33.4* |
| Tumor volume mean ± S.D. (range) cm3 | 0.91 ± 2.1 (0.01–10.95) | 2.25 ± 1.95 (0.01–7.7) |
| Median tumor volume cm3 | 0.18 | 1.47* |
| Total number of individual tumors | 165 | 141 |
| Median number tumors/prostate (range) | 1 (1–9) | 4 (1–7)* |
| Patients with organ-confined cancer | 71 (83%) | 21 (52%)** |
| Patients with clinically threatening cancer | 32 (37%) | 32 (80%)* |
Statistically significant differences with p = 0.0001
Statistically significant difference with p = 0.0004
Only 44/84 autopsy patients had pre-mortem serum available to determine PSA
AP = autopsy prostates; RP = radical prostatectomy; PSA = prostate-specific antigen; S.D. = standard deviation
Prostate cancer detection rates for SB and TAB are given in Table 2. For the AP series, the TAB detected significantly more patients with prostate cancer than SB. Both methods detected 26 while TAB also detected 21 that SB missed and failed to detect only two that SB detected (McNemar’s Tests p = 0.00003). TAB detected a significantly higher proportion of patients with clinically threatening prostate cancer than with SB. For the 32 patients with clinically threatening disease, both methods detected 20, while the TAB detected nine that the SB didn’t and missed only one case detected by SB (McNemar’s Test p = 0.011). For the 54 patients with non-threatening disease, TAB also detected significantly more prostate cancer than SB (McNemar’s Test p = 0.002). As a result, sensitivity for TAB was higher (91% versus 66%), but specificity was lower compared to SB (67% versus 87%).
TABLE 2.
Comparison of prostate cancer diagnosis
| Performance parameter | AP series (n = 86)* | RP series (n = 40)* | ||
|---|---|---|---|---|
| SB | TAB | SB | TAB | |
| Overall cancer detected | 28 (33%) | 47 (55%) | 24 (60%) | 35 (88%) |
| Clinically threatening cancer detected* | 21 (66%) | 29 (91%) | 21 (66%) | 31 (97%) |
| Clinically non-threatening cancer detected** | 7 (13%) | 18 (33%) | 3 (38%) | 4 (50%) |
| Sensitivity (for detecting threatening cases) | 66% | 91% | 66% | 97% |
| Specificity (not detecting non-threatening cases) | 87% | 67% | 63% | 50% |
| PPV (detected cases are clinically threatening) | 75% | 62% | 88% | 89% |
| NPV (not detected cases are non-threatening) | 81% | 92% | 31% | 80% |
32 patients had clinically-threatening cancer in each series
54 patients in AP series and 8 patients in RP series had clinically non-threatening cancer
AP = autopsy prostates; RP = radical prostatectomy; SB = sextant biopsy; TAB = transrectal anterior biopsy; PPV = positive predictive values; NPV = negative predictive values
For the RP series, both methods detected 21 while TAB also detected 14 that SB missed and failed to detect only three that SB detected (McNemar’s Tests p = 0.006). TAB also detected significantly higher proportion of patients with clinically threatening prostate cancer than with SB. For the 32 patients with clinically threatening disease, both methods detected 20, while the TAB detected 11 that SB did not and missed only one case detected by SB (McNemar’s Test p = 0.004). TAB detected 96% (45/47) and 100% (35/35) of patients with prostate cancer in AP and RP series, respectively, when the biopsy needle is inserted 5 mm to 15 mm beyond prostate capsule. Therefore, prostate cancer of those patients were located within 5 mm to 30 mm distance from the prostatic capsule measured at an angle of 45° inside the prostate, Figure 1b.
