Abstract
Although behavioral lateralization is known to correlate with certain aspects of brain asymmetry in primates, there are limited data concerning hemispheric biases in the microstructure of the neocortex. In the present study, we investigated whether there is asymmetry in synaptophysin-immunoreactive puncta density and protein expression levels in the region of hand representation of the primary motor cortex in chimpanzees (Pan troglodytes). Synaptophysin is a presynaptic vesicle-associated protein found in nearly all synapses of the central nervous system. We also tested whether there is a relationship between hand preference on a coordinated bimanual task and the interhemispheric distribution of synaptophysin as measured by both stereologic counts of immunoreactive puncta and by Western blotting. Our results demonstrated that synaptophysin-immunoreactive puncta density is not asymmetric at the population level, whereas synaptophysin protein expression levels are significantly higher in the right hemisphere. Handedness was correlated with interindividual variation in synaptophysin-immunoreactive puncta density. As a group, left-handed and ambidextrous chimpanzees showed a rightward bias in puncta density. In contrast, puncta densities were symmetrical in right-handed chimpanzees. These findings support the conclusion that synapse asymmetry is modulated by lateralization of skilled motor behavior in chimpanzees.
Keywords: asymmetry, handedness, plasticity, primary motor cortex, synapse
Introduction
The neuroanatomical organization underlying lateralized behavior provides a model for understanding the interaction among structure, genetics, experience and function in the nervous system (Rogers & Andrew, 2002). In addition, because the human brain shows pronounced hemispheric dominance (Toga & Thompson, 2003; Corballis, 2007), interpreting the evolution of our species’ distinctive behavioral abilities requires tracing neural asymmetries through phylogeny to reveal the anatomical substrates of behaviors such as language and dexterous manual control (Sherwood et al., 2008).
Chimpanzees (Pan troglodytes), one of our closest living relatives, display population-level lateralization for a number of behaviors, including bimanual coordinated actions, tool use, throwing, and the perception and production of species-specific vocal calls (Hopkins et al., 2007). Correlative magnetic resonance imaging studies in chimpanzees have further demonstrated that anatomical asymmetries of particular brain structures relate to interindividual variation in functional directional bias (Hopkins & Cantalupo, 2004; Dadda et al., 2006). For example, interhemispheric differences in the volume of the ‘hand knob’ of the precentral gyrus are associated with handedness for coordinated bimanual actions.
Considerably less research, however, has addressed the microstructural correlates of lateralization in primates. In the current study, we examined the presence of synaptic asymmetry in the region of hand representation of the primary motor cortex in chimpanzees as revealed by immunostaining and Western blotting against synaptophysin (SY), and we investigated whether individual variation in these asymmetries shows a relationship to handedness. SY is a 38-kDa calcium-binding vesicular glycoprotein present in nearly all presynaptic boutons of the nervous system and is often used as a marker of synaptic density and synaptogenesis (Calhoun et al., 1996; Li et al., 2002; Eastwood et al., 2006; Derksen et al., 2007; Glantz et al., 2007). We focused on synaptic organization because it comprises a key substrate of plasticity in the context of motor skill acquisition and maintenance (Greenough & Chang, 1988; Jones et al., 1999; Rioult-Pedotti et al., 2000). For example, motor learning due to training induces increased synapse numbers in layers II and III of the motor cortex in rats (Kleim et al., 1996, 1998). In addition, SY protein expression during the acquisition phase of motor skill learning in rats has been shown to increase in response to elevated activation in the motor cortex (Derksen et al., 2007).
Handedness for coordinated bimanual actions is stable over time and induces strong hand preferences at the individual level (i.e. most individuals prefer to use either the left or the right hand) (Hopkins et al., 2001). Because previous research has shown that SY expression is sensitive to activity-dependent experience, we predicted that the interhemispheric distribution of SY in the primary motor cortex of chimpanzees would reflect an individual’s handedness on a task of bimanual coordination, as synaptic connections may be modified in response to the repeated performance of skilled motor actions.
Materials and methods
Subjects
Thirty-three chimpanzees were used in this study (Table 1), including 15 females (mean age at death ± SD = 33.9 ± 11.8 years, range = 13–48 years) and 18 males (23.8 ± 9.5 years, 10–41 years). Ten of the subjects were wild-caught and had lived in captivity since the time of capture. The remaining 23 chimpanzees were born in captivity. All subjects lived in social groups at Yerkes National Primate Research Center in Atlanta, Georgia, or University of Texas M.D. Anderson Cancer Center in Bastrop, Texas. Subjects were not part of any research protocol that may have contributed to their death and postmortem brains were normal upon routine neuropathology evaluation. All experimental protocols were caried out according to the Naional Institutes of Health (NIH) guidelines for animal research and were approved by the Institutional Animal Care and Use Committee (IACUC) at The George Washington University.
Table 1.
Data used in this study
| Subject | Sex | Age at death (years) | PMI (h) | Rearing history | SY-immunoreactive puncta density AQ | SY protein expression level AQ | Handedness index | Handedness category |
|---|---|---|---|---|---|---|---|---|
| C0630 | F | 13 | 0.5 | C | −0.18 | – | 0.51 | R |
| C5625 | F | 15 | 18.0 | C | 0.12 | – | 0.48 | R |
| C0594 | F | 23 | N/A | C | 0.02 | 0.23 | 0.40 | R |
| C4751 | F | 23 | 19.0 | C | 0.15 | 0.05 | 1.00 | R |
| C0368 | F | 25 | 5.0 | C | −0.09 | – | 0.72 | R |
| C3001 | F | 29 | 6.0 | W | −0.36 | 0.32 | −0.62 | L |
| C0342 | F | 35 | N/A | C | −0.05 | 0.39 | 0.36 | R |
| C2108 | F | 38 | 3.0 | W | 0.22 | – | 0.52 | R |
| C0320 | F | 39 | 0.5 | W | 0.35 | – | −0.69 | L |
| C0406 | F | 42 | 0.5 | W | 0.10 | 0.27 | 0.91 | R |
| C2105 | F | 42 | N/A | W | 0.61 | 0.16 | 0.56 | R |
| C0336 | F | 44 | 0.5 | W | 0.47 | −0.01 | −0.16 | NP |
| C0408 | F | 45 | 0.5 | W | −0.29 | 0.14 | 1.00 | R |
| C0200 | F | 48 | N/A | C | −0.12 | 0.90 | 0.84 | R |
| C0242 | F | 48 | 3.0 | C | −0.08 | – | 0.68 | R |
| C0645 | M | 10 | 18.0 | C | −0.04 | 0.02 | 0.45 | R |
| C5960 | M | 11 | 7.0 | C | 0.22 | – | 0.10 | NP |
| C5872 | M | 13 | 14.0 | C | 0.37 | – | −0.79 | L |
| C5531 | M | 16 | 3.5 | C | −0.91 | – | 0.65 | R |
| C0507 | M | 17 | 17.0 | C | 0.38 | −0.28 | 0.35 | R |
| C0491 | M | 18 | 0.5 | C | 0.12 | −0.08 | 0.34 | R |
| C0576 | M | 19 | 0.5 | C | 0.03 | 0.07 | 0.34 | R |
| C4630 | M | 20 | 7.0 | C | 0.61 | – | −0.18 | NP |
| C5389 | M | 20 | 8.0 | C | −0.28 | 0.10 | 0.61 | R |
| C0429 | M | 24 | 1.0 | C | −0.23 | 0.00 | 0.50 | R |
| C0423 | M | 25 | 1.5 | C | 0.56 | 0.21 | 0.36 | R |
| C3961 | M | 26 | 3.5 | C | 0.42 | – | 1.00 | R |
| C0573 | M | 28 | 8.0 | C | −0.04 | −0.23 | 0.83 | R |
| C0369 | M | 32 | 2.0 | C | 0.22 | 0.07 | 0.77 | R |
| C9698 | M | 34 | 5.0 | W | 0.17 | – | 0.75 | R |
| C0301 | M | 35 | 7.0 | C | 0.45 | 0.32 | −0.71 | L |
| C0273 | M | 40 | 0.5 | W | −0.89 | 0.32 | 0.65 | R |
| C0367 | M | 41 | 2.0 | W | 0.54 | −0.14 | −1.00 | L |
AQ, asymmetry quotient; F, female; M, male; C, captive born; W, wild born; L, left; R, right; NP, no preference; N/A, not available.