Tumor volume and anatomical location as well as prostate volume can influence prostate cancer diagnosis via prostate biopsies. There was no difference in the mean volumes of tumors detected by TAB versus undetected tumors in both the AP and RP series patients with clinically threatening disease. However, there were statistically significant differences in the mean volumes of tumors detected by SB versus undetected tumors for the AP series patients with clinically threatening (3.12 cc versus 0.58 cc; p = 0.01) and non-threatening disease (0.20 cc versus 0.10 cc; p = 0.04). Table 3 provides a summary of clinically threatening tumors diagnosed by anatomical locations. The majority of the largest tumors in the clinically threatening cases from both the AP (63%) and RP (72%) series were located in the posterior peripheral zone (PPZ). For both series, SB tended to detect only PPZ tumors since the biopsy needle often does not penetrate into the anterior prostate. In addition to detecting PPZ tumors, however, TAB also detected all clinically threatening anterior tumors in the anterior peripheral zone (APZ) and anterior transitions zone (ATZ) and missed only one APZ tumor in the AP series. There was no difference in the mean prostate volumes of detected versus undetected threatening cases using TAB in either series. SB, however, tended to detect clinically threatening disease in smaller prostates (AP series: 22.4 cc versus 29.6 cc, p = 0.04; RP series: 36.5 cc versus 42.0 cc). Therefore, prostate cancer diagnosis by TAB may be influenced only by location of tumor but not the tumor or prostate volumes whereas SB may be influenced by all three parameters.
TABLE 3.
Comparison of clinically-threatening cancer diagnosis
| Anatomical location | AP series (n = 32) | RP series (n = 32) | ||||
|---|---|---|---|---|---|---|
| Pts | SB | TAB | Pts | SB | TAB | |
| Posterior peripheral zone | 20 | 17 (85%) | 18 (90%) | 23 | 20 (87%) | 22 (96%) |
| Anterior transition zone | 6 | 2 (33%) | 6 (100%) | 5 | 0 (0%) | 5 (100%) |
| Anterior peripheral zone | 4 | 1 (25%) | 3 (75%) | 4 | 1 (25%) | 4 (100%) |
| Posterior central zone | 2 | 1 (50%) | 2 (100%) | --- | --- | --- |
AP = autopsy prostates; RP = radical prostatectomy; SB = sextant biopsy; TAB = transrectal anterior biopsy
Table 4 shows results for biopsy schemes with the addition of bilateral apical TAB, (Figure 1: biopsy sites 1 and 2) or bilateral mid TAB (Figure 1: biopsy sites 3 and 4) to SB. Given that additional biopsy cores were taken, detection rates for the extended schemes are higher compared to SB alone, with higher proportions of both threatening and non-threatening prostate cancer detected. Thus, the sensitivities for these schemes are higher with a trade-off of lower specificities. While differences were not statistically significant, addition of bilateral apical TAB appears to have a better overall detection rate of clinically threatening prostate cancer compared to bilateral mid TAB for both the AP and RP series.
TABLE 4.
Performance of combined sextant biopsy and transrectal anterior biopsy
| Performance parameter | AP series (n = 86) | RP series (n = 40) | ||
|---|---|---|---|---|
| SB + bilateral mid TAB | SB + bilateral apical TAB | SB + bilateral mid TAB | SB + bilateral apical TAB | |
| Overall cancer detected | 42% | 45% | 78% | 80% |
| Sensitivity | 72% | 84% | 81% | 91% |
| Specificity | 76% | 78% | 38% | 63% |
| PPV | 64% | 69% | 84% | 91% |
| NPV | 82% | 89% | 33% | 63% |
AP = autopsy prostates; RP = radical prostatectomy; SB = sextant biopsy; TAB = transrectal anterior biopsy; PPV = positive predictive values; NPV = negative predictive values
A total of 114 patients were enrolled in a non-randomized phase-I clinical trial for initial screening.20 The mean age, PSA, and prostate volume of patients were 62 years, 8 ng/mL, and 47 cc, respectively. According to the extended biopsy protocol, five patients with prostate volumes ≤ 15 cc did not receive TAB. Remaining 109 patients with prostate volume > 15 cc received bilateral apical TAB and 31 patients with prostate volume > 50 cc received additional bilateral mid TAB. Overall, the extended-biopsy protocol diagnosed cancer in 38/114 (33%) patients. The patients diagnosed by each method based on serum PSA values are shown in Table 5. When four unique patients diagnosed by TAB alone were excluded, the overall cancer detection rate decreased to 30%. Of the 38 total patients with cancer, 29, 25, and 15 patients were diagnosed by SB, LDB, and TAB, respectively. Bilateral apical TAB (Figure 2: TAB1 and TAB2) detected cancer in 15/109 patients. Bilateral mid TAB (Figure 2: TAB3 and TAB4) detected only 2/31 patients who were already diagnosed by bilateral apical TAB. Statistically significant differences were observed between unique patients diagnosed by bilateral apical TAB versus SB and LDB (McNemar’s test p = 0.01 and p = 0.02, respectively). For patients with PSA ≤ 14.0, TAB diagnosed 8/31 (26%) with prostate cancer whereas for patients with PSA > 14.0, TAB detected 7/7 (100%). In a multivariate LR analysis, only TAB was significant for patients diagnosed by PSA (β = 7.4, p = 0.01). There was no significant relationship between prostate volume and cancer diagnosed by any of the biopsy methods.
TABLE 5.
Clinical trial results of biopsy protocols
| Biopsy protocol | Patients diagnosed with cancer based on PSA | ||
|---|---|---|---|
| ≥ 7.0 (n = 66) | 7.1–14.0 (n = 30) | > 14.0 (n = 18) | |
| Extended biopsy | 17 | 14 | 7 |
| Sextant biopsy | 14 (4) | 8 (4) | 7 (0) |
| Laterally-directed biopsy | 12 (2) | 6 (2) | 7 (0) |
| Transrectal anterior biopsy | 5 (1) | 3 (2) | 7 (1) |
Unique patients diagnosed by each biopsy method is indicated within ()
PSA = prostate-specific antigen
Discussion
The diagnosis of prostate cancer is solely dependent on biopsy results. Factors that define the chances of better detection rate and true sampling of clinically relevant disease include: tumor volume, presence of high grade Gleason patterns (4 or 5) or evidence of neurovascular invasion. Currently, there are no direct methods to determine tumor volume based on biopsy cores. Unless the patient undergoes subsequent prostatectomy surgery, biomorphometry (e.g., volume and location of tumors) information cannot be obtained. Finally, it is difficult to account for false-negative biopsy results without prostatectomy to determine the prevalence of cancer in the study population. Therefore, the lack of accurate information prior radical prostatectomy, it is impossible to determine sensitivity, specificity, PPV, and NPV of clinically relevant disease.
Advancements in computer modeling allow effectiveness of different prostate biopsy protocols to be determined. Recently, several investigators used whole-mount sections of prostates to develop three dimensional computer models.4,9,22,23 These models were then subjected to simulated biopsy protocols that can provide reliable estimates for sensitivity, specificity, PPV, and NPV. However, these estimates represent the best case scenario since it is rather a difficult task to duplicate accuracy and precision of computer simulations in clinical practice. Nevertheless, computer simulations can provide valuable information regarding performance of biopsy protocols that may be virtually impossible to obtain from clinical trials.
Radical prostatectomy, autopsy, and cystoprostatectomy specimens can be used to generate 3D computer models of the prostate. Unfortunately, none of these specimens accurately represent the screening population in clinical situations. Because radical prostatectomy specimens represent a biased sample of men already diagnosed with prostate cancer, autopsy specimens with their incidental findings of cancer may give a more accurate representation of incidental prostate cancer in the general population. Although the men in the AP series died of causes other than prostate cancer, it is possible that these men would have had clinically detectable disease had they been screened for prostate cancer. Because limited screening information (e.g., PSA, DRE) is available for these men, we cannot determine whether they should have been biopsied. Given that about 10% of the tumors in the autopsy specimens were large (> 1 cm3) and some had high PSA values, it is likely that if they were screened for prostate cancer, they could have been positively diagnosed.