Behavioral measurements
Prior to death, hand preference in each subject was considered for a task measuring coordinated bimanual actions, called the tube task (Hopkins, 1995). Handedness data from these subjects have been previously reported (Hopkins, 1995; Hopkins et al., 2004). For this task, peanut butter was smeared on the inside edges of polyvinyl chloride tubes approximately 15 cm in length and 2.5 cm in diameter. Each time the subjects reached into the tube with their finger, extracted peanut butter and brought it to their mouth, the hand used was recorded. We used measurements of hand preference on this task because it is stable across the life span and the strength of handedness elicited in chimpanzees by the tube task is significantly higher than for other actions such as bimanual feeding or simple reaching (Hopkins, 2010). Although the number of responses obtained from each subject differed for this task, a minimum of 30 responses was obtained for each individual.
Binomial z scores were calculated for each subject on the basis of the frequency of left- and right-hand use. Subjects with z scores > 1.95 or < −1.95 were classified as right- and left-handed, respectively. Subjects with z scores between −1.95 and 1.95 were classified as having no preference. In addition, a handedness index (HI) was derived for each subject by subtracting the number of left-handed responses from the number of right-handed responses and dividing by the total number of responses: HI = (R−L)/(R + L). Positive HI values reflect right-hand preference and negative values represent left-hand preference. The absolute value of HI corresponds to the consistency of directional hand preference. Of the 33 chimpanzees in the current study, five were classified as left-handed, three showed no preference and 25 were right-handed. Measurements of SY expression were performed blind to HI score and handedness classification of the subjects.
Tissue preparation and immunohistochemistry
Within 19 h of each subject’s death, the brain was removed and immersed in 10% formalin at necropsy. In most cases, the brain was transferred to 0.1 m phosphate-buffered saline (PBS) with 0.1% sodium azide solution after 10 days and stored at 4°C. Notably, SY protein concentrations, as measured by ELISA assay, have been shown to be unaffected by post-mortem intervals (PMI) up to 72 h in both human and rat cerebral cortex (Siew et al., 2004).
The region of hand representation in primary motor cortex was dissected from each hemisphere as a block approximately 2–4 cm thick containing the pre- and postcentral gyri. The location of hand representation was estimated based on the morphology of the hand knob of the precentral gyrus as described previously (Sherwood et al., 2007b). After dissection, tissue blocks were cryoprotected by immersion in buffered sucrose solutions up to 30%, embedded in Tissue-Tek medium, frozen in a slurry of dry ice and isopentane, and sectioned at 40 μm with a sliding microtome either perpendicular to the axis of the central sulcus or in the coronal plane. Every 10th section (i.e. 400 μm apart) was stained for Nissl substance with a solution of 0.5% cresyl violet to visualize the cytoarchitecture of primary motor cortex (Brodmann’s area 4).
Immunohistochemistry was performed on a 1 : 20 series of sections. Free-floating sections were stained with a rabbit polyclonal IgG1 antibody to the synthetic human SY peptide coupled to ovalbumin (A 0010; DakoCytomation, Ely, UK). The antibody was used at a dilution of either 1 : 50 or 1 : 100 depending on tissue preservation quality; the antibody dilution was always consistent within an individual for both hemispheres. When divided according to handedness categories, indices of asymmetry (see below) did not significantly differ between samples that were prepared with different antibody dilutions (independent-samples t-tests P > 0.3). Prior to immunostaining, sections were rinsed thoroughly in PBS and pretreated for antigen retrieval by incubation in 10 mm sodium citrate buffer (pH 3.5) at 37°C in an oven for 30 min. Sections were then immersed in a solution of 0.75% hydrogen peroxide in 75% methanol to eliminate endogenous peroxidase activity. After rinsing again, sections were incubated in the primary antibody diluted in PBS with 2% normal horse serum and 0.1% Triton X-100 detergent for approximately 48 h on a rotating table at 4°C. After rinsing in PBS, sections were incubated in biotinylated anti-rabbit IgG (Vector Laboratories, Burlingame, CA, USA; dilution 1 : 200) and processed with the avidin–biotin–peroxidase method using a Vectastain Elite ABC kit (Vector Laboratories). Immunoreactivity was revealed using 3,3′-diaminobenzidine and nickel enhancement. Specificity of the reaction was confirmed by processing negative control sections as described, excluding the primary antibody. No immunostaining was observed in control sections. Each set of sections from each hemisphere was stained together to control for inter-experiment variation.