In both AP and RP series, TAB detected a higher proportion of cancers, both threatening and non-threatening, when compared to SB alone, Table 2. As a result, TAB had higher sensitivity and lower specificity. If the goal is to detect threatening cancer, TAB has the disadvantage of a lower PPV (from the AP series; no difference seen in RP series). Therefore, inclusion of TAB in the biopsy plan should be based on additional information such as PSA, DRE, prostate volume, etc. We found that the best overall detection rate was achieved when we used an extended eight core biopsy protocol combining either bilateral TAB from the apical or mid region with the SB. In practice, some urologists include bilateral TAB from the mid region in the biopsy template. However, our analysis in Table 4, shows that SB with bilateral TAB from the apex has higher sensitivity than SB with bilateral TAB from mid prostate. Opell et al reported the base region had a significantly lower distribution rate of cancer (37%) than either the mid (56%) or the apical region (54%), while in the anterior half, both the left and right medial apical regions had a higher distribution rate of cancer than the corresponding medial mid regions.8 This supports the improved detection rate using bilateral TAB from the apex rather than the mid in combination with SB. For repeat biopsies, however, inclusion of TAB from apical region is highly recommended. This recommendation supports the findings and conclusions of Presti.24
Results from our clinical trial also confirm the above findings regarding the efficacy of TAB from the apical region as a part of the initial biopsy plan.20 Fifteen of the 109 (14%) patients were diagnosed by bilateral apical TAB. Two of the 31 (7%) patients diagnosed by bilateral mid TAB were also diagnosed by bilateral apical TAB. However, only 4/38 (10%) patients were uniquely diagnosed by bilateral apical TAB and they were significantly different from those diagnosed either by SB or LDB. Each patient had one positive core; three had Gleason score 6 and one had Gleason score 7 cancer. One patient opted for watchful-waiting; two had laparoscopic radical prostatectomy, and one open prostatectomy. TAB also provided additional diagnostic information used to decide therapeutic options for 11/38 (29%) patients and 3/11 opted for watchful-waiting. Hence, 39% (15/38) of patients with prostate cancer received valuable diagnostic and prognostic information from TAB. Though the reduction in overall cancer detection rate is 3% (33%→30%) when TAB is excluded, it is difficult to underestimate the value of bilateral apical TAB as part of the initial biopsy plan.
Location plays an important role in cancer detection since TAB samples slightly different areas of the prostate than SB, Table 3. Majority of tumors in AP and RP series detected by TAB were physically located 5 mm–30 mm from the prostate capsule when measured at a 45° angle. Using standard length biopsy needle with a 15 mm core, these were diagnosed by inserting the biopsy needle 5 mm–15 mm through the prostate capsule. The regions sampled by TAB include parts of the PPZ, anterior medial apical region, central zone, anterior portions of the prostatic horns, and the ATZ. Hence, because ATZ cancers are relatively uncommon compared to PPZ cancers, routine ATZ biopsies (TAB from the mid region) do not significantly increase the detection rates of clinically threatening prostate cancer.25 Results from our computer simulation studies and clinical trial support this observation. However, Fowler et al reported that at least 30% of men with PSA greater than 10 ng/mL and a negative PPZ biopsy actually have prostate cancer with approximately 50% of these cancers residing in the ATZ.26 Additionally, the diagnosis of ATZ cancer is an important factor in predicting biochemical PSA failure.27 From a consecutive series of 148 radical prostatectomy specimens, Noguchi et al found that the biochemical PSA cure rate for ATZ carcinoma (72%) was significantly higher than the cure rate for PPZ cancers (49%).28 If only ATZ cancers are found, patients become strong candidates for watchful-waiting, active surveillance, or focal therapy which eliminates the serious side effects (erectile dysfunction, incontinence, etc.) associated with surgery or radiation.29 Therefore, some other key parameters such as prostate volume, PSA, etc., must be taken into consideration prior to the inclusion of TAB from either the apical or mid regions or both as part of patient’s initial biopsy plan.
Prostate volume can influence prostate cancer detection rates. Computer simulations demonstrated that prostate cancer diagnosis by TAB is influenced only by location of tumor but not by the prostate or tumor volumes whereas SB is influenced by all three parameters. One study found a statistically significant inverse relationship between prostate volume and prostate cancer detection by SB.30 They concluded that for men with large prostates defined by either a 37.5 cc or 50 cc cut off volume, SB may not be adequate to diagnosed prostate cancer. Our data indicate that TAB has a better detection rate than SB in prostates greater than 37.5 cc (AP series: 64% versus 36%; RP series: 94% versus 50%). Our clinical trial data showed that prostate volume had no significant impact on prostate cancer diagnosed by SB, LDB, or TAB. Overall, the cancer detection rate decreased as the size of the prostate increased. Prostate volumes ≤ 15 cc, > 15 cc and ≤ 50 cc, and > 50 cc had cancer detection rates of 40%, 36%, and 26%, respectively. For prostates > 50 cc, 20 biopsy cores including bilateral TAB from the apical and mid regions, appear to be inadequate to diagnose prostate cancer. Chen et al contended that the lower detection rate they found in large prostates (> 50 cc) was due to a higher proportion of small tumors (< 0.5 cc) found in the larger prostates.31 We had insufficient numbers to look at the data using cut off of 50 cc, but using the 37.5 cc value, we found no significant differences in the proportions of small tumors in small or large prostates in both the AP and RP series. More importantly, after adding Gleason grade to size to define significant tumors, the large and small prostates contained the same proportion of threatening cancers. Although higher PSA values resulting from benign diseases such as BPH probably result in larger prostates being biopsied unnecessarily, large prostates contain clinically threatening carcinoma.
Clinical trial data show that the prostate cancer detection rate was 15% among patients with PSA < 3 and increased to 58% when PSA > 9. By univariate LR analysis, PSA significantly predicted prostate cancer detected by both LDB and TAB (p = 0.044 and p = 0.01, respectively). By multivariate LR analysis, however, PSA was a significant factor only for prostate cancer diagnosed by TAB. By univariate LR analysis, PSA density (PSA/prostate gland volume) was another significant factor for prostate cancer diagnosed by LDB and TAB (p = 0.0496 and p = 0.014, respectively) but PSA density did not retain significance by multivariate LR analysis. Abdel-Khalek et al evaluated the importance of TZ biopsies in BPH patients with PSA > 10 and a prior negative PZ biopsy.32 They found that TZ biopsies improved the detection rate by 14% while the majority of TZ cancers (74%) were detected at the apex site. Hence, inclusion of bilateral TAB from the apex in patients with PSA above 10 ng/mL should be considered.
Conclusions
We have demonstrated that by adding bilateral TAB from the apex to the standard biopsy protocols improves the overall cancer detection rates. TAB detected majority of tumors when a standard length biopsy needle was inserted between 5 mm–15 mm through the prostate capsule at a 45° angle. The anterior apical region is one of the primary sites that must be targeted during repeat biopsies in patients with increasing PSA. For patients with PSA > 10 ng/mL, inclusion of TAB increased the overall cancer detection rates. For prostate volumes > 50 cc, 20 biopsy cores including bilateral TAB from the apex and mid prostate appear insufficient to diagnose cancer. Moreover, TAB may increase detection of clinically non-threatening cancer and hence has a lower specificity. The limitations of our study include a lack of PSA data in the AP series and small study population in the RP series and clinical trial. Consequently, it is challenging to precisely determine the cut off PSA values and prostate volumes for inclusion of TAB to standard biopsies from the peripheral sites. Therefore, clinicians may consider other risk factors such as family history, ethnicity, other derivatives of PSA, etc., prior to inclusion of TAB during initial prostate biopsies.