To characterize further the specificity of SY immunoreactivity in these samples, we also performed double-labeling immunofluorescence experiments in four individuals using the SY antibody and a mouse monoclonal antibody against MAP2 (MAB3418, clone AP20; Chemicon International, Temecula, CA, USA; dilution 1 : 200). MAP2 protein is a component of neuronal microtubules, allowing visualization of neuron somata and dendrites. In these experiments, immunoreactivity was revealed using an Alexa Fluor 488 anti-rabbit secondary antibody to label SY and an Alexa Fluor 568 anti-mouse secondary antibody to label MAP2. Sudan Black B (1% in 70% ethanol) was used to quench the autofluorescence generated by lipofuscin pigments. Double-labeled tissue was imaged with a Bio-Rad MRC 1024 confocal laser scanning system coupled to an Olympus IX-70 microscope, using a 60 × objective lens and a slice thickness of 1.5 μm.
Paraformadehyde perfusion-fixed cerebral cortical tissue from macaque monkeys was also used in single and double-labeling experiments to evaluate the effect of the fixation method on staining quality. In general, the qualitative appearance of staining was similar in chimpanzees and perfused macaque monkey brains (data not shown).
Stereologic analyses
We identified the region of interest for quantitative measurements in adjacent Nissl-stained sections as primary motor cortex (Brodmann’s area 4) based on previous descriptions of the cytoarchitecture of this area in chimpanzees (Sherwood et al., 2007b). Quantification of the numerical density of SY-immunoractive (ir) puncta in layers I–VI was performed using a Zeiss Axioplan 2 photomicroscope equipped with a Ludl XY motorized stage, Heidenhain z-axis encoder, an Optronics MicroFire color videocamera, a Dell PC workstation and stereoinvestigator software (MBF Bioscience, Williston, VT, USA). We did not attempt to subdivide the cortex further into layers because they were not readily apparent on SY-stained sections (Fig. 1). Quantification of total SY-ir puncta was not feasible because distinct boundaries cannot be established to separate the hand representation from the motor representation of adjacent body parts. Therefore, SY-ir presynaptic puncta densities were estimated using the optical disector with fractionator sampling following methods described by Sherwood et al. (2007a,b). Beginning at a random starting point, three equidistantly spaced sections were quantified from each hemisphere. After outlining the boundaries of the cortex at low magnification, a set of optical disector frames (3 × 3 μm) were placed in a systematic random fashion with a scan grid size of 700 × 700 μm. Disector analysis was performed under Koehler illumination using a 100 × objective (Zeiss Plan-Apochromat, NA 1.4). Because of the limited depth of immunostaining penetration, the thickness of optical disectors was set to 1 μm, with a 1-μm guard zone at the top of the section. Synapse densities were derived from these stereologic counts and corrected for shrinkage from histological processing by the number-weighted mean section thickness. On average, the coefficient of error (Schmitz & Hof, 2000) of measurements was 0.07 ± 0.03 (SD). A second observer also measured a subset of ten randomly selected specimens to obtain an estimate of inter-rater reliability. The intraclass correlation coefficient (ICC) between the two observers indicated a high degree of agreement (ICC = 0.74, P = 0.03).
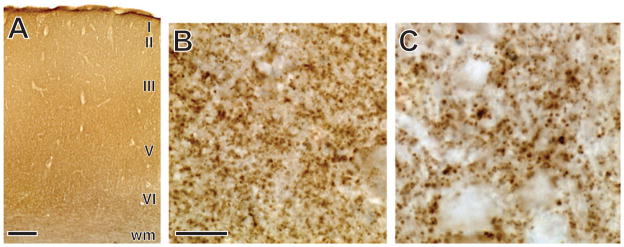
Fig. 1.
Immunostaining against SY in the primary motor cortex of a chimpanzee. The pattern of staining across all layers of the cortex is shown in A. Higher magnification views of SY-immunoreactive puncta in layer III are shown in B and C. Scale bar = 500 μm in panel A. Scale bar in panel B represents 40 μm in B and 20 μm in C. wm, white matter.
Western blotting
After determining the location of primary motor cortex based on cytoarchitecture, an adjacent section was selected for use in Western blot analyses. Using a scalpel, the gray matter was dissected, yielding approximately 0.075 mg of sample tissue.
Three different protocols were used to examine the protein expression level of SY in these formalin-fixed samples. In Protocol I, tissue was hand-homogenized in buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl and 1 mm EDTA) containing 0.1% Nonidet P-40, 0.1% Triton X-100, 1 mm PMSF and protease inhibitor cocktail tablets (Complete Mini, EDTA-free, Roche Diagnostics, Mannheim, Germany). Lysates were left on ice for 60 min and then sonicated twice by using the VibraCell Ultrasonic Processor (Sonics and Materials, Inc., Newton, CT, USA). Samples were kept on ice for at least 30 min between sonications. Protein concentrations were measured using the Bradford assay (Bio-Rad, Hercules, CA, USA). Laemmli sample buffer was added to the brain homogenate and the sample was boiled for 10 min.
In Protocol II, the sample preparation procedure was based on the principle of heat-induced antigen retrieval (HIAR) (Ikeda et al., 1998). Briefly, tissue was homogenized in 200 μL of radioimmunoprecipitation assay buffer (pH 7.6) containing 2% sodium dodecyl sulfate (SDS) and protease inhibitors, and the contents were incubated at 100°C for 20 min followed by incubation at 60°C for 2 h. The tissue lysates were then centrifuged at 15 000 g for 20 min at 4°C. Protein concentrations were measured with the DC protein assay (Bio-Rad) after detergent solubilization. Protein samples were diluted 1 : 1 in Laemmli sample buffer and boiled for 10 min.
In Protocol III, the sample preparation procedure was based on the HIAR strategy using Laemmli buffer containing SDS. Briefly, tissue homogenates were prepared as per Protocol I, Laemmli sample buffer was added to the brain homogenates and the samples were heated at 105°C for 20 min (Nirmalan et al., 2009). Protein estimation was carried out using the DC protein assay (Bio-Rad), with the bovine serum albumin standard set (Bio-Rad) used to generate standard curves.
Samples obtained from all protocols were analysed by Western blots with 4–12% SDS-polyacrylamide gel electrophoresis (SDS-PAGE). Primary antibodies that were used included anti-SY rabbit polyclonal antibody (1 : 300; AbCam, Cambridge, MA, USA), anti-α-synuclein mouse monoclonal antibody (1 : 300; BD Transduction Laboratories, San Jose, CA, USA), anti-synapsin I rabbit polyclonal antibody (1 : 300; AbCam), anti-MAP2 mouse monoclonal antibody (1 : 300; Chemicon International) and anti-β-tubulin rabbit polyclonal antibody (1 : 300; AbCam). Protein bands were scanned on an Epson Perfection 5000 Photo Scanner (Epson America, Long Beach, CA, USA) and quantitatively analysed by Scion Image (Scion Corp., Frederick, MD, USA). When reprobing was necessary, the membranes were stripped of bound antibodies by incubating in stripping buffer (Thermo Scientific, Waltham, MA, USA) at room temperature with agitation according to the manufacturer’s protocol.
Human prefrontal cortex samples were used as positive controls and were homogenized as indicated for the fixed chimpanzee samples and then analysed by SDS-PAGE and Western blot. The human control samples came from individuals who were neurologically normal on routine pathology examination, including one non-fixed, frozen sample (female, 78 years old at death, 8 h PMI) and seven formalin-fixed samples (three females and four males, 37–90 years old at death, 2–12 h PMI).
Normalization of gel loading between samples based on estimations of total protein was shown to be reliable when probed with Western blotting, but additionally, MAP2 and β-tubulin were also examined as internal loading controls. The correlation between SY protein levels when using β-tubulin and MAP2 as loading controls was high (r = 0.99, P < 0.001, n = 20).
To determine whether SY protein level parallels the expression of other presynaptic proteins, we also performed immunoblotting for synapsin I and α-synuclein in a subset of the total chimpanzee sample. Because these presynaptic proteins are implicated in regulation of neurotransmitter release and synapse formation, they share common functional properties with SY (Petersen et al., 1999). We found a significant correlation between the expression level of SY and synapsin I (r = 0.62, P = 0.02, n = 13) and between SY and α-synuclein (r = 0.69, P = 0.006, n = 14).
Data analysis
Hemispheric lateralization in SY-ir puncta density and SY protein expression level were calculated as an asymmetry quotient (AQ) using the formula: AQ = (R−L)/[(R + L) × 0.5]. Positive AQ values signify right hemisphere dominance, negative values signify left hemisphere dominance and zero denotes symmetry. The absolute value of the AQ indicates the degree of asymmetry. Because the AQ was the dependent variable in subsequent statistical tests, we assume that any interindividual differences in duration of fixation, storage conditions and PMI affected both hemispheres equally. The original SY-ir puncta densities used to calculate the AQs are given in Table S1.
Quantifiable Western blot results for SY were obtained from 21 subjects. Of these, one subject had results from only one protocol, nine subjects had results from two protocols, and 11 subjects had results from all three protocols. The correlation between AQs from the different protocols was r = 0.60, P = 0.05, n = 11 (Protocols I and II); r = 0.71, P = 0.03, n = 9 (Protocols I and III); and r = 0.60, P = 0.07, n = 10 (Protocols II and III). We used the average AQ from all available experiments in each subject for further analyses of SY protein expression level.
Non-parametric Spearman rank order correlations were used to examine associations between SY-ir puncta density and protein expression AQs with handedness because HI data were not normally distributed. Finally, SY-ir puncta density and protein expression AQs were also investigated using a two-way anova with handedness category and sex as factors. Statistical significance is reported at α = 0.05 (two-tailed).
Results
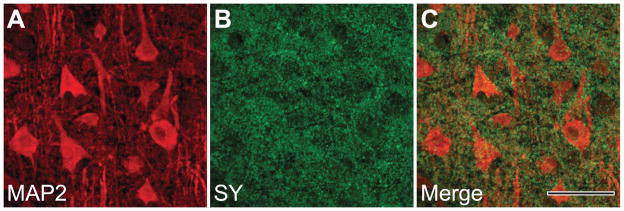
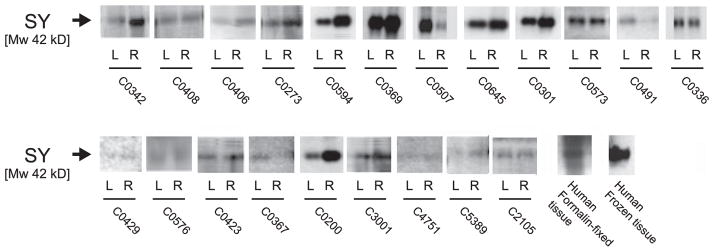
Immunohistochemistry against SY in primary motor cortex of chimpanzees yielded a distinct punctate staining pattern (Fig. 1). Double-immunofluorescence labeling against MAP2 demonstrated that SY-ir presynaptic puncta were most highly concentrated in the neuropil surrounding MAP2-labeled neuronal perikarya and dendrites (Fig. 2). Western blots of chimpanzee tissue homogenates showed SY immunolabeling at the expected molecular weight of 38 kDa (Fig. 3). Notably, the same molecular weight was also detected for SY in human frozen and formalin-fixed prefrontal cortex samples.
Fig. 2.
Double-labeling immunofluorescence against MAP2 (A) and synaptophysin (SY) (B) in layer III of the primary motor cortex of a chimpanzee, showing the pattern of punctate SY distribution that surrounds the MAP2-immunoreactive somata and dendrites (C). Scale bar = 50 μm.
Fig. 3.
Representative Western blots of SY expression in the primary motor cortex of chimpanzees. An equal amount of total protein was added to each lane. The SY-immunoreactive bands migrated to 38 kDa. Note that although there was inter-subject variation in the quality of immunoblots, asymmetry quotients were calculated as a within-subject index of the difference in protein expression between hemispheres.
SY-ir puncta density and protein expression AQs did not correlate with age at death (SY-ir puncta density AQ – Spearman’s rho: rs = 0.12, P = 0.53, n = 29; SY protein expression AQ – rs = 0.45, P = 0.07, n = 21) or with PMI (SY-ir puncta density AQ – rs = 0.04, P = 0.81, n = 33; SY protein expression AQ – rs = −0.23, P = 0.37, n = 17). In addition, there were no significant differences between data from wild-born vs. captive-born chimpanzees (SY-ir puncta density AQ – independent-samples t-test: t31 = 1.26, P = 0.91; SY protein expression AQ – t19 = 0.21, P = 0.84).
Population-level asymmetry
SY-ir puncta density was not significantly asymmetric at the population level (mean AQ = 0.078, t32 = 1.21, P = 0.24; Fig. 4). In contrast, SY protein expression showed population-level rightward asymmetry (mean AQ = 0.135, t20 = 2.43, P = 0.03). There was no significant correlation between SY-ir puncta density and protein expression AQs within subjects (rs = −0.35, P = 0.12, n = 21).
Fig. 4.
The correlation between handedness index on the tube task and SY-immunoreactive puncta density asymmetry quotient (A). Boxplots of the asymmetry quotients for SY-immunoreactive puncta density and SY protein expression level according to handedness category (B). Positive AQ and HI values indicate rightward asymmetry and negative values indicate leftward asymmetry. The box encompasses 75–25%, with the line indicating the 50% point. Whiskers indicate the maximum and minimum values, excluding outliers and extremes. Circles indicate outliers and asterisk indicates extreme. All data, including outliers and extremes, were used in the statistical analyses.