Acknowledgments
This research study was supported by United States Public Health Service Grant CA66161. Authors wish to thank Kathy Lux for preparation of biopsy slides and Lisa Litzenberger for the drawings in this manuscript. Preliminary results were presented in abstract format at the 13th and 17th International Prostate Cancer Update Meeting, Vail Colorado in 2003 and 2007.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Fine SW, Reuter VE. Anatomy of the prostate revisited: implications for prostate biopsy and zonal origins of prostate cancer. Histopathology. 2012;60(1):142–152. doi: 10.1111/j.1365-2559.2011.04004.x. [DOI] [PubMed] [Google Scholar]
- 3.Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4. 0 ng per milliliter. N Engl J Med. 2004;350(22):2239–2246. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- 4.Crawford ED, Hirano D, Werahera PN, et al. Computer modeling of prostate biopsy: tumor size and location--not clinical significance--determine cancer detection. J Urol. 1998;159(4):1260–1264. doi: 10.1016/s0022-5347(01)63576-6. [DOI] [PubMed] [Google Scholar]
- 5.Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA. 2005;293(17):2095–2101. doi: 10.1001/jama.293.17.2095. [DOI] [PubMed] [Google Scholar]
- 6.Kawata N, Miller GJ, Crawford ED, et al. Laterally directed biopsies detect more clinically threatening prostate cancer: computer simulated results. Prostate. 2003;57(2):118–128. doi: 10.1002/pros.10285. [DOI] [PubMed] [Google Scholar]
- 7.Ochiai A, Babaian RJ. Update on prostate biopsy technique. Curr Opin Urol. 2004;14(3):157–162. doi: 10.1097/00042307-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Opell MB, Zeng J, Bauer JJ, et al. Investigating the distribution of prostate cancer using three-dimensional computer simulation. Prostate Cancer Prostatic Dis. 2002;5(3):204–208. doi: 10.1038/sj.pcan.4500577. [DOI] [PubMed] [Google Scholar]
- 9.Chen ME, Troncoso P, Johnston DA, Tang K, Babaian RJ. Optimization of prostate biopsy strategy using computer based analysis. J Urol. 1997;158(6):2168–2175. doi: 10.1016/s0022-5347(01)68188-6. [DOI] [PubMed] [Google Scholar]
- 10.Babaian RJ, Toi A, Kamoi K, et al. A comparative analysis of sextant and an extended 11-core multisite directed biopsy strategy. J Urol. 2000;163(1):152–157. [PubMed] [Google Scholar]
- 11.Bott SR, Young MP, Kellett MJ, Parkinson MC. Anterior prostate cancer: is it more difficult to diagnose? BJU Int. 2002;89(9):886–889. doi: 10.1046/j.1464-410x.2002.02796.x. [DOI] [PubMed] [Google Scholar]
- 12.Crawford ED, Wilson SS, Torkko KC, et al. Clinical staging of prostate cancer: a computer-simulated study of transperineal prostate biopsy. BJU Int. 2005;96(7):999–1004. doi: 10.1111/j.1464-410X.2005.05801.x. [DOI] [PubMed] [Google Scholar]
- 13.Crawford ED, Rove KO, Barqawi AB, et al. Clinical-pathologic correlation between transperineal mapping biopsies of the prostate and three-dimensional reconstruction of prostatectomy specimens. Prostate. 2013;73(7):778–787. doi: 10.1002/pros.22622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hossack T, Patel MI, Huo A, et al. Location and pathological characteristics of cancers in radical prostatectomy specimens identified by transperineal biopsy compared to transrectal biopsy. J Urol. 2012;188(3):781–785. doi: 10.1016/j.juro.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Miller GJ, Cygan JM. Morphology of prostate cancer: the effects of multifocality on histological grade, tumor volume and capsule penetration. J Urol. 1994;152(5 Pt 2):1709–1713. doi: 10.1016/s0022-5347(17)32368-6. [DOI] [PubMed] [Google Scholar]
- 16.Gleason DF. Histologic grading and clinical staging of prostatic carcinoma. In: Tannenbaum MD, editor. Urologic Pathology: The Prostate. Philadelphia: Lea & Febiger; 1977. pp. 171–198. [Google Scholar]