Relationships to handedness
Interindividual variation in SY-ir puncta density AQ exhibited a significant negative correlation with handedness index on the tube task (rs = −0.40, P = 0.02, n = 33; Fig. 4). However, there was no correlation between SY protein expression AQ and handedness index (rs = 0.15, P = 0.51, n = 21).
When each chimpanzee was categorized as either right-handed or non-right-handed (i.e. left-handed and no preference grouped together), a two-way anova of SY-ir puncta density AQ revealed a significant effect of handedness category (F1,29 = 4.15, P = 0.05), but no significant main effect of sex (F1,29 = 0.53, P = 0.47), and no interaction between sex and handedness (F1,29 = 1.49, P = 0.23). As a group, the right-handed chimpanzees had a nearly symmetrical density of SY-ir puncta (mean AQ = −0.003), whereas the non-right-handed individuals showed a rightward bias (mean AQ = 0.331). In contrast, the anova of SY protein expression AQ did not reveal any significant main effects or interactions (handedness: F1,17 = 0.88, P = 0.77; sex: F1,17 = 1.84, P = 0.19; handedness × sex: F1,17 = 0.66, P = 0.43).
Discussion
This study demonstrates that SY protein expression in the chimpanzee primary motor cortex is asymmetric at the population level and that the distribution of SY-ir puncta displays a correlation with handedness. In the context of previous data indicating that handedness in chimpanzees also correlates with asymmetry of the volume of the hand knob region of the precentral gyrus (Hopkins & Cantalupo, 2004) and parvalbumin-containing interneuron densities in primary motor cortex (Sherwood et al., 2007b), these findings support the conclusion that the neural underpinning of lateralized behavior involves multiple levels of anatomical asymmetry.
We found that SY protein expression level, as measured by Western blotting, tended to be greater in the right hemisphere primary motor cortex in chimpanzees, regardless of handedness. In contrast, the density of SY-ir puncta did not show significant laterality at the population level. Because comparable data on hemispheric asymmetry of synapse-associated proteins do not currently exist from other cortical regions and species, the functional significance of the observed population-level rightward bias in SY expression level is uncertain, especially considering that 76% of the chimpanzees in our study had a right-hand preference on the bimanual coordination task. Accordingly, these data suggest that the greater expression of SY protein in the right hemisphere may not be directly related to behavioral lateralization. Indeed, it is noteworthy that several studies in humans have shown that the primary motor cortex of the right hemisphere has a relatively higher resting and active motor threshold than the left, regardless of handedness (Netz et al., 1995; Baumer et al., 2007). Furthermore, other microstructural asymmetries in the neocortex of humans have been reported which favor the right hemisphere in the absence of a clear link to lateralized function. In particular, despite the fact that left-hemisphere-dominant language functions are mediated by the orofacial representation in the primary motor cortex (Salmelin & Sams, 2002), Scheibel et al. (1985) found that the length of proximal dendritic segments of supragranular pyramidal neurons was greater in the right hemisphere. Similarly, Anderson & Rutledge (1996) showed that supragranular pyramidal neurons in the posterior superior temporal gyrus (Wernicke’s area) of brains from ten human males displayed rightward asymmetry in a number of parameters, including total dendritic length and spine density. Finally, a previous study that included many of the same chimpanzee subjects as the current report found a significantly lower total neuron density in the right hemisphere of the primary motor at the population level (Sherwood et al., 2007b). Taken together, these data from the chimpanzee motor cortex suggest that there may be an inverse relationship between the volume occupied by neuronal somata and the expression of synaptic proteins. The functional implications of such asymmetries of cellular compartments, however, remain unclear.
We found that asymmetry in SY-ir puncta density was not correlated with asymmetry in SY protein levels. Because SY is involved in multiple functions at the synapse, including induction of synaptic vesicle formation, regulation of exocytosis and vesicle recycling (Valtorta et al., 2004), SY protein levels are related to a combination of dynamic factors such as the number of molecules per synaptic vesicle, the number of synaptic vesicles per bouton and the total density of synapses. SY protein is specifically located in the membrane of small round or flat synaptic vesicles, which are present in 95–100% of neocortical presynaptic terminals. Thus, variation in SY protein expression does not necessarily have a simple linear relationship to the number of synaptic contacts. In this context, it is interesting to note that Calhoun et al. (1996) compared Western blotting, stereology and optical density measurement for quantifying SY in subregions of the rat hippocampal formation and found that only stereologic counting of puncta was able to reveal greater synapse density in the dentate gyrus compared with the stratum oriens. Hence, the density of synapses may be more sensitive than SY protein expression as an indicator of specializations for the maintenance of complex motor behaviors as reflected in handedness (Kleim et al., 2004). Alternatively, the discrepancy between the immunohistochemical and immunoblotting results might stem from the fact that the Western blot analysis used total tissue homogenates, not the synaptic vesicular fraction in particular. Additionally, because we used different primary antibodies to detect SY in immunohistochemistry and Western blotting, it is possible that some antigenic sites were altered in these archival formalin-fixed materials, making the epitopes differentially recognized by the specific antibodies.
Despite the lack of population-level asymmetry of SY-ir puncta density among the subjects in our study, this measure showed a significant correlation with interindividual variability in hand preference. In general, right-handed chimpanzees had symmetrical densities of synaptic boutons, whereas the majority of left-handed and ambidextrous chimpanzees (i.e. seven of eight) had a greater density of SY-ir puncta in the right hemisphere. Congruent with these results, it is notable that a recent review of neuroimaging studies in humans concluded that right-hand dominance may not necessarily correspond with greater activation of the opposite hemisphere’s motor cortex representation (Hammond, 2002). Similarly, Cahn et al. (2003) found no difference between hemispheres in the excitability of primary motor cortex of humans based on transcranial magnetic stimulation. The contribution of interhemispheric inhibition may complicate a straightforward relationship between dominant hand recruitment and local cortical activity (Newton et al., 2005; Baumer et al., 2007). Our data are consistent with the possibility that such factors may mitigate enhanced levels of synaptogenesis in one hemisphere relative to the other in right-handed chimpanzees. The relatively increased numbers of SY-ir puncta in the contralateral hemisphere of non-right-handed chimpanzees, however, suggests that there is an interaction between synapse establishment in the motor cortex and an individual’s handedness, which remains to be explored further.
Viewed from this perspective, our results are consistent with other studies demonstrating that neocortical organization can be refined in development and adulthood through activity-dependent experience (Galvan & Weinberger, 2002; Svensson et al., 2003; Rosenkranz et al., 2007). Such plasticity may occur either through re-mapping territories of sensorimotor representations (Nudo et al., 1996; Schwenkreis et al., 2007; Driemeyer et al., 2008) or by more subtle changes in synaptic densities and strength (Niemann et al., 1991; Gandolfo et al., 2000; Rioult-Pedotti et al., 2000). Although the present study is the first to establish a relationship between synaptic asymmetry and known handedness in any primate species, previous research has demonstrated other structural correlates of hand preference in the primary motor cortex. For example, forelimb muscle representations were shown to be more extensive contralateral to the hand which was used for a skilled digit movement task in squirrel monkeys (Nudo et al., 1992). Asymmetry in the depth of the central sulcus has been shown to correlate with handedness in humans, chimpanzees and capuchin monkeys (Amunts et al., 2000; Phillips & Sherwood, 2005; Dadda et al., 2006). Additionally, in humans, greater fractional anisotropy in the white matter underlying the precentral gyrus characterizes the hemisphere contralateral to the preferred hand (Büchel et al., 2004).
Finally, our findings are significant because they challenge the assumption that synapse density is invariant in the neocortex due to biophysical constraints (Stevens, 1989; Abeles, 1991; Changizi, 2001). Although some empirical data appear to support the claim that synapse density is relatively constant in the neocortex (Stevens, 1989), other studies have reported significant variation across cortical areas, layers and species (e.g. humans, rats and mice; DeFelipe et al., 2002). Congruent with the notion that the organization of neocortical synapses shows functionally relevant variation, it has also been demonstrated that the spine density of pyramidal neurons differs among cytoarchitectonic areas in humans (Jacobs et al., 2001; Travis et al., 2005; Anderson et al., 2009) and macaques (Elston, 2000; Elston & Rockland, 2002; Elston et al., 2005), as well as among species (Elston et al., 2001; Ballesteros-Yañez et al., 2006). Synapse density, furthermore, differs between sexes in the temporal neocortex of humans (Alonso-Nanclares et al., 2008). Our data complement these studies in showing that synapse density asymmetry interacts with handedness in chimpanzees. It will be important to determine whether similar synaptic asymmetry, and its relationship to behavioral lateralization, also characterizes other species, including humans.
Supplementary Material
Acknowledgments
We thank Dr Anastas Popratiloff for assistance with confocal imaging and Drs Carol Fowler and David Evers for assistance with Western blotting techniques. Brains used in this study were provided by the University of Texas M.D. Anderson Cancer Center (U42-RR-015090), the Great Ape Aging Project (NIH grant AG14308), the Foundation for Comparative and Conservation Biology, Dr Larry Walker and Dr Todd M. Preuss. This work was supported by the National Science Foundation (BCS-0515484, BCS-0549117, BCS-0827531, DGE-0801634), the National Institutes of Health (NS42867) and the James S. McDonnell Foundation (22002078).
Abbreviations
- AQ
asymmetry quotient
- HI
handedness index
- HIAR
heat-induced antigen retrieval
- ir
immunoreactive
- PMI
post-mortem interval
- SDS
sodium dodecyl sulfate
- SY
synaptophysin
Footnotes
Additional supporting information may be found in the online version of this article: Table S1. SY-immunoraective puncta density data used to calculate the asymmetry quotient.
Please note: As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset by Wiley-Blackwell. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
References
- Abeles M. Corticonics: Neural Circuits of the Cerebral Cortex. Cambridge University Press; Cambridge: 1991. [Google Scholar]
- Alonso-Nanclares L, Gonzalez-Soriano J, Rodriguez JR, DeFelipe J. Gender differences in human cortical synaptic density. Proc Natl Acad Sci USA. 2008;105:14615–14619. doi: 10.1073/pnas.0803652105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K, Jancke L, Mohlberg H, Steinmetz H, Zilles K. Interhemispheric asymmetry of the human motor cortex related to handedness and gender. Neuropsychologia. 2000;38:304–312. doi: 10.1016/s0028-3932(99)00075-5. [DOI] [PubMed] [Google Scholar]
- Anderson B, Rutledge V. Age and hemisphere effects on dendritic structure. Brain. 1996;119:1983–1990. doi: 10.1093/brain/119.6.1983. [DOI] [PubMed] [Google Scholar]
- Anderson K, Bones B, Robinson B, Hass C, Lee H, Ford K, Roberts TA, Jacobs B. The morphology of supragranular pyramidal neurons in the human insular cortex: a quantitative Golgi study. Cereb Cortex. 2009;19:2131–2144. doi: 10.1093/cercor/bhn234. [DOI] [PubMed] [Google Scholar]
- Ballesteros-Yañez I, Benavides-Piccione R, Elston GN, Yuste R, DeFelipe J. Density and morphology of dendritic spines in mouse neocortex. Neuroscience. 2006;138:403–409. doi: 10.1016/j.neuroscience.2005.11.038. [DOI] [PubMed] [Google Scholar]
- Baumer T, Dammann E, Bock F, Kloppel S, Siebner HR, Munchau A. Laterality of interhemispheric inhibition depends on handedness. Exp Brain Res. 2007;180:195–203. doi: 10.1007/s00221-007-0866-7. [DOI] [PubMed] [Google Scholar]
- Büchel C, Raedler T, Sommer M, Sach M, Weiller C, Koch MA. White matter asymmetry in the human brain: a diffusion tensor MRI study. Cereb Cortex. 2004;14:945–951. doi: 10.1093/cercor/bhh055. [DOI] [PubMed] [Google Scholar]
- Cahn SD, Herzog AG, Pascual-Leone A. Paired-pulse transcranial magnetic stimulation: effects of hemispheric laterality, gender, and handedness in normal controls. J Clin Neurophysiol. 2003;20:371–374. doi: 10.1097/00004691-200309000-00009. [DOI] [PubMed] [Google Scholar]
- Calhoun ME, Jucker M, Martin LJ, Thinakaran G, Price DL, Mouton PR. Comparative evaluation of synaptophysin-based methods for quantification of synapses. J Neurocytol. 1996;25:821–828. doi: 10.1007/BF02284844. [DOI] [PubMed] [Google Scholar]
- Changizi MA. Principles underlying mammalian neocortical scaling. Biol Cybern. 2001;84:207–215. doi: 10.1007/s004220000205. [DOI] [PubMed] [Google Scholar]