- 17.McNeal JE. Origin and development of carcinoma in the prostate. Cancer. 1969;23(1):24–34. doi: 10.1002/1097-0142(196901)23:1<24::aid-cncr2820230103>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 18.Werahera PN, Miller GJ, Torkko K, et al. Biomorphometric analysis of human prostatic carcinoma by using three-dimensional computer models. Hum Pathol. 2004;35(7):798–807. doi: 10.1016/j.humpath.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 19.Hodge KK, McNeal JE, Terris MK, Stamey TA. Random systematic versus directed ultrasound guided transrectal core biopsies of the prostate. J Urol. 1989;142(1):71–74. doi: 10.1016/s0022-5347(17)38664-0. [DOI] [PubMed] [Google Scholar]
- 20.Werahera PN, Sullivan K, La Rosa FG, et al. Optimization of prostate cancer diagnosis by increasing the number of core biopsies based on gland volume. Int J Clin Exp Pathol. 2012;5(9):892–899. [PMC free article] [PubMed] [Google Scholar]
- 21.Chan TY, Chan DY, Stutzman KL, Epstein JI. Does increased needle biopsy sampling of the prostate detect a higher number of potentially insignificant tumors? J Urol. 2001;166(6):2181–2184. [PubMed] [Google Scholar]
- 22.Loughlin M, Carlbom I, Busch C, et al. Three-dimensional modeling of biopsy protocols for localized prostate cancer. Comput Med Imaging Graph. 1998;22(3):229–238. doi: 10.1016/s0895-6111(98)00019-6. [DOI] [PubMed] [Google Scholar]
- 23.Bauer JJ, Zeng J, Weir J, et al. Three-dimensional computer-simulated prostate models: lateral prostate biopsies increase the detection rate of prostate cancer. Urology. 1999;53(5):961–967. doi: 10.1016/s0090-4295(99)00068-0. [DOI] [PubMed] [Google Scholar]
- 24.Presti JC., Jr Repeat prostate biopsy--when, where, and how. Urol Oncol. 2009;27(3):312–314. doi: 10.1016/j.urolonc.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 25.Liu IJ, Macy M, Lai YH, Terris MK. Critical evaluation of the current indications for transition zone biopsies. Urology. 2001;57(6):1117–1120. doi: 10.1016/s0090-4295(01)00944-x. [DOI] [PubMed] [Google Scholar]
- 26.Fowler JE, Jr, Condon MA, Terrell FL. Cancer diagnosis with prostate specific antigen greater than 10 ng./ml and negative peripheral zone prostate biopsy. J Urol. 1996;156(4):1370–1374. [PubMed] [Google Scholar]
- 27.Stamey TA, Yemoto CM, McNeal JE, Sigal BM, Johnstone IM. Prostate cancer is highly predictable: a prognostic equation based on all morphological variables in radical prostatectomy specimens. J Urol. 2000;163(4):1155–1160. doi: 10.1016/s0022-5347(05)67713-0. [DOI] [PubMed] [Google Scholar]
- 28.Noguchi M, Stamey TA, Neal JE, Yemoto CE. An analysis of 148 consecutive transition zone cancers: clinical and histological characteristics. J Urol. 2000;163(6):1751–1755. [PubMed] [Google Scholar]
- 29.Barqawi AB, Crawford ED. The current use and future trends of focal surgical therapy in the management of localized prostate cancer. Cancer J. 2007;13(5):313–317. doi: 10.1097/PPO.0b013e318156eb99. [DOI] [PubMed] [Google Scholar]
- 30.Basillote JB, Armenakas NA, Hochberg DA, Fracchia JA. Influence of prostate volume in the detection of prostate cancer. Urology. 2003;61(1):167–171. doi: 10.1016/s0090-4295(02)02103-9. [DOI] [PubMed] [Google Scholar]
- 31.Chen ME, Troncoso P, Johnston D, Tang K, Babaian RJ. Prostate cancer detection: relationship to prostate size. Urology. 1999;53(4):764–768. doi: 10.1016/s0090-4295(98)00574-3. [DOI] [PubMed] [Google Scholar]
- 32.Abdel-Khalek M, Sheir KZ, El-Baz M, Ibrahiem E. Is transition zone biopsy valuable in benign prostatic hyperplasia patients with serum prostate-specific antigen >10 ng/mL and prior negative peripheral zone biopsy? Scand J Urol Nephrol. 2005;39(1):49–55. doi: 10.1080/00365590410002555. [DOI] [PubMed] [Google Scholar]