- Corballis MC. Cerebral asymmetry and human uniqueness. In: Hopkins WD, editor. The Evolution of Hemispheric Specialization in Primates: Special Topics in Primatology. Vol. 5. Academic Press; Oxford: 2007. pp. 1–21. [Google Scholar]
- Dadda M, Cantalupo C, Hopkins WD. Further evidence of an association between handedness and neuroanatomical asymmetries in the primary motor cortex of chimpanzees (Pan troglodytes) Neuropsychologia. 2006;44:2582–2586. doi: 10.1016/j.neuropsychologia.2006.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J, Alonso-Nanclares L, Arellano JI. Microstructure of the neocortex: comparative aspects. J Neurocytol. 2002;31:299–316. doi: 10.1023/a:1024130211265. [DOI] [PubMed] [Google Scholar]
- Derksen MJ, Ward NL, Hartle KD, Ivanco TL. MAP2 and synaptophysin protein expression following motor learning suggests dynamic regulation and distinct alterations coinciding with synaptogenesis. Neurobiol Learn Mem. 2007;87:404–415. doi: 10.1016/j.nlm.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Driemeyer J, Boyke J, Gaser C, Buchel C, May A. Changes in gray matter induced by learning—revisited. PLoS ONE. 2008;3:e2669. doi: 10.1371/journal.pone.0002669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood SL, Weickert CS, Webster MJ, Herman MM, Kleinman JE, Harrison PJ. Synaptophysin protein and mRNA expression in the human hippocampal formation from birth to old age. Hippocampus. 2006;16:645–654. doi: 10.1002/hipo.20194. [DOI] [PubMed] [Google Scholar]
- Elston GN. Pyramidal cells of the frontal lobe: all the more spinous to think with. J Neurosci. 2000;20:RC95. doi: 10.1523/JNEUROSCI.20-18-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elston GN, Rockland KS. The pyramidal cell of the sensorimotor cortex of the macaque monkey: phenotypic variation. Cereb Cortex. 2002;12:1071–1078. doi: 10.1093/cercor/12.10.1071. [DOI] [PubMed] [Google Scholar]
- Elston GN, Benavides-Piccione R, DeFelipe J. The pyramidal cell in cognition: a comparative study in human and monkey. J Neurosci. 2001;21:RC163. doi: 10.1523/JNEUROSCI.21-17-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elston GN, Benavides-Piccione R, DeFelipe J. A study of pyramidal cell structure in the cingulate cortex of the macaque monkey with comparative notes on inferotemporal and primary visual cortex. Cereb Cortex. 2005;15:64–73. doi: 10.1093/cercor/bhh109. [DOI] [PubMed] [Google Scholar]
- Galvan V, Weinberger N. Long-term consolidation and retention of learning-induced tuning plasticity in the auditory cortex of the guinea pig. Neurobiol Learn Mem. 2002;77:78–108. doi: 10.1006/nlme.2001.4044. [DOI] [PubMed] [Google Scholar]
- Gandolfo F, Li C, Benda BJ, Schioppa CP, Bizzi E. Cortical correlates of learning in monkeys adapting to a new dynamical environment. Proc Natl Acad Sci USA. 2000;97:2259–2263. doi: 10.1073/pnas.040567097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz LA, Gilmore JH, Hamer RM, Lieberman JA, Jarskog LF. Synaptophysin and postsynaptic density protein 95 in the human prefrontal cortex from mid-gestation into early adulthood. Neuroscience. 2007;149:582–591. doi: 10.1016/j.neuroscience.2007.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough WT, Chang F-L. Plasticity of synapse structure and pattern in the cerebral cortex. In: Peters A, Jones EG, editors. Cerebral Cortex: Development and Maturation of Cerebral Cortex. Plenum Press; New York: 1988. pp. 391–440. [Google Scholar]
- Hammond G. Correlates of human handedness in primary motor cortex: a review and hypothesis. Neurosci Biobehav Rev. 2002;26:285–292. doi: 10.1016/s0149-7634(02)00003-9. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Hand preferences for a coordinated bimanual task in 110 chimpanzees (Pan troglodytes): cross-sectional analysis. J Comp Psychol. 1995;109:291–297. doi: 10.1037/0735-7036.109.3.291. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Hemispheric specialization in chimpanzees: evolution of hand and brain. In: Platek SM, Keenan JP, Shackelford T, editors. Evolutionary Cognitive Neuroscience. MIT Press; Boston: 2010. pp. 95–120. [Google Scholar]
- Hopkins WD, Cantalupo C. Handedness in chimpanzees (Pan troglodytes) is associated with asymmetries of the primary motor cortex but not with homologous language areas. Behav Neurosci. 2004;118:1176–1183. doi: 10.1037/0735-7044.118.6.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Fernanadez-Carriba S, Wesley MJ, Hostetter A, Pilcher D, Poss S. The use of bouts and frequencies in the evaluation of hand preferences for a coordinated bimanual task in chimpanzees (Pan troglodytes): an empirical study comparing two different indices of laterality. J Comp Psychol. 2001;115:294–299. doi: 10.1037//0735-7036.115.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Wesley MJ, Izard MK, Hook M, Schapiro SJ. Chimpanzees (Pan troglodytes) are predominantly right-handed: replication in three populations of apes. Behav Neurosci. 2004;118:659–663. doi: 10.1037/0735-7044.118.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Russell JL, Lambeth S, Schapiro SJ. Handedness and neuroanatomical asymmetry in captive chimpanzees: a summary of 15 years of research. In: Hopkins WD, editor. The Evolution of Hemispheric Specialization in Primates: Special Topics in Primatology. Vol. 5. Academic Press; Oxford: 2007. pp. 147–181. [Google Scholar]
- Ikeda K, Monden T, Kanoh T, Tsujie M, Izawa H, Haba A, Ohnishi T, Sekimoto M, Tomita N, Shioaki H, Monden M. Extraction and analysis of diagnostically useful proteins from formalin-fixed, paraffin-embedded tissue sections. J Histochem Cytochem. 1998;46:397–404. doi: 10.1177/002215549804600314. [DOI] [PubMed] [Google Scholar]
- Jacobs B, Schall M, Prather M, Kapler E, Driscoll L, Baca S, Jacobs J, Ford K, Wainwright M, Treml M. Regional dendritic and spine variation in human cerebral cortex: a quantitative Golgi study. Cereb Cortex. 2001;11:558–571. doi: 10.1093/cercor/11.6.558. [DOI] [PubMed] [Google Scholar]
- Jones TA, Chu CJ, Grande LA, Gregory AD. Motor skills training enhances lesion-induced structural plasticity in the motor cortex of adult rats. J Neurosci. 1999;19:10153–10163. doi: 10.1523/JNEUROSCI.19-22-10153.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim JA, Lussnig E, Schwarz ER, Comery TA, Greenough WT. Synaptogenesis and Fos expression in the motor cortex of the adult rat after motor skill learning. J Neurosci. 1996;16:4529–4535. doi: 10.1523/JNEUROSCI.16-14-04529.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim JA, Barbay S, Nudo RJ. Functional reorganization of the rat motor cortex following motor skill learning. J Neurophysiol. 1998;80:3321–3325. doi: 10.1152/jn.1998.80.6.3321. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Hogg TM, VandenBerg PM, Cooper NR, Bruneau R, Remple M. Cortical synaptogenesis and motor map reorganization occur during late, but not early, phase of motor skill learning. J Neurosci. 2004;24:628–633. doi: 10.1523/JNEUROSCI.3440-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Reinprecht I, Fahnestock M, Racine RJ. Activity-dependent changes in synaptophysin immunoreactivity in hippocampus, piriform cortex, and entorhinal cortex of the rat. Neuroscience. 2002;115:1221–1229. doi: 10.1016/s0306-4522(02)00485-2. [DOI] [PubMed] [Google Scholar]
- Netz J, Ziemann U, Homberg V. Hemispheric asymmetry of transcallosal inhibition in man. Exp Brain Res. 1995;104:527–533. doi: 10.1007/BF00231987. [DOI] [PubMed] [Google Scholar]
- Newton JM, Sunderland A, Gowland PA. fMRI signal decreases in ipsilateral primary motor cortex during unilateral hand movements are related to duration and side of movement. NeuroImage. 2005;24:1080–1087. doi: 10.1016/j.neuroimage.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Niemann J, Winker T, Gerling J, Landwehrmeyer B, Jung R. Changes of slow cortical negative DC-potentials during the acquisition of a complex finger motor task. Exp Brain Res. 1991;85:417–422. doi: 10.1007/BF00229418. [DOI] [PubMed] [Google Scholar]
- Nirmalan NJ, Harnden P, Selby PJ, Banks RE. Development and validation of a novel protein extraction methodology for quantitation of protein expression in formalin-fixed paraffin-embedded tissues using western blotting. J Pathol. 2009;217:497–506. doi: 10.1002/path.2504. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Jenkins WM, Merzenich MM, Prejean T, Grenda R. Neurophysiological correlates of hand preference in primary motor cortex of adult squirrel monkeys. J Neurosci. 1992;12:2918–2947. doi: 10.1523/JNEUROSCI.12-08-02918.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci. 1996;16:785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen K, Olesen OF, Mikkelsen JD. Developmental expression of alpha-synuclein in rat hippocampus and cerebral cortex. Neuroscience. 1999;91:651–659. doi: 10.1016/s0306-4522(98)00596-x. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Sherwood CC. Primary motor cortex asymmetry is correlated with handedness in capuchin monkeys (Cebus apella) Behav Neurosci. 2005;119:1701–1704. doi: 10.1037/0735-7044.119.6.1701. [DOI] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science. 2000;290:533–536. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- Rogers LJ, Andrew RJ. Comparative Vertebrate Lateralization. Cambridge University Press; Cambridge: 2002. [Google Scholar]
- Rosenkranz K, Kacar A, Rothwell JC. Differential modulationm of motor cortical plasticity and excitabilty in early and late phases of human motor learning. J Neurosci. 2007;27:12058–12066. doi: 10.1523/JNEUROSCI.2663-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmelin R, Sams M. Motor cortex involvement during verbal versus non-verbal lip and tongue movements. Hum Brain Mapp. 2002;16:81–91. doi: 10.1002/hbm.10031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibel AB, Paul LA, Fried I, Forsythe AB, Tomiyasu U, Wechsler A, Kao A, Slotnick J. Dendritic organization of the anterior speech area. Exp Neurol. 1985;87:109–117. doi: 10.1016/0014-4886(85)90137-2. [DOI] [PubMed] [Google Scholar]
- Schmitz C, Hof PR. Recommendations for straightforward and rigorous methods of counting neurons based on a computer simulation approach. J Chem Neuroanat. 2000;20:93–114. doi: 10.1016/s0891-0618(00)00066-1. [DOI] [PubMed] [Google Scholar]
- Schwenkreis P, El Tom S, Ragert P, Pleger B, Tegenthoff M, Dinse HR. Assessment of sensorimotor cortical representation asymmetries and motor skills in violin players. Eur J Neurosci. 2007;26:3291–3302. doi: 10.1111/j.1460-9568.2007.05894.x. [DOI] [PubMed] [Google Scholar]
- Sherwood CC, Raghanti MA, Stimpson CD, Bonar CJ, de Sousa AA, Preuss TM, Hof PR. Scaling of inhibitory interneurons in areas V1 and V2 of anthropoid primates as revealed by calcium-binding protein immunohistochemistry. Brain Behav Evol. 2007a;69:176–195. doi: 10.1159/000096986. [DOI] [PubMed] [Google Scholar]
- Sherwood CC, Wahl E, Erwin JM, Hof PR, Hopkins WD. Histological asymmetries of primary motor cortex predict handedness in chimpanzees (Pan troglodytes) J Comp Neurol. 2007b;503:525–537. doi: 10.1002/cne.21399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood CC, Subiaul F, Zawidzki TW. A natural history of the human mind: tracing evolutionary changes in brain and cognition. J Anat. 2008;212:426–454. doi: 10.1111/j.1469-7580.2008.00868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siew LK, Love S, Dawbarn D, Wilcock GK, Allen SJ. Measurement of pre- and post-synaptic proteins in cerebral cortex: effects of post-mortem delay. J Neurosci Methods. 2004;139:153–159. doi: 10.1016/j.jneumeth.2004.04.020. [DOI] [PubMed] [Google Scholar]
- Stevens CF. How cortical interconnectedness varies with network size. Neural Comput. 1989;1:473–479. [Google Scholar]
- Svensson P, Romaniello A, Wang K, Arendt-Nielsen L, Sessle BJ. Plasticity in corticomotor control of the human tongue musculature induced by tongue-task training. Exp Brain Res. 2003;152:42–51. doi: 10.1007/s00221-003-1517-2. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM. Mapping brain asymmetry. Nat Rev Neurosci. 2003;4:37–48. doi: 10.1038/nrn1009. [DOI] [PubMed] [Google Scholar]
- Travis K, Ford K, Jacobs B. Regional dendritic variation in neonatal human cortex: a quantitative Golgi study. Dev Neurosci. 2005;27:277–287. doi: 10.1159/000086707. [DOI] [PubMed] [Google Scholar]
- Valtorta F, Pennuto M, Bonanomi D, Benfenati F. Synaptophysin: leading actor or walk-on role in synaptic vesicle exocytosis? Bioessays. 2004;26:445–453. doi: 10.1002/bies.20012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